Abstract
The population structure of endangered species is one of the main criteria for assessing their state in their habitats. Representatives of the Ericaceae family are sensitive to environmental changes, including anthropogenic pressure; thus, they are considered the indicator species in assessing phytocenose stability. The population structure and density of the threatened species green-flowered wintergreen, Pyrola chlorantha Sw., have been described at the southern range margin (south-east of the European part of Russia, Samara Region). The observations were performed here in 2006–2021, and the main parameters of the age and spatial structure of P. chlorantha populations were revealed for the first time. Green-flowered wintergreen populations were studied at monitoring study sites and at temporarily established study plots. A bush part (ramet) was set as a counting unit. In total, 27 sub-populations were surveyed, with 1520 individuals registered. The age structure of populations was characterized using common demographic indicators: the recovery index and the population age index. The age structure of the population was associated with the efficiency of both vegetative and seed reproduction. Generally, the share of pre-generative individuals was 32.3%, generative, 66.9%, and senile, 1.8%. The studied populations were stable due to low anthropogenic impact at the growth sites.
1. Introduction
Currently, large-scale anthropogenic pressure on ecosystems causes significant changes in the structure and organization of natural complexes, including vegetation cover (communities, plant populations) [1,2,3,4,5,6,7]. At the same time, rare plant species (mostly stenobionts) disappear from plant communities first, so they are included in the lists of protected plants [8,9,10].
The protection of rare species is impossible without knowledge of their bioecological characteristics. In this regard, population-age methods are relevant and serve as the basis for assessing the current state of vulnerable plant species populations [11,12,13,14,15]. Monitoring of threatened plant populations is one of the main approaches in conservation biology and plant ecology [16,17,18,19,20]. These populations are studied as a part of the monitoring of rare species included in the Red Lists [21,22,23,24].
Many authors pay special attention to the structural and functional features of plant species and their populations near the range margin [25,26,27,28,29,30,31]. A targeted study of plant populations helps to identify factors limiting the natural succession of plant populations and to propose appropriate conservation measures.
For a long time, both the biology and ecology of Pyrola species attracted much attention [32,33,34,35,36,37,38]. As a rule, Palaearctic range of green-flowered wintergreen, Pyrola chlorantha Sw., coincides with that of Pinus sylvestris L. Green-flowered wintergreen is less moisture-demanding in comparison with other representatives of the genus Pyrola, so it serves as an indicator of dry soils [39].
Despite the fairly wide distribution of P. chlorantha in Russia, the populations of this species have been studied quite rarely. There are some data on the population structure for the Mordovia State Nature Reserve [40] and the Volzhsko-Kamsky State Nature Reserve [41], but they are insufficient to assess the current population state in natural habitats. In Eastern Europe, the populations of green-flowered wintergreen have been studied in the Desnyansko-Starogutsky National Park (north-eastern Ukraine), where rather stable seed reproduction by plants was observed [42].
The Samara Region (Russia) is the southern range margin of P. chlorantha [43,44]. Here, green-flowered wintergreen is rare due to the small number of suitable habitats and rather high anthropogenic load [44]. No studies of the spatial-age structure of P. chlorantha populations in Samara Region have been previously conducted.
The study aims to describe the spatial and age structure of P. chlorantha populations at the southern range margin (Samara Region, Russian Federation), assess the dynamics of age structure and total population size, and search for the main regularities of the spatial distribution.
2. Materials and Methods
2.1. Species Description
Green-flowered wintergreen Pyrola chlorantha is found in Scandinavia, Atlantic, Middle and Eastern Europe, the Mediterranean, the Caucasus, Western and Eastern Siberia, the Far East, Asia Minor, and North America [43,44,45].
P. chlorantha is a herbaceous, evergreen, rhizomatous perennial, mesophyte, sciophyte, and mycotroph. It grows in pine (Pinus sylvestris L.) and pine-broadleaved forests. In the Samara Region, total species abundance is not high, population density is low; and the specimens grow in groups, usually not more than 5–15 individuals per 100 m2 [44].
The peculiarities of the life form of green-flowered wintergreen are well described [7,46]. Most species of the Ericaceae family are usually represented by a bushy part; in P. chlorantha, the latter develops for 5–8 years. The life span of the main module is approximately 10 years. In green-flowered wintergreen, all axillary buds of one of the elementary shoots are often initiated into growth simultaneously [46].
2.2. Study Area
The Zhiguli State Nature Reserve and the Buzuluksky Bor National Park are federally protected areas within the borders of the Samara Region. The main forest types, where Pyrola chlorantha has been registered are lichen-green-moss pine forests and tall grass pine forests (Buzuluksky Bor National Park), pine forests, and oak (Querqus robur L.) forests (Zhiguli State Nature Reserve). The Zhiguli State Nature Reserve is located in the Pre-Volga area in the forest-steppe zone (53.405796° N, 49.779342° E). The climate is moderately continental. Average annual precipitation is about 580 mm. The area is represented by a thick formation of limestones and dolomites of Carboniferous and Permian ages. The bedrock, apart from the overlying clay loam layer, is often covered with rubbly clastic weathering products up to 5–20 cm thick.
The Buzuluksky Bor National Park is located in the Samara Trans-Volga Region in the steppe zone (53.011025° N, 51.982755° E); the climate here is also moderately continental. Average annual precipitation is 530 mm, but its uneven nature predetermines extreme moisture conditions, manifested either in extreme drainage of the territory and lowering of the groundwater level or in significant humidification. The soils are sandy and loamy-sandy loam.
In general, habitat conditions are characterized by sufficient aridity, mosaic grasses and tiers, and sparse tree cover. Anthropogenic pressure in the form of fires, recreation, and grazing is often present in the study area, thus affecting the structure and dynamics of P. chlorantha populations.
2.3. Experimental Design
P. chlorantha populations were studied during the growing seasons of 2006–2021 in accordance with standard methods [5,47,48]. The populations were searched using the route method and then studied. The main techniques of this method were direct observation, assessing the state of a sub-population, measuring and describing plants, and plotting the diagrams and maps. Populations were studied at monitoring and temporarily established sites (from 10 to 150 m2). The population characteristics included the population size, the total area covered, the absolute number of individuals and their population density, the actual contour of the phytocenoses, and the degree of anthropogenic transformation of the phytocenoses.
The age structure of populations was characterized using standard demographic indicators: the recovery index [49] and the population age index [6,50]. The population state was estimated using the “delta-omega” criterion of Zhivotovsky [51]. The replacement index (Irep.), recovery index (Irec.), index of aging (IAg), age index (Δ), and index efficiency (ω) are primary indicators of age structure dynamics in plant populations [47,48,49,50,51].
A bush part (ramet) was set as a counting unit. The number of leaves in the rosette was counted, the length and width of the leaf plates were measured, the height of the flower stalk, and the number of flowers were determined for generative plants.
The age state of individuals of P. chlorantha was assessed with regard to that of the closely related species P. rotundifolia L. [52].
2.4. Statistical Analysis
The life states of the registered individuals were specified, the age structure was assessed, and the main demographic parameters of the populations were calculated based on the measured morphological parameters of individuals. The analysis was performed using PAST 4.04 software (Øyvind Hammer, University of Oslo, Oslo, Norway) [53] and Microsoft Excel 2010 (Microsoft, Redmond, WA, USA).
3. Results
Fifteen populations of P. chlorantha in the Zhiguli State Nature Reserve and twelve populations in the Buzuluksky Bor National Park were surveyed. The total number of recorded individual plants was 1520:980 in Zhiguli State Nature Reserve and 540 in Buzuluksky Bor National Park, respectively. The average number of individuals (aboveground shoots) in the populations of the Zhiguli State Nature Reserve was ~65 specimens (from 20 to 84); in the Buzuluksky Bor National Park, 45 plants (from 33 to 79). The peculiarities of the population structure of green-flowered wintergreen, important for environmental planning, have been described for the first time.
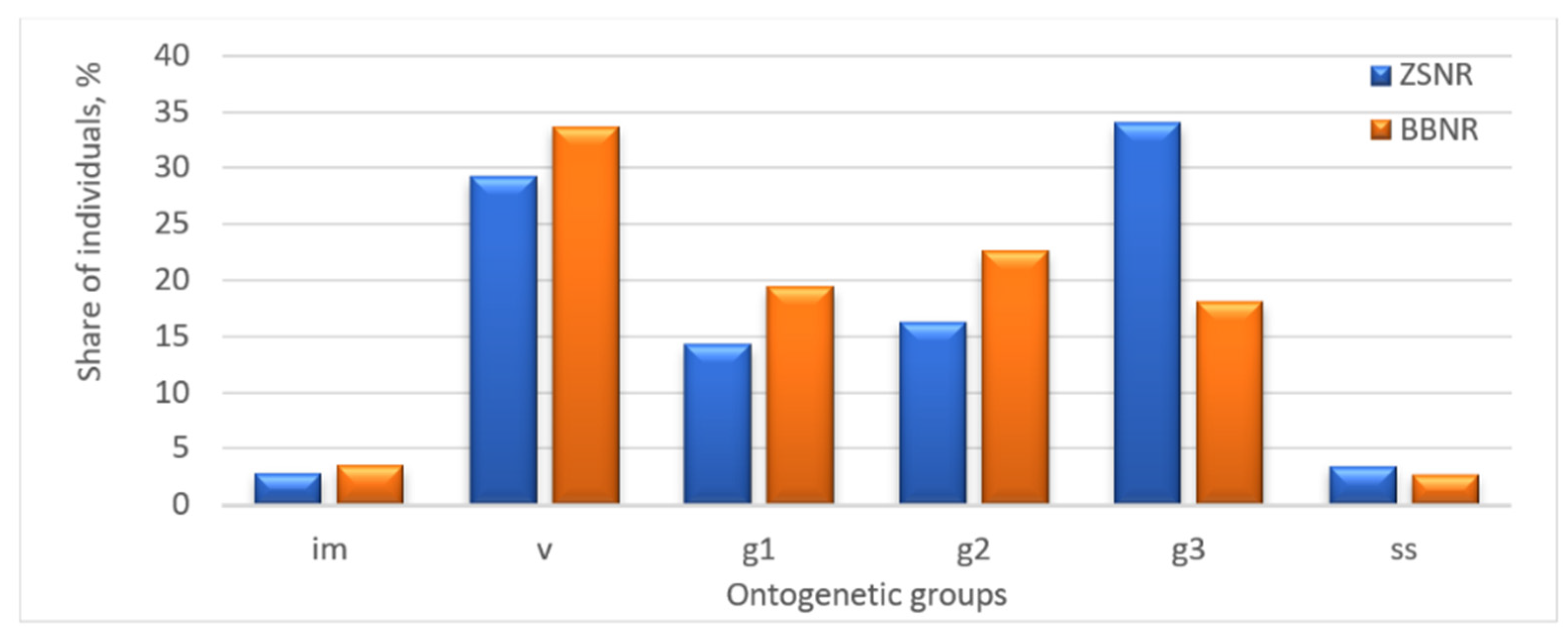
The age structure of the studied populations of P. chlorantha differed in two surveyed geographical locations. In the Zhiguli State Nature Reserve, the age structure was right modal, with a predominance of old generative plants (34.0%). In the Buzuluksky Bor National Park, the spectrum was characterized as bimodal, with the predominance of virginal individuals (33.7%). In both cases, the populations included all age groups (Figure 1). In the Zhiguli State Nature Reserve, a smaller share of young and mature generative plants was noted than that of old generative plants, which might indicate a gradual suppression of the population. The Buzuluksky Bor National Park population exhibited a basic age structure that formed under optimal habitat conditions.
Figure 1.
Age structure of P. chlorantha populations in the Zhiguli State Nature Reserve (ZSNR) and in the Buzuluksky Bor National Park (BBNR): im—immature; v—virginal; g1—young generative; g2—mature generative; g3—old generative; ss—sub-senile individuals (compiled by the authors).
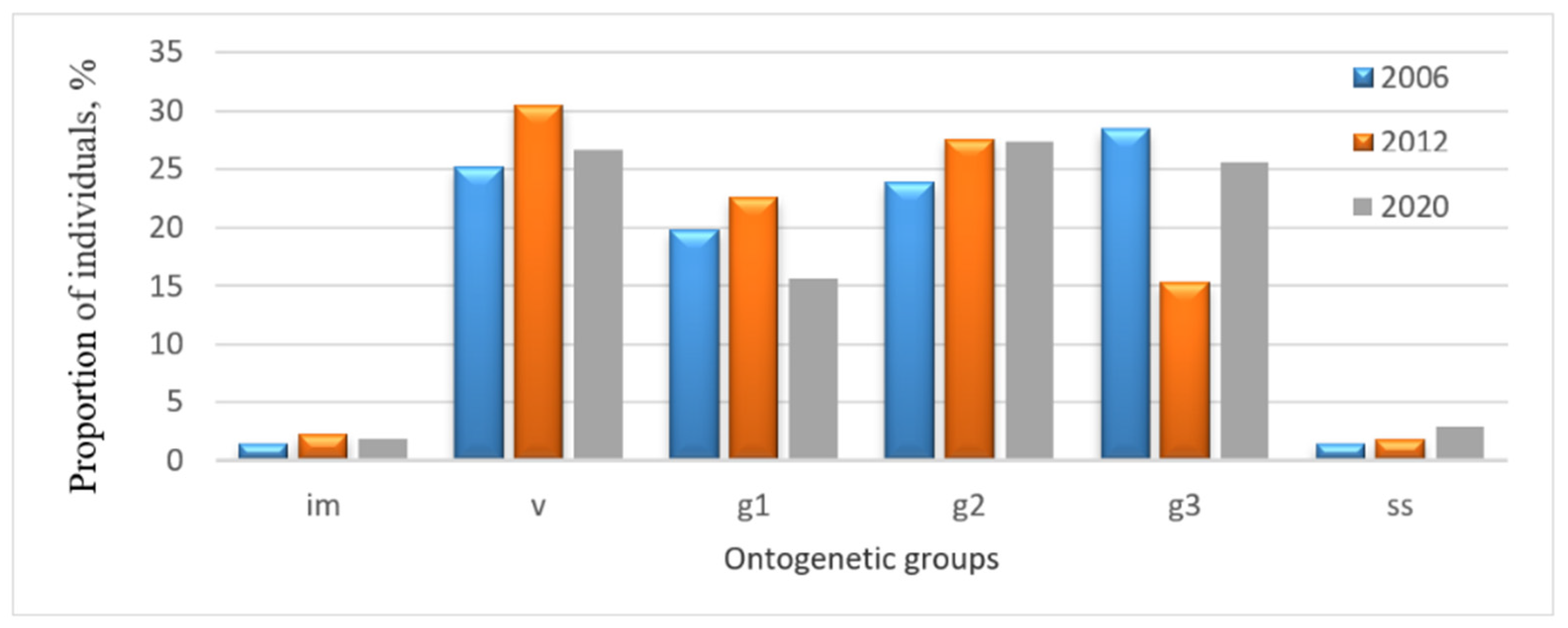
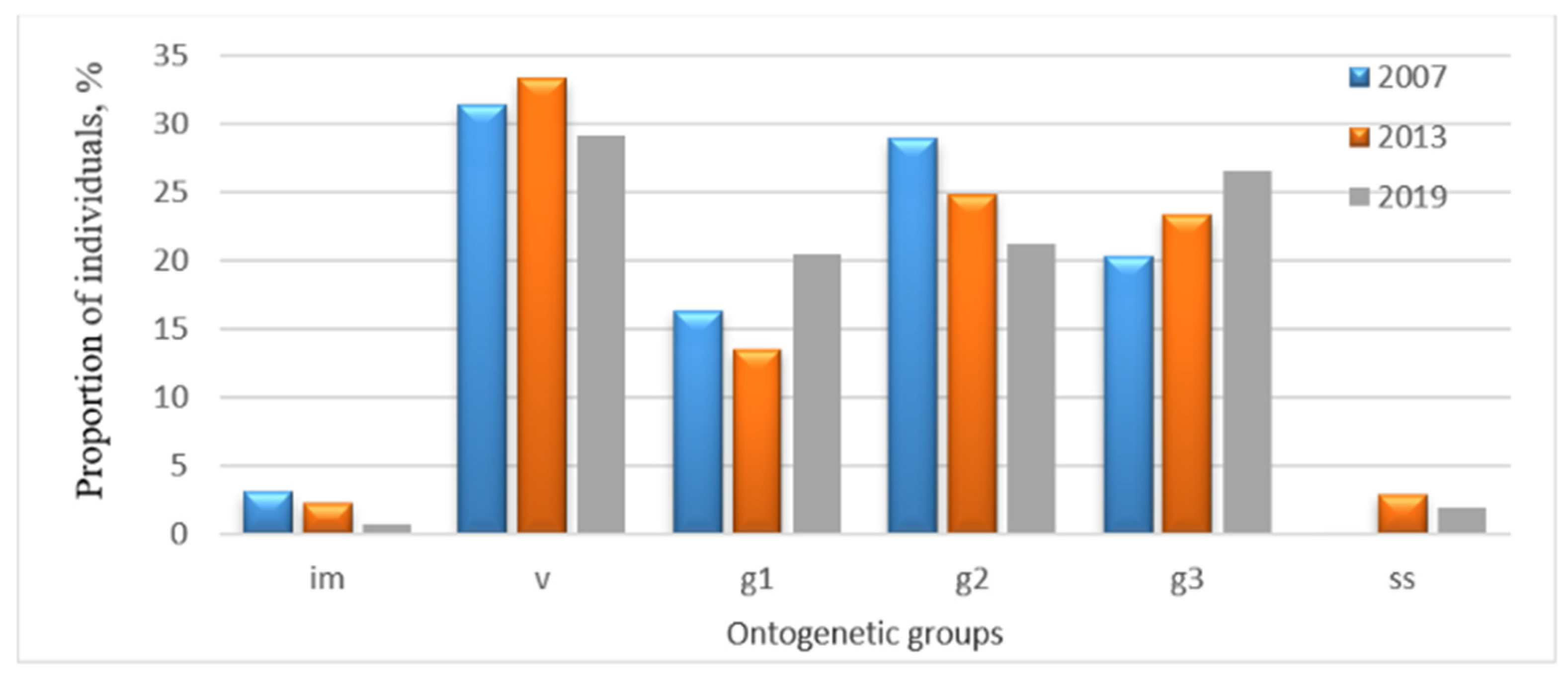
The dynamics of the age structure of P. chlorantha populations were worth analyzing separately in the Zhiguli State Nature Reserve and in the Buzuluksky Bor National Park (Figure 2 and Figure 3). In order to estimate the composition and dynamics of populations, we chose the data for the years with the highest number of recorded individuals.
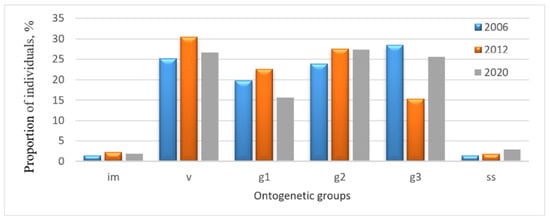
Figure 2.
Age structure dynamics of the P. chlorantha population in the Zhiguli State Nature Reserve: im—immature; v—virginal; g1—young generative; g2—mature generative; g3—old generative; ss—sub-senile individuals.
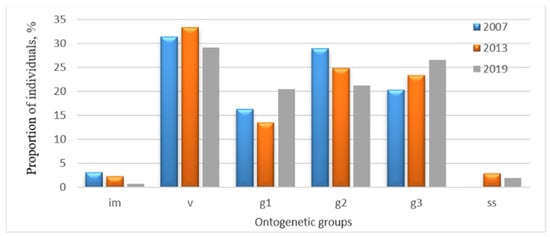
Figure 3.
Age structure dynamics of the P. chlorantha population in the Buzuluksky Bor National Park: im—immature; v—virginal; g1—young generative; g2—mature generative; g3—old generative; ss—sub-senile individuals.
Pronounced dynamics of the age structure were observed in the Zhiguli State Nature Reserve. From 2006 to 2012, the group of old generative individuals experienced the greatest change, with their share nearly halving, from 28.5% to 15.3% (Figure 2).
In the Buzuluksky Bor National Park, the dynamics of the age structure of the population was close to normal. However, the proportion of mature generative individuals decreased (from 28.9% to 21.2%), but the share of old generative plants has increased (from 20.3% to 26.6%).
The age structure of populations and referring indexes allowed for some assumptions (Table 1). Generally, pre-generative individuals represented about a third of the population (32.3%), generative individuals made up two-thirds (66.9%), and senile individuals made up a minor portion(1.8%). The population indices were: (1) the index of replacement (IRep) of 0.46; (2) the population recovery index (IRec) of 0.47; (3) the aging index (IAg) of 0.02; (4) the ageing index (Δ) of 0.40; and (5) the efficiency index (ω) of 0.71. According to the delta-omega criterion [48,51], the populations of both Buzuluksky Bor National Park and the Zhiguli State Nature Reserve belonged to the mature type, but transitional populations were noted in this area in some years. The studied parameters differed significantly (Student’s t-test, tSt = 2.08), but did not differ statistically according to the nonparametric test (p < 0.05).

Table 1.
Age structure of populations of P. chlorantha at the southern range margin (ZSNR—the Zhiguli State Nature Reserve, BBNP—the Busuluksky Bor National Park): p–v—pre-generative plants; g1–g3—generative plants; ss–s—sub-senile and senile individuals.
The spatial structure of P. chlorantha populations was similar at all studied sites due to the peculiarities of its vegetative reproduction in the Samara Region. In each population, there was a cluster formed by 10–50 individuals. The distance between clusters was from 1.5 to 30 m in both the Zhiguli State Nature Reserve (2006, 2012, 2020) and the Buzuluksky Bor National Park (2007, 2013, 2019) (Table 2). Cluster formation was predominantly due to the vegetative reproduction and mosaic nature of the grass layer. The average population density was calculated for the plots of 1, 10, and 100 m2. Single individuals or the small groups of two or three specimens (mostly individuals of seed origin) were found very rarely between the clusters. However, in most cases, other P. chlorantha plants were not registered between the clusters.

Table 2.
Spatial distribution of individual P. chlorantha at the southern range margin (ZSNR—the Zhiguli State Nature Reserve, BBNP—the Busuluksky Bor National Park).
Vegetative reproduction served as the main way of replenishment in the studied populations of P. chlorantha, as evidenced by the high share of virginal individuals: 29.2% in the Zhiguli State Nature Reserve and 33.7% in the Buzuluksky Bor National Park. A small number of immature plants of vegetative origin (2.8% and 3.5%, respectively) was recorded as well. In general, the individuals that appeared from the seeds accounted for about 3% of the total population in the Zhiguli State Nature Reserve and about 4.5% in the Buzuluksky Bor National Park.
In some years, forest fires led to the deterioration of P. chlorantha populations. Large forest fires were recorded in Zhiguli State Nature Reserve in 2010 and in Buzuluksky Bor National Park in 2010 and 2021.
4. Discussion
The predominance of vegetative reproduction over seed reproduction in P. chlorantha is observed in both the Zhiguli State Reserve and the Buzuluksky Bor National Park. During vegetative propagation, new young individuals are characterized by an elongated plagiotropic shoot; the individuals emerged from the seeds as orthotropic rosette shoots. Low shares of seed reproduction in the Zhiguli State Nature Reserve may be explained by the peculiarities of the limestone substrate and the weak development of the humus layer [54]. Low rates of Pyrola seed reproduction have been reported earlier [55]. Low potential seed productivity affects the morphogenesis of individuals, abundance, renewal processes, and the age structure of many rare plant species populations [56,57,58,59].
The green-flowered wintergreen population in the Buzuluksky Bor National Park is characterized by better renewal due to replenishment by immature and virgin individuals. The species that reproduce vegetatively usually have a high potential for recovery [60,61,62]. However, the number of individuals in the studied populations of green-flowered wintergreen is low, indicating a low level of recovery associated with the elimination of plants in the early stages. Most often, the limiting factors for population growth are anthropogenic pressure [63] and the ecological and coenotic conditions of habitats [64]. In the Zhiguli State Nature Reserve, green-flowered wintergreen populations gradually accumulate generative individuals that become the dominant group after some time. The accumulation of individuals in the generative group is typical for plant species with a long life span [63,65].
Numerous publications repeatedly emphasize the rather narrow ecological and coenotic niches of representatives of the Ericaceae family and the pronounced effect of climate changes on their bioecological characteristics [66,67]. The presence of mycorrhizal fungi is indispensable for seed germination and further plant growth [27,68]. Differences in the environmental conditions of P. chlorantha habitats in the Zhiguli State Nature Reserve and the Buzuluksky Bor National Park are reflected in the population structure.
The ageing of the Pyrola population in the Buzuluksky Bor National Park, as manifested by a gradual increase in the share of old generative individuals, is most likely due to recreational impact on vegetation cover, which is influenced by changes in ecological and coenotic conditions reported in different regions [69]. However, the total, absolute abundance of green-flowered wintergreen has not undergone strong changes. A decrease in the number of individuals in clusters and an increase in the distance between them are associated with partial disturbance of the ground cover in some areas due to timber activities and cattle grazing.
The age structure dynamics of green-flowered wintergreen in the Zhiguli State Nature Reserve are close to normal for the species. The decrease in the share of old generative plants is associated with the disturbance of the soil and vegetation cover during the catastrophic forest fires of 2010, which have significantly affected the oak and pine forests of the reserve [70]. After the fires of 2010, the number of green-flowered wintergreen individuals in the studied area have also decreased significantly, but the species began to recover its numbers subsequently.
Forest lowland fires reduce the vitality and abundance of P. chlorantha and cause changes in the spatial and age structure of populations, resulting in a decrease in the number of individuals in clusters and an increase in the distance between the preserved plant clusters. This is due both to the elimination of existing individuals and the severity of affecting the surface soil layer. Fire exposure often leads to the elimination of the mycelium of the fungus mycorrhiza associated with P. chlorantha. According to some data, recovery of mycorrhizal fungi in soil takes a long time [71,72,73], which also affects population growth, age structure, and population state in general. Comprehensive monitoring is a prerequisite for assessing the fire effects on the populations of rare plants [74].
5. Conclusions
The age structure of P. chlorantha populations indicates that they are more stable in the Buzuluksky Bor National Park compared to those from the Zhiguli State Nature Reserve. The plants are characterized mainly by vegetative reproduction, predetermining the specimen clusters in plant communities as well as the predominance of pre-generative individuals in populations. The basic spectrum of the left modular type was revealed in the P. chlorantha populations in the Buzuluksky Bor National Park. This type is characteristic of many long-rhizome plant species. The differences in the age structure of populations in the Zhiguli State Nature Reserve are apparently related to the features of the stony substrate, which complicate both vegetative and seed reproduction. At the same time, the populations are characterized by lower rates of self-renewal, so the emergence and successful settlement of young individuals are difficult.
Author Contributions
Conceptualization, V.I. and S.S.; methodology, V.I.; data acquisition and analysis, V.I., A.M., O.K. and I.K.; writing—original draft preparation V.I., A.M. and S.S.; writing—review and editing, V.I. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Institutional research project No. 122042700002-6 of the Tsitsin Main Botanical Garden, Russian Academy of Sciences. We thank the Ministry of Science and Higher Education of the Russian Federation for their support of the Center of Collective Use “Herbarium MBG RAS”, grant 075-15-2021-678.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ames, G.M.; Wall, W.A.; Hohmann, M.G.; Wright, J.P. Trait space of rare plants in a fire-dependent ecosystem. Conserv. Biol. 2017, 31, 903–911. [Google Scholar] [CrossRef]
- Bernardo, H.L.; Goad, R.; Vitt, P.; Knight, T.M. Nonadditive effects among threats on rare plant species. Conserv. Biol. 2020, 34, 1029–1034. [Google Scholar] [CrossRef]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jiménez-Alfaro, B.; Hennekens, S.M.; Botta-Dukát, Z.; Chytrý, M.; Field, R.; Jansen, F.; et al. Global trait-environment relationships of plant communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef]
- Salerno, J.; Bailey, K.; Gaughan, A.E.; Stevens, F.R.; Hilton, T.; Cassidy, L.; Drake, M.D.; Pricope, N.G.; Hartter, J. Wildlife impacts and vulnerable livelihoods in a transfrontier conservation landscape. Conserv. Biol. 2020, 34, 891–902. [Google Scholar] [CrossRef]
- Coenopopulations of Plants: Essays of Population Biology; Nauka: Moscow, Russia, 1988. (In Russian)
- Uranov, A.A. Age spectrum of phytocenopopulations as a function of time and energy wave processes. Biol. Sci. 1975, 2, 7–34. (In Russian) [Google Scholar]
- Tonkova, N.A. Biomorphological characteristics of representatives of the subfamily Pyroloideae Jeps. (Ericaceae) in Primorsky Krai. Komar. Read. 2013, 61, 81–118. (In Russian) [Google Scholar]
- IUCN. 2017. The IUCN Red List of Threatened Species v. 2017-1. Available online: http://www.iucnredlist.org (accessed on 8 November 2022).
- Wraith, J.; Pickering, C. Quantifying anthropogenic threats to orchids using the IUCN Red List. Ambio 2018, 47, 307–317. [Google Scholar] [CrossRef]
- Borgelt, J.; Sicacha-Parada, J.; Skarpaas, O.; Verones, F. Native range estimates for red-listed vascular plants. Sci. Data 2022, 9, 117. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; Acad. Press.: London, UK, 1977; 892p. [Google Scholar]
- Schemske, D.W. Plant populations. Perspectives on plant population ecology. Science 1985, 227, 405–406. [Google Scholar] [CrossRef]
- Villellas, J.; Garcia, M.B.; Morris, W.F. Variation in stochastic demography between and within central and peripheral regions in a widespread short-lived herb. Ecology 2013, 94, 1378–1388. [Google Scholar] [CrossRef]
- Dahlgren, J.P.; Roach, D.A. Demographic senescence in herbaceous plants. In The Evolution of Senescence in the Tree of Life; Cambridge University Press: Cambridge, UK, 2017; pp. 303–319. [Google Scholar] [CrossRef]
- Edelfeldt, S.; Dahlgren, J.P.; Bengtsson, K. Demographic senescence and effects on population dynamics of a perennial plant. Ecology 2019, 100, 27–42. [Google Scholar] [CrossRef]
- Kull, T. Population dynamics of north temperate orchids. In Orchid Biology: Review, and Perspectives; VIII; Kull, T., Arditti, J., Eds.; Kluwer: Amsterdam, The Netherlands, 2002; pp. 139–165. [Google Scholar]
- Rodriguez-Riaio, T.; Ortega-Olivencia, A.; Devesa, J.A. Reproductive biology in Cytisus multiflorus (Fabaceae). Ann. Bot. Fenn. 2004, 41, 179–188. [Google Scholar] [CrossRef]
- Vaz, A.S.; Alves, P.; Vicente, J.R.; Caldas, F.B.; Honrado, J.P.; Lomba, A.; Silva, D. Evaluating populations and community structure against climate and land-use determinants to improve the conservations of the rare Narcissus pseudonarcissus subsp. nobilis. An. Jard. Bot. Madr. 2016, 73, e027. [Google Scholar] [CrossRef]
- Lohvynenko, I.P.; Lyko, S.M.; Trochymchuk, I.M.; Portukhay, O.I.; Glinska, S.O. Structure of some rare flora species populations in conditions of Volhynian Upland. Ukr. J. Ecol. 2019, 9, 102–114. [Google Scholar]
- Esina, I.G.; Khapugin, A.A.; Esin, M.N.; Popov, S.Y. New data on vascular plants of the Mordovian State Reserve. Proc. Mordovian State Nat. Reserve 2021, 27, 15–38. (In Russian) [Google Scholar]
- Karimova, O.A.; Abramova, L.M.; Golovanov, Y.M. Analysis of the current status of populations of rare plant species of nature monument Troicki chalk mountains (Orenburg Region). Arid. Ecosyst. 2017, 7, 41–48. (In Russian) [Google Scholar] [CrossRef]
- Plikina, N.V.; Efremov, A.N.; Samoylova, G.V. Evaluation of the population state of rare plant species of the Omsk Region (Isilkulsky and Krutinsky municipal districts). Vestn. Omsk SAU 2017, 1, 49–59. (In Russian) [Google Scholar]
- Golovanov, Y.M.; Abramova, L.M. Chalky highlands in Orenburg Region, a uniqe habitat for rare plant communities. Arid Ecosyst. 2019, 9, 89–96. (In Russian) [Google Scholar] [CrossRef]
- Mitroshenkova, A.E.; Ilyina, V.N.; Kazantsev, I.V.; Rogov, S.A. Current state, population structure and population dynamics of rare plants under economic and recreational use of natural-territorial complexes in the Middle Volga basin (Russia). IOP Conf. Ser. Earth Environ. Sci. 2021, 723, 042054. [Google Scholar] [CrossRef]
- Abramova, L.M.; Karimova, O.A.; Andreeva, I.Z. On the ecology and biology of Althea officinalis L. (Malvaceae) et the northern border of its range (Republic of Bashkortostan). Contemp. Probl. Ecol. 2013, 6, 415–425. (In Russian) [Google Scholar] [CrossRef]
- Abramova, L.M.; Karimova, O.A.; Mustafina, A.N. Evaluation of the state of marginal populations of some rare plant species of the Southern Urals. Proc. Natl. Acad. Sci. Belarus. Biol. Sci. Ser. 2014, 4, 23–27. (In Russian) [Google Scholar]
- Johansson, V.A.; Eriksson, O. Recruitment limitation, germination of dust seeds, and early development of underground seedlings in six Pyroleae species. Botany 2013, 91, 17–24. [Google Scholar] [CrossRef]
- Puzyrkina, M.V.; Silaeva, T.B. The population state of Scabiosa isetensis L. (Dipsacaceae) at the northwestern border of the range. Proc. Samara Sci. Cent. Russ. Acad. Sci. 2013, 15, 98–102. (In Russian) [Google Scholar]
- Selyutina, I.Y.; Konichenko, E.S.; Rupyshev, Y.A. Ontogenesis and age structure of a rare species Oxytropis nitens (Fabaceae) at the northern border of its range. Bot. Zhurnal 2014, 99, 1001–1009. [Google Scholar]
- Klimenko, G.; Kovalenko, I.; Likholat, Y.; Khromikh, N.; Dydur, O.; Alexeeva, A. Integral assessment of the population state of rare species of plants. Ukr. J. Ecol. 2017, 2, 201–208. (In Ukrainian) [Google Scholar] [CrossRef] [PubMed]
- Ilyina, V.; Zenkina, T.; Sagalaev, V.; Senator, S.; Mitroshenkova, A.; Kozlovskaya, O.; Kalmykova, O. Structure of cenopopulations Clausia aprica (Stephan) Korn.-Tr. on the border of the area. BIO Web Conf. 2021, 38, 00043. [Google Scholar] [CrossRef]
- Proner, M. Investigation of the melanogenesis in Pyrola secunda L. Wiadomosci. Farm. 1937, 64, 623–628. [Google Scholar]
- Copeland, H.F. Observations on the structure and classification of the Pyroleae. Madrono 1947, 9, 65–102. [Google Scholar]
- Knaben, G.; Engelskjon, T. Studies in Pyrolaceae, especially in the Pyrola rotundifolia complex. Acta Univ. Bergen. Ser. Math Natur. 1968, 4, 68–71. [Google Scholar]
- Holub, J.; Krisa, B. Pyrola carpatica Holub et Krisa, a new species among European wintergreens; with remarks on the name “Pyrola intermedia”. Folia Geobot. Et Phytotaxon. 1971, 6, 81–92. [Google Scholar] [CrossRef]
- Stevanovic, V. Ekolosko-cenoloska analiza stanista zelene kruscice (Pirola chlorantha Sw.) u Deliblatskoj pescari. Glas. Inst. Bot. 1972, 7, 97–102. [Google Scholar]
- Haber, E.; Cruise, J.E. Generic limits in the Pyroloideae. Canad. Jurn. Bot. 1974, 52, 877–883. [Google Scholar] [CrossRef]
- Haber, E.; Takahashi, H. A comparative study of the North American Pyrola asarifolia and its Asien vicariad, P. incarnata (Ericaceae). Bot. Mag. Tokyo 1988, 101, 483–495. [Google Scholar] [CrossRef]
- Bagdasarova, T.V.; Vakhrameeva, M.G.; Nikitina, S.V.; Denisova, L.V. Genus Pyrola. In Biological Flora of the Moscow Region. Vol. 7; Publishing house of Moscow State University: Moscow, Russia, 1983; pp. 153–176. (In Russian) [Google Scholar]
- Khapugin, A.A.; Korochkina, A.M.; Kitina, A.V. Pyrola chlorantha (Ericaceae) in the Mordovsky State Nature Reserve. Proc. Mordovian State Nat. Reserve 2018, 20, 192–202. (In Russian) [Google Scholar]
- Chakhireva, E.V. Ecological and biological peculiarities of the Pyrola chlorantha in the Volga-Kama Nature Reserve. In Biodiversity and Rational use of Natural Resources; Proceedings of the All-Russian Scientific and Practical Conference; Dagestan State Pedagogical University: Makhachkala, Russia, 2013; pp. 67–69. (In Russian) [Google Scholar]
- Klimenko, A.; Kovalenko, I. Reproduction as a Factor of the Sustainability of Rare Plant Species. Notes Curr. Biol. 2016, 7, 49–54. (In Ukrainian) [Google Scholar] [CrossRef]
- Skvortsov, A.K. Family 73. Pyrolaceae Dum. In Flora of the European part of the USSR. Vol. 5; Nauka: Leningrad, Russia, 1981; pp. 52–57. (In Russian) [Google Scholar]
- Senator, S.A.; Saksonov, S.V. (Eds.) Red Data Book of the Samara Region. Rare Species of Plants and Fungi; Publishing House of the Samara State Regional Academy: Samara, Russia, 2017; Volume 1, 384p. (In Russian) [Google Scholar]
- Krisa, B. Pyrolaceae. In Flora Europaea; Cambridge University Press: Cambridge, UK, 1972; Volume 3, p. 34. [Google Scholar]
- Bobrov, Y.A. On the early stages of development of individuals of European species of the Pyrolaceae family. Bot. Zhurnal 2004, 89, 1342–1351. (In Russian) [Google Scholar]
- Zhukova, L.A. Diversity of ontogenic pathways in plant populations. Russ. J. Ecol. 2001, 32, 151–158. (In Russian) [Google Scholar] [CrossRef]
- Osmanova, G.O.; Zhivotovsky, L.A. Age structure as an indicator of the state of plant populations. Biol. Bull. 2020, 2, 144–152. (In Russian) [Google Scholar] [CrossRef]
- Zhukova, L.A. Population Life of Meadow Plants; Lanar: Yoshkar-Ola, Russia, 1995; 224p. (In Russian) [Google Scholar]
- Glotov, N.V. On the estimation of the parameters of the age structure of plant populations. In Life of Populations in Heterogeneous Environment; Part 1; Periodika Marii El: Yoshkar-Ola, Russia, 1998; pp. 146–149. (In Russian) [Google Scholar]
- Zhivotovsky, L.A. Age states, effective density and classification of plant populations. Russ. J. Ecol. 2001, 21, 3–7. [Google Scholar]
- Polyanskaya, T.A.; Romanova, O.Y.; Vedernikova, O.P. Ontogenesis Pyrola rotundifolia L. In An Age Atlas of Medicinal Plants; Mari State University: Yoshkar-Ola, Russia, 2004; Volume IV, pp. 161–168. (In Russian) [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Abakumov, E.V.; Gagarina, E.I. Soils of Samarskaya Luka: Diversity, Genesis, Protection; Publishing House of St. Petersburg University: Saint-Petersburg, Russia, 2008; 153p. (In Russian) [Google Scholar]
- Nikiforova, A.A. Comparison of ontogenesis of the Pyrola in central and northwestern Yakutia as an indicator of adaptation to climate change. Nat. Sci. 2014, 1, 48–52. (In Russian) [Google Scholar]
- Andronova, E.V.; Evdokimova, E.E.; Semenov, A.V. Fruiting, heterospermia and seed quality of Orchis purpurea subsp. caucasica (Orchidaceae). Bot. Zhurnal 2015, 100, 359–372. (In Russian) [Google Scholar]
- Guseva, A.A.; Cheryomushkina, V.A. Morphogenesis and state of coenopopulations of the endemic species Scutellaria tuvensis (Lamiaceae). Bull. Mosc. Soc. Naturalists. Biol. Ser. 2017, 122, 68–77. (In Russian) [Google Scholar]
- Krjukova, A.V.; Abramova, L.M. On the biology of Iris scariosa Willd. ex Link, a rare species of the Republic of Bashkortostan. Izv. Ufim. Nauchnogo Cent. RAN 2015, 3, 49–52. (In Russian) [Google Scholar]
- Shokhina, N.K.; Gordeeva, N.I.; Pshenichkina, Y.A. Assessment of the reproductive potential of Hierochloe odorata (L.) Beauv. and H. repens (Host) Beauv. (Poaceae) at different levels of organization. Contemp. Probl. Ecol. 2020, 13, 95–103. [Google Scholar] [CrossRef]
- Zheleznaya, E.L. Changes in the structure of a Dactylorhiza incarnata (L.) Soó population during the overgrowing of a meadow-bog community complex in the Moscow Region. Russ. J. Ecol. 2009, 40, 39–43. [Google Scholar] [CrossRef]
- Mayorova, O.Y.; Hrytsak, L.R.; Drobyk, N.M. The strategy of Gentiana lutea L. populations in the Ukrainian Carpathians. Russ. J. Ecol. 2015, 46, 43–50. [Google Scholar] [CrossRef]
- Sultangazina, G.J.; Kuprijanov, A.N.; Kupriyanov, O.A.; Raimbekov, E.B. The ontogenesis and structure of coenopopulations of Pulsatilla uralensis in the conditions of Northern Kazakhstan. Biol. Med. Geogr. Ser. 2020, 99, 140–148. (In Russian) [Google Scholar] [CrossRef]
- Ilyina, V.N. Age structure and types of cenopopulation of Laser trilobum (L.) Borkh. (Apiaceae) in the Middle Volga Basin. Vestsi Natsyyanal’nai Akade. Navuk Belarusi. Seryya Biyalagichnych Navuk 2018, 63, 99–106. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Selyutina, I.Y.; Zibzeev, E.G. Age structure and vitality of the cenopopulations of Oxytropis sulphurea (Fisch. ex DC.) Ledeb. in different ecocoenotic conditions of Rudny Altai and the Saur Ridge. Contemp. Probl. Ecol. 2016, 9, 355–365. [Google Scholar] [CrossRef]
- Egorova, N.Y. Influence of ecological factors on the population-age parameters of Vaccinium vitis-idaea L. in forest ecosystems of the European Northeast of Russia. Contemp. Probl. Ecol. 2020, 13, 656–662. [Google Scholar] [CrossRef]
- Maslova, S.P.; Tabalenkova, G.N. Physiological and biochemical characteristics of Pyrola rotundifolia (Pyrolaceae) in Middle Taiga. Rastit. Resur. 2011, 47, 27–33. (In Russian) [Google Scholar]
- Revchuk, N.A.; Brizhataya, A.A. Ecological and coenotic peculiarities of pearl-grass growth in the black fir-broadleaved forest in the south of Primorsky Krai. Bull. Bot. Gard.-Inst. FEB RAS 2017, 18, 27–31. (In Russian) [Google Scholar]
- Malysheva, V.F.; Malysheva, E.F.; Voronina, E.Y.; Fedosova, A.G.; Bibikov, N.M.; Kiseleva, D.S.; Tiunov, A.V.; Kovalenko, A.E. Mycorrhiza Pyrolaceae (Pyrola rotundifolia, P. media and Orthilia secunda): Composition of fungal symbionts and trophic status of plants. Mycol. Phytopathol. 2017, 51, 350–364. (In Russian) [Google Scholar]
- Arnesen, T. Vegetation dynamics following trampling in grassland and heathland in Sølendet Nature Reserve, a Boreal Upland Area in Central Norway. Nord. J. Bot. 1999, 19, 47–69. [Google Scholar] [CrossRef]
- Bykov, E.V.; Kuzmina, M.V. The comprehensive analisis of pyrogenic impact on ecosistems of the Samarskaya Luka National Park. Vestn. Volzhsky Univ. Named V.N. Tatishchev 2011, 12, 17–21. (In Russian) [Google Scholar]
- Lukina, N.V.; Chibrik, T.S.; Glazyrina, M.A.; Filimonova, E.I. The dynamics of vegetation and mycorrhizal restoration on recultivated and non-recultivated sites of the ash dump. Ekosistems 2019, 20, 188–196. (In Russian) [Google Scholar]
- Horn, S.; Caruso, T.; Verbruggen, E.; Rillig, M.C.; Hempel, S. Arbuscular mycorrhizal fungal communities are phylogenetically clustered at small scales. ISME J. 2014, 8, 2231–2242. [Google Scholar] [CrossRef]
- Lukina, N.V.; Ryazanova, S.V. Tre peculiarity of mycorrhisa in technogenic ecosistems. Optim. Prot. Ecosyst. 2012, 7, 261–269. (In Russian) [Google Scholar]
- Suleymanova, G.F.; Boldyrev, V.A.; Savinov, V.A. Post-fire restoration of plant communities with Paeonia tenuifolia in the Khvalynsky National Park (Russia). Nat. Conserv. Res. 2019, 4, 57–77. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).