Abstract
Carbapenemase-producing Klebsiella pneumoniae is responsible for multiple serious infections with high mortality rates. K. pneumoniae carbapenemases (KPCs) are the most commonly isolated carbapenemases worldwide. To study the epidemiological and molecular characteristics of KPC-producing K. pneumoniae (KPC-KP), we conducted a retrospective study at the University General Hospital of Ioannina, Greece. A total of 177 K. pneumoniae clinical strains from the period 2014–2015 were confirmed as KPC producers by polymerase chain reaction (PCR) and were further examined for the presence of blaVIM, blaNDM, blaTEM, blaSHV, and blaCTX-M genes. Using the amplification refractory mutation system (ARMS) method, we identified the presence of the KPC-2 allele in 130 strains and the KPC-9 allele in 47. Strains from both allele groups belonged to the sequence type 258 (ST258). KPC-9 was responsible for a distinct outbreak, considered part of the broader KPC-2 outbreak. Molecular characterization of selected KPC-KP isolates from the period 2021–2022 revealed their continued presence in our hospital. Comparison of the antimicrobial susceptibility profiles of the two alleles showed a statistically significant increase in minimum inhibitory concentration (MIC) for ceftazidime (p = 0.03) and higher resistance to amikacin (p = 0.012) and colistin (p < 0.001) for KPC-9 compared to the KPC-2 allele. The two KPC alleles had similar mortality rates. This study demonstrates the heterogeneity of resistance genes in carbapenem-resistant K. pneumoniae (CR-KP) within a single-hospital setting and underscores the need for immediate containment measures.
1. Introduction
The increasing prevalence of multidrug resistance among bacteria responsible for serious infections has become a major global concern [1]. The widespread use of carbapenems has significantly contributed to a rise in carbapenem-resistant Enterobacterales infections, posing a substantial public health challenge [2]. Klebsiella pneumoniae is capable of producing carbapenemases, periplasmic enzymes that efficiently hydrolyze beta-lactam antibiotics, including carbapenems [3]. This capability has greatly restricted the therapeutic options available for treating infections caused by carbapenem-resistant K. pneumoniae (CR-KP), leading to increased mortality rates [1,4].
K. pneumoniae carbapenemases (KPCs) are class A carbapenemase enzymes with a serine residue at their active site [3]. They are the most commonly isolated carbapenemases worldwide, exhibiting high-level resistance to penicillins and cephalosporins, as well as variable resistance to carbapenems [5]. Interestingly, the blaKPC gene is located on self-conjugative plasmids, often accompanied by genetic elements conferring resistance to other groups of antibiotics such as aminoglycosides, trimethoprim, sulfonamides, quinolones, and tetracyclines [6]. CR-KP infections are currently treated with tigecycline, aminoglycosides, polymyxins, and fosfomycin. However, the effectiveness of the existing treatments for infections caused by KPC-producing K. pneumoniae (KPC-KP) appears to be compromised by their increasing resistance to polymyxin and tigecycline [7]. To strengthen the antimicrobial drug arsenal, the European Medicines Agency approved the clinical use of ceftazidime/avibactam, meropenem/vaborbactam, and cefiderocol in 2016, 2018, and 2020, respectively [7].
Within a few years of the identification of the first KPC-KP strain, several outbreaks caused by KPC-KP were reported, initially in the United States and Israel, and later in many European countries and South America [8,9]. The subsequent spread of KPC-KP led to its endemicity in Greece, Italy, China, the Middle East, as well as North and South America, making it the most prevalent type of CR-KP [10]. To date, more than 200 different KPC alleles have been identified, with KPC-2 and KPC-3 being the most common [11]. In European countries and the United States, the spread of KPC is mainly associated with the ST258 clone, whereas in Asia—particularly China and Taiwan—the ST11 clone is the most prevalent clone [12]. The rapid and efficient dissemination of blaKPC is attributed to its association with the Tn4401 transposon, which has the ability to integrate into a variety of plasmids of non-clonal species [12,13]. Moreover, global concern regarding the dissemination of KPC alleles is heightened by the fact that they have also been detected in bacterial species other than K. pneumoniae. A wide range of Gram-negative pathogens—including Escherichia coli, Enterobacter spp., Citrobacter spp., Serratia spp., Proteus spp., Morganella spp., and even non-fermenting Gram-negative bacteria such as Pseudomonas aeruginosa and Acinetobacter spp.—have been recognized as KPC carriers [14].
Given their known transmissibility, resistance to antibiotics and the impact of KPC-KP infections on health care systems, high vigilance and thorough investigation of KPC-KP cases are required for the identification of new variants associated with outbreaks. Recently, we identified a KPC-9-producing Klebsiella pneumoniae (KPC-9-KP) ST258 cluster closely related to the current KPC-2 outbreak in Greece [15]. KPC-9-KP was detected in clinical isolates from patients in the hematology ward and represents the first reported outbreak of KPC-9 in Greece. To our knowledge, a previous case report from Israel was the first report of KPC-9 [16]. Here, we describe the characteristics of KPC-KP infections, highlighting the outbreak caused by KPC-9-KP at the settings of our hospital during a two-year study period.
2. Materials and Methods
The study was conducted at the University General Hospital of Ioannina, an 845-bed tertiary care hospital located in Ioannina, the capital and largest city of the Epirus region, in northwestern Greece. The isolates were evaluated after two distinct referrals. From January 2014 to December 2015, K. pneumoniae strains isolated from clinical samples and exhibiting resistance to at least one carbapenem according to updated Clinical And Laboratory Standards Institute (CLSI) criteria [17], were phenotypically screened for carbapenemase production. The screening process included the use of the modified Hodge test (MHT) to detect carbapenemase activity due to its excellent sensitivity for detecting enterobacterial isolates producing KPC [18]. The combined phenylboronic acid (PBA) and ethylenediaminetetraacetic acid (EDTA) zone inhibition enhancement double disc test, with meropenem as the substrate [19], was simultaneously performed. During patient hospitalization, KPC-KP isolates with identical antimicrobial susceptibility profiles obtained either from different infection sites or from the same site with an interval between isolations of less than two weeks were counted only once. All K. pneumoniae isolates positive for KPC carbapenemase production were included in the study and subsequently subjected to molecular testing. Although the majority of the isolates were KPC-2 producers, there were isolates with different or unusual KPC variant and subsequently the study was extended to a recent KPC study (2021–2022) intended to clarify whether the presence of this type continues. Due to the retrospective and non-interventional nature of the study, informed consent was not required. The scientific ethics committee approved that the data collected will be recorded anonymously, strictly adhering to the applicable national and international legislation for the protection of the participants’ personal data, in accordance with the principles of ethics and research ethics. Information on patients’ demographic characteristics, hospital course, clinical diagnosis, medical history, comorbidities, type of infection, isolation site, prior hospitalizations, transfer to other hospital wards and hospitalization outcomes were obtained from patients’ electronic medical records.
Bacterial identification and determination of minimum inhibitory concentrations (MICs) were performed using the VITEK 2 automated identification system (bioMérieux, Marcy-l’Étoile, France). MICs for tigecycline were re-evaluated using the Etest (bioMérieux), while those for colistin were confirmed by broth microdilution (ComASP® Colistin, Liofilchem, Italy). MICs for tigecycline and colistin were interpreted according to U.S. Food and Drug Administration (FDA) recommendations (susceptible, ≤2 mg/L; resistant, ≥8 mg/L) [20] and European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (susceptible, ≤2 mg/L; resistant, >2 mg/L), respectively [21]. Susceptibility to other antimicrobials was determined according to the updated CLSI criteria [17]. An isolate was considered non-susceptible to an antimicrobial agent when it tested resistant or intermediate using clinical breakpoints as interpretive criteria, provided by CLSI [22]. Screening for class A and B carbapenemases was conducted using the MHT and combined PBA and EDTA double disc test [18,19] as previously described. Extended-spectrum β-lactamase (ESBL) co-production was assessed using a modified CLSI ESBL combined-disc test [17,23].
Total DNA from the clinical isolates was extracted by the boiling method [24]. Polymerase chain reactions (PCRs) with specific primers were used to detect genes encoding carbapenemases (blaKPC, blaNDM, blaVIM) [25,26,27], as well as extended-spectrum β-lactamases (ESBLs) (blaSHV, blaTEM, blaCTX-M group 1) [28]. The reactions contained 100 ng DNA, 1 x HotStarTaq PCR buffer [Tris pH 8.7, KCl,, (NH4)2SO4], 1.5 mM MgCl2, 200 μM dNTP, 40 pmol of each specific primer, and 1U of HotStarTaq DNA polymerase (Qiagen, Germatown, USA) in a final volume of 50 μL. The amplification protocol consisted of an initial denaturation step at 95 °C for 5 min followed by 35 cycles of 94 °C for 45 s, 55 °C for 45 s, 72 °C for 1 min and a final extension step at 72 °C for 10 min. All PCR reactions were performed in a Pelter Thermal Cycler (PTC-200, MJ Research, Inc., Waltham, MA, USA) and the products were analyzed by electrophoresis on a 2% (wt/vol) agarose gel in 1 x TBE buffer stained with ethidium bromide (10 μΜ) and visualized under UV light. A 100bp DNA ladder (Invitrogen, ThermoFisher Scientific Baltics UAB, Vilnius, Lithuania) was used for the size estimation of each amplicon.
In those isolates where blaKPC was detected, a second PCR-based technique, the amplification refractory mutation system (ARMS) method, was applied to distinguish the KPC-9 allele from other KPC alleles. The KPC-9-ARMS-specific PCR was performed according to the protocol described elsewhere [29].
Sequence type (ST) identification by multi-locus sequence typing (MLST) was performed on a representative subset of K. pneumoniae isolates. Thirty-two isolates from the 2014–2015 period of study were selected based on their year of isolation, disease type, and the type of bla genes expressed. Additionally, as part of monitoring the continued emergence of KPC-9 strains, 16 randomly selected KPC-KP strains from the years 2021–2022 were further tested to determine KPC variant and sequence type. Amplicons of seven housekeeping genes (gapA, infB, mdh, pgi, phoE, rpoB, and tonB) were generated by PCR following a previously described protocol [30]. PCR products were purified using the NucleoSpin Extract II kit (Macherey-Nagel, Düren, Germany) and sequenced on an ABI PRISM 3130 Genetic Analyzer (Applied Biosystems Japan Ltd., Tokyo, Japan). The resulting sequences were compared with the MLST database for K.pneumoniae (https://bigsdb.pasteur.fr/klebsiella/) (accessed on 12 December 2022).
The chi-square test or Fisher’s exact test was used to compare categorical variables. The 30-day survival functions of patients carrying the KPC-2 and the KPC-9 strains were illustrated graphically using the Kaplan–Meier method and compared using the log-rank test. The starting point (time of origin) was defined as the day of the first positive culture of the KPC strain. The event of interest was death, with censoring applied at the end of follow-up (hospital discharge or Day 30, whichever occurred first). Data analysis was performed using SPSS v.27 (IBM) statistical software. Statistical significance was set at p < 0.05.
3. Results
3.1. Bacterial Isolates
Over the two-year study period (2014–2015), 306 CR-KP clinical isolates were detected. Following initial screening with phenotypic assays and molecular detection of blaKPC gene, 177 of them were found to produce carbapenemase class A, primarily originating from hospitalized patients (92%), with a distribution of 54.8% in 2014 and 45.2% in 2015. The majority of clinical strains were isolated from urine (49.7%, 88/177) followed by blood (14.7%, 26/177), bronchial secretion/BAL (13.5%, 24/177), trauma/pus (10.2%, 18/177), central venous catheter (7.9%, 14/177), surveillance samples (2.8%, 5/177), pleural fluid (0.6%, 1/177), and female genital tract specimen (0.6%, 1/177).
All 177 isolates were PCR confirmed for the presence of the blaKPC gene. Among these, 47 (26.6%) K. pneumoniae strains were found to harbor KPC-9 carbapenemase, as determined by ARMS-PCR results, while the remaining strains belonged to the KPC-2-like group. Additionally, 5 out of 16 KPC-KP strains originated from the 2021–2022 study period identified as KPC-9 (31.25%), highlighting that the KPC-9 variant does not appear to have been transient. MLST comparison classified all blaKPC-2 and blaKPC-9 strains to ST258.
Genotypic characterization also revealed the presence of both blaKPC and blaNDM genes in only one strain. The majority of KPC strains co-harbored the blaTEM gene (79.1%), followed by the blaSHV gene (28.2%) and the blaCTX-M group 1 gene (10.7%). Among the combinations of ESBL genes, blaTEM/blaSHV, and blaCTX-M group 1/blaTEM were the most frequently observed, while the combination blaCTX-M group 1/blaTEM/blaSHV was detected in only 2.4% of KPC strains. The distribution of β-lactamase genes tested within the KPC-2, and KPC-9 groups is shown in Table 1. KPC-9 strains almost exclusively co-harbored the blaTEM gene (93.6%) demonstrating a statistically significant difference compared to the KPC-2 strains (x2 = 8.161, p = 0.003).

Table 1.
Distribution of carbapenemase genes (blaNDM, blaVIM) and extended-spectrum β-lactamase (ESBL) genes (blaTEM, blaCTX-M group 1, blaSHV) detected in KPC-2 and KPC-9 carriers by PCR.
3.2. Clinical Characteristics of the Patients and Antibiotic Susceptibility of KPC-2 and KPC-9
The clinical and demographic characteristics of hospitalized patients (N = 149) infected with KPC strains are summarized in Table 2. Significant percentage of patients (24.1%) were hospitalized in Ιntensive Care Units (ICUs). KPC-9 strains (N = 47) were isolated from 40 hospitalized patients and one outpatient. Detailed demographic and clinical data for these patients are provided in the Supplementary Materials Table S1.

Table 2.
Demographic and clinical data of the hospitalized patients colonized/infected with KPC strains (N = 149).
All KPC-KP strains were non-susceptible to all available beta-lactam antibiotics and their combinations with beta-lactamase inhibitors and aztreonam. In addition, KPC strains were also tested for susceptibility to a range of non-beta-lactam antibiotics as shown in Figure 1.
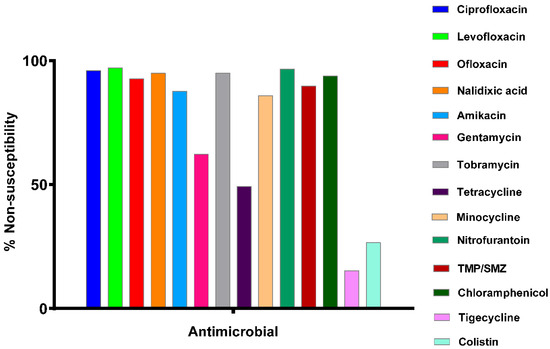
Figure 1.
Non-susceptibility profile of 177 KPC-producing K. pneumoniae (KPC-KP) strains to non-beta-lactam antibiotics and combinations (TMP/SMZ = trimethoprim/sulfamethoxazole). On the y-axis, the term “non-susceptibility” includes both resistant and intermediate isolates.
According to the results in Figure 1, these strains exhibit high resistance (>90%) to quinolones, nitrofurantoin, and trimethoprim/sulfamethoxazole combination. On the other hand, the KPC strains exhibit moderate sensitivity to gentamicin (38.8%) while showing lower sensitivity to the remaining aminoglycosides (amikacin: 12.4% and tobramycin: 5.1%). Also, high sensitivity is observed for tigecycline (84.8%) and colistin (73.4%).
The MIC distributions and cumulative percentages of KPC-2 and KPC-9 isolates for selected antimicrobials are shown in Figure 2 (detailed MIC data are provided in Supplementary Materials Table S2). Among the antibiotics tested, ceftazidime was the only one with a statistically significant different MIC for KPC-2 compared to KPC-9 (x2 = 8.614, p = 0.03). Additionally, the two alleles showed significant differences in the cumulative percentages (susceptible, non-susceptible) for amikacin and colistin (Figure 3).
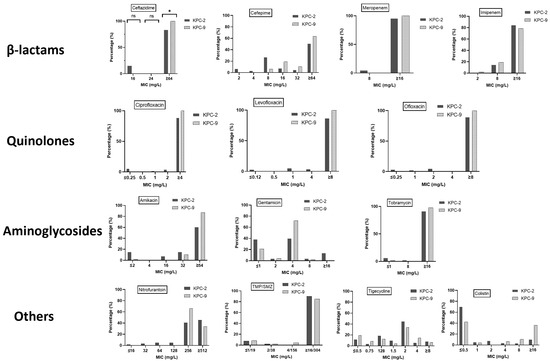
Figure 2.
Minimum inhibitory concentration (MIC) distributions and cumulative percentage of KPC-2 and KPC-9 isolates inhibited by medically important antimicrobial agents (TMP/SMZ = trimethoprim/sulfamethoxazole). Significance in the diagrams is indicated using asterisks (ns = non-significant, * = p < 0.05).
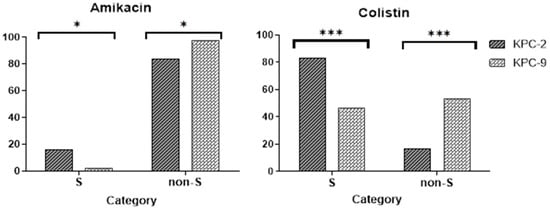
Figure 3.
Histograms showing the statistically significant differences (chi-square test) in the percentage of KPC strains being either susceptible (S) or non-susceptible (non-S) to amikacin and colistin. Significance in the diagrams is indicated using asterisks (* = p < 0.05; *** = p < 0.001).
The susceptibility of all KPC strains to tigecycline increased from 2014 to 2015 (x2 = 18.369, p < 0.001). Specifically, the susceptibility of KPC-2 strains was reduced for tetracycline (61.8% vs. 40.7%, x2 = 5.644, p = 0.021) and increased for tigecycline (78.9% vs. 98.1%, x2 = 10.239, p = 0.001). In contrast, KPC-9 strains were found to be less susceptible to gentamicin (42.9% vs. 11.5%, x2 = 5.993, p = 0.02) and more susceptible to tigecycline (57.1% vs. 96.2%, x2 = 10.555, p = 0.03). No significant change in susceptibility was observed for the remaining antimicrobials tested.
3.3. Outbreak Description
An outbreak of KPC-9-KP, as part of an ongoing KPC-2 outbreak in our hospital, was recorded. During the study period (2014–2015), 47 K. pneumoniae isolates harboring the KPC-9 carbapenemase were obtained from 41 patients. The time course of the isolation of KPC-9 strains and the distribution of patients between ICUs and non-ICU wards are shown in Figure 4a,b, respectively.
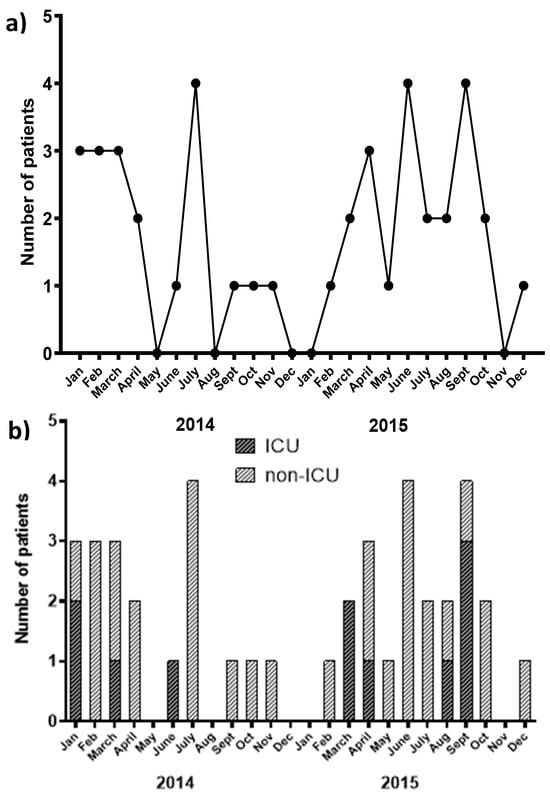
Figure 4.
(a) Graph of the KPC-9 outbreak time course [number of KPC-9-producing Klebsiella pneumoniae (KPC-9-KP) positive patients vs. time]. (b) Chart showing the number of patients carrying a KPC-9 strain hospitalized in ICU or other hospital ward during the time period of the study.
As can be deduced from the outbreak time course, KPC-9-KP cases occurred in two distinct waves, the first wave spanning from January 2014 to November 2014 and the second from February 2015 to December 2015 (Figure 4a). The first wave began with two isolates recovered from the ICU, one with a history of transfer to the neurology and pulmonary ward. During this wave, an apparent dissemination to wards other than ICUs was observed (Figure 4b). It is noteworthy that the first wave included a small cluster of isolates (five of the seven recorded cases) retrieved from the hematology department. The second wave started with a patient from the neurosurgical ward on day 32 of hospitalization, followed by two patients hospitalized in the ICUs. The second wave continued with sporadic cases in other hospital wards.
Some epidemiological data support the intra-hospital dissemination of the KPC-9 outbreak strain. Firstly, there is a significant number (N = 23) of cases with a history of transfer between ICUs, neurology and neurosurgical wards, as well as the physical medicine and rehabilitation center. Secondly, a notable cluster of cases was identified in the hematology ward (7 out of the total 47 cases), while the majority of the patients (61.7%, 29/47) involved in the outbreak developed infection/colonization with the KPC-9 strain more than two days after admission. Furthermore, six cases involved patients with chronic diseases and multiple hospital admissions during the study period.
3.4. Analysis of Mortality Rate in Relation to KPC Allele
The total all-cause mortality among patients with an isolation of KPC-KP was 39.6%, escalating to 61.5% in the case of bloodstream infection. The mean time to death was approximately 25 ± 3 days, whereas 18% of patients died within 14 days of KPC strain isolation. Interestingly, 78% of the patients who died had been transferred to the ICU during their hospitalization. Figure 5 illustrates the 30-day cumulative survival of patients carrying KPC-2 versus those carrying the KPC-9 strain. No significant difference was found between the 30-day cumulative survival functions of KPC-2 vs. KPC-9 carriers (x2 = 0.023, p = 0.880).
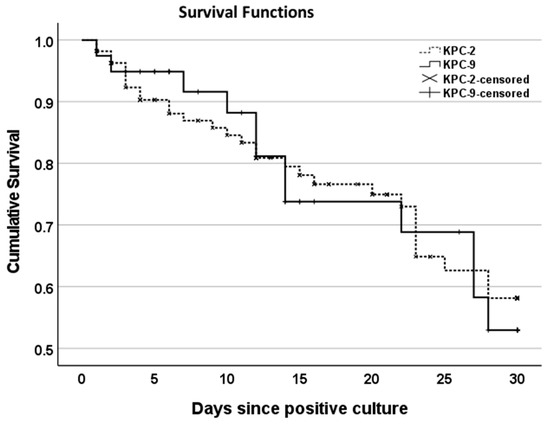
Figure 5.
Kaplan–Meier curves showing the 30-day cumulative survival of patients with isolated strains of KPC-2-KP vs. KPC-9-KP. Censoring has been applied at the end of follow-up period defined as hospital discharge or Day 30 (whichever occurred first).
4. Discussion
In this study, we report the dissemination of a KPC-9-KP outbreak strain, which appears to have emerged as part of a prolonged KPC-2 outbreak. Τhe persistence of this outbreak until recently is suggested by the identification of selected isolates from the 2021–2022 period that also belonged to the ST258 clone. Among the known KPC alleles, KPC-2 is predominantly endemic in Greece [31]. The KPC-9 variant was first reported in Israel in 2012 and was found to differ from KPC-3, the epidemic clone in the country at the time, by a single amino acid substitution. Both variants were typed as ST258 [16]. The first report of the KPC-9 variant in Greece came from clinical isolates in our hospital during the 2014–2015 period [15]. To gain insight into the characteristics of the KPC-KP outbreak during this period, we isolated a total of 177 KPC strains, of which 130 (73.4%) belonged to the KPC-2 variant and 47 (26.6%) belonged to the KPC-9 variant. The dissemination of KPC-9-KP was significant in our hospital during this two-year period, occurring in two outbreak waves. Given that 31.25% of the selected strains tested in the most recent period (2021–2022) demonstrated KPC-9 persistence at a rate comparable to the prior period, its presence does not appear to be temporary. MLST analysis classified all isolates in this study to the same sequence type (ST258). This, along with additional findings as the intra-hospital patient’s transfer, the identification of a cluster of isolates in the hematology ward during the first outbreak wave, and the post-admission isolation of KPC-9-KP in the majority of cases, strongly supports intra-hospital transmission of KPC-9-KP. Furthermore, as previously shown by core genome MLST (cgMLST), the KPC-9-KP cluster differed from KPC-2-KP by only 15 allelic mismatches while sharing an identical capsular type and a highly similar plasmid [15]. These data strongly suggest that the emergence of the KPC-9-KP strains was closely linked to the ongoing KPC-2 outbreak and did not originate from external sources.
According to ECDC surveillance data from 15 Greek hospitals during the period 2013–2022, more than 90% of K. pneumoniae strains with resistance or reduced susceptibility to carbapenems belonged mainly to five clones: ST258/512, ST11, ST39, ST147, and ST323 [32]. Among these, ST258/512, ST11, and ST147 are considered high-risk clones dominating also in other European countries and worldwide. In Greece, while ST258/512 was the predominant clone during the decade 2009–2019, it seems to have been replaced by ST11 in 2022 [32]. ST39 and ST323 emerged in 2019 and 2022, respectively [32]. Despite these facts, KPC-KP strains recently isolated from our hospital (2021–2022) also belonged to the ST258 clone, indicating the persistence of this clone in our hospital.
KPC-KP strains were also examined for the presence of ESBL genes. Besides blaKPC, KPC-KP strains co-harbored blaTEM (79.1%), blaSHV (33.3%), and blaCTX-M group 1 (10.7%). The combination TEM+SHV (20.9%) was the most frequent in the present study. During the 1980s and 1990s, TEM and SHV represented the predominant ESBL types among K. pneumoniae isolates involved in nosocomial outbreaks [30]. However, during the 2000s and beyond, CTX-M spread rapidly not only into hospital settings but also in the community [33]. According to the results of the Antimicrobial Testing Leadership and Surveillance (ATLAS) surveillance database, CTX-M genotypes comprised the majority of ESBLs in several regions of Europe (83.9%) and worldwide (86.7%) [34]. Another study, based on the International Network for Optimal Resistance Monitoring (INFORM) for the period 2013–2017, reported that CTX-M prevailed in all European countries except for Greece, where SHV was the most common type of ESBL [35]. Nevertheless, KPC-KP strains of our study co-harbor TEM as the dominant ESBL type, which, in the case of KPC-9-KP strains, is present almost exclusively (93.6%).
All KPC-KP isolates exhibited elevated MICs for carbapenems. Specifically, for meropenem, 96.6% (171/177) had MIC values ≥16 mg/L, consistent with findings from similar studies [36,37]. Among the aminoglycosides, gentamicin demonstrated the highest efficacy (38.8%), showing a potential therapeutic agent, whereas amikacin (12.4%) and tobramycin (5.1%) were significantly less effective against KPC-KP isolates. Similar overall results or slightly better performance of gentamicin have also been reported in other Greek hospitals [36,38]. According to EARS-Net (European Antimicrobial Resistance Surveillance Network) data for Greece, the overall resistance of K. pneumoniae strains to aminoglycosides remained constant at approximately 50–55% for the period 2015–2019 [39]. However, a slightly increasing trend was observed, with resistance reaching an average of 64.7% in 2023 [40]. We detected high resistance rates (>90%) for all fluoroquinolones, a common finding for KPC-KP isolates [40,41]. In contrast, tigecycline and colistin—last-resort antibiotics for infections caused by multidrug-resistant pathogens—retain satisfactory activity against KPC-KP isolates with sensitivity rates being 84.8% and 73.4%, respectively. Similarly, a multicenter study by Galani et al. for the period 2014–2016 reported sensitivity rates of 88.5% (232/262) and 65% (171/262) for tigecycline and colistin, respectively [36].
The comparison of KPC-2 and KPC-9 susceptibility rates revealed that KPC-9 exhibited higher MIC for ceftazidime and higher resistance to amikacin and colistin than KPC-2. This phenomenon is not uncommon among KPC variants. For example, the KPC-3 variant has demonstrated greater ceftazidime hydrolysis capacity while maintaining a similar hydrolysis rate for carbapenems compared to KPC-2 [42]. Kinetic studies on the hydrolytic activity of several KPC-2 variants against ceftazidime have shown that amino acid substitutions in KPC-2 result in alleles encoding less stable enzymes, yet with significantly higher catalytic activity—up to a 25-fold increase—in ceftazidime hydrolysis [43]. Additionally, point mutations at residues D179 and N170 have been implicated in altering enzymatic specificity and broadening the resistance spectrum [44]. The KPC-44 variant, which carries mutations in the Ω-loop (residues 164 to 179), has been associated with resistance to ceftazidime–avibactam, similar to the KPC-93 variant, which is characterized by a five-amino-acid insertion (Pro-Asn-Asn-Arg-Ala) between Ambler positions 267 and 268 of KPC-2 [45,46]. However, ceftazidime–avibactam-resistant variants have also exhibited reduced resistance to other β-lactams, such as carbapenems, which has been interpreted as an evolutionary trade-off [47]. On the other hand, the KPC-55 variant, which carries the Y264N substitution, has been found to exhibit reduced activity against ampicillin while displaying enhanced hydrolysis of aztreonam and meropenem [48]. Regarding aminoglycosides, studies have shown that strains harboring different alleles—or even the same allele—can exhibit varying antibiotic susceptibility patterns due to the high heterogeneity of the aminoglycoside-modifying enzymes (AMEs) they express [49]. This is particularly true for strains of the ST258 type [49,50]. Variable resistance to aminoglycosides can also be interpreted as heteroresistance, which results from the presence of subpopulations with different susceptibilities to antimicrobials [51]. Moreover, a multicenter study of the heteroresistance in colistin of KPC-KP strains by 13 hospitals from six countries in Europe has demonstrated that heteroresistant strains have high heterogeny regarding the sequence type of and the insertion sequences in the mgrB gene [52]. Interestingly, the insertion sequence IS5 in the mgrB gene was identified in two out of eight KPC-9-KP strains characterized by NGS in our previous study [15].
No significant difference was found in mortality rates between the two KPC variants, KPC-2 and KPC-9. Mortality rates associated with infections caused by KPC-KP strains vary across studies. This has been attributed to differences in patient populations, including age and comorbidities, as well as the challenge of distinguishing colonization from infection [53]. Regarding this last challenge, existing approaches to address it include methods that are imperfect and lack the ability to effectively differentiate between infection and sterile inflammation or colonization [54]. Systematic reviews and meta-analyses by Ramos-Castaneda et al. and Xu et al. have estimated mortality rates for KPC-KP infections at approximately 41% and 47.66%, respectively, findings similar to our results [4,55]. In our study, a total of 78.0% of the outbreak patients who died had been transferred to ICUs. This is to be expected, as an ICU stay exceeding 24 h has been associated with a fourfold increased risk of acquiring CR-KP strains [56]. Additionally, many studies have reported high mortality rates in cases of bloodstream infections [57,58].
Although less common than KPC-2 or KPC-3, KPC-9-producing K. pneumoniae strains have been detected in recent years not only in Greece but also in countries such as India, Italy, and China [59,60,61]. This geographical distribution raises concerns for a wider dissemination of the variant, particularly in high-risk clones such as ST258. A number of specific infection control measures are required to prevent further transmission within the healthcare system. Such measures, which have been reviewed elsewhere, include the screening of high-risk patients (i.e., upon hospital admission and transfer to the ICU), isolation or grouping of colonized/infected patients, strict implementation of contact precautions, vigorous hand hygiene, and thorough environmental cleaning [62]. In addition, antimicrobial stewardship programs and the communication of carrier status among different facilities within the hospital are essential components in reducing the spread of CRE strains [63]. The latter is known to be facilitated by plasmid dynamics and horizontal gene transfer [64]. As previously mentioned, the blaKPC alleles are found on various conjugative plasmids within the integrated transposon Tn4401 sequences. These plasmids have been shown to be responsible for the efficient horizontal gene transfer of carbapenem-resistance determinants among K. pneumoniae strains across different hospital settings in Europe [65]. Thus, plasmid surveillance and molecular epidemiology are crucial in the formulation of infection control strategies.
While providing valuable insights into the epidemiology of KPC-KP, the main limitation of this study is that KPC-KP isolates were not tested for susceptibility to combinations of antibiotics such as ceftazidime–avibactam, imipenem/cilastatin/relebactam, and meropenem/vaborbactam, which had not yet been introduced into clinical practice during the study period. Despite this limitation, this study provides powerful insights into the resistance gene epidemiology of KPC-KP and the potential implications for the evolution of multidrug-resistant K. pneumoniae strains in the future.
5. Conclusions
This study describes the characteristics of the dissemination of two KPC variants during the same period in our hospital. Our report highlights the heterogeneity of resistance genes present in K. pneumoniae strains, as well as the urgent need for the implementation of preventive and containment measures to limit the spread of multidrug-resistant pathogens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres16040081/s1, Table S1: Demographic and clinical data of 41 patients infected with Klebsiella pneumonia-producing KPC-9 carbapenemase. Table S2: MIC distributions and cumulative percentage of KPC-2 and KPC-9 isolates inhibited by medically important antimicrobial agents.
Author Contributions
Conceptualization, K.G.; methodology, A.M., P.B., G.V. and K.G.; formal analysis, A.M.; investigation, A.M., P.B., E.P. and K.G.; resources, E.P., E.K., V.K. and K.G.; data curation, A.M. and P.B.; writing—original draft preparation A.M., P.B. and K.G.; writing—review and editing, A.M., P.B., G.V. and K.G.; visualization, A.M. supervision, K.G.; project administration, P.B. and K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Scientific Ethics Committee of University General Hospital of Ioannina (approval reference number: 88/18-02-2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data will be available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Murray, C.; Ikuta, K.; Sharara, F.; Swetschinski, L.; Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Caliskan-Aydogan, O.; Alocilja, E.C. A Review of Carbapenem Resistance in Enterobacterales and Its Detection Techniques. Microorganisms 2023, 11, 1491. [Google Scholar] [CrossRef] [PubMed]
- Queenan, A.M.; Bush, K. Carbapenemases: The Versatile Beta-Lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Castañeda, J.A.; Ruano-Ravina, A.; Barbosa-Lorenzo, R.; Paillier-Gonzalez, J.E.; Saldaña-Campos, J.C.; Salinas, D.F.; Lemos-Luengas, E.V. Mortality Due to KPC Carbapenemase-Producing Klebsiella Pneumoniae Infections: Systematic Review and Meta-Analysis: Mortality Due to KPC Klebsiella Pneumoniae Infections. J. Infect. 2018, 76, 438–448. [Google Scholar] [CrossRef]
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Porreca, A.M.; Sullivan, K.V.; Gallagher, J.C. The Epidemiology, Evolution, and Treatment of KPC-Producing Organisms. Curr. Infect. Dis. Rep. 2018, 20, 13. [Google Scholar] [CrossRef]
- Campos, A.C.; Albiero, J.; Ecker, A.B.; Kuroda, C.M.; Meirelles, L.E.F.; Polato, A.; Tognim, M.C.B.; Wingeter, M.A.; Teixeira, J.J.V. Outbreak of Klebsiella Pneumoniae Carbapenemase-Producing K Pneumoniae: A Systematic Review. Am. J. Infect. Control 2016, 44, 1374–1380. [Google Scholar] [CrossRef]
- Nordmann, P.; Naas, T.; Poirel, L. Global Spread of Carbapenemase-Producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef]
- Girmenia, C.; Serrao, A.; Canichella, M. Epidemiology of Carbapenem Resistant Klebsiella Pneumoniae Infections in Mediterranean Countries. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016032. [Google Scholar] [CrossRef]
- Chen, L.F.; Anderson, D.J.; Paterson, D.L. Overview of the Epidemiology and the Threat of Klebsiella Pneumoniae Carbapenemases (KPC) Resistance. Infect. Drug Resist. 2012, 5, 133–141. [Google Scholar] [CrossRef]
- Reference Gene Catalog—Pathogen Detection—NCBI. Available online: https://www.ncbi.nlm.nih.gov/pathogens/refgene/#gene_family:(blaKPC) (accessed on 16 February 2025).
- Lee, C.-R.; Lee, J.H.; Park, K.S.; Kim, Y.B.; Jeong, B.C.; Lee, S.H. Global Dissemination of Carbapenemase-Producing Klebsiella Pneumoniae: Epidemiology, Genetic Context, Treatment Options, and Detection Methods. Front. Microbiol. 2016, 7, 895. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi Halat, D.; Ayoub Moubareck, C. The Current Burden of Carbapenemases: Review of Significant Properties and Dissemination among Gram-Negative Bacteria. Antibiotics 2020, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Alvisi, G.; Curtoni, A.; Fonnesu, R.; Piazza, A.; Signoretto, C.; Piccinini, G.; Sassera, D.; Gaibani, P. Epidemiology and Genetic Traits of Carbapenemase-Producing Enterobacterales: A Global Threat to Human Health. Antibiotics 2025, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Gartzonika, K.; Rossen, J.W.A.; Sakkas, H.; Rosema, S.; Priavali, E.; Friedrich, A.W.; Levidiotou, S.; Bathoorn, E. Identification of a KPC-9-Producing Klebsiella Pneumoniae ST258 Cluster among KPC-2-Producing Isolates of an Ongoing Outbreak in Northwestern Greece: A Retrospective Study. Clin. Microbiol. Infect. 2018, 24, 558–560. [Google Scholar] [CrossRef]
- Hidalgo-Grass, C.; Warburg, G.; Temper, V.; Benenson, S.; Moses, A.E.; Block, C.; Strahilevitz, J. KPC-9, a Novel Carbapenemase from Clinical Specimens in Israel. Antimicrob. Agents Chemother. 2012, 56, 6057–6059. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2024. [Google Scholar]
- Girlich, D.; Poirel, L.; Nordmann, P. Value of the Modified Hodge Test for Detection of Emerging Carbapenemases in Enterobacteriaceae. J. Clin. Microbiol. 2012, 50, 477–479. [Google Scholar] [CrossRef]
- Tsakris, A.; Poulou, A.; Pournaras, S.; Voulgari, E.; Vrioni, G.; Themeli-Digalaki, K.; Petropoulou, D.; Sofianou, D. A Simple Phenotypic Method for the Differentiation of Metallo-Beta-Lactamases and Class A KPC Carbapenemases in Enterobacteriaceae Clinical Isolates. J. Antimicrob. Chemother. 2010, 65, 1664–1671. [Google Scholar] [CrossRef]
- Tigecycline—Injection Products. FDA-Identified Interpretive Criteria. Available online: https://www.fda.gov/drugs/development-resources/tigecycline-injection-products (accessed on 15 December 2024).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters v.14.0. 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_14.0_Breakpoint_Tables.pdf (accessed on 15 December 2024).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Poulou, A.; Grivakou, E.; Vrioni, G.; Koumaki, V.; Pittaras, T.; Pournaras, S.; Tsakris, A. Modified CLSI Extended-Spectrum β-Lactamase (ESBL) Confirmatory Test for Phenotypic Detection of ESBLs among Enterobacteriaceae Producing Various β-Lactamases. J. Clin. Microbiol. 2014, 52, 1483–1489. [Google Scholar] [CrossRef]
- Dashti, A.A.; Jadaaon, M.M.; Abdulsamad, A.M.; Dashti, H.M. Heat Treatment of Bacteria: A Simple Method of DNA Extraction for Molecular Techniques. Kuwait Med. J. 2009, 41, 117–122. [Google Scholar]
- Lomaestro, B.M.; Tobin, E.H.; Shang, W.; Gootz, T. The Spread of Klebsiella Pneumoniae Carbapenemase–Producing K. Pneumoniae to Upstate New York. Clin. Infect. Dis. 2006, 43, e26–e28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poirel, L.; Naas, T.; Nicolas, D.; Collet, L.; Bellais, S.; Cavallo, J.D.; Nordmann, P. Characterization of VIM-2, a Carbapenem-Hydrolyzing Metallo-Beta-Lactamase and Its Plasmid- and Integron-Borne Gene from a Pseudomonas Aeruginosa Clinical Isolate in France. Antimicrob. Agents Chemother. 2000, 44, 891–897. [Google Scholar] [CrossRef]
- Manchanda, V.; Rai, S.; Gupta, S.; Rautela, R.S.; Chopra, R.; Rawat, D.S.; Verma, N.; Singh, N.P.; Kaur, I.R.; Bhalla, P. Development of TaqMan Real-Time Polymerase Chain Reaction for the Detection of the Newly Emerging Form of Carbapenem Resistance Gene in Clinical Isolates of Escherichia Coli, Klebsiella Pneumoniae, and Acinetobacter Baumannii. Indian J. Med. Microbiol. 2011, 29, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Tzelepi, E.; Magana, C.; Platsouka, E.; Sofianou, D.; Paniara, O.; Legakis, N.J.; Vatopoulos, A.C.; Tzouvelekis, L.S. Extended-Spectrum Beta-Lactamase Types in Klebsiella Pneumoniae and Escherichia Coli in Two Greek Hospitals. Int. J. Antimicrob. Agents 2003, 21, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Gartzonika, K.; Bozidis, P.; Priavali, E.; Sakkas, H. Rapid Detection of blaKPC-9 Allele from Clinical Isolates. Pathogens 2021, 10, 487. [Google Scholar] [CrossRef]
- Institut Pasteur MLST Databases and Software. Available online: https://bigsdb.pasteur.fr/klebsiella/primers-used/ (accessed on 16 February 2025).
- Hansen, G.T. Continuous Evolution: Perspective on the Epidemiology of Carbapenemase Resistance Among Enterobacterales and Other Gram-Negative Bacteria. Infect. Dis. Ther. 2021, 10, 75–92. [Google Scholar] [CrossRef]
- Tryfinopoulou, K.; Linkevicius, M.; Pappa, O.; Alm, E.; Karadimas, K.; Svartström, O.; Polemis, M.; Mellou, K.; Maragkos, A.; Brolund, A.; et al. Emergence and Persistent Spread of Carbapenemase-Producing Klebsiella Pneumoniae High-Risk Clones in Greek Hospitals, 2013 to 2022. Eurosurveillance 2023, 28, 2300571. [Google Scholar] [CrossRef]
- Cantón, R.; González-Alba, J.M.; Galán, J.C. CTX-M Enzymes: Origin and Diffusion. Front. Microbiol. 2012, 3, 110. [Google Scholar] [CrossRef]
- Gales, A.C.; Stone, G.; Sahm, D.F.; Wise, M.G.; Utt, E. Incidence of ESBLs and Carbapenemases among Enterobacterales and Carbapenemases in Pseudomonas Aeruginosa Isolates Collected Globally: Results from ATLAS 2017-2019. J. Antimicrob. Chemother. 2023, 78, 1606–1615. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; de Jonge, B.L.M.; Stone, G.G.; Sahm, D.F. Longitudinal Analysis of ESBL and Carbapenemase Carriage among Enterobacterales and Pseudomonas Aeruginosa Isolates Collected in Europe as Part of the International Network for Optimal Resistance Monitoring (INFORM) Global Surveillance Programme, 2013–2017. J. Antimicrob. Chemother. 2020, 75, 1165–1173. [Google Scholar] [CrossRef]
- Galani, I.; Karaiskos, I.; Karantani, I.; Papoutsaki, V.; Maraki, S.; Papaioannou, V.; Kazila, P.; Tsorlini, H.; Charalampaki, N.; Toutouza, M.; et al. Epidemiology and Resistance Phenotypes of Carbapenemase-Producing Klebsiella Pneumoniae in Greece, 2014 to 2016. Eurosurveillance 2018, 23, 1700775. [Google Scholar] [CrossRef] [PubMed]
- Bathoorn, E.; Tsioutis, C.; da Silva Voorham, J.M.; Scoulica, E.V.; Ioannidou, E.; Zhou, K.; Rossen, J.W.; Gikas, A.; Friedrich, A.W.; Grundmann, H. Emergence of Pan-Resistance in KPC-2 Carbapenemase-Producing Klebsiella Pneumoniae in Crete, Greece: A Close Call. J. Antimicrob. Chemother. 2016, 71, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Galani, I.; Nafplioti, K.; Adamou, P.; Karaiskos, I.; Giamarellou, H.; Souli, M. Study Collaborators Nationwide Epidemiology of Carbapenem Resistant Klebsiella Pneumoniae Isolates from Greek Hospitals, with Regards to Plazomicin and Aminoglycoside Resistance. BMC Infect. Dis. 2019, 19, 167. [Google Scholar] [CrossRef]
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2019. Country Summaries-AER-EARS. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Country%20summaries-AER-EARS-Net%20202019.pdf (accessed on 16 February 2025).
- Country Summaries—Antimicrobial Resistance in the EU/EEA. 2023. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Country_profiles_2023_2024_1.pdf (accessed on 16 February 2025).
- Zhan, Q.; Xu, Y.; Wang, B.; Yu, J.; Shen, X.; Liu, L.; Cao, X.; Guo, Y.; Yu, F. Distribution of Fluoroquinolone Resistance Determinants in Carbapenem-Resistant Klebsiella Pneumoniae Clinical Isolates Associated with Bloodstream Infections in China. BMC Microbiol. 2021, 21, 164. [Google Scholar] [CrossRef]
- Oueslati, S.; Iorga, B.I.; Tlili, L.; Exilie, C.; Zavala, A.; Dortet, L.; Jousset, A.B.; Bernabeu, S.; Bonnin, R.A.; Naas, T. Unravelling Ceftazidime/Avibactam Resistance of KPC-28, a KPC-2 Variant Lacking Carbapenemase Activity. J. Antimicrob. Chemother. 2019, 74, 2239–2246. [Google Scholar] [CrossRef]
- Mehta, S.C.; Rice, K.; Palzkill, T. Natural Variants of the KPC-2 Carbapenemase Have Evolved Increased Catalytic Efficiency for Ceftazidime Hydrolysis at the Cost of Enzyme Stability. PLoS Pathog. 2015, 11, e1004949. [Google Scholar] [CrossRef]
- Parwana, D.; Gu, J.; Chen, S.; Bethel, C.R.; Marshall, E.; Hujer, A.M.; Bonomo, R.A.; Haider, S. The Structural Role of N170 in Substrate-Assisted Deacylation in KPC-2 β-Lactamase. Angew. Chem. Int. Ed. 2024, 63, e202317315. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, H.; Hu, L.; Neetu, N.; Sankaran, B.; Wang, J.; Prasad, B.V.V.; Palzkill, T. Klebsiella Pneumoniae Carbapenemase Variant 44 Acquires Ceftazidime-Avibactam Resistance by Altering the Conformation of Active-Site Loops. J. Biol. Chem. 2024, 300, 105493. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Liu, C.; Zhang, Y.; Cheung, Y.C.; Wai Chi Chan, E.; Chen, S.; Zhang, R. Identification of a KPC Variant Conferring Resistance to Ceftazidime-Avibactam from ST11 Carbapenem-Resistant Klebsiella Pneumoniae Strains. Microbiol. Spectr. 2022, 10, e02655-21. [Google Scholar] [CrossRef]
- Hobson, C.A.; Pierrat, G.; Tenaillon, O.; Bonacorsi, S.; Bercot, B.; Jaouen, E.; Jacquier, H.; Birgy, A. Klebsiella Pneumoniae Carbapenemase Variants Resistant to Ceftazidime-Avibactam: An Evolutionary Overview. Antimicrob. Agents Chemother. 2022, 66, e00447-22. [Google Scholar] [CrossRef]
- Yoon, E.-J.; Choi, Y.J.; Park, S.H.; Shin, J.H.; Park, S.G.; Choi, J.R.; Jeong, S.H. A Novel KPC Variant KPC-55 in Klebsiella Pneumoniae ST307 of Reinforced Meropenem-Hydrolyzing Activity. Front. Microbiol. 2020, 11, 561317. [Google Scholar] [CrossRef] [PubMed]
- Almaghrabi, R.; Clancy, C.J.; Doi, Y.; Hao, B.; Chen, L.; Shields, R.K.; Press, E.G.; Iovine, N.M.; Townsend, B.M.; Wagener, M.M.; et al. Carbapenem-Resistant Klebsiella Pneumoniae Strains Exhibit Diversity in Aminoglycoside-Modifying Enzymes, Which Exert Differing Effects on Plazomicin and Other Agents. Antimicrob. Agents Chemother. 2014, 58, 4443–4451. [Google Scholar] [CrossRef] [PubMed]
- Deleo, F.R.; Chen, L.; Porcella, S.F.; Martens, C.A.; Kobayashi, S.D.; Porter, A.R.; Chavda, K.D.; Jacobs, M.R.; Mathema, B.; Olsen, R.J.; et al. Molecular Dissection of the Evolution of Carbapenem-Resistant Multilocus Sequence Type 258 Klebsiella Pneumoniae. Proc. Natl. Acad. Sci. USA 2014, 111, 4988–4993. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Q.; Bai, J.; Ding, M.; Yan, X.; Wang, G.; Zhu, B.; Zhou, Y. Heteroresistance to Amikacin in Carbapenem-Resistant Klebsiella Pneumoniae Strains. Front. Microbiol. 2021, 12, 682239. [Google Scholar] [CrossRef] [PubMed]
- Braspenning, A.J.M.M.; Rajakani, S.G.; Sey, A.; El Bounja, M.; Lammens, C.; Glupczynski, Y.; Malhotra-Kumar, S. Assessment of Colistin Heteroresistance among Multidrug-Resistant Klebsiella Pneumoniae Isolated from Intensive Care Patients in Europe. Antibiotics 2024, 13, 281. [Google Scholar] [CrossRef]
- Tumbarello, M.; Trecarichi, E.M.; De Rosa, F.G.; Giannella, M.; Giacobbe, D.R.; Bassetti, M.; Losito, A.R.; Bartoletti, M.; Del Bono, V.; Corcione, S.; et al. Infections Caused by KPC-Producing Klebsiella Pneumoniae: Differences in Therapy and Mortality in a Multicentre Study. J. Antimicrob. Chemother. 2015, 70, 2133–2143. [Google Scholar] [CrossRef]
- Jeffrey, M.; Denny, K.J.; Lipman, J.; Conway Morris, A. Differentiating Infection, Colonisation, and Sterile Inflammation in Critical Illness: The Emerging Role of Host-Response Profiling. Intensive Care Med. 2023, 49, 760–771. [Google Scholar] [CrossRef]
- Xu, L.; Sun, X.; Ma, X. Systematic Review and Meta-Analysis of Mortality of Patients Infected with Carbapenem-Resistant Klebsiella Pneumoniae. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 18. [Google Scholar] [CrossRef]
- Soares de Moraes, L.; Gomes Magalhaes, G.L.; Material Soncini, J.G.; Pelisson, M.; Eches Perugini, M.R.; Vespero, E.C. High Mortality from Carbapenem-Resistant Klebsiella Pneumoniae Bloodstream Infection. Microb. Pathog. 2022, 167, 105519. [Google Scholar] [CrossRef]
- Zarkotou, O.; Pournaras, S.; Tselioti, P.; Dragoumanos, V.; Pitiriga, V.; Ranellou, K.; Prekates, A.; Themeli-Digalaki, K.; Tsakris, A. Predictors of Mortality in Patients with Bloodstream Infections Caused by KPC-Producing Klebsiella Pneumoniae and Impact of Appropriate Antimicrobial Treatment. Clin. Microbiol. Infect. 2011, 17, 1798–1803. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, M.; Wu, J.; Chen, S.; Yang, H.; Long, J.; Duan, G. Mortality and Genetic Diversity of Antibiotic-Resistant Bacteria Associated with Bloodstream Infections: A Systemic Review and Genomic Analysis. BMC Infect. Dis. 2024, 24, 1385. [Google Scholar] [CrossRef]
- Shankar, C.; Karunasree, S.; Manesh, A.; Veeraraghavan, B. First Report of Whole-Genome Sequence of Colistin-Resistant Klebsiella quasipneumoniae subsp. Similipneumoniae Producing KPC-9 in India. Microb. Drug Resist. 2019, 25, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Piccirilli, A.; Cherubini, S.; Azzini, A.M.; Tacconelli, E.; Lo Cascio, G.; Maccacaro, L.; Bazaj, A.; Naso, L.; Amicosante, G.; LTCF-Veneto Working Group; et al. Whole-Genome Sequencing (WGS) of Carbapenem-Resistant K. Pneumoniae Isolated in Long-Term Care Facilities in the Northern Italian Region. Microorganisms 2021, 9, 1985. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Yuan, Y.; Li, Q.; Wu, S.; Liu, Y.; Zhang, W.; Xiao, Y.; Kang, M. Comparing Three Different Phenotypic Methods for Accurate Detection of Carbapenemase-Producing Enterobacterales. J. Infect. Chemother. 2021, 27, 794–799. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Burns, K.; Rodríguez Baño, J.; Borg, M.; Daikos, G.; Dumpis, U.; Lucet, J.C.; Moro, M.L.; Tacconelli, E.; Simonsen, G.S.; et al. Infection Prevention and Control Measures and Tools for the Prevention of Entry of Carbapenem-Resistant Enterobacteriaceae into Healthcare Settings: Guidance from the European Centre for Disease Prevention and Control. Antimicrob. Resist. Infect. Control 2017, 6, 113. [Google Scholar] [CrossRef]
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs; CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef]
- Ding, L.; Shen, S.; Chen, J.; Tian, Z.; Shi, Q.; Han, R.; Guo, Y.; Hu, F. Klebsiella pneumoniae Carbapenemase Variants: The New Threat to Global Public Health. Clin. Microbiol. Rev. 2023, 36, e00008-23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).