Fluid Overload-Associated Large B-Cell Lymphoma: A Case Report and Review of Literature
Abstract
1. Introduction
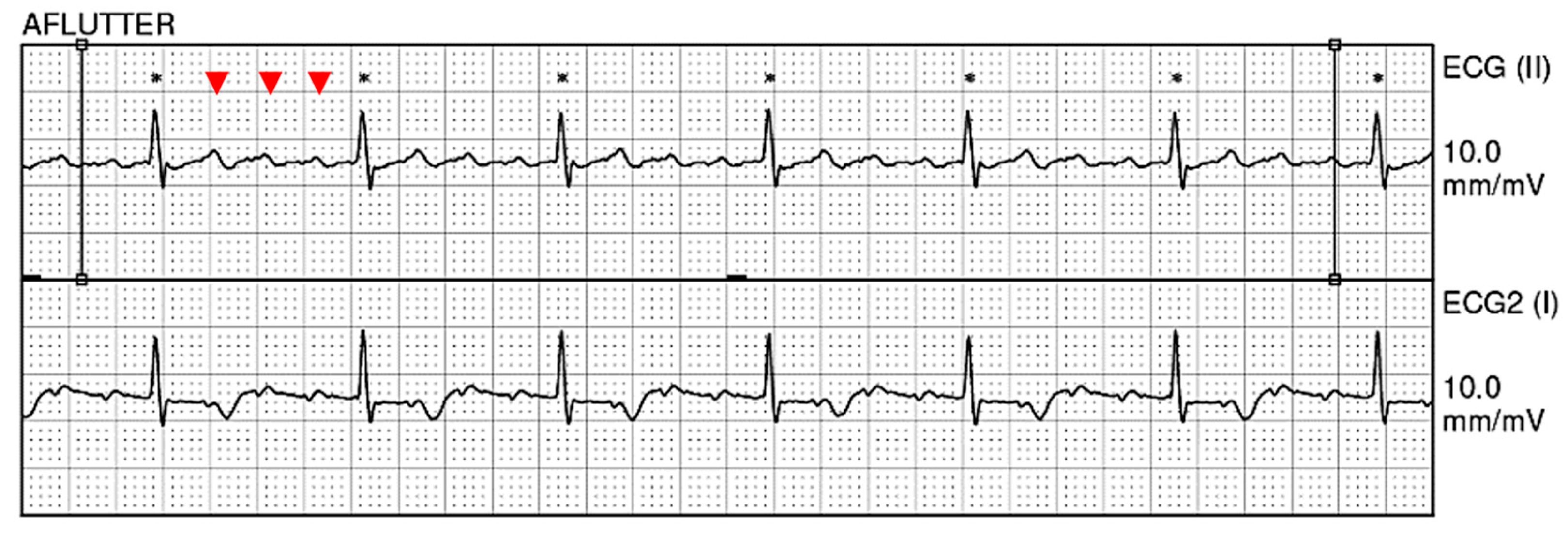
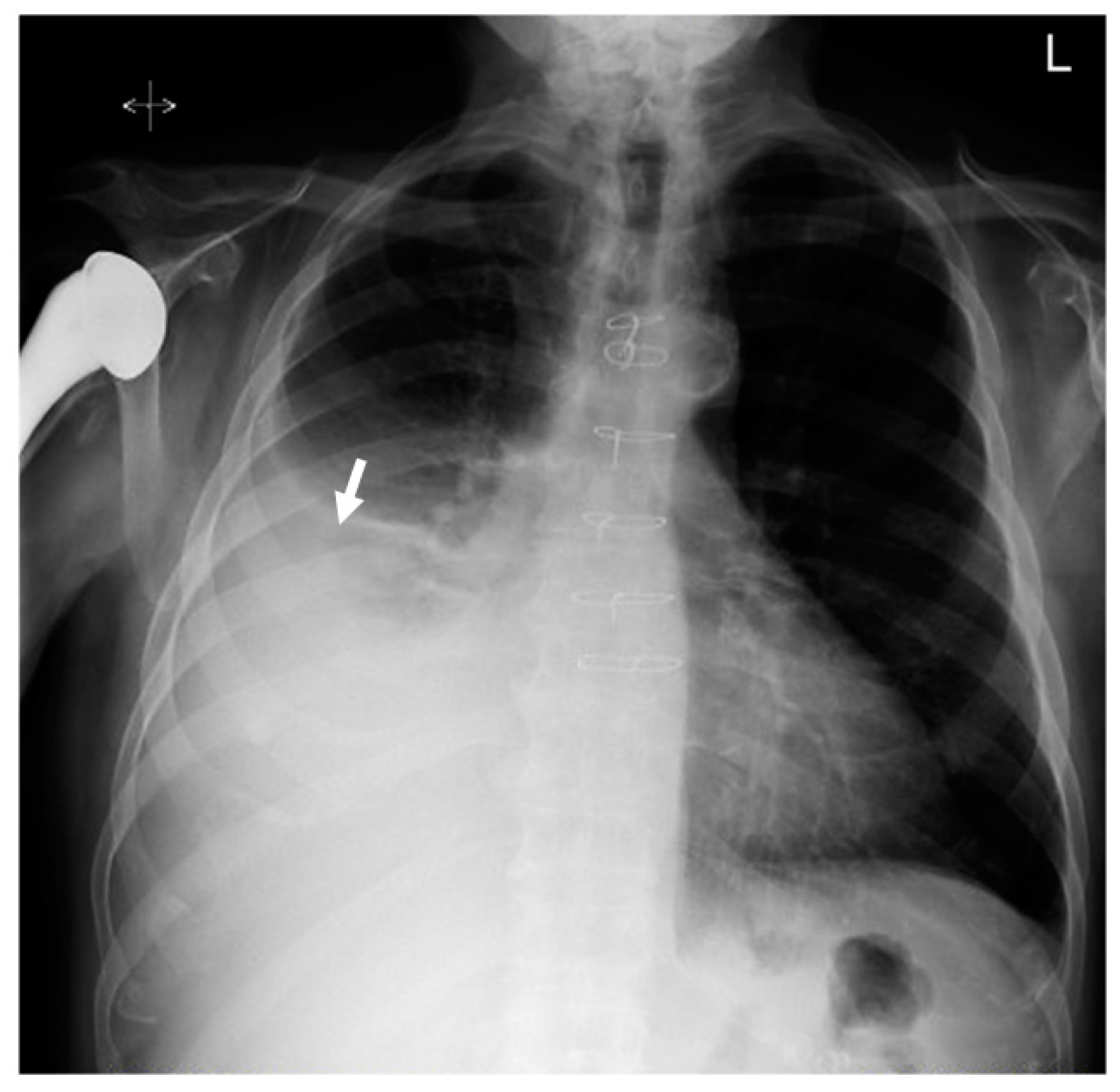
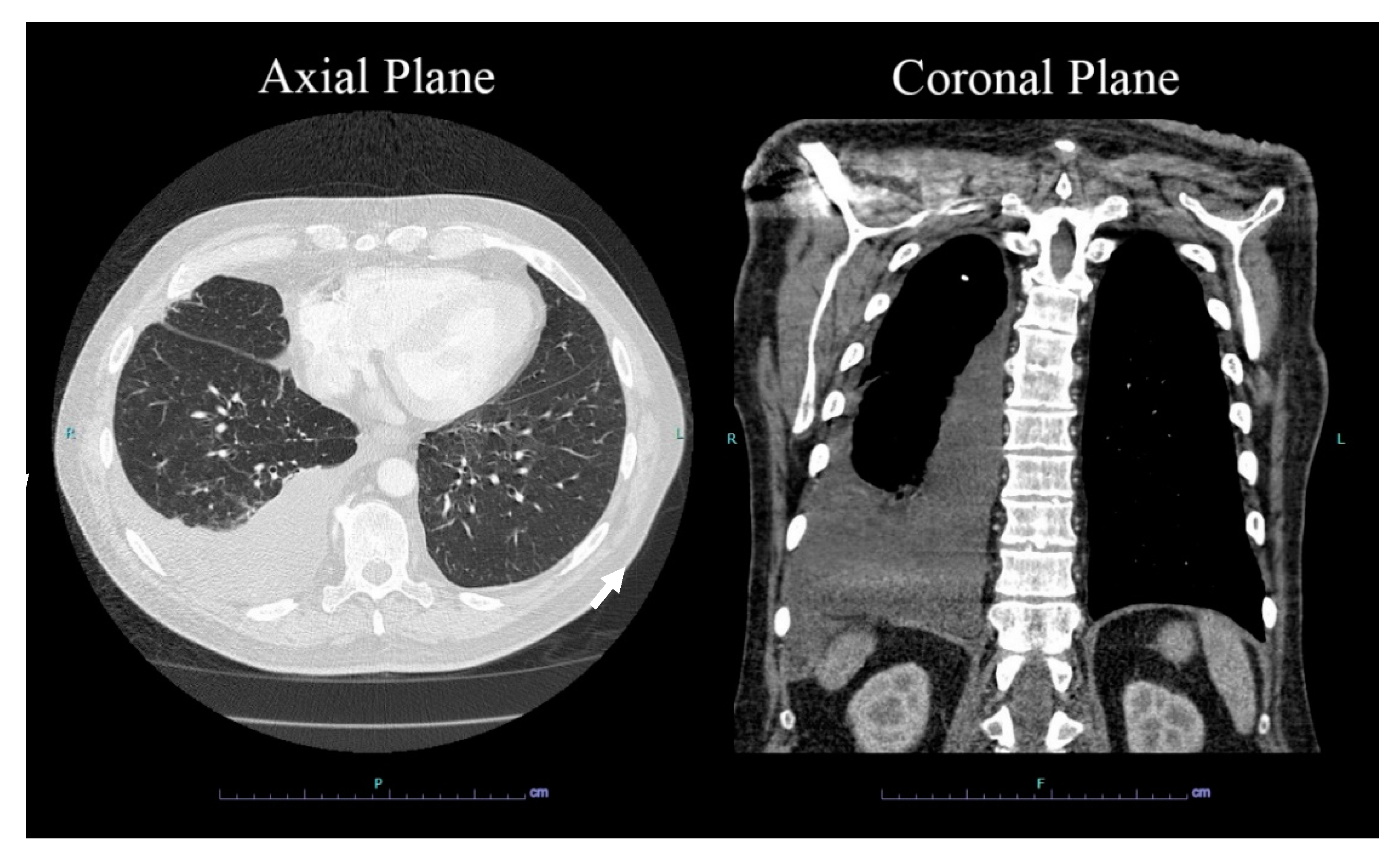
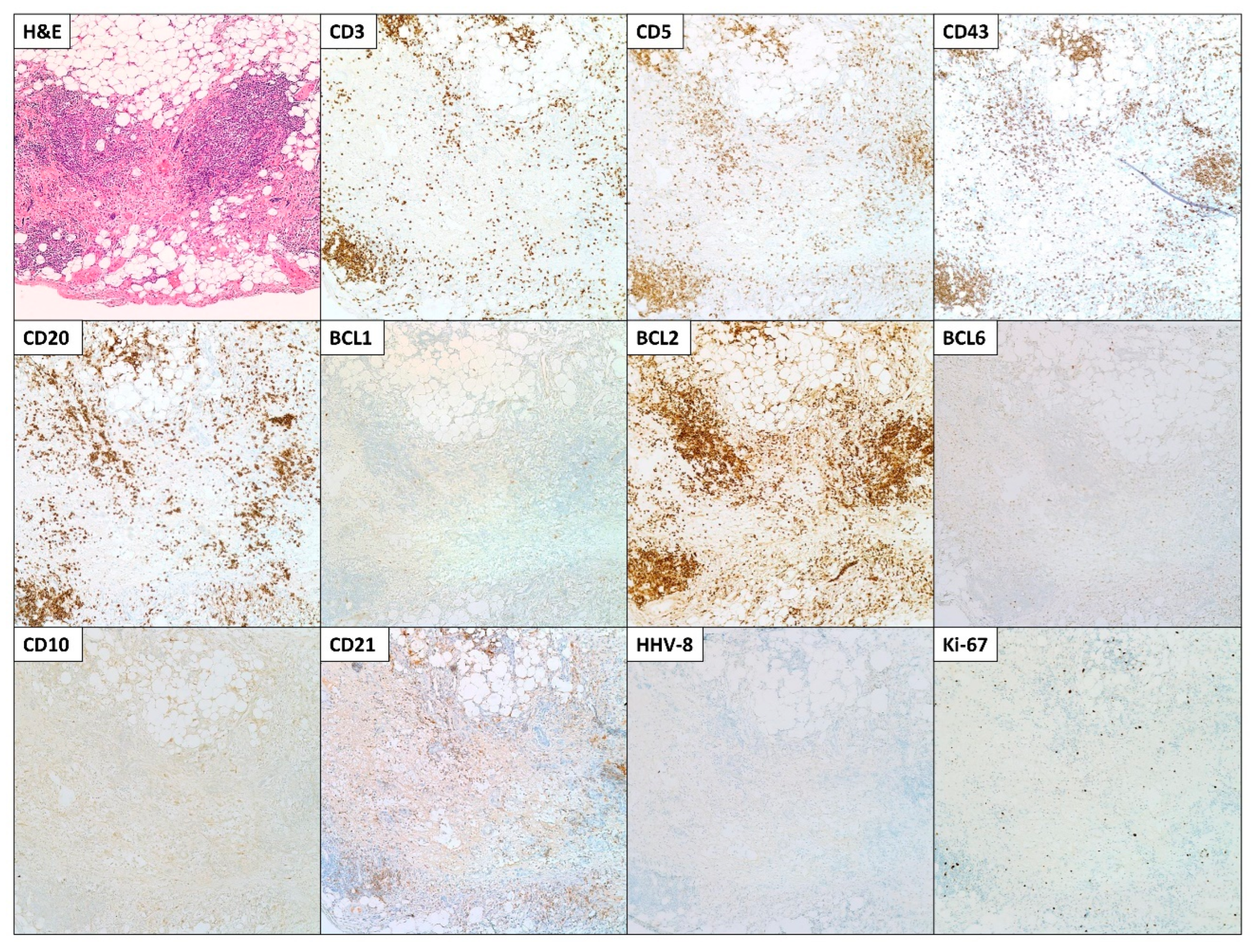
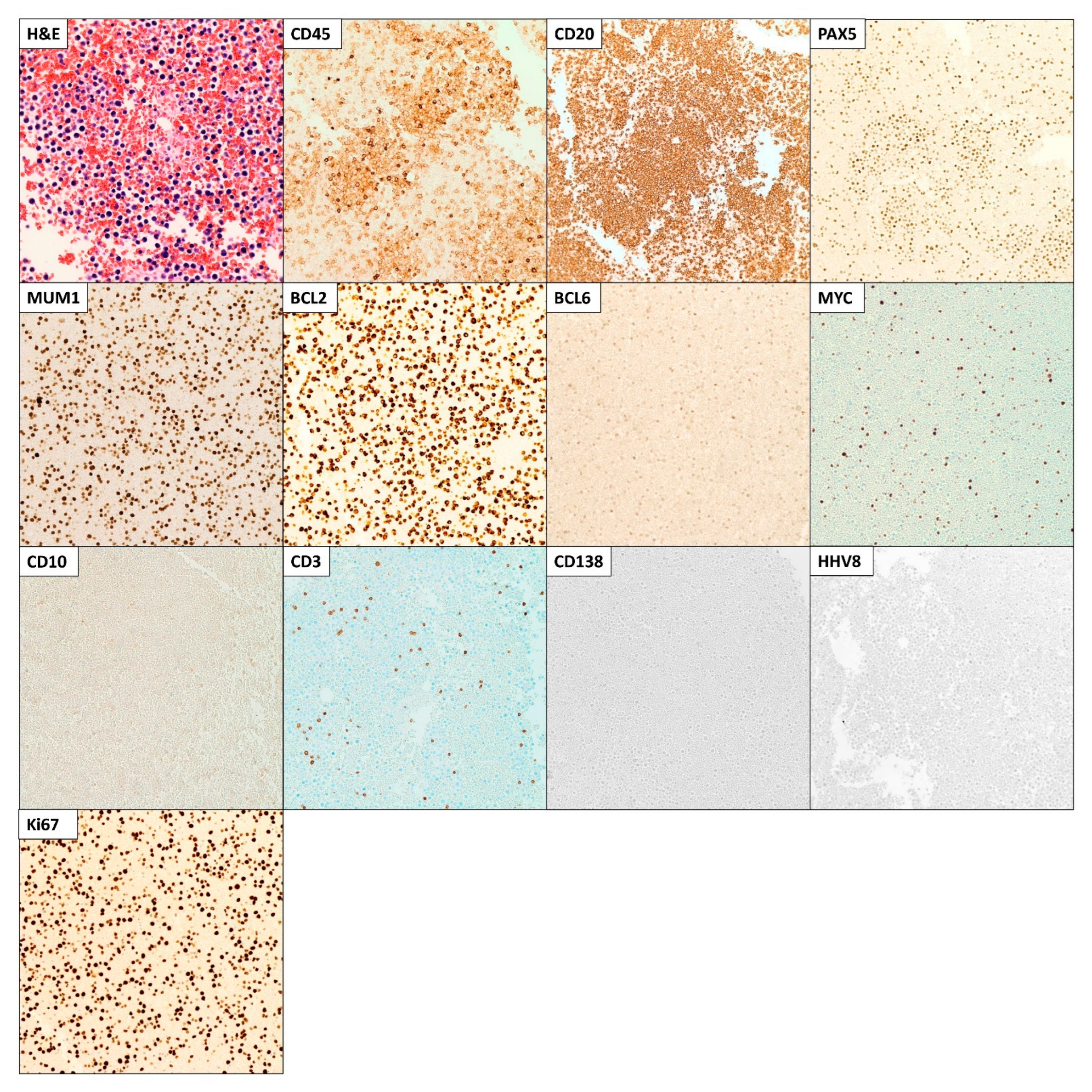
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Mendeville, M.; Roemer, M.G.M.; van den Hout, M.; Los-de Vries, G.T.; Bladergroen, R.; Stathi, P.; Hijmering, N.J.; Rosenwald, A.; Ylstra, B.; de Jong, D. Aggressive genomic features in clinically indolent primary HHV8-negative effusion-based lymphoma. Blood 2019, 133, 377–380. [Google Scholar] [CrossRef]
- Wu, W.; Youm, W.; Rezk, S.A.; Zhao, X. Human herpesvirus 8-unrelated primary effusion lymphoma-like lymphoma: Report of a rare case and review of 54 cases in the literature. Am. J. Clin. Pathol. 2013, 140, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Adiguzel, C.; Bozkurt, S.U.; Kaygusuz, I.; Uzay, A.; Tecimer, T.; Bayik, M. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma: Report of a rare case and review of the literature. Apmis 2009, 117, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Alexanian, S.; Said, J.; Lones, M.; Pullarkat, S.T. KSHV/HHV8-negative effusion-based lymphoma, a distinct entity associated with fluid overload states. Am. J. Surg. Pathol. 2013, 37, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Shum, H.; Lahijani, M.; Winer, E.S.; Butera, J.N. Prognosis in primary effusion lymphoma is associated with the number of body cavities involved. Leuk Lymphoma 2012, 53, 2378–2382. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2008; Volume 2. [Google Scholar]
- Patel, S.; Xiao, P. Primary effusion lymphoma. Arch. Pathol. Lab. Med. 2013, 137, 1152–1154. [Google Scholar] [CrossRef]
- Gisriel, S.D.; Yuan, J.; Braunberger, R.C.; Maracaja, D.L.V.; Chen, X.; Wu, X.; McCracken, J.; Chen, M.; Xie, Y.; Brown, L.E.; et al. Human herpesvirus 8-negative effusion-based large B-cell lymphoma: A distinct entity with unique clinicopathologic characteristics. Mod. Pathol. 2022, 35, 1411–1422. [Google Scholar] [CrossRef]
- Sarkozy, C.; Hung, S.S.; Chavez, E.A.; Duns, G.; Takata, K.; Chong, L.C.; Aoki, T.; Jiang, A.; Miyata-Takata, T.; Telenius, A.; et al. Mutational landscape of gray zone lymphoma. Blood 2021, 137, 1765–1776. [Google Scholar] [CrossRef]
- Kubota, T.; Sasaki, Y.; Shiozawa, E.; Takimoto, M.; Hishima, T.; Chong, J.M. Age and CD20 Expression Are Significant Prognostic Factors in Human Herpes Virus-8-negative Effusion-based Lymphoma. Am. J. Surg. Pathol. 2018, 42, 1607–1616. [Google Scholar] [CrossRef]
- Cooper, A.R.; Burack, W.R.; Allerton, J.P. A case of Kaposi sarcoma-associated herpesvirus/human herpesvirus 8-unrelated but Epstein-Barr virus-positive primary effusion lymphoma-like lymphoma in the setting of human immunodeficiency virus and hepatitis C virus infection. Leuk Lymphoma 2010, 51, 2303–2305. [Google Scholar] [CrossRef]
- Kaji, D.; Ota, Y.; Sato, Y.; Nagafuji, K.; Ueda, Y.; Okamoto, M.; Terasaki, Y.; Tsuyama, N.; Matsue, K.; Kinoshita, T.; et al. Primary human herpesvirus 8-negative effusion-based lymphoma: A large B-cell lymphoma with favorable prognosis. Blood Adv. 2020, 4, 4442–4450. [Google Scholar] [CrossRef]
- Chen, B.J.; Wang, R.C.; Ho, C.H.; Yuan, C.T.; Huang, W.T.; Yang, S.F.; Hsieh, P.P.; Yung, Y.C.; Lin, S.Y.; Hsu, C.F.; et al. Primary effusion lymphoma in Taiwan shows two distinctive clinicopathological subtypes with rare human immunodeficiency virus association. Histopathology 2018, 72, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Gandhiraj, D.; Miller, A.; Zhang, C.; Mwangi, J.; Picker, D.; Charbek, E.; Stoeckel, D. Human Herpes Virus-8 Negative Primary Effusion Lymphoma. In D62. Thoracic Oncology Case Reports II; American Thoracic Society: New York, NY, USA, 2019; p. A6971. Available online: https://10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A6971 (accessed on 20 March 2023).
- Chak, E.; Talal, A.H.; Sherman, K.E.; Schiff, E.R.; Saab, S. Hepatitis C virus infection in USA: An estimate of true prevalence. Liver Int. 2011, 31, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kamitsuji, Y.; Kuroda, J.; Tsunoda, S.; Uoshima, N.; Kimura, S.; Wada, K.; Matsumoto, Y.; Nomura, K.; Horiike, S.; et al. Comparison of human herpes virus 8 related primary effusion lymphoma with human herpes virus 8 unrelated primary effusion lymphoma-like lymphoma on the basis of HIV: Report of 2 cases and review of 212 cases in the literature. Acta Haematol 2007, 117, 132–144. [Google Scholar] [CrossRef]
- Ohshima, K.; Ishiguro, M.; Yamasaki, S.; Miyagi, J.; Okamura, S.; Sugio, Y.; Muta, T.; Sasaki, H.; Tuchiya, T.; Kawasaki, C.; et al. Chromosomal and comparative genomic analyses of HHV-8-negative primary effusion lymphoma in five HIV-negative Japanese patients. Leuk Lymphoma 2002, 43, 595–601. [Google Scholar] [CrossRef]
- Ascoli, V.; Lo Coco, F.; Artini, M.; Levrero, M.; Fruscalzo, A.; Mecucci, C. Primary effusion Burkitt’s lymphoma with t(8;22) in a patient with hepatitis C virus-related cirrhosis. Hum. Pathol. 1997, 28, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Romaguera, J.E.; Katz, R.L.; Said, J.; Cabanillas, F. Primary effusion lymphoma in an HIV-negative patient with no serologic evidence of Kaposi’s sarcoma virus. Leuk Lymphoma 2001, 41, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, E.; Shimazaki, C.; Hirai, H.; Inaba, T.; Hasegawa, G.; Mori, S.; Nakagawa, M. Human herpes virus 8-negative primary effusion lymphoma in a patient with a ventriculoperitoneal shunt tube. Int. J. Hematol. 2001, 74, 327–332. [Google Scholar] [CrossRef]
- Xiao, J.; Selvaggi, S.M.; Leith, C.P.; Fitzgerald, S.A.; Stewart, J., 3rd. Kaposi sarcoma herpesvirus/human herpesvirus-8-negative effusion-based lymphoma: Report of 3 cases and review of the literature. Cancer Cytopathol. 2013, 121, 661–669. [Google Scholar] [CrossRef]
- Zaimoku, Y.; Takahashi, W.; Iwaki, N.; Saito, C.; Yoshida, A.; Aoki, G.; Yamaguchi, M.; Nakao, S. Human herpesvirus-8-unrelated primary effusion lymphoma of the elderly not associated with an increased serum lactate dehydrogenase level: A benign sub-group of effusion lymphoma without chemotherapy. Leuk Lymphoma 2016, 57, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
| Blood Test | Patient Value | Reference Range |
|---|---|---|
| White blood cell (WBC) count | 7.89 × 103/μL | 4.8–10.8 × 103/μL |
| Segmented neutrophils | 43.3% | 42–75% |
| Absolute neutrophil count | 3.42 × 103/μL | 1.8–7.2 × 103/μL |
| Red blood cell (RBC) count | 6.3 × 106/μL | 3.93–5.22 × 106/μL |
| Hemoglobin | 11.1 g/dL | 12.0–16.0 g/dL |
| Hematocrit | 39.6% | 37.0–47.0% |
| MCV | 65.3 fL | 79.0–92.2 fL |
| MCH | 18.3 pg | 25.6–32.2 pg |
| MCHC | 28.0 g/dL | 32.0–36.0 g/dL |
| Platelet count | 65 × 103/uL | 150–450 × 103/uL |
| Serum creatinine level | 0.69 mg/dL | 0.55–1.02 mg/dL |
| Blood urea nitrogen (BUN) level | 8.0 mg/dL | 7–18 mg/dL |
| Prothrombin time (PT) | 16.1 s | 12.4–15.2 s |
| INR | 1.3 | 0.1–1.1 |
| AP thromboplastin time (PTT) | 34.9 s | 24.7–39.8 s |
| pH | 7.38 | 7.35–7.45 |
| PCO2 | 42.0 mmHg | 35.0–45.0 mmHg |
| PO2 | 91.0 mmHg | 75.0–100.0 mmHg |
| HCO3 | 24.8 mmol/L | 22.0–26.0 mmol/L |
| FO-LBCL | PEL | DLBCL | |
|---|---|---|---|
| Morphology | Variable morphology between large immunoblastic, plasmablastic, or anaplastic large cell lymphoma | Variable morphology between large immunoblastic, plasmablastic, or anaplastic large cell lymphoma | Diffuse sheets of large, atypical cells with vesicular chromatin and prominent nucleoli |
| Pan B-cell markers | Positive | Negative | Positive |
| EBV | +ve (13–30% [5,11]) | +ve (80% [1]) | +/− (<6% [1]) |
| HHV-8 | Negative | Positive | Negative |
| Lymph node involvement | No | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahmad, H.F.; Gomez, A.S.; Deb, A.; Safdie, F.M.; Sriganeshan, V. Fluid Overload-Associated Large B-Cell Lymphoma: A Case Report and Review of Literature. Hematol. Rep. 2023, 15, 411-420. https://doi.org/10.3390/hematolrep15030042
Bahmad HF, Gomez AS, Deb A, Safdie FM, Sriganeshan V. Fluid Overload-Associated Large B-Cell Lymphoma: A Case Report and Review of Literature. Hematology Reports. 2023; 15(3):411-420. https://doi.org/10.3390/hematolrep15030042
Chicago/Turabian StyleBahmad, Hisham F., Aaron S. Gomez, Arunima Deb, Fernando Martin Safdie, and Vathany Sriganeshan. 2023. "Fluid Overload-Associated Large B-Cell Lymphoma: A Case Report and Review of Literature" Hematology Reports 15, no. 3: 411-420. https://doi.org/10.3390/hematolrep15030042
APA StyleBahmad, H. F., Gomez, A. S., Deb, A., Safdie, F. M., & Sriganeshan, V. (2023). Fluid Overload-Associated Large B-Cell Lymphoma: A Case Report and Review of Literature. Hematology Reports, 15(3), 411-420. https://doi.org/10.3390/hematolrep15030042







