Abstract
Watercress is a nutrient-dense, aquatic leafy vegetable with significant public health and economic potential. Hydroponically cultivated watercress can offer greater nutritional benefits due to the controlled delivery of specific nutrients. From an agronomist’s perspective, watercress has the advantage of optimized environmental resource efficiency, achieved through reduced energy, chemical, and water consumption, as well as its short cultivation cycle. Glucosinolates (GSLs) in watercress enhance sustainable agriculture by naturally protecting crops from pests and diseases, reducing the need for chemical inputs. They also increase market value and shelf-life, supporting resource-efficient and profitable farming. Within the pharmaceutical space, GSLs are well-known for their chemo preventive and anti-inflammatory properties. This review aims to summarize research findings, critically evaluate existing studies to highlight current knowledge, and identify research gaps, and to guide future investigations. The synthesis of the reviewed literature demonstrates that increased sulphate generally improves GSL content. However, not many studies have looked specifically at how magnesium sulphate (MgSO4) affects watercress. This review highlights the specific impact of MgSO4 on GSL production in watercress, which could provide valuable insights for optimizing nutrient management in hydroponic systems and enhancing the health benefits of this nutrient-dense crop.
1. Introduction
Different cultivation approaches, including hydroponics, have been employed to evaluate the accumulation of key metabolites and plant performance metrics such as fresh and dry biomass, leaf area, and root hair development [1]. Glucosinolates (GSLs), the principal sulphur (S) containing secondary metabolites in the Brassicaceae family, exhibit variable accumulation depending on environmental S availability and S fertilization levels [2]. Studies in model and crop species like Arabidopsis and Brassica have demonstrated that S-containing metabolites, including GSLs, are sensitive to variations in nutrient composition, especially S availability [3]. Magnesium sulphate (MgSO4) is a widely used nutrient in hydroponic systems. Magnesium (Mg2+) acts as a cofactor in numerous enzymatic reactions within secondary metabolism, while sulphate (SO42−) contributes directly to GSL biosynthetic precursors [4]. Although hydroponic systems provide the advantage of precise nutrient manipulation and can be used to evaluate the biochemical sensitivity of watercress to cultivation conditions, few studies have specifically investigated how MgSO4 influences GSL biosynthesis in watercress. Preliminary findings suggest that hydroponic conditions, including the use of microbubbles and tailored nutrient regimes, can modulate GSL accumulation in watercress [5].
Watercress’s long-standing use in traditional medicine is supported by numerous pharmacological studies highlighting its therapeutic potential [6,7]. This nutrient-dense crop exhibits a range of therapeutic effects, such as antioxidants, anti-inflammatory, heart-protective, skin-healing, antibacterial, and anticancer activities [8]. Additionally, watercress leaves can serve as natural remedies, acting as diuretic, cleansing, cough-relieving, toothache-soothing, and blood sugar-lowering agents [9]. Experimental and human trials suggest that watercress intake may enhance lipid metabolism and influence inflammatory responses associated with cardiovascular and respiratory diseases [10]. Within cancer models, watercress extracts demonstrate growth-inhibiting, cell death-promoting, and cancer-preventive activities, thus exhibiting targeted toxicity against malignant cells while safeguarding the healthy ones [11,12]. The hydrolysis product formed from GSL breakdown by myrosinases (2-Phenethyl isothiocyanate) has demonstrated anticancer properties by inhibiting phase I metabolic enzymes and enhancing the activity of phase II detoxification enzymes [13]. These medicinal properties are primarily attributed to watercress’s high content of GSLs, flavonoids, vitamin C, and carotenoids, which collectively help to promote human and animal health [14,15]. Beyond their medicinal value, these bioactive constituents are closely linked to the crop’s characteristic flavor profile. Brassica family crops, including watercress, are abundant sources of (GSLs).
Watercress is characterized by its distinctive peppery flavor and abundant phytonutrients, particularly GSLs [16]. Garden cress is incorporated into soups, sandwiches, and salads for its sharp, zesty taste. It is also consumed as sprouts, and both the fresh and dried seed pods can serve as a spicy condiment [17]. In a culinary assessment by Renna et al. [18], watercress that grows devoid of soil was regarded as the initial step in the culinary process for preparing the dishes that were part of the assessment process. Vegetables like watercress produce a wide range of specialized compounds, such as phenolics, terpenoids, alkaloids, and S-containing substances, which enhance their nutritional benefits, taste, and fragrance. Watercress particularly, is a remarkable provider of essential minerals such as Mg, iodine, copper, phosphorus, calcium, and iron, along with flavonoids, particularly kaempferol, quercetin, and rutin which are well known for their antioxidant, anti-inflammatory, and heart-protective properties [19,20]. The characteristic odor and pungency of Brassica crops are caused by isothiocyanates, which are degradation products of GSLs [21]. Importantly, these biochemical pathways are central to the health-promoting and ecological functions of GSLs.
The biosynthesis of GSLs occurs through three distinct stages as shown in Figure 1. The first stage begins with the corresponding amino acid. The carbon backbone of amino acids other than tryptophan may be extended via a series of iterative reactions that can repeat as many as six cycles. Next, the fundamental GSL scaffold is assembled. Finally, the resulting GSLs may experience numerous downstream tailoring reactions, including hydroxylation, methylation, oxidation, desaturation, or benzoyl substitution, most of which modify the side chain [22,23]. These GSLs are then subject to hydrolysis by the enzyme myrosinase, which is a critical step in the breakdown process (Figure 2a). The biosynthesis of GSLs culminates in a crucial sulfation step which provides the key sulfated functional group required for GSL biological activity (Figure 2b). Please see Section 4 “The Science Behind GSLs” for details.
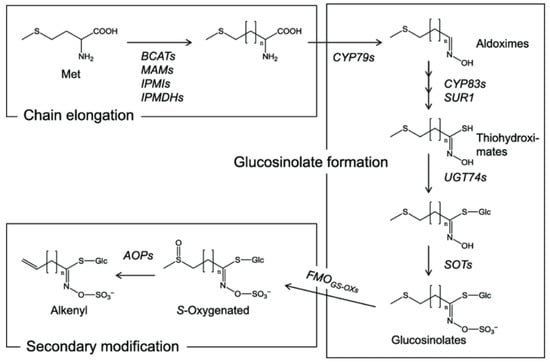
Figure 1.
Biosynthesis of GSLs in Brassicaceae: chain elongation stage, reconfiguration step that produces the core GSL structure, and side-chain modification stage of the GSL core. Adapted from Graser et al. [24].
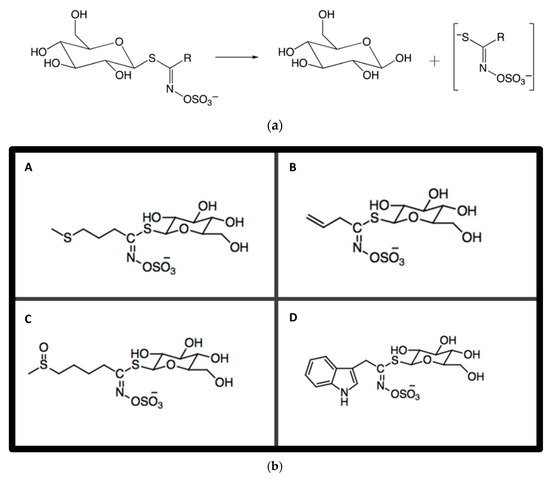
Figure 2.
(a) The hydrolysis of glucosinolates by myrosinase upon tissue disruption. R represents alkyl aryl or indole. (b) Chemical structures of the main GSLs in cruciferous plants. The Figure shows (A) Glucoiberverin, (B) Sinigrin, (C) Glucoraphanin (D) Glucobrassicin.
2. Review Methodology
This study employs a review methodology characterized by a rigorous, transparent, and methodologically sound process for GSL biosynthesis in hydroponically grown watercress in response to. The approach encompasses the formulation of explicit inclusion and exclusion criteria, the execution of an exhaustive search across multiple peer-reviewed academic databases, critical evaluation of the methodological quality of selected studies, and the synthesis of findings to derive evidence-based conclusions or to elucidate unresolved gaps within the current body of knowledge. In preparing this review, the authors conducted a literature search using these databases: Google Scholar, Web of Science, ScienceDirect, Scopus and PubMed. The keywords used were “Brassica”, “watercress”, “glucosinolates”, “hydroponics”, magnesium sulphate and sulphur assimilation. Inclusion criteria were limited to English-language studies published between 1 January 2000 and 1 October 2025. Data extraction focused on the effects of MgSO4 fertilization on GSL accumulation in Brassica vegetables. Additional data were extracted on genetic factors and environmental determinants of GSL levels in Brassica. The GSL biosynthesis, composition, and their implications for agriculture and human health were also explored.
The biochemical modulation of GSL biosynthesis in hydroponically grown watercress in response to sulphate fertilization remains an underexplored area, despite its significant implications for functional food production and plant secondary metabolism. While GSL pathways have been partially characterized in watercress through transcriptomic and metabolic profiling, previous studies have largely overlooked the effects of mineral nutrient inputs, particularly sulphate, within controlled hydroponic systems. Previous studies have predominantly focused on genetic manipulation, model species such as Arabidopsis thaliana, or broad comparisons between cultivation systems (e.g., aquaponics vs. hydroponics), without dissecting the role of targeted sulphate enrichment, but suggesting the potential of sulphate modulation as a strategic lever to enhance the nutritional and health-promoting properties of watercress.
In this review, we highlight recent advancements regarding the role of GSLs in watercress, drawing primarily from studies on S fertilization effects, the biosynthetic pathways of GSLs within the Brassicaceae family, and the influence of environmental, epigenetic, and mineral nutrient factors, particularly S and Mg, on GSL biosynthesis and metabolism. Our objective is to critically assess how sulphate fertilization influences GSLs profiles in hydroponically grown watercress, thereby linking plant physiological responses with targeted biochemical regulation.
3. History of Watercress
Based on data from the Food and Agriculture Organization of the United Nations, cruciferous vegetable cultivation spanned over 0.48 million hectares in 2020 with significant year-on-year growth, particularly for cabbage, Chinese cabbage, and cauliflower, although production remains geographically limited [25]. According to the USDA [26], a total of 638 acres of watercress were harvested for the fresh market across the United States. Of this, 22 acres were harvested in California, while Kentucky accounted for 7 acres of freshly harvested watercress. The origins of watercress trace back to ancient Greece and Rome, where it was utilized as both a nutritional staple and a remedy for various ailments, such as digestive problems and scurvy [27]. Its continued popularity throughout the Middle Ages and Victorian era for its nutritional and therapeutic benefits laid the foundation for modern studies [28]. Watercress is identified by the US Centers for Disease Control and Prevention (CDC) as the crop offering the greatest nutrient density per calorie [22]. Interest in watercress is driven in part by its unique phytochemical profile, particularly its content of GSLs, which are chemically stable and biologically inert, comprising a fundamental framework containing a sulphated oxime moiety linked to thioglucose.
4. The Science Behind Glucosinolates
The GSLs are S and nitrogen (N) containing secondary metabolites found mainly in cruciferous vegetables like watercress, broccoli, and mustard. The GSLs are typically water-soluble and remain stable within undamaged plant tissues. However, high intake of raw cruciferous vegetables can interfere with thyroid function, due to goitrogenic compounds. The GSLs are composed of a fundamental framework containing a sulphated oxime moiety linked to thioglucose, with side chains originating from amino acids (Figure 2) [23,29]. Amino acid chain elongation (Figure 3) only happens for certain GSLs (mainly methionine-derived). At this chain elongation stage, GSL biosynthesis pathway begins in the cytosol, where methionine or phenylalanine undergo transamination via branched-chain amino acid aminotransferase 4 (BCAT4), resulting in the formation of 2-oxo acids [30]. These intermediate compounds are subsequently transported into the chloroplast by Bile Acid Transporter 5 (BAT5), where the enzymes involved in side-chain extensions are situated [31]. Once inside the chloroplast, the enzyme methylthioalkylmalate synthase (MAM) facilitates the condensation of 2-oxo acids with acetyl-CoA, yielding a 2-malate derivative [32]. In the elongation of aromatic GSLs, MAM3 was identified as a substrate for phenylalanine, indicating a potential involvement in the biosynthesis of aromatic GSLs [33]. As illustrated in Figure 3, the resulting 2-malate derivative is then converted to a 3-malate derivative by the isopropylmalate isomerase 1 (IPM1) complex [34]. During the concluding phase of the elongation process, the extended 2-oxo-acid either proceeds through additional rounds of elongation or is converted via transamination by branched-chain amino acid aminotransferase 3 (BCAT3) into the respective amino acid, which subsequently participates in the formation of the core structure [35].
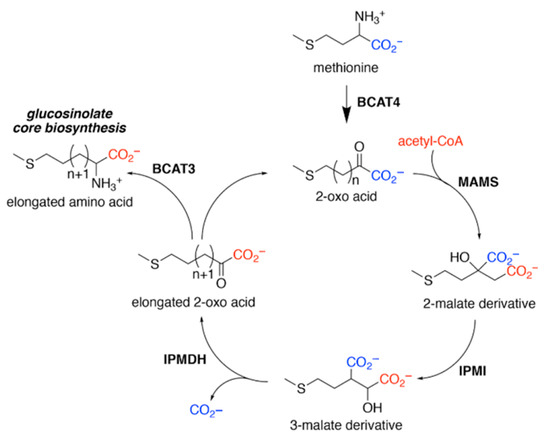
Figure 3.
Chain elongation in GSLs. The transformation of methionine into its corresponding 2-oxo acid (4-(methylsulfanyl)-2-oxobutanoate) by branched-chain amino transferase 4 (BCAT4) initiates the chain elongation process. Subsequently, a sequence of enzymatic reactions involving methylthioalkylmalate synthase (MAMS), isopropylmalate isomerase (IPMI), and isopropylmalate dehydrogenase (IPMDH) extends the aliphatic chain by a single methylene unit. The elongated 2-oxo acid can either undergo transamination to form the corresponding amino acid via BCAT3 or re-enter the elongation cycle for further chain extension.
After chain elongation, Met is activated to aldoxime and nitrile oxide by cytochrome P450 (CYP83) and conjugated with glutathione (GSH) by GSH-S-transferase. Following these initial activation and conjugation steps, the pathway advances into the metabolic rearrangement phase. The process of metabolic rearrangement initiates with the oxidation of the elongated amino acid into aldoximes which are converted to thiohydroximic acids. The activated aldoxime is subsequently linked to glutathione (GSH), which serves as a S source, resulting in the formation of the corresponding thiohydroximate intermediate (Figure 4). The GSL core structure is then formed and a third stage of biosynthesis proceeds with various R-group modifications, such as hydroxylation, methoxylation, oxidation, desaturation, and benzoylation [30].
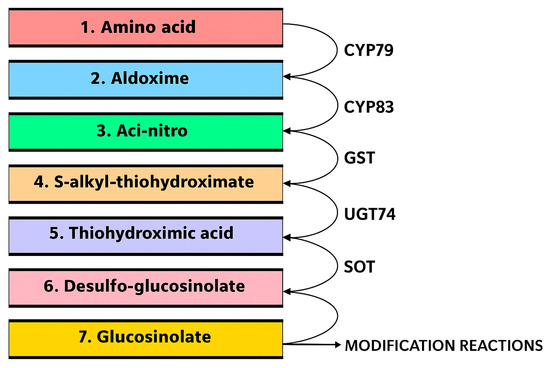
Figure 4.
Enzymes involved in the biosynthesis of the core glucosinolate structure of Brassicaceae.
The biosynthesis of GSLs in Brassicaceae vegetables involves a sequence of five enzymatic reactions: oxidation, sequential oxidation, carbon-S bond cleavage, glycosylation, and sulfation. The key enzymes involved in Brassicaceae GSL biosynthesis include CYP79, CYP83, UGTs, and sulfotransferases as shown in Figure 4. Firstly, the oxidation of the precursor amino acid (e.g., tryptophan, phenylalanine, methionine) into the corresponding aldoxime is catalyzed by cytochrome P450 monooxygenases of the CYP79 family, in a NADPH- and O2-dependent mechanism, yielding the aldoxime intermediate [36]. Second, a sequential oxidation of the aldoxime by CYP83 enzymes generates reactive aci-nitro or nitrile oxide intermediates, which are conjugated to glutathione via glutathione-S-transferases to produce S-alkyl-thiohydroximates (Figure 1) [37]. Third, carbon–S bond cleavage is carried out by the lyase SUR1 which processes the glutathione-conjugated intermediate into thiohydroximic acid (Figure 1), effecting release of glutamate residues and forging the C–S bond essential to the thioglucose attachment [38]. Fourth, a glycosylation step is performed by UDP-glucosyltransferases of the UGT74 family (e.g., UGT74B1, UGT74C1), which transfers UDP-glucose to the thiohydroximate to yield desulfoglucosinolate, forming the S-glycosidic linkage [39,40]. Finally, a sulfation reaction, mediated by desulfoglucosinolate sulfotransferase enzymes, transfers a sulphate moiety from 3′-phosphoadenylyl sulphate (PAPS) onto the desulfoglucosinolate substrate to yield the mature GSL, releasing adenosine 3′,5′-bisphosphate (PAP) as a by-product [41]. This is a rate-limiting step since the availability of PAPS and sulfotransferase activity can constrain GSL accumulation.
5. Glucosinolates in Agriculture
GSLs are of immediate practical importance to farmers because they underpin multiple key benefits, primarily through their roles in plant defense, soil health, and breeding strategies. They act as inducible chemical defenses that reduce herbivore damage and pathogen incidence, providing biofumigant activity when Brassicaceae residues are incorporated into soil, and offering clear targets for breeding programs aimed at enhancing host resistance or tailoring seed/forage quality. Together these effects can improve yield stability, reduce reliance on synthetic pesticides, and contribute to integrated pest-management strategies. Growth of cruciferous crops is affected by a variety of fungi, bacteria, aphids, and other harmful insects. GSL-mediated defense mechanisms against bacterial infections have been demonstrated, whereby exposure to Burkholderia cepacia, Pseudomonas syringae, and Xanthomonas campestris pv. campestris (Xcc) triggered an increase in the expression of GSL biosynthetic pathways [42]. Likewise, GSL-driven defense mechanisms against fungal pathogens have been proven, whereby water-based plant extracts rich in isothiocyanates are can reduce the in vitro growth of Alternaria brassicicola, a fungal pathogen that infects cruciferous crops, by approximately 50% [43]. Additionally, a comparison of GSL-deficient biosynthetic (gsm1-1) mutants with wild-type Arabidopsis plants showed that isothiocyanates are key contributors to Arabidopsis defense against infections by Plectosphaerella cucumerina, Botrytis cinerea, Fusarium oxysporum, and Peronospora parasitic [44].
Despite long-lived knowledge that plants rich in GSL can suppress soil-dwelling plant pests [45], scientists were eager to investigate chemical dynamics within and around roots during pest suppression. They found that B. nigra, B. juncea, and B. napus plants immediately release m/z 60, a compound which indicates formation of an isothiocyanate from sinigrin, when their roots are injured or attacked by cabbage root fly larvae [46]. Both farming practices and breeding research have shown that GSL levels can be adjusted to balance pest resistance with food and feed safety for example, low-GSL varieties for seed meal and feed, or higher levels for pest control and biofumigation. However, some herbivores may take advantage of certain GSL profiles, so the choice of cultivar should consider the local pest issues. Further, GSLs aide farmers to enhance crop protection and breeding strategies are optimized to local soils, climates and production goals [47]. Beyond their agronomic relevance, the significance of GSLs extends to human nutrition, where their unique biochemical properties underpin the health-promoting qualities of cruciferous vegetables.
6. Glucosinolates in the Human Diet
The GSLs contribute to the health benefits of cruciferous vegetables and distinguish them from other plant types as shown in Table 1. The watercress plant harbors a diverse range of bioactive compounds, such as quercetin, lutein, β-carotene, phenolic acids, and GSLs [48,49]. GSLs may increase or decrease in vegetables during storage. They can be broken down or leached out during processing, or retained through heat-induced inactivation of myrosinase. These compounds are metabolized by plant-derived myrosinase in the small intestine or by microbial myrosinase in the colon. Isothiocyanates are taken up from both the small intestine and the colon, and their byproducts can be detected in human urine within two to three hours following the consumption of Brassica vegetables. Further, 4-methylsulfinylbutyl (glucoraphanin) GSL, present in broccoli, breaks down into the isothiocyanate sulforaphane, a compound that inhibits the cell cycle and induces programmed cell death, helping to suppress tumor development [50]. Given the structural and functional diversity of GSLs across plant species, it is important to consider the distinct physiological and pharmacological roles of individual compounds.

Table 1.
Glucosinolate content in Brassicaceae plants.
7. Pharmacological Potential of Watercress
Among the five individual GSLs, gluconasturtiin (phenethyl GSL) is the major GSL in leaves and stems of watercress. Gluconasturtiin has historically been employed in managing diabetes [64]. Watercress extracts enriched with gluconasturtiin or polyphenolic compounds have been reported to exert cytotoxic effects on a wide range of human cancer cell lines, including those of the breast, colon, lung, liver, prostate, cervix, melanoma, leukemia, and myeloma [65]. The medicinal leaf is also well known for its mucus-clearing, toothache-relieving, and estrogen-inhibiting properties [66]. Consumption of raw watercress has also been shown to elevate plasma antioxidant concentrations, particularly lutein and β-carotene [67]. Watercress can also be used in phytotherapy, rhinitis, and homeopathic medicines [16]. As a natural remedy, leaves of watercress can serve as a diuretic, detoxifier, expectorant, pain reliever for toothaches, and blood sugar-lowering agent [9]. In hypercholesterolemic rats, watercress intake was associated with increased activities of superoxide dismutase and catalase, alongside reduced hepatic levels of glutathione (GSH), glutathione reductase (GR), glutathione peroxidase (GPx), and malondialdehyde (MDA) [68]. Watercress has also been documented to possess antimicrobial activity [69] and anti-tubercular activity [70]. Furthermore, watercress juice has been found to regulate hyperglycemia by inhibiting α-glucosidase activity while enhancing the functions of lipase and α-amylase [71]. The use of oil in treating heat and chemical-induced burn wounds in rabbits has been demonstrated [72]. The antihypertensive activity and the anti-ulcer capability of watercress have also been reported [73,74], documenting the effectiveness of the ethanol-based extract of watercress as a supportive agent in the recovery of injury-induced ulcers on the dorsal surface of rat tongues. An antihyperlipidemic activity study demonstrated that flavonoids, phenolic constituents, and glycosides from the plant contribute to cholesterol-lowering, lipid-reducing effects [75] and improved urinary system health [76]. Building on these pharmacological insights, it is critical to examine the biosynthetic pathways and accumulation patterns of GSLs, which underpin many of the health-related properties of watercress.
8. Literature Gaps Relevant to Glucosinolates in Watercress
8.1. Glucosinolate Accumulation Under Soilless Conditions
Hydroponic systems offer controlled nutrient management that shapes plant metabolic profiles, making the understanding of flow rate effects essential for maximizing both crop performance and system efficiency. A study by Hameed et al. [77] substantiates this, since altering ammonium/nitrate (NH4+/NO3−) ratios generates distinct metabolomic profiles in lettuce across soil, substrate, and hydroponic systems, offering candidate markers for enhancing N efficiency. Moreover, studies have probed watercress growth, nutrient uptake efficiency, and antioxidative enzyme activity within hydroponic environments [78,79,80]. In Brassica vegetables, results from an analysis identified 23 principal metabolites, chiefly GSLs and flavonoids, which played a pivotal role in differentiating the metabolic profiles of watercress leaves cultivated in hydroponic systems [81]. Despite increasing interest in biofortification and controlled-environment agriculture, the biochemical response of watercress to mineral nutrient modulation in hydroponic systems, particularly sulphate fertilization, remains poorly understood. Existing studies have explored GSL biosynthesis through genetic manipulation and environmental perturbation in model species or under soil-based cultivation [82], yet there is a lack of consolidated evidence addressing how sulphate availability influences GSL accumulation in watercress under soilless conditions. This gap limits the development of nutrient management strategies aimed at optimizing both yield and phytochemical richness in controlled environments. By synthesizing cross-disciplinary literature on sulphate-driven modulation of GLSs in hydroponically grown watercress, this review provides a critical foundation for targeted nutrient interventions, offering new insights for functional food production, sustainable horticulture, and public health nutrition. Thus, insights into nutrient modulation in watercress directly intersect with the growing emphasis on functional foods in public health and agriculture.
8.2. Impact of Magnesium Sulphate on Glucosinolate Accumulation
Mg is essential for numerous enzymatic functions, including energy metabolism, while sulphate is employed as a major pathway for producing various S-containing metabolites, including amino acids (cysteine and methionine), glutathione (GSH) and GSLs as shown in Figure 5. A study by Kopsell et al. [83] shows that MgSO4 fertilization promotes GSL synthesis in watercress. One key finding across multiple studies was that moderate concentrations of MgSO4 can increase the total GSL content in plants [84]. This effect is likely due to the activation of S assimilation pathways and gene regulation mechanisms involving v-myb avian myeloblastosis viral oncogene homolog (MYB) transcription factors [85]. Analogous work in broccoli sprouts showed that 50 mM MgSO4 treatment increased total GSLs by approximately 42% within 24 h, though with some yield penalty [86]. This reduced biomass result aligns with previous findings in Brassica species, where increased S availability enhances secondary metabolite accumulation while concurrently impairing growth through resource reallocation or stress signaling [87]. Together, these examples illustrate a classic biofortification trade-off: enhanced secondary metabolism achieved at the expense of primary growth (both fresh and dry biomass). Conversely, a study by Lu et al. [88] showed that Mg fertilization significantly improved the yield but reduced nutritional quality. From an agronomic perspective, enhancing GSLs while minimizing biomass loss requires careful management to balance nutritional quality with yield and production efficiency [89]. Further, success depends on crop-specific optimization of dosage, timing, and genotype to achieve a favorable balance between enhanced nutraceutical value and agronomic productivity [90]. Thus, understanding how MgSO4 modulates this pathway could offer novel strategies to enhance the nutritional and functional value of watercress cultivated in controlled environments. To effectively investigate these nutrient–metabolite interactions, cultivation platforms with controlled growing conditions are essential.
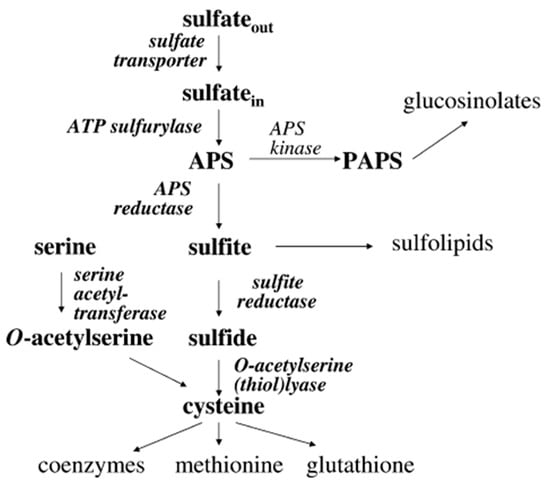
Figure 5.
Schematic representation of plant sulphate assimilation. Adapted from Kopriva et al., [91].
8.3. Impact of Magnesium Sulphate on Glucosinate Accumulation in Hydroponically Grown Watercress
Hydroponic systems offer an ideal platform for investigating biochemical interactions due to their accuracy in nutrient delivery and environmental control [92]. MgSO4 contributes about 13% S by weight in the commonly used hydroponic solutions such as Hoagland solution [93]. It is a low-cost hydroponic fertilizer containing 10% Mg and 16% kieserite, which are both highly water-soluble and quick-acting sources of Mg [94]. It is chosen for its high solubility, nutrient supply duality, and compatibility with other salts [95,96], and its ability to trigger secondary metabolites including GSLs [97]. Other sources of hydroponic S fertilization such as sodium sulphate (Na2SO4), ammonium sulphate ((NH4)2SO4), potassium sulphate (K2SO4) and calcium sulphate (CaSO4) are less soluble, may introduce cations that can unbalance the nutrient solution and may be toxic to plant stress if supplied in high doses [98,99]. Further, a study by Ali et al. [100] investigated the relationship between S availability and GSL biosynthesis. In their experiment, (Lepidium latifolium, which hails from the mustard family and first formally described by Carl Linnaeus) was supplied with varying levels (0.5 and 1.0 mM) of sulphate sources. Further, MgSO4, zinc sulphate (ZnSO4), and their mixtures were also added. Application of these salts led to marked increases in GSL concentrations, reaching up to 29% and 38% with MgSO4 and ZnSO4, respectively.
9. Factors Influencing Glucosinolate Biosynthesis and Accumulation
9.1. Effect of Sulphur Fertilization on the Glucosinolate Content
S and N have been demonstrated to impact GSL concentrations across a range of vegetable crops. A review by Falk et al. [2] concluded that sulphur fertilization commonly results in marked increases in GSL content across Brassica species, sometimes up to a 50% increase, depending on species and treatment. A study by Barickman et al. [61] confirms this since both N and S applications showed a positive association with gluconasturtiin levels, which varied between 1.5 and 19.6 µmol per gram of dry weight. Sulphur, an essential nutrient for plant growth and development, fulfills various functions in metabolic processes and adaptation to stress within plants. The elements’ contribution to improving the yield and quality of cruciferous crops has attracted growing interest in agricultural studies [101]. Given that GSLs can constitute as much as 30% of the total S pool within plant tissues, their synthesis and accumulation are likely to be closely linked to the plant’s overall S nutritional status [2]. Further, S is found in protein-related amino acids like methionine and cysteine, as well as in vitamins such as biotin and thiamine, phytochelatins, chlorophyll, coenzyme A, and S-adenosylmethionine [102]. The S nutrient is frequently used as a soil amendment and is particularly crucial for the growth of crops such as onions, garlic, and members of the cruciferous family like broccoli and cauliflower [101]. This nutrient is an integral part of the GSL’s core structure, and it is directly incorporated into the thioglucoside bond. Haneklaus et al. [103], along with Zenda et al. [3], provide an overview of how S supplementation influences the GSL levels in various agricultural crops. Further studies by Krumbein et al. [104] show that higher S availability enhances the levels of certain GSLs, such as glucobrassicanapin, progoitrin, gluconapin, and sinigrin, in Brassica juncea L., as well as the overall GSL content in Brassica rapa. Sulphur fertilization can satisfy the nutrient demand of the crop and increase the GSL content. This is shown by a study by Traka et al. [105] which shows that broccoli hybrids with elevated glucoraphanin levels had 2.5–3 times more glucoraphanin content, due to improved S uptake. A study by Kopsell et al. [83] showed that, in watercress tissues, the levels of aromatic, indole, and overall GSLs were influenced by variations in N supply, while aliphatic, aromatic, and total GSL contents were affected by adjustments in S nutrition. A study by Kim et al. [106] found that when MgSO4 was applied through the roots of broccoli, the levels of glucoraphanin rose significantly within 24 h. Additionally, a study by Szulc et al. [107] agrees with previous findings, in that their study found the overall GSLs content, along with the combined amount of alkenyl GSL, is significantly greater in oilseed rape seeds treated with 60 kg·ha−1 of elemental S applied to the soil. Further, another study showed that S application led to an increase in indole GSL levels in cabbage [108]. In germinating broccoli sprouts, S fertilization enhances the GSL glucoraphanin [109]. Treatments which use amino acids alone or with S and boron, culminate in the highest GSL concentration [86]. Conversely, when S fertilizer is not used, the total amount of aliphatic GSLs in the plants is notably reduced compared to treatments with sulphur fertilization [110]. These findings highlight the broader influence of environmental conditions on GSL accumulation, extending beyond nutrient supplementation alone.
9.2. Effect of Magnesium on Glucosinate Content
Generally, Mg enhances carbon assimilation, which in turn leads to production of carbon-based secondary metabolites such as GSLs. Mg’s role as a central atom in chlorophyll and as a cofactor in GSL biosynthesis may explain its dual influence on yield and nutritional quality [111]. Research by Gui-Xiao et al. [112] showed that an appropriate combination of nitrogen and Mg significantly enhanced photosynthetic performance, stabilized antioxidant activity and internal hormone balance, thereby promoting plant development and boosting the fresh biomass of Chinese kale. Notwithstanding, in Brassica species, excessive application of N fertilizer lowers the relative content of GSLs, thereby diminishing the nutritional and therapeutic significance of these vegetables [113]. Specifically, Mg acts as a key cofactor for enzymes needed for side-chain elongation of methionine-derived GSLs biosynthesis such as MAM [114]. Further, it acts as a divalent ion necessary to stimulate sulfotransferase enzymes in the process of GSL biosynthesis [115]. Conversely, elevated Mg levels suppress ATP-sulphurylase activity, the enzyme responsible for catalyzing the initial step of sulphate assimilation [116]. Therefore, Mg is crucial for playing a dual role by controlling S assimilation and GSL production in plants.
9.3. Environmental Factors Affecting Glucosinolate Biosynthesis
The level of GSLs is influenced by the surrounding environmental factors [117] which affect the conversion of GSLs into isothiocyanates, including low pH, iron (II) ions, and proteins involved in thiocyanate formation. Temperature, light, physical damage, and insect herbivory also affect GSL production and composition [118]. A study by Antonious et al. [119] showed that environmental pressure on developing plants can elevate the levels of GSLs, vitamin C, and overall phenolic compounds in Brassica shoots, but it does not enhance the total output of these phytochemicals per unit of land area. In Arabidopsis thaliana there is regulatory influence of photoperiod and S availability, where diurnal variation, HY5-mediated light signaling, and nutrient stress distinctly modulate GSL biosynthesis [120]. Additionally, GSL synthesis is regulated by phytohormones, plant growth regulators, agrochemicals, pesticides, metals, atmospheric gases, and other stimuli [121]. Further, agricultural conditions such as soil composition, fertilizers, cultivation methods, and habitat changes, along with environmental variables like season, light, temperature, water availability, humidity, CO2 levels, and nutrient supply, further impact the levels of GSLs in Brassicaceae crops [122] and gluconasturtiin in watercress [64]. Beyond these external and agronomic factors, intrinsic regulatory mechanisms, such as epigenetic modifications, also play a critical role in determining GSL content by modulating gene expression.
9.4. Transcriptomic, Epigenetic and Metabolomic Factors Affecting Glucosinolate Biosynthesis
De novo transcriptome sequencing and organ-specific GSL profiling in watercress identified approximately 33 candidate biosynthetic genes and quantified eight GSLs, establishing a foundational molecular framework, though lacking integration with hydroponic or nutrient-focused systems [49]. Transgenic approaches employing Brassica oleracea-derived transcription factors (e.g., IQD1, MYB29) in watercress hairy root cultures achieved significant GSLs enhancement of 2.6–4.7-fold yet were restricted to non-soil systems without nutrient modulation [123]. The amount of GSLs is influenced by epigenetic changes, which have been shown to affect metabolic composition by regulating the expression of metabolism-related genes [39]. At the epigenetic level, factors such as DNA methylation, histone modification patterns, chromatin structure, and non-coding RNAs influence the transcriptional accessibility of gene clusters involved in biosynthetic pathways [124]. As shown in Figure 6, epigenetic regulation modulates GSL biosynthetic pathways, influencing both the quantity and profile of GSLs produced in Brassica species [125]. Like Heterochromatin Protein (LHP1), a component of the polycomb repressive complex 1 that modifies histones, plays a role in both development and stress adaptation. In the LHP1 mutant, levels of indolic GSLs are moderately decreased, while aliphatic GSLs are significantly increased [106]. Comparative metabolomics of watercress cultivated under hydroponic, aquaponic, and soil-based systems revealed higher GSL diversity and abundance, particularly gluconasturtiin, in aquaponics [81]. Understanding how the transcriptome, epigenetic regulation, and the metabolome influence GSL profiles provides a foundation for exploring how targeted nutrient interventions, such as MgSO4 supplementation, can optimize phytochemical accumulation in watercress.
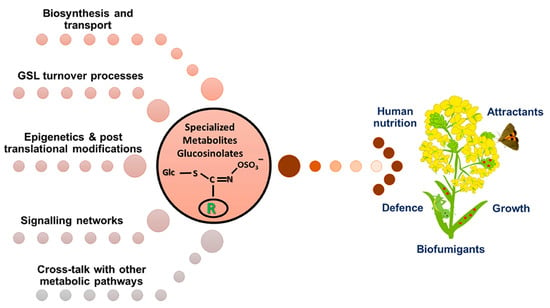
Figure 6.
Application of GSLs in agriculture and in the human diet. In agricultural contexts, GSLs act as natural biofumigants, enhancing soil health, while in human nutrition, GSL-derived isothiocyanates possess anticancer, antioxidant, and anti-inflammatory properties, are mediated via epigenetic regulation [59]. Figure adapted from Malhotra and Bisht [60].
9.5. Intra-Genic Variation in Glucosinolate Biosynthesis
In 12 commonly eaten Brassicaceae vegetables, precursor GSL levels strongly correlate with their resulting metabolites, and GSL types and amounts vary significantly among plant genera [47]. These variations align with morphological classifications and suggest genus-specific biosynthetic pathways. Understanding these genus-specific biosynthetic variations underscores the importance of key nutrients, particularly S, in modulating GSL profiles and overall plant development. A study by Kittipol et al. [126] further exemplifies intragenic variation in regulatory genes such as MYB28/HAG1 and its specific homologues Bna.HAG1.A9 and Bna.HAG1.C2 modulates aliphatic GSL levels across tissues, reinforcing the link between genotype-specific biosynthesis and resulting metabolite accumulation. Robin and co-workers [127] also researched how, intragenic differences, in this case differences in expression of specific biosynthetic genes like ST5b and GSL-OH, translate into distinct GSL profiles among cabbage genotypes. Building on these genotype-specific insights in model Brassicaceae crops, focus on watercress, integrating broader physiological and agronomic perspectives.
10. Conclusions
This narrative review synthesizes current research on GSL biosynthesis and accumulation in watercress and related cruciferous species, highlighting genetic, environmental, and nutritional influences while addressing a critical gap: the role of targeted sulphate fertilization in hydroponically grown watercress. Further, the review revealed the impact of organic and inorganic amendments on the GSL concentrations in various vegetable crops; however, very few studies investigated the effect of MgSO4. Additionally, this review highlights the increasing recognition of watercress as a nutrient-dense, environmentally efficient crop with substantial economic and public health potential, particularly through hydroponic cultivation. The relevance of this review lies in the fact that watercress contains bioactive compounds such as GSLs, which is why watercress has anti-inflammatory, antimicrobial, hepatoprotective and cardioprotective effects. Supplementation with Mg sulphate can boost GSL accumulation by supporting both S metabolism and the activity of key biosynthetic enzymes. Given the established influence of sulphate supply on GSL profiles in multiple Brassica crops, the sulphate levels employed in analogous experiments, such as 0.5–1.0 mM sulphate or 50 mM MgSO4 in broccoli, offer a biologically meaningful starting point for designing future hydroponic studies in watercress. Concentrations of at least 20 mM can impair growth and alter S homeostasis. Future research should define the dose–response thresholds for GSL accumulation. Further, the respective roles of Mg and sulphate should be elucidated through multivariable nutrient interaction designs, coupled with isotope-based metabolic tracking to determine how S assimilation responds to environmental factors such as light quality and temperature. Elucidating the role of MgSO4 in the GSL biochemical pathway can enable the development of targeted strategies to enhance phytochemical accumulation under controlled hydroponic cultivation of watercress, leading to improved agricultural productivity and public health benefits.
Author Contributions
Conceptualization, H.H.M. and T.N.; literature survey, preparing the manuscript, figures, and tables, H.H.M.; review and editing, R.K., T.N., and G.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Evans Allen Grant: FOAP: 210242-206002-3100.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GSH | Glutathione |
| GSLs | Glucosinolates |
| MAM | Methylthioalkylmalate synthase |
| PAPS | 3′-phosphoadenylyl sulphate |
References
- Balliu, A.; Zheng, Y.; Sallaku, G.; Fernández, J.A.; Gruda, N.S.; Tuzel, Y. Environmental and cultivation factors affect the morphology, architecture and performance of root systems in soilless grown plants. Horticulturae 2021, 7, 243. [Google Scholar] [CrossRef]
- Falk, K.L.; Tokuhisa, J.G.; Gershenzon, J. The Effect of Sulfur Nutrition on Plant Glucosinolate Content: Physiology and Molecular Mechanisms. Plant Biol. 2007, 9, 573–581. [Google Scholar] [CrossRef]
- Zenda, T.; Liu, S.; Dong, A.; Duan, H. Revisiting sulphur—The once neglected nutrient: It’s roles in plant growth, metabolism, stress tolerance and crop production. Agriculture 2021, 11, 626. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Ismoilov, K.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. In Plant Ionomics, Sensing, Signaling, and Regulation; Singh, V.P., Siddiqui, M.H., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 1–18. [Google Scholar]
- Bok, G.; Choi, J.; Lee, H.; Lee, K.; Park, J. Microbubbles increase glucosinolate contents of watercress (Nasturtium officinale R. Br.) grown in hydroponic cultivation. J. Bio-Environ. Control 2019, 28, 158–165. [Google Scholar] [CrossRef]
- Khalid, M.; Amayreh, M.; Sanduka, S.; Salah, Z.; Al-Rimawi, F.; Al-Mazaideh, G.M.; Alanezi, A.A.; Wedian, F.; Alasmari, F.; Shalayel, M.H.F. Assessment of antioxidant, antimicrobial, and anticancer activities of Sisymbrium officinale plant extract. Heliyon 2022, 8, e10477. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Hassanin, S.O.; Hamza, S.; Abdalla, A.; Amin, A. Polyphenolic-enriched olive leaf extract attenuated doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress and inflammation. J. Basic Appl. Zool. 2021, 82, 54. [Google Scholar] [CrossRef]
- Zafar, R.; Zahoor, M.; Shah, A.B.; Majid, F. Determination of antioxidants and antibacterial activities, total phenolic, polyphenol and pigment contents in Nasturtium officinale. Pharmacologyonline 2017, 1, 11–18. [Google Scholar]
- Amiri, H. Volatile constituents and antioxidant activity of flowers, stems and leaves of Nasturtium officinale R. Br. Nat. Prod. Res. 2012, 26, 109–115. [Google Scholar] [CrossRef]
- Maluwa, C.; Zinan’dala, B.; Chuljerm, H.; Parklak, W.; Kulprachakarn, K. Watercress (Nasturtium officinale) as a Functional Food for Non-Communicable Diseases Prevention and Management: A Narrative Review. Life 2025, 15, 1104. [Google Scholar] [CrossRef]
- Nilash, A.B.; Jahanbani, J.; Jolehar, M. Effect of nasturtium extract on oral cancer. Adv. Biomed. Res. 2023, 12, 53. [Google Scholar] [CrossRef]
- Voutsina, N.; Hancock, R.D.; Becerra-Sanchez, F.; Qian, Y.; Taylor, G. Characterization of a new dwarf watercress (Nasturtium officinale R Br.) ‘Boldrewood’ in commercial trials reveals a consistent increase in chemopreventive properties in a longer-grown crop. Euphytica 2024, 220, 106. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Jin, L.; Lin, S. Anticarcinogenic effects of isothiocyanates on hepatocellular carcinoma. Int. J. Mol. Sci. 2022, 23, 13834. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Hisham, H.; Mohamed, D. A review on phytochemical and pharmacological potential of watercress plant. Asian J. Pharm. Clin. Res. 2018, 11, 102–107. [Google Scholar] [CrossRef]
- Kyriakou, S.; Michailidou, K.; Amery, T.; Stewart, K.; Winyard, P.G.; Trafalis, D.T.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Polyphenolics, glucosinolates and isothiocyanates profiling of aerial parts of Nasturtium officinale (Watercress). Front. Plant Sci. 2022, 13, 998755. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Chemical composition, traditional and professional use in medicine, application in environmental protection, position in food and cosmetics industries, and biotechnological studies of Nasturtium officinale (watercress)—A review. Fitoterapia 2018, 129, 283–292. [Google Scholar] [CrossRef]
- Khan, F.A.; Bhat, S.A.; Narayan, S. Wild edible plants as a food Resource: Traditional Knowledge. Preprint 2017. [Google Scholar] [CrossRef]
- Renna, M.; Di Gioia, F.; Leoni, B.; Mininni, C.; Santamaria, P. Culinary Assessment of Self-Produced Microgreens as Basic Ingredients in Sweet and Savory Dishes. J. Culin. Sci. Technol. 2017, 15, 126–142. [Google Scholar] [CrossRef]
- Kyriakou, S.; Potamiti, L.; Demosthenous, N.; Amery, T.; Stewart, K.; Winyard, P.G.; Franco, R.; Pappa, A.; Panayiotidis, M.I. A naturally derived watercress flower-based phenethyl isothiocyanate-enriched extract induces the activation of intrinsic apoptosis via subcellular ultrastructural and Ca2+ efflux alterations in an in vitro model of human malignant melanoma. Nutrients 2023, 15, 4044. [Google Scholar] [CrossRef]
- Kijkuokool, P.; Stepanov, I.; Ounjaijean, S.; Koonyosying, P.; Rerkasem, K.; Chuljerm, H.; Parklak, W.; Kulprachakarn, K. Effects of Drying Methods on the Phytochemical Contents, Antioxidant Properties, and Anti-Diabetic Activity of Nasturtium officinale R. Br. (Betong Watercress) from Southern Thailand. Life 2024, 14, 1204. [Google Scholar] [CrossRef]
- Wittstock, U.; Burow, M. Glucosinolate breakdown in Arabidopsis: Mechanism, regulation and biological significance. Arab. Book 2010, 8, E0134. [Google Scholar] [CrossRef]
- Di Noia, J. Defining powerhouse fruits and vegetables: A nutrient density approach. Prev. Chronic Dis. 2014, 11, E95. [Google Scholar] [CrossRef]
- Grubb, C.D.; Abel, S. Glucosinolate metabolism and its control. Trends Plant Sci. 2006, 11, 89–100. [Google Scholar] [CrossRef]
- Graser, G.; Oldham, N.J.; Brown, P.D.; Temp, U.; Gershenzon, J. The biosynthesis of benzoic acid glucosinolate esters in Arabidopsis thaliana. Phytochemistry 2001, 57, 23–32. [Google Scholar] [CrossRef]
- FAO. Food Balance Sheet 2002; Food and Agriculture Organization of United Nation: Rome, Italy, 2005; Available online: http://faostat.fao.org/faostat (accessed on 9 October 2025).
- NASS. Quick Stats. 2023. Available online: https://quickstats.nass.usda.gov/ (accessed on 22 October 2023).
- Howard, H.W.; Lyon, A.G. Nasturtium officinale R. Br. (Rorippa Nasturtium-Aquaticum (L.) Hayek). J. Ecol. 1952, 40, 228–245. [Google Scholar] [CrossRef]
- Clemente, M.; Miguel, M.; Gribner, C.; Moura, P.F.; Angelica, A.; Rigoni, R.; Fernandes, L.C.; Miguel, O.G. Watercress, as a functional food, with protective effects on human health against oxidative stress: A review study. Int. J. Med. Plants Nat. Prod. 2019, 5, 12–16. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and Biochemistry of Glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Harun, S.; Abdullah-Zawawi, M.R.; Goh, H.H.; Mohamed-Hussein, Z.A. A Comprehensive Gene Inventory for Glucosinolate Biosynthetic Pathway in Arabidopsis thali-ana. J. Agric. Food Chem. 2020, 68, 7281–7297. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Yatusevich, R.; Rollwitz, I.; Humphry, M.; Gershenzon, J.; Flügge, U.I. The plastidic bile acid transporter 5 is required for the biosynthesis of methionine-derived glucosinolates in Arabidopsis thaliana. Plant Cell 2009, 21, 1813–1829. [Google Scholar] [CrossRef] [PubMed]
- Textor, S.; De Kraker, J.W.; Hause, B.; Gershenzon, J.; Tokuhisa, J.G. MAM3 catalyzes the formation of all aliphatic glucosinolate chain lengths in Arabidopsis. Plant Physiol. 2007, 144, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.; Hansen, L.G.; Mirza, N.; Crocoll, C.; Mirza, O.; Halkier, B.A. Changing substrate specificity and iteration of amino acid chain elongation in glucosinolate biosynthesis through targeted mutagenesis of Arabidopsis methylthioalkylmalate synthase 1. Biosci. Rep. 2019, 39, BSR20190446. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, B.; Pang, Q.; Strul, J.M.; Chen, S. Functional specification of Arabidopsis isopropylmalate isomerases in glucosinolate and leucine biosynthesis. Plant Cell Physiol. 2010, 51, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Knill, T.; Reichelt, M.; Paetz, C.; Gershenzon, J.; Binder, S. Arabidopsis thaliana encodes a bacterial-type heterodimeric isopropylmalate isomerase involved in both Leu biosynthesis and the Met chain elongation pathway of glucosinolate formation. Plant Mol. Biol. 2009, 71, 227–239. [Google Scholar] [CrossRef]
- Ravilious, G.E.; Jez, J.M. Structural biology of plant sulfur metabolism: From assimilation to biosynthesis. Nat. Prod. Rep. 2012, 29, 1138–1152. [Google Scholar] [CrossRef] [PubMed]
- Naur, P.; Petersen, B.L.; Mikkelsen, M.D.; Bak, S.; Rasmussen, H.; Olsen, C.E.; Halkier, B.A. CYP83A1 and CYP83B1, two nonredundant cytochrome P450 enzymes metabolizing oximes in the biosynthesis of glucosinolates in Arabidopsis. Plant Physiol. 2003, 133, 63–72. [Google Scholar] [CrossRef]
- Mikkelsen, M.D.; Naur, P.; Halkier, B.A. Arabidopsis mutants in the C–S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant J. 2004, 37, 770–777. [Google Scholar] [CrossRef]
- Douglas Grubb, C.; Zipp, B.J.; Ludwig-Müller, J.; Masuno, M.N.; Molinski, T.F.; Abel, S. Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J. 2004, 40, 893–908. [Google Scholar] [CrossRef]
- Gachon, C.M.; Langlois-Meurinne, M.; Henry, Y.; Saindrenan, P. Transcriptional co-regulation of secondary metabolism enzymes in Arabidopsis: Functional and evolutionary implications. Plant Mol. Biol. 2005, 58, 229–245. [Google Scholar] [CrossRef]
- Sonderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates–gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef]
- Tinte, M.M.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Lipopolysaccharide perception in Arabidopsis thaliana: Diverse LPS chemotypes from Burkholderia cepacia, Pseudomonas syringae and Xanthomonas campestris trigger differential defence-related perturbations in the metabolome. Plant Physiol. Biochem. 2020, 156, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Tierens, K.F.J.; Thomma, B.P.; Brouwer, M.; Schmidt, J.; Kistner, K.; Porzel, A.; Mauch-Mani, B.; Cammue, B.P.; Broekaert, W.F. Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of Arabidopsis to microbial pathogens. Plant Physiol. 2001, 125, 1688–1699. [Google Scholar] [CrossRef]
- Sellam, A.; Dongo, A.; Guillemette, T.; Hudhomme, P.; Simoneau, P. Transcriptional responses to exposure to the brassicaceous defence metabolites camalexin and allyl-isothiocyanate in the necrotrophic fungus Alternaria brassicicola. Mol. Plant Pathol. 2007, 8, 195–208. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J. Control of soil-borne plant pests using glucosinolate-containing plants. Adv. Agron. 1997, 61, 167–231. [Google Scholar]
- van Dam, N.M.; Samudrala, D.; Harren, F.J.; Cristescu, S.M. Real-time analysis of sulfur-containing volatiles in Brassica plants infested with root-feeding Delia radicum larvae using proton-transfer reaction mass spectrometry. AoB Plants 2012, 2012, PLS021. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Xie, J.; Lv, J.; Zhang, G.; Hu, L.; Luo, S.; Li, L.; Yu, J. The roles of cruciferae glucosinolates in disease and pest resistance. Plants 2021, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Carvalho, R.; Rosa, E.A.; Saavedra, M.J. Phytochemical characterization and antioxidant properties of baby-leaf watercress produced under organic production system. CyTA J. Food 2013, 11, 343–351. [Google Scholar] [CrossRef]
- Jeon, J.; Bong, S.J.; Park, J.S.; Park, Y.K.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. De novo transcriptome analysis and glucosinolate profiling in watercress (Nasturtium officinale R. Br.). BMC Genom. 2017, 18, 401. [Google Scholar] [CrossRef]
- Keum, Y.S.; Jeong, W.S.; Kong, A.T. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat. Res. /Fundam. Mol. Mech. Mutagen. 2004, 555, 191–202. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yoshimoto, M.; Murata, Y.; Shimoishi, Y.; Asai, Y.; Park, E.Y.; Sato, K.; Nakamura, Y. Papaya seed represents a rich source of biologically active isothiocyanate. J. Agric. Food Chem. 2007, 55, 4407–4413. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2001, 7, 213–229. [Google Scholar] [CrossRef]
- Sarkar, R.; Mukherjee, S.; Biswas, J.; Roy, M. Sulphoraphane, a naturally occurring isothiocyanate induces apoptosis in breast cancer cells by targeting heat shock proteins. Biochem. Biophys. Res. Commun. 2012, 427, 80–85. [Google Scholar] [CrossRef]
- Clarke, D.B. Glucosinolates, structures and analysis in foods. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Wang, T.T.; Schoene, N.W.; Milner, J.A.; Kim, Y.S. Broccoli-derived phytochemicals indole-3-carbinol and 3,3′-diindolylmethane exerts concentration-dependent pleiotropic effects on prostate cancer cells: Comparison with other cancer preventive phytochemicals. Mol. Carcinog. 2012, 51, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Nagraj, G.S.; Chouksey, A.; Jaiswal, S.; Jaiswal, A.K. Broccoli. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Elsevier: Amsterdam, The Netherland; Academic Press: Cambridge, MA, USA, 2020; pp. 5–17. [Google Scholar]
- Saha, M.; Chatterjee, S. Strategies to Control Cancer by Chinese Cabbage and Anise Seed. In Seeds: Anti-Proliferative Storehouse for Bioactive Secondary Metabolites; Springer Nature: Singapore, 2024; pp. 707–726. [Google Scholar]
- Ben Ammar, H. Epigenetic Regulation of Glucosinolate Biosynthesis: Mechanistic Insights and Breeding Prospects in Brassicaceae. DNA 2025, 5, 51. [Google Scholar] [CrossRef]
- Malhotra, B.; Bisht, N.C. Glucosinolates: Regulation of biosynthesis and hydrolysis. Front. Plant Sci. 2020, 11, 620965. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Impact of nitrogen and sulfur fertilization on the phytochemical concentration in watercress, Nasturtium officinal R. Br. In Proceedings of the II International Symposium on Human Health Effects of Fruits and Vegetables: FAVHEALTH, Houston, TX, USA, 9–13 October 2007; Volume 841, pp. 479–482. [Google Scholar]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in Brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016, 7, 248–258. [Google Scholar] [CrossRef]
- Alibrahem, W.; Nguyen, D.H.; Kharrat Helu, N.; Tóth, F.; Nagy, P.T.; Posta, J.; Prokisch, J.; Oláh, C. Health Benefits, Applications, and Analytical Methods of Freshly Produced Allyl Isothiocyanate. Foods 2025, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Engelen-Eigles, G.; Holden, G.; Cohen, J.D.; Gardner, G. The Effect of Temperature, Photoperiod, and Light Quality on Gluconasturtiin Concentration in Watercress (Nasturtium officinale R. Br.). J. Agric. Food Chem. 2006, 54, 328–334. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Anestopoulos, I.; Kyriakou, S.; Trafalis, D.T.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Benzyl and phenethyl isothiocyanates as promising epigenetic drug compounds by modulating histone acetylation and methylation marks in malignant melanoma. Investig. New Drugs 2021, 39, 1460–1468. [Google Scholar] [CrossRef]
- Shahani, S.; Behzadfar, F.; Jahani, D.; Ghasemi, M.; Shaki, F. Antioxidant and anti-inflammatory effects of Nasturtium officinale involved in attenuation of gentamicin-induced nephrotoxicity. Toxicol. Mech. Methods 2017, 27, 107–114. [Google Scholar] [CrossRef]
- Gill, C.I.; Haldar, S.; Boyd, L.A.; Bennett, R.; Whiteford, J.; Butler, M.; Pearson, J.R.; Bradbury, I.; Rowland, I.R. Watercress supplementation in diet reduces lymphocyte DNA damage and alters blood antioxidant status in healthy adults. Am. J. Clin. Nutr. 2007, 85, 504–510. [Google Scholar] [CrossRef]
- Yazdanparast, R.; Bahramikia, S.; Ardestani, A. Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem. Biol. Interact. 2008, 172, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Szopa, A.; Korona-Głowniak, I.; Ekiert, H. Evaluation of antimicrobial activity of nasturtium officinale (watercress)–rita® bioreactor grown in vitro cultures and herb extracts. In Proceedings of the 3-rd ICPMS Martin, Kraków, Szeged, 24–26 September 2020; p. 134. [Google Scholar]
- Camacho-Corona, M.D.R.; Ramírez-Cabrera, M.A.; Santiago, O.G.; Garza-González, E.; Palacios, I.D.P.; Luna-Herrera, J. Activity against drug resistant-tuberculosis strains of plants used in Mexican traditional medicine to treat tuberculosis and other respiratory diseases. Phytother. Res. 2008, 22, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Pinto, J.; Castilho, P.C. In vitro studies on the effect of watercress juice on digestive enzymes relevant to type 2 diabetes and obesity and antioxidant activity. J. Food Biochem. 2017, 41, E12335. [Google Scholar] [CrossRef]
- Abu-Zinadah, O.A. Using nigella sativa oil to treat and heal chemical induced wound of rabbit skin. JKAU Sci. 2009, 21, 335–346. [Google Scholar] [CrossRef]
- Bettega, P.V.C.; Johann, A.C.B.R.; Alanis, L.R.A.; Bazei, I.F.; Miguel, O.G. Experimental confirmation of the utility of Nasturtium officinale used empirically as mouth lesion repairing promotor. Clin. Exp. Pharmacol. 2016, 5, 1161–1459. [Google Scholar]
- Yaricsha, C.A. ACE inhibitory activity, total phenolic and flavonoid content of watercress (Nasturtium officinale R. Br.) extract. Pharmacogn. J. 2017, 9, 249–251. [Google Scholar] [CrossRef]
- Mousa-Al-Reza Hadjzadeh, Z.R.; Moradi, R.; Ghorbani, A. Effects of hydroalcoholic extract of watercress (Nasturtium officinale) leaves on serum glucose and lipid levels in diabetic rats. Indian J. Physiol. Pharmacol. 2015, 59, 223–230. [Google Scholar]
- Chaudhary, R.; Kumar, S.; Malik, J.; Singh, G.; Siroliya, V.K. A Review of the Phytochemical and Pharmacological Potential of the Watercress Plant (Nasturitium Officinale): A Medicinal Plant. Int. J. Pharm. Biol. Sci. Arch. 2023, 11, 1–10. [Google Scholar]
- Hameed, M.K.; Umar, W.; Razzaq, A.; Aziz, T.; Maqsood, M.A.; Wei, S.; Niu, Q.; Huang, D.; Chang, L. Differential metabolic responses of lettuce grown in soil, substrate and hydroponic cultivation systems under NH4+/NO3− application. Metabolites 2022, 12, 444. [Google Scholar] [CrossRef]
- Vardar, G.; Altıkatoğlu, M.; Ortaç, D.; Cemek, M.; Işıldak, İ. Measuring calcium, potassium, and nitrate in plant nutrient solutions using ion-selective electrodes in hydroponic greenhouse of some vegetables. Biotechnol. Appl. Biochem. 2015, 62, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Lira, R.M.; Silva, Ê.F.; Silva, G.F.; Soares, H.R.; Willadino, L.G. Growth, water consumption and mineral composition of watercress under hydroponic system with brackish water. Hortic. Bras. 2018, 36, 13–19. [Google Scholar] [CrossRef]
- Irhayyim, T.; Fehér, M.; Lelesz, J.; Bercsényi, M.; Bársony, P. Nutrient removal efficiency and growth of watercress (Nasturtium officinale) under different harvesting regimes in integrated recirculating aquaponic systems for rearing common carp (Cyprinus carpio L.). Water 2020, 12, 1419. [Google Scholar] [CrossRef]
- Buitrago-Villanueva, I.; Barbosa-Cornelio, R.; Coy-Barrera, E. Specialized Metabolite Profiling-Based Variations of Watercress Leaves (Nasturtium officinale R. Br.) from Hydroponic and Aquaponic Systems. Molecules 2025, 30, 406. [Google Scholar] [CrossRef]
- Hirani, A.H.; Li, G.; Zelmer, C.D.; McVetty, P.B.; Asif, M.; Goyal, A. Molecular genetics of glucosinolate biosynthesis in Brassicas: Genetic manipulation and application aspect. In Crop Plant; IntechOpen Limited: London, UK, 2012; pp. 189–216. [Google Scholar]
- Kopsell, D.A.; Barickman, T.C.; Sams, C.E.; McElroy, J.S. Influence of Nitrogen and Sulfur on Biomass Production and Carotenoid and Glucosinolate Concentrations in Watercress (Nasturtium officinale R. Br.). J. Agric. Food Chem. 2007, 55, 10628–10634. [Google Scholar] [CrossRef] [PubMed]
- Chu Ting, C.T.; Peng Chang, P.C.; Guo LiPing, G.L. Effect of MgSO4 treatment on bioactive compounds and antioxidant activity in broccoli sprouts. Food Sci. 2018, 39, 53–59. [Google Scholar]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef]
- Li, L.; Ma, P.; Nirasawa, S.; Liu, H. Formation, immunomodulatory activities, and enhancement of glucosinolates and sulforaphane in broccoli sprouts: A review for maximizing the health benefits to human. Crit. Rev. Food Sci. Nutr. 2024, 64, 7118–7148. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Glucosinolates in broccoli sprouts (Brassica oleracea var. italica) as conditioned by sulphate supply during germination. J. Food Sci. 2010, 75, C673–C677. [Google Scholar] [CrossRef]
- Lu, Z.; Degryse, F.; Wu, J.; Huang, C.; Yu, Y.; Mclaughlin, M.J.; Zhang, F. Slow-and fast-release magnesium-fortified macronutrient fertilizers improve plant growth with lower Mg leaching loss. J. Soils Sediments 2024, 24, 1507–1515. [Google Scholar] [CrossRef]
- Malhotra, B.; Kumar, P.; Bisht, N.C. Defense versus growth trade-offs: Insights from glucosinolates and their catabolites. Plant Cell Environ. 2023, 46, 2964–2984. [Google Scholar] [CrossRef]
- Singh, V.K.; Gautam, P.; Sen, S. Soil Test Crop Response-Based Fertilizer Application Improves Soil Fertility and Wheat (Triticum Aestivum L.) Productivity Grown After Direct-Seeded Rice in Mollisols of Northern India. Commun. Soil Sci. Plant Anal. 2025, 56, 1767–1789. [Google Scholar] [CrossRef]
- Kopriva, S.; Mugford, S.G.; Matthewman, C.; Koprivova, A. Plant sulfate assimilation genes: Redundancy versus specialization. Plant Cell Rep. 2009, 28, 1769–1780. [Google Scholar] [CrossRef]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic solutions for soilless production systems: Issues and opportunities in a smart agriculture perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef]
- Landis, T.D.; Pinto, J.R.; Davis, A.S. Fertigation-Injecting soluble fertilizers into the irrigation system. In Forest Nursery Notes, Volume 29, Issue 2; Kasten, D.R., Tom, L.D., Eds.; US Department of Agriculture, Forest Service, Natural Resources Conservation Service, National Agroforestry Center: Lincoln, NE, USA, 2009; Volume 4, pp. 4–13. [Google Scholar]
- Morgan, L. Hydroponics and Protected Cultivation: A Practical Guide; CABI: Wallingford, UK, 2021. [Google Scholar]
- Morgan, L. Plant nutrition and nutrient formulation. In Hydroponics and Protected Cultivation: A Practical Guide; CABI: Wall-ingford, UK, 2021; pp. 136–169. [Google Scholar]
- Yilmaz, Ö. Velocity Analysis and Statics Corrections. In Seismic Data Analysis; Society of Exploration Geophysicists: Houston, TX, USA, 2001; pp. 271–461. [Google Scholar]
- Qiao, Z.; Zhou, P.C.; Fan, Z.T.; Wei, F.; Qin, S.S.; Wang, J.; Liang, Y.; Chen, L.Y.; Wei, K.H. Multi-omics analysis uncovers the transcriptional regulatory mechanism of magnesium Ions in the synthesis of active ingredients in Sophora tonkinensis. Sci. Rep. 2024, 14, 25527. [Google Scholar] [CrossRef] [PubMed]
- Reginato, M.; Luna, V.; Papenbrock, J. Current knowledge about Na2SO4 effects on plants: What is different in comparison to NaCl? J. Plant Res. 2021, 134, 1159–1179. [Google Scholar] [CrossRef]
- Rivero-Marcos, M.; Lasa, B.; Neves, T.; Zamarreño, Á.M.; García-Mina, J.M.; García-Olaverri, C.; Aparicio-Tejo, P.M.; Cruz, C.; Ariz, I. Plant ammonium sensitivity is associated with external pH adaptation, repertoire of nitrogen transporters, and nitrogen requirement. J. Exp. Bot. 2024, 75, 3557–3578. [Google Scholar] [CrossRef]
- Ali, V.; Faiz, S.; Jamwal, S.; Bhagnyal, D.; Bhasin, S.; Rashid, A.; Vyas, D. Sulfate and chloride ions differentially affect sulfur and glucosinolate metabolism in Lepidium latifolium L. Food Biosci. 2024, 62, 105034. [Google Scholar] [CrossRef]
- Mithen, R. Sulphur-containing compounds. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Wiley: Hoboken, NJ, USA, 2008; pp. 25–46. [Google Scholar]
- Nakai, Y.; Maruyama-Nakashita, A. Biosynthesis of sulfur-containing small biomolecules in plants. Int. J. Mol. Sci. 2020, 21, 3470. [Google Scholar] [CrossRef] [PubMed]
- Haneklaus, S.; Bloem, E.; Schnug, E. History of sulfur deficiency in crops. In Sulfur: A Missing Link between Soils, Crops, and Nutrition; Book and Multimedia Publishing Committee: Singapore, 2008; Volume 50, pp. 45–58. [Google Scholar]
- Krumbein, A.; Schonhof, I.; Rühlmann, J.; Widell, S. Influence of sulphur and nitrogen supply on flavour and health-affecting compounds in Brassicaceae. In Plant Nutrition: Food Security and Sustainability of Agro-Ecosystems Through Basic and Applied Research; Springer Netherlands: Dordrecht, The Netherlands, 2001; pp. 294–295. [Google Scholar]
- Traka, M.H.; Saha, S.; Huseby, S.; Kopriva, S.; Walley, P.G.; Barker, G.C.; Moore, J.; Mero, G.; van den Bosch, F.; Constant, H.; et al. Genetic regulation of glucoraphanin accumulation in Beneforté® broccoli. New Phytol. 2013, 198, 1085–1095. [Google Scholar] [CrossRef]
- Kim, Y.C.; Hussain, M.; Anarjan, M.B.; Lee, S. Examination of glucoraphanin content in broccoli seedlings over growth and the impact of hormones and sulfur-containing compounds. Plant Biotechnol. Rep. 2020, 14, 491–496. [Google Scholar] [CrossRef]
- Szulc, P.M.; Drozdowska, L.; Kachlicki, P. Effect of sulphur on the yield and content of glucosinolates in spring oilseed rape seeds. Electron. J. Pol. Agric. Univ. 2003, 6, 1–8. [Google Scholar]
- Kacjan Maršić, N.; Može, K.S.; Mihelič, R.; Nečemer, M.; Hudina, M.; Jakopič, J. Nitrogen and sulphur fertilisation for marketable yields of cabbage (Brassica oleracea L. var. Capitata), leaf nitrate and glucosinolates and nitrogen losses studied in a field experiment in Central Slovenia. Plants 2021, 10, 1304. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Q.; Zhang, L.; Chen, Z.; Han, Y.; Gu, Z. Physiological and biochemical metabolism of germinating broccoli seeds and sprouts. J. Agric. Food Chem. 2012, 60, 209–213. [Google Scholar] [CrossRef]
- Serafin-Andrzejewska, M.; Marcin, K.; Kotecki, A. Effect of different sulfur fertilizer doses on the glucosinolate content and profile of white mustard seeds. J. Elem. 2020, 25, 1413–1422. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Bozdar, B.; Chachar, S.; Rai, M.; Li, J.; Li, Y.; Hayat, F.; Chachar, Z.; Tu, P. The power of magnesium: Unlocking the potential for increased yield, quality, and stress tolerance of horticultural crops. Front. Plant Sci. 2023, 14, 1285512. [Google Scholar] [CrossRef]
- Gui-xiao, L.A.; Ping, F.A.N.G.; Yi-bo, T.E.N.G.; Ya-juan, L.I.; Xian-yong, L.I.N. Effect of nitrogen level on growth and glucosinolates content of Chinese kale. J. Plant Nutr. Fertil. 2009, 15, 429–434. [Google Scholar]
- Chen, X.J.; Zhu, Z.J.; Ni, X.L.; Qian, Q.Q. Effect of nitrogen and sulfur supply on glucosinolates in Brassica campestris ssp. chinensis. Agric. Sci. China 2006, 5, 603–608. [Google Scholar] [CrossRef]
- Kitainda, V.; Jez, J.M. Kinetic and catalytic mechanisms of the methionine-derived glucosinolate biosynthesis enzyme methylthioalkylmalate synthase. J. Biol. Chem. 2024, 300, 107814. [Google Scholar] [CrossRef] [PubMed]
- Glendening, T.M.; Poulton, J.E. Glucosinolate Biosynthesis: Sulfation of Desulfobenzylglucosinolate by Cell-Free Extracts of Cress (Lepidium sativum L.) Seedlings. Plant Physiology. 1988, 86, 319–321. [Google Scholar] [CrossRef]
- Wójcik-Augustyn, A.; Johansson, A.J.; Borowski, T. Mechanism of Sulfate Activation Catalyzed by ATP Sulfurylase—Magnesium Inhibits the Activity. Comput. Struct. Biotechnol. J. 2019, 17, 770–784. [Google Scholar] [CrossRef]
- Bohinc, T.; Trdan, S. Environmental factors affecting the glucosinolate content in Brassicaceae. J. Food Agric. Env. 2012, 10, 357. [Google Scholar]
- Lin, P.; Di, H.; Li, Z.; Wang, Y.; Zhou, W.; Huang, S.; Zhang, C.; Li, H.; Zhang, F.; Sun, B. Light irradiation maintains the sensory quality, health-promoting phytochemicals, and antioxidant capacity of post-harvest baby mustard. J. Food Sci. 2020, 87, 112–123. [Google Scholar] [CrossRef]
- Antonious, G.F.; Bomford, M.; Vincelli, P. Screening Brassica species for glucosinolate content. J. Environ. Sci. Health Part B 2009, 44, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Huseby, S.; Koprivova, A.; Lee, B.R.; Sahar, S.; Mithen, R.; Wold, A.B.; Bengtsson, G.B.; Kopriva, S. Diurnal and light regulation of sulphur assimilation and glucosinolate biosynthesis in Arabidopsis. J. Exp. Bot. 2013, 64, 1039–1048. [Google Scholar] [CrossRef]
- Teng, Z.; Yu, Y.; Zhu, Z.; Hong, S.B.; Yang, B.; Zang, Y. Melatonin elevated Sclerotinia sclerotiorum resistance via modulation of ATP and glucosinolate biosynthesis in Brassica rapa ssp. pekinensis. J. Proteom. 2021, 243, 104264. [Google Scholar] [CrossRef] [PubMed]
- Björkman, M.; Klingen, I.; Birch, A.N.; Bones, A.M.; Bruce, T.J.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health–Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Cuong, D.M.; Park, C.H.; Bong, S.J.; Kim, N.S.; Kim, J.K.; Park, S.U. Enhancement of glucosinolate production in watercress (Nasturtium officinale) hairy roots by overexpressing cabbage transcription factors. J. Agric. Food Chem. 2019, 67, 4860–4867. [Google Scholar] [CrossRef]
- Vaschetto, L.M. DNA Methylation, Histone Modifications, and Non-coding RNA Pathways. In Epigenetics in Crop Improvement: Safeguarding Food Security in an Ever-Changing Climate; Springer Nature: Cham, Switzerland, 2024; pp. 15–27. [Google Scholar]
- Mitreiter, S.; Gigolashvili, T. Regulation of glucosinolate biosynthesis. J. Exp. Bot. 2021, 72, 70–91. [Google Scholar] [CrossRef]
- Kittipol, V.; He, Z.; Wang, L.; Doheny-Adams, T.; Langer, S.; Bancroft, I. Genetic architecture of glucosinolate variation in Brassica napus. J. Plant Physiol. 2019, 240, 152988. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Yi, G.E.; Laila, R.; Yang, K.; Park, J.I.; Kim, H.R.; Nou, I.S. Expression profiling of glucosinolate biosynthetic genes in Brassica oleracea L. var. capitata inbred lines reveals their association with glucosinolate content. Molecules 2016, 21, 787. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).