Malignant Phyllodes Tumor of the Breast: A Practice Review
Abstract
1. Introduction
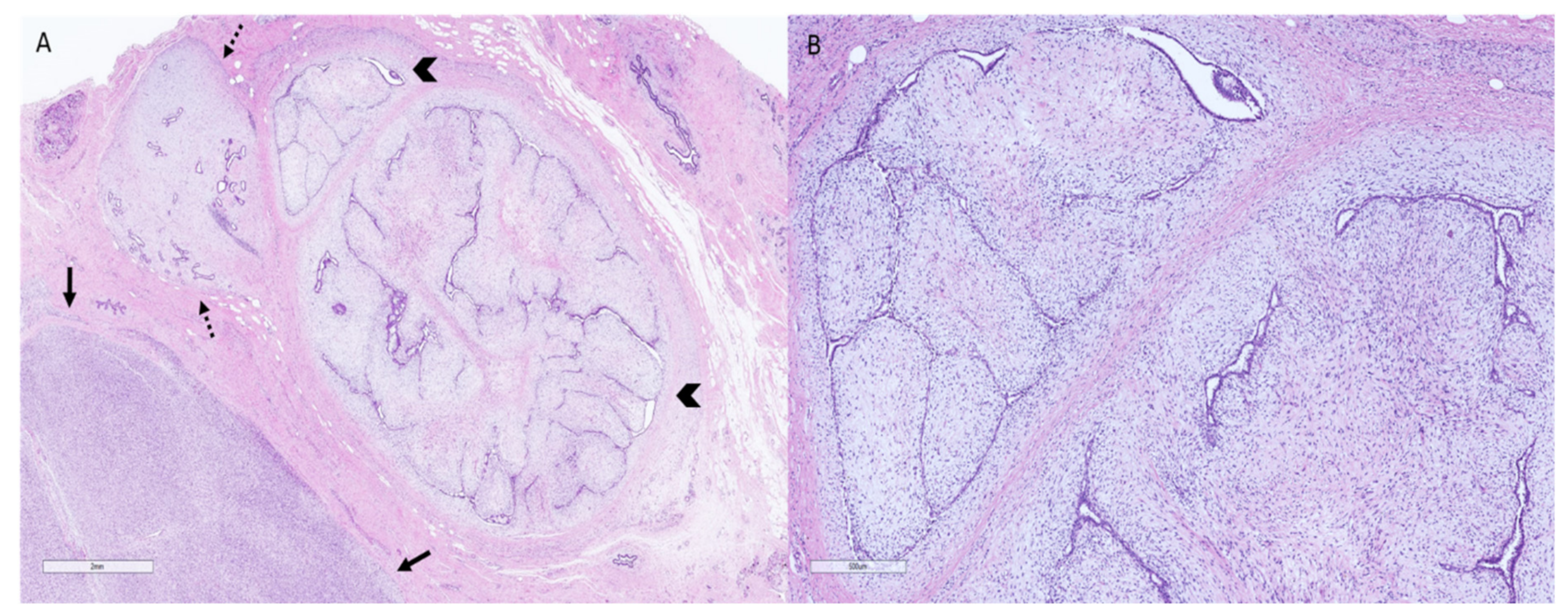
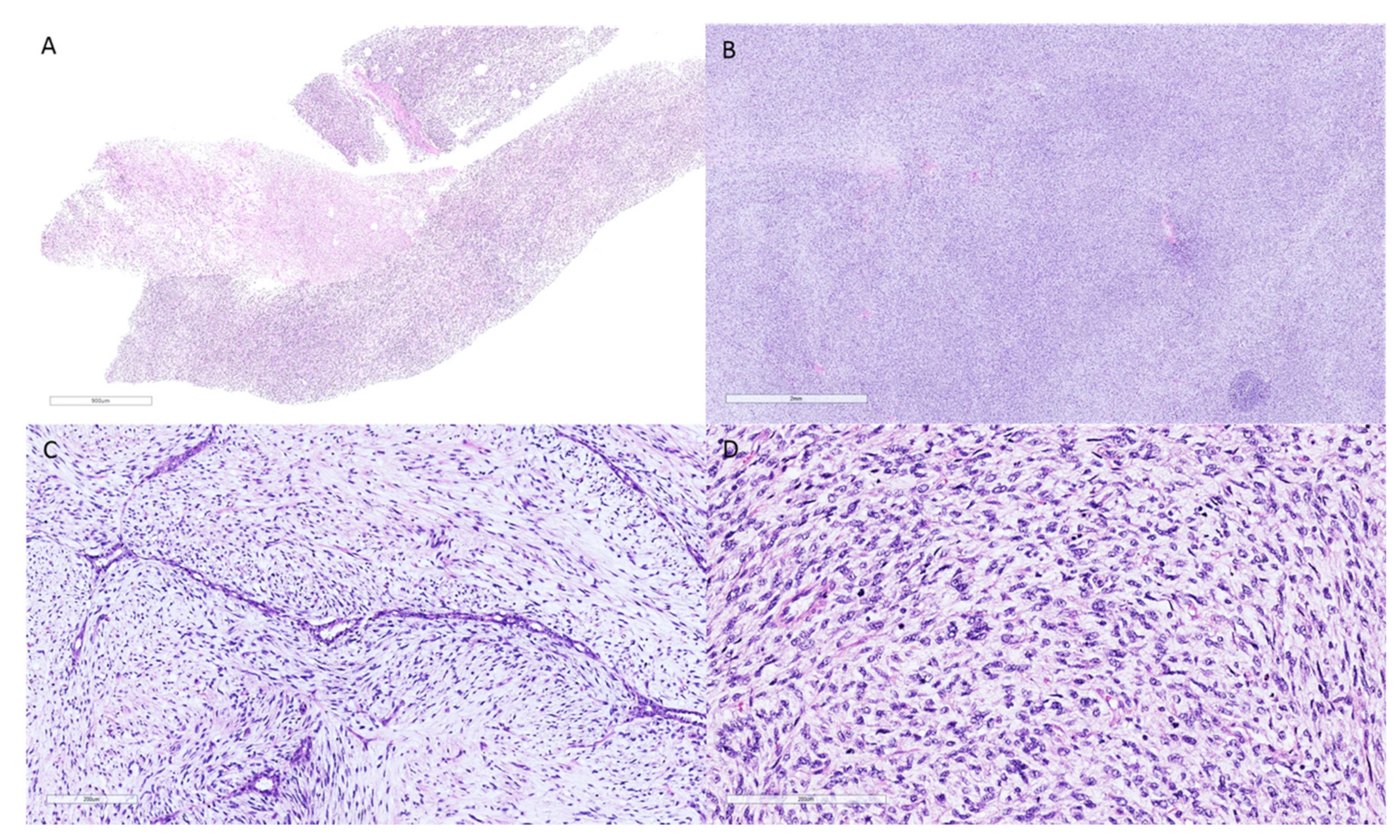
2. Phyllodes Tumors—Pathologic Features
3. Phyllodes Tumors—Pathogenesis and Molecular Findings
4. Hereditary Genetic Features
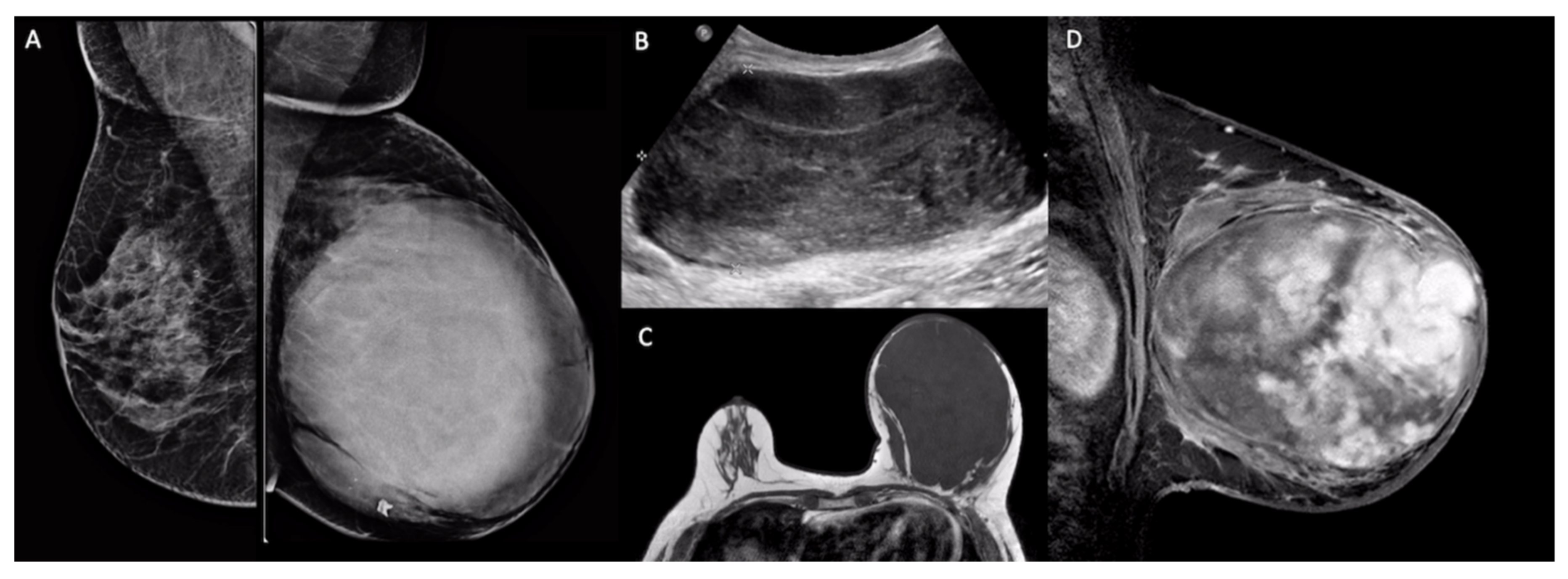
5. Imaging Findings
6. Clinical Findings
7. Surgical Treatment
8. Adjuvant Radiotherapy in Malignant Phyllodes Tumors
9. Chemotherapy in Early Stage and Metastatic Disease
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Kleer, C.G. Phyllodes Tumor of the Breast: Histopathologic Features, Differential Diagnosis, and Molecular/Genetic Updates. Arch. Pathol. Lab. Med. 2016, 140, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Publication of the WHO Classification of Tumours, 5th ed.; Volume 2: Breast Tumours—IARC. Available online: https://www.iarc.fr/news-events/who-classification-of-tumours-5th-edition-volume-2-breast-tumours/ (accessed on 23 March 2021).
- Tan, B.Y.; Acs, G.; Apple, S.K.; Badve, S.; Bleiweiss, I.J.; Brogi, E.; Calvo, J.P.; Dabbs, D.J.; Ellis, I.O.; Eusebi, V.; et al. Phyllodes tumours of the breast: A consensus review. Histopathology 2016, 68, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.; Thike, A.A.; Cheok, P.Y.; Chong, L.Y.-Z.; Tse, G.M.-K.; Tan, P.H. Stromal keratin expression in phyllodes tumours of the breast: A comparison with other spindle cell breast lesions. J. Clin. Pathol. 2012, 65, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Cimino-Mathews, A.; Sharma, R.; Illei, P.B.; Vang, R.; Argani, P. A subset of malignant phyllodes tumors express p63 and p40: A diagnostic pitfall in breast core needle biopsies. Am. J. Surg. Pathol. 2014, 38, 1689. [Google Scholar] [CrossRef]
- Md Nasir, N.D.; Ng, C.C.Y.; Rajasegaran, V.; Wong, S.F.; Liu, W.; Ng, G.X.P.; Lee, J.Y.; Guan, P.; Lim, J.Q.; Thike, A.A.; et al. Genomic characterisation of breast fibroepithelial lesions in an international cohort. J. Pathol. 2019, 249, 447–460. [Google Scholar] [CrossRef]
- Pareja, F.; Geyer, F.C.; Kumar, R.; Selenica, P.; Piscuoglio, S.; Ng, C.K.Y.; Burke, K.A.; Edelweiss, M.; Murray, M.P.; Brogi, E.; et al. Phyllodes tumors with and without fibroadenoma-like areas display distinct genomic features and may evolve through distinct pathways. NPJ Breast Cancer 2017, 3, 40. [Google Scholar] [CrossRef]
- Kuijper, A.; Snijders, A.M.; Berns, E.M.; Kuenen-Boumeester, V.; Van Der Wall, E.; Albertson, D.G.; Van Diest, P.J. Genomic profiling by array comparative genomic hybridization reveals novel DNA copy number changes in breast phyllodes tumours. Cell. Oncol. Off. J. Int. Soc. Cell. Oncol. 2009, 31, 31–39. [Google Scholar]
- Jones, A.M.; Mitter, R.; Springall, R.; Graham, T.; Winter, E.; Gillett, C.; Hanby, A.M.; Tomlinson, I.P.M.; Sawyer, E.J. A comprehensive genetic profile of phyllodes tumours of the breast detects important mutations, intra-tumoral genetic heterogeneity and new genetic changes on recurrence. J. Pathol. 2008, 214, 533–544. [Google Scholar] [CrossRef]
- Fortarezza, F.; Pezzuto, F.; Cazzato, G.; Punzo, C.; D’Amati, A.; Lettini, T.; Gentile, M.; Buonadonna, A.L.; Mariano, M.; Pezzolla, A.; et al. Bilateral Phyllodes Giant Tumor. A Case Report Analyzed by Array-CGH. Diagnostics 2020, 10, 825. [Google Scholar] [CrossRef]
- Garcia-Dios, D.A.; Levi, D.; Shah, V.; Gillett, C.; Simpson, M.A.; Hanby, A.; Tomlinson, I.; Sawyer, E.J. MED12, TERT promoter and RBM15 mutations in primary and recurrent phyllodes tumours. Br. J. Cancer 2018, 118, 277–284. [Google Scholar] [CrossRef]
- Yeong, J.; Thike, A.A.; Ng, C.C.Y.; Nasir, N.D.M.; Loh, K.; Teh, B.T.; Tan, P.H. A genetic mutation panel for differentiating malignant phyllodes tumour from metaplastic breast carcinoma. Pathology 2017, 49, 786–789. [Google Scholar] [CrossRef]
- Kersting, C.; Kuijper, A.; Schmidt, H.; Packeisen, J.; Liedtke, C.; Tidow, N.; Gustmann, C.; Hinrichs, B.; Wülfing, P.; Tio, J.; et al. Amplifications of the epidermal growth factor receptor gene (egfr) are common in phyllodes tumors of the breast and are associated with tumor progression. Lab. Investig. 2005, 86, 54–61. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Joseph, N.M.; Ravindranathan, A.; Stohr, B.A.; Greenland, N.Y.; Vohra, P.; Hosfield, E.; Yeh, I.; Talevich, E.; Onodera, C.; et al. Genomic profiling of malignant phyllodes tumors reveals aberrations in FGFR1 and PI-3 kinase/RAS signaling pathways and provides insights into intratumoral heter-ogeneity. Mod. Pathol. 2016, 29, 1012–1027. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Yu, J.H.; Nam, S.J.; Kim, S.W.; Lee, S.K.; Park, W.-Y.; Noh, D.-Y.; Nam, D.-H.; Park, Y.H.; Han, W.; et al. Genetic and Clinical Characteristics of Phyllodes Tumors of the Breast. Transl. Oncol. 2018, 11, 18–23. [Google Scholar] [CrossRef]
- Cani, A.K.; Hovelson, D.H.; McDaniel, A.S.; Sadis, S.; Haller, M.J.; Yadati, V.; Amin, A.M.; Bratley, J.; Bandla, S.; Williams, P.D.; et al. Next-Gen Sequencing Exposes Frequent MED12 Mutations and Actionable Therapeutic Targets in Phyllodes Tumors. Mol. Cancer Res. 2015, 13, 613–619. [Google Scholar] [CrossRef]
- Bougeard, G.; Renaux-Petel, M.; Flaman, J.-M.; Charbonnier, C.; Fermey, P.; Belotti, M.; Gauthier-Villars, M.; Stoppa-Lyonnet, D.; Consolino, E.; Brugières, L.; et al. Revisiting Li-Fraumeni Syndrome From TP53 Mutation Carriers. J. Clin. Oncol. 2015, 33, 2345–2352. [Google Scholar] [CrossRef]
- Mai, P.L.; Malkin, D.; Garber, J.E.; Schiffman, J.D.; Weitzel, J.N.; Strong, L.C.; Wyss, O.; Locke, L.; Means, V.; Achatz, M.I.; et al. Li-Fraumeni syndrome: Report of a clinical research workshop and creation of a research consortium. Cancer Genet. 2012, 205, 479–487. [Google Scholar] [CrossRef]
- Birch, J.M.; Alston, R.D.; McNally, R.J.Q.; Evans, D.G.R.; Kelsey, A.M.; Harris, M.; Eden, O.B.; Varley, J.M. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene 2001, 20, 4621–4628. [Google Scholar] [CrossRef]
- Giacomazzi, J.; Koehler-Santos, P.; Palmero, E.I.; Graudenz, M.S.; Rivero, L.F.; Lima, E.; Pütten, A.C.K.; Hainaut, P.; Camey, S.A.; Michelli, R.D.; et al. A TP53 founder mutation, p.R337H, is associated with phyllodes breast tumors in Brazil. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 2013, 463, 17–22. [Google Scholar] [CrossRef]
- Pinto, E.M.; Figueiredo, B.C.; Chen, W.; Galvao, H.C.; Formiga, M.N.; Fragoso, M.C.B.; Ashton-Prolla, P.; Ribeiro, E.M.; Felix, G.; Costa, T.E.; et al. XAF1 as a modifier of p53 function and cancer susceptibility. Sci. Adv. 2020, 6, eaba3231. [Google Scholar] [CrossRef]
- Rhiem, K.; Flucke, U.; Engel, C.; Wappenschmidt, B.; Reinecke-Lüthge, A.; Büttner, R.; Schmutzler, R.K. Association of the BRCA1 missense variant R1699W with a malignant phyllodes tumor of the breast. Cancer Genet. Cytogenet. 2007, 176, 76–79. [Google Scholar] [CrossRef]
- Shearer, D.D.; Askeland, R.W.; Park, J.M.; Fajardo, L.L.; Yang, L. Malignant phyllodes tumor in a patient with hereditary retinoblastoma: A case report and literature review. Proc. Obstet. Gynecol. 2012, 3, 1–9. [Google Scholar] [CrossRef]
- Kazmi, S.; Wagner, S.; Heintzelman, R.; Corbman, M. Malignant phyllodes tumor in Lynch syndrome: A case report. J. Med. Case Rep. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Mitus, J.; Adamczyk, A.; Majchrzyk, K.; Kowalik, A.; Ryś, J.; Niemiec, J. Comparison of mutation profile between primary phyllodes tumors of the breast and their paired local recurrences. Pol. J. Pathol. 2020, 71, 7–12. [Google Scholar] [CrossRef]
- Rosenberger, L.H.; Thomas, S.M.; Nimbkar, S.N.; Hieken, T.J.; Ludwig, K.K.; Jacobs, L.K.; Miller, M.E.; Gallagher, K.K.; Wong, J.; Neuman, J.B.; et al. Germline Genetic Mutations in a Mul-ti-center Contemporary Cohort of 550 Phyllodes Tumors: An Opportunity for Expanded Multi-gene Panel Testing. Ann. Surg. Oncol. 2020, 27, 3633–3640. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, S.; Liu, H.; Peng, W.; Li, R.; Gu, Y.; Wang, X.; Mao, J.; Shen, X. Imaging findings in phyllodes tumors of the breast. Eur. J. Radiol. 2012, 81, e62–e69. [Google Scholar] [CrossRef]
- Yabuuchi, H.; Soeda, H.; Matsuo, Y.; Okafuji, T.; Eguchi, T.; Sakai, S.; Kuroki, S.; Tokunaga, E.; Ohno, S.; Nishiyama, K.; et al. Phyllodes Tumor of the Breast: Correlation between MR Findings and Histologic Grade. Radiology 2006, 241, 702–709. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, W.-J.; Kong, Q.-C.; Liang, Y.-Y.; Han, X.-R.; Zheng, B.-J.; Sun, L.; Wei, X.-H.; Jin, Z.; Liu, C.-L. Can whole-tumor apparent diffusion coefficient histogram analysis be helpful to evaluate breast phyllode tumor grades? Eur. J. Radiol. 2019, 114, 25–31. [Google Scholar] [CrossRef]
- Bernstein, L.; Deapen, D.; Ross, R.K. The descriptive epidemiology of malignant cystosarcoma phyllodes tumors of the breast. Cancer 1993, 71, 3020–3024. [Google Scholar] [CrossRef]
- Reinfuss, M.; Mituś, J.; Duda, K.; Stelmach, A.; Ryś, J.; Smolak, K. The treatment and prognosis of patients with phyllodes tumor of the breast: An analysis of 170 cases. Cancer 1996, 77, 910–916. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Breast Cancer (Version 2.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 23 March 2021).
- Tan, P.H.; Thike, A.A.; Tan, W.J.; Thu, M.M.M.; Busmanis, I.; Li, H.; Chay, W.Y.; Tan, M.; Singapore, T.P.T.N. Predicting clinical behaviour of breast phyllodes tumours: A nomogram based on histological criteria and surgical margins. J. Clin. Pathol. 2011, 65, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, O.K.; Lee, C.M.; Tward, J.D.; Chappel, C.D.; Gaffney, D.K. Malignant phyllodes tumor of the female breast: Association of primary therapy with cause-specific survival from the Surveillance, Epidemiology, and End Results (SEER) program. Cancer 2006, 107, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Cheng, S.-P.; Tzen, C.-Y.; Yang, T.-L.; Jeng, K.-S.; Liu, C.-L.; Liu, T.-P. Surgical treatment of phyllodes tumors of the breast: Retrospective review of 172 cases. J. Surg. Oncol. 2005, 91, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, Y.; Zhu, L.; Cartwright, P.; Song, E.; Jacobs, L.; Chen, K. Local Recurrence of Benign, Borderline, and Malignant Phyllodes Tumors of the Breast: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2019, 26, 1263–1275. [Google Scholar] [CrossRef]
- Spanheimer, P.M.; Do, M.P.M.; Ms, E.C.Z.; Stempel, M.; Morrow, M.; Van Zee, K.J.; Barrio, A.V. Long-Term Outcomes After Surgical Treatment of Malignant/Borderline Phyllodes Tumors of the Breast. Ann. Surg. Oncol. 2019, 26, 2136–2143. [Google Scholar] [CrossRef]
- Barth, R.J., Jr.; Wells, W.A.; Mitchell, S.E.; Cole, B.F. A Prospective, Multi-Institutional Study of Adjuvant Radiotherapy After Resection of Malignant Phyllodes Tumors. Ann. Surg. Oncol. 2009, 16, 2288–2294. [Google Scholar] [CrossRef]
- Gnerlich, J.L.; Williams, R.T.; Yao, K.; Jaskowiak, N.; Kulkarni, S.A. Utilization of Radiotherapy for Malignant Phyllodes Tumors: Analysis of the National Cancer Data Base, 1998–2009. Ann. Surg. Oncol. 2013, 21, 1222–1230. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, X.; Yang, D.; Wang, X.; Ren, G. Effects of adjuvant radiotherapy on borderline and malignant phyllodes tumors: A systematic review and meta-analysis. Mol. Clin. Oncol. 2015, 3, 663–671. [Google Scholar] [CrossRef]
- Spitaleri, G.; Toesca, A.; Botteri, E.; Bottiglieri, L.; Rotmensz, N.; Boselli, S.; Sangalli, C.; Catania, C.; Toffalorio, F.; Noberasco, C.; et al. Breast phyllodes tumor: A review of literature and a single center retrospective series analysis. Crit. Rev. Oncol. 2013, 88, 427–436. [Google Scholar] [CrossRef]
- Guillot, E.; Couturaud, B.; Reyal, F.; Curnier, A.; Ravinet, J.; Laé, M.; Bollet, M.; Pierga, J.-Y.; Salmon, R.; Fitoussi, A.; et al. Management of Phyllodes Breast Tumors. Breast J. 2011, 17, 129–137. [Google Scholar] [CrossRef]
- Chao, X.; Chen, K.; Zeng, J.; Bi, Z.; Guo, M.; Chen, Y.; Yao, Y.; Wu, W.; Liang, S.; Nie, Y. Adjuvant radiotherapy and chemotherapy for patients with breast phyllodes tumors: A systematic review and meta-analysis. BMC Cancer 2019, 19, 372. [Google Scholar] [CrossRef]
- Paula, B.H.R.; de Guerra Sousa, R.; de Sousa, C.A.M.; Crocamo, S. Adjuvant chemotherapy for malignant phyllodes tumor of the breast. World J. Adv. Res. Rev. 2020, 5, 48–54. [Google Scholar]
- Morales-Vásquez, F.; Gonzalez-Angulo, A.M.; Broglio, K.; Lopez-Basave, H.N.; Gallardo, D.; Hortobagyi, G.N.; De La Garza, J.G. Adjuvant chem-otherapy with doxorubicin and dacarbazine has no effect in recurrence-free survival of malignant phyllodes tumors of the breast. Breast J. 2007, 13, 551–556. [Google Scholar] [CrossRef]
- De Roos, W.K.; Kaye, P.; Dent, D.M. Factors leading to local recurrence or death after surgical resection of phyllodes tumours of the breast. Br. J. Surg. 1999, 86, 396–399. [Google Scholar] [CrossRef]
- Abdalla, H.M.; Sakr, M.A. Predictive factors of local recurrence and survival following primary surgical treatment of phyllodes tumors of the breast. J. Egypt. Natl. Cancer Inst. 2006, 18, 125–133. [Google Scholar]
- Wei, J.; Tan, Y.-T.; Cai, Y.-C.; Yuan, Z.-Y.; Yang, N.; Wang, S.-S.; Peng, R.-J.; Teng, X.-Y.; Liu, D.-G.; Shi, Y.-X. Predictive factors for the local recurrence and distant metastasis of phyllodes tumors of the breast: A retrospective analysis of 192 cases at a single center. Chin. J. Cancer 2014, 33, 492–500. [Google Scholar] [CrossRef]
- Chaney, A.W.; Pollack, A.; Mcneese, M.D.; Zagars, G.K.; Pisters, P.W.; Pollock, R.E.; Hunt, K.K. Primary treatment of cystosarcoma phyllodes of the breast. Cancer 2000, 89, 1502–1511. [Google Scholar] [CrossRef]
- Casali, P.; Abecassis, N.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; Broto, J.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv51–iv67. [Google Scholar] [CrossRef]
- Ca-Treatment-Phyllodes-Web-Algorithm.pdf. Available online: https://www.mdanderson.org/content/dam/mdanderson/documents/for%20physi-cians/algorithms/cancer-treatment/ca-treatment-phyllodes-web-algorithm.pdf (accessed on 23 March 2021).
- Amanda, M.P.; Shreyaskumar, P.; Cheuk, H.L.; Heather, Y.; Lin, A.P.C.; Neeta, S.; Dejka, M.A.; Maria, A.Z.; JAndrew, L.; Joseph, A.L.; et al. Systemic therapy regimen outcomes in metastatic phyllodes tumors of the breast. J. Clin. Oncol. 2018, 36, 11554. [Google Scholar]
- Kyriazoglou, A.; Zagouri, F.; Dimopoulos, M.A. Olaratumab administered in two cases of phyllodes tumour of the breast: End of the beginning? ESMO Open 2019, 4, e000479. [Google Scholar] [CrossRef]
- Asoglu, O.; Ugurlu, M.M.; Blanchard, K.; Grant, C.S.; Reynolds, C.; Cha, S.S.; Donohue, J.H. Risk factors for recurrence and death after primary surgical treatment of malignant phyllodes tumors. Ann. Surg. Oncol. 2004, 11, 1011–1107. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fede, Â.B.d.S.; Pereira Souza, R.; Doi, M.; De Brot, M.; Aparecida Bueno de Toledo Osorio, C.; Rocha Melo Gondim, G.; Casali-da-Rocha, J.C.; Jbili, R.; Bitencourt, A.G.V.; Alves de Souza, J.; et al. Malignant Phyllodes Tumor of the Breast: A Practice Review. Clin. Pract. 2021, 11, 205-215. https://doi.org/10.3390/clinpract11020030
Fede ÂBdS, Pereira Souza R, Doi M, De Brot M, Aparecida Bueno de Toledo Osorio C, Rocha Melo Gondim G, Casali-da-Rocha JC, Jbili R, Bitencourt AGV, Alves de Souza J, et al. Malignant Phyllodes Tumor of the Breast: A Practice Review. Clinics and Practice. 2021; 11(2):205-215. https://doi.org/10.3390/clinpract11020030
Chicago/Turabian StyleFede, Ângelo Bezerra de Souza, Ronaldo Pereira Souza, Mauricio Doi, Marina De Brot, Cynthia Aparecida Bueno de Toledo Osorio, Guilherme Rocha Melo Gondim, Jose Claudio Casali-da-Rocha, Rima Jbili, Almir Galvao Vieira Bitencourt, Juliana Alves de Souza, and et al. 2021. "Malignant Phyllodes Tumor of the Breast: A Practice Review" Clinics and Practice 11, no. 2: 205-215. https://doi.org/10.3390/clinpract11020030
APA StyleFede, Â. B. d. S., Pereira Souza, R., Doi, M., De Brot, M., Aparecida Bueno de Toledo Osorio, C., Rocha Melo Gondim, G., Casali-da-Rocha, J. C., Jbili, R., Bitencourt, A. G. V., Alves de Souza, J., Caparica Bitton, R., Baroni Alves Makdissi, F., & Moraes Sanches, S. (2021). Malignant Phyllodes Tumor of the Breast: A Practice Review. Clinics and Practice, 11(2), 205-215. https://doi.org/10.3390/clinpract11020030




