Abstract
Diffuse large B-cell lymphoma (DLBCL) and angioimmunoblastic T-cell lymphoma (AITL) are two subtypes of non-Hodgkin lymphoma (NHL). The simultaneous occurrence of DLBCL and AITL in a composite lymphoma is very rare, and there are no established treatment regimens. We present the case of an 85-year-old male admitted to the intensive care unit with distributive shock, lymphocytosis, and lymphadenopathy, who was subsequently diagnosed with composite AITL and DLBCL, and treated with brentuximab vedotin (BV) and rituximab. To our knowledge, this is the first case of composite lymphoma presenting with distributive shock and treated with BV and rituximab, with successful resolution of shock.
1. Introduction
Non-Hodgkin lymphomas (NHLs) are classified by their cell of origin: B-, T-, or natural killer (NK)-cell. B-cell lymphomas comprise 85% of NHLs, with diffuse large B-cell lymphoma (DLBCL) being the most common [1,2]. NK/T-cell lymphomas comprise approximately 15% of all NHLs [1,2]. Angioimmunoblastic T-cell lymphoma (AITL) is a rare, aggressive peripheral T-cell lymphoma (PTCL) with a median overall survival of less than three years. It is characterized by intense inflammation and immune dysregulation [3]. Composite AITL and DLBCL is exceedingly rare, and its description has been limited to case series. It is still unclear how this composite lymphoma develops, but the presence of Epstein–Barr virus (EBV) in the setting of immune dysregulation from pre-existing AITL suggests that EBV-mediated B-cell lymphomagenesis may be the causative agent [3]. The optimal treatment remains uncertain. Here, we present a patient who was found to have a new diagnosis of composite AITL and DLBCL in the setting of distributive shock.
2. Case Presentation
An 85-year-old male with a history of coronary artery disease and heart failure with preserved ejection fraction was hospitalized for suspected heart failure exacerbation. He had a nine-year history of leukocytosis, and in the year prior to admission, developed peripheral eosinophilia and lymphadenopathy, with a previously non-diagnosed fine needle aspiration of a hilar lymph node.
On admission, initial labs were notable for worsened leukocytosis to 34,400/mm3 (absolute lymphocytes 6900/mm3, absolute eosinophils 1400/mm3), and Epstein–Barr virus (EBV) viral load of 1300 IU/mL (Table 1). Due to worsening leukocytosis and peripheral eosinophilia, Hematology was consulted. CT imaging demonstrated diffuse lymphadenopathy throughout the thorax, abdomen, and pelvis, the largest of which was a 2.4 cm right paratracheal lymph node. Bone marrow biopsy and excisional biopsy of two left iliac lymph nodes were obtained on hospital day (HD) 7. Eosinophilia work-up showed PDGFR-β rearrangement in 6–7% of peripheral blood cells. The work-up was negative for peripheral blood mutations in KIT, JAK2 (Janus Kinase 2), MPL (myeloproliferative leukemia), and CALR (calreticulin), and for Strongyloides immunoglobulin G (IgG).

Table 1.
Complete blood count at admission and discharge.
On HD8, the patient developed hypotension requiring two vasopressors and transfer to the intensive care unit. An extensive work-up for undifferentiated shock did not reveal an etiology. The infectious work-up showed only a right lower lobe lung opacity. Thoracentesis revealed transudative pleural fluid. The transthoracic echocardiogram was not consistent with cardiac eosinophilic infiltration. Despite empiric treatment with broad-spectrum antibiotics, there was no improvement in pressor requirements.
On HD14, the lymph node biopsy results returned, demonstrating composite lymphoma: AITL and DLBCL (Figure 1). T-cell receptor gamma gene rearrangement and gain of chromosome 3/3q were noted in both the bone marrow and the lymph node. IgH and IgK clonal rearrangements were noted in the lymph node alone. Bone marrow was involved by AITL but not DLBCL.
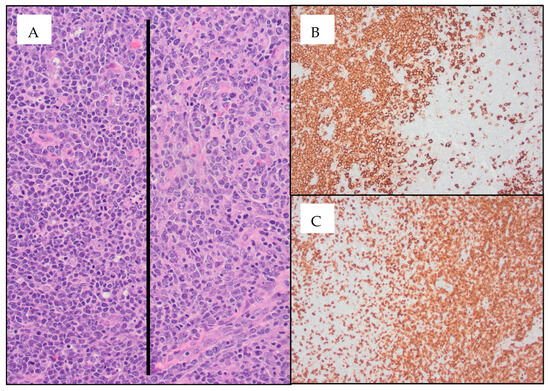
Figure 1.
Diffuse large B-cell lymphoma (DLBCL) arising in the setting of angioimmunoblastic T-cell lymphoma (AITL) ((A) 20× image; AITL cells are seen to the right of the black line and DLBCL cells are seen to the left of the black line) within the same inguinal lymph node specimen. CD20 immunohistochemistry (IHC) stains highlight the DLBCL cells ((B) 10× image), which are seen next to the AITL cells highlighted using CD3 IHC stains ((C) 10× image). IHC was positive in AITL cells for CD2, CD3, CD4, CD5, CD10, CD45, BCL6, and PD1, with patchy CD30 staining of 15–20% of atypical cells. Focal involvement by large B-cells (germinal center phenotype) was positive for CD10, CD20, CD30, PAX5, BCL6, BCL2, MUM1, and EBER.
Given persistent distributive shock with inability to wean vasopressors for seven days, suspicions increased that the cause of distributive shock was secondary to lymphoma. Methylprednisolone 1 mg/kg daily was initiated on HD14, and vasopressor requirements began to decrease. Given the patient’s age, shock status, and co-morbidities, he was deemed not to be a candidate for systemic chemotherapy, and thus brentuximab vedotin (BV) and rituximab were administered on HD20 and HD21, respectively. By HD22, all vasopressors were weaned off. He was discharged on HD31. The patient was recovering well at a one-week follow-up appointment, with plans for a second cycle of BV and rituximab. Unfortunately, the patient unexpectedly expired at home nineteen days after hospital discharge.
3. Discussion
This is an atypical case of a new diagnosis of composite AITL/DLBCL presenting with distributive shock and responding to initial treatment with rituximab and BV.
While multiple cases of composite AITL and DLBCL have been described, the pathophysiology of its development is not well-understood, though various hypotheses have been proposed. AITL is characterized by immune dysregulation which may lead to EBV reactivation and subsequent B-cell lymphomagenesis. In one study, 72% of cases with composite AITL and DLBCL were EBV-positive by in situ hybridization for EBER, suggesting that EBV plays a role in pathogenesis [4]. One suggested theory is that the development of AITL leads to EBV reactivation from dysfunctional, decreased T-cell immune surveillance. Because EBV preferentially infects B-cells [5], EBV-infected B-cells then proliferate in the absence of adequate immune surveillance [4,6,7]. This proposed mechanism is analogous to the pathogenesis of post-transplant lymphoproliferative disorders (PTLD), in which the iatrogenic immunosuppression of T-cells leads to the development of EBV-associated B-cell lymphomas [8,9].
It has also been proposed that AITL may develop by EBV-infected B-cells causing malignant transformation of T-cells. EBV-infected B-cells can activate T-cells via upregulation of the CD28 co-stimulatory ligand B7 found on B-cells. This upregulates T-cell expression of CXCL13, a B-cell chemokine, recruiting more B-cells to the affected lymph node, creating a co-stimulatory loop driving the development of both AITL and DLBCL [10]. It is notable that 3% of reported composite AITL and DLBCL cases are EBV-negative [6], suggesting that other pathogenic mechanisms may exist. For example, Fujisawa proposed that one of these mechanisms may include mutations in TET2, an enzyme involved in DNA methylation. TET2-mutated germinal B-cells may undergo clonal hematopoiesis and create a tumor microenvironment conducive to the clonal proliferation of T follicular helpers (TFH) cells, the cell of origin in AITL, leading to concurrent B- and T-cell lymphoma [11,12].
Our case is unique in its presentation of distributive shock. Shock as a presenting symptom of any NHL is uncommon. We identified 15 such cases in the literature (Table 2). It is notable that of these fifteen cases, six were PTCLs (three AITL, one PTCL NOS with AITL features, one PTCL NOS, and one anaplastic lymphoma).
Proposed mechanisms for the development of shock include massive cytokine release from both malignant cells and activated macrophages, resembling cytokine storm [13,14,15]. Cytokine release may be further heightened in PTCL and AITL given the immune dysfunction prevalent in these lymphomas [15]. This is suggested by the increased proportion of PTCLs and AITL in cases of shock. Despite PTCL and AITL comprising 5–10% and 1–2% of all NHLs, respectively [2], they made up 40% and 20% of the fifteen NHL cases we identified presenting in shock. Systemic capillary leak syndrome (SCLS) should also be considered on the differential. While typically associated with paraproteinemia, there have been a small number of cases of PTCL in association with SCLS [16,17]. However, as these patients typically present with anasarca, this was considered less likely in our patient.
Our patient’s clinical condition began to improve following the initiation of steroids, with the resolution of shock and weaning off all pressors after the administration of BV and rituximab, supporting that lymphoma was driving his shock state.
There is little guidance on the optimal treatment of composite AITL and DLBCL given its rarity. Because most cases arise sequentially, patients are often first treated with an AITL-directed regimen, and following the development of DLBCL, a separate DLBCL-directed regimen. While rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is the standard treatment for DLBCL, frontline treatment for AITL ranges from autologous stem cell transplant (SCT), to chemotherapy with CHOP, to immunosuppression. BV, cyclophosphamide, doxorubicin, and prednisone (BV-CHP) has also emerged as standard treatment for certain PTCLs such as systemic anaplastic large-cell lymphoma, though efficacy in other histologic subtypes such as AITL remains unclear [18]. Histone deacetylase (HDAC) inhibition may also have a role in AITL treatment, as HDAC inhibition has had efficacy in PTCL with the TFH phenotype, a disease entity similar to AITL with the same cell of origin [19].
In composite lymphoma, treatment is directed to the more aggressive lymphoma. For example, patients with concurrent AITL and indolent B-cell lymphomas such as SLL or FL were treated with CHOP-based regimens to target AITL [4,20]. Composite AITL and DLBCL poses a challenge since both lymphomas are considered aggressive, and treatment must take both into account. Of the 24 cases of composite AITL and DLBCL we identified in the literature, 14 received treatment, including rituximab, pirarubicin, cyclophosphamide, vincristine, and prednisone (R-THP-COP), R-CHOP, CHOP, R-fludarabine, auto-SCT, and thalidomide [4,7,21,22].
Patients presenting with shock pose a unique challenge given the concern that intensive chemotherapy may worsen their already critically ill state. In one case series, all eleven NHL patients in shock received chemotherapy, with or without rituximab, but only three patients survived the hospitalization [13]. In an effort to initiate conservative treatment given our patient’s multi-pressor requirement, we opted to treat initially with steroids, BV, and rituximab to target the respective T- and B- cell components of AITL and DLBCL. While this combination has been safely used in CD30+ B-cell lymphomas [23], to our knowledge, this is the only case of composite AITL and DLBCL treated with two immunotherapy agents targeting the respective T- and B-cells. While our patient had CD30 expression in over 10% of cells, studies suggest that BV efficacy may be independent of CD30 expression and could remain a treatment option even in CD30-negative PTCL [18,24]. Our patient was successfully weaned off vasopressors and discharged from the hospital soon after treatment. Our case suggests that rituximab and BV may represent a safe and well-tolerated alternative for patients, especially those in which critical illness is a concern, until further clinical improvement allows the addition of chemotherapy.

Table 2.
Treatment and outcomes of Non-Hodgkin lymphoma patients presenting with shock.
Table 2.
Treatment and outcomes of Non-Hodgkin lymphoma patients presenting with shock.
| Age/Sex | Diagnosis | Treatment | Outcome | Reference |
|---|---|---|---|---|
| 35F | PTCL NOS | Antibiotics (lymphoma diagnosed post-mortem) | Died inpatient | [25] |
| 70M | PTCL NOS (with features of AITL) | Stress-dose steroids | Off pressors within one day and discharged | [26] |
| 59M | AITL | Stress-dose steroids | Died inpatient | [12] |
| 73F | AITL | Steroids, alemtuzumab | Discharged | [13] |
| 79F | CLL | CHOP | Died inpatient | [11] |
| 53M | DLBCL | COP | Died inpatient | [11] |
| 76M | DLBCL | R-CHOP | Died inpatient | [11] |
| 79M | DLBCL | BR, followed by R-CHOP | Died inpatient | [11] |
| 82F | DLBCL | CHOP | Discharged | [11] |
| 68F | DLBCL with HLH | Etoposide | Discharged | [11] |
| 58F | B-cell endovascular lymphoma | R-CHOP, etoposide | Died inpatient | [11] |
| 56M | FL | COP | Discharged | [11] |
| 78F | AITL | CHOP | Died inpatient | [11] |
| 57F | T-cell lymphoma | Carmustine, cytarabine, etoposide, melphalan | Died inpatient | [11] |
| 19F | T-cell anaplastic lymphoma | CHOP | Died inpatient | [11] |
AITL, angioimmunoblastic T-cell lymphoma; BR, bendamustine, rituximab; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; NOS, not otherwise specified; PTLC, peripheral T-cell lymphoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone.
4. Conclusions
As more cases emerge describing composite AITL with B-cell lymphomas (most commonly DLBCL), it is important to quickly recognize and initiate appropriate treatment. In patients with hematologic abnormalities and persistent, undifferentiated shock, lymphoma should be considered on the differential. For composite lymphomas in critically ill patients, immunotherapy may represent a well-tolerated and effective initial treatment.
Author Contributions
Writing—original draft preparation, N.H. and B.H.; writing—review and editing, N.H., B.H., A.K., M.D. and M.O.; figure preparation, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study as case reports containing three or fewer subjects are not considered Human Subject’s Research.
Informed Consent Statement
Informed consent was obtained from subjects involved in the study. Written informed consent has been obtained from next of kim in order to publish this paper.
Data Availability Statement
Additional information and data is available upon request.
Conflicts of Interest
B.H. has received research funding from Oncternal Therapeutics. He is an institutional principal investigator for AstraZeneca. He serves on Scientific Advisory Boards for Epizyme, Beigene, and TG therapeutics.
References
- The Non-Hodgkin’s Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood 1997, 89, 3909–3918. [Google Scholar] [CrossRef]
- Perry, A.M.; Diebold, J.; Nathwani, B.N.; MacLennan, K.A.; Müller-Hermelink, H.K.; Bast, M.; Boilesen, E.; Armitage, J.O.; Weisenburger, D.D. Non-Hodgkin lymphoma in the developing world: Review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica 2016, 101, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Iannitto, E.; Ferreri, A.J.M.; Minardi, V.; Tripodo, C.; Kreipe, H.H. Angioimmunoblastic T-cell lymphoma. Crit. Rev. Oncol. Hematol. 2008, 68, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Suefuji, N.; Niino, D.; Arakawa, F.; Karube, K.; Kimura, Y.; Kiyasu, J.; Takeuchi, M.; Miyoshi, H.; Yoshida, M.; Ichikawa, A.; et al. Clinicopathological analysis of a composite lymphoma containing both T- and B- cell lymphomas. Pathol. Int. 2012, 62, 690–698. [Google Scholar] [CrossRef]
- Kuppers, R. B cells under influence: Transformation of B cells by Epstein-Barr virus. Nat. Rev. Immunol. 2003, 3, 801–812. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, P.; Gao, Y.; Qiu, L. Sequential development of diffuse large B-cell lymphoma in a patient with angioimmunoblastic T-cell lymphoma. Diagn. Cytopathol. 2012, 40, 346–351. [Google Scholar] [CrossRef]
- Xu, Y.; McKenna, R.W.; Hoang, M.P.; Collins, R.H.; Kroft, S.H. Composite angioimmunoblastic T-cell lymphoma and diffuse large B-cell lymphoma: A case report and review of the literature. Am. J. Clin. Pathol. 2002, 118, 848–854. [Google Scholar] [CrossRef]
- Naresh, K.N.; Menasce, L.P.; Shenjere, P.; Banerjee, S.S. Precursors of classical Hodgkin lymphoma in samples of angioimmunoblastic T-cell lymphoma. Br. J. Haematol. 2008, 141, 124–126. [Google Scholar] [CrossRef]
- Zettl, A.; Lee, S.-S.; Rüdiger, T.; Starostik, P.; Marino, M.; Kirchner, T.; Ott, M.; Müller-Hermelink, H.K.; Ott, G. Epstein-Barr virus-associated B-cell lymphoproliferative disorders in angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. Am. J. Clin. Pathol. 2002, 117, 368–379. [Google Scholar] [CrossRef]
- Dunleavy, K.; Wilson, W.H.; Jaffe, E.S. Angioimmunoblastic T cell lymphoma: Pathobiological insights and clinical implications. Curr. Opin. Hematol. 2007, 14, 348–353. [Google Scholar] [CrossRef]
- Fujisawa, M.; Nguyen, T.B.; Abe, Y.; Suehara, Y.; Fukumoto, K.; Suma, S.; Makishima, K.; Kaneko, C.; Nguyen, Y.T.M.; Usuki, K.; et al. Clonal germinal center B cells function as a niche for T-cell lymphoma. Blood 2022, 140, 1937–1950. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.E. A Tet-a-Tet in T follicular helper cell lymphoma. Blood 2022, 140, 1919–1921. [Google Scholar] [CrossRef] [PubMed]
- Cherruault, M.; Le Goff, M.; Tamburini, J.; Pene, F. Urgent chemotherapy in sepsis-like shock related to hematologic malignancies. Crit. Care Med. 2018, 46, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Yadav, H. Refractory and progressive distributive shock due to angioimmunoblastic T cell lymphoma. Crit. Care Med. 2016, 44, 507. [Google Scholar] [CrossRef]
- Halene, S.; Zieske, A.; Berliner, N. Sustained remission from angioimmunoblastic T-cell lymphoma induced by alemtuzumab. Nat. Clin. Pract. Oncol. 2006, 3, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, T.; Watanabe, T.; Ogose, T.; Kondo, R.; Nakatsu, T.; Sakata, A.; Yamashita, M.; Gotho, T. Capillary leak syndrome: Initial presentation in a patient with ALK+ anaplastic large cell lymphoma associated with increased levels of serum cytokines. Leuk. Lymphoma 2011, 52, 1139–1142. [Google Scholar] [CrossRef]
- Lourdes, L.S.; Al-Quran, S.Z.; Dang, N.H.; Markham, M. Systemic capillary leak syndrome as an initial presentation of ALK-negative anaplastic large cell lymphoma. Case Rep. Hematol. 2012, 2012, 954201. [Google Scholar] [CrossRef]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.; Christensen, J.H.; Morschhauser, F.; Domenech, E.D.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef]
- Ghione, P.; Faruque, P.; Mehta-Shah, N.; Seshan, V.; Ozkaya, N.; Bhaskar, S.; Yeung, J.; Spinner, M.A.; Lunning, M.; Inghirami, G.; et al. T follicular helper phenotype predicts response to histone deacetylase inhibitors in relapsed/refractory peripheral T-cell lymphoma. Blood Adv. 2020, 4, 4640–4647. [Google Scholar] [CrossRef]
- Campidelli, C.; Sabattini, E.; Piccioli, M.; Rossi, M.; De Blasi, D.; Miraglia, E.; Rodriguez-Abreu, D.; Franscini, L.L.; Bertoni, F.; Mazzucchelli, L.; et al. Simultaneous occurrence of peripheral T-cell lymphoma unspecified and B-cell small lymphocytic lymphoma. Report of 2 cases. Hum. Pathol. 2007, 38, 787–792. [Google Scholar] [CrossRef]
- Hong, R.; Sheng, L.; Ouyang, G. Composite angioimmunoblastic T cell/diffuse large B-cell lymphoma treated with reduced-intensity conditioning HLA-haploidentical allo-HSCT: A case report and review of the literature. Int. J. Clin. Exp. Pathol. 2018, 11, 5473–5480. [Google Scholar]
- Attygalle, A.D.M.; Kyriakou, C.; Dupuis, J.; Grogg, K.L.; Diss, T.C.; Wotherspoon, A.C.M.; Chuang, S.S.; Cabeçadas, J.; Isaacson, P.G.F.; Du, M.-Q.M.; et al. Histologic evolution of angioimmunoblastic T-cell lymphoma in consecutive biopsies: Clinical correlation and insights into natural history and disease progression. Am. J. Surg. Pathol. 2007, 31, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, J.; Bair, S.M.; Landsburg, D.J.; Nasta, S.D.; Nagle, S.J.; Barta, S.K.; Khan, N.; Filicko-O’Hara, J.; Gaballa, S.; Strelec, L.; et al. Brentuximab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone as frontline treatment for patients with CD30-positive B-cell lymphomas. Haemtologica 2021, 106, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Stefoni, V.; Pellegrini, C.; Argnani, L.; Corradini, P.; Dodero, A.; Orsucci, L.; Volpetti, S.; Zinzani, P.L. Brentuximab vedotin in the treatment of relapsed/refractory CD30+ peripheral T-cell lymphoma: A FIL phase 2 study. Hematol. Oncol. 2022, 40, 307–309. [Google Scholar] [CrossRef]
- Kumar, K.; Saad, M.; Chime, C.; Badipatla, K.; Tariq, H.; Nayudu, S.; Niazi, M.; Chilimuri, S. Refractory septic shock due to underlying immunocompromised disease: A case of fatal peripheral T-cell lymphoma not otherwise specified in a young Hispanic woman. Case Rep. Oncol. 2018, 11, 404–411. [Google Scholar] [CrossRef]
- Imamura, H.; Kashima, Y.; Hattori, M.; Mori, K.; Takeshige, K.; Nakazawa, H. Unexplained recurrent shock in peripheral T-cell lymphoma: A case report. Clin. Case Rep. 2021, 9, 04612. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).