Abstract
Pouchitis is one of the most common long-term complications after ileal pouch–anal anastomosis (IPAA) surgery, with a reported incidence rate of up to 50%. Identifying specific bacteria involved in the pathogenesis has important implications for clinical management. Indeed, antibiotic therapy is a common treatment option, but antibiotic choice and treatment duration can vary depending on the severity of symptoms and the bacteria involved. Ansamycins are effective in the management of antibiotic-dependent pouchitis. Therefore, this study aimed to test the in vitro antibacterial activity of a novel rifamycin gel solution, specifically intended for treating infections associated with pouchitis, with the agar diffusion assay. Furthermore, the in vitro antibacterial activity of rifamycin sodium salt against several bacterial strains involved in pouchitis was compared with the gel formulation. Rifamycin’s in vitro anti-microbial characteristics were not affected by the gel formulation. These results, although preliminary, support the potential of the Rifamycin Gel formulation as a valuable addition to the therapeutic armamentarium for this challenging condition.
1. Introduction
Pouchitis is an inflammatory condition that occurs in patients who have undergone ileal pouch–anal anastomosis (IPAA) surgery for ulcerative colitis (UC) or familial adenomatous polyposis (FAP). It has been observed to occur up to 50% of the time after IPAA surgery, making it one of the most frequent long-term consequences. The symptoms of pouchitis, which frequently include increased bowel frequency, urgency, stomach pain, and occasionally fever, can significantly lower patients’ quality of life [1].
Depending on how long and often the symptoms last, pouchitis may develop as an acute, chronic, or recurrent disease. While chronic and recurring pouchitis can be more challenging to manage and may require prolonged antibiotic medication or other measures, acute pouchitis often responds well to antibiotic treatment [2].
Clinical signs, endoscopic findings, and histologic analysis all contribute to diagnosing pouchitis. The gold standard for diagnosis is endoscopy with biopsy, since it can identify inflammation and rule out other pouch disorders, such as cuffitis, Crohn’s disease, or ischemic pouchitis [3].
Although the exact cause of pouchitis is unknown, it has been proposed to result from altered gut microbiota composition and immunological dysregulation. After IPAA surgery, the pouch, which is created from the ileum, is exposed to new environmental stimuli (including fecal stasis) and nutrients, which alters the microbial community and metabolic activity. Following surgery, the host immune system also experiences alterations that may modify the interaction between the host and bacteria [4].
Studies have shown that individuals with pouchitis have different gut microbiota from healthy controls. These changes are characterized by a rise in the quantity of potentially harmful bacteria such as Enterococcus, Streptococcus, and Escherichia coli, along with a decrease in the microorganism’s diversity [5].
Investigators do not fully understand the specific mechanisms by which these bacteria contribute to the pathophysiology of pouchitis. The delicate balance between the host immune system and the commensal microbiota may be altered, causing chronic inflammation and tissue damage [4].
In patients with multiple-stage IPAA surgery, biopsies obtained from the upstream ileostomy site at the time of stoma closure have predominately facultative anaerobes (such as lactobacilli, enterococci, and coliforms), a scarcity of sulfate-reducing bacteria (SRB), and low levels of Clostridium perfringens [6]. On the other hand, during pouchitis episodes, an increase in the abundance of Enterobacteriaceae and reduced prevalence of Bacteroides and Faecalibaterium prausnitzii have been described [7,8,9].
The awareness of the crucial importance of fecal stasis and bacterial overgrowth in the pathogenesis of acute pouchitis has led clinicians to treat patients with antibiotics, which have become the mainstay of treatment, even without controlled trials [3]. Pouchitis is frequently treated with antibiotic courses; however, the type of antibiotic and the treatment course can change depending on the severity of the symptoms and the type of bacteria involved [10]. For example, metronidazole and ciprofloxacin are commonly used as first-line agents for treating pouchitis. The two-drug combination may be more effective than either agent alone. Current disease management guidelines and therapeutic protocols foresee that patients who experience their first episode of acute pouchitis following IPAA surgery are treated with antibiotics, either ciprofloxacin at 500 mg B.I.D. or metronidazole at 500 mg T.I.D. for 2 to 4 weeks [11].
Rifaximin is effective in the management of antibiotic-dependent pouchitis [12]. In vitro studies have demonstrated that rifamycins are effective against a broad range of bacteria commonly found in the gut, and they are particularly effective against antibiotic-resistant strains commonly found in patients with pouchitis [13].
Rifamycin is a semi-synthetic macrocyclic antibiotic belonging to the ansamycin class originating from the fermentation broth of Amycolatopsis rifamycinica (previously Streptomyces mediterranei). Rifamycin exhibits a broad spectrum of antibiotic activity against Gram-positive bacteria (e.g., Staphylococcus aureus, Streptococcus pyogenes, Clostridium difficile, Klebsiella spp., and Enterococcus spp.) and Gram-negative bacteria (e.g., Escherichia coli, Yersinia enterocolitica, and Bacteroides spp.) [14,15].
This study aimed to test the in vitro antibacterial activity of a novel rifamycin in situ gelling solution (Rifamycin Gel), specifically intended for treating infections associated with pouchitis, with the agar diffusion assay for drug permeation evaluation. The new formulation, which is liquid and suitable for rectal administration via an enema, has a composition comprising three different biocompatible polymers responsible for the transition from solution to gel in situ upon administration to the target area. This new formula’s in vitro antibacterial activity was compared with rifamycin sodium salt against several bacterial strains involved in pouchitis.
2. Materials and Methods
2.1. Tested Formulation
Rifamycin Gel was prepared by dissolving rifamycin sodium into an aqueous composition comprising the three polymers and brought to a physiological pH of 7.
This in situ gelling formulation is based on the combination of three polymers: one thermo-responsive polymer that jellifies at body temperature to create a structure with the bio-relevant medium; an ion-sensitive polymer able to jellify in the presence of specific ions to create a stronger gel structure; and a bio-adhesive polymer that improves adhesion to the target tissue. The combination of these effects allows for a longer residence time at the site of action and the release of the active ingredient.
To verify the possible interference of the carrier gel, a vehicle formulation (Vehicle) with the same composition but without the active ingredient was tested.
2.2. Bacterial Strains
All anaerobic strains (Table 1), except Bifidobacterium spp., were grown in Brucella agar medium supplemented with 5 µg/mL hemin, 1 µg/mL vitamin K1, and defibrinated horse blood (5% v/v) [16]. Bifidobacterium strains were grown in MRS agar supplemented with 0.05% w/v L-cysteine hydrochloride [17,18]. All bacterial cultures were incubated in an anaerobic atmosphere (AnaeroGen 2.5 L AGS; Oxoid Basingstoke, UK) at 37 °C until visible growth.

Table 1.
Selected bacterial strains for in vitro antimicrobial activity testing. DSMZ: Leibniz-Institut Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH Braunschweig Germany. ATCC: American Type Culture Collection Manassas, Virginia, USA.
Regarding the aerobic bacteria, Klebsiella oxytoca strains were grown in Muller Hinton (MH) agar. Rothia dentocariosa was grown in Columbia agar supplemented with 5% defibrinated horse blood. Bacterial cultures were incubated at 37 °C for 24 h (Table 2) [19].

Table 2.
In vitro antimicrobial activity of Rifamycin Gel formulation and Vehicle tested by agar diffusion (results are expressed as inhibition halos in millimeters). DSMZ: Leib-niz-Institut Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH. ATCC: American Type Culture Collection.
2.3. Agar Diffusion Test
The in vitro antimicrobial activity of Rifamycin Gel and Vehicle was determined by an agar diffusion–agar permeation assay in solid inoculated culture media.
Agar media were prepared according to the manufacturer’s instructions and autoclaved at 121 °C at 1 atm pressure for 15 min.
Bacterial inocula at a final 1 × 105 CFU/mL concentration were added slightly above the agarization point to ensure microorganism vitality. Rifamycin Gel (with a final concentration of rifamycin equal to 500 µg/mL) and the Vehicle solution were dispensed into 6 mm diameter wells to allow for diffusion.
Inoculated plates were incubated at the appropriate temperature and oxygen concentration for the bacterial species tested (aerobic or anaerobic) to allow for bacterial growth and compound diffusion through the agar plate. Incubation times ranged from a minimum of 24 to a maximum of 72 h. Antimicrobial susceptibility was assessed as the extent of the tested compound’s halo of inhibition (mm) in counteracting microbial growth. Experiments were conducted in duplicate, and the results are expressed as means and standard deviations.
2.4. MIC (Minimal Inhibitory Concentration) Determination by Broth Microdilution Test
In vitro antimicrobial susceptibility for Rifamycin sodium salt was assessed by the microdilution method according to the NCCLS guidelines [16,19].
Two-fold dilutions of the tested compounds were dispensed in 96-well round-bottom plates (Biowell, Biogenerica Srl, Rovereto, Italy), and each dilution was processed in the liquid culture medium suitable for the tested bacterial species. Bacterial inocula were prepared by a suspension of the microbial strain in sterile water saline and adjusted to a concentration of 0.5 McFarland standard corresponding to 1.5 × 108 CFU/mL. A final bacterial concentration of 0.5–1 × 105 CFU/mL was distributed in each well. Each plate was incubated at the appropriate temperature and oxygen concentration for the bacterial species tested (aerobic or anaerobic). The MIC was recorded when a visible growth appeared in the positive growth control column (bacterial inoculum without test substance).
2.5. Cell Viability Assay
A human colorectal carcinoma cell line derived from an adult male tumor (HCT-116) was used for the viability assay. The adherent cells have an epithelial morphology and were maintained in cell culture in 75 cm2 flasks in McCoy’s 5a Medium with the following supplements: fetal bovine serum at a final concentration of 10%, penicillin (100 units/mL), and streptomycin (100 μg/mL). Cells were cultured at 37 °C and 5% CO2, changing the medium 2 to 3 times per week.
The HCT-116 cell-seeded microplate was incubated at 37°C and 5% CO2 for 24 h before treatment. The following day, the medium was replaced with 180 μL of fresh, complete medium. Rifamycin Gel and Vehicle were tested at final concentrations starting at 50 µM down to 00.5 nM with 6-point log dose titration measurements. An aliquot of 20 μL of 10× stock solution in medium was added to a final volume of 200 μL per well. The treated cultures were incubated for 48 h at 37 °C and 5% CO2. The CellTiter-Glo™ luminescent cell viability assay was used to measure cell proliferation and cytotoxicity. This assay relies on a stable form of luciferase to measure ATP as an indicator of cell viability. The luminescent signal produced is proportional to the number of viable cells present in the culture. After the 48 h incubation, the medium was removed, and the remaining adherent cells were lysed in 50 μL of 30% CellTiter-Glo reagent for 10 min at room temperature. Lysates were transferred into 96-well white opaque plates, and luminescence was measured by using a BMG Labtech ClarioStar plate reader (BMG Labtech, Offenburg, Germany). Luminescence values from treated cells were normalized to the values obtained for the untreated cells to determine percent survival.
2.6. Data Analysis
Data were fitted with GraphPad Prism software version 9.0 by using Rifamycin Gel and Vehicle log concentration versus relative luminescent units. Background values were subtracted from all readings. Each experiment was repeated at least three times and performed in triplicate for each concentration tested. Statistical analysis was carried out by using Wilcoxon matched-pairs signed rank test for parametric variables.
3. Results
The in vitro antimicrobial susceptibility results, determined by the agar diffusion of the selected bacteria (aerobes and anaerobes) involved in pouchitis pathogenesis, are shown in Table 2. The findings provided evidence that rifamycin’s in vitro anti-microbial characteristics were not affected by the gel formulation.
Table 3 shows the MIC values (µg/mL) for rifamycin sodium salt versus the selected bacteria (aerobes and anaerobes) involved in pouchitis pathogenesis by the broth microdilution method. The values are in agreement with the inhibition halo results reported in Table 2, further demonstrating the non-interference of the gel formulation with the antimicrobial activity of rifamycin.

Table 3.
In vitro antimicrobial activity of rifamycin sodium salt tested by microdilution method (results are expressed as MIC in µg/mL). DSMZ: Leib-niz-Institut Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH. ATCC: American Type Culture Collection.
The formulations’ effect on cell viability was obtained in HCT 116 cells. The toxicity of Rifamycin Gel solutions and the Vehicle was tested by six-point log dose titrations from as high as 50 µM to as low as 0.5 nM of the Vehicle and Rifamycin Gel solutions. Figure 1 shows that both formulations were not significantly toxic to HCT116 cells after 48 h of treatment across the six concentrations tested (p = 0.156), suggesting that the active compound (rifamycin) does not add any cell toxicity.
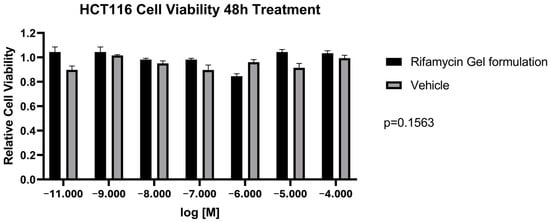
Figure 1.
Cell viability assay on HCT116 cell line after 48 h treatment of Rifamycin Gel formulation and Vehicle.
4. Discussion
An imbalance or overgrowth of particular bacterial taxa could cause pouchitis. Although derived from the ileum, upon IPAA surgery, the pouch’s bacterial population changes to resemble that naturally present in the colon, probably in reaction to fecal stasis. Following ileostomy closure, the bacterial community changes, becoming more like that found in the colon by having less facultative anaerobes and more obligate anaerobes [4].
For this reason, the strains tested in this study were chosen by a bibliographic search according to specific criteria. Both major taxa with higher abundance in UC ileal pouches than in FAP ileal ones (i.e., Clostridium spp. [20]) and taxa with higher abundance in FAP ileal pouches than in UC ileal ones (Ruminococcaceae [7], Faecalibacterium prausnitzii [7,20], and Streptococcus spp. [20]) were selected. Indeed, a comparison between the microbiota of UC patients, who have high incidence of pouchitis, and FAP patients, who have low incidence, can be useful to understand how the composition of the bacterial population may influence the risk of pouchitis. Furthermore, during a pouchitis episode, a reduction in the bacterial diversity of the ileal pouch has been described [7,9], including an increase in specific taxa (i.e., Clostridium perfringens [21,22], Enterobacteriaceae [7,8], and Fusobacterium spp. [23]) and a decrease in others (Bifidobacterium spp. [22] and Ruminococcaceae [8,23]). Lastly, several bacterial species involved in oral dysbiosis (i.e., Streptococcus pyogenes and Rothia dentocariosa) were chosen. According to a recent 2022 study by Molinero et al., there is a correlation between oral dysbiosis and UC [24], so given the significance of this condition in pouchitis onset, these strains were also taken into account.
As well as the type of antibiotic prescribed, effective therapy for pouchitis depends on the antibiotic formulations’ pharmacokinetic characteristics. These findings are crucial to improving dose regimens for treatment. Different studies investigated factors like absorption, distribution, metabolism, and excretion to find the best dose regimens to ensure the infection site received enough medication exposure. Formulations must, therefore, ensure that the drug substance remains in contact with the inflamed mucosa for a prolonged time, especially considering that patients with pouches often have high stool frequency (four to eight bowel movements per day) even when in remission [25,26,27].
In vitro, methods play a crucial role in evaluating drug permeation and diffusion across biological membranes. These studies provide valuable insights into the absorption, distribution, and elimination of drugs, aiding in developing effective drug delivery systems. Among the various in vitro techniques available, the agar diffusion method is an established technique for studying in vitro antimicrobial activity by agar permeation. The agar diffusion method utilizes a solid agar medium, which mimics the biological membrane, to measure the diffusion of drugs through the matrix. It offers several advantages, such as simplicity, cost-effectiveness, reproducibility, and the ability to test a wide range of drugs. This method has extensive applications in pharmaceutical research, including the antibacterial assessment of transdermal drug delivery systems, topical formulations, and ocular drug permeation [28].
The data reported in this study demonstrate that the Rifamycin Gel formulation shows remarkable growth inhibition activity of the bacterial panel involved in pouchitis pathogenesis. Importantly, the Vehicle formulation did not exert any antimicrobial activity against the tested microbial strains, confirming specific rifamycin antibacterial activity (Table 3).
Furthermore, the gel formulation did not decrease the antibacterial activity of rifamycin. In vitro results confirmed a correspondence between the MIC values obtained for rifamycin (Table 3) and the inhibition halos of Rifamycin Gel (Table 3). Indeed, for the same bacterial strains, lower MIC values correspond to inhibition halos with larger diameters.
The safety profile of the tested formulations was assessed by evaluating their impact on cell viability by using the HCT 116 cell line. The results, as illustrated in Figure 1, demonstrated that neither the Rifamycin Gel formulation nor the Vehicle exhibited significant toxicity after 48 h of treatment. Statistical analysis further supported this observation, with a p-value of 0.156, indicating no statistically significant difference in cell viability between the treated and control groups across all tested concentrations. These findings are particularly noteworthy, as they suggest that the active compound, rifamycin, does not contribute to cytotoxic effects, thereby reinforcing its safety profile. The absence of significant cytotoxicity in the Rifamycin Gel solution is a critical finding for its potential clinical application, especially considering the importance of preserving the integrity of intestinal epithelial cells in patients with pouchitis. The gel formulation’s compatibility with epithelial cells highlights its suitability for localized drug delivery within the gastrointestinal tract, where maintaining cellular health is paramount. Furthermore, the vehicle’s lack of cytotoxic effects underscores the biocompatibility of the gel matrix itself, which is essential to ensuring that the formulation does not induce unintended adverse effects independent of the active compound. This characteristic is particularly advantageous for patients with pouchitis, who often experience compromised mucosal integrity and may be more susceptible to additional epithelial damage. In summary, the findings from the cell viability assay provide robust evidence for the safety of the Rifamycin Gel formulation, both in terms of its active compound and its vehicle. These results lay a solid foundation for further preclinical and clinical investigations, emphasizing the formulation’s potential as a safe and effective therapeutic option for managing pouchitis.
5. Conclusions
To sum up, the Rifamycin Gel formulation’s in vitro antimicrobial activity against bacteria involved in pouchitis pathogenesis demonstrates good growth inhibition with values comparable to those obtained for rifamycin sodium salt and rifamixin.
Furthermore, this study introduces a novel rifamycin in situ gelling formulation, which has the potential to significantly improve patient care and outcomes for individuals suffering from pouchitis, a common and challenging postoperative complication of ileal pouch–anal anastomosis. By demonstrating in vitro antibacterial activity against key bacterial strains implicated in pouchitis pathogenesis, the formulation offers a targeted and localized therapeutic option that aligns with the specific needs of this condition. Its in situ gelling mechanism enhances drug retention at the site of action, which may reduce the frequency of dosing and improve treatment adherence, particularly for patients experiencing high stool frequency. These advancements hold promise for enhancing quality of life for patients while providing clinicians with a more effective tool for managing this complex condition.
Although the study presents encouraging in vitro evidence for the potential efficacy of the rifamycin in situ gelling formulation in managing pouchitis, certain aspects warrant further exploration. The absence of in vivo experiments means that additional research is needed to confirm these findings in the more complex biological environment of the gastrointestinal system. Furthermore, the long-term effects of the formulation, including its sustained efficacy and safety over extended use, have not yet been investigated. While the antibacterial activity against key bacterial strains is promising, its broader clinical relevance should be validated in diverse patient settings. Additionally, the study does not address pharmacokinetics or bioavailability, which are important considerations for optimizing dosing regimens and ensuring effective delivery of the drug to the site of action. These areas of investigation will be crucial to fully understanding and demonstrating the therapeutic potential of this formulation. Nonetheless, the evidence presented thus far supports the potential of the Rifamycin Gel formulation as a valuable addition to the therapeutic armamentarium for this challenging condition.
Author Contributions
Conceptualization, R.D.M.; methodology, R.D.M.; validation, R.D.M., R.M. and G.P.P.; formal analysis, D.N. and G.P.P.; investigation, C.A. and C.Q.; resources, R.M., C.A. and C.Q.; data curation, C.A., C.Q. and D.N.; writing—original draft preparation, C.A., D.N. and C.Q.; writing—review and editing, G.P.P., R.M. and R.D.M.; supervision, G.P.P., D.N. and R.D.M.; project administration, R.D.M.; funding acquisition, R.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Cosmo SPA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Katia Aiello and Cinzia Quattrocchi were employed by Cosmo SPA. Roberto Di Marco was Scientific Advisor for Cosmo SPA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the study’s design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lightner, A.L. Are We Able to Accurately Assess Post IPAA Pathology? Crohn’s Colitis 2020, 360, otaa038. [Google Scholar] [CrossRef] [PubMed]
- Gionchetti, P.; Calabrese, C.; Laureti, S.; Poggioli, G.; Rizzello, F. Pouchitis: Clinical Features, Diagnosis, and Treatment. Int. J. Gen. Med. 2021, 14, 3871–3879. [Google Scholar] [CrossRef] [PubMed]
- Calvino-Suarez, C.; Ferreiro-Iglesias, R.; Baston Rey, I.; Barreiro-de Acosta, M. Managing Ulcerative Colitis after Surgery. Front. Med. 2023, 9, 3774. [Google Scholar] [CrossRef]
- Schieffer, K.M.; Williams, E.D.; Yochum, G.S.; Koltun, W.A. The Pathogenesis of Pouchitis. Aliment. Pharmacol. Ther. 2016, 44, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Sabino, J.; Vandermosten, L.; Joossens, M.; Arijs, I.; de Bruyn, M.; Eeckhaut, V.; Van Assche, G.; Ferrante, M.; Verhaegen, J. Specific Members of the Predominant Gut Microbiota Predict Pouchitis Following Colectomy and IPAA in UC. Gut 2017, 66, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Maharshak, N.; Cohen, N.A.; Reshef, L.; Tulchinsky, H.; Gophna, U.; Dotan, I. Alterations of Enteric Microbiota in Patients with a Normal Ileal Pouch Are Predictive of Pouchitis. J. Crohn’s Colitis 2017, 11, 314–320. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.D.; Walker, A.W.; Churcher, C.; Clark, S.K.; Tekkis, P.P.; Johnson, M.W.; Parkhill, J.; Ciclitira, P.J.; Dougan, G.; Nicholls, R.J. The Bacteriology of Pouchitis: A Molecular Phylogenetic Analysis Using 16S rRNA Gene Cloning and Sequencing. Ann. Surg. 2010, 252, 90. [Google Scholar] [CrossRef] [PubMed]
- Reshef, L.; Kovacs, A.; Ofer, A.; Yahav, L.; Maharshak, N.; Keren, N.; Konikoff, F.M.; Tulchinsky, H.; Gophna, U.; Dotan, I. Pouch Inflammation Is Associated with a Decrease in Specific Bacterial Taxa. Gastroenterology 2015, 149, 718–727. [Google Scholar] [CrossRef]
- Tyler, A.D.; Knox, N.; Kabakchiev, B.; Milgrom, R.; Kirsch, R.; Cohen, Z.; McLeod, R.S.; Guttman, D.S.; Krause, D.O.; Silverberg, M.S. Characterization of the Gut-Associated Microbiome in Inflammatory Pouch Complications Following Ileal Pouch-Anal Anastomosis. PLoS ONE 2013, 8, e66934. [Google Scholar] [CrossRef]
- Rabbenou, W.; Chang, S. Medical Treatment of Pouchitis: A Guide for the Clinician. Ther. Adv. Gastroenterol. 2021, 14, 17562848211023376. [Google Scholar] [CrossRef] [PubMed]
- Segal, J.P.; Kayal, M.; Ardalan, Z.; Garg, M.; Sparrow, M.; Barnes, E. Acute Pouchitis: The Condition That Time Forgot About. Ther. Adv. Gastroenterol. 2022, 15, 17562848221124057. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, Z.S.; Sparrow, M.P. A Personalized Approach to Managing Patients with an Ileal Pouch-Anal Anastomosis. Front. Med. 2020, 6, 337. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, K.L.; Sandler, R.S.; Abreu, M.; Picco, M.F.; Hanauer, S.B.; Bickston, S.J.; Present, D.; Farraye, F.A.; Wolf, D.; Sandborn, W.J. Rifaximin for the Treatment of Active Pouchitis: A Randomized, Double-blind, Placebo-controlled Pilot Study. Inflamm. Bowel Dis. 2007, 13, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.B.; Rosani, A.; Wadhwa, R. Rifampin. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Bergamini, N.; Fowst, G. Rifamycin SV. A Review. Arzneim. Forsch. 1965, 15, S951–S1002. [Google Scholar]
- CLSI. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, 9th ed.; Clinical Standard Laboratory Institute: Wayne, PA, USA, 2018; Volume M11, ISBN 1-56238-987-4. [Google Scholar]
- Yildirim, Z.; Johnson, M.G. Characterization and Antimicrobial Spectrum of Bifidocin B, a Bacteriocin Produced by Bifidobacterium Bifidum NCFB 1454. J. Food Prot. 1998, 61, 47–51. [Google Scholar] [CrossRef]
- Blandino, G.; Fazio, D.; Petronio, G.P.; Inturri, R.; Tempera, G.; Furneri, P.M. Labeling Quality and Molecular Characterization Studies of Products Containing lactobacillus spp. Strains. Int. J. Immunopathol. Pharmacol. 2016, 29, 121–128. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 7th ed.; CLinical and Laboratory Standard Institute, Ed.; CLinical and Laboratory Standard Institute: Wayne, PA, USA, 2006; Volume M7-A7. [Google Scholar]
- Zella, G.C.; Hait, E.J.; Glavan, T.; Gevers, D.; Ward, D.V.; Kitts, C.L.; Korzenik, J.R. Distinct Microbiome in Pouchitis Compared to Healthy Pouches in Ulcerative Colitis and Familial Adenomatous Polyposis. Inflamm. Bowel Dis. 2011, 17, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, M.P.; Schouten, W.R.; van Lieshout, L.M.; Hop, W.C.; Laman, J.D.; Ruseler-van Embden, J.G. Eradication of Pathogenic Bacteria and Restoration of Normal Pouch Flora: Comparison of Metronidazole and Ciprofloxacin in the Treatment of Pouchitis. Dis. Colon Rectum 2004, 47, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Ruseler-van Embden, J.G.; Schouten, W.R.; Van Lieshout, L.M. Pouchitis: Result of Microbial Imbalance? Gut 1994, 35, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Komanduri, S.; Gillevet, P.M.; Sikaroodi, M.; Mutlu, E.; Keshavarzian, A. Dysbiosis in Pouchitis: Evidence of Unique Microfloral Patterns in Pouch Inflammation. Clin. Gastroenterol. Hepatol. 2007, 5, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Molinero, N.; Taladrid, D.; Zorraquín-Peña, I.; de Celis, M.; Belda, I.; Mira, A.; Bartolomé, B.; Moreno-Arribas, M.V. Ulcerative Colitis Seems to Imply Oral Microbiome Dysbiosis. Curr. Issues Mol. Biol. 2022, 44, 1513–1527. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-Intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-Anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Shen, B.; Lashner, B.A. Diagnosis and Treatment of Pouchitis. Gastroenterol. Hepatol. 2008, 4, 355. [Google Scholar]
- Akiyama, S.; Rai, V.; Rubin, D.T. Pouchitis in Inflammatory Bowel Disease: A Review of Diagnosis, Prognosis, and Treatment. Intest. Res. 2021, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).