Abstract
Klebsiella oxytoca originating from shellfish Scapharca subcrenata contains a number of virulence-related genes. In this study, we investigated its pathogenicity using a murine intestinal infection model and predicted its antibacterial compounds and targets via molecular docking analysis. The results revealed that the intake of K. oxytoca 8-2-11 strain (109 CFU/day) via oral gavage for 7 days reduced the average body weight of the mice. The bacterium was present in fecal samples but absent from blood, lung, and liver samples from the mice. The intake of K. oxytoca 8-2-11 significantly altered colon bacteriota, with reduced abundance of Firmicutes, Lachnospiraceae, Lactobacillaceae, Lactobacillus, and Lactobacillus murinus, and increased in Bacteroidota, Muribaculaceae, and Alistipes (p < 0.05). Forty-four bioactive compounds in Scutellaria baicalensis and Forsythia suspensa were screened for docking with 117 potential virulence factors (VFs) in K. oxytoca 8-2-11. The compound baicalin displayed higher binding affinity toward these VFs, with the lowest mean binding energy (−8.4 kcal/mol). Baicalin was able to bind to key VFs in biofilm formation and adherence/motility (e.g., Mrks and EcpA) via forming stable hydrogen bonds, π-stacking, and π-cation interaction. In vitro, baicalin inhibited the bacterial growth and biofilm formation. This study establishes the first murine infection model using aquatic animal-derived K. oxytoca, and it provides candidate antibacterial compounds and targets for control of K. oxytoca infections.
1. Introduction
After Klebsiella pneumoniae, Klebsiella oxytoca is the second most severe pathogen in the Klebsiella genus. K. oxytoca is found growing in soil, water, plants, and animals [1], and is also a member of the intestinal flora in humans, colonizing 2–9% of healthy adults and over 70% of healthy infants [2,3]. It has increasingly been reported that K. oxytoca can cause nosocomial infection (e.g., bloodstream, urinary tract, respiratory tract, skin and soft tissue and intra-abdominal infection), infectious endocarditis, and antibiotic-associated hemorrhagic colitis (AAHC) [1,2,3,4]. Nevertheless, the pathogenesis of K. oxytoca remains largely uncovered. Particularly, recent studies on the impact of K. oxytoca on host intestinal flora have been rare. The study by Hua et al. showed that the microbiota in digestive and respiratory tracts of C57BL/6 mice were altered after the gavaging of a clinical strain of K. oxytoca (5 × 108 CFU) every other day for one or two weeks [5].
Many commonly used antimicrobial drugs, including β-lactams (e.g., cephalosporins, carbapenems, and piperacillin-tazobactam) and non-β-lactams (e.g., amikacin, mucins, quinolones, tigecycline, and trimethoprim-sulfamethoxazole), may be good therapeutic options for infectious diseases caused by K. oxytoca and even more severe K. oxytoca complexes, which share carbapenem resistance mechanisms with other carbapenem-resistant Enterobacterales (CRE) [4]. The emergence and transmission of antimicrobial resistant pathogens pose a major threat to human health. Therefore, the search for and discovery of new antimicrobial agents and therapeutic options are imperative.
Medicinal plants provide a rich resource of naturally occurring antimicrobial compounds [6]. In addition to their safety, low cost, and good efficacy, nutrient phytochemicals have demonstrated anti-oxidant, anti-inflammatory, anti-hypertensive, anti-tumor and anti-microbial activities [7,8,9]. For instance, Scutellaria baicalensis has been used for reducing fever and dampness and for detoxification, for thousands of years in China [10,11,12]. This plant belongs to the family of Labiatae and is widely distributed in China, Japan, Korea, Russia, and Mongolia [13]. Modern pharmaceutical studies have demonstrated that extracts of S. baicalensis could treat hypertension, hyperlipidemia, dysentery, pneumonia, liver cancer, respiratory disorders, and Alzheimer’s disease [10,14,15,16,17]. For instance, Cui et al. reported that a homogeneous polysaccharide SP2-1 (50 and 200 mg/kg) isolated from S. baicalensis ameliorated colonic pathology of ulcerative colitis (UC) of C57BL/6 mice. SP2-1 inhibited the production of pro-inflammatory cytokines (e.g., IL-6, IL-1β, and tumor necrosis factor (TNF)-α), strengthened the function of the intestinal barrier, promoted the proliferation of Bifidobacterium, Lactobacillus and Roseburia, and inhibited harmful bacteria (e.g., Staphylococcus), thus effectively eliminating the symptoms of colitis [11]. S. baicalensis also effectively relieved symptoms of clostridium difficile-associated diarrhea (CDAD), reduced inflammatory response, and beneficially reorganized intestinal microbiota of CDAD mice [18].
Similarly, Forsythia suspensa is also a traditional medicine for reducing fever, and detoxification [19]. This plant belongs to the family of Oleaceae and widely distributes in East Asia (e.g., China, Japan, and Korea) [20,21,22,23]. Modern pharmacological studies have revealed that monomeric compounds extracted from F. suspensa have anti-inflammatory, anti-oxidant, anti-endotoxin, antibacterial, and antiviral activities [22,24,25,26,27]. For example, phillygenin (PHI) extracted from F. suspensa could treat UC through inhibiting pro-inflammatory cytokines, and reducing macrophage infiltration into colonic tissues [24].
Network pharmacology and molecular docking technique are very useful to predict potential protein receptors targeted by bioactive compounds in medicinal plants [28]. For example, forsythoside A (FTA) in F. suspensa for treating cholestasis was predicted to target potential receptors such as Toll-like receptor 4 (TLR4), myeloid differentiation primary response 88 (MyD88), nuclear factor-kappa B (NF-κB)1, and matrix metalloproteinase-2 (MMP-2) in the liver. Their binding free energies were found to be less than −5.0 kcal/mol, suggesting that FTA may effectively bind to these receptors and regulate TLR4/MyD88/NF-κB signaling pathways, potentially improving liver damage caused by cholestasis [29]. Baicalein in S. baicalensis for treating osteoarthritis was also predicted to target AKT serine/threonine kinase 1 (Akt1), epidermal growth factor receptor (EGFR), vascular endothelial cell growth factor receptor (VEGFA), hypoxia inducible factor 1 alpha (HIF1α), and prostaglandin G/H synthase 2 (PTGS2) according to molecular docking analysis [30]. Molecular docking techniques have also been used to screen new drugs targeting key antimicrobial resistance and virulence proteins in bacteria, such as the penicillin binding protein 2a (PBP-2a) of methicillin-resistant Staphylococcus aureus (MRSA), and SdiA protein involved in quorum sensing (QS) [31].
K. oxytoca has recently been detected in food animals such as poultry and fish [32,33,34]. In our previous research, K. oxytoca was for the first time isolated from 10 species of mollusks, three species of fish, and one species of crustacean [32]. Notably, K. oxytoca 8-2-11 originating from Scapharca subcrenata displayed a strong capability to form biofilm and tolerated gentamycin (GEN)/sulfamethoxazole-trimethoprim (SXT) and heavy metals (Cr3+/Cu2+/Hg2+/Mn2+/Pb2+/Zn2+). K. oxytoca 8-2-11 belongs to a new sequence type (ST), and its genome harbors a number of strain-specific genes (n = 403) and virulence-associated genes (n = 117) [35]. Based on those findings, in this study, we further evaluated the pathogenicity of K. oxytoca 8-2-11 using a murine intestinal infection model and employed molecular docking technique to predict bioactive ingredients in S. baicalensis and F. suspensa targeting key virulence factors (VFs) in K. oxytoca 8-2-11.
2. Materials and Methods
2.1. Bacteria Strain and Cultural Condition
K. oxytoca 8-2-11 was isolated and characterized in our recent study [32,35]. The bacterium was routinely incubated in sterile Tryptic Soy Broth (TSB) medium (Beijing Land Bridge Technology, Beijing, China) at 37 °C with shaking at 180 rpm. A single colony grown on Tryptic Soy Agar (TSA) plates (Land Bridge, Beijing, China) was inoculated in fresh TSB, and incubated at 37 °C to middle logarithmic growth phase (mid-LGP) (OD600 = 0.8–1.0). The bacterial cells were harvested by centrifugation at 5000 rpm at 4 °C for 3 min, and resuspended with sterile 1 × phosphate-buffered saline (PBS, pH 7.4–7.6, Shanghai Sangon Biological Engineering Technology and Services Co., Ltd., Shanghai, China). Aliquots of the bacterial resuspension (100 μL, 1 × 1010 colony forming unit (CFU)/mL) were stored at 4 °C for the mouse infection model.
2.2. Mouse Infection Model
The mice (C57BL/6J, female, 6–8 week) were purchased from Shanghai Jie Si Jie Laboratory Animal Co., Ltd. (Production License Number: SCXK2023-0004, Shanghai, China). This strain (C57BL/6J) was used in a mouse model orally gavaged with clinical K. oxytoca [5]. The mice were acclimatized for one week in a controlled environment at 22 ± 2 °C, 60 ± 5% humidity and 12 h light–dark cycle in the animal house at Shanghai Ocean University (Shanghai, China). The mice had free access to distilled water and sterilized feed (Si Pei Fu (Beijing) Biotechnology Co., Ltd., Beijing, China). All the experimental mice were used in compliance with laws and regulations on test animal welfare and ethics, and this experiment was approved by Ethical Review Committee for Laboratory Animals of Shanghai Ocean University (Ethics Certificate No. SHOU-DW-2024-154).
The mice were randomly allocated into two groups (n = 10/group): the normal control (NC) group and the model group. Each mouse in the model group was orally gavaged with a 100 µL (1010 CFU/mL) of K. oxytoca 8-2-11 daily at 11:00 a.m. for 7 days; and the NC group was gavaged with a 100 µL of sterile 0.86% NaCl (Sangon, Shanghai, China) instead (Figure 1A). Daily observation of each mouse’s behavior was recorded, and the body weight of each mouse was measured daily.
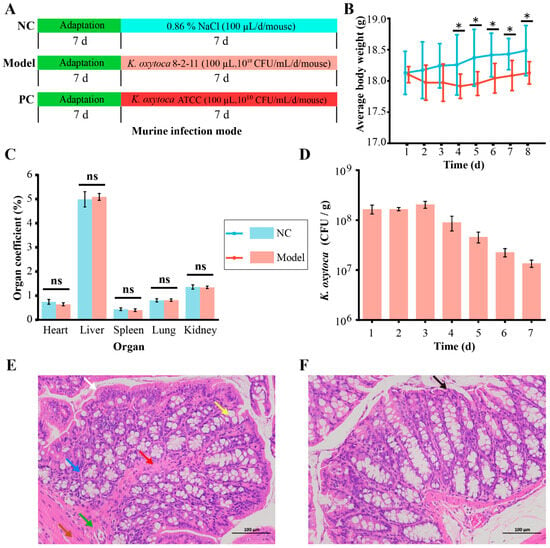
Figure 1.
The effects of intake of K. oxytoca 8-2-11 on average body weight, the major organ coefficients, and surface structure of colon tissue of the mice. (A) The mouse infection mode experiment design. (B) The average body weight changes of the mice. (C) The changes in the major organ coefficients of the mice. (D) The concentrations of K. oxytoca in fecal samples in the model group. (E,F) The histological analysis of colon samples of the mice in the model and the NC groups, respectively (observed by 200 ×). The statistical difference was shown between the NC and model groups. *: p < 0.05; and ns: no statistical difference. The color arrows represented monolayer columnar epithelium (white), mucosal muscularis propria (red), intestinal crypts (yellow), submucosa (blue), muscular layer (green), plasma membrane layer (brown), and necrotic epithelial cells (black), respectively.
On the day 8 of the trail, all the mice were euthanized with sodium pentobarbital, and blood was collected from the retro-orbital vein of each mouse in each group in the sterile environment. By strictly aseptically controlled manipulation, the mice were dissected along the midline of abdomen using sterilized scalpel and tweezers, and the entire heart, lung, spleen, liver, kidney, and colon of the mice in each group were sampled, placed in sterile petri dishes, and weighted. Organ coefficients were calculated according to the following formula: organ coefficient (%) = (mean weight of the organ/mean body weight) × 100 [36].
To avoid cross-contamination during the gavage of K. oxytoca, we chose to perform the positive control (PC) group and the model group in batches. K. oxytoca ATCC 700324 was used as a positive control strain. The trail was the same as described above, except that the mice (ICR, male, 4–8 week) (Shanghai Jie Si Jie Laboratory Animal Co., Ltd. (Shanghai, China) was used (Production License Number: SCXK2023-0004, Shanghai, China), in order to observe the difference of mouse strains (C57BL/6J, and ICR) to the intake of aquatic K. oxytoca.
2.3. Detection of K. oxytoca in the Fecal, Serum and Tissue Samples of the Mice
Fresh fecal samples were collected daily from each group of the mice and weighed. The sterile 1 × PBS was added into the fecal samples at 1:9 (wt:vol, g/mL) and mixed for 2–5 min by vortexing. The fecal supernatants were serially diluted and spread (100 µL/plate) on MacConkey Inositol Adonitol Carbenicillin Agar plates (Qingdao HaiBo Bio Co., Ltd., Qingdao, China). The plates were incubated at 37 °C for 16–20 h. Viable counts of K. oxytoca were calculated as CFU per gram of the fecal sample.
Similarly, the lung and liver samples (0.2 and 0.4 g/each) of each mouse in each group were gently pinched and mixed with the 1 × PBS at 1:9 (wt:vol). The mixture was individually diluted for viable counts of K. oxytoca as described above. The blood samples (100 µL/each) were also serially diluted and spread on MacConkey Inositol Adonitol Carbenicillin Agar plates for viable counts of K. oxytoca.
2.4. Hematoxylin-Eosin (H&E) Staining of Colon Tissue Samples of the Mice
The colon tissue samples of the mice in each group were fixed with 4% paraformaldehyde (Sangon, Shanghai, China) for 24 h. The samples were dehydrated with 70–95% gradient alcohol and embedded in paraffin. Histopathological analysis was conducted by Wuhan Service Biotechnology Co., Ltd. (Wuhan, China). The morphological structures of the colon samples were visualized using Eclipse Ci-L microscope (Nikon, Tokyo, Japan) (200×).
2.5. 16S rRNA Sequencing and Analysis
The colon contents of each mouse in each group were aseptically collected, frozen in dry ice, and transferred to Hong Shun Biotechnology Co., Ltd. (Shanghai, China) for 16S rRNA sequencing. Genomic DNA was extracted from the samples using E.Z.N.A.® soil DNA kit (Omega Bio-Tek, Norcross, GA, USA), according to the manufacturer’s protocol. A NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, DE, USA) was used to determine concentrations and purity of the extracted DNA samples. The V3-V4 hypervariable regions of the bacterial 16S rRNA gene were amplified by polymerase chain reaction (PCR) using the primer pair 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Paired-end sequencing (PE300) was performed using an Illumina MiSeq platform (Illumina, San Diego, CA, USA).
Sequencing reads were demultiplexed and quality filtered for denoise sequence, and chimeric sequences. The taxonomy of each amplicon sequence variant (ASV) representative sequence was assigned using a Ribosomal Database Project (RDP) classifier against the reference database SILVA138, with a minimum confidence score of 0.7 [37].
Statistical analysis and visualization were performed using R software packages including Vegan (version 2.6-4), phyloseq (version 1.38.0), tidyverse (version 1.3.2), ggpubr (version 0.5.0), ComplexHeatmap (version 2.10.0) and corrplot (version 0.92). Alpha diversity was evaluated using the Ace, Chao1, Shannon, and Simpson indices, while beta diversity analysis was conducted using principal coordinate analysis (PCoA) and non-metric multidimensional scaling (NMDS) based on Bray–Curtis distance [37]. The Wilcoxon rank-sum test was used to compare differences in bacterial community composition between groups. Linear discriminant analysis effect size (LEfSe) was used to compare relative abundances of different taxa between groups for identifying signature bacterial populations in the colon samples, with a Kruskal–Wallis test p-value threshold of < 0.05 and an effect size threshold of logarithmic linear discriminant analysis (LDA) score > 3.0 [38].
2.6. Screening of Bioactive Compounds in S. baicalensis and F. suspensa
The Traditional Chinese Medicine Systems Pharmacology (TCMSP) database (https://old.tcmsp-e.com/tcmsp.php, accessed on 20 May 2024) is a platform focusing on systemic pharmacology of herbal medicines, encompassing 499 herbal medicines and over 29,000 ingredients [30]. The TCMSP database was used to identify bioactive ingredients in S. baicalensis and F. suspensa, of which 143 and 150 compounds were obtained, respectively. Compounds with oral bioavailability (OB) ≥ 30% and drug-like properties (DL) ≥ 0.18 were screened based on the pharmacokinetic body absorption of exogenous chemicals (absorption distribution metabolism excretion/toxicology, ADME/T) [39]. The Bioinformatics Analysis Tool for Molecular Mechanism of Traditional Chinese Medicine (BATMAN-TCM) database (http://bionet.ncpsb.org.cn/batman-tcm/index.php, accessed on 21 May 2024) [39], the Encyclopedia of Traditional Chinese Medicine (ETCM) database (http://www.tcmip.cn/ETCM/, accessed on 22 May 2024 ) [40], and the current literature (https://pubmed.ncbi.nlm.nih.gov/, accessed on 24 May 2024) were also utilized for supplementation of compounds in S. baicalensis and F. suspensa. Finally, PubChem database (https://pubchem.ncbi.nlm.nih.gov/, accessed on 26 May 2024) and ChemDraw (version 20.0) software were used to obtain and drew 2D structure diagrams of the compounds.
2.7. Homology Modeling of VFs in K. oxytoca
The genome sequence of K. oxytoca 8-2-11 was determined in our recent research [35]. The Virulence Factor Database (VFDB) (http://www.mgc.ac.cn/VFs/, accessed on 29 May 2024) offers a robust analysis platform for bacterial VFs [41]. Based on the VFDB, potential VFs in K. oxytoca 8-2-11 were searched and compared to obtain corresponding amino acid sequences, and the duplicates were removed. A total of 117 amino acid sequences of the VFs in K. oxytoca 8-2-11 were obtained. SWISS-MODEL (https://swissmodel.expasy.org/interactive, accessed on 1 June 2024) is an automated protein comparative modeling server [42], to which the 117 amino acid sequences were uploaded for template search. We screened templates for closely related homologous with high similarity (>50%) and sequence coverage (>90%), allowing us to construct and save homologous 3D structures of the proteins.
2.8. Molecular Docking Analysis
3D structures of bioactive compounds in S. baicalensis and F. suspensa were downloaded from PubChem database as sdf format files and then converted to mol2 format using OpenBabel (version 3.1.1) software (http://openbabel.org/, accessed on 8 June 2024). Processing of the receptors (potential VFs) and ligands (bioactive compounds), including the removal of water molecules, hydrogenation, and assigning atomic charges, was performed using Autodock Tools (version 1.5.7) software (https://ccsb.scripps.edu/mgltools/, accessed on 5 July 2024). Semi-flexible docking was performed using Autodock Vina (version 1.2.3) software with the default parameters [39,43]. Binding energy heat map was constructed using GraphPad Prism (version 9) software (https://www.graphpad.com/, accessed on 7 July 2024). The 3D and 2D structure of the binding site was visualized using Pymol (version 2.5.7) software (https://pymol.org/, accessed on 9 August 2024) and Ligplot (version 2.2) software (https://www.ebi.ac.uk/thornton-srv/software/LigPlus/, accessed on 22 January 2025), respectively.
2.9. Susceptibility of K. oxytoca to Baicalin and Biofilm Formation Assays
The susceptibility of K. oxytoca 8-2-11 to baicalin (95%, Macklin, Shanghai, China) was examined using the agar Disco diffusion method [44]. K. oxytoca 8-2-11 at mid-LGD was spread on Mueller Hinton Agar (MHA, OXOID, Basingstoke, UK) plates. Then, 10 μL of baicalin (50 mg/mL), dissolved in dimethyl sulfoxide (DMSO, Beyotime, Shanghai, China), was dropped onto each sterile Disco (6 mm in diameter) and then kept at 4 °C for 30 min for diffusion. The plates were then incubated at 37 °C for 8–12 h. DMSO and sterile water were used as negative control, and antibiotic drug sulfamethoxazole-trimethoprim (SXT, OXOID, Basingstoke, UK) (25 μg/mL) as a positive control.
The biofilm formation of K. oxytoca 8-2-11 was determined using crystal violet staining method [45]. A 1 mL of K. oxytoca 8-2-11 culture (OD600 = 0.4) was inoculated into sterile 24-well plates and mixed with baicalin at 1 × minimum inhibitory concentration (MIC) concentration (5 mg/mL, diluted with sterilized water). After incubation at 37 °C for 12 h, 24 h, 36 h, 48 h, and 60 h, respectively, each well was gently washed three times with the sterile 1 × PBS to remove suspended bacterial cells. The biofilms were stained with 0.1% crystal violet solution for 15 min in the dark, followed by three additional gentle washes with the 1 × PBS to remove excess dye. After air-drying at room temperature, 2 mL of 95% ethanol was added into each well to dissolve the crystal violet, and OD595 values of each well were then measured.
2.10. Data Analysis
The obtained data were statistically analyzed using SPSS (version 26) software (https://www.ibm.com/spss/, accessed on 19 February 2025), and one-way analysis of variance (ANOVA) was performed using the least-significant difference (LSD) method and variance chi-square test. All tests in this study were performed in three replications.
3. Results and Discussion
3.1. Intake of K. oxytoca Reduced the Body Weight of the Mice
As shown in Figure 1B, in the NC group, the average body weight of the mice increased by 1.93% on the day 8 (18.48 ± 0.40 g) at the end of the trail, but showing no significant difference compared to that on the day 1 (18.13 ± 0.35 g) (p > 0.05). In the model group orally gavaged with K. oxytoca 8-2-11 (109 CFU) daily for 7 days, the average body weight of the mice decreased by 1.05% on the day 4 (17.91 ± 0.20 g) (p > 0.05), and then increased by 0.17% on day 8 (18.13 ± 0.18 g) compared with day 1 (18.10 ± 0.13 g), but also showing no significant difference (p > 0.05).
Between the model and NC groups, during the first three days of the trail, there was no significant difference in the average body weight of the mice (p > 0.05). However, from the day 4 to day 8, the average body weight in the model group decreased significantly by 1.88%, 2.26%, 2.02%, 1.86%, 1.93% compared to those of the NC group, respectively (p < 0.05) (Figure 1B). Although the weight changes were minor, our data suggested that they were biologically relevant, because the intake of K. oxytoca 8-2-11 significantly altered structure and composition of colon bacteriota in the mice (see below). Moreover, the trial period (one week) may have been too short to show the body weight change in the mice. The body weight loss may have been a sign of colitis [46].
To avoid cross-contamination risk during the trail, we carried out the PC group orally gavaged with K. oxytoca ATCC 700324 in batches. As shown in Figure S1A, similar results were observed as that in the model group.
3.2. The Intake of K. oxytoca Did Not Affect the Major Organ Coefficients of the Mice
As shown in Figure 1C, compared with the NC group, the coefficients of heart, spleen and kidney tissue samples of the mice in the model group decreased by 13.74% (p > 0.05), 9.02% (p > 0.05), and 0.98% (p > 0.05), respectively, while those of live and lung samples increased by 2.08% (p > 0.05) and 0.83% (p > 0.05), respectively. However, there was no significant difference in these organ coefficients between the model group and the NC group.
It has been reported that dextran sulfate sodium (DSS)-induced colitis significantly increases the spleen index of mice [47]. Our results in this study showed that the coefficient of spleen in the model group showed no significant difference from the NC group (p > 0.05).
Similarly, compared with the NC group, the coefficients of heart, liver, spleen, lung and kidney samples of the mice in the PC group increased by 3.15% (p > 0.05), 5.47% (p > 0.05), 30.93% (p > 0.05), 2.80% (p > 0.05) and 2.02% (p > 0.05), respectively, but also showing no significant difference from those in the NC group (Figure S1B).
3.3. K. oxytoca Was Present in Fecal Samples but Absent from Blood, Lung, and Liver Samples of the Mice
In the NC group, K. oxytoca was detected negative in all the fecal samples of the mice collected throughout the entire trail, demonstrating that the NC group was in a sterile environment with no cross-contamination with the other groups.
As shown in Figure 1D, however, in the model group, K. oxytoca was detected positive in the fecal samples of the mice from day 1 (1.7 × 108 CFU/g) to the day 7 (1.4 × 107 CFU/g) of the trail. Moreover, the abundance of K. oxytoca in the fecal samples slightly increased by 0.24-fold on day 3 (2.1 × 108 CFU/g, p > 0.05), but then showed a decrease trend from day 4 to day 7 (p < 0.001), in comparison to that on day 1.
The reduced K. oxytoca in the fecal samples may have resulted from its transfer from the intestinal tract to other organs, as it may also cause opportunistic infections in the other tissues [3]. Therefore, we analyzed the blood samples of the mice from the model group. Notably, K. oxytoca was absent from neither the blood samples nor the lung and liver samples of the mice throughout the entire trail. Moreover, no significant change in morphological structure of the lung and liver samples was observed between the model group and NC group.
It has been reported that the gavage infection with a clinical K. oxytoca strain did not show the bacterial entry into the bloodstream of the mice either [5]. However, different from our results of this study, the clinical K. oxytoca strain was reported present in the lungs of the mice [5]. One possibility could be the different virulence between the K. oxytoca strains of clinical and aquatic animal origins.
Similarly, in the PC group, K. oxytoca was also detected positive in the fecal samples of the mice from day 1 (3.1 × 1010 CFU/g) to day 7 (3.8 × 108 CFU/g) during the trail. The bacterium significantly increased by 15.81-fold on day 2 (4.9 × 1011 CFU/g) (p < 0.001) compared to that on day 1 (p < 0.001), and then gradually decreased (Figure S1C). Like the model group, K. oxytoca was detected negative in the blood, lung, and liver samples in the PC group, and no significant change in morphological structure of these tissues was observed between the PC group and NC group.
3.4. The Effects of the Intake of K. oxytoca on the Colon Tissue of the Mice
As shown in Figure 1E, in the NC group, there were no villi on the surface structure of the colon tissue samples of the mice and the crypts were deeper. The surface was a single layer of columnar epithelium (white arrow); the mucosal muscularis propria (red arrow) composed of two layers of smooth muscle cells, separated the intestinal crypts (yellow arrow) from the submucosa (blue arrow); the remainder of the intestinal wall consisted of the muscular layer (green arrow), composed of smooth muscle cells and the plasma membrane layer (brown arrow).
In the model group, however, the mucosal layer of colon tissue samples showed a small number of necrotic epithelial cells (black arrow) with nuclear consolidation and deep staining, but there was no obvious infiltration of inflammatory cells (Figure 1F).
In the PC group, cytosolic consolidation of mucosal epithelial cells and focal aggregation of lymphocytes were occasionally seen, indicating a mild inflammatory response (Figure S1D,E).
3.5. The Intake of K. oxytoca 8-2-11 Altered Structure and Composition of Colon Bacteriota in the Mice
In this study, we further investigated the impact of the intake of K. oxytoca 8-2-11 of aquatic animal origin on colon bacteriota in the mice by 16S rRNA sequencing of colon content samples. The sequencing coverage of all the samples was more than 99.95%, indicating that all the samples had sufficient sequencing depth.
As shown in Figure 2A, in the model group, the bacterial community richness Chao1 and Ace indices (280.22 and 279.16) decreased by 2.31% and 2.86% compared with the NC group (286.85 and 287.38), respectively (p > 0.05); the Shannon index (3.75) decreased by 2.09% compared to the NC group (3.83) (p > 0.05); and the Simpson index (0.95) was slightly higher than that in the NC group (0.94) (p > 0.05) (Figure 2A,D). Chao1 and Ace indices represent species abundance [48], while Shannon and Simpson indices reflect species diversity in terms of distributional evenness and concentration, respectively [49]. It has been reported that gut microbial diversity and richness are reduced in patients with inflammatory bowel disease (IBD) and UC [50], following the similar trend as our results. Although the gavage of K. oxytoca 8-2-11 had no significant influence on the species richness and diversity in the colon bacteriota and did not lead to colitis, we could not rule out the possibility of precolitis.
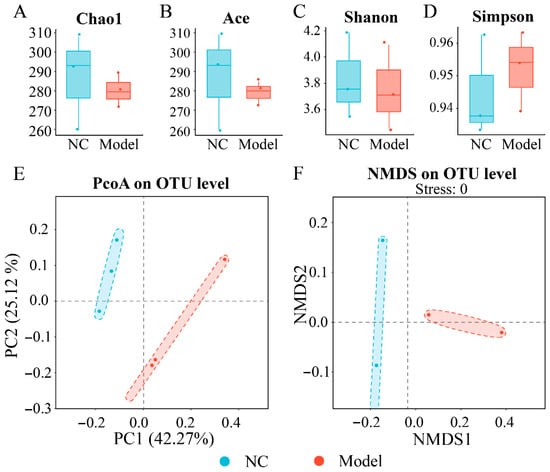
Figure 2.
The effects of intake of K. oxytoca on α and β diversity of the colon bacteriota in the mice. (A–D) The Chao1, Ace, Shannon, and Simpson indices of bacterial community in the mice, respectively. (E,F) Principal coordinate analysis (PCoA) and nonmetric multidimensional scaling (NMDS) plot of bacteriota between the NC and model groups.
As shown in Figure 2E,F, the PCoA analysis showed that the colon bacteriota of the model group clustered away from the NC group. In addition, the NMDS analysis showed significant differences in the structure of colon bacteriota between the model group and the NC group (p < 0.05). These results indicated that the intake of K. oxytoca 8-2-11 significantly influenced the structural of the colon bacteriota to a certain extent compared with the NC group. Additionally, the hierarchical cluster analysis also revealed remarkable differences in the colon content samples between the NC and model groups (see below).
At the phylum level, the major phyla of bacterial community in the colon content samples in the NC and model groups were Bacteroidota (61.82% and 83.27%), Firmicutes (33.87% and 15.09%), Proteobacteria (1.59% and 0.81%), and Desulfobacterota (1.17% and 0.37%) (Figure 3A). Remarkably, in the model group, the abundance of Bacteroidota (83.27%) was significantly increased by 21.45% compared with the NC group (61.82%) (p < 0.01); and conversely, Firmicutes (15.09%) significantly decreased by 18.78% compared with the NC group (33.87%) (p < 0.01). It is noteworthy that the Firmicutes/Bacteroidetes ratio in the model group (0.18) was significantly decreased by 67.3% compared with the NC group (0.55) (p < 0.01). Bacteroidota and Firmicutes are the major predominant phyla in human gut microbiota and are involved in many intestinal and extra-intestinal physiological functions [51]. Moreover, the Firmicutes/Bacteroidetes ratio is defined as a biomarker of obesity [52]. In this study, the reduced Firmicutes/Bacteroidetes ratio in the model group could be an explanation for the observed body weight loss of the mice.
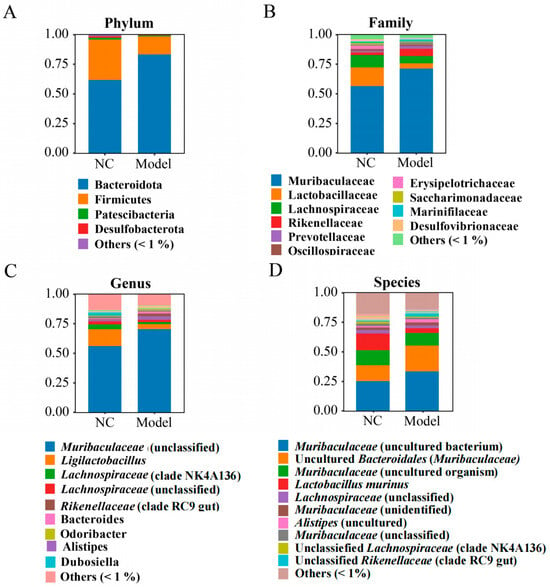
Figure 3.
The effects of intake of K. oxytoca on the composition of the colon bacteriota in the mice. (A–D) The relative bacterial abundance at the phylum, family, genus, and species levels, respectively.
At the family level, the NC and model groups mainly consisted of Muribaculaceae, Lactobacillaceae, Lachnospiraceae, Rikenellaceae, Prevotellaceae, Oscillospiraceae, Erysipelotrichaceae, Saccharimonadaceae, Marinifilaceae, and Desulfovibrionaceae, which accounted for more than 94.00% of the bacterial community in the both groups (Figure 3B). Of these, Muribaculaceae has a strong ability to metabolize both endogenous (mucin glycans) and exogenous (dietary fibers) polysaccharides to produce short-chain fatty acids (SCFAs) and exhibits cross-feeding relationships with Bifidobacterium and Lactobacillus [53]. Interestingly, the abundance of Muribaculaceae in the model group (71.34%) was significantly increased by 14.74% compared with the NC group (56.60%) (p < 0.01). Lachnospiraceae is an important producer of SCFAs and linked to various intestinal and extra-intestinal disorders, underscoring its significance for overall health of the host [54]. In this study, the abundance of Lachnospiraceae in the model group (6.23%) was significantly reduced by 4.19% compared with the NC group (10.42%) (p < 0.05). Remarkably, the abundance of Lactobacillaceae in the model group (4.31%) was also significantly reduced by 11.35% compared with the NC group (15.66%) (p < 0.05). As known as probiotics, the properties of Lactobacillaceae, such as competitive adhesion, self-aggregation, and co-aggregation, may affect the colonization and clearance of pathogenic bacteria in the gastrointestinal tract [55].
At the genus level, the NC and model groups were predominated by Muribaculaceae (norank), Lactobacillus, Lachnospiraceae (group NK4A136), Lachnospiraceae (unclassified), Alistipes, Rikenellaceae (group RC9 gut), Bacteroides, Odoribacter, and Dubosiella, which accounted for more than 80% of the bacteriota in both groups (Figure 3C). Of these, in the model group, the abundance of Muribaculaceae (norank), Alistipes, and Rikenellaceae (group RC9 gut) was significantly increased by 14.35%, 1.53%, and 1.99%, respectively; and conversely, Lactobacillus, Lachnospiraceae (group NK4A136), and Dubosiella were significantly decreased by 10.08%, 2.35%, and 2.10%, respectively, compared with the NC group (p < 0.05). Alistipes has been reported to contribute to diseases e.g., anxiety, myalgic encephalomyelitis/chronic fatigue syndrome, depression, pervasive developmental disorder—not otherwise specified (PDD-NOS), and colorectal cancer (CRC) [56]. Lactobacillus plays very important roles in food digestion, nutritional absorption, inflammatory modulation, gut flora management, and preventing bacterial infection [57]. Lactobacillus can inhibit the adhesive colonization of pathogenic bacteria toward intestinal epithelial cells by secreting metabolites that competed for the same receptors shared by pathogenic bacteria [58].
At the species level, the NC and model groups were dominated by Muribaculaceae (uncultured bacterium) (25.51% and 33.73%, p < 0.05), Muribaculaceae (uncultured Bacteroidales) (13.36% and 21.82%, p < 0.05), Muribaculaceae (uncultured_organisms) (12.76% and 10.66%, p > 0.05), Lactobacillus murinus (14.16% and 4.07%, p < 0.05), and Lachnospiraceae (unclassified) (2.86% and 2.27%, p > 0.05) (Figure 3D). As a potential probiotic, L. murinus is important in maintaining intestinal immune balance by regulating T-lymphocyte activity [59]. In this study, the abundance of L. murinus in the model group was significantly reduced by 10.08% compared with the NC group (p < 0.05).
The phylogenetic relationships analysis also revealed significant differences in the taxa of the colon bacteriota between the NC and the model groups (Figure 4A). Based on the LEfSe analysis, we found that fourteen taxa were over-represented in the model group compared with the NC group, e.g., Bacteroides, Rikenellaceae (group RC9_gut), Alistipes, Odoribacter, and Muribaculum. Conversely, 19 taxa were under-represented in the model group, e.g., Lactobacillales, Dubosiella, Faecalibaculum rodentium, Saccharimonadaceae, and Eggerthellaceae (Figure 4B).
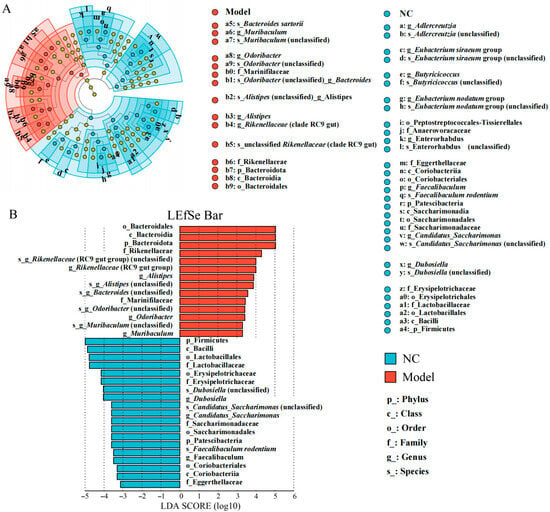
Figure 4.
The effects of the gavage of K. oxytoca on the composition of the colon bacteriota in the mice. (A) The linear discriminant analysis effect size (LEfSe) analysis of the taxa with the greatest differences in abundance in the colon bacteriota between the NC and the model groups. The letters and numbers represented different taxa clade. (B) The LDA scores of the colon bacteriota of the NC and Model groups. The NC group is in blue, and the model group in red. Only the taxa with a significant LDA threshold value > 3 are shown. Letters p, c, o, f, g, and s before the taxa names represent phylum, class, order, family, genus, and species, respectively.
Taken together, these results revealed that the intake of K. oxytoca 8-2-11 (109/day, 7 days) of aquatic animal origin significantly altered bacterial community structure and composition, and inhibited probiotic bacterial growth (such as Lactobacillus) in the colons of the mice.
3.6. The 44 Bioactive Compounds Were Identified in S. baicalensis and F. suspensa
Based on the TCMSP database, a total of 61 compounds in S. baicalensis and F. suspensa were identified to meet the criteria of OB ≥ 30% and DL ≥ 0.18. Similarly, seven compounds in S. baicalensis and F. suspensa were identified from the BATMAN-TCM and ETCM databases. In addition, we also searched the current literature and identified baicalin and oroxindin. The former is a major bacteriostatic component in S. baicalensis [40], while the latter can be absorbed by the colon and attenuate inflammatory responses by repressing NLRP3 inflammasome formation [60]. Although these two compounds did not meet the criteria for OB, they were also chosen and subjected to the Autodock pre-treatment. After removing compounds without a PubChem CID or with a PubChem CID mismatch, we finally identified 44 bioactive compounds as potential ligands for the molecular docking analysis, of which 29 were from S. baicalensis and 17 from F. suspensa (including 2 (wogonin and β-sitosterol) shared with S. baicalensis) (Table S1).
3.7. The 117 Potential VFs in K. oxytoca 8-2-11 Were Identified and Classified
Approximately 117 VFs-associated genes in the K. oxytoca 8-2-11 genome were identified [35]. They were mainly classified into five catalogues according to the VFDB database: (1) biofilm formation (n = 10) e.g., type 3 fimbrial major pilin subunit MrkA, fimbrial adhesin protein precursor MrkD, and type 3 fimbrial minor pilin subunit MrkF; (2) adherence and motility (n = 23) e.g., type 1 major fimbrial subunit precursor, elongation factor Tu (EF-Tu), and E. coli common pilus usher EcpC; (3) nutritional/metabolic factor (n = 36) e.g., yersiniabactin transcriptional regulator YbtA, outer membrane receptor FepA, and yersiniabactin biosynthetic protein Irp1; (4) immune modulation (n = 28) e.g., O-antigen export ABC transporter permease RfbA, ADP-L-glycero-D-mannoheptose-6-epimerase, and NADP-dependent phosphogluconate dehydrogenase; and (5) others (n = 20) e.g., urease alpha subunit UreA, outer membrane protein A, and RNA polymerase sigma factor RpoS (Tables S2–S4).
3.8. The Binding Capacity of the Bioactive Compounds in S. baicalensis and F. suspensa with the VFs Receptors in K. oxytoca
3.8.1. Potential Interaction Between the Bioactive Compounds and the VFs in Biofilm Formation
As shown in Figure 5A,C, among the 10 VFs involved in biofilm formation in K. oxytoca 8-2-11, the transcriptional activator (MrkH) (VFG048327, gb|WP_004152886) and phosphodiesterase (MrkJ) (VFG048348, gb|WP_004149658) had the lower free binding energies to coptisine, baicalin, oroxindin, betulinic acid, and bicuculline compounds in S. baicalensis and F. suspensa, ranging from −9.9 kcal/mol to −8.4 kcal/mol, indicating their higher binding affinity. In contrast, the binding ability of fibrillar chaperone protein precursor MrkB (VFG043626, gb|WP_000820818) and S-ribosylhomocysteinase (VFG018241, gb|WP_001130227) to these compounds was relatively weak, ranging from −8.5 kcal/mol to −7.1 kcal/mol.
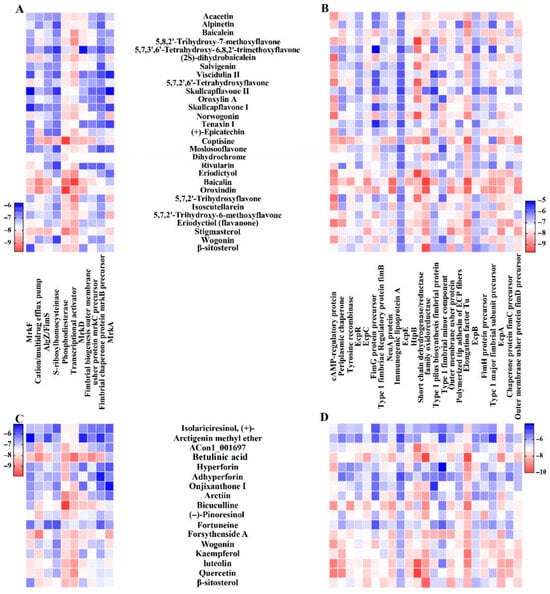
Figure 5.
The docking scores of the bioactive compounds in S. baicalensis and F. suspensa with the major VFs in K. oxytoca 8-2-11. The red color represents stronger binding ability than the blue color, and the darker colors represent stronger binding ability than the lighter colors. (A,B) The binding energies (kcal/mol) of bioactive compounds in S. baicalensis with the VFs in biofilm formation and adherence/motility in K. oxytoca 8-2-11, respectively. (C,D) The binding energies (kcal/mol) of bioactive compounds in F. suspensa with the VFs in biofilm formation and adherence/motility in K. oxytoca 8-2-11, respectively. Dihydrochromen: (2R)-5,7-dihydroxy-2-(4-hydroxyphenyl)-6-methoxy-2,3-dihydrochromen-4-one; ACon1_001697: 4-[(3S,3aR,6S,6aR)-6-(3,4-dimethoxyphenyl)-1,3,3a,4,6,6a-hexahydrofuro [3,4-c]furan-3-yl]-2-methoxyphenol.
Among all the tested compounds (n = 29) in S. baicalensis, baicalin had the lowest mean binding energy (−8.4 kcal/mol) and the strongest binding affinity to all the biofilm-associated VFs; baicalin, coptisine, oroxindin and stigmasterol all showed good binding ability to MrkH (−9.7, −8.5, −8.6 and −8.7 kcal/mol), MrkJ (−9.2, −9.7, −9.7 and −9.1 kcal/mol), alginate biosynthesis protein AlgZ/FimS (VFG000121, gb|NP_249453) (−8.6, −8.8, −8.4 and −8.5 kcal/mol), and cation/multidrug efflux pump (VFG037720, gb|WP_001027056) (−9.0, −8.5, −9.0 and −8.4 kcal/mol) in K. oxytoca 8-2-11 (Figure 5A).
Among all the tested compounds (n = 17) in F. suspensa, betulinic acid had the lowest mean binding energy (−8.4 kcal/mol). The binding energies of betulinic acid, forsythenside A, and luteolin to all the biofilm-associated VFs in K. oxytoca 8-2-11 were lower than −6.9 kcal/mol. Notably, MrkB (−8.5 kcal/mol), fimbrial biogenesis outer membrane usher protein precursor MrkC (VFG043625, gb|WP_000813718) (−9 kcal/mol), MrkD (VFG043624, gb|WP_004149659) (−8.5 kcal/mol) and MrkH (−9.4 kcal/mol) docked with betulinic acid at lower binding energies than the other compounds. Besides, bicuculline, forsythenside A, luteolin and betulinic acid also showed good binding ability to MrkD (−8.2, −8.0, −7.9 and −8.5 kcal/mol), MrkH (−8.4, −8.4, −8.8 and −9.4 kcal/mol) and MrkJ (−9.9, −8.1, −8.4 and −8.7 kcal/mol) (Figure 5C).
3.8.2. Potential Interaction Between the Bioactive Compounds and the Adherence/Motility-Related VFs
Among the 29 compounds in S. baicalensis, baicalin had the lowest binding energy (below −6.8 kcal/mol) to all the adherence/motility-associated VFs (n = 23) in K. oxytoca 8-2-11 (Figure 5B). Interestingly, the 5,8,2′-trihydroxy-7-methoxyflavone had the lowest binding energy (−9.7 kcal/mol) docking with HspB. The coptisine, oroxindin, eriodyctiol (flavanone) and baicalin also showed good binding ability to the tested VFs such as outer membrane usher protein precursor FimD (VFG000876, gb|WP_000120946) (−8.9, −8.8, −7.8 and −8.7 kcal/mol), E. coli common pilus structural subunit EcpA (VFG002414, gb|WP_000730972) (−8.6, −8.4, −8.4 and −8.5 kcal/mol), EF-Tu (−9.3, −9.2, −9.0 and −9.5 kcal/mol), short chain dehydrogenase/reductase (SCD/R) family oxidoreductase (−9.2, −9.4, −8.5 and −9.7 kcal/mol), cAMP regulatory protein (−8.4, −8.1, −9.0 and −8.7 kcal/mol) and N-acetylneuraminic acid synthase (VFG038845, gb|WP_016351249) (−8.3, −9.1, −7.9, −9.2 kcal/mol), respectively.
Among the 17 compounds in F. suspensa, luteolin displayed the lowest mean binding energies (−7.7 kcal/mol) and the strongest binding affinity to the 23 adherence/motility-associated VFs (Figure 5D). The betulinic acid had binding energies below −6.6 kcal/mol to all these proteins. Notably, EcpA (−8.8 kcal/mol), EF-Tu (−9.8 kcal/mol), SCD/R (−9.9 kcal/mol), and EcpC (VFG002415, gb|WP_001131063) (−8.4 kcal/mol) docked with betulinic acid at significantly lower binding energies than the other compounds. Moreover, betulinic acid, bicuculline, quercetin and luteolin also bound well to EcpA (−8.8, −7.7, −8.4 and −8.4 kcal/mol), EF-Tu (−9.8, −9.2, −8.2 and −9,0 kcal/mol), SDR (−9.9, −8.9, −8.5 and −8.6 kcal/mol), NeuA (−8.2, −8.6, −7.9 and −7.9 kcal/mol), and cAMP regulatory protein (−7.8, −8.7, −9.1 and −9.1 kcal/mol), respectively.
Additionally, the docking scores of potential bioactive compounds in S. baicalensis and F. suspensa with other VFs in K. oxytoca 8-2-11 are shown in Figures S2 and S3.
3.9. Baicalin Could Form Non-Covalent Interaction Forces with VFs in K. oxytoca 8-2-11
3.9.1. The Non-Covalent Interaction Between Baicalin and the Key VFs in Biofilm Formation
Based on the above findings, in this study, baicalin in S. baicalensis was chosen for further investigation of non-covalent interaction with potential receptors, particularly VFs involved in biofilm formation and adherence/motility, in K. oxytoca 8-2-11.
Biofilm enables pathogens to thrive in a variety of environments and contributes to their colonization, virulence and persistence in hosts [61]. As shown in Figure 6 and Table S2, baicalin can form hydrogen bonds, π-stacking, and π-cation interaction with active site residues of key VFs in biofilm formation, e.g., MrkA, MrkD, MrkF (VFG043623, gb|WP_002916122), MrkH, and MrkJ. The binding energies were all less than −7 kcal/mol, indicating stable binding patterns between baicalin and these VFs.
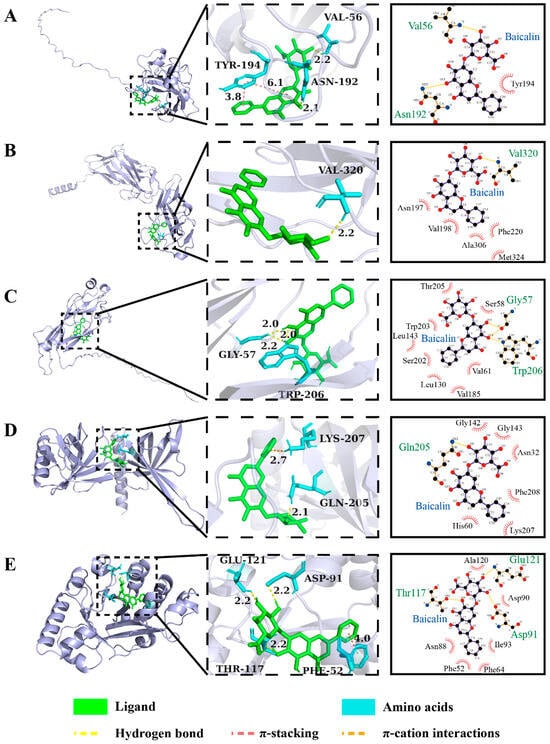
Figure 6.
The 3D and 2D structure of interaction forces of baicalin with the key VFs in biofilm formation in K. oxytoca 8-2-11. (A–E) MrkA, MrkD, MrkF, MrkH, and MrkJ, respectively.
For instance, MrkA (20 KDa) has a conserved amino acid sequence among the Enterobacteriaceae. It has been recognized as a common protein antigen functioning in biofilm formation and establishment of infection of K. pneumoniae [62]. As shown in Figure 6A, baicalin formed two hydrogen bonds with active site residues VAL 56 and ASN 192 of MrkA at distances of 2.2 Å and 2.1 Å, respectively. The aromatic ring center distance of the π-stacking interaction force between TYR 194 of MrkA and baicalin was 3.8 Å and 6.1 Å, respectively.
MrkD is a Type 3 fimbrial adhesin binding to extracellular matrix proteins (e.g., collagen), or abiotic surfaces [63,64]. As shown in Figure 6B, baicalin formed one hydrogen bond with VAL 320 of MrkD at a distance of 2.2 Å. The intermittent incorporation of MrkF to MrkD influences fimbrial assembly, potentially modulating the activity of Type 3 fimbriae essential for maintaining stability of fimbrial filament on the cell surface [65,66]. As shown in Figure 6C, baicalin formed three hydrogen bonds with GLY 57, GLY 57 and TRP 206 of MrkF at distances of 2.2 Å, 2.0 Å, and 2.0 Å, respectively.
Additionally, baicalin formed one hydrogen bond with GLN 205 of MrkH at distance of 2.1 Å, and a π-cation interaction with the LYS 207 of MrkH (Figure 6D). Baicalin also formed three hydrogen bonds with GLU 121, ASP 91, and THR 117 of MrkJ at same distances of 2.2 Å, and the aromatic ring center distance of the π-stacking interaction force between PHE 52 of MrkJ and baicalin was 4.0 Å (Figure 6E).
3.9.2. The Non-Covalent Interaction Between Baicalin and the Key VFs in Adherence/Motility
As shown in Figure 7 and Table S3, baicalin can form hydrogen bonds and π-cation interaction with active site residues of key VFs in adherence/motility, such as EcpA, EcpB, regulator protein EcpR (VFG002413, gb|WP_000389022), periplasmic chaperone (VFG048267, gb|WP_015875145), EF-Tu, SCD/R and HspB. The binding energies of all the dockings were less than −7 kcal/mol.
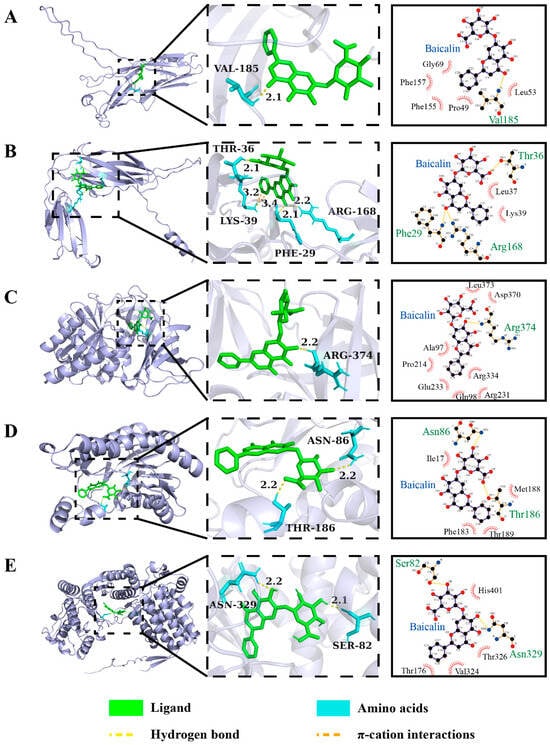
Figure 7.
The 3D and 2D structure of interaction forces of baicalin with the key VFs in adherence/motility in K. oxytoca 8-2-11. (A–E) EcpA, EcpB, EF-Tu, SCD/R, and HspB, respectively.
E. coli common pilus (ECP) plays a crucial role in bacteria-to-bacteria interactions within biofilms and in cell adherence. The ECP is encoded by the ecpRABCDE operon, and consists of a major pilin subunit EcpA (21 kDa) [67]. Yi et al. reported that EcpA is specifically involved in regulating swimming motility of Dickeya dadantii 3937 [68]. As shown in Figure 7A, baicalin could form one hydrogen bond with VAL 185 of EcpA at a distance of 2.1 Å. The chaperone EcpB is involved in the biogenesis of filament in uropathogenic E. coli [69]. As shown in Figure 7B, baicalin formed three hydrogen bonds with ARG 168, PHE 29, and THR 36 of EcpB at distances of 2.2 Å, 2.1 Å, and 2.1 Å, respectively, and a π-cation interaction with LYS 39 of EcpB.
EF-Tu plays a critical role in protein biosynthesis. It has been demonstrated that EF-Tu is also secreted as a cell surface protein of bacteria binding to extracellular matrix proteins (e.g., collagen, fibronectin, and laminin), and host proteins enhancing bacterial adhesion and invasion to host cells [70]. As shown in Figure 7C, baicalin formed one hydrogen bond with ARG 374 of EF-Tu at a distance of 2.2 Å.
The SCD/R uses NAD(H) or NADP(H) as a cofactor and has broad substrate spectra involved in primary and secondary metabolism [71]. As shown in Figure 7D, baicalin formed two hydrogen bonds with THR 186 and ASN 86 of SCD/R at the same distance of 2.2 Å.
HspB is both a secreted and cell surface-associated protein that may play a role in pathogenesis [72]. As shown in Figure 7E, we found that baicalin formed two hydrogen bonds with ASN 329 and SER 82 of Hsp60 at a distance of 2.2 Å and 2.1 Å, respectively.
Additionally, as a transcriptional activator, EcpR self-regulates and activates the transcription of the entire ecpRABCDE operon, including the ecpA gene [67]. The binding energy of EcpR to baicalin was relatively high (−7.4 kcal/mol), but there was no predicted interaction force (e.g., hydrogen bonding) between EcpR and baicalin.
Recently, Akbarzadeh et al. reported in silico and in vitro analysis of gentamicin (GENT)-EDTA encapsulated niosomes to enhance antibacterial activity and biofilm inhibition in drug-resistant K. pneumoniae [73]. Mrks are very important VFs in K. pneumoniae. In this study, the molecular docking results revealed that baicalin can specifically bind to MrkA/MrkD/MrkF in K. oxytoca through hydrogen bonding, π-stacking, and π-cation interaction with active site residues of these Mrks, with binding energies all less than −7 kcal/mol, suggesting that baicalin possibly affects biofilm formation of K. oxytoca.
3.10. Baicalin Inhibited Growth and Biofilm Formation of K. oxytoca In Vitro
To verify the molecular docking results, we assessed the inhibitory activity of baicalin against K. oxytoca 8-2-11 in vitro. As shown in Figure 8A and Table S5, we observed that the DIZ and MIC values of baicalin against K. oxytoca 8-2-11 were 9.1 ± 0.1 mm and 5 mg/mL, respectively, indicating that this bacterium was sensitive to baicalin. We observed that baicalin relied on DMSO for dissolution and showed poor solubility in water. This could be the reason for the observed high MIC value.
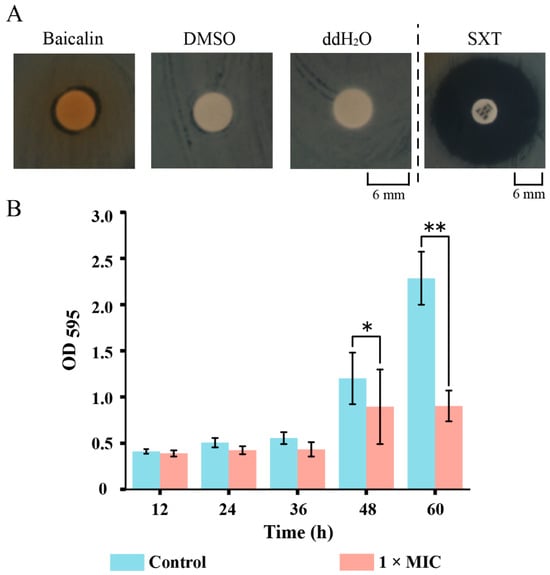
Figure 8.
Baicalin inhibited the growth and biofilm formation of K. oxytoca 8-2-11 in vitro. (A,B) The Disco diffusion, and biofilm formation assays, respectively. *: p < 0.05; and **: p < 0.01.
As shown in Figure 8B, compared to the control group, the biomass of biofilm formed by K. oxytoca 8-2-11 significantly decreased by 0.26-fold, and 0.61-fold after being treated with 1 × MIC baicalin for 48 h (p < 0.05), and 60 h (p < 0.01), respectively.
K. oxytoca is the second most severe pathogen following K. pneumoniae in the Klebsiella genus [74,75]. Recently, Han et al. reported that the combination of baicalin and levofloxacin significantly reduced the early biofilm formation of Hypervirulent K. pneumoniae [76]. Baicalin also inhibited the adhesion and aggregation phases of biofilm formation of Streptococcus saprophyticus [77]. In this study, the combined molecular docking in silico and in vitro experimental results highlighted that the inhibited biofilm formation of K. oxytoca possibly resulted from the blocked expression of Mrk gene cluster by baicalin.
A limitation of this study was that the molecular docking results were not verified by protein inhibition assays and/or gene knockout studies. In the future, the binding of baicalin to the expressed Mrks proteins should be further investigated through in vitro and in vivo experiments.
4. Conclusions
In this study, we investigated for the first time the pathogenicity of K. oxytoca originating from shellfish S. subcrenata using a murine intestinal infection model. The results revealed that the intake of K. oxytoca 8-2-11 (109 CFU/day) via oral gavage for 7 days reduced the average body weight but did not change the major organ coefficients of the mice. The bacterium was present in fecal samples but absent from blood, lung, and liver tissue samples of the mice. The intake of K. oxytoca 8-2-11 led to minor epithelial cell necrosis in the intestinal mucosa, characterized by pyknotic nuclei and deep staining, but without significant inflammatory cell infiltration in the mice. The intake of K. oxytoca 8-2-11 significantly altered the structure and composition of the colon bacteriota in the mice, with reduced abundance of Firmicutes, Lachnospiraceae, Lactobacillaceae, Lactobacillus, and Lactobacillus murinus, and increased Bacteroidota, Muribaculaceae, and Alistipes.
We screened 44 active compounds in medical plants S. baicalensis and F. suspensa, and predict their potential interaction with 117 VFs in K. oxytoca 8-2-11 through molecular docking analysis. The results revealed that baicalin showed higher binding affinity with the VFs with lower mean binding energy (−8.4 kcal/mol). Moreover, baicalin can bind to key biofilm formation, and adherence/motility-related VFs (e.g., MrkA/MrkD/MrkF, and EcpA) via formation of stable hydrogen bonds, π-stacking, and π-cation interaction. In vitro, baicalin inhibited the bacterial growth and biofilm formation.
Overall, the results of this study address a gap in the research into in vivo infection of aquatic animal-derived K. oxytoca, paving the way to the screening of therapeutic drugs to control K. oxytoca infections.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres16080189/s1, Table S1: Basic information of the 44 bioactive compounds in S. baicalensis and F. suspensa; Table S2: The affinity and interaction forces between baicalin and the key VFs in biofilm formation in K. oxytoca 8-2-11; Table S3: The affinity and interaction forces between baicalin and the key VFs in adherence/motility in K. oxytoca 8-2-11; Table S4: The affinity and interaction forces between baicalin, coptisine, oroxindin, and bicuculline and other VFs in K. oxytoca 8-2-11; Table S5: The DIZ value of baicalin against K. oxytoca 8-2-11; Figure S1: The effects of intake of K. oxytoca ATCC 700324 on average body weight, the major organ coefficients, and surface structure of colon tissue of the mice. (A) The average body weight changes of the mice. (B): The changes in the major organ coefficients of the mice. (C) The concentrations of K. oxytoca in fecal samples of the PC group mice. (D and E) The surface structure of colon tissues of the mice in the NC and PC groups, respectively (observed by 200×). ns: no statistical difference; Figure S2: The docking scores of the bioactive compounds in S. baicalensis with the other VFs in K. oxytoca 8-2-11. (A–C): The binding energies (kcal/mol) of bioactive compounds in S. baicalensis with the VFs in nutritional/metabolism, immunomodulation, and others in K. oxytoca 8-2-11, respectively; Figure S3: The docking scores of the bioactive compounds in F. suspensa with the other VFs in K. oxytoca 8-2-11. (A–C): The binding energies (kcal/mol) of bioactive compounds in F. suspensa with the VFs in nutritional/metabolism, immunomodulation, and others in K. oxytoca 8-2-11, respectively.
Author Contributions
Y.M.: major experiments, data analysis, and writing—original draft; X.Q.: molecule docking analysis; Y.W. and L.X.: resource and supervision; L.C.: funding acquisition, conceptualization, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Science and Technology Commission of Shanghai Municipality, grant number 17050502200.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The 16S rRNA sequencing data are available in the NCBI SDR database under the accession numbers PRJNA1271959.
Acknowledgments
We would like to thank L.Y., Y.O., T.Y., E.T., Y.L., Y.D., J.X., H.F., and F.C. at Shanghai Ocean University for their help with the animal experiment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wan, W.; Yang, X.; Yu, H.; Wang, M.; Jia, W.; Huang, B.; Qu, F.; Shan, B.; Tang, Y.W.; Chen, L.; et al. Genomic characterization of carbapenem-resistant Klebsiella oxytoca complex in China: A multi-center study. Front. Microbiol. 2023, 14, 1153781. [Google Scholar] [CrossRef] [PubMed]
- Osbelt, L.; Almási, É.D.H.; Wende, M.; Kienesberger, S.; Voltz, A.; Lesker, T.R.; Muthukumarasamy, U.; Knischewski, N.; Nordmann, E.; Bielecka, A.A.; et al. Klebsiella oxytoca inhibits Salmonella infection through multiple microbiota-context-dependent mechanisms. Nat. Microbiol. 2024, 9, 1792–1811. [Google Scholar] [CrossRef] [PubMed]
- Neog, N.; Phukan, U.; Puzari, M.; Sharma, M.; Chetia, P. Klebsiella oxytoca and emerging nosocomial infections. Curr. Microbiol. 2021, 78, 1115–1123. [Google Scholar] [CrossRef]
- Yang, J.; Long, H.; Hu, Y.; Feng, Y.; McNally, A.; Zong, Z. Klebsiella oxytoca complex: Update on taxonomy, antimicrobial resistance, and virulence. Clin. Microbiol. Rev. 2022, 35, e0000621. [Google Scholar] [CrossRef]
- Hua, M.; Duan, A.; Li, Q.; Yue, J.; Liu, X.; Yuan, L.; Liu, J.; Chen, C. Alteration of microbiota and immune response of mice gavaged with Klebsiella oxytoca. Microbes Infect. 2022, 24, 104977. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. An overview on antimicrobial potential of edible terrestrial plants and marine macroalgae Rhodophyta and Chlorophyta extracts. Mar. Drugs 2023, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Archana, H.; Geetha Bose, V. Evaluation of phytoconstituents from selected medicinal plants and its synergistic antimicrobial activity. Chemosphere 2022, 287, 132276. [Google Scholar] [CrossRef]
- López Villarreal, S.M.; Elizondo Luévano, J.H.; Pérez Hernández, R.A.; Sánchez García, E.; Verde Star, M.J.; Castro Ríos, R.; Garza Tapia, M.; Rodríguez Luis, O.E.; Chávez Montes, A. Preliminary study of the antimicrobial, anticoagulant, antioxidant, cytotoxic, and anti-inflammatory activity of five selected plants with therapeutic application in dentistry. Int. J. Environ. Res. Public Health 2022, 19, 7927. [Google Scholar] [CrossRef]
- Boy, F.R.; Casquete, R.; Martínez, A.; Córdoba, M.G.; Ruíz-Moyano, S.; Benito, M.J. Antioxidant, antihypertensive and antimicrobial properties of phenolic compounds obtained from native plants by different extraction methods. Int. J. Environ. Res. Public Health 2021, 18, 2475. [Google Scholar] [CrossRef]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S.; Zhang, J.; Wu, J. Scutellaria baicalensis georgi is a promising candidate for the treatment of autoimmune diseases. Front. Pharmacol. 2022, 13, 946030. [Google Scholar] [CrossRef]
- Cai, J.; Hu, Q.; He, Z.; Chen, X.; Wang, J.; Yin, X.; Ma, X.; Zeng, J. Scutellaria baicalensis Georgi and their natural flavonoid compounds in the treatment of ovarian cancer: A review. Molecules 2023, 28, 5082. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, X.; Zhang, H.; Cheong, M.S.; Chen, X.; Farag, M.A.; Cheang, W.S.; Xiao, J. Baicalin ameliorates insulin resistance and regulates hepatic glucose metabolism via activating insulin signaling pathway in obese pre-diabetic mice. Phytomedicine 2024, 124, 155296. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhao, J.X.; Qin, X.M.; Zhao, J. The ethanol extract of Scutellaria baicalensis Georgi attenuates complete Freund’s adjuvant (CFA)-induced inflammatory pain by suppression of P2X3 receptor. J. Ethnopharmacol. 2023, 317, 116762. [Google Scholar] [CrossRef]
- Wu, T.H.; Lin, T.Y.; Yang, P.M.; Li, W.T.; Yeh, C.T.; Pan, T.L. Scutellaria baicalensis induces cell apoptosis and elicits mesenchymal-epithelial transition to alleviate metastatic hepatocellular carcinoma via modulating HSP90β. Int. J. Mol. Sci. 2024, 25, 3073. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, Q.; Ding, S.; Chen, Y.; Song, T.; Shang, Y. Scutellaria baicalensis Georgi stems and leaves flavonoids promote neuroregeneration and ameliorate memory loss in rats through cAMP-PKA-CREB signaling pathway based on network pharmacology and bioinformatics analysis. Heliyon 2024, 10, e27161. [Google Scholar] [CrossRef]
- Tang, Z.W.; Zhang, C.E.; Ma, F.Z.; Cui, Y.T.; Ye, R.H.; Pu, S.B.; Ma, Z.J. Scutellaria baicalensis Georgi alleviates Clostridium difficile associated diarrhea and its modulatory effects on the gut microbiota. Fitoterapia 2024, 176, 105973. [Google Scholar] [CrossRef]
- Zhou, C.; Xia, Q.; Hamezah, H.S.; Fan, Z.; Tong, X.; Han, R. Efficacy of Forsythia suspensa (Thunb.) Vahl on mouse and rat models of inflammation-related diseases: A meta-analysis. Front. Pharmacol. 2024, 15, 1288584. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Zhao, W.; Meng, Z.; Ji, W.; Wang, C. Transcriptomic and lipidomic analysis of lipids in Forsythia suspensa. Front. Genet. 2021, 12, 758326. [Google Scholar] [CrossRef]
- Tan, X.; Chen, J.; Zhang, J.; Guo, G.; Zhang, H.; Zhao, X.; Lv, S.; Xu, H.; Hou, D. Gene expression and interaction analysis of FsWRKY4 and FsMAPK3 in Forsythia suspensa. Plants 2023, 12, 3415. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, X.; Li, A.; Fan, A.; He, L.; Sun, Z.; Niu, Y.; Qiao, Y. Assembly and analysis of the complete mitochondrial genome of Forsythia suspensa (Thunb.) Vahl. BMC Genomics 2023, 24, 708. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, Q.; Liu, X.; Liu, W.; Huang, W.; Mei, X.; Luo, J.; Shan, M.; Lin, R.; Zou, D.; et al. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: A review. J. Ethnopharmacol. 2018, 210, 318–339. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Chen, L.; He, S.; Liu, S.; Yao, J.; Shao, Z.; Ye, Y.; Yao, S.; Lin, Z.; Zuo, J. Forsythia suspensa (Thunb.) Vahl extract ameliorates ulcerative colitis via inhibiting NLRP3 inflammasome activation through the TLR4/MyD88/NF-κB pathway. Immun. Inflamm. Dis. 2023, 11, e1069. [Google Scholar] [CrossRef]
- Lv, W.; Jin, W.; Lin, J.; Wang, Z.; Ma, Y.; Zhang, W.; Zhu, Y.; Hu, Y.; Qu, Q.; Guo, S. Forsythia suspensa polyphenols regulate macrophage M1 polarization to alleviate intestinal inflammation in mice. Phytomedicine 2024, 125, 155336. [Google Scholar] [CrossRef]
- Zhou, M.; Huo, J.; Wang, C.; Wang, W. UPLC/Q-TOF MS screening and identification of antibacterial compounds in Forsythia suspensa (Thunb.) Vahl Leaves. Front. Pharmacol. 2021, 12, 704260. [Google Scholar] [CrossRef] [PubMed]
- Nishibe, S.; Mitsui-Saitoh, K.; Sakai, J.; Fujikawa, T. The biological effects of Forsythia leaves containing the cyclic AMP phosphodiesterase 4 inhibitor phillyrin. Molecules 2021, 26, 2362. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Li, N.; Chen, J.; Xu, H.; Wang, Y.; Liang, Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J. Ethnopharmacol. 2023, 309, 116306. [Google Scholar] [CrossRef]
- Fu, K.; Li, Y.; Dai, S.; Li, Y. Exploration of the molecular basis of Forsythia fruit in the prevention and treatment of cholestatic liver injury through network pharmacology and molecular docking. Nutrients 2023, 15, 2065. [Google Scholar] [CrossRef]
- Hou, F.; Yu, Z.; Cheng, Y.; Liu, Y.; Liang, S.; Zhang, F. Deciphering the pharmacological mechanisms of Scutellaria baicalensis Georgi on oral leukoplakia by combining network pharmacology, molecular docking and experimental evaluations. Phytomedicine 2022, 103, 154195. [Google Scholar] [CrossRef]
- Verma, A.K.; Ahmed, S.F.; Hossain, M.S.; Bhojiya, A.A.; Mathur, A.; Upadhyay, S.K.; Srivastava, A.K.; Vishvakarma, N.K.; Barik, M.; Rahaman, M.M.; et al. Molecular docking and simulation studies of flavonoid compounds against PBP-2a of methicillin-resistant Staphylococcus aureus. J. Biomol. Struct. Dyn. 2022, 40, 10561–10577. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Xu, Y.; Chen, L. First experimental evidence for the presence of potentially virulent Klebsiella oxytoca in 14 species of commonly consumed aquatic animals, and phenotyping and genotyping of K. oxytoca isolates. Antibiotics 2021, 10, 1235. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, J.; Czokajło, I.; Gańko, M.; Śmiałek, M.; Koncicki, A. Identification and antimicrobial resistance in Klebsiella spp. isolates from Turkeys in Poland between 2019 and 2022. Animals 2022, 12, 3157. [Google Scholar] [CrossRef]
- Phetburom, N.; Boueroy, P.; Chopjitt, P.; Hatrongjit, R.; Nuanualsuwan, S.; Kerdsin, A. Phenotypic and molecular characterization of β-lactamase and plasmid-mediated quinolone resistance genes in Klebsiella oxytoca isolated from slaughtered pigs in Thailand. Vet. World 2022, 15, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Gu, T.; Ou, Y.; Wang, Y.; Xie, L.; Chen, L. Environmental compatibility and genome flexibility of Klebsiella oxytoca isolated from eight species of aquatic animals. Diversity 2024, 16, 30. [Google Scholar] [CrossRef]
- Ruan, G.Y.; Ye, L.X.; Lin, J.S.; Lin, H.Y.; Yu, L.R.; Wang, C.Y.; Mao, X.D.; Zhang, S.H.; Sun, P.M. An integrated approach of network pharmacology, molecular docking, and experimental verification uncovers kaempferol as the effective modulator of HSD17B1 for treatment of endometrial cancer. J. Transl. Med. 2023, 21, 204. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.; Feng, J.; Li, Z.; Zhang, Z.; Zhu, L.; Zhou, R.; Wang, H.; Dai, X.; Liu, Y. Exploring changes in metabolites and fecal microbiota of advanced gastric cancer based on plasma metabolomics and 16S rDNA sequencing. Heliyon 2025, 11, e41715. [Google Scholar] [CrossRef]
- Zhu, S.; Han, M.; Liu, S.; Fan, L.; Shi, H.; Li, P. Composition and diverse differences of intestinal microbiota in ulcerative colitis patients. Front. Cell Infect. Microbiol. 2022, 12, 953962. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Shi, C.; Sun, Y. Unraveling the role of Scutellaria baicalensis for the treatment of breast cancer using network pharmacology, molecular docking, and molecular dynamics simulation. Int. J. Mol. Sci. 2023, 24, 3594. [Google Scholar] [CrossRef]
- Qin, X.; Wu, Y.; Zhao, Y.; Qin, S.; Ji, Q.; Jia, J.; Huo, M.; Zhao, X.; Ma, Q.; Wang, X.; et al. Revealing active constituents within traditional Chinese Medicine used for treating bacterial pneumonia, with emphasis on the mechanism of baicalein against multi-drug resistant Klebsiella pneumoniae. J. Ethnopharmacol. 2024, 321, 117488. [Google Scholar] [CrossRef]
- Yu, M.; Hou, Y.; Cheng, M.; Liu, Y.; Ling, C.; Zhai, D.; Zhao, H.; Li, Y.; Chen, Y.; Xue, X.; et al. Antibacterial activity of squaric amide derivative SA2 against methicillin-resistant Staphylococcus aureus. Antibiotics 2022, 11, 1497. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Cai, X.; Peng, S.; Wang, L.; Tang, D.; Zhang, P. Scutellaria baicalensis in the treatment of hepatocellular carcinoma: Network pharmacology analysis and experimental validation. Evid. Based Complement. Alternat. Med. 2023, 2023, 4572660. [Google Scholar] [CrossRef]
- Barhouchi, B.; Menacer, R.; Bouchkioua, S.; Mansour, A.; Belattar, N. Compounds from myrtle flowers as antibacterial agents and SARS-CoV-2 inhibitors: In-vitro and molecular docking studies. Arab. J. Chem. 2023, 16, 104939. [Google Scholar] [CrossRef] [PubMed]
- Bianchini Fulindi, R.; Domingues Rodrigues, J.; Lemos Barbosa, T.W.; Goncalves Garcia, A.D.; de Almeida La Porta, F.; Pratavieira, S.; Chiavacci, L.A.; Pessoa Araújo Junior, J.; da Costa, P.I.; Martinez, L.R. Zinc-based nanoparticles reduce bacterial biofilm formation. Microbiol. Spectr. 2023, 11, e0483122. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.K.; Hsiao, P.Y.; Liu, Y.Y.; Tang, H.L.; Chiou, C.S.; Lu, M.C.; Lai, Y.C. Two ST11 Klebsiella pneumoniae strains exacerbate colorectal tumorigenesis in a colitis-associated mouse model. Gut Microbes 2021, 13, 1980348. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef]
- Yu, X.; Li, X.; Yang, H. Unraveling intestinal microbiota’s dominance in polycystic ovary syndrome pathogenesis over vaginal microbiota. Front. Cell Infect. Microbiol. 2024, 14, 1364097. [Google Scholar] [CrossRef]
- Konopiński, M.K. Shannon diversity index: A call to replace the original Shannon’s formula with unbiased estimator in the population genetics studies. PeerJ 2020, 8, e9391. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef]
- Ahlawat, S.; Asha; Sharma, K.K. Gut-organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae family in the gut microbiota: Diversity, metabolism, and function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The controversial role of human gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Turpin, W.; Humblot, C.; Noordine, M.L.; Thomas, M.; Guyot, J.P. Lactobacillaceae and cell adhesion: Genomic and functional screening. PLoS ONE 2012, 7, e38034. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The genus Alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.B.; Baiseitova, A.; Zahoor, M.; Ahmad, I.; Ikram, M.; Bakhsh, A.; Shah, M.A.; Ali, I.; Idress, M.; Ullah, R.; et al. Probiotic significance of Lactobacillus strains: A comprehensive review on health impacts, research gaps, and future prospects. Gut Microbes 2024, 16, 2431643. [Google Scholar] [CrossRef] [PubMed]
- Sribuathong, S.; Saengprakai, J.; Trevanich, S. In vitro anti-adherent assessment of selected lactic acid bacteria isolates against Salmonella Typhimurium and listeria monocytogenes to caco-2 cells. J. Food Safety 2014, 34, 270–282. [Google Scholar] [CrossRef]
- Hu, J.; Deng, F.; Zhao, B.; Lin, Z.; Sun, Q.; Yang, X.; Wu, M.; Qiu, S.; Chen, Y.; Yan, Z.; et al. Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling. Microbiome 2022, 10, 38. [Google Scholar] [CrossRef]
- Liu, Q.; Zuo, R.; Wang, K.; Nong, F.F.; Fu, Y.J.; Huang, S.W.; Pan, Z.F.; Zhang, Y.; Luo, X.; Deng, X.L.; et al. Oroxindin inhibits macrophage NLRP3 inflammasome activation in DSS-induced ulcerative colitis in mice via suppressing TXNIP-dependent NF-κB pathway. Acta Pharmacol. Sin. 2020, 41, 771–781. [Google Scholar] [CrossRef]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella pneumoniae biofilms and their role in disease pathogenesis. Front. Cell Infect. Microbiol. 2022, 12, 877995. [Google Scholar] [CrossRef]
- Monaci, V.; Gasperini, G.; Banci, L.; Micoli, F.; Cantini, F. (1)H, (13)C and (15)N assignment of self-complemented MrkA protein antigen from Klebsiella pneumoniae. Biomol. NMR Assign. 2024, 18, 171–179. [Google Scholar] [CrossRef]
- Jagnow, J.; Clegg, S. Klebsiella pneumoniae MrkD-mediated biofilm formation on extracellular matrix- and collagen-coated surfaces. Microbiology 2003, 149, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Nucci, A.; Rocha, E.P.C.; Rendueles, O. Latent evolution of biofilm formation depends on life-history and genetic background. NPJ Biofilms Microbiomes 2023, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Liao, H.W.; Wu, C.C.; Peng, H.L. MrkF is a component of type 3 fimbriae in Klebsiella pneumoniae. Res. Microbiol. 2009, 160, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.L.; Gerlach, G.F.; Clegg, S. Nucleotide sequence and functions of mrk determinants necessary for expression of type 3 fimbriae in Klebsiella pneumoniae. J. Bacteriol. 1991, 173, 916–920. [Google Scholar] [CrossRef][Green Version]
- Alcántar-Curiel, M.D.; Blackburn, D.; Saldaña, Z.; Gayosso-Vázquez, C.; Iovine, N.M.; De la Cruz, M.A.; Girón, J.A. Multi-functional analysis of Klebsiella pneumoniae fimbrial types in adherence and biofilm formation. Virulence 2013, 4, 129–138. [Google Scholar] [CrossRef]
- Yi, X.; Yamazaki, A.; Biddle, E.; Zeng, Q.; Yang, C.H. Genetic analysis of two phosphodiesterases reveals cyclic diguanylate regulation of virulence factors in Dickeya dadantii. Mol. Microbiol. 2010, 77, 787–800. [Google Scholar] [CrossRef]
- Garnett, J.A.; Diallo, M.; Matthews, S.J. Purification, crystallization and preliminary X-ray diffraction analysis of the Escherichia coli common pilus chaperone EcpB. Acta Crystallogr. F. Struct. Biol. Commun. 2015, 71, 676–679. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Li, J.; Xue, F.; Tang, F.; Dai, J. Extraintestinal pathogenic Escherichia coli utilizes the surface-expressed elongation factor Tu to bind and acquire iron from holo-transferrin. Virulence 2022, 13, 698–713. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, Y.; Mao, X.; Jiang, M.; Wei, Y.; Lian, L.; Xu, H.; Chen, L.; Xie, H.; Lu, G.; et al. SDR7-6, a short-chain alcohol dehydrogenase/reductase family protein, regulates light-dependent cell death and defence responses in rice. Mol. Plant Pathol. 2022, 23, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Valderas, K.N.; Moreno-Hagelsieb, G.; Rohde, J.R.; Garduño, R.A. The functional differences between the GroEL chaperonin of Escherichia coli and the HtpB chaperonin of Legionella pneumophila can be mapped to specific amino acid residues. Biomolecules 2021, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, I.; Rezaei, N.; Bazzazan, S.; Mezajin, M.N.; Mansouri, A.; Karbalaeiheidar, H.; Ashkezari, S.; Moghaddam, Z.S.; Lalami, Z.A.; Mostafavi, E. In silico and in vitro studies of GENT-EDTA encapsulated niosomes: A novel approach to enhance the antibacterial activity and biofilm inhibition in drug-resistant Klebsiella pneumoniae. Biomater. Adv. 2023, 149, 213384. [Google Scholar] [CrossRef]
- Pruss, A.; Miładowska, K.; Masiuk, H.; Kwiatkowski, P.; Jursa-Kulesza, J.; Wojciuk, B.; Giedrys-Kalemba, S.; Dołęgowska, B. Epidemiological analysis of a K. pneumoniae NDM outbreak in a temporary ward for patients with primary COVID-19 infection. Microbiol. Res. 2025, 16, 17. [Google Scholar] [CrossRef]
- Kuinkel, S.; Acharya, J.; Dhungel, B.; Adhikari, S.; Adhikari, N.; Shrestha, U.T.; Banjara, M.R.; Rijal, K.R.; Ghimire, P. Biofilm formation and phenotypic detection of ESBL, MBL, KPC and AmpC enzymes and their coexistence in Klebsiella spp. isolated at the national reference laboratory, Kathmandu, Nepal. Microbiol. Res. 2021, 12, 683–697. [Google Scholar] [CrossRef]
- Han, J.; Luo, J.; Du, Z.; Chen, Y.; Liu, T. Synergistic effects of baicalin and levofloxacin against hypervirulent Klebsiella pneumoniae biofilm in vitro. Curr. Microbiol. 2023, 80, 126. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.; Meng, J.; Qiu, T.; Wang, W.; Wang, R.; Liu, J. Baicalin inhibits biofilm formation by influencing primary adhesion and aggregation phases in Staphylococcus saprophyticus. Vet. Microbiol. 2021, 262, 109242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).