Effects of Obesity and Feeding Avocado Extract on Gut Microbiota and Fecal Metabolomic Profile in Overweight/Obese Cats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Avocado Dietary Supplement, Placebo
2.3. Study Design
2.4. Analysis of Fecal Microbiota (DNA Extraction and Sequencing of 16S rRNA Genes)
2.5. Untargeted Fecal Metabolomics
2.6. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Gut Microbiota Composition
3.2.1. Univariate Statistics
3.2.2. Linear Discriminant Analysis Effect Size (LEfSe)
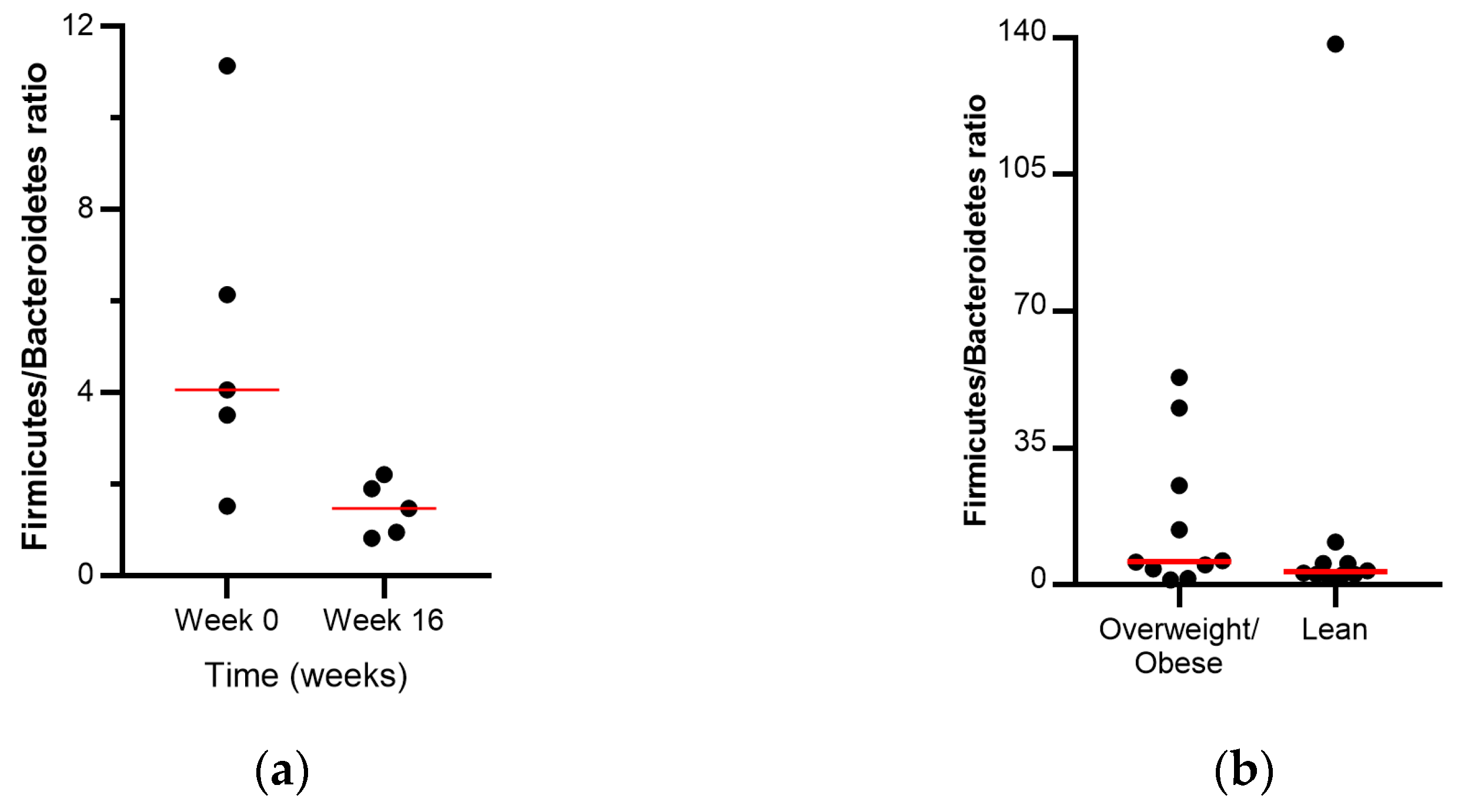
3.2.3. Firmicutes/Bacteroidetes Ratio
3.2.4. Diversity Within Samples (Alpha Diversity)
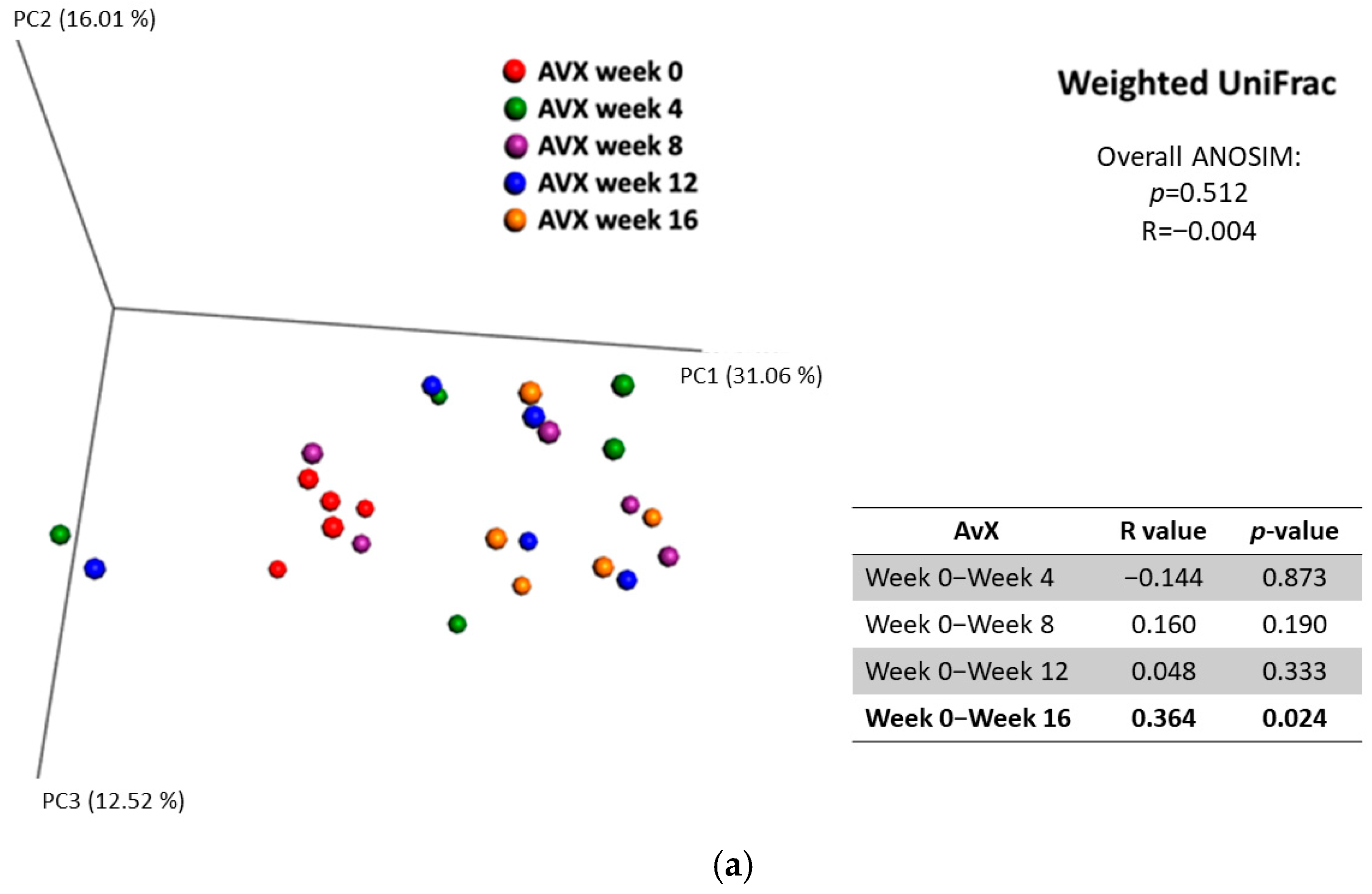
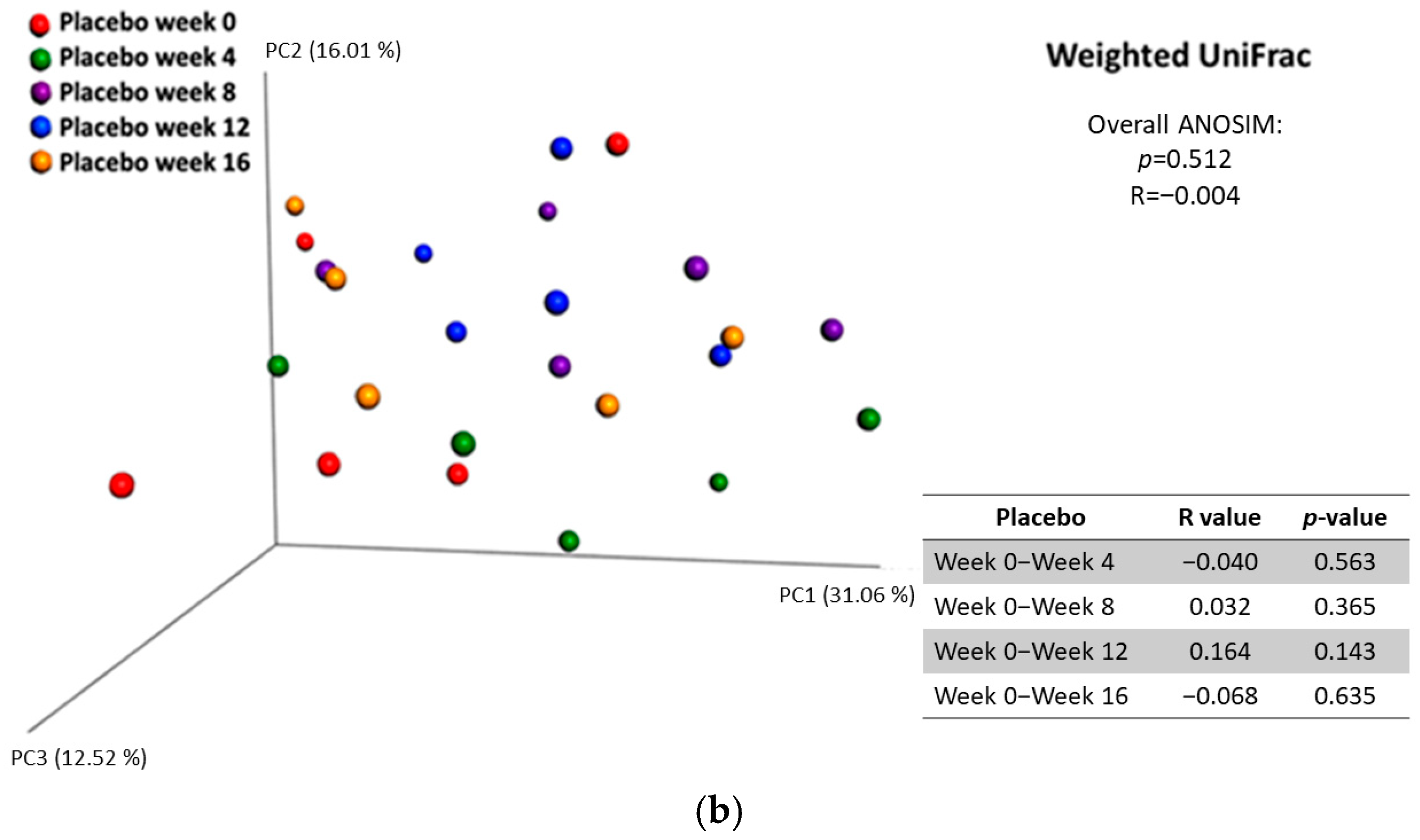
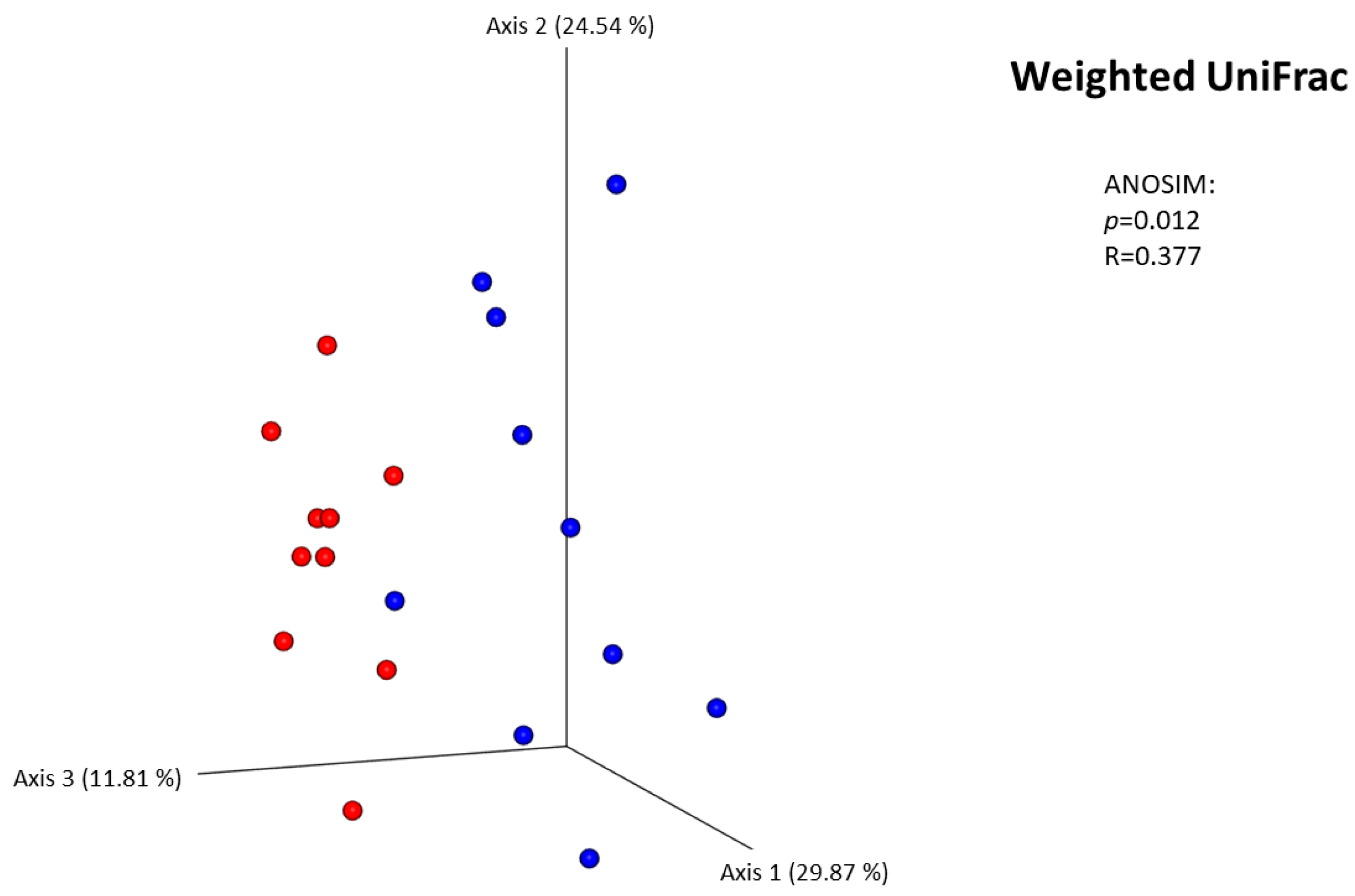
3.2.5. Diversity Between Samples (Beta Diversity)
3.3. Analysis of the Fecal Metabolome
3.4. Correlation Between the Concentration of Metabolites Significantly Different Between the AvX and Placebo Groups and the Abundances of Detected Bacterial Taxa in the Group of Overweight/Obese Cats Receiving AvX
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
References
- Emmerich, S.D.; Fryar, C.D.; Stierman, B.; Ogden, C.L. Obesity and Severe Obesity Prevalence in Adults: United States, August 2021–August 2023. NCHS Data Briefs 2024. [Google Scholar] [CrossRef]
- German, A.J. The growing problem of obesity in dogs and cats. J. Nutr. 2006, 136 (Suppl. 7), 1940S–1946S. [Google Scholar] [CrossRef]
- Scarlett, J.M.; Donoghue, S. Associations between body condition and disease in cats. J. Am. Vet. Med. Assoc. 1998, 212, 1725–1731. Available online: https://www.ncbi.nlm.nih.gov/pubmed/9621878 (accessed on 9 August 2025). [CrossRef] [PubMed]
- Russell, K.; Sabin, R.; Holt, S.; Bradley, R.; Harper, E.J. Influence of feeding regimen on body condition in the cat. J. Small Anim. Pract. 2000, 41, 12–17. [Google Scholar] [CrossRef]
- Lund, E.M.; Armstrong, P.J.; Kirk, C.A.; Klausner, J.S. Prevalence and risk factors for obesity in adult cats for private US veterinary practices. Int. J. Appl. Res. Vet. Med. 2005, 3, 88–96. [Google Scholar]
- Courcier, E.A.; Mellor, D.J.; Pendlebury, E.; Evans, C.; Yam, P.S. An investigation into the epidemiology of feline obesity in Great Britain: Results of a cross-sectional study of 47 companion animal practises. Vet. Rec. 2012, 171, 560. [Google Scholar] [CrossRef]
- Cave, N.J.; Allan, F.J.; Schokkenbroek, S.L.; Metekohy, C.A.; Pfeiffer, D.U. A cross-sectional study to compare changes in the prevalence and risk factors for feline obesity between 1993 and 2007 in New Zealand. Prev. Vet. Med. 2012, 107, 121–133. [Google Scholar] [CrossRef]
- Colliard, L.; Paragon, B.M.; Lemuet, B.; Benet, J.J.; Blanchard, G. Prevalence and risk factors of obesity in an urban population of healthy cats. J. Feline Med. Surg. 2009, 11, 135–140. [Google Scholar] [CrossRef]
- MClark, H.; Hoenig, M.; Ferguson, D.C.; Dirikolu, L. Pharmacokinetics of pioglitazone in lean and obese cats. J. Vet. Pharmacol. Ther. 2012, 35, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Pla, J.J. Obesity. In Ettinger’s Textbook of Veterinary Internal Medicine, 9th ed.; Ettinger, S.J., Feldman, E.C., Cote, E., Eds.; Elsevier: Philadelphia, PA, USA, 2024; Volume 1, pp. 758–763, ch. 150. [Google Scholar]
- Masood, B.; Moorthy, M. Causes of obesity: A review. Clin. Med. 2023, 23, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, M. The cat as a model for human nutrition and disease. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 584–588. [Google Scholar] [CrossRef]
- Hoenig, M. The cat as a model for human obesity and diabetes. J. Diabetes Sci. Technol. 2012, 6, 525–533. [Google Scholar] [CrossRef]
- Henson, M.S.; O’Brien, T.D. Feline models of type 2 diabetes mellitus. ILAR J. 2006, 47, 234–242. [Google Scholar] [CrossRef]
- Santos, V.M.D.; Muller, M.; de Vos, W.M. Systems biology of the gut: The interplay of food, microbiota and host at the mucosal interface. Curr. Opin. Biotechnol. 2010, 21, 539–550. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef]
- Nerurkar, P.V.; Orias, D.; Soares, N.; Kumar, M.; Nerurkar, V.R. Momordica charantia (bitter melon) modulates adipose tissue inflammasome gene expression and adipose-gut inflammatory cross talk in high-fat diet (HFD)-fed mice. J. Nutr. Biochem. 2019, 68, 16–32. [Google Scholar] [CrossRef]
- Agus, A.; Clement, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Barko, P.C.; Williams, D.A.; Wu, Y.-A.; Steiner, J.M.; Suchodolski, J.S.; Gal, A.; Marsilio, S. Chronic Inflammatory Enteropathy and Low-Grade Intestinal T-Cell Lymphoma Are Associated with Altered Microbial Tryptophan Catabolism in Cats. Animals 2023, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated With Systemic Inflammation. Front. Immunol. 2020, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Mallmann, N.H.; Lima, E.S.; Lalwani, P. Dysregulation of Tryptophan Catabolism in Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 135–142. [Google Scholar] [CrossRef]
- Liu, M.; Nieuwdorp, M.; de Vos, W.M.; Rampanelli, E. Microbial Tryptophan Metabolism Tunes Host Immunity, Metabolism, and Extraintestinal Disorders. Metabolites 2022, 12, 834. [Google Scholar] [CrossRef]
- Laurans, L.; Venteclef, N.; Haddad, Y.; Chajadine, M.; Alzaid, F.; Metghalchi, S.; Sovran, B.; Denis, R.G.P.; Dairou, J.; Cardellini, M.; et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat. Med. 2018, 24, 1113–1120. [Google Scholar] [CrossRef]
- Mondanelli, G.; Albini, E.; Orecchini, E.; Pallotta, M.T.; Belladonna, M.L.; Ricci, G.; Grohmann, U.; Orabona, C. Pathogenetic Interplay Between IL-6 and Tryptophan Metabolism in an Experimental Model of Obesity. Front. Immunol. 2021, 12, 713989. [Google Scholar] [CrossRef]
- Ahmad, A.; Yang, W.; Chen, G.; Shafiq, M.; Javed, S.; Zaidi, S.S.A.; Shahid, R.; Liu, C.; Bokhari, H.; Jacobs, J. Analysis of gut microbiota of obese individuals with type 2 diabetes and healthy individuals. PLoS ONE 2019, 14, e0226372. [Google Scholar] [CrossRef]
- Graham, C.; Mullen, A.; Whelan, K. Obesity and the gastrointestinal microbiota: A review of associations and mechanisms. Nutr. Rev. 2015, 73, 376–385. [Google Scholar] [CrossRef]
- Rajani, C.; Jia, W. Disruptions in gut microbial-host co-metabolism and the development of metabolic disorders. Clin. Sci. 2018, 132, 791–811. [Google Scholar] [CrossRef]
- Deng, P.; Swanson, K.S. Gut microbiota of humans, dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2015, 113, S6–S17. [Google Scholar] [CrossRef]
- Loftus, J.P.; Wakshlag, J.J. Canine and feline obesity: A review of pathophysiology, epidemiology, and clinical management. Vet. Med. Res. Rep. 2014, 6, 49–60. [Google Scholar] [CrossRef]
- Kieler, I.N.; Molbak, L.; Hansen, L.L.; Hermann-Bank, M.L.; Bjornvad, C.R. Overweight and the feline gut microbiome—A pilot study. J. Anim. Physiol. Anim. Nutr. 2016, 100, 478–484. [Google Scholar] [CrossRef]
- Tal, M.; Weese, J.S.; Gomez, D.E.; Hesta, M.; Steiner, J.M.; Verbrugghe, A. Bacterial fecal microbiota is only minimally affected by a standardized weight loss plan in obese cats. BMC Vet. Res. 2020, 16, 112. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.M.; Kessler, A.M.; Kieffer, D.A.; Knotts, T.A.; Kim, K.; Wei, A.; Ramsey, J.J.; Fascetti, A.J. Effects of obesity, energy restriction and neutering on the faecal microbiota of cats. Br. J. Nutr. 2017, 118, 513–524. [Google Scholar] [CrossRef]
- Pallotto, M.R.; de Godoy, M.R.C.; Holscher, H.D.; Buff, P.R.; Swanson, K.S. Effects of weight loss with a moderate-protein, high-fiber diet on body composition, voluntary physical activity, and fecal microbiota of obese cats. Am. J. Vet. Res. 2018, 79, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.K.; Roth, G.S. Glycolytic inhibition as a strategy for developing calorie restriction mimetics. Exp. Gerontol. 2010, 46, 148–154. [Google Scholar] [CrossRef]
- Ingram, D.K.; Pistell, P.J.; Wang, Z.Q.; Yu, Y.; Massimino, S.; Davenport, G.M.; Hayek, M.; Roth, G.S. Characterization and Mechanisms of Action of Avocado Extract Enriched in Mannoheptulose as a Candidate Calorie Restriction Mimetic. J. Agric. Food Chem. 2021, 69, 7367–7376. [Google Scholar] [CrossRef] [PubMed]
- Pistell, P.J.; Utsuki, T.; Francis, J.; Ebenezer, P.J.; Terrebonne, J.; Roth, G.S.; Ingram, D.K. An Avocado Extract Enriched in Mannoheptulose Prevents the Negative Effects of a High-Fat Diet in Mice. Nutrients 2021, 14, 155. [Google Scholar] [CrossRef]
- Stone, A.E.; Brummet, G.O.; Carozza, E.M.; Kass, P.H.; Petersen, E.P.; Sykes, J.; E Westman, M. 2020 AAHA/AAFP Feline Vaccination Guidelines. J. Feline Med. Surg. 2020, 22, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, D. Development and validation of a body condition score system for cats: A clinical tool. Feline Pract. 1997, 25, 13–17. [Google Scholar]
- Isaiah, A.; Parambeth, J.C.; Steiner, J.M.; Lidbury, J.A.; Suchodolski, J.S. The fecal microbiome of dogs with exocrine pancreatic insufficiency. Anaerobe 2017, 45, 50–58. [Google Scholar] [CrossRef]
- Kasiraj, A.C.; Harmoinen, J.; Isaiah, A.; Westermarck, E.; Steiner, J.M.; Spillmann, T.; Suchodolski, J.S.; Marchesi, J. The effects of feeding and withholding food on the canine small intestinal microbiota. FEMS Microbiol. Ecol. 2016, 92, fiw085. [Google Scholar] [CrossRef]
- Honneffer, J.B.; Steiner, J.M.; Lidbury, J.A.; Suchodolski, J.S. Variation of the microbiota and metabolome along the canine gastrointestinal tract. Metabolomics 2017, 13, 26. [Google Scholar] [CrossRef]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 2015, 6, 33–47. [Google Scholar] [CrossRef]
- Pilla, R.; Gaschen, F.P.; Barr, J.W.; Olson, E.; Honneffer, J.; Guard, B.C.; Blake, A.B.; Villanueva, D.; Khattab, M.R.; AlShawaqfeh, M.K.; et al. Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs. J. Vet. Intern. Med. 2020, 34, 1853–1866. [Google Scholar] [CrossRef]
- Arnold, C.E.; Isaiah, A.; Pilla, R.; Lidbury, J.; Coverdale, J.S.; Callaway, T.R.; Lawhon, S.D.; Steiner, J.; Suchodolski, J.S.; Ratnasekhar, C. The cecal and fecal microbiomes and metabolomes of horses before and after metronidazole administration. PLoS ONE 2020, 15, e0232905. [Google Scholar] [CrossRef]
- Park, M.J.; Pilla, R.; Panta, A.; Pandey, S.; Sarawichitr, B.; Suchodolski, J.; Sohrabji, F. Reproductive Senescence and Ischemic Stroke Remodel the Gut Microbiome and Modulate the Effects of Estrogen Treatment in Female Rats. Transl. Stroke Res. 2020, 11, 812–830. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Zakrzewski, M.; Proietti, C.; Ellis, J.J.; Hasan, S.; Brion, M.-J.; Berger, B.; Krause, L.; Wren, J. Calypso: A user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics 2017, 33, 782–783. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Napolitano, M.; Covasa, M. Microbiota Transplant in the Treatment of Obesity and Diabetes: Current and Future Perspectives. Front. Microbiol. 2020, 11, 590370. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Castaner, O.; Goday, A.; Park, Y.-M.; Lee, S.-H.; Magkos, F.; Shiow, S.-A.T.E.; Schröder, H. The Gut Microbiome Profile in Obesity: A Systematic Review. Int. J. Endocrinol. 2018, 2018, 4095789. [Google Scholar] [CrossRef]
- Chanda, D.; De, D. Meta-analysis reveals obesity associated gut microbial alteration patterns and reproducible contributors of functional shift. Gut Microbes 2024, 16, 2304900. [Google Scholar] [CrossRef] [PubMed]
- Pinart, M.; Dötsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef]
- Louis, S.; Tappu, R.M.; Damms-Machado, A.; Huson, D.H.; Bischoff, S.C. Characterization of the Gut Microbial Community of Obese Patients Following a Weight-Loss Intervention Using Whole Metagenome Shotgun Sequencing. PLoS ONE 2016, 11, e0149564. [Google Scholar] [CrossRef]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Karlsson, C.L.; Onnerfalt, J.; Xu, J.; Molin, G.; Ahrne, S.; Thorngren-Jerneck, K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity 2012, 20, 2257–2261. [Google Scholar] [CrossRef]
- Bervoets, L.; Van Hoorenbeeck, K.; Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE 2009, 4, e7125. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- De Bandt, J.P.; Waligora-Dupriet, A.J.; Butel, M.J. Intestinal microbiota in inflammation and insulin resistance: Relevance to humans. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 334–340. [Google Scholar] [CrossRef]
- Zou, Y.; Ju, X.; Chen, W.; Yuan, J.; Wang, Z.; Aluko, R.E.; He, R. Rice bran attenuated obesity via alleviating dyslipidemia, browning of white adipocytes and modulating gut microbiota in high-fat diet-induced obese mice. Food Funct. 2020, 11, 2406–2417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Verdam, F.J.; Fuentes, S.; de Jonge, C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; de Vos, W.M.; Rensen, S.S. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013, 21, E607–E615. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Hoisington, A.J.; Stamper, C.E.; Ellis, J.C.; Lowry, C.A.; Brenner, L.A. Quantifying variation across 16S rRNA gene sequencing runs in human microbiome studies. Appl. Microbiol. Biotechnol. 2024, 108, 367. [Google Scholar] [CrossRef]
- Forry, S.P.; Servetas, S.L.; Kralj, J.G.; Soh, K.; Hadjithomas, M.; Cano, R.; Carlin, M.; de Amorim, M.G.; Auch, B.; Bakker, M.G.; et al. Variability and bias in microbiome metagenomic sequencing: An interlaboratory study comparing experimental protocols. Sci. Rep. 2024, 14, 9785. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Iakhno, S.; Umu, Ö.C.O.; Håkenåsen, I.M.; Åkesson, C.P.; Mydland, L.T.; Press, C.M.; Sørum, H.; Øverland, M. Effect of Cyberlindnera jadinii yeast as a protein source on intestinal microbiota and butyrate levels in post-weaning piglets. Anim. Microbiome 2020, 2, 13. [Google Scholar] [CrossRef]
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes. Rev. 2013, 14, 950–959. [Google Scholar] [CrossRef]
- Chen, X.; Sun, H.; Jiang, F.; Shen, Y.; Li, X.; Hu, X.; Shen, X.; Wei, P. Alteration of the gut microbiota associated with childhood obesity by 16S rRNA gene sequencing. PeerJ 2020, 8, e8317. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Gut microbiota is associated with obesity and cardiometabolic disease in a population in the midst of Westernization. Sci. Rep. 2018, 8, 11356. [Google Scholar] [CrossRef]
- Kieler, I.N.; Osto, M.; Hugentobler, L.; Puetz, L.; Gilbert, M.T.P.; Hansen, T.; Pedersen, O.; Reusch, C.E.; Zini, E.; Lutz, T.A.; et al. Diabetic cats have decreased gut microbial diversity and a lack of butyrate producing bacteria. Sci. Rep. 2019, 9, 4822. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyötyläinen, T.; Hämäläinen, A.-M.; Peet, A.; Tillmann, V.; Pöhö, P.; Mattila, I.; et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Huang, X.; Fang, S.; Xin, W.; Huang, L.; Chen, C. Uncovering the composition of microbial community structure and metagenomics among three gut locations in pigs with distinct fatness. Sci. Rep. 2016, 6, 27427. [Google Scholar] [CrossRef]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef] [PubMed]
- Nirmalkar, K.; Murugesan, S.; Pizano-Zárate, M.L.; Villalobos-Flores, L.E.; García-González, C.; Morales-Hernández, R.M.; Nuñez-Hernández, J.A.; Hernández-Quiroz, F.; Romero-Figueroa, M.D.S.; Hernández-Guerrero, C.; et al. Gut Microbiota and Endothelial Dysfunction Markers in Obese Mexican Children and Adolescents. Nutrients 2018, 10, 2009. [Google Scholar] [CrossRef]
- Lai, Z.-L.; Tseng, C.-H.; Ho, H.J.; Cheung, C.K.Y.; Lin, J.-Y.; Chen, Y.-J.; Cheng, F.-C.; Hsu, Y.-C.; Lin, J.-T.; El-Omar, E.M.; et al. Fecal microbiota transplantation confers beneficial metabolic effects of diet and exercise on diet-induced obese mice. Sci. Rep. 2018, 8, 15625. [Google Scholar] [CrossRef]
- Horie, M.; Miura, T.; Hirakata, S.; Hosoyama, A.; Sugino, S.; Umeno, A.; Murotomi, K.; Yoshida, Y.; Koike, T. Comparative analysis of the intestinal flora in type 2 diabetes and nondiabetic mice. Exp. Anim. 2017, 66, 405–416. [Google Scholar] [CrossRef]
- Dong, T.S.; Mayer, E.A.; Osadchiy, V.; Chang, C.; Katzka, W.; Lagishetty, V.; Gonzalez, K.; Kalani, A.; Stains, J.; Jacobs, J.P.; et al. A Distinct Brain-Gut-Microbiome Profile Exists for Females with Obesity and Food Addiction. Obesity 2020, 28, 1477–1486. [Google Scholar] [CrossRef]
- 94. Murugesan, S.; Ulloa-Martínez, M.; Martínez-Rojano, H.; Galván-Rodríguez, F.M.; Miranda-Brito, C.; Romano, M.C.; Pi-ña-Escobedo, A.; Pizano-Zárate, M.L.; Hoyo-Vadillo, C.; García-Mena, J. Study of the diversity and short-chain fatty acids pro-duction by the bacterial community in overweight and obese Mexican children. Eur. J. Clin. Microbiol. Infect Dis. 2015, 34, 1337–1346. [Google Scholar] [CrossRef]
- Naderpoor, N.; Mousa, A.; Gomez-Arango, L.F.; Barrett, H.L.; Nitert, M.D.; de Courten, B. Faecal Microbiota Are Related to Insulin Sensitivity and Secretion in Overweight or Obese Adults. J. Clin. Med. 2019, 8, 452. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.E.; Kim, H.B.; Isaacson, R.E.; Seo, K.W.; Song, K.H. Association of obesity with serum leptin, adiponectin, and serotonin and gut microflora in beagle dogs. J. Vet. Intern. Med. 2015, 29, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Handl, S.; German, A.J.; Holden, S.L.; Dowd, S.E.; Steiner, J.M.; Heilmann, R.M.; Grant, R.W.; Swanson, K.S.; Suchodolski, J.S. Faecal microbiota in lean and obese dogs. FEMS Microbiol. Ecol. 2013, 84, 332–343. [Google Scholar] [CrossRef]
- Hou, Y.-P.; He, Q.-Q.; Ouyang, H.-M.; Peng, H.-S.; Wang, Q.; Li, J.; Lv, X.-F.; Zheng, Y.-N.; Li, S.-C.; Liu, H.-L.; et al. Human Gut Microbiota Associated with Obesity in Chinese Children and Adolescents. Biomed. Res. Int. 2017, 2017, 7585989. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Kunath, J.; Loh, G.; Blaut, M. Human intestinal microbiota: Characterization of a simplified and stable gnotobiotic rat model. Gut Microbes 2011, 2, 25–33. [Google Scholar] [CrossRef]
- Ibrahim, K.S.; Bourwis, N.; Dolan, S.; Lang, S.; Spencer, J.; Craft, J.A. Characterisation of gut microbiota of obesity and type 2 diabetes in a rodent model. Biosci. Microbiota Food Health 2021, 40, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, S.; Yang, L.; Huang, P.; Li, W.; Wang, S.; Zhao, G.; Zhang, M.; Pang, X.; Yan, Z.; et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat. Commun. 2013, 4, 2163. [Google Scholar] [CrossRef]
- LeComte, V.; Kaakoush, N.O.; Maloney, C.A.; Raipuria, M.; Huinao, K.D.; Mitchell, H.M.; Morris, M.J. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS ONE 2015, 10, e0126931. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Kim, H.-N.; Kim, S.E.; Heo, S.G.; Chang, Y.; Ryu, S.; Shin, H.; Kim, H.-L. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol. 2017, 17, 151. [Google Scholar] [CrossRef]
- Adeshirlarijaney, A.; Gewirtz, A.T. Considering gut microbiota in treatment of type 2 diabetes mellitus. Gut Microbes 2020, 11, 253–264. [Google Scholar] [CrossRef]
- Ganesh, B.P.; Klopfleisch, R.; Loh, G.; Blaut, M. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PLoS ONE 2013, 8, e74963. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Minamoto, Y.; Kawas, J.R.; Suchodolski, J.S.; de Vos, W.M. Akkermansia and Microbial Degradation of Mucus in Cats and Dogs: Implications to the Growing Worldwide Epidemic of Pet Obesity. Vet. Sci. 2020, 7, 44. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Nitert, M.D. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef]
- Selma, M.V.; Romo-Vaquero, M.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A.; Espin, J.C. The human gut microbial ecology associated with overweight and obesity determines ellagic acid metabolism. Food Funct. 2016, 7, 1769–1774. [Google Scholar] [CrossRef]
- Mitev, K.; Taleski, V. Association between the Gut Microbiota and Obesity. Open Access Maced. J. Med. Sci. 2019, 7, 2050–2056. [Google Scholar] [CrossRef]
- Hooda, S.; Boler, B.M.V.; Kerr, K.R.; Dowd, S.E.; Swanson, K.S. The gut microbiome of kittens is affected by dietary protein:carbohydrate ratio and associated with blood metabolite and hormone concentrations. Br. J. Nutr. 2013, 109, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Sunagawa, S.; Mende, D.R.; Bork, P. Inter-individual differences in the gene content of human gut bacterial species. Genome Biol. 2015, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Péan, N.; Le Lay, A.; Brial, F.; Wasserscheid, J.; Rouch, C.; Vincent, M.; Myridakis, A.; Hedjazi, L.; Dumas, M.-E.; Grundberg, E.; et al. Dominant gut Prevotella copri in gastrectomised non-obese diabetic Goto-Kakizaki rats improves glucose homeostasis through enhanced FXR signalling. Diabetologia 2020, 63, 1223–1235. [Google Scholar] [CrossRef]
- Jiao, N.; Baker, S.S.; Nugent, C.A.; Tsompana, M.; Cai, L.; Wang, Y.; Buck, M.J.; Genco, R.J.; Baker, R.D.; Zhu, R.; et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: A meta-analysis. Physiol. Genomics 2018, 50, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Crott, J.W.; Lyu, L.; Pfalzer, A.C.; Li, J.; Choi, S.-W.; Yang, Y.; Mason, J.B.; Liu, Z. Diet- and Genetically-induced Obesity Produces Alterations in the Microbiome, Inflammation and Wnt Pathway in the Intestine of Apc(+/1638N) Mice: Comparisons and Contrasts. J. Cancer 2016, 7, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Ross, R.P.; O’Toole, P.W.; Shanahan, F.; Cotter, P.D. Targeting the microbiota to address diet-induced obesity: A time dependent challenge. PLoS ONE 2013, 8, e65790. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, H.; Zhan, L.; Lu, X.; Zhang, L. Dynamic Development of Fecal Microbiome During the Progression of Diabetes Mellitus in Zucker Diabetic Fatty Rats. Front. Microbiol. 2019, 10, 232. [Google Scholar] [CrossRef]
- Marsilio, S.; Pilla, R.; Sarawichitr, B.; Chow, B.; Hill, S.L.; Ackermann, M.R.; Estep, J.S.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Characterization of the fecal microbiome in cats with inflammatory bowel disease or alimentary small cell lymphoma. Sci. Rep. 2019, 9, 19208. [Google Scholar] [CrossRef]
- Johnson, A.M.F.; Costanzo, A.; Gareau, M.G.; Armando, A.M.; Quehenberger, O.; Jameson, J.M.; Olefsky, J.M.; Nerurkar, P.V. High fat diet causes depletion of intestinal eosinophils associated with intestinal permeability. PLoS ONE 2015, 10, e0122195. [Google Scholar] [CrossRef]
- Rossi, G.; Pengo, G.; Caldin, M.; Piccionello, A.P.; Steiner, J.M.; Cohen, N.D.; Jergens, A.E.; Suchodolski, J.S.; Chamaillard, M. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS ONE 2014, 9, e94699. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Tang, Z.; Yan, X.; Feng, B. Structural modulation of the gut microbiota and the relationship with body weight: Compared evaluation of liraglutide and saxagliptin treatment. Sci. Rep. 2016, 6, 33251. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.V.; Bailey, M.A.; Taylor, A.M.; Kaczmarek, J.L.; Mysonhimer, A.R.; Edwards, C.G.; Reeser, G.E.; Burd, N.A.; Khan, N.A.; Holscher, H.D. Avocado Consumption Alters Gastrointestinal Bacteria Abundance and Microbial Metabolite Concentrations among Adults with Overweight or Obesity: A Randomized Controlled Trial. J. Nutr. 2021, 151, 753–762. [Google Scholar] [CrossRef]
- Kelder, T.; Stroeve, J.H.; Bijlsma, S.; Radonjic, M.; Roeselers, G. Correlation network analysis reveals relationships between diet-induced changes in human gut microbiota and metabolic health. Diabetes 2014, 4, e122. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J.; Sanz, Y. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE 2013, 8, e65465. [Google Scholar] [CrossRef]
- Walker, A.W.; Duncan, S.H.; McWilliam Leitch, E.C.; Child, M.W.; Flint, H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef]
- Sanchez-Alcoholado, L.; Gutierrez-Repiso, C.; Gomez-Perez, A.M.; Garcia-Fuentes, E.; Tinahones, F.J.; Moreno-Indias, I. Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surg. Obes. Relat. Dis. 2019, 15, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Adamberg, K.; Adamberg, S.; Ernits, K.; Larionova, A.; Voor, T.; Jaagura, M.; Visnapuu, T.; Alamäe, T. Composition and metabolism of fecal microbiota from normal and overweight children are differentially affected by melibiose, raffinose and raffinose-derived fructans. Anaerobe 2018, 52, 100–110. [Google Scholar] [CrossRef]
- Forster, G.M.; Stockman, J.; Noyes, N.; Heuberger, A.L.; Broeckling, C.D.; Bantle, C.M.; Ryan, E.P. A Comparative Study of Serum Biochemistry, Metabolome and Microbiome Parameters of Clinically Healthy, Normal Weight, Overweight, and Obese Companion Dogs. Top. Companion Anim. Med. 2018, 33, 126–135. [Google Scholar] [CrossRef]
- Kageyama, A.; Benno, Y. Catenibacterium mitsuokai gen. nov., sp. nov., a gram-positive anaerobic bacterium isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 4, 1595–1599. [Google Scholar] [CrossRef]
- Fayfman, M.; Flint, K.; Srinivasan, S. Obesity, Motility, Diet, and Intestinal Microbiota-Connecting the Dots. Curr. Gastroenterol. Rep. 2019, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Petriz, B.A.; Castro, A.P.; Almeida, J.A.; Gomes, C.P.; Fernandes, G.R.; Kruger, R.H.; Pereira, R.W.; Franco, O.L. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics 2014, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.G.; Skov, M.N.; Justesen, U.S. Two cases of Ruminococcus gnavus bacteremia associated with diverticulitis. J. Clin. Microbiol. 2013, 51, 1334–1336. [Google Scholar] [CrossRef]
- Strate, L.L.; Liu, Y.L.; Aldoori, W.H.; Syngal, S.; Giovannucci, E.L. Obesity increases the risks of diverticulitis and diverticular bleeding. Gastroenterology 2009, 136, 115–122.e1. [Google Scholar] [CrossRef]
- Wijaya, A.; Hermann, A.; Abriouel, H.; Specht, I.; Yousif, N.M.K.; Holzapfel, W.H.; Franz, C.M.A.P. Cloning of the bile salt hydrolase (bsh) gene from Enterococcus faecium FAIR-E 345 and chromosomal location of bsh genes in food enterococci. J. Food Prot. 2004, 67, 2772–2778. [Google Scholar] [CrossRef]
- Wang, X.; Ota, N.; Manzanillo, P.; Kates, L.; Zavala-Solorio, J.; Eidenschenk, C.; Zhang, J.; Lesch, J.; Lee, W.P.; Ross, J.; et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 2014, 514, 237–241. [Google Scholar] [CrossRef]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.-L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e4. [Google Scholar] [CrossRef] [PubMed]
- Ihekweazu, F.D.; Engevik, M.A.; Ruan, W.; Shi, Z.; Fultz, R.; Engevik, K.A.; Chang-Graham, A.L.; Freeborn, J.; Park, E.S.; Venable, S.; et al. Bacteroides ovatus Promotes IL-22 Production and Reduces Trinitrobenzene Sulfonic Acid-Driven Colonic Inflammation. Am. J. Pathol. 2021, 191, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Ruane, D.T.; Lavelle, E.C. The role of CD103(+) dendritic cells in the intestinal mucosal immune system. Front. Immunol. 2011, 2, 25. [Google Scholar] [CrossRef]
- Su, X.; Zhang, M.; Qi, H.; Gao, Y.; Yang, Y.; Yun, H.; Zhang, Q.; Yang, X.; Zhang, Y.; He, J.; et al. Gut microbiota-derived metabolite 3-idoleacetic acid together with LPS induces IL-35(+) B cell generation. Microbiome 2022, 10, 13. [Google Scholar] [CrossRef]
- Shen, P.; Roch, T.; Lampropoulou, V.; O’cOnnor, R.A.; Stervbo, U.; Hilgenberg, E.; Ries, S.; Dang, V.D.; Jaimes, Y.; Daridon, C.; et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014, 507, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Su, L.C.; Liu, X.Y.; Huang, A.F.; Xu, W.D. Emerging role of IL-35 in inflammatory autoimmune diseases. Autoimmun. Rev. 2018, 17, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.; Struys, E.A.; van Gaal, N.; Bakkali, A.; Jansen, E.W.; Diederen, K.; Benninga, M.A.; Mulder, C.J.; de Boer, N.K.; de Meij, T.G. Fecal Amino Acid Analysis Can Discriminate De Novo Treatment-Naive Pediatric Inflammatory Bowel Disease From Controls. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Huang, S.L. The Commensal Anaerobe Veillonella dispar Reprograms Its Lactate Metabolism and Short-Chain Fatty Acid Production during the Stationary Phase. Microbiol. Spectr. 2023, 11, e0355822. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Young, W.; Butowski, C.F.; Moon, C.D.; Maclean, P.H.; Rosendale, D.; Cave, N.J.; Thomas, D.G. The Fecal Microbiota in the Domestic Cat (Felis catus) Is Influenced by Interactions Between Age and Diet; A Five Year Longitudinal Study. Front. Microbiol. 2018, 9, 1231. [Google Scholar] [CrossRef]
- Deusch, O.; O’Flynn, C.; Colyer, A.; Swanson, K.S.; Allaway, D.; Morris, P. A Longitudinal Study of the Feline Faecal Microbiome Identifies Changes into Early Adulthood Irrespective of Sexual Development. PLoS ONE 2015, 10, e0144881. [Google Scholar] [CrossRef]

| AvX Group | Placebo Group | |
|---|---|---|
| Number | 5 | 5 |
| Sex | ||
| neutered males | 2 | 3 |
| spayed females | 3 | 2 |
| Age (mean ± SD) | 3.7 ± 1.7 years | 4.7 ± 2.6 years |
| (Range: 3 to 7 years) | (Range: 2.5 to 9.5 years) | |
| Body weight (mean ± SD) | 5.7 ± 1.4 kg | 5.5 ± 1 kg |
| (Range: 4.8 to 8.2 kg) | (Range: 4.6 to 7.5 kg) | |
| BCS (median) | 7/9 | 8/9 |
| (Distribution: three cats with BCS 7, one cat with BCS 8, one cat with BCS 9) | (Distribution: one cat with BCS 6, one cat with BCS 7, one cat with BCS 8, two cats with BCS 9) | |
| Fecal score (median) | Week 0 2/7 (range, 2/7–3/7), week 8 2/7 (range, 2/7–4/7), week 16 2/7 (range, 2/7–3/7) | Week 0 2/7 (range, 2/7–2/7), week 8 2/7 (range, 2/7–2/7), week 16 3/7 (range, 2/7–3/7) |
| Overweight/Obese | Lean | |
|---|---|---|
| Number | 10 | 10 |
| Sex | ||
| neutered males | 5 | 5 |
| spayed females | 5 | 5 |
| Age (mean ± SD) | 4.2 ± 1.9 years | 1.3 ± 0.08 years |
| (Range: 2.5 to 9.5 years) | (Range: 1.3 to 1.5 years) | |
| Body weight (mean ± SD) | 5.7 ± 1.1 kg | 4.7 ± 0.9 kg |
| (Range: 4.6 to 8.2 kg) | (Range: 3.2 to 5.72 kg) | |
| BCS (median) | 8/9 | 5/9 |
| (Distribution: one cat with BCS 6, four cats with BCS 7, two cats with BCS 8, three cats with BCS 9) | (Distribution: four cats with BCS 4/9 and six cats with BCS 5/9) |
| Phylotypes with Altered Abundances | p/q Value | |
|---|---|---|
| At the species level, abundances of the following bacteria were trending up compared to baseline in the AvX group at the end of the 16-week period in overweight/obese cats (n = 5) At the species level, abundances of the following bacteria were trending down compared to baseline in the AvX group at the end of the 16-week period in overweight/obese cats (n = 5) At the species level, abundances of the following bacteria were trending down in the AvX group compared to the placebo group at week 16. | Dialister sp. Rickettsiella sp. SMB53 Roseburia sp. Blautia producta Helicobacter sp. Vibrio sp. Acidaminococcus sp. Akkermansia sp. Adlercreutzia sp. Collinsella aerofaciens | (p = 0.04, q = 0.6) (p = 0.02, q = 0.6) (p = 0.02, q = 0.6) (p = 0.02, q = 0.6) (p = 0.04, q = 0.6) (p = 0.001, q = 0.07) (p = 0.04, q = 0.6) (p = 0.01, q = 0.4) (p = 0.01, q = 0.4) (p = 0.02, q = 0.4) (p = 0.02, q = 0.4) |
| Phylotypes with Altered Abundances | p/q Value | |
|---|---|---|
| At the species level, abundances of the following bacteria were significantly higher in lean cats (n = 10) At the species level, abundances of the following bacteria were significantly higher in overweight/obese cats (n = 10) | Prevotella sp. Turicibacter sp. Clostridium sp. Veillonella sp. Dialister sp. Catenibacterium sp. Eubacterium biforme Desulfovibrio sp. Campylobacter sp. Coriobacterium sp. Ruminococcus gnavus | (p = 0.001, q = 0.01) (p = 0.003, q = 0.02) (p = 0.003, q = 0.02) (p = 0.01, q = 0.04) (p = 0.001, q = 0.02) (p = 0.002, q = 0.02) (p = 0.01, q = 0.04) (p = 0.001, q = 0.01) (p = 0.001, q = 0.01) (p = 0.004, q = 0.03) (p = 0.001, q = 0.01) |
| Enriched Phylotypes | LDA Score | |
|---|---|---|
| Study 1, phylotypes differentially enriched in AvX compared to the placebo group (week 16) Study 2, naturally lean cats Study 2, naturally overweight/obese cats | Dialister Rickettsiella Dialister Prevotella Ruminococcus Campylobacter Catenibacterium Clostridium Helicobacter Eubacterium Pseudoramibacter Veillonella S247 Turicibacter Phascolarctobacterium Coriobacterium Enterococcus | (LDA score > 3) (LDA score > 3) (LDA score > 3) (LDA score > 3) (LDA score > 3) (LDA score > 3) (LDA score > 3) (LDA score > 3) (LDA score > 3) (LDA score > 3) (LDA score > 3) (LDA score > 3) (LDA score > 3) (LDA score > 3) (LDA score = 3) (LDA score > 3) (LDA score > 3) |
| Alpha-Diversity Parameter | p Value | |
|---|---|---|
| Study 1, AvX vs. placebo group at baseline Study 1, AvX vs. placebo group on week 16 Study 1, placebo group over 16-week period Study 1, AvX group over 16-week period Study 2, lean vs. overweight/obese cats | Chao1 Observed ASVs Shannon index Chao1 Observed ASVs Shannon index Chao1 Observed ASVs Shannon index Chao1 Observed ASVs Shannon index Chao1 Observed ASVs Shannon index | p = 1 p = 0.4 p = 0.5 p = 0.8 p = 0.4 p = 0.8 p = 0.8 p = 0.6 p = 0.7 p = 0.8 p = 0.6 p = 0.7 p = 0.4 p = 0.4 p = 0.8 |
| Metabolite | Concentration in AvX Group (Median and Range)) | Concentration in Placebo Group (Median and Range) | p/q Value |
|---|---|---|---|
| Tryptophan Nicotianamine Indole-3-acetate Glycyl-proline | 7449 (2476 to 20,805) 279 (158 to 379) 108,255 (78,679 to 146,151) 8826 (1251 to 16,732) | 26,731 (24,431 to 62,562) 1251 (609 to 2157) 44,404 (7841 to 59,547) 1310 (418 to 15,538) | p = 0.0006, q = 0.02 p = 0.002, q = 0.04 p = 0.0004, q = 0.02 p = 0.0003, q = 0.02 |
| Metabolite | Bacterial Taxa | r Value | p Value |
|---|---|---|---|
| Bacterial taxa significantly positively correlated with tryptophan Bacterial taxa significantly negatively correlated with tryptophan Bacterial taxa significantly positively correlated with indole-3-acetate Bacterial taxa significantly negatively correlated with indole-3-acetate Bacterial taxa significantly positively correlated with glycyl-proline Bacterial taxa significantly negatively correlated with glycyl-proline Bacterial taxa significantly positively correlated with nicotianamine Bacterial taxa significantly negatively correlated with nicotianamine | Bifidobacterium Eubacterium Blautia Roseburia Veillonella Desulfovibrio Veillonella Eubacterium Lactobacillus Roseburia Mitsuokella Bulleidia Rickettsiella Bacteroides Odoribacter Veillonella Desulfovibrio Bifidobacterium Eubacterium Blautia | 0.9 1 0.9 0.9 −0.9 −0.9 0.9 −0.9 0.9 0.9 0.9 0.9 0.9 −0.9 −0.9 1 0.9 −0.9 −1 −0.9 | p = 0.04 p = 0.005 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.04 p = 0.005 p = 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husnik, R.; Fletcher, J.; Pilla, R.; Ingram, D.; Gaschen, F.; Roth, G.; Chen, C.-C.; Suchodolski, J. Effects of Obesity and Feeding Avocado Extract on Gut Microbiota and Fecal Metabolomic Profile in Overweight/Obese Cats. Microbiol. Res. 2025, 16, 190. https://doi.org/10.3390/microbiolres16080190
Husnik R, Fletcher J, Pilla R, Ingram D, Gaschen F, Roth G, Chen C-C, Suchodolski J. Effects of Obesity and Feeding Avocado Extract on Gut Microbiota and Fecal Metabolomic Profile in Overweight/Obese Cats. Microbiology Research. 2025; 16(8):190. https://doi.org/10.3390/microbiolres16080190
Chicago/Turabian StyleHusnik, Roman, Jon Fletcher, Rachel Pilla, Donald Ingram, Frederic Gaschen, George Roth, Chih-Chun Chen, and Jan Suchodolski. 2025. "Effects of Obesity and Feeding Avocado Extract on Gut Microbiota and Fecal Metabolomic Profile in Overweight/Obese Cats" Microbiology Research 16, no. 8: 190. https://doi.org/10.3390/microbiolres16080190
APA StyleHusnik, R., Fletcher, J., Pilla, R., Ingram, D., Gaschen, F., Roth, G., Chen, C.-C., & Suchodolski, J. (2025). Effects of Obesity and Feeding Avocado Extract on Gut Microbiota and Fecal Metabolomic Profile in Overweight/Obese Cats. Microbiology Research, 16(8), 190. https://doi.org/10.3390/microbiolres16080190







