Abstract
The United States is facing outbreaks of highly pathogenic avian influenza H5N1 in birds and dairy cattle, along with threats of African swine fever, classical swine fever, and foot-and-mouth disease. While the National Animal Health Laboratory Network (NAHLN) depends on high-throughput testing, the KingFisher Duo Prime, IndiMag 48s, and IndiMag 2 are viable alternatives to aid in outbreak assessments. This study evaluates extraction platforms and the IndiMag Pathogen Kit for detecting the previous listed pathogens. Samples and reference materials were extracted using the MagMAX Viral RNA Isolation Kit, MagMAX CORE Nucleic Acid Purification Kit, and IndiMag Pathogen Kit. Real-time RT-PCR was performed following NAHLN protocols to assess analytical and diagnostic performance. Comparable limits of detection across extraction chemistries, instrumentation, and pathogens were demonstrated, with PCR efficiency ranging between 82.5% and 107.6%. The precision variability was low, with the coefficient of variation ranging from 0.16% to 1.76%. Diagnostic sensitivity and specificity were 100%, with a kappa coefficient of 1.0, indicating strong agreement between methods. These findings support the KingFisher Duo Prime, IndiMag 48s, IndiMag 2, and IndiMag Pathogen Kits as reliable options for NAHLN-approved testing, increasing equipment and reagent alternatives to enhance diagnostic resilience and improve response capabilities to emerging animal health threats.
1. Introduction
The United States (U.S.) is currently facing significant outbreaks of the H5N1 strain of highly pathogenic avian influenza (HPAI). Since February 2022, HPAI has been detected in both wild and domestic bird populations across all 50 states, leading to substantial poultry losses and economic impacts [1]. Notably, in 2024, the B3.13 subtype of H5N1 HPAI was identified in U.S. dairy cattle for the first time [2], and the subsequent detection of subtype D1.1 [3] raises concerns about cross-species transmission [4]. Additionally, human cases have emerged, including 70 cases at the time of this manuscript preparation and the first death reported in Louisiana in January 2025 [5]. These concurrent outbreaks underscore the critical need for vigilant surveillance and comprehensive response strategies to mitigate the spread and impact of these viruses on both animal and public health.
African swine fever, classical swine fever, and foot-and-mouth disease are significant foreign animal diseases (FADs) to the United States, with severe economic consequences to the livestock industries [6]. The spread of African swine fever virus (ASFV) to the Dominican Republic in 2021 heightens the potential risk to U.S. domestic and feral swine populations [7]. Classical swine fever virus (CSFV) remains a global concern, with outbreaks reported in regions of Asia, parts of Europe, and South America [8]. The recent detections of foot-and-mouth disease virus (FMDV) in a water buffalo farm in Germany (January 2025), a cattle farm in Hungary (March 2025) and five cattle farms in Slovakia (March 2025) [9]—marked the first significant detections in the European Union since 2011—highlight the need for continued surveillance, early detection, and reliable diagnostic methods to prevent outbreaks.
The continued HPAI outbreak highlights the effective efforts of the National Animal Health Laboratory Network (NAHLN), established in the U.S. in 2002, to coordinate diagnostic testing and respond to U.S. high consequence animal disease outbreaks. The NAHLN’s mission is to enhance the nation’s animal health infrastructure by providing reliable, rapid diagnostic testing, ensuring the early detection of significant animal diseases, and supporting emergency response efforts to protect animal and public health [10]. High-throughput (96-well format) molecular testing solutions are the backbone of high-volume diagnostic and surveillance testing and are approved by the NAHLN. In addition to high-throughput platforms, low- and medium-throughput equipment are required when testing a new suspected outbreak, as only a few samples are submitted, and these samples require immediate results. Magnetic particle processors, such as the KingFisher Duo Prime (KF Duo Prime), IndiMag 48s (IM48s), and IndiMag 2 (IM2), enhance flexibility in laboratory workflows for PCR testing, particularly for low sample volumes in NAHLN assays. Several magnetic-based extraction kits are approved for sample extraction in the NAHLN assays, requiring the reagents to be manually pipetted into the extraction plates for each piece of equipment. The IndiMag Pathogen prefilled extraction is available for the KingFisher 96-well family of extractors, the IM48s and IM2, offering improved workflow efficiency, reduced technical errors, and providing time and cost savings by eliminating reagent-dispensing steps.
At the start of this study in 2022, the approved extraction protocols for influenza A virus (IAV) and avian paramyxovirus type-1 (APMV-1) included TRIzol LS Reagent, RNeasy Kit (spin column-based), and the MagMAX Viral RNA Isolation Kit, and MagMAX Pathogen RNA/DNA Kit on the Biosprint 96, MagMAX Express 96, KingFisher 96, and KingFisher Flex magnetic particle processors. The approved extraction protocols for FADs are the MagMAX Pathogen RNA/DNA Kit, MagMAX CORE Nucleic Acid Purification Kit, and IndiMag Pathogen Kit on the Biosprint 96, MagMAX Express 96, KingFisher 96, and KingFisher Flex magnetic particle processors. The IndiMag Pathogen Kit is NAHLN-approved for FAD detection assays but lacks NAHLN approval for IAV and APMV-1 surveillance testing and was evaluated in this study. Aside from the approved KingFisher 96-well family of extractors, additional low- and medium-throughput nucleic acid extractors and reagents should be suggested to provide reliable alternatives for equipment and reagents, thereby minimizing the risk of supply chain issues and the monopolization of governmental testing. In this study, the KF Duo Prime, IM48s, IM2, and IndiMag Pathogen Kits were investigated as alternate solutions to NAHLN IAV, APMV-1, ASFV, CSFV, and FMDV testing.
2. Materials and Methods
2.1. Field and Reference Samples
For diagnostic sensitivity evaluation, a total of 31 previously positive field samples containing low pathogenic influenza A (LPAI) or APMV-1 viruses were used. These consisted of three tracheal swabs from domestic turkeys, four oropharyngeal swabs from pigeons, and twenty-four pooled oropharyngeal or cloacal swabs from waterfowl. Ten of the positive pooled oropharyngeal or cloacal swabs were spiked into ten negative IAV tissue homogenates from turkeys for testing. Thirty previously IAV and APMV-1 negative field samples (seven tissue homogenates and twenty-three trachea, oropharyngeal, or cloacal swabs) were used for diagnostic specificity evaluation. All samples were dispensed and stored in an ultralow freezer at −80 °C until ready for use. Field samples were not evaluated for the three FADs due to the lack of availability.
Three LPAI and three APMV-1 reference strains were kindly provided by the National Veterinary Service Laboratories (NVSL) Diagnostic Virology Laboratory (DVL), (Ames, IA). The Foreign Animal Disease Diagnostic Laboratory (FADDL) (Plum Island, NY) provided a single synthetic phage construct each for the ASFV, CSFV, and FMDV (Appendix A Table A1) for the analytical sensitivity evaluation. Ten-fold serial dilutions of the references were prepared in preferred media (brain heart infusion (BHI) or tris-buffered tryptose broth (TBTB)), and multiple aliquots were made and stored in an ultralow freezer at −80 °C until they were ready for use.
2.2. Extraction Chemistries and Equipment
The Thermo Fisher SCIENTIFIC MagMAX Viral RNA Isolation Kit (MagMAX Viral) and the MagMAX CORE Nucleic Acid Purification Kit (MagMAX CORE) (Thermo Fisher SCIENTIFIC, Waltham, MA, USA) were the approved extraction chemistries for the IAV and APMV-1 assays at the time of this study [11]. The MagMAX CORE and the INDICAL IndiMag Pathogen Kits (INDICAL BIOSCIENCE, Leipzig, Germany) were approved for FAD testing [12,13]. The study involved comparing the manual fill and prefilled versions of the IndiMag Pathogen Kit to the MagMAX Viral or MagMAX CORE extraction kits.
The Kingfisher Flex (KF Flex) (Thermo Fisher SCIENTIFIC, Waltham, MA, USA) is NAHLN-approved for the extraction of IAV and APMV-1 [11] and FADs [12,13,14]. The KF Duo Prime (Thermo Fisher SCIENTIFIC, Waltham, MA, USA, cat # 5400110), IndiMag 48s (INDICAL BIOSCIENCE, Leipzig, Germany, cat # IN943048s), and IndiMag 2 (INDICAL BIOSCIENCE, Leipzig, Germany, cat # IN950048) were not NAHLN-approved equipment in the previous SOPs at the time of this study and were evaluated as alternative extraction platforms.
For the IAV and APMV-1 extraction evaluation, each of the serial diluted reference strains and samples were extracted using the MagMAX Viral on the KF Flex according to the approved protocol [11], which was considered the gold standard for the performance comparison of extraction chemistry and equipment. The six serially diluted reference strains and samples were each extracted on the KF Flex and IM48s using manual and/or prefilled versions of the IndiMag Pathogen Kits to evaluate the reagents and IM48s equipment performance. Subsequently, the test samples and a single IAV and APMV-1 reference strain were extracted with the IndiMag Pathogen Kit on the IM2 and the MagMAX CORE on the KF Duo Prime to evaluate the equipment performance. The manufacturer’s instructions for the MagMAX Viral, MagMAX CORE, and IndiMag Pathogen Kits were followed, except for a single modification. The MagMAX Viral has a recommended 50 µL sample input, while the MagMAX CORE and IndiMag Pathogen Kits have a recommended 200 µL input sample volume. Thus, a mixture of a 50 µL sample input with 150 µL of phosphate-buffered saline (PBS) was used as starting material for the MagMAX CORE and IndiMag Pathogen Kits for an equivalent input sample volume between the extraction kits.
For the FAD extraction evaluation, each reference construct (200 µL) was extracted using the MagMAX CORE on KF Flex according to the approved protocol [12], which served as the gold standard for comparing the performance of the equipment under evaluation. Each reference construct was also extracted with the IndiMag Pathogen Kit on the IM48s and IM2, and the MagMAX CORE on the KF Duo Prime. Extraction was performed according to the manufacturer’s instructions.
2.3. Polymerase Chain Reactions (PCR)
All IAV and APMV-1 extracts were evaluated using the AgPath-ID One Step PCR reagents (Thermo Fisher SCIENTIFIC, Waltham, MA, USA) on the ABI 7500 with version 2.3 software (Thermo Fisher SCIENTIFIC, Waltham, MA, USA), following the harmonized IAV protocol and the APMV-1 protocol as approved [11]. The current harmonized IAV protocol indicates analysis at a 5% manual threshold value, while the APMV-1 protocol utilizes an auto threshold setting for analyzing results. This study compared data across multiple PCR runs, and the auto threshold fluctuated from run to run as the samples influenced the algorithm in each run. We performed multiple analyses and found that the 5% manual threshold value for IAV runs was within the proximity of 0.2, and a threshold of 0.2 also accurately positioned the assays around the midpoint of the log linear phase for the APMV-1 assays. Based on this observation, all the IAV and APMV-1 PCR runs were normalized with a manual threshold at 0.2 (with auto baseline setting), to allow for the ease of comparison of all data points across the runs.
All the FAD extractions were evaluated using the TaqMan Fast Virus One-Step Master Mix (Thermo Fisher SCIENTIFIC, Waltham, MA, USA) on the ABI 7500 with version 2.3 software, following the approved protocol [15,16]. The FAD pathogen targets and the VetMAX Xeno Internal Positive Control RNA (Xeno RNA) (Thermo Fisher SCIENTIFIC, Waltham, MA, USA) exogenous control were analyzed at the set threshold per protocols (ASFV and FMDV at 0.2, CSFV at 0.05, and Xeno RNA at 0.1).
The NAHLN approved protocols are available online: https://www.aphis.usda.gov/animal_health/lab_info_services/downloads/ApprovedSOPList.pdf (accessed 27 February 2025). The NAHLN program office controls the distribution of protocols and the detailed information in the previously listed protocols and can be requested by emailing NAHLN@usda.gov.
2.4. Analytical Sensitivity
A standard curve was generated using the ten-fold diluted materials for each of the references. The end-point dilution of the reference strains determined the limit of detection (LOD). The PCR efficiency and the coefficient of correlation of the standard curve (R2) were obtained from the standard curve generated by the ABI 7500 software.
The repeatability within a single assay for IAV and APMV-1 was assessed by running both high and low concentrations of the references. The low concentration was prepared at approximately ten times before the concentration of the reported LOD endpoint, and the high concentration was a dilution with a 25–30 cycle threshold (CT) range. Each sample was extracted and subjected to PCR in 5 replicates to assess single-run repeatability. This was repeated one more time (a total of two runs) to evaluate the inter-run repeatability.
2.5. Diagnostic Sensitivity and Specificity
For the IAV and APMV-1 assays, diagnostic sensitivity was evaluated using 31 positive samples (20 LPAI and 11 APMV-1) from waterfowl or domestic poultry. Samples were tested as collected (oropharyngeal, trachea, or cloacal swabs) or spiked into a pooled tissue matrix. Diagnostic specificity was evaluated using 30 negative diagnostic samples as collected (oropharyngeal, trachea, or cloacal swabs and tissues).
2.6. Statistical Analysis
The assay performance was evaluated using the LOD, R2, PCR efficiency, diagnostic sensitivity, and diagnostic specificity. The standard deviation and coefficient of variant (CV) were calculated for assay precision. Standard F-tests or analysis of variance (ANOVA) tests were used to infer statistical significance among various methods. The statistical analysis was computed using the Prism software (version 10.4.1) (GraphPad, Boston, MA, USA). Figures were generated using Tableau software (Public Edition) (Salesforce, San Francisco, CA, USA).
3. Results
This study was divided into sections due to the availability of testing materials and the launch of the IM2. The first study compared the manual fill and prefilled versions of the IndiMag Pathogen Kit on the KF Flex and IM48s to the MagMAX Viral on KF Flex. The LOD was the same across all extraction chemistries and instrumentation (Table 1, Supplemental Data in Table S1, LOD plots in Appendix A Figure A1). When LOD differences occurred, the last CT value did not represent a ten-fold dilution and was deemed non-repeatable. The R2 values ranged from 0.910 to 1.000 across the six references and instruments. The PCR efficiency ranged between 82.5% and 107.6%, with the lowest value occurring with the prefilled IndiMag Pathogen Kit on the IM48s and the highest value occurring with the MagMAX Viral on the KF Flex.

Table 1.
The influenza A virus (IAV) and avian paramyxovirus type-1 (APMV-1) limit of detection, coefficient of correlation of the standard curve (R2), and percent PCR efficiency for extraction with the manual fill and prefilled versions of the IndiMag Pathogen Kit on the KingFisher (KF) Flex and IndiMag 48s (IM48s), the MagMAX CORE Nucleic Acid Purification Kit (MagMAX CORE) on KF Duo Prime, and the IndiMag Pathogen Kit on the IndiMag 2 (IM2) compared to the MagMAX Viral RNA Isolation Kit (MagMAX Viral) on KF Flex.
The CT values of the manual fill and prefilled versions of the IndiMag Pathogen Kit on the KF Flex and IM48s for each dilution of the individual reference were averaged and compared to the MagMAX Viral on the KF Flex to investigate CT value differences in the format of the IndiMag Pathogen Kit (Figure 1a). Both the manual fill and prefilled versions of the IndiMag Pathogen Kit have the same dynamic range as the NAHLN-approved MagMAX Viral. The mean CT value differences between the manual fill and prefilled versions of the IndiMag Pathogen Kit to MagMAX Viral on the KF Flex were−0.06 and −0.33, respectively. The standard error of the means (SEM) was −0.2691 ± 1.391, indicating no significant difference between the formats of the IndiMag Pathogen Kit (F-test, p = 0.9061).
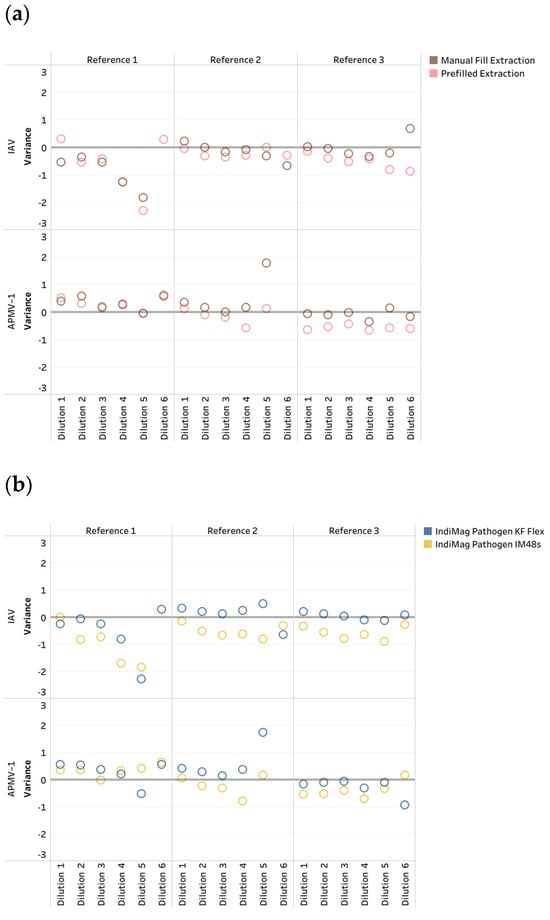
Figure 1.
The influenza A virus (IAV) and avian paramyxovirus type-1 (APMV-1) CT variation across dilution levels of references. (a) Comparison of the manual and prefilled IndiMag Pathogen Kits versus the MagMAX Viral RNA Kit (MagMAX Viral); (b) comparison of the IndiMag Pathogen Kit using the KingFisher (KF) Flex and IndiMag 48s (IM48s) versus the MagMAX Viral on the KF Flex.
Next, the CT values of the KF Flex and IM48s for the two versions of the IndiMag Pathogen Kit for each dilution of the individual reference were averaged and compared to the MagMAX Viral on the KF Flex to investigate differences in instrumentation (Figure 1b). The mean CT value differences between the KF Flex and IM48s with the two versions of the IndiMag Pathogen Kit to the MagMAX Viral on the KF Flex were 0.00 and −0.39, respectively. The SEM was −0.3932 ± 1.391, with no significant difference observed using the IndiMag Pathogen Kit on the KF Flex and IM48s (F-test, p = 0.9269).
Subsequently, both versions of the IndiMag Pathogen Kit using the KF Flex and IM48s became approved by NAHLN for the IAV and APMV-1 assays during the study. Thus, only a single IAV and APMV-1 reference strain (per NAHLN requirements) was evaluated using the IndiMag Pathogen Kit on the IM2 and using the MagMAX CORE on the KF Duo Prime as a verification of these instruments. Similar to the previous LOD results, the last CT value did not represent a ten-fold dilution and was deemed non-repeatable (Table 1, also Supplemental Data in Table S2, LOD plots in Appendix A Figure A1). The R2 ranged between 0.995 and 1.00 across the two reference strains and two instruments. The PCR efficiency ranged between 96.62% and 104.4%, with the lowest value occurring with the IndiMag Pathogen Kit on the IM2 and the highest value occurring with the MagMAX CORE on the KF Duo Prime. All three of the low- and medium-throughput instruments have the same dynamic range as the NAHLN-approved high-throughput KF Flex. The CT value differences between the IM2 using the IndiMag Pathogen Kit and the KF Duo Prime using the MagMAX CORE, compared to the MagMAX Viral on the KF Flex, were 0.91 and 0.60, respectively. When the average CT values for all four new chemistry/instrument combinations were compared to the MagMAX Viral on the KF Flex (Figure 2), the SEM was 0.6249 ± 1.405, 0.2958 ± 1.405, 1.852 ± 1.958 and 1.361 ± 1.901 for the IndiMag Pathogen Kit on the KF Flex, IM48s, IM2, and the MagMAX CORE on the KF Duo Prime, respectively, with a lack of significant differences (ANOVA test, p = 0.8738) observed.
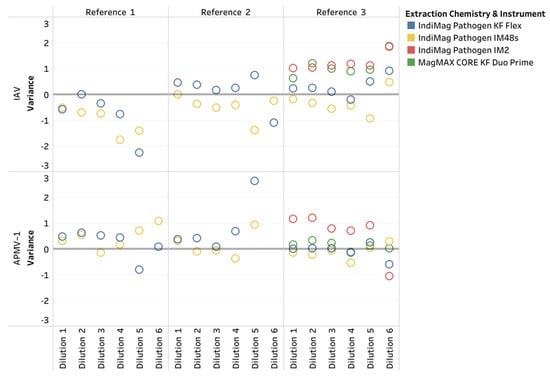
Figure 2.
The influenza A virus (IAV) and avian paramyxovirus type-1 (APMV-1) CT variations across dilution levels for the IndiMag Pathogen Kit, using the KingFisher (KF) Flex, IndiMag 48s (IM48s), and IndiMag 2 (IM2), and the MagMAX CORE Nucleic Acid Purification Kit (MagMAX CORE) using the KF Duo Prime, compared to the MagMAX Viral RNA Kit (MagMAX Viral) using the KF Flex.
The precision was evaluated using the IndiMag Pathogen Kit on the KF Flex, IM48s, and IM2, as well as using the MagMAX CORE on the KF Duo Prime. Five replicates at high and low concentrations for both IAV and APMV-1 were run on different days, totaling 10 replicates at high and low concentrations for IAV and APMV-1 for each instrument (Figure 3, Supplemental Data Table S3). The average CT values for the high and low concentrations of IAV were 26.42 and 33.22, respectively. The average inter-run standard deviations for the high and low concentrations of IAV were 0.16 and 0.45, respectively. The coefficient of variation (CV) ranged between 0.16% and 1.76% for IAV. The average CT values for the high and low concentrations of APMV-1 were 27.08 and 33.72, respectively. The average inter-run standard deviations for the high and low concentrations of APMV-1 were 0.18 and 0.42, respectively. The CV ranged between 0.50% and 1.52% for APMV-1.
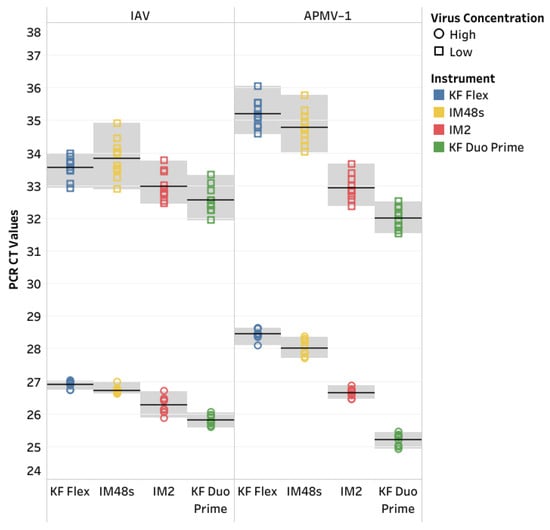
Figure 3.
Precision for the influenza A virus (IAV) and avian paramyxovirus type-1 (APMV-1) 1 reference strains was determined using the IndiMag Pathogen Kit with the KingFisher (KF) Flex, IndiMag 48s (IM48s), and IndiMag 2 (IM2), as well as the MagMAX CORE Nucleic Acid Purification Kit (MagMAX CORE) with the KF Duo Prime, using varying concentrations on each instrument.
A total of 32 positive and 30 negative samples were tested to evaluate the diagnostic sensitivity and specificity of the IndiMag Pathogen Kit on the KF Flex, IM48s, and IM2, as well as the MagMAX CORE on the KF Duo Prime, compared to the MagMAX Viral on the KF Flex (Figure 4, Supplemental Data in Table S4). The kappa coefficient was k = 1.0, indicating 100% agreement for both the diagnostic sensitivity and specificity of the extraction chemistry on its respective instrumentation. The average CT value was slightly lower, with a positive skew using the IndiMag Pathogen Kit on the IM2. However, a significant difference was lacking between the average CT values, regardless of the extraction chemistry and instrumentation (ANOVA test, p = 0.8602).
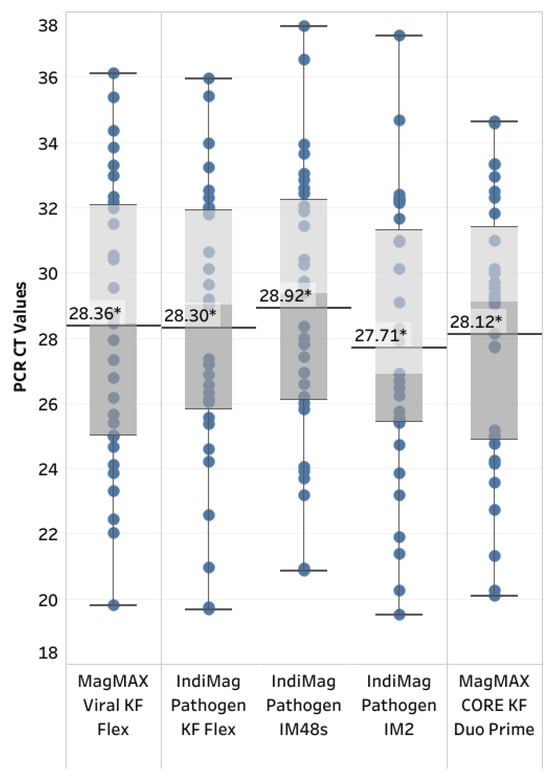
Figure 4.
Box and whisker plot illustrating the diagnostic sensitivity of the IndiMag Pathogen Kit using the KingFisher (KF) Flex, IndiMag 48s (IM48s), and IndiMag 2 (IM2), as well as the MagMAX CORE Nucleic Acid Purification Kit using the KF Duo Prime. The * indicates a lack of significant differences among the average CT values reported in the chart.
Finally, single references for ASFV, CSFV, and FMDV were evaluated using the IndiMag Pathogen Kit on the IM48s and IM2 and the MagMAX CORE on the KF Duo Prime compared to the MagMAX CORE on the KF Flex. Both extraction chemistries were NAHLN-approved for ASFV, CSFV, and FMDV. Similar to the IAV and APMV-1 LOD results, the CT value did not represent a ten-fold dilution when a difference in dilution level occurred and was deemed non-repeatable (Table 2, also Supplemental Material of CT values in Table S5, LOD plots in Appendix A Figure A1). The R2 values ranged from 0.986 to 1.000 across the three references and instruments. The PCR efficiency ranged between 95.6% and 104.5%. All three of the low- and medium-throughput instruments have the same dynamic range as the NAHLN-approved high-throughput KF Flex. The CT value difference using the IndiMag Pathogen Kit on the IM48s and IM2, and the MagMAX CORE on the KF Duo Prime, compared to the MagMAX CORE on the KF Flex, was −0.4, 0.44, and 1.32, respectively (Figure 5, Supplemental Material of CT values in Table S5). A significant difference was not observed between the average CT values for the FADs, regardless of the extraction chemistry and instrumentation (ANOVA test, p = 0.8579). The Xeno RNA was included in the ASFV, CSFV, and FMDV PCR assays; the CT value ranges are provided in Figure A1. The CT value differences for Xeno RNA were −1.36, 0.09, and −0.05, respectively, with no significant differences (ANOVA test, p = 0.7777).

Table 2.
Analytical sensitivity is presented, including the limit of detection, correlation coefficient (R2), and percent PCR efficiency, for African swine fever virus (ASFV), classical swine fever virus (CSFV), and foot-and-mouth disease virus (FMDV) with the IndiMag Pathogen Kit on the IndiMag 48s (IM48s) and IndiMag 2 (IM2) and the MagMAX CORE Nucleic Acid Purification Kit (MagMAX CORE) on the KingFisher (KF) Flex and KF Duo Prime.
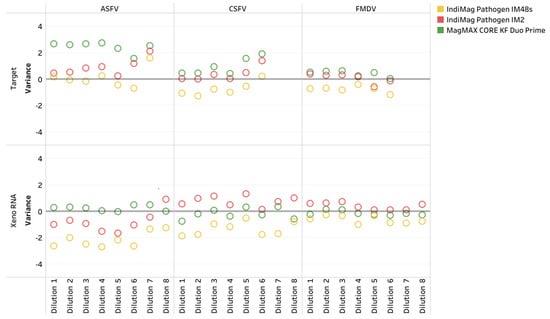
Figure 5.
The African swine fever virus (ASFV), classical swine fever virus (CSFV), and foot-and-mouth disease virus (FMDV), and the Xeno Internal Positive Control RNA (Xeno RNA) CT variations across dilution levels for the IndiMag Pathogen Kit using the IndiMag 48s (IM48s) and IndiMag 2 (IM2), and the MagMAX CORE Nucleic Acid Purification Kit (MagMAX CORE) using the KingFisher (KF) Duo Prime versus the MagMAX CORE using the KF Flex.
4. Discussion
The ongoing U.S. H5N1 HPAI outbreak in wild and domestic bird populations across all 50 states and the detection of the B3.13 and D1.1 subtypes of H5N1 in dairy cattle pose substantial challenges to the U.S. agricultural industries. In addition to the HPAI outbreaks, the U.S. livestock industry faces ongoing threats from FADs, especially with ASFV in the Dominican Republic and the detection of FMDV in Germany, which highlights the critical need for vigilant surveillance, early detection, and robust diagnostic methods to prevent potential U.S. outbreaks, all of which is supported by NAHLN ensuring the nation’s animal health infrastructure and U.S. food supply through reliable and rapid diagnostic testing methods.
High-throughput molecular testing solutions have become essential to handle large volumes of samples during the HPAI outbreak. This study evaluated the performance of the IndiMag Pathogen Kit (both manual fill and prefilled versions) on the KF Flex and IM48s, compared to the NAHLN-approved MagMAX Viral extraction on the KF Flex. Similar to previously reported findings [17], no significant differences were observed between the manual fill and prefilled versions of the IndiMag Pathogen Kit and the MagMAX Viral. The prefilled extraction significantly improves the laboratory workflow in multiple ways. First, the prefilled extraction eliminates the need for numerous pipetting steps, as only the sample and lysis buffer require pipetting. The reduced amount of manual handling leads to a reduced risk of sample contamination, failed extractions, and inaccurate and delayed results due to pipetting errors. Second, reducing the number of pipetting steps reduces the repetitive strain on the technician’s hands, providing an overall better working environment for the laboratory staff [18]. Lastly, prefilled extraction streamlines the workflow and increases efficiency, as technicians save time by not having to fill the extraction plates, resulting in faster results.
Low- and medium-throughput equipment, such as the IM48s, IM2, and KF Duo Prime, provide flexibility for time and cost savings when testing a small number of samples. Previous studies have reported excellent performances of several low- and medium-throughput equipment, including the IndiMag 48, IM48s, and KF Duo Prime [19,20,21,22]. This study evaluated the performance of IM48s, IM2, and KF Duo Prime, which are very useful for providing immediate results in suspected farm breaks, compared to the NAHLN-approved KF Flex. Our results illustrate that these instruments generate comparable results to the KF Flex. Samples from farms suspected of HPAI, APMV-1, and FADs are routinely driven to the laboratory for immediate testing. In HPAI-suspected infections, up to 11 swabs are collected and pooled into a single sample for testing. For ASFV- or CSFV-suspected infections, tissue samples from a few field necropsy animals or a pool of up to five whole blood samples may be submitted, while lesion swabs from a few animals suspected of FMDV infection may be submitted. Testing these small numbers of individual or pooled samples on a 96-well extraction instrument is wasteful of resources due to the cost and plastic waste associated with the unused plastics in the 96-well extraction. In addition, the prefilled IndiMag Pathogen eight-sample cartridge is perfect for up to six samples and two extraction controls. The prefilled IndiMag Pathogen eight-sample cartridge offers time savings by eliminating the need to aliquot four extraction buffers per sample into extraction plates, thereby reducing potential technical errors and improving workflow efficiency. This makes it a valuable addition to NAHLN-approved assays.
Diagnostic sensitivity and specificity assessments using 32 positive and 30 negative samples demonstrated 100% agreement across all extraction chemistries and instruments, with a kappa coefficient of 1.0. Precision evaluations revealed consistent CT values across instruments and extraction methods, with coefficients of variation of less than 3% across different concentrations for the IAV and APMV-1 assays. The average CT values were slightly lower when using the IndiMag Pathogen Kit on the IM2, although the difference was not statistically significant. Similar to previous reports, our data confirmed the reliability of these methods [19,20,21,22]. At the time of manuscript preparation, the manual fill and prefilled version of the IndiMag Pathogen Kit and the IM48s, IM2, and KF Duo Prime platforms were approved by the NAHLN program for use in testing IAV and APMV-1 and have been utilized for research and testing of the current HPAI outbreak [23,24].
One limitation of this study was the inability to use actual samples for the assessment of FADs, which is limited to only reference laboratories and select agent-registered entities. Our study of analytical sensitivity was limited to the use of a phage clone of the PCR target segment of the FADs (provided by the FADDL reference laboratory), and diagnostic sensitivity and specificity were not evaluated using field samples. Currently, the IM48s, IM2, and KF Duo Prime platforms have not been accepted for use in FAD detection protocols. Our preliminary data suggests the suitability of these platforms for consideration, and further validation should be conducted using the actual virus and field samples to complete the validation process.
5. Conclusions
Overall, the study supports the adoption of both versions of the IndiMag Pathogen Kit and the IM48s, IM2, and KF Duo Prime platforms as reliable alternatives for NAHLN diagnostic assays, enhancing flexibility, efficiency, and preparedness for managing current and emerging animal health threats.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres16040080/s1. Table S1. Raw data and statistical analysis for manual fill and prefilled versions of the IndiMag Pathogen Kit versus the MagMAX Viral RNA Isolation Kit (MagMAX Viral). The IndiMag Pathogen Kit utilizes the KingFisher (KF) Flex and IndiMag 48s (IM48s), as opposed to the MagMAX Viral on the KF Flex. Table S2. Raw data and statistical analysis for the IndiMag Pathogen Kit utilizing the KF Flex, IM48s, IM2, and the MagMAX CORE utilizing the KF Duo Prime versus the MagMAX Viral utilizing the KF Flex. Table S3. Raw data and statistical analysis for precision of the IAV and APMV-1 reference strains using the IndiMag Pathogen Kit on the KF Flex, IM48s, and IM2, as well as the MagMAX CORE on the KF Duo Prime. Table S4. Raw data and statistical analysis for the diagnostic sensitivity for the IndiMag Pathogen Kit on the KF Flex, IM48s, and IM2 and using the MagMAX CORE on the KF Duo Prime. Table S5. Raw data and statistical analysis for the IndiMag Pathogen Kit utilizing the IM48s, IM2, and the MagMAX CORE utilizing the KF Duo Prime versus the MagMAX CORE utilizing the KF Flex.
Author Contributions
Conceptualization, D.G.M. and A.L.; methodology, A.L.; software, A.V.-C.; validation, A.V.-C., D.G.M. and A.L.; formal analysis, A.V.-C., D.G.M. and A.L.; investigation, D.G.M. and A.L.; resources, D.G.M. and A.L.; data curation, D.G.M. and A.L.; writing—original draft preparation, A.V.-C., D.G.M. and A.L.; writing—review and editing, A.V.-C., D.G.M. and A.L.; visualization, A.V.-C.; supervision, A.L.; project administration, A.L.; funding acquisition, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
The portion of the research for KingFisher Duo Prime evaluation was funded by the NAHLN Farm Bill, grant number AP21VSD&B000C029 provided by U.S. Department of Agriculture, and the portion for the validation of the IndiMag Pathogen Kit, IndiMag 48s, and IndiMag 2 were funded by INDICAL BIOSCIENCE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data and the supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres16040080/s1.
Acknowledgments
We thank the U.S. Department of Agriculture National Veterinary Services Laboratory for providing reference materials for this study (Diagnostic Virology Laboratory for the IAV and APMV-1 reference strains, and the Foreign Animal Disease Diagnostic Laboratory for the phage constructs for ASFV, CSFV and FMDV); the U.S. Geological Survey National Wildlife Health Center for providing positive field samples; and Joanna Colovas and Eryn Opgenorth from Wisconsin Veterinary Diagnostic Laboratory for their technical assistance.
Conflicts of Interest
The authors (A.V.C. and A.L.) declare no conflicts of interest. D.M. is employed by INDICAL BIOSCIENCE, which partially funded the project, and participates in the design of the study, in the analyses of data, and in the writing of the manuscript.
Abbreviations
The following abbreviations are used in this manuscript:
| ANOVA | Analysis of Variance |
| APMV-1 | Avian paramyxovirus type-1 |
| ASFV | African swine fever virus |
| BHI | Brain heart infusion |
| CSFV | Classical swine fever virus |
| CT | Cycle threshold |
| CV | Correlation of variance |
| FMDV | Foot-and-mouth disease virus |
| FADs | Foreign animal diseases |
| HPAI | Highly Pathogenic Avian Influenza |
| IAV | Influenza A virus |
| IM2 | IndiMag 2 |
| IM48s | IndiMag 48s |
| KF Duo Prime | KingFisher Duo Prime |
| KF Flex | KingFisher Flex |
| LOD | Limit of detection |
| LPAI | Low Pathogenic Avian Influenza |
| MagMAX CORE | MagMAX CORE Nucleic Acid Purification Kit |
| MagMAX Viral | MagMAX Viral RNA Isolation Kit |
| NAHLN | National Animal Health Laboratory Network |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reactions |
| R2 | Coefficient of correlation of the standard curve |
| SEM | Standard error of the mean |
| TBTB | Tris-buffered tryptose broth |
| Xeno RNA | VetMAX Xeno Internal Positive Control RNA |
Appendix A

Table A1.
Information of the reference viruses used for the analytical evaluation.
Table A1.
Information of the reference viruses used for the analytical evaluation.
| Reference Strains | Virus |
|---|---|
| IAV Reference Strain 1 | Influenza A Virus A/Turkey/CA/6889/1980 (H9N2) LPAI |
| IAV Reference Strain 2 | Influenza A Virus A/Turkey/Oregon/1971 (H7N3) LPAI |
| IAV Reference Strain 3 | Influenza A Virus A/Turkey/Wisconsin/1968 (H5N9) LPAI |
| APMV-1 Reference Strain 1 | Newcastle disease virus APMV-1 Texas GB vNDV |
| APMV-1 Reference Strain 2 | Newcastle disease virus Pigeon-44407 strain (APMV-1) |
| APMV-1 Reference Strain 3 | Newcastle disease virus LaSota strain (APMV-1) |
| ASFV Reference Construct | Synthetic phage construct for ASFV PCR assay control |
| CSFV Reference Construct | Synthetic phage construct for CSFV PCR assay control |
| FMDV Reference Construct | Synthetic phage construct for FMDV PCR assay control |
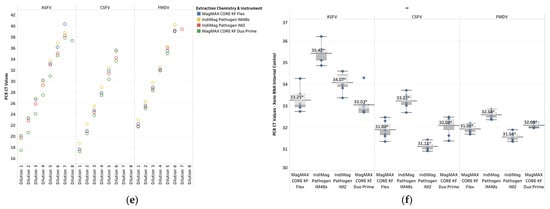
Figure A1.
Standard curves for references and CT values for the VetMAX Xeno Internal Positive Control RNA (Xeno RNA) extracted using various combination of extraction chemistries and instrumentations, the MagMAX Viral RNA Isolation Kit (MagMAX Viral) on the KingFisher (KF) Flex, the IndiMag Pathogen Kit on the KF Flex, IndiMag 48s (IM48s), and IndiMag 2 (IM2), as well as the MagMAX CORE Nucleic Acid Purification Kit (MagMAX CORE) on the KF Duo Prime. (a) Standard curves for influenza A virus (IAV) reference strains extracted using manual fill and prefilled versions of the IndiMag Pathogen Kit; (b) standard curves for avian paramyxovirus type-1 (APMV-1) reference strains extracted using manual fill and prefilled versions of the IndiMag Pathogen Kit; (c) standard curves for IAV reference strains extracted using instrumentations under evaluation; (d) standard curves for APMV-1 reference strains extracted using instrumentations under evaluation; and (e) standard curves for FADs reference constructs; and (f) CT values for Xeno RNA run with FADs assays. The * indicates no significant difference among the average CT values reported in the chart.
References
- Animal and Plant Health Inspection Service|U.S. Department of Agriculture. Detections of Highly Pathogenic Avian Influenza. Available online: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections?utm_source=chatgpt.com (accessed on 27 February 2025).
- Burrough, E.R.; Magstadt, D.R.; Petersen, B.; Timmermans, S.J.; Gauger, P.C.; Zhang, J.; Siepker, C.; Mainenti, M.; Li, G.; Thompson, A.C.; et al. Early Release—Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg. Infect. Dis. 2024, 30, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Animal and Plant Health Inspection Service|U.S. Department of Agriculture. APHIS Confirms D1.1 Genotype in Dairy Cattle in Nevada. Available online: https://www.aphis.usda.gov/news/program-update/aphis-confirms-d11-genotype-dairy-cattle-nevada-0 (accessed on 27 February 2025).
- American Veterinary Medical Association. Avian Influenza Virus Type A (H5N1) in U.S. Dairy Cattle. Available online: https://www.avma.org/resources-tools/animal-health-and-welfare/animal-health/avian-influenza/avian-influenza-virus-type-h5n1-us-dairy-cattle?utm_source=chatgpt.com (accessed on 27 February 2025).
- H5N1 Bird Flu: Current Situation. Avian Influenza (Bird Flu)|CDC. Available online: https://www.cdc.gov/bird-flu/situation-summary/index.html (accessed on 27 February 2025).
- Brown, V.R.; Miller, R.S.; McKee, S.C.; Ernst, K.H.; Didero, N.M.; Maison, R.M.; Grady, M.J.; Shwiff, S.A. Risks of introduction and economic consequences associated with African swine fever, classical swine fever and foot-and-mouth disease: A review of the literature. Transbound. Emerg. Dis. 2021, 68, 1910–1965. [Google Scholar] [CrossRef] [PubMed]
- Animal and Plant Health Inspection Service|U.S. Department of Agriculture. Disease Alert: African Swine Fever. Available online: https://www.aphis.usda.gov/livestock-poultry-disease/swine/african-swine-fever (accessed on 27 February 2025).
- WOAH—World Organisation for Animal Health. Classical Swine Fever. Available online: https://www.woah.org/en/disease/classical-swine-fever/ (accessed on 27 February 2025).
- WOAH—World Organisation for Animal Health. Foot and Mouth Disease (FMD) Official Disease Status. Available online: https://www.woah.org/en/disease/foot-and-mouth-disease/#ui-id-2 (accessed on 31 March 2025).
- Animal and Plant Health Inspection Service|U.S. Department of Agriculture. National Animal Health Laboratory Network. Available online: https://www.aphis.usda.gov/labs/nahln (accessed on 27 February 2025).
- National Animal Health Laboratory Network. Standard operating procedure for Real-time RT-PCR Detection of Influenza A and Avian Paramyxovirus Type-1. (NVSL-SOP-0068). Available online: https://www.aphis.usda.gov/animal_health/lab_info_services/downloads/ApprovedSOPList.pdf (accessed on 27 February 2025).
- National Animal Health Laboratory Network. Standard operating procedure for Nucleic Acid Extraction Using the MagMAX Core Nucleic Acid Purification Kit on a Magnetic Particle Processor. (NVSL-SOP-0643). Available online: https://www.aphis.usda.gov/animal_health/lab_info_services/downloads/ApprovedSOPList.pdf (accessed on 27 February 2025).
- National Animal Health Laboratory Network. Standard operating procedure for Nucleic Acid Extraction Using the INDICAL BIOSCIENCE IndiMag Pathogen Kit on a Magnetic Particle Processor. (NVSL-SOP-0645). Available online: https://www.aphis.usda.gov/animal_health/lab_info_services/downloads/ApprovedSOPList.pdf (accessed on 27 February 2025).
- National Animal Health Laboratory Network. Standard operating procedure for Nucleic Acid Extraction Using the MagMAX Pathogen RNA/DNA Kit on a Magnetic Particle Processor. (NVSL-SOP-0644). Available online: https://www.aphis.usda.gov/animal_health/lab_info_services/downloads/ApprovedSOPList.pdf (accessed on 27 February 2025).
- National Animal Health Laboratory Network. Standard operating procedure for Classical Swine Fever and Foot and Mouth Disease rRT-PCR using the Taqman Fast Virus 1-Step Master Mix on the Applied Biosystems 7500 Real-time PCR System. (NVSL-SOP-0642). Available online: https://www.aphis.usda.gov/animal_health/lab_info_services/downloads/ApprovedSOPList.pdf (accessed on 27 February 2025).
- National Animal Health Laboratory Network. Standard operating procedure for Preparation, Performance, and Interpretation of the African Swine Fever rPCR Assay on the Applied Biosystems 7500 Real-time PCR System. (NVSL-SOP-0646). Available online: https://www.aphis.usda.gov/animal_health/lab_info_services/downloads/ApprovedSOPList.pdf (accessed on 27 February 2025).
- Elnagar, A.; Pikalo, J.; Beer, M.; Blome, S.; Hoffmann, B. Swift and Reliable “Easy Lab” Methods for the Sensitive Molecular Detection of African Swine Fever Virus. Int. J. Mol. Sci. 2021, 22, 2307. [Google Scholar] [CrossRef] [PubMed]
- Caskey, C.R. Ergonomics in the clinical laboratory. Clinical laboratory science. J. Am. Soc. Med. Technol. 1999, 12, 140–144. [Google Scholar]
- Aebischer, A.; Beer, M.; Hoffmann, B. Development and Validation of Rapid Magnetic Particle Based Extraction Protocols. Virol. J. 2014, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Korthase, C.; Elnagar, A.; Beer, M.; Hoffmann, B. Easy Express Extraction (TripleE)—A Universal, Electricity-Free Nucleic Acid Extraction System for the Lab and the Pen. Microorganisms 2022, 10, 1074. [Google Scholar] [CrossRef] [PubMed]
- Dei Giudici, S.; Loi, F.; Ghisu, S.; Angioi, P.P.; Zinellu, S.; Fiori, M.S.; Carusillo, F.; Brundu, D.; Franzoni, G.; Zidda, G.M.; et al. The Long-Jumping of African Swine Fever: First Genotype II Notified in Sardinia, Italy. Viruses 2023, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, K.; Świętoń, E.; Kozak, E.; Wyrostek, K.; Tarasiuk, K.; Styś-Fijoł, N.; Śmietanka, K. Phylogenetic and Molecular Characteristics of Wild Bird-Origin Avian Influenza Viruses Circulating in Poland in 2018−2022: Reassortment, Multiple Introductions, and Wild Bird–Poultry Epidemiological Links. Transbound. Emerg. Dis. 2024, 2024, 6661672. [Google Scholar] [CrossRef]
- Caserta, L.C.; Frye, E.A.; Butt, S.L.; Laverack, M.; Nooruzzaman, M.; Covaleda, L.M.; Thompson, A.C.; Koscielny, M.P.; Cronk, B.; Johnson, A.; et al. Spillover of Highly Pathogenic Avian Influenza H5N1 Virus to Dairy Cattle. Nature 2024, 634, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Nooruzzaman, M.; Covaleda, L.M.; Martin, N.H.; Koebel, K.; Ivanek, R.; Alcaine, S.D.; Diel, D.G. Thermal Inactivation Spectrum of Influenza a H5N1 Virus in Raw Milk. bioRxiv 2024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).