Abstract
Background: Three-dimensional (3D) printing technology has rapidly emerged as a transformative tool in medicine, enabling the conversion of two-dimensional scans into highly accurate 3D models. This technology, especially when combined with artificial intelligence (AI) and advanced materials, offers numerous applications in surgical planning, simulation-based training, and patient-specific care. Methods: This review examines current literature and case studies on the use of 3D printing technology in various fields of medicine, especially in surgical specialties. Key applications include surgical planning, mock surgeries, biopsy guide creation, and customized implant fabrication across various surgical fields. Results: 3D printing is transforming surgery by enabling precise visualization of tumors and critical structures, significantly enhancing preoperative planning for conditions such as bone, soft tissue (e.g., neuroblastomas), renal, and maxillofacial tumors. In reconstruction surgeries, patient-specific 3D-printed implants ensure better anatomical compatibility, particularly in maxillofacial, neurosurgical, and vascular applications. Puncture guides improve procedural accuracy in interventions like percutaneous nephrolithotripsy. Detailed anatomical models aid in simulation-based training, increasing preparedness for complex procedures. Additionally, patient-specific implants and AI-integrated decision support systems are paving the way for more personalized and efficient surgical care. Conclusions: 3D printing technology, especially when combined with AI, is reshaping modern surgery by improving both accuracy, safety, and personalized healthcare. Its applications extend across multiple specialties, offering new possibilities in surgical planning, training, and patient-specific treatments. As AI and bioprinting continue to evolve, the potential for real-time applications, such as live-printed tissue implants and enhanced decision support, could drive the next phase of innovation in various fields.
1. Introduction
In recent years, 3D printing technology has experienced rapid growth across various fields, including medicine, driven by advancements in print quality, reduced material costs, and increasing technological capabilities. This surge in additive manufacturing (AM), which has been in existence for over 30 years, has also been propelled by developments in artificial intelligence and machine learning [1]. These advancements have made 3D printing more accessible, fostering its application in fields such as medical implants, anatomical models, and tissue engineering. As a result, healthcare has undergone a significant transformation, with 3D printing allowing the creation of highly detailed surgical planning prototypes, aiding in preoperative planning, and improving surgical precision.
AM techniques involve layering materials such as plastics, rubber, metals, ceramics, resin, and even biocompatible materials to build three-dimensional models from imaging data, such as Computed Tomography (CT) and Magnetic Resonance Imagining (MRI) scans (Figure 1). This process, known as segmentation, allows for the creation of patient-specific models that can be manipulated digitally to analyse anatomical relationships [1]. Surgeons can rotate, decompose, and explore these models in virtual space, providing them with enhanced understanding and planning capabilities for complex procedures [2]. These models are then 3D-printed for a hands-on review [3].
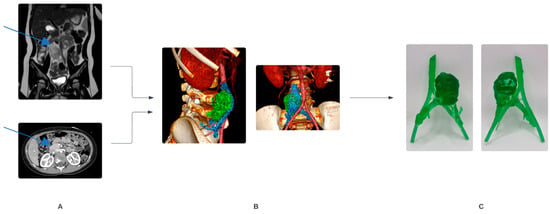
Figure 1.
Process of creating 3D models, example illustrating retroperitoneal tumor in a child. (A) Contrast-enhanced MRI (top) and CT (bottom). Blue arrows show the tumor. (B) Obtained radiological data is segmented, and a 3D reconstruction is created. (C) Printed 3D model, front and back view.
In pediatric surgery, where anatomy is restricted to small cavities and often influenced by unique congenital features, the need for personalized treatment is particularly critical. Three-dimensional printed models offer distinct advantages for clinicians, enabling precise visualization of congenital defects, improving communication with patients’ families, and enhancing decision-making in complex cases [3]. This technology holds great promise for the future, particularly as its integration with AI and machine learning continues to advance [4].
This review aims to explore current applications and challenges of 3D printing in the medical field, with a focus on its transformative potential in surgery, particularly in pediatric care, and its future role in personalized medicine (Table 1).

Table 1.
Systematic Summary of simulation methods, evaluation techniques, and analysis approaches in 3D printing technology.
2. Origins of 3D Printing
Three-dimensional printing (3D printing), also known as rapid prototyping or AM, traces its origins back to the 1970s, when early patents and prototypes were first developed. The 1980s saw the emergence of practical AM equipment and materials, including the groundbreaking invention of stereolithography (SLA) by Charles Hull. This marked the beginning of a transformative technology that would later be widely recognized as 3D printing.
During its initial development, 3D printing was primarily adopted by the automotive and aerospace industries for rapid prototyping. It was a time-intensive and costly process, limiting its application to industrial and research settings. The foundational principles of 3D printing involved the layer-by-layer deposition of material to create three-dimensional objects, with materials ranging from polymers and ceramics to metals and, more recently, living cells.
2.1. Technological Advancements and Accessibility
The 1990s and early 2000s witnessed the refinement of 3D printing technologies, including selective laser sintering (SLS), fused deposition modelling (FDM), and thermal inkjet printing (TIJ). Despite these advancements, widespread adoption was hindered by high costs and restrictive patents.
A pivotal moment occurred around 2010 when many relevant patents expired, leading to the development of affordable desktop 3D printers. This democratization of 3D printing made the technology more accessible. Researchers and clinicians began exploring its use in creating patient-specific anatomical models, surgical guides, and custom implants.
2.2. 3D Printing in Medicine
While early mentions of 3D printing in medical literature appeared before 2000, its adoption surged in the following decades. By the 2010s, 3D printing was being utilized in a wide range of specialties, including dentistry, orthopedics, and spinal surgery [31].
These fields are now best known for using 3D printing techniques and have contributed to the tremendous development and spread of the technique to other branches of medicine.
In particular, the field of orthopedics has been one of the earliest medical specialties to implement 3D printing for surgical planning and the development of patient-specific implants (PSI), with studies and procedures starting as early as 2010s [32].
More advanced, successful examples of PSI are also resounding in neurosurgery. They include reconstructive titanium-polyethylethylketone alloy cranial prostheses, a range of unique spinal implants for complex congenital, traumatic, and neoplastic pathologies [33]. Such reports and first procedures have already been performed by the Neuro Spine Surgery Research Group (NSURG), Australia, in 2016 and subsequent years [34].
3. Current 3D Printing Technology
The 3D model printing process starts with a CT or MRI carried out with a patient positioned for a planned procedure. A contrast-enhanced radiological examination is performed to visualize the lesion, associated anatomy, and vessels. Data derived from CT and MRI is then analyzed layer after layer to mark the structures chosen for imaging. At this point, using visualization software, the reconstructed model can be manipulated in space.
Regardless of the modality used, excellent image fidelity is essential for high-quality 3D printing. In some cases, multimodal imaging is used to exploit the advantages of more than one imaging modality. In our department, we often use CT and MRI image fusion.
This segmented data set is converted to an StereoLithography (STL) file and configured in printer software to set model parameters.
The created file is sent to the 3D printer. There are many 3D printing techniques and a wide range of materials with unique properties affecting the printing process, time, print durability, conformability, flexibility, and transparency. They are suitable for different applications. In our department, UV-sensitive resin works best for 3D printing, as it allows us to obtain prints with high resolution and preservation of details.
Once the printer is set up, printing begins, and little or no input is required. The newly printed model is not usually finished at this point. Post-production is required before it is ready. This almost always consists of support removal, sometimes a bit of polishing or smoothing, curing in an UV chamber, or assembly of multi-part models.
Once printed, optimally in 1:1 scale and polished, these models allow for further hands-on practice, mock surgeries, and real-time intraoperative guidance, contributing to better outcomes and reducing surgery times.
4. Discipline-Specific Applications
The introduction of 3D printing technology has provided groundbreaking opportunities for preoperative planning and simulation in various medical disciplines, especially surgical ones.
Pre-prepared models created using 3D printers allow for more precise and patient-specific approaches, improving outcomes and enhancing surgical precision, particularly in complex cases.
4.1. Vascular Surgery and Interventional Radiology
3D printing applications remain particularly valuable in vascular surgery, given the intricate nature of vascular structures and the difficulty of surgical interventions. By providing a tangible representation of vascular networks, 3D models improve preoperative planning and decision-making. This technology allows for the creation and visualization of complex vascular anatomy, enabling surgeons to simulate and plan procedures with greater accuracy.
4.1.1. Aortic and Renal Aneurysms
In vascular surgery, 3D-printed models have proven particularly beneficial for the planning and simulation of procedures such as aneurysm repair, stent graft implantation, and other interventions involving complex vascular anatomy. Traditional preoperative planning relied heavily on two-dimensional (2D) imaging from CT or MRI scans viewed on a monitor. However, interpreting the intricate structures of blood vessels from these images has limitations, especially when dealing with tortuous or highly branched vasculature.
By utilizing 3D printing, patient-specific vascular models are created directly from imaging data. These physical models allow surgeons to tangibly interact with the vascular structures, providing a clear understanding of the distances, angles, and complexities of the branches. This haptic feedback is crucial when planning catheter or stent navigation. Preoperative use of these models aids in selecting the optimal size and positioning of stent grafts, ultimately reducing surgery time, enhancing accuracy, and improving patient safety [11]. A great example of pre-planning and simulation are provided by work of Tomohiro Komada et al. [10]. They report a case of a woman with a 3 cm splenic artery aneurysm. During the initial procedure, the catheter and the wire were rotating in the aneurysm and could not be inserted into the outflow artery once embolization was attempted. A 3D vascular model was created, and the treatment plan was re-designed. The pre-planning and simulation performed on the model showed that it was impossible to select the outflow artery by merely bending the wire. The next simulation and then procedure itself was performed using a steerable microcatheter with a satisfying outcome [5].
3D-printed vascular models are also valuable in the management of complex cases, such as thoracoabdominal aortic aneurysms (TAAA) and renal artery aneurysms. For TAAA cases, 3D printing, combined with mechanical analysis, enables precise simulation of graft deformation in the aorta, allowing surgeons to optimize the placement of fenestrations in the graft. Wen-Dong Li et al. prepared for operation of 67-year-old male with enormous TAAA using a 3D model [17]. A stent graft was deployed in the 3D model to make a physician-modified stent graft (PMSGs) on the table. The locations of the opening of branches were marked twice during the operation. The PMSG was successfully deployed during the surgery and repaired the TAAA, providing a safe outcome [17]. Similar conclusions were derived from an unblinded, multicenter, non-randomized, prospective study from Anthony Le Bras et al., where simulations and radiological procedures were performed on 21 of 22 enrolled patients with cerebral aneurysms [11]. This individualized approach ensures more accurate and safer outcomes, particularly when dealing with the complex vascular anatomy [12].
4.1.2. Cerebral Aneurysms and Preoperative Simulations
In interventional radiology, 3D-printed vascular models offer significant advantages for planning endovascular procedures. In the treatment of cerebral aneurysms, preoperative simulation using 3D-printed models has demonstrated improved outcomes by allowing neurointerventionalists to rehearse the procedure. By performing simulations on these models, practitioners can optimize device selection, ensuring better wall apposition and closure of aneurysms without device misplacement or complications.
In a study on embolization procedures, patient-specific 3D models provided high concordance between the preoperative simulations and the actual procedure. Surgeons could accurately predict the necessary catheter or stent size and simulate how devices would interact with the aneurysm or vessel anatomy. The pre-procedural experience with a 3D-printed model led to reduced fluoroscopy time, radiation exposure, and overall procedure duration, demonstrating the tangible benefits of this technology in complex vascular interventions [4].
Additionally, these models can be combined with blood-mimicking fluids and hydraulic systems to simulate real-world blood flow dynamics, closely replicating density, viscosity, and body temperature. This allows for more accurate assessments of catheter maneuverability and device deployment. This is a greatly improved training model [12,35].
4.1.3. Application in Pediatric Vascular Surgery
In pediatric vascular surgery, the compact and evolving anatomy of children poses unique challenges. Procedures such as renal artery auto-transplantation or treatment of renovascular hypertension (RVH) greatly benefit from the use of 3D-printed models. These models allow surgeons to practice complex interventions in a controlled environment and pre-plan surgeries.
For children with RVH, 3D-printed models of renal arteries help clinicians visualize the stenosed segments more clearly [13]. It facilitates decision-making for endovascular or surgical interventions [4].
4.1.4. Challenges and Future Directions
While 3D printing has clear advantages in vascular surgery and interventional radiology, there are some limitations to consider [36]. Creating hollow-structure vascular models that simulate the inner lumen of blood vessels requires additional data processing, and even then, the models may not perfectly replicate the tactile feel or friction characteristics of real vessels (Figure 2). Despite these challenges, advances in material science, such as the use of flexible photopolymers, are improving the pliability and realism of 3D-printed models [11].

Figure 2.
3D liver model (transparent resin) with vessels (red colour). Blue arrow shows pathological finding. Printed at a scale of 1:3.
4.2. Urology
3-D printing is becoming a useful tool in urology. It is used for precise, patient-specific anatomical visualization, which is critical for accurately identifying and differentiating structures such as renal parenchyma, renal vasculature, urinary tract, and pathologies, e.g., tumors [14]. It demonstrates significant utility in the preparation for partial nephrectomy surgery in cases of small kidney tumors, where the primary objective is complete tumor excision while preserving renal function. In such cases, 3D printing customized surgical guides facilitate tumor location and indicate incision lines, optimizing the likelihood of complete tumor excision [14]. The guides are usually external circular models providing an outline of excision for the operator.
More often, 3D printing is valuable for surgical planning in cases involving congenital renal anomalies, such as horseshoe kidney. These patients present with complex vascular and anatomical variations that complicate standard surgical approaches. Preoperative 3D models in these cases provide critical insights into the unique anatomical configuration [14].
Another evolving application of 3D models in urology is during percutaneous nephrolithotripsy (PCNL). Patient-specific 3D-printed puncture guides have been shown to improve the accuracy of channel positioning, reduce operative time, and minimize blood loss. For instance, Gao Keyu et al. reported that these guides, which include a sterile 3D-printed tube attached to a molded reference surface (e.g., rib or hip bone), direct the needle precisely to the target location using pre-determined CT or MRI measures [16]. This has resulted in a reduction in the number of punctures needed and improved outcomes [15,16,37]. Furthermore, Lorenzo Bianchi et al. demonstrated that the use of 3D-printed models significantly enhances surgical training and planning [37]. Urologist using these models experienced improved procedural accuracy and confidence, particularly in challenging cases [16].
4.3. Oncological Surgery
3D planning has found application in many fields of oncological surgery. It facilitates the visualization of the tumor and essential pathological anatomy. Studies have shown that it provides more information than 2D imaging alone and can increase surgical efficiency, enabling more accurate resections, sparing critical anatomical structures, and shortening the time under general anesthesia. 3D printing is also being used for reconstructing structures removed along with the tumor, which impair aesthetics and function. In our department, we often use 3D models for planning oncological surgeries, mainly in cases of retroperitoneal tumors implicating large, critical vessels like neuroblastomas and pheochromocytomas, chest lesions, liver metastasis, sarcomas, and vascular malformations.
4.3.1. Head and Neck Oncological Surgery
Surgery is still the main choice of treatment for head and neck cancer. Surgeons dealing with large tissue defects after ablative surgery choose microvascular free tissue grafts as standard when primary closure and regional grafts are not possible. In recent years, advancements in 3D printing have enabled the use of bony free flaps. 3D tissue reconstruction and modeling now enable total virtual planning preceding the surgery and adjusting functionality. One of the first uses of 3D technology in head and neck surgery was for preoperative planning and designing reconstructive flaps prior to commencing the procedure. 3D models were used for planning exact measurements of reconstructive plates. Currently, some teams use sterilized plates intraoperatively as guides for instrumental planning, whereas others report more benefits using anatomical 3D models to pre-bend the plates prior to the operation and sterilization.
Also, 3D-printed cutting guides are being designed to fit the bone, illustrating intended osteotomy angles for resections, and shaping of donor reconstructive grafts, both designed in a way to protect the surrounding tissue. Guides are screwed to the bone and enable complete and precise lesion excision. These guides provide the exact angle and location for each screw before tumor resection. Tarsitano et al. used 3D-designed and printed surgical guides to dissect the mandible and fibula in 18 tumor cases [18]. The survival rate of the reconstructive fibula microvascular flap was 100%, and no microvascular or donor site complications occurred [19,20].
Many reports have compared the length of intraoperative time and ischemia between conventional surgery and methods using 3D models for free fibula flap mandibular reconstruction, and the results have consistently shown an improvement with 3D models. In the study by Hanasono and Skoracki on 38 patients, the mean operative time was 8.8 ± 1.0 h, whereas in the control group without the use of 3D models, it was 10.5 ± 1.4 h (p = 0.0006). The total intraoperative time in the 3D modelling group was significantly shorter (562 min) compared with the conventional group (662 min) in the study by Mahendru et al [38]. Reduction in free flap ischemia time is associated with fewer postoperative complications, including surgical site infections and partial or complete flap failure [20].
4.3.2. Bone Sarcoma
In osteosarcoma, complete surgical resection remains essential for treatment. The widespread use of 3D visualization in the surgical treatment of osteosarcoma aims to minimize the incidence of inadequate resection margins. At the planning stage, the created 3D bone-tumor model allows the surgeon to virtually perform bone tumor resection cuts and then design the cutting guides as well as the prosthesis. Based on the 3D model, patient-specific instruments (PSI) and implants are designed according to the planned resection strategy. The individual anatomy of the patient and the individual shape of the PSI allow for the cutting guide to be placed only in a defined position. These contact surfaces must be determined by both surgeon and engineer, considering the surgical approach, bone exposure, and tumor extension. This makes the use of PSI particularly useful in cases of bone deformities and tumors, where the anatomy is often abnormal. The final PSI is commonly printed in nylon or polyamide and provided to the surgeon sterile or to be sterilized. The virtual prosthesis is created, allowing for optimal fixation with reconstruction of the individual bone anatomy. Evrard et al. performed resection with PSI in 9 patients with primary pelvic sarcomas and compared the results with those in a historical control group of 19 patients with primary bone sarcomas who underwent resection in the same hospital without PSI [20]. After a mean follow-up of 52 months, none of the cases had local bone or soft tissue recurrence, compared with 7 of 19 controls (p = 0.03). Bone resection margins were R0 in 8 cases, and in one patient, R1 resection was intentionally performed to preserve the S1 root. All 9 cases had soft tissue resection margins of R0. In contrast, in the control group, bone resection margins were R0 in 13 patients, R1 in 5 patients, and R2 in 1 patient (p = 0.47). The lower local recurrence rate in the studied cases showed that the improved accuracy of resection provided by PSI directly affects the risk of local recurrence. Furthermore, R0 bone margins in 8 cases confirmed that PSI is effective in improving the accuracy of resection [21].
3D reconstruction-guided tumor surgery is an innovation that can improve the accuracy of osteosarcoma resection surgery and offer a reduction in recurrences. This is of particular use with complex resection and reconstructions, such as those in the pelvis and periarticular resections of the appendicular skeleton. 3D models provide precision, which is especially beneficial in an era of constantly increasing rates of limb-sparing surgeries in osteosarcoma. 3D planning-guided tumor surgery has shown benefits for reconstructive methods using both allografts and custom prostheses. In addition, it has been shown to reduce intraoperative time [22]. Gouin et al. described 11 cases of pelvic bone tumor resection [39]. Based on the fusion of MR and CT images, segmentation was performed to create a 3D model of the tumor and bone. Resection planning consisted of defining the desired cutting planes around the tumor, including a safe margin. The instruments were designed to fit a unique position on the bony structure and to indicate the desired resection planes. Histopathological analysis of the resected specimens showed tumor-free bone resection margins in all cases [39]. Docquier et al. described the case of a 32-year-old man with synovial sarcoma of the ischium [40]. Preoperative CT scans of the pelvis and MRI were performed. A CT scan of the allograft (hip bone) was also performed. A 3D model of the patient’s hip with tumor and a model of the allograft were created by rapid prototyping. A preoperative attempt at tumor resection was performed using a computer-assisted navigation system. Identical cuts were made in 2 hip prototypes: the patient’s and the allograft. Finally, the fit of the graft model to the patient’s hip model was checked. Histological examination showed that all resection margins were free of tumor. Postoperative CT revealed perfect plane-to-plane contact between the graft and the host pelvic bone [40].
4.3.3. Pediatric Tumors
Pediatric literature is mainly focused on studies on 3D printing applied to the analysis of cardiovascular anomalies, orthopedics, and dentistry. Only a few studies have reported cases of childhood tumors using 3D printing, and they mainly concern the most common ones: neuroblastoma and nephroblastoma. Since most surgical problems in these cases are due to their anatomical location, close to large blood vessels and critical organs, 3D printing has become a useful tool for surgical practice. Pereira et al. conducted a comprehensive review of the literature on this topic [41]. In addition to planning neuroblastoma surgery and Wilms’ tumor resection, 3D printing has also been successfully used for other cancers, including heart, brain, bone, and soft tissue tumors (Figure 3). The main benefits of 3D visualization in complex neuroblastoma cases included estimating the total resectable tumor volume and understanding the anatomy of the tumor, the adjacent vital structures, and their relations. It is essential for predicting risks and complications. Krauel et al. and Sánchez-Sánchez et al. used 3D-printed models to plan surgery in children with complex abdominal neuroblastomas encasing major vessels [22,23]. All patients underwent successful surgery as planned, achieving gross total resection. Souzaki et al. developed a method for preoperative surgical simulation using a 3D-printed model before laparoscopic adrenalectomy for adrenal neuroblastomas [42]. They used the 3D model to discuss port layout, simulate the laparoscopic view, and range of forceps movement [3,7,8]. In our department, based on resin 3D-printed models, we planned surgeries of retroperitoneal abdominal and pelvic neuroblastomas associated with the aorta, inferior vena cava, renal or iliac vessels. In one case of cervical neuroblastoma in a 5-month-old girl, we printed a single-color 3D model of the tumor and the adjacent common carotid artery with its division into internal and external carotid arteries. In all children, we obtained complete or gross total resection [23,24,42]. In our department, we use resin 3D-printed models in almost all neuroblastoma cases. In Wilms tumors, models provide more information than 2D images, which helps the team to decide between nephron-sparing surgery and nephrectomy. Sánchez-Sánchez et al. and Girón-Vallejo et al. presented cases of bilateral Wilms tumor [23,43]. Surgical planning and volumetric reconstruction using a 3D-printed model of the kidney: renal parenchyma, renal pelvis, greater calyces, renal artery, and renal vein of the tumor allowed bilateral nephron-sparing surgery [24,43]. The reviewed studies also showed that 3D models allow for understanding and visualizing the relationships between the tumor and adjacent anatomical regions in bone tumors: osteochondroma of the scapula, osteosarcomas, osteoid pedicle tumors, glioma, and craniopharyngioma. In osteosarcoma cases, printing the femur and cutting guide shortened the time of surgery and blood loss. It also reduced exposure to X-radiation [41].
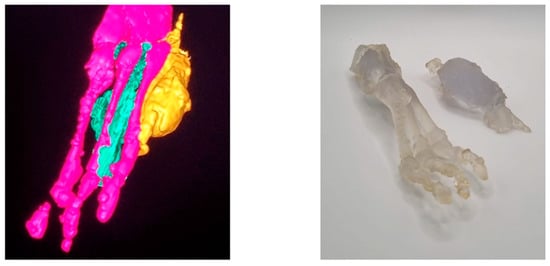
Figure 3.
3D reconstruction and model of soft tissue sarcoma (yellow), adjacent bones (pink), and selected muscle structures (green).
4.3.4. Others
Chen et al. investigated the application of 3D printing technology in local segmental thoracoscopic resection of lung cancer [44]. For the experimental group A, 3D chest CT reconstruction combined with the 3D printing technique was used in simulating a model to analyze the anatomical relationship and variations. For control group B, only a 3D CT scan was performed. All patients underwent uniport video-assisted thoracoscopic segmentectomy and systemic lymphadenectomy. Between groups A and B, there were significant differences in the surgical approach, operative time, blood loss, and the conversion rate. In group A, vascular variability was identified and avoided by performing 3D CT reconstruction combined with 3D printing. In group B, four patients underwent conversion to thoracotomy due to vascular variability, whereas in group A, there were no conversions because of that reason [44].
The 3D-printed model was also utilized in a female patient’s breast cancer. Initially, mastectomy was preferred for treatment, but after reviewing the 3D-printed model, the patient and surgeon often agreed to breast-conserving surgery. Preoperative use of the 3D-printed model provided accurate information on anatomical relationships, which facilitated the implementation of negative surgical margins around the tumor [45].
In turn, Zhang et al. described total thyroidectomy for giant nodular goiter guided by preoperative 3D computed tomography reconstruction and 3D printing [46]. According to the authors, 3D image-guided thyroidectomy facilitates precise and safe resection with fewer complications [46].
4.4. Liver Surgery
Liver surgery is difficult to perform due to its complex anatomy, and treatment results are not always satisfactory. Successful liver resection requires safety, precision, and adequate liver volume assessment to avoid liver failure after resection. 3D and 3D-printed models allow for optimal identification of the three types of hepatic vessels and bile ducts, their common anatomical anomalies, and correlations with pathological lesions. Patient-specific 3D liver models are used to plan resection and calculate parenchyma volumes to optimize a parenchyma-sparing approach while maintaining sufficient surgical margins [25].
4.4.1. Hepatobiliary Surgery
Lopez-Lopez et al. performed a multicenter study of complex hepatobiliary tumors, analyzing data from eight centers in Spain and Germany for patients undergoing hepatobiliary resection [25]. The endpoint was to validate the accuracy of the 3D model from the original image sources for use in teaching, patient communication, and surgical planning. The results confirmed a reliable correlation between the 3D model, radiological imaging, and surgical specimen, and also showed that this technique is useful for education, understanding, and surgical planning, with a proven advantage of 3D-printed models over 3D visualization [26].
A pilot study was conducted in Japan on the effect of patient-specific 3D-printed liver models on the safety of liver surgery. Thirteen patients underwent advanced hepatobiliary surgery using 3D models. The difference between patient-specific 3D liver models and the original data was less than 0.6 mm in the 90% region. The 3D model helped to identify the intrahepatic vein and determine the incision line. This study showed that the patient-specific 3D-printed liver model contributed to the safety of liver surgery. To evaluate the usefulness of the model, the surgeon’s psychological stress during surgery was selected as a surrogate indicator. According to the subjective postoperative evaluation, the surgeons found that these models improved safety and reduced psychological stress during surgery [47].
4.4.2. Liver Transplantation and Biliary Stenosis
The 3D-printed liver model was used in a 1-year-10-month-old girl with complete visceral inversion who underwent a living donor liver transplant for biliary atresia. 3D models of each liver part were printed from colored polyurethane resins and assembled together, resulting in an individualized liver model. Industrial CT scanning of the 3D model showed that the gaps between the model and the original data were <0.4 mm in the 90% region and 1.53 mm maximum. The printed model was introduced to the OR and used as an intraoperative navigation for total liver resection. The procedure was successful [48].
Pereira da Silva et al. described the use of a 3D model for surgery in a selected case of iatrogenic biliary stenosis [26]. The created model was used in surgical planning, increasing surgeons’ confidence in this delicate procedure, thanks to detailed preoperative information about the patient’s anatomy [26].
4.5. Respiratory Tract
3D printing has expanded its applications beyond surgical planning and is increasingly proving valuable for anesthesiologists in managing complex airway cases. In patients with congenital anomalies affecting the head and neck, which can lead to laryngo-tracheal abnormalities, 3D-printed airway models offer critical insights during pre-anesthesia planning [27]. These models allow anesthesiologists to visualize airway abnormalities in the upper respiratory tract, enabling them to practice intubation techniques and optimize their approach prior to the procedure. Furthermore, 3D printing allows the selection of an appropriate endotracheal tube size in patients with a history of tracheostomy or other respiratory tract problems [27,49].
In addition, 3D airway models are becoming valuable in preoperative planning for patients with airway obstructions, such as tumors, tracheomalacia, tracheobronchomalacia, or tracheal stenosis. These patient-specific models allow the development of tailored stent designs, enabling surgeons to achieve precise stenting [28,50]. Using advanced biodegradable materials for 3D printing also holds promise for novel interventions in pediatric airway obstructions, offering new therapeutic options [28]. Zopf et al. report the use of a PCL external splint in an eight-week-old infant with tracheobronchomalacia, ultimately leading to weaning off respiratory support [30]. Huang et al. [51] also used PCL external splints for tracheobronchomalacia in a case series, all with improved airway symptoms. The largest case series published by far with pediatric patients, by Les et al., [52] used PCL Hydroxyapatite to 3D print external splints with varied results. Finally, polyetherketoneketone (PEKK) was used by Morrison et al. [53] as an external splint for tracheomalacia with near-complete resolution of symptoms at the one-year follow-up.
4.6. Other Applications of 3D Printing in Surgery
4.6.1. Guide Plates: VPS Placement
In neurosurgery, 3D printing aids in the accurate placement of ventricular catheter tips during ventriculoperitoneal shunt (VPS) placement. Surgeons can ensure precise ventricle catheter insertion using printed guide tubes with reference surface mold individualized for each patient’s skull bone. This technique has proven to be simple and efficient in avoiding improper catheter positioning [54].
4.6.2. Maxillofacial Surgery (MFS)
The use of 3D printing is widely accepted in MFS. Surgical guides, anatomic models, and custom implants are printed to enhance precision in procedures such as dental implant placement and mandibular reconstruction. Anatomic 3D models enable a better understanding of complex facial procedures. The main challenges are the cost, manufacturing time, and regulatory issues associated with medical devices [55].
4.6.3. Chest Wall Reconstruction
In cases of large chest wall defects, 3D-printed prostheses—often made from biocompatible materials like titanium—are becoming more common. These prostheses not only improve structural integrity but also enhance respiratory function by preserving the chest wall curvature. The integration of 3D printing in these procedures reduces morbidity associated with traditional reconstruction methods [29]. Tan et al. [56] randomized 34 patients with complicated thoracic tumors to having preoperative operative planning with 3D reconstruction and 3D-printed translucent resin models versus standard CT images. 3D reconstruction and 3D-printed translucent resin models were associated with shorter operative time, less change in approach, and less blood loss.
4.6.4. Craniofacial Reconstruction in Low-Resource Settings
In low- and middle-income countries (LMICs), 3D printing offers a cost-effective solution for craniofacial reconstruction. The Bosnia and Herzegovina model showcases how 3D-based technologies and PMMA implants can be used to address cranial defects in resource-constrained environments. Multidisciplinary collaboration and 3D printing help provide effective reconstruction solutions that would otherwise be unavailable due to financial or logistical limitations [57].
4.6.5. Breast Reconstruction
After mastectomy, 3D printing is employed to create personalized external silicone breast prostheses. Particularly, Fused Filament Fabrication (FFF), provides a customizable method for producing prosthetic devices. In developing countries, this approach is instrumental in overcoming the barriers to reconstructive surgery by offering accessible and personalized alternatives [58].
4.6.6. Pediatric Vitreoretinal Surgery
In pediatric vitreoretinal (VR) surgery, 3D printing has led to the development of spacer devices that shorten trocar length, ensuring safer procedures in small eyes. The 3D-printed spacers are very simple, but precisely measured toruses. This technique minimizes the risk of iatrogenic injury and offers a reproducible solution that can be adapted for various clinical scenarios. Low cost, provided a department owns a 3D printer, and great efficacy in practice enhances the safety of pediatric vitreoretinal surgeries [59].
4.6.7. Conjoined Twin Separation
One of the most complex cases involving the use of 3D printing is in the separation of conjoined twins, particularly in cases involving the liver. 3D-printed models of the patients’ anatomy help surgeons plan the separation, reducing the risks associated with vascular and biliary complications. The role of 3D printing in such high-stakes operations continues to evolve, with the potential for future integration of virtual reality simulation [6]. Joshua A. Villarreal started recording cases of conjoined twin separations between January 2015 and December 2019, 17 individual separation procedures in total. Over a 20-year period, 3D modalities have ranged from 3D-printed models from CT and MRI reconstructions, plaster cast molds to virtual reality surgical simulation software in more recent cases. These complex patients require extensive preoperative imaging for planning the separation procedure and 3D-printed models are strongly recommended in preparation for better surgeon and family understanding of the procedure.
4.7. Medical Education and Patient Information
Medical education constantly seeks innovative methods to enhance learning. Particularly in fields like surgery, hands-on practice is crucial. Traditionally, students have relied on cadaveric dissection, animal models, and 2D imaging, but these have limitations in terms of cost, availability, and realism. The advent of 3D printing has introduced a new frontier, offering high-fidelity models that allow for better understanding of complex anatomy and rare pathologies (Figure 4).

Figure 4.
3D models of kidney.
4.7.1. Simulation and Practice
Obtaining procedural experience outside of the operating room is vital for developing surgical expertise, improving confidence, and enhancing clinical outcomes. However, access to realistic practice opportunities is often limited. Traditional simulators used for surgical simulation do not always mimic real-world pathology. 3D printing offers a solution by creating anatomically accurate models that can be used to simulate complex procedures. These models are especially valuable for rehearsing rare or advanced pediatric surgeries where cadaveric models are unavailable.
In specialties such as otolaryngology, 3D-printed models have been used to simulate surgeries like myringotomy, sinonasal and skull base surgeries, and direct laryngoscopy, providing trainees with diverse, risk-free practice opportunities. These models allow surgeons to practice complex techniques in a controlled environment. Unlike cadaveric and animal models, 3D-printed simulators are ethically sound, cost-effective, and reproducible. Furthermore, they enable repetition of procedures until mastery is achieved, a crucial advantage in pediatric and congenital cases where cadaveric options are limited [30].
4.7.2. Visualization and Understanding
Studies have demonstrated that 3D-printed models significantly improve anatomic visualization compared to traditional 2D imaging techniques like CT scans and anatomy atlases. These models offer a tangible, 3D understanding of complex anatomical structures, making it easier for medical students and surgical trainees to grasp spatial relationships within the body. The tactile feedback provided by 3D models is particularly beneficial for understanding vascular and neural anatomy, where spatial orientation is crucial for surgical planning and execution [29].
4.7.3. Limitations of 3D Printing in Education
Despite its promise, 3D printing does have limitations. Not all institutions have the resources to implement 3D printing into their medical education curricula. The process of creating patient-specific models can be time-consuming and require specialized personnel and equipment. Furthermore, although many studies have reported positive feedback from the use of 3D-printed models, there remains a need for higher-quality, quantitative evidence to demonstrate that these models are superior to traditional training methods, particularly cadaveric dissections. Some studies have shown that while 3D models are valuable educational tools, they are not yet viewed as replacements for cadaveric models, which are still considered the gold standard in surgical training [29,30].
4.7.4. Future Directions in Medical Education
As 3D printing technology continues to evolve, it holds great potential for expanding its role in medical education. Current studies suggest that 3D printing could be integrated more formally into surgical training programs. For instance, advanced pediatric fellowship programs have reported significant benefits from 3D-printed models, and future iterations of these programs could include formal evaluation tools to further validate their effectiveness. The development of affordable, high-fidelity models could democratize access to advanced surgical training, especially in institutions with limited resources [30]. Konkrety Brian Chang et al. suggests including in the fellowship programs high-fidelity simulator prep courses [30]. Such courses are already available, for example, for otolaryngology fellows. They report a statistically relevant increase in self-reported confidence (average and expertise) [34].
4.7.5. Patient Information and Understanding
There is potential for expanding the use of 3D printing beyond surgical education. For example, realistic 3D models could be used in outpatient clinics to help patients better understand their conditions. Studies have shown that patients who are presented with 3D-printed models of their anatomy report improved understanding of their disease and proposed surgical treatments. This could be particularly valuable in educating patients about complex or rare conditions. It is also a valuable tool in pediatric surgery during preoperative talks with parents and children [60]. Nusrat Iqbal et al. report in their research on 52 patients that 96% of patients are satisfied with the 3D model presentation and would like that method to be used in outpatient educational setting [60].
4.8. Ethics, Data Privacy and Patient Consent
The most urgent ethical aspects of 3D printing concern data privacy. Patients are informed that a 3D model will be created based on preoperative CT and MRI examinations. The benefits of this form of 3D visualization are explained. Printing 3D models often requires the cooperation of several departments, surgeons, radiologists, radiology technicians and engineers. Therefore, CT and MRI results, most often in the form of DICOM files, should be anonymized and digital content can be sent in this form. The cooperating units are bound by data confidentiality agreements. The use of patient-specific instruments and implants is included in the surgical consent form, with a description of the benefits and most common complications. Rizzo et al. indicate that the introduction of 3D printing in medicine is a change for the healthcare system, which is facing certain challenges, including the need for regulation and patient safety [61]. According to them, it is necessary to create unified standards for 3D printing, strengthen market surveillance of clinical applications, and encourage communication between hospitals, companies, and research institutes. In addition, they point out that ethical issues regarding 3D bioprinting must be assessed before it can be routinely introduced into the healthcare system. A ban on bioprinting would inhibit basic scientific achievements in the medical sector. On the one hand, not regulating bioprinting is the least safe option, on the other—providing too stringent regulations will limit the availability of this technology, encouraging lawbreaking and the development of the black market. The authors emphasize the need to adopt clear standards, prepare training programs, and define the roles and possible responsibilities of all those involved in the different stages of application. In the era of globalization, it is necessary to build a network of shared skills and experiences [61]. Deane and Byers proposed a new and simplified framework for categorizing donor-derived materials and the appropriate level of consent required for digital sharing. This framework proposes an equivalent minimum level of specific consent for materials from human donors and from human donors to generalized, non-identical artificial plastic models. They also suggested the creation of a centralized, secure repository for 3D digital content from human donors to collect, regulate, and control the distribution of appropriately approved 3D digital content from human donors. This would increase the availability of ethically produced teaching materials from human donors while discouraging commodification [62].
4.9. Costs and Accessibility
Accessibility to 3D printing remains limited because of high costs and resource demands. The process of segmentation and 3D printing requires specialized personnel, software, and other tools, which are currently costly [2]. In the literature, data on the precise costs of creating 3D models and cost savings from using these models during perioperative planning and intraoperative use are still limited. A wide range of materials and types of 3D printers enables the production of 3D models even in resource-constrained environments. Partnering with established 3D printing companies or non-profit organizations can offer access to cutting-edge technology and expertise, eliminating the need for hospitals to invest heavily in their own infrastructure [63]. Additionally, shared 3D printing centers within healthcare networks or regional collaborations can help spread costs across multiple institutions, making the technology more economically viable [64].
Building local capacity by training personnel in 3D modeling and segmentation reduces dependency on external specialists, while adopting open-source software and cost-effective printers helps further lower expenses [5,65]. Public–private partnerships and funding from government or international organizations can also play a vital role in facilitating the adoption of 3D printing in healthcare. Some analyses suggest that using 3D models in surgical fields can reduce overall costs by decreasing surgery time, enabling precise equipment selection, and shortening hospital stays [66]. As advancements in 3D continue, it is expected to become more affordable and accessible, which is crucial for patient-specific healthcare [67].
5. Discussion
The integration of 3D printing in surgery has seen rapid advancements yet remains underutilized, particularly in pediatric cases. The ease of operation and accessibility of modern 3D printers allow physicians to create patient-specific models without requiring extensive engineering knowledge. This is especially beneficial for novice physicians, who may struggle with interpreting complex vascular or anatomical structures from traditional imaging. For pediatric patients with intricate anatomical conditions, 3D models significantly improve preoperative planning and decision-making [36], enhancing both surgical outcomes and patient-family understanding (Figure 5).
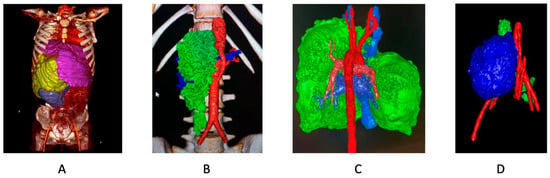
Figure 5.
3D reconstructions. (A) Enormous retroperitoneal sarcoma (yellow), translocating liver (pink), kidney (purple). (B) Retroperitoneal paraganglioma (green), abdominal aorta and arteries (red). (C) Thymoma (green), thoracic aorta and (red). (D) Retroperitoneal neuroblastoma (blue), arteries (red).
One critical benefit of 3D printing in pediatric surgery is its ability to refine and optimize surgical planning. Studies have shown its profound impact, such as a case series where 3D models modified surgical plans for nearly half of 40 children undergoing procedures for congenital heart disease [68]. Some of these children, previously considered inoperable, had successful outcomes following the surgeries guided by the models. Other studies, like those involving living donor kidney and liver transplants in children, demonstrated fewer complications and reduced anesthesia times, confirming the models’ value in enhancing procedural safety [69].
Moreover, 3D printing supports the visualization of complex anatomical structures, allowing surgeons to simulate surgeries before entering the operating room. This often leads to altered intraoperative management, contributing to improved clinical outcomes. 3D models enhance communication with patients and their families by providing a tangible representation of the disease and proposed intervention, improving consent and overall understanding. This is supported by studies in adult renal tumor resections where patient comprehension improved significantly after the introduction of patient-specific 3D models [29].
Looking ahead, the future of 3D printing in vascular surgery lies in its integration with other cutting-edge technologies, such as AI and real-time imaging. AI and machine learning used in international settings are introduced in many ways, some as open-source projects focused on gathering data and programming solutions for automated segmentations, tumor and metastasis detection, vascular analysis, and others. As those are very promising ideas, there are obstacles on the way concerning data privacy or, what can seem even more important, effectiveness of AI learning on a specific group of patients that can translate poorly onto other ethnicities or biological sexes. On the other hand, there’s a matter of medical certifications as most of those solutions are used ‘off-label’. These technology combinations may allow for even more precise simulations, enabling surgeons to practice and perfect techniques before entering the operating room. The development of point-of-care manufacturing and the increasing availability of 3D printers in hospitals further enhances the potential for this technology to become a routine tool in complex vascular and interventional procedures [36]. There are ongoing projects exploring 3D bioprinting in pediatric surgery, mostly used in urology. There are ongoing trials testing urethral bioprinting capabilities [70,71], and other promising studies explore technologies for substance supplementation using 3D-bioprinted pancreatic petals in type 1 diabetes mellitus [72] or Antimicrobial and Biodegradable 3D-Printed Scaffolds for orthopedic infections [73].
Technical Limitations
Despite these advancements, several challenges persist. The quality of 3D reconstructions is highly dependent on the diagnostic imaging used, requiring precise imaging protocols and artifact-free scans. Cooperation between radiologists and surgeons is essential to create accurate virtual models that capture all necessary details, particularly when dealing with complex vascular anatomy. Another challenge is when reconstructing soft tissue anatomy altered by pathological abnormalities. Currently, the common answer is to perform the initial radiological investigations in predetermined surgical positioning that will ensure the model’s usefulness. The future may equip us with new technologies such as flexible, soft, adaptive materials mimicking soft-tissue anatomy [74].
Furthermore, 3D printing in surgery remains costly and lacks standardization, both in the production of models and in their reimbursement within clinical practice. These issues, along with the inherent limitations in material properties—such as hardness or lack of flexibility—continue to limit the technology’s broader clinical adoption. Although some data suggest that overall costs of patient care are decreased while using 3D models due to shorter operative time, shorter hospital stay, and lower blood loss.
Finally, 3D printing holds great promise in medical education and training. For young surgeons, these models offer hands-on experience, reducing the risks associated with learning curves. While research continues to explore the full educational potential, the preliminary evidence suggests that 3D-printed models offer an affordable, effective tool for improving both practical skills and patient outcomes. As the technology evolves, its role in surgical education will likely expand, aiding in the development of novel treatment techniques and enhancing patient care [9].
6. Conclusions
3D printing is poised to revolutionize surgery, particularly in pediatric applications, where precision and anatomical complexities present significant challenges. It offers a unique opportunity for personalized patient care, surgical planning, and education. In pediatrics, where the margin for error is smaller, 3D printing enables surgeons to simulate procedures, reduce operative risk, and enhance patient outcomes. Additionally, the use of 3D models improves patient-family communication, facilitating informed consent and helping families better understand the medical conditions and interventions.
However, while the potential benefits of 3D printing in surgery are clear, challenges such as high costs, lack of standardization, and limitations in material properties hinder its widespread adoption. For the technology to reach its full potential, further research and standardization of 3D printing protocols are needed, along with an exploration of reimbursement structures to make the technology more accessible. Below we present a summary from our point of view of the advantages and obstacles of 3D printing and its near future (Table 2). As the field continues to grow, the possibilities for bioprinting, customized implants, and patient-specific models will likely expand, offering even greater advancements in personalized medicine.

Table 2.
Conclusions—advantages and obstacles of 3D printing and its near future.
Author Contributions
J.K. (Jakub Kopeć): literature search, writing—original draft preparation, review & editing, J.K. (Justyna Kukulska): writing—original draft preparation, review & editing, M.L.: writing—original draft preparation, review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Segaran, N.; Saini, G.; Mayer, J.L.; Naidu, S.; Patel, I.; Alzubaidi, S.; Oklu, R. Application of 3D Printing in Preoperative Planning. J. Clin. Med. 2021, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, A.; Chen, D.; Elumalai, A.; Albers, B.; Tappa, K.; Jammalamadaka, U.; Hoegger, M.J.; Ballard, D.H. Guide for Starting or Optimizing a 3D Printing Clinical Service. Methods 2022, 206, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Szary, J.; Luis, M.S.; Mikulski, S.; Patel, A.; Schulz, F.; Tretiakow, D.; Fercho, J.; Jaguszewska, K.; Frankiewicz, M.; Pawłowska, E.; et al. The Role of 3D Printing in Planning Complex Medical Procedures and Training of Medical Professionals & Mdash; Cross-Sectional Multispecialty Review. Available online: https://www.mdpi.com/1660-4601/19/6/3331 (accessed on 19 October 2024).
- Sukanya, V.S.; Panigrahy, N.; Rath, S.N. Recent Approaches in Clinical Applications of 3D Printing in Neonates and Pediatrics. Eur. J. Pediatr. 2021, 180, 323–332. [Google Scholar] [CrossRef]
- Ganguli, A.; Pagan-Diaz, G.J.; Grant, L.; Cvetkovic, C.; Bramlet, M.; Vozenilek, J.; Kesavadas, T.; Bashir, R. 3D Printing for Preoperative Planning and Surgical Training: A Review. Biomed. Microdevices 2018, 20, 65. [Google Scholar] [CrossRef]
- Villarreal, J.A.; Yoeli, D.; Masand, P.M.; Galvan, N.T.N.; Olutoye, O.O.; Goss, J.A. Hepatic Separation of Conjoined Twins: Operative Technique and Review of Three-Dimensional Model Utilization. J. Pediatr. Surg. 2020, 55, 2828–2835. [Google Scholar] [CrossRef]
- Habermann, A.C.; Timmerman, W.R.; Cohen, S.M.; Burkhardt, B.W.; Amendola, M.F. Clinical Applications of 3D Printing in Colorectal Surgery: A Systematic Review. Int. J. Colorectal. Dis. 2024, 39, 127. [Google Scholar] [CrossRef]
- Francoisse, C.A.; Sescleifer, A.M.; King, W.T.; Lin, A.Y. Three-Dimensional Printing in Medicine: A Systematic Review of Pediatric Applications. Pediatr. Res. 2021, 89, 415–425. [Google Scholar] [CrossRef]
- Tejo-Otero, A.; Buj-Corral, I.; Fenollosa-Artés, F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann. Biomed. Eng. 2020, 48, 536–555. [Google Scholar] [CrossRef]
- Komada, T.; Kamomae, T.; Matsushima, M.; Hyodo, R.; Naganawa, S. Embolization Using Patient-Specific Vascular Models Created by a 3D Printer for Difficult Cases: A Report of Two Cases. Nagoya J. Med. Sci. 2022, 84, 477–483. [Google Scholar] [CrossRef]
- Le Bras, A.; Boustia, F.; Janot, K.; Le Pabic, E.; Ouvrard, M.; Fougerou-Leurent, C.; Ferre, J.-C.; Gauvrit, J.-Y.; Eugene, F. Rehearsals Using Patient-Specific 3D-Printed Aneurysm Models for Simulation of Endovascular Embolization of Complex Intracranial Aneurysms: 3D SIM Study. J. Neuroradiol. 2023, 50, 86–92. [Google Scholar] [CrossRef]
- Coles-Black, J.; Barber, T.; Bolton, D.; Chuen, J. A Systematic Review of Three-Dimensional Printed Template-Assisted Physician-Modified Stent Grafts for Fenestrated Endovascular Aneurysm Repair. J. Vasc. Surg. 2021, 74, 296–306.e1. [Google Scholar] [CrossRef]
- Mercader, C.; Vilaseca, A.; Moreno, J.L.; López, A.; Sebastià, M.C.; Nicolau, C.; Ribal, M.J.; Peri, L.; Costa, M.; Alcaraz, A. Role of the Three-Dimensional Printing Technology in Complex Laparoscopic Renal Surgery: A Renal Tumor in a Horseshoe Kidney. Int. Braz. J. Urol. 2019, 45, 1129–1135. [Google Scholar] [CrossRef]
- Lim, B.; Lee, S.; Kim, T.; Ock, J.; Song, C.; Kim, N.; Kyung, Y.S. Clinical Utility of a 3D-Printed Patient-Specific Surgical Guide for Partial Nephrectomy. Urol. Int. 2023, 107, 591–594. [Google Scholar] [CrossRef]
- Ai, G.Y.; Zhou, Z.; Huang, Z.; Zhong, J.; Liu, S.; Liu, W.; Pang, X.; Zhu, W. The Value of 3D Printing Model Combined PCNL in Kidney Stones: A Systematic Review and Meta-Analysis. Minerva Urol. Nephrol. 2024, 76, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Keyu, G.; Shuaishuai, L.; Raj, A.; Shuofeng, L.; Shuai, L.; Yuan, Z.; Haitao, Z.; Junqi, W. A 3D Printing Personalized Percutaneous Puncture Guide Access Plate for Percutaneous Nephrolithotomy: A Pilot Study. BMC Urol. 2021, 21, 184. [Google Scholar] [CrossRef]
- Li, W.-D.; Keyoumu, R.; Wang, C.; Liu, Z. 3D Printing-Guided Endovascular Repair of Enormous Twisted Thoracoabdominal Aortic Aneurysm with Branch Stenosis and Occlusion. Catheter. Cardiovasc. Interv. 2023, 101, 813–816. [Google Scholar] [CrossRef]
- Tarsitano, A.; Mazzoni, S.; Cipriani, R.; Scotti, R.; Marchetti, C.; Ciocca, L. The CAD-CAM Technique for Mandibular Reconstruction: An 18 Patients Oncological Case-Series. J. Cranio Maxillofac. Surg. 2014, 42, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Nyirjesy, S.C.; Heller, M.; von Windheim, N.; Gingras, A.; Kang, S.Y.; Ozer, E.; Agrawal, A.; Old, M.O.; Seim, N.B.; Carrau, R.L.; et al. The Role of Computer Aided Design/Computer Assisted Manufacturing (CAD/CAM) and 3-Dimensional Printing in Head and Neck Oncologic Surgery: A Review and Future Directions. Oral Oncol. 2022, 132, 105976. [Google Scholar] [CrossRef] [PubMed]
- Evrard, R.; Schubert, T.; Paul, L.; Docquier, P.-L. Resection Margins Obtained with Patient-Specific Instruments for Resecting Primary Pelvic Bone Sarcomas: A Case-Control Study. Orthop. Traumatol. Surg. Res. 2019, 105, 781–787. [Google Scholar] [CrossRef]
- McCulloch, R.A.; Frisoni, T.; Kurunskal, V.; Donati, D.M.; Jeys, L. Computer Navigation and 3D Printing in the Surgical Management of Bone Sarcoma. Cells 2021, 10, 195. [Google Scholar] [CrossRef]
- Krauel, L.; Fenollosa, F.; Riaza, L.; Pérez, M.; Tarrado, X.; Morales, A.; Gomà, J.; Mora, J. Use of 3D Prototypes for Complex Surgical Oncologic Cases. World J. Surg. 2016, 40, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, Á.; Girón-Vallejo, Ó.; Ruiz-Pruneda, R.; Fernandez-Ibieta, M.; García-Calderon, D.; Villamil, V.; Giménez-Aleixandre, M.C.; Montoya-Rangel, C.A.; Bermejo, J.P.H. Three-Dimensional Printed Model and Virtual Reconstruction: An Extra Tool for Pediatric Solid Tumors Surgery. Eur. J. Pediatr. Surg. Rep. 2018, 6, e70. [Google Scholar] [CrossRef]
- Gavriilidis, P.; Edwin, B.; Pelanis, E.; Hidalgo, E.; de’Angelis, N.; Memeo, R.; Aldrighetti, L.; Sutcliffe, R.P. Navigated Liver Surgery: State of the Art and Future Perspectives. Hepatobiliary Pancreat. Dis. Int. 2022, 21, 226–233. [Google Scholar] [CrossRef]
- Lopez-Lopez, V.; Robles-Campos, R.; García-Calderon, D.; Lang, H.; Cugat, E.; Jiménez-Galanes, S.; Férnandez-Cebrian, J.M.; Sánchez-Turrión, V.; Fernández-Fernández, J.M.; Barrera-Gómez, M.Á.; et al. Applicability of 3D-Printed Models in Hepatobiliary Surgey: Results from “LIV3DPRINT” Multicenter Study. HPB 2021, 23, 675–684. [Google Scholar] [CrossRef]
- Pereira da Silva, N.; Abreu, I.; Serôdio, M.; Ferreira, L.; Alexandrino, H.; Donato, P. Advanced Hepatic Vasculobiliary Imaging Segmentation and 3D Reconstruction as an Aid in the Surgical Management of High Biliary Stenosis. BMC Med. Imaging 2020, 20, 120. [Google Scholar] [CrossRef]
- Park, S.; Ahn, J.; Kim, H.-J.; Choi, E.-J.; Kim, H.Y. Endotracheal Intubation Using a Three-Dimensional Printed Airway Model in a Patient with Pierre Robin Sequence and a History of Tracheostomy -a Case Report. Korean J. Anesthesiol. 2021, 74, 262–265. [Google Scholar] [CrossRef]
- Young, J.S.; McAllister, M.; Marshall, M.B. Three-Dimensional Technologies in Chest Wall Resection and Reconstruction. J. Surg. Oncol. 2023, 127, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Langridge, B.; Momin, S.; Coumbe, B.; Woin, E.; Griffin, M.; Butler, P. Systematic Review of the Use of 3-Dimensional Printing in Surgical Teaching and Assessment. J. Surg. Educ. 2018, 75, 209–221. [Google Scholar] [CrossRef]
- Chang, B.; Powell, A.; Ellsperman, S.; Wehrmann, D.; Landry, A.; Jabbour, N.; Goudy, S.; Zopf, D. Multicenter Advanced Pediatric Otolaryngology Fellowship Prep Surgical Simulation Course with 3D Printed High-Fidelity Models. Otolaryngol. Neck Surg. 2020, 162, 658–665. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, C.; Xu, X.; Wang, J.; Hou, X.; Li, K.; Lu, X.; Shi, H.; Lee, E.-S.; Jiang, H.B. A Review of 3D Printing in Dentistry: Technologies, Affecting Factors, and Applications. Scanning 2021, 2021, 9950131. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Colon, R.; Nayak, V.V.; Parente, P.E.L.; Leucht, P.; Tovar, N.; Lin, C.C.; Rezzadeh, K.; Hacquebord, J.H.; Coelho, P.G.; Witek, L. The Presence of 3D Printing in Orthopedics: A Clinical and Material Review. J. Orthop. Res. 2023, 41, 601–613. [Google Scholar] [CrossRef]
- Wilcox, B.; Mobbs, R.J.; Wu, A.-M.; Phan, K. Systematic Review of 3D Printing in Spinal Surgery: The Current State of Play. J. Spine Surg. 2017, 3, 433–443. [Google Scholar] [CrossRef]
- Phan, K.; Sgro, A.; Maharaj, M.M.; D’Urso, P.; Mobbs, R.J. Application of a 3D Custom Printed Patient Specific Spinal Implant for C1/2 Arthrodesis. J. Spine Surg. 2016, 2, 314–318. [Google Scholar] [CrossRef]
- Yamaki, V.N.; Cancelliere, N.M.; Nicholson, P.; Rodrigues, M.; Radovanovic, I.; Sungur, J.-M.; Krings, T.; Pereira, V.M. Biomodex Patient-Specific Brain Aneurysm Models: The Value of Simulation for First in-Human Experiences Using New Devices and Robotics. J. Neurointerventional Surg. 2021, 13, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Soliński, D.; Celer, M.; Dyś, K.; Witkiewicz, W.; Wiewiora, M. 3D Printing in the Endovascular Treatment of Visceral Artery Aneurysms. Medicine 2023, 102, e35844. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Schiavina, R.; Barbaresi, U.; Angiolini, A.; Pultrone, C.V.; Manferrari, F.; Bortolani, B.; Cercenelli, L.; Borghesi, M.; Chessa, F.; et al. 3D Reconstruction and Physical Renal Model to Improve Percutaneous Punture during PNL. Int. Braz. J. Urol. 2019, 45, 1281–1282. [Google Scholar] [CrossRef]
- Mahendru, S.; Jain, R.; Aggarwal, A.; Aulakh, H.S.; Jain, A.; Khazanchi, R.K.; Sarin, D. CAD-CAM vs conventional technique for mandibular reconstruction with free fibula flap: A comparison of outcomes. Surg. Oncol. 2020, 34, 284–291. [Google Scholar] [CrossRef]
- Gouin, F.; Paul, L.; Odri, G.A.; Cartiaux, O. Computer-Assisted Planning and Patient-Specific Instruments for Bone Tumor Resection within the Pelvis: A Series of 11 Patients. Sarcoma 2014, 2014, 842709. [Google Scholar] [CrossRef]
- Docquier, P.-L.; Paul, L.; Cartiaux, O.; Delloye, C.; Banse, X. Computer-Assisted Resection and Reconstruction of Pelvic Tumor Sarcoma. Sarcoma 2010, 2010, 125162. [Google Scholar] [CrossRef][Green Version]
- Pereira, H.R.; Barzegar, M.; Hamadelseed, O.; Esteve, A.V.; Munuera, J. 3D Surgical Planning of Pediatric Tumors: A Review. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Souzaki, R.; Kinoshita, Y.; Ieir, S.; Kawakubo, N.; Obata, S.; Jimbo, T.; Koga, Y.; Hashizume, M.; Taguchi, T. Preoperative Surgical Simulation of Laparoscopic Adrenalectomy for Neuroblastoma Using a Three-Dimensional Printed Model Based on Preoperative CT Images—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26440294/ (accessed on 31 October 2024).
- Girón-Vallejo, Ó.; García-Calderón, D.; Ruiz-Pruneda, R.; Cabello-Laureano, R.; Doménech-Abellán, E.; Fuster-Soler, J.L.; Ruiz-Jiménez, J.I.; Girón-Vallejo, Ó.; García-Calderón, D.; Ruiz-Pruneda, R.; et al. Three-Dimensional Printed Model of Bilateral Wilms Tumor: A Useful Tool for Planning Nephron Sparing Surgery. Pediatr. Blood Cancer 2018, 65, e26894. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Chen, Q.; Li, T.; Chen, K.; Yu, Q.; Lin, X. Three-Dimensional Printing Technology for Localised Thoracoscopic Segmental Resection for Lung Cancer: A Quasi-Randomised Clinical Trial. World J. Surg. Oncol. 2020, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.; Adrada, B.E.; Caudle, A.S.; Clemens, M.W.; Black, D.M.; Arribas, E.M. The Role of Three-Dimensional Printing in the Surgical Management of Breast Cancer. J. Surg. Oncol. 2019, 120, 897–902. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Zhang, Q.; Zhao, C.; Li, J.; Li, X.; Li, G.; Chen, J.; Peng, D.; Wang, Y.; et al. Total Thyroidectomy for Giant Nodular Goiter Guided by Pre-Operative 3D Computed Tomography Reconstruction and 3D Printing: A Case Report. Medicine 2022, 101, e32456. [Google Scholar] [CrossRef]
- Fukumitsu, K.; Ishii, T.; Ogiso, S.; Yoh, T.; Uchida, Y.; Ito, T.; Seo, S.; Hata, K.; Uemoto, S.; Hatano, E. Impact of Patient-Specific Three-Dimensional Printed Liver Models on Hepatic Surgery Safety: A Pilot Study. HPB 2023, 25, 1083–1092. [Google Scholar] [CrossRef]
- Ishii, T.; Fukumitsu, K.; Ogawa, E.; Okamoto, T.; Uemoto, S. Living Donor Liver Transplantation in Situs Inversus Totalis with a Patient-Specific Three-Dimensional Printed Liver Model. Pediatr. Transplant. 2020, 24, e13675. [Google Scholar] [CrossRef]
- Stramiello, J.A.; Saddawi-Konefka, R.; Ryan, J.; Brigger, M.T. The Role of 3D Printing in Pediatric Airway Obstruction: A Systematic Review. Int. J. Pediatr. Otorhinolaryngol. 2020, 132, 109923. [Google Scholar] [CrossRef]
- Hatachi, G.; Matsumoto, K.; Miyazaki, T.; Tsuchiya, T.; Taniguchi, D.; Doi, R.; Watanabe, H.; Nakatsukasa, T.; Matsuo, N.; Nagayasu, T. Enhanced Airway Stenting Using a Preoperative, Three-Dimensionally Printed Airway Model Simulation. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 1591–1593. [Google Scholar] [CrossRef]
- Huang, L.; Wang, L.; He, J.; Zhao, J.; Zhong, D.; Yang, G.; Guo, T.; Yan, X.; Zhang, L.; Li, D.; et al. Tracheal suspension by using 3-dimensional printed personalized scaffold in a patient with tracheomalacia. J. Thorac. Dis. 2016, 8, 3323–3328. [Google Scholar] [CrossRef]
- Les, A.S.; Ohye, R.G.; Filbrun, A.G.; Ghadimi Mahani, M.; Flanagan, C.L.; Daniels, R.C.; Kidwell, K.M.; Zopf, D.A.; Hollister, S.J.; Green, G.E. 3D-printed, externally-implanted, bioresorbable airway splints for severe tracheobronchomalacia. Laryngoscope 2019, 129, 1763–1771. [Google Scholar] [CrossRef]
- Morrison, R.J.; Hollister, S.J.; Niedner, M.F.; Mahani, M.G.; Park, A.H.; Mehta, D.K.; Ohye, R.G.; Green, G.E. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci. Transl. Med. 2015, 7, 285ra64. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Yi, J.; Wu, Y.; Wang, S.; Qu, Y.; Cai, Q. Precise Location of the Ventricular Catheter Tip in Ventriculoperitoneal Shunt Placement Guided by 3D Printed Individualized Guide. Clin. Neurol. Neurosurg. 2023, 229, 107730. [Google Scholar] [CrossRef] [PubMed]
- Louvrier, A.; Marty, P.; Barrabé, A.; Euvrard, E.; Chatelain, B.; Weber, E.; Meyer, C. How Useful Is 3D Printing in Maxillofacial Surgery? J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 206–212. [Google Scholar] [CrossRef]
- Tan, D.; Yao, J.; Hua, X.; Li, J.; Xu, Z.; Wu, Y.; Wu, W. Application of 3D modeling and printing technology in accurate resection of complicated thoracic tumors. Ann. Transl. Med. 2020, 8, 1342. [Google Scholar] [CrossRef]
- Bečulić, H.; Spahić, D.; Begagić, E.; Pugonja, R.; Skomorac, R.; Jusić, A.; Selimović, E.; Mašović, A.; Pojskić, M. Breaking Barriers in Cranioplasty: 3D Printing in Low and Middle-Income Settings—Insights from Zenica, Bosnia and Herzegovina. Medicina 2023, 59, 1732. [Google Scholar] [CrossRef] [PubMed]
- Leme, J.C.; Spinosa, R.M.d.O.; Leal, S.O.; Hirsch, A.B.B.; Lodovico, A.; Stramandinoli-Zanicotti, R.T.; Kunkel, M.E.; Moura, F.A. Development of Low-Cost and Personalized External Silicone Breast Prosthesis Produced by Additive Manufacturing for Women Who Have Undergone Mastectomy: A Pilot Study. Clin. Biomech. 2023, 110, 106123. [Google Scholar] [CrossRef]
- Di-Luciano, A.; Gómez-Nuñez, R.; Acosta, F.; Rivas-Vega, L.; Morales-Cantón, V.; Trujillo-Alvarez, M.; Cernichiaro-Espinosa, L. Trocar Shortening for Pediatric Vitreoretinal Surgery with a 3D Printed Trocar Spacer: Report of Two Cases. Arch. Soc. Española Oftalmol. 2022, 97, 473–476. [Google Scholar] [CrossRef]
- Iqbal, N.; Fletcher, J.; Bassett, P.; Hart, A.; Lung, P.; Tozer, P. Exploring Methods of Improving Patient Understanding and Communication in a Complex Anal Fistula Clinic: Results from a Randomized Controlled Feasibility Study. Colorectal. Dis. 2024, 26, 518–526. [Google Scholar] [CrossRef]
- Rizzo, M.L.; Turco, S.; Spina, F. 3D Printing and 3D Bioprinting Technology in Medicine: Ethical and Legal Issues. Clin. Ter. 2023, 174, 80–84. [Google Scholar] [CrossRef]
- Deane, A.S.; Byers, K.T. A Review of the Ethical Considerations for the Use of 3D Printed Materials in Medical and Allied Health Education and a Proposed Collective Path Forward. Anat. Sci. Educ. 2024, 17, 1164–1173. [Google Scholar] [CrossRef]
- Hellman, S.; Frisch, P.; Platzman, A.; Booth, P. 3D Printing in a hospital: Centralized clinical implementation and applications for comprehensive care. Digital Health 2023, 9, 20552076231221899. [Google Scholar] [CrossRef]
- Awuah, W.A.; Tenkorang, P.O.; Adebusoye, F.T.; Ng, J.C.; Wellington, J.; Abdul-Rahman, T.; Nazir, A.; Mustapha, M.J.; Bulut, H.; Papadakis, M. 3D Printing in Surgery: Revolutionizing Trauma and Fracture Care in Low and Middle-Income Countries. Postgrad. Med. J. 2024, 100, 1–3. [Google Scholar] [CrossRef]
- Beroza, A. 3D Printing in Low Resource Healthcare Settings: Analysis of Potential Implementations. Master’s Thesis, Michigan Technological University, Houghton, MI, USA, 2019. [Google Scholar] [CrossRef]
- Thorn, C.; Ballard, J.; Lockhart, C.; Crone, A.; Aarvold, A. The Perioperative Utility of 3D Printed Models in Complex Surgical Care: Feedback from 106 Cases. Ann. R. Coll. Surg. Engl. 2023, 105, 747–753. [Google Scholar] [CrossRef]
- Bastawrous, S. Utility and Costs Benchmarked in a New 3D Printing Service—Optimizing the Path Forward. J. Am. Coll. Radiol. 2023, 20, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Valverde, I.; Gomez-Ciriza, G.; Hussain, T.; Suarez-Mejias, C.; Velasco-Forte, M.N.; Byrne, N.; Ordoñez, A.; Gonzalez-Calle, A.; Anderson, D.; Hazekamp, M.G.; et al. Three-Dimensional Printed Models for Surgical Planning of Complex Congenital Heart Defects: An International Multicentre Study European Journal of Cardio-Thoracic Surgery Oxford Academic. Available online: https://academic.oup.com/ejcts/article/52/6/1139/3925909 (accessed on 21 October 2024).
- Paessler, A.; Forman, C.; Minhas, K.; Patel, P.A.; Carmichael, J.; Smith, L.; Jaradat, F.; Assia-Zamora, S.; Arslan, Z.; Calder, F.; et al. 3D Printing: A Useful Tool for Safe Clinical Practice in Children with Complex Vasculature—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/38627026/ (accessed on 21 October 2024).
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D Bioprinting of Urethra with PCL/PLCL Blend and Dual Autologous Cells in Fibrin Hydrogel: An in Vitro Evaluation of Biomimetic Mechanical Property and Cell Growth Environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef]
- Booth, D.; Afshari, R.; Ghovvati, M.; Shariati, K.; Sturm, R.; Annabi, N. Advances in 3D Bioprinting for Urethral Tissue Reconstruction. Trends Biotechnol. 2024, 42, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Klak, M.; Wszoła, M.; Berman, A.; Filip, A.; Kosowska, A.; Olkowska-Truchanowicz, J.; Rachalewski, M.; Tymicki, G.; Bryniarski, T.; Kołodziejska, M.; et al. Bioprinted 3D Bionic Scaffolds with Pancreatic Islets as a New Therapy for Type 1 Diabetes—Analysis of the Results of Preclinical Studies on a Mouse Model. J. Funct. Biomater. 2023, 14, 371. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Vahabi, H.; Kumaravel, V. Antimicrobial and Biodegradable 3D Printed Scaffolds for Orthopedic Infections. ACS Biomater. Sci. Eng. 2023, 9, 4020–4044. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Qiao, Y.; Deng, H.; Zhang, Y.; Lu, S.; Zhao, Y.; Yang, X.; Wang, Z.; Li, M.; et al. Light-weight Triangular Mesh Deformable Reconstruction for Low-Cost 3D Surface Imaging in Minimally Invasive Surgery. Comput. Methods Programs Biomed. 2025, 241, 107152. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).