Abstract
Venomous snakes constitute ecologically significant and medically relevant organisms due to the risks associated with their bites, which frequently result in secondary infections. The oral microbiota of these reptiles plays a crucial role in the pathogenesis of such infections; however, its diversity and clinical implications remain insufficiently characterized. This is the first comprehensive review to systematically trace the methodological evolution in snake oral microbiota research, documenting the paradigm shift from traditional culture-dependent techniques to advanced culture-independent approaches, including next-generation sequencing and metagenomics. Our analysis uniquely demonstrates the transformative impact of these technological advances on bacterial diversity identification and antimicrobial resistance gene detection in venomous species. Environmental factors, captivity conditions, and venom composition significantly influence microbial community structure and resistance profiles. These intricate interactions are essential for improving clinical management of snakebite infections, informing empirical antibiotic therapy protocols, and guiding antivenom production strategies. Additionally, the potential of snake oral microbiota as a source of novel bioactive compounds represents an emerging area of bioprospecting research. This review uniquely bridges microbiology, venomics, and clinical medicine, demonstrating the necessity for integrative, multidisciplinary approaches to fully elucidate the ecological and biomedical significance of oral microbial communities in venomous snakes.
1. Introduction
Venomous snakebites constitute a major global public health concern, with consequences ranging from immediate local effects to severe systemic complications [1]. According to the World Health Organization (WHO), snakebite envenomation is a global health priority, especially in areas with the highest incidence rates, primarily among rural populations in tropical and subtropical regions [2,3]. Recent estimates suggest that snakebite affects approximately 1.8 million persons each year, leading to approximately 94,000 fatalities every year [4,5]. This massive burden is further compounded by the complex interplay among ecological, socioeconomic, and human factors that affect the frequency of such events [5,6].
The WHO’s strategy to reduce snakebite morbidity and mortality emphasizes education and training for both healthcare workers and communities, aiming for faster, more effective responses [7]. Snakebites also have lasting psychological consequences, including post-traumatic stress disorder and depression, often aggravated by disabilities such as amputations. Comprehensive care must therefore address both physical and mental health outcomes [4,8].
Secondary infections post-envenomation, such as cellulitis and necrotizing fasciitis, represent significant complications that can exacerbate patient morbidity [9,10]. Although some venoms have antibacterial activity, snake oral microbiota increase infection risk [11]. Studies reveal that bacterial infections after snakebite frequently require surgery and can cause permanent impairments for those who suffer from them [12]. These insights underline the urgency of addressing snakebite as a public health issue with multifaceted implications. While snake venoms generally possess some antibacterial properties [13], the actual risks associated with the oral flora of snakes have gained attention in recent years. There is evidence that bacteriological profiles of snakebite wounds frequently contain both contaminants and possibly snake salivary microbiome-derived pathogens [11].
A major shift is underway from culture-based to culture-independent methods for studying the oral microbiota of venomous snakes. Traditional microbiological culture-based methods formed the basis of early research but were constrained by special growth requirements that can suppress the growth of certain microbiota [14]. Next-generation sequencing (NGS) now enables comprehensive exploration of snake oral microbiota, overcoming the limitations of traditional culturing methods due to advances in molecular biology [11,13]. These advanced approaches clarify the links between snake venoms, oral bacteria, and post-envenomation complications. High-throughput sequencing now reveals not only the composition of oral microbiota but also how these communities interact with venoms during envenomation [13].
Furthermore, insights gained from this research may guide clinical practices for managing snakebite-related infections and lead to improved outcomes for victims. Culture-based methods provided early insights, its limitations excluded much microbial diversity. Advances such as 16S rRNA gene sequencing and metagenomics now allow a deeper, more accurate understanding of oral microbial communities, surpassing culture-dependent methods in taxonomic resolution (Table 1). Following the development of culture-independent to culture-dependent methods for characterizing the oral microbiota of venomous snakes, this review aims to demonstrate the transformative power of next-generation sequencing technologies in expanding our knowledge of these intricate microbial communities.

Table 1.
Comparative Analysis of Microbiological Methods for Snake Oral Microbiota Research. Comprehensive comparison of traditional and modern approaches used to study microbial communities in venomous snake oral cavities, highlighting the evolution from culture-dependent to culture-independent methodologies and their respective advantages and limitations in characterizing intricate microbiomes.
2. Snakebite Complications and the Role of Oral Microbiota
Secondary infections are particularly concerning due to the nature of the wounds and the environment prone to bacterial invasion that occurs at the bite site. Snake venom can cause significant damage to local tissues, creating a fertile environment for bacterial colonization [18,19]. When a snake bites, the venom that is introduced not only contains toxins that affect the body’s normal function, but can also induce necrosis of nearby tissues that facilitates bacterial entry. This is often observed in wounds from snakes of the genus Bothrops, where complications such as thrombotic microangiopathy have been reported that can lead to acute renal injury and subsequent tissue infection [18,19].
The relationship between snakebites and bacterial superinfection must be considered in the context of wound management. Often, bites can be treated with a combination of antivenom and antibiotics to prevent bacterial colonization and subsequent infections. However, research finds that treatment is often delayed, which can lead to a significant increase in morbidity associated with secondary infections [20]. Poor wound management and unhygienic conditions at the time of injury further elevate infection risk [18]. Wounds that require surgical intervention are more prone to secondary infections, especially if adequate measures are not taken to prevent contamination [21].
2.1. Microbial Diversity in Snake Oral Flora and Post-Envenomation Infection
Numerous microbial species are implicated in secondary snakebite wound infections, based on epidemiological data of snake oral flora. Infected wounds often harbor bacteria such as Enterobacter, Proteus, Morganella morganii, and Aeromonas hydrophila [10,22,23,24,25,26]. These microorganisms have been isolated both from wounds and directly from snake oral cavities, underscoring their clinical relevance and the link between snake microbiota and post-envenomation infections [27].
Analyses of snakebite infections reveal a wide range of Gram-positive and Gram-negative species in wound microbiota. Studies of snake oral flora report pathogens such as Clostridium, coagulase-negative staphylococci, and Pseudomonas aeruginosa [27,28]. Snakebites involving hemotoxic Bothrops snakes often cause significant tissue damage and necrosis, facilitating colonization by pathogens such as P. aeruginosa and Staphylococcus aureus, which are more prevalent due to the extensive necrotic tissue compared to bites from neurotoxic snakes [29]. In contrast, bites from smaller snakes like some Micrurus species may pose different risks; although tissue damage is less severe, their fast-acting venom can lead to respiratory paralysis, complicating both clinical management and infection risk [30].
Anaerobic microorganisms also play a crucial role in the development of secondary infections. The most prominent include Bacteroides fragilis, which is part of the normal intestinal flora and can cause severe infections when it contacts subcutaneous tissue due to injuries such as bites [31]. Clostridium perfringens is known for its association with wound infections and can cause gas gangrene, with its presence being particularly dangerous in the context of snakebites that cause tissue necrosis, although evidence specific to snakebite contexts remains limited [32]. Additionally, Fusobacterium spp. represents another group of anaerobic bacteria potentially involved in secondary post-bite infections, especially in necrotic tissues [31]. These anaerobic pathogens pose significant clinical challenges due to their ability to thrive in the oxygen-depleted environments created by tissue necrosis following envenomation.
Because diet influences microbial composition, bacterial load and diversity vary across snake species and environments [33]. Studies from different regions show that both the snake species and the microbiological profile of infections can vary significantly. For example, in South America, where Bothrops venom is predominant, infections by Pseudomonas and Staphylococcus are more prevalent. In contrast, in Africa and Asia, snakes such as Naja and Crotalus are involved, and the bacterial flora presents a wider variety of potentially pathogenic microorganisms [34,35].
2.2. Clinical Translation and Therapeutic Implications
Monitoring and controlling post-snakebite infections remains challenging, particularly in resource-limited tropical settings where snakebite envenomation represents a significant public health burden. Although empirical advice varies according to local bacterial profiles, empirical antibiotic treatment is usually employed to cover a broad spectrum of likely pathogens [36,37]. Recent research made particular emphasis on the inefficacy of preemptive antibiotics against different local bacterial strains and their corresponding patterns of resistance [38].
Current medical consensus supports empirical antibiotic therapy as essential for managing snakebite complications. The Infectious Diseases Society of America (IDSA) guidelines recommend amoxicillin/clavulanate as standard empirical treatment to mitigate secondary infection risk following animal bites, including snakebites [39]. However, recent evidence suggests that amoxicillin/clavulanate is often ineffective for Bothrops bites because many involved pathogens display resistance [25,37]. In regions such as Taiwan, alternatives such as piperacillin/tazobactam, ciprofloxacin, or combinations of cephalosporins and aminoglycosides have been proposed due to the presence of resistant microorganisms like Enterococcus spp. [26,33]. Geographical specificity of bacterial flora further necessitates customizing empirical antibiotic strategies, as bacterial profiles vary between snake species and habitats [23,40].
The prophylactic use of antibiotics remains debated. WHO guidelines suggest prophylaxis may be indicated in cases with extensive necrosis or when non-sterile instruments have been used [41]. Overuse is a concern, as most snakebite victims do not develop infections requiring antibiotics, increasing the risk of resistance [24]. These findings underscore the importance of understanding the snake oral microbiota and its role in guiding secondary infection management [39,42]. To enhance prevention and treatment and ensure timely, effective care that reduces venom toxicity and infection, future research should focus on establishing comprehensive microbial profiles of snakebite wounds.
Wound infections following snakebites can be prevalent and insidious due to complexities introduced by the venom itself. Snake venoms possess antibacterial properties that may mask ongoing infection and affect the microbiota [22]. Studies show that wounds inflicted by snakes, such as the Taiwan cobra (Naja atra), harbor diverse bacterial populations, often dominated by pathogens like Morganella morganii and Enterococcus faecalis [43,44]. If untreated or mismanaged, such infections may lead to significant morbidity.
Wound culture and antimicrobial sensitivity testing becomes critical following broad-spectrum antibiotic initiation. When empirical treatment fails or infections persist, these diagnostic approaches provide essential information for targeted therapy. Notably, up to 80% of snakebite patients initially diagnosed with cellulitis lack confirmed bacterial infections [24], emphasizing the importance of accurate microbiological assessment to prevent unnecessary antibiotic exposure. The frequent involvement of M. morganii, often resistant to first-line antibiotics, necessitates precise identification through culture-based methods [33,45], as resistance patterns can lead to treatment failures and prolonged hospitalization [46,47].
Species-specific microbial profiles require tailored management approaches, as demonstrated by distinct patterns observed in Bothrops envenomations [25,48]. Case series from Martinique illustrate specific associations between snake species and bacterial infection outcomes, emphasizing the necessity for localized studies of post-envenomation infection dynamics [25]. Optimal culture collection timing requires obtaining specimens before antibiotic administration to accurately capture native microbiota and guide effective therapy [49].
Traditional healing practices and non-evidence-based interventions further complicate clinical outcomes, reflecting critical gaps in health education and antibiotic stewardship awareness [50,51]. Integrating systematic resistance testing into clinical protocols can guide immediate therapeutic decisions while supporting community education initiatives. Culture-guided targeted therapies enable de-escalation from broad-spectrum antibiotics, reducing resistance development risk [47,52]. Continuous surveillance of resistance patterns remains essential for adapting treatment protocols and preserving therapeutic options [48].
Optimal management requires considering wound characteristics and patient health status alongside microbiological findings. Multidisciplinary approaches involving infectious disease specialists provide comprehensive care addressing both envenomation pathophysiology and secondary infectious complications, ultimately improving patient outcomes in this complex clinical scenario.
3. Early Studies Using Culture-Dependent Methods
Initial research with culture-dependent methods has yielded immense information on microbial populations of snakebite infection. However, these methods have significant weaknesses with serious limitations that are critical to bias the accuracy and completeness of estimates of microbial diversity. One of the most significant limitations of traditional culture methods is perhaps a low resolution of microbial diversity (Figure 1). Typically, culture-based techniques isolate only a minuscule percentage, usually cited at 1–10%, of the total microbial variety that exists within an ecosystem, and in challenging ones like infected tissue following a snakebite, where several various bacterial groups coexist but few are identifiable using culturing [53].
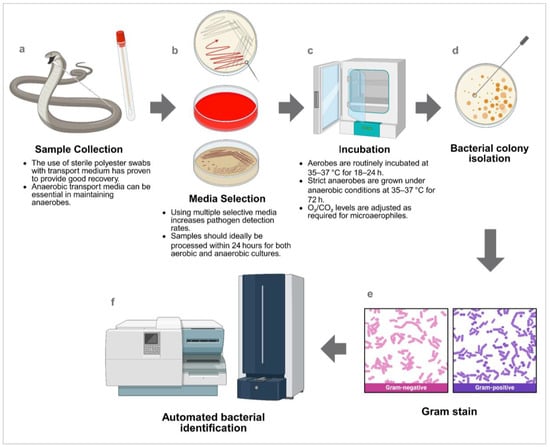
Figure 1.
General workflow for bacterial identification using culture-dependent methods. (a) a biological sample is collected and (b) inoculated onto appropriate culture media, followed by (c) incubation under suitable conditions. After incubation, (d) isolated bacterial colonies are obtained and (e) subjected to Gram staining to differentiate between Gram-positive and Gram-negative bacteria. (f) Pure isolates are then identified using automated systems. This scheme is a general outline; specific steps may vary depending on the bacterial species’ nutritional and atmospheric requirements, as well as the laboratory’s identification equipment. Created in https://BioRender.com. Note: Transport and storage conditions are medium-dependent and must ensure bacterial viability. Incubation parameters (temperature, atmosphere, duration) should be optimized according to target bacterial species and their specific growth requirements. Automated identification platforms utilize diverse methodologies including metabolic profiling, biochemical characterization, and mass spectrometry analysis to achieve species-level classification. Quality control measures and appropriate reference strains are essential for result validation and diagnostic accuracy.
Also, culture-dependent studies are significantly influenced by sampling and transportation methodologies. Sampling strategies critically shape microbiota profiles: individual sampling yields a more representative assessment of taxon richness than pooled sampling, which can dilute rare yet significant populations [54]. Increased sampling effort uncovers greater microbial diversity [55], while storage and transport conditions profoundly affect microbial viability and community composition, necessitating containment measures such as refrigeration to preserve sample integrity [56]. Moreover, contamination from reagents or processing steps can compromise microbiome analyses and misrepresent community structure [57], and temporal variability further influences microbiota composition, indicating that consistent sampling schedules enhance study reliability [58]. Although these methodological insights derive mainly from human and plant microbiome research, their principles remain applicable to snake oral microbiota studies despite limited direct evidence in reptilian systems.
The second fundamental deficiency of culture-based approaches lies in their inbuilt prejudice in favor of aerobic and fast-growing strains. Culture media and conditions employed are generally optimized for specific populations of bacteria that are unrepresentative of the real diversity present in nature. Most of the pathogenic bacteria that cause snakebite are anaerobes or slow growers, hence easily overlooked under standard culture conditions [59]. Consequently, this selectivity will lead to significant underrepresentation of such important pathogens as M. morganii, Providencia sp., and A. hydrophila, which most frequently have been isolated from infected wounds and are causative agents of severe post-envenomation complications [53]. Further, awareness of the existing bacterial community may also influence surgical decision-making and management of complications such as necrotizing fasciitis [60].
Regarding antivenom production, insights from microbial research stress the need for rigorous microbiological investigation. Since antivenoms are designed to counteract the toxic action of snake venoms, they must also be designed to compensate for the possibility of secondary bacterial infection that might complicate the clinical condition of patients. Bacterial population resistance genes could influence treatment efficacy and necessitate multi-target strategies in antivenom formulation [61,62]. By integrating data from both cultured and uncultured populations, researchers can better understand patient pathophysiology and develop improved healthcare strategies.
The substantial limitations of culture-dependent methods, particularly their ability to cultivate only a small fraction of microbial diversity and their bias toward aerobic, fast-growing species, necessitated the development of molecular approaches. These constraints led researchers to adopt culture-independent techniques that could reveal the true diversity of snake oral microbiota communities previously hidden from traditional methods.
4. Transition to Molecular and Culture-Independent Techniques
The shift from culture-dependent to culture-independent methods represents a major advance in studying microbial diversity. Polymerase chain reaction (PCR) and Sanger sequencing of 16S rRNA gene fragments enabled the detection and identification of bacteria previously uncultivable in the lab. By amplifying and sequencing these fragments, Sanger sequencing revealed the structure of microbial communities in diverse environments, including infected snakebite wounds [63,64]. The method has provided the specific taxa present in these samples and established a foundation for the understanding of bacterial infection dynamics.
However, both PCR and Sanger sequencing are constrained in that they do not sequence the entire 16S rRNA gene economically and relatively lack resolution for differentiating between closely related bacteria. Later, next-generation sequencing (NGS) technologies supplied remedies with the capacity to sequence numerous samples in parallel at high throughput. NGS has transformed microbial ecology through the ability to sequence longer amplicons and deeper coverage of microbial diversity, hence solving issues encountered with traditional sequencing methods [65].
Specifically noted is the use of 16S rRNA amplicon sequencing of the targeted hypervariable regions, such as V3–V4 (Figure 2). These are significant when it comes to taxonomic classification and are most widely used in microbiome studies. By applying a two-step PCR method and leveraging high-throughput sequencing technologies, researchers can amplify and sequence such regions to obtain fine-grained insights into microbial diversity among varying samples, e.g., snakebite wounds [63,66]. Sequencing data can be interpreted using alternative frameworks, namely amplicon sequence variants (ASVs) or operational taxonomic units (OTUs). ASVs, or unique sequences at single-nucleotide resolution, have better resolution for classification than OTUs, which cluster sequences by similarity and can lead to oversimplification or loss of some taxonomic information [67,68]. Both approaches are widely used in culture-independent molecular techniques to characterize microbial community structure and function, with OTUs offering simplicity and familiarity but risking inflated richness estimates [69], while ASVs overcome these limitations and enable robust cross-study comparisons, though they demand greater computational resources and careful filtering to avoid sequencing artifacts [70,71].
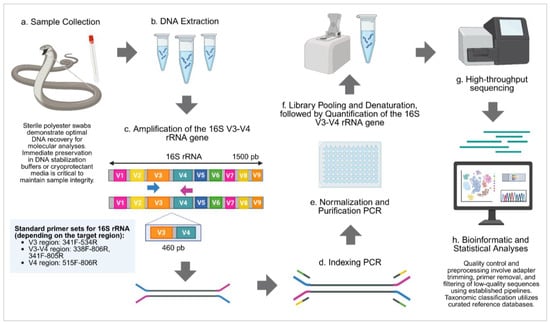
Figure 2.
General workflow for bacterial identification using 16S rRNA amplicon sequencing targeting the V3–V4 hypervariable regions. (a) A biological sample is collected, and (b) DNA is extracted from the sample. Library preparations: (c) The V3–V4 regions of the 16S rRNA gene are amplified, followed by (d) indexing PCR to add adapter sequences. (e) Libraries are then normalized and purified, and (f) pooled and denatured, followed by quantification of the 16S V3–V4 amplicons. (g) The prepared libraries are subjected to high-throughput sequencing. (h) Sequencing data are analyzed through bioinformatic and statistical approaches to determine microbial composition. This scheme is a general outline; specific steps may vary depending on the target microbiome, reagents, and sequencing platform used. Created in https://BioRender.com. Note: Primer selection and protocol optimization depend on the target 16S rRNA hypervariable region, sequencing platform capabilities, and study objectives. Different primer pairs provide varying taxonomic resolution, with V3–V4 offering balanced coverage, V4 maximizing phylogenetic diversity detection, and full-length 16S providing superior species-level discrimination. Platform-specific considerations include read length limitations, amplicon size constraints, and sequencing chemistry compatibility.
It has advantages in having increased higher taxonomic resolution, in which discrimination among closely related bacterial species that might be clumped together in classical culture-based studies can be made. For example, functional amplification and sequencing of the whole 16S rRNA gene can facilitate differentiation at the species level, providing more insight into the richness in microbial infections ensuing from snakebites [72,73]. In addition, examination of the whole gene might provide leeway for debating functional niches of specific microbial taxa, and these can inform treatment protocols and clinical care strategies.
High taxonomic resolution and the broad scope of next-generation sequencing (NGS) greatly advance microbial ecology in snakebite disease by enabling detection of rare and uncultivated species. This knowledge is clinically valuable, as identifying pathogenic organisms helps guide antibiotic therapy and intervention after envenomation. Additionally, microbiome analysis can improve antivenom safety and efficacy by revealing interactions between bacterial infections and venom components, ultimately impacting patient outcomes [74,75].
4.1. Current Advances: Full-Length 16S rRNA Gene Sequencing and Metagenomics
Recent studies using next-generation sequencing have more accurately identified bacterial communities in venomous snakes’ oral cavities. Technologies like Oxford Nanopore and PacBio now enable full-length 16S rRNA gene sequencing, allowing improved resolution in microbial taxonomic identification. For example, in Lin et al.’s work in 2023 [42], the authors used high-throughput sequencing to explore the oral microbiota of certain species of venomous snakes by sequencing and amplifying the full-length 16S rRNA gene, including hypervariable regions V1–V9. Rich microbial diversity was observed, which was responsible for the ecological roles of these bacteria within the snakes’ environments [42].
Current research describes the dominant microbial phyla of the oral and fecal microbiota of snakes, such as Bacteroidetes, Proteobacteria, Firmicutes, and Fusobacteria [76,77]. The fecal microbiota of the Red Back Pine Root Snake (Oligodon formosanus) and Chinese Slug-Eating Snake (Pareas chinensis) have been specifically characterized, with research describing these dominant phyla and reporting significant differences in the composition of the microbiome between these two species, suggesting ecological and evolutionary adaptation [76].
Shotgun metagenomics enables taxonomic and functional profiling, revealing metabolic potential critical to host-microbiota interactions [78]. Applying shotgun metagenomics to reptilian oral microbiomes has enhanced understanding of both pathogenic potential and community resilience. Recent studies have identified specific pathogens and revealed dynamics related to their ecological niches [6]. The studies are fundamental since they not only document the microbial diversity but also establish connections to potential health effects due to microbiome dysbiosis in snakes.
High-throughput methods are vital for understanding reservoirs of zoonotic pathogens and ecological impacts, as snake oral microbiota may harbor antibiotic resistance genes of public health concern. Metagenomic research also demonstrates that specific feeding habits shape oral microbiota, answering key questions about the relationship between diet and microbial ecology in snakes [79].
Furthermore, the exploration of dietary correlates with metagenomic research has identified how specific feeding habits determine oral microbiota structure in snakes [80]. This resolves fundamental questions concerning diet interaction with microbial ecology, where specific dietary specialization can enhance microbial taxa development that facilitates adaptability to their environments.
4.2. Limitations and Challenges of Molecular and Culture-Independent Techniques
The study of snake oral microbiota has been revolutionized by culture-independent molecular approaches, particularly 16S rRNA amplicon sequencing and shotgun metagenomics. These methods enable comprehensive exploration of microbial diversity within snake oral cavities, revealing complex ecological dynamics, pathogen interactions, and potential sources of novel antimicrobial compounds. However, methodological factors such as DNA extraction protocols and the selection of hypervariable regions for 16S rRNA sequencing significantly influence bacterial community recovery and representation. Understanding these technical considerations is essential for accurate interpretation of microbial diversity patterns and their biological implications in snake oral ecosystems.
Various DNA extraction methods present differing efficiencies in lysing bacterial cells. Mechanical lysis is effective in breaking down the robust cell walls of Gram-positive bacteria, characterized by thick peptidoglycan layers, which can be resistant to enzymatic lysis alone [81]. Studies indicate that mechanical disruption methods yield higher total DNA concentrations and more representative microbial community profiles than purely enzymatic methods [81,82]. Techniques involving bead beating or mechanical disruption are essential for extracting DNA from bacteria like Bifidobacterium and Lactobacillus, which are abundant in the oral microbiome but notoriously difficult to lyse [33,83]. In contrast, enzymatic lysis may result in incomplete cell lysis, leading to biased representation, especially toward Gram-negative bacteria, which are generally more susceptible to lysis under these conditions [81,82].
Amplification of 16S rRNA gene regions also plays a critical step, as primer choice strongly influences bacterial representation. Universal primers may unevenly amplify taxa, biasing community profiles and obscuring rare or novel bacteria with ecological relevance [84,85]. The hypervariable regions selected for sequencing—such as V3–V4, V1–V9, or V4—play a critical role in determining the taxonomic resolution of bacterial communities detected. Different hypervariable regions exhibit varying degrees of sequence variability and thus provide differing levels of resolution across bacterial phyla [33,83]. For example, the V3–V4 region is widely adopted due to its balance of coverage and resolution, often yielding a more representative view of microbial diversity. However, selecting other regions, like V1–V9 (Full-length 16S rRNA), may provide insights into additional taxa that the V3–V4 region might miss, emphasizing the importance of the choice of region on community profiling [86]. Sequencing the V4 region alone generally enables genus-level identification but often lacks species-level resolution, particularly among closely related taxa [87]. Although it provides a broad overview of microbial communities, it may overlook subtle taxonomic differences essential for understanding ecological roles, interactions, and health implications [88]. Similarly, the V3 region captures high variability and partially overlaps with V4 but also struggles to discriminate closely related species [89].
The choice of sequencing technology, whether Sanger sequencing, Illumina, or newer platforms like Oxford Nanopore, can also define the depth and breadth of microbial diversity captured. Illumina sequencing tends to provide higher throughput and more precise taxonomic resolution, which is beneficial for detailed community analyses [90]. On the other hand, while long-read technologies such as Oxford Nanopore or PacBio can facilitate the assembly of full-length genes, they may introduce a higher error rate, influencing downstream analyses and the accuracy of phylogenetic placement [91].
To further illustrate the interplay between hypervariable region selection impacting bacterial community analysis in snakes, the following Table 2 summarizes some key studies:

Table 2.
Comparative outcomes of different 16S rRNA gene regions in snake oral microbiota analysis. Comparative analysis of snake microbiota studies across different 16S rRNA regions reveals significant methodological influence on community characterization outcomes. V3 region sequencing achieved high species-level resolution but exhibited limited phylogenetic breadth, while V4 region studies demonstrated superior taxonomic coverage with broader phylogenetic diversity. Full-length 16S rRNA sequencing provided the most comprehensive taxonomic resolution from phylum to species level.
Besides the need for methodological rigor, notorious limitations remain in data interpretations. Bioinformatics workflows such as ASaiM integrate multiple steps for user-friendly microbiota analysis [96], while gNOMO merges metagenomic and metatranscriptomic data for in-depth functional study [97]. Reliable results depend on proper sampling and sequencing quality control, including k-mer analysis and mapping to gene catalogs [98], ensuring that sample profiles truly represent underlying microbiota [99]. Despite progress, important metagenomic limitations persist. Short-read sequencing, though affordable, results in fragmented assemblies and struggles with intricate or repetitive genomes [100,101]. Long-read sequencing improves continuity and the resolution of complex regions, but its higher costs and lower throughput limit broader application [101,102].
Interpreting the functional impact of microbial community composition remains difficult. While metagenomics identifies which microbes are present, connecting their presence to functional roles such as pathogenicity or antimicrobial resistance requires further experimental validation [46,103]. Ecosystem interactions are especially multifaceted in dynamic environments, complicating analysis [104]. Moving forward, it is essential to standardize methodologies across research studies to allow for better comparability of results, particularly in the context of oral microbiota from diverse species of venomous snakes. Additionally, enhancing the databases used for comparison and improving computational tools for data analysis will be paramount in leveraging metagenomic insights.
5. Microbiota-Host-Venom Interactions and Ecological Considerations
The relationships among microbiota, host species, and venom in reptiles, especially venomous snakes, offer insights into ecology, evolution, and health. Oral microbiota composition responds to factors like captivity, diet, and habitat quality. Research shows that captivity significantly reduces oral microbiota diversity and richness compared to wild conditions (Figure 3). For example, studies found the intestinal bacterial communities of red-crowned cranes differ markedly between captive and semi-free-range settings due to variations in diet and habitat [105]. This shows captivity is not only crucial for microbial diversity but also for host health and ecological adaptability.
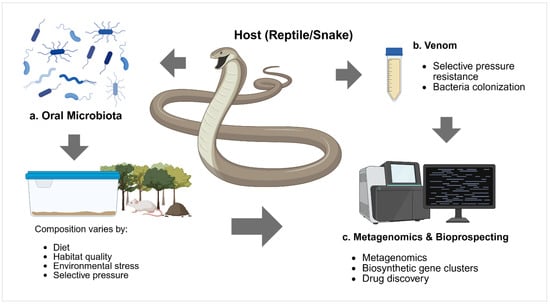
Figure 3.
Conceptual framework illustrating the complex interactions between oral microbiota, host (reptile/snake), and venom in the context of metagenomics and bioprospecting research. (a) Oral microbiota consists of diverse bacterial communities whose composition varies according to multiple factors, including diet, habitat quality, environmental stress, and selective pressure from the host’s biological systems. (b) Venom represents a unique ecological niche that exerts selective pressure on microbial communities, leading to bacterial colonization and potential development of resistance mechanisms. (c) Metagenomics and bioprospecting encompass the application of culture-independent molecular techniques, including next-generation sequencing, identification of biosynthetic gene clusters, and drug discovery approaches to explore the untapped potential of venom-associated microbiomes for novel antimicrobial compounds and therapeutic applications. Created in https://BioRender.com.
Similarly, extreme variations in microbial diversity and function have been reported in captive alpine musk deer versus their free animals, demonstrating the extent to which captivity conditions and dietary supplies may fundamentally alter the oral microbiome [106].
The host genus’s role on oral microbiota has also been highlighted, demonstrating that there are unique microbial signatures to different primate species, as a result of diet and being captive [107]. Snake venom may exert selective pressure on oral microbial communities, as venom components affect not only prey but also the viability and structure of bacteria colonizing the snake’s mouth.
Environmental stresses like venom profile and diet might have significant effects on microbial resistance profiles of snakes [108,109]. The dynamic interaction between venom and microbiota can lead to the creation of new resistant strains, either through natural selection or horizontal gene transfer, potentially enabling these bacteria to grow in extreme conditions. Microbial resistance mechanisms may reveal new bioactive compounds for therapeutic development [110,111], as snake venoms and microbiota form a biological niche with unexploited bioprospecting potential [112].
As new methods illuminate the diversity and functions of these microbial communities, opportunities arise to explore their antimicrobial potential, genetic capacity for bioactive compounds, and evolutionary parallels with other vertebrates.
5.1. Antimicrobial Potential & Biosynthetic Gene Clusters in Snake Oral Microbiota
Venomous snake oral microbiota includes diverse taxa capable of producing antimicrobial compounds, especially antimicrobial peptides (AMPs) that help maintain ecological balance and prevent infections from prey or the environment [113]. These molecules are instrumental not only in defending snakes against infections but also in aiding them in their ecological roles. AMPs have garnered considerable interest in both ecological and medical research due to their evolutionarily conserved mechanisms and potential applications in combating antibiotic-resistant infections.
Species such as Bothrops asper and Bothrops jararaca produce venoms with well-documented antimicrobial properties, including enzymes like L-amino acid oxidase, which represent promising sources of unique molecular targets against resistant pathogens [114,115]. Beyond venom components, the Bothrops microbiome itself contributes to antimicrobial potential. Studies have identified biosynthetic gene clusters (BGCs) encoding antimicrobial peptides (AMPs), revealing natural biosynthetic pathways unique to these microorganisms [77]. Enzymes from these BGCs directly shape microbial community composition and may influence host health. Genomic and metagenomic approaches enable precise mapping of these clusters, highlighting bacteria from venom environments as promising candidates for novel antimicrobial discovery [116].
AMPs such as cathelicidins are central to innate immune defense in sea snakes, with strong protective activity against infections [117]. By analogy, cathelicidin-like peptides in Bothrops microbiota may provide similar protective and therapeutic functions. A notable example is cathelicidin-BF, active against Streptococcus pneumoniae and with additional anti-inflammatory potential [118]. These findings underscore the versatility of snake-derived cathelicidins, combining antimicrobial efficacy with immune modulation for multifunctional therapeutics.
Other venom-related enzymes also contribute antimicrobial effects. Phospholipases A2 (PLA2s), abundant in Bothrops venoms, disrupt bacterial membranes and significantly impair viability [119]. Novel PLA2 variants with enhanced antibacterial potency could further expand options for antibiotic development. In addition, venom peptides such as disintegrins and metalloproteinases display bactericidal activity against pathogens like Escherichia coli and Staphylococcus aureus [120], while rattlesnake-derived fragments have shown antimicrobial properties suitable for topical and systemic applications [121].
The composition of snake oral microbiota varies across species, reflecting co-evolutionary adaptations that optimize antimicrobial traits [38,122]. Ecological interactions between pathogenic and beneficial bacteria within their oral cavities demonstrate a balance that may inspire strategies to promote beneficial strains while suppressing pathogens. Moreover, bacteria colonizing snake fangs can modulate venom activity, creating selective pressures favoring the emergence of more potent AMPs [123].
These evolutionary and ecological dynamics underline the value of interdisciplinary approaches. Metagenomic and proteomic studies provide critical insights into functional microbial capacities and venom-microbiota interactions, facilitating the identification of bioactive compounds capable of augmenting or mimicking conventional antimicrobials. Collectively, venomous snakes emerge as reservoirs of bioactive molecules with significant potential for antimicrobial drug development.
5.2. Parallels with Amphibian Skin Microbiota
The antimicrobial properties observed in snake oral microbiota draw notable parallels with the skin microbiota of amphibians, which have long been acknowledged for their rich repertoire of AMPs that serve as chemical defenses against pathogens [113]. Amphibians, similarly to snakes, live in environments where microbial exposure is significant, necessitating the evolution of robust antimicrobial strategies. Research has revealed that amphibian skin secretions contain diverse bioactive peptides that exhibit potent antimicrobial and antifungal activities. These compounds represent evolutionary adaptations that protect against threats posed by numerous microorganisms [113,124]. Studying microbial communities in snakes and amphibians provides valuable opportunities for cross-species bioprospecting and reveals shared evolutionary traits underlying antimicrobial capabilities.
5.3. The Role of Snake Venoms in Antimicrobial Defense
The microbiota and venoms of snakes both offer promising sources for bioprospecting antimicrobial agents. Snake venoms contain proteins and peptides, such as phospholipases A2 (PLA2s) and secreted phospholipases, with broad-spectrum antimicrobial activity that may be developed for therapeutic use [125,126]. Venom’s ability to inhibit or kill bacteria reflects evolutionary pressures and suggests potential applications not only for antivenom production but also for combating resistant pathogens [113,124,125].
To effectively harness these antimicrobial resources, advanced genomic and metagenomic techniques are needed to identify and characterize biosynthetic gene clusters involved in antimicrobial production [77]. Comparative studies between snake oral microbiomes and amphibian skin microbiomes can reveal conserved antimicrobial pathways and peptide variations with potent activity [113,124]. Researchers should systematically investigate venom diversity across snake species, focusing on structure-function relationships that influence antimicrobial effectiveness [125]. Pharmacological studies are essential to evaluate the efficacy and safety profiles of these novel compounds and inform potential clinical applications for treating multidrug-resistant infections [127].
6. Research Gaps and Future Directions
Tropical and subtropical snake oral microbiota remain under-studied despite advances such as shotgun metagenomics and full-length 16S rRNA gene sequencing, creating a geographic bias favoring temperate ecosystems and limiting a comprehensive understanding of global microbiome diversity [128,129,130]. Consequently, medically important species, particularly African and Indian Elapidae and Viperidae, remain poorly characterized, constraining insights into host–microbe interactions during envenomation [23,77,131]. Functional diversity also remains overlooked, despite evidence that geographic differences significantly shape microbial composition and ecological roles [42]. This sampling bias not only restricts our understanding of tropical ecosystems but also limits interpretation of microbial interactions and their impacts on host organisms within these regions.
Methodological discrepancies further hinder progress, as inconsistent sampling, sequencing, and processing methods yield markedly different microbial profiles, complicating replication and cross-study comparisons [132,133]. Standardized protocols are therefore essential to ensure reproducibility, enable comparative analyses, and support broader ecological conclusions.
A particularly neglected area is the link between oral microbiota and venom composition. Microbes may metabolically interact with venom molecules, influencing their efficacy, potency, and ecological functions in prey immobilization or defense [134,135]. Exploring these dynamics could provide novel insights into snake biology, envenomation, and therapeutic strategies in humans. This is especially critical in tropical regions, where venomous species are highly diverse and snakebite remains a neglected public health issue [134]. Moreover, environmental changes such as climate shifts may alter microbial interactions and species distributions, emphasizing the need to anticipate future ecological and health impacts [136].
Metagenomic exploration of biosynthetic gene clusters (BGCs) further expands the scope of snake oral microbiome research. Advances in bioinformatics enable the prediction of BGCs encoding secondary metabolites with pharmaceutical and ecological importance, including antibiotics and antifungals [137]. Recent studies show that workflows integrating long- and short-read sequencing can successfully uncover novel BGCs [138], while pipelines such as antiSMASH facilitate their identification and functional characterization [137]. These tools add significant value by highlighting the metabolic potential of snake-associated microbiota and their possible roles in producing unique compounds with ecological and biomedical applications.
To advance this field effectively and address the critical knowledge gaps identified throughout this review, we propose the following prioritized research roadmap that integrates methodological improvements, geographical expansion, and clinical translation:
- Expand Geographical Representation: There is an urgent need to broaden the geographical scope of microbiome studies to encompass regions with high incidences of snake envenomations, such as Sub-Saharan Africa, where annual cases exceed 435,000, and Latin America, which reports approximately 57,500 cases yearly [11,40]. This expansion will facilitate a nuanced understanding of regional variations in snake oral microbiota and their ecological roles in post-bite infections. Systematic studies focusing on medically important venomous species from underrepresented regions will illuminate the global picture of snake microbiomes and their clinical implications [40,139].
- Establish Standardized Methodological Protocols: To enhance reproducibility and enable meaningful cross-study comparisons, international consensus guidelines must encompass sampling techniques, DNA extraction methods (mechanical vs. enzymatic lysis), hypervariable region selection (V1–V3 vs. V3–V4 vs. full-length 16S), and bioinformatics pipelines [140,141]. Standardization will ensure consistency across laboratories and regions, advancing field quality and data reliability in snake oral microbiota research.
- Characterize Antimicrobial Resistance Profiles and Clinical Implications: The growing prevalence of multidrug-resistant infections necessitates systematic documentation of resistance patterns in snake oral microbiota, with current studies suggesting 60–69% resistance to common antibiotics. Further investigation into venom-resistant bacterial strains and development of evidence-based antibiotic protocols for post-bite management is crucial to combat increasing antibiotic-resistant infections [11,23,84,142].
- Investigate Venom-Microbiome-Host Interactions and Temporal Dynamics: Understanding the complex relationships among venom composition, microbial communities, and envenomation outcomes requires longitudinal studies capturing seasonal variations, developmental changes, and environmental influences on microbiome stability and pathogenicity. These dynamic interactions may reveal how microbial communities modulate venoms and contribute to clinical outcomes following snakebites, leading to improved clinical care protocols and therapeutic strategies [77,143].
- Develop Microbiome-Guided Precision Medicine Approaches: Integration of functional metagenomics and host-specific microbiome profiling can enable personalized medicine for snake envenomation treatment. By characterizing individual patient microbiomes and identifying functional capabilities, clinicians can optimize antibiotic selection based on predicted resistance profiles and potentially harness microbial metabolites for therapeutic applications [144,145]. This precision medicine approach could revolutionize snakebite management and improve patient outcomes.
Addressing these pressing research gaps through expanded geographic representation, standardized protocols, antimicrobial resistance characterization, dynamic interaction investigations, and precision medicine development will enhance our understanding of snake oral microbiota and significantly impact public health efforts in snakebite prevention and treatment. The concerted effort to study ecological interactions using an integrated approach will elucidate microbiota functions in reptile biology and inform broader ecological and conservation objectives in tropical biodiverse environments.
7. Conclusions
This review demonstrates that the methodological evolution from culture-dependent to culture-independent techniques has fundamentally transformed our understanding of snake oral microbiota, revealing complex ecosystems with direct clinical and ecological implications. The transition to shotgun metagenomics and full-length 16S rRNA sequencing has unveiled previously hidden microbial diversity, identifying core pathogenic taxa such as Enterobacter, Morganella morganii, and Aeromonas hydrophila that directly contribute to post-envenomation infection risk. Snake oral microbiomes harbor species-specific bacterial signatures influenced by diet, habitat, and evolutionary history, with venom composition creating unique selective pressures that foster antimicrobial resistance mechanisms. Current empirical antibiotic protocols often fail due to intrinsic resistance patterns to common antibiotics necessitating species-specific treatment guidelines. Furthermore, these microbiomes represent untapped reservoirs of biosynthetic gene clusters encoding novel antimicrobial compounds, offering promising pharmaceutical opportunities for combating multidrug-resistant infections.
Critical knowledge gaps persist, particularly the geographic bias toward Asian species that limits global applicability concerning medically important African and Latin American species responsible for over 490,000 annual envenomations. Methodological inconsistencies across studies impede cross-comparative analyses and reproducibility, while the complex relationships between venom composition, microbial communities, and temporal dynamics remain poorly understood. The prioritized research agenda outlined requires expanding geographical representation to high-burden regions, establishing standardized protocols for sampling and analysis, characterizing antimicrobial resistance profiles for evidence-based treatment, investigating venom–microbiome–host interactions through longitudinal studies, and developing microbiome-guided precision medicine approaches.
Future research must integrate venomics, microbiome science, and clinical medicine to address the growing threat of antibiotic-resistant post-bite infections while harnessing the therapeutic potential of these unique microbial ecosystems. This multidisciplinary approach will transform snakebite management from empirical treatment to precision medicine, ultimately improving patient outcomes in regions where snakebite remains a neglected public health crisis.
Author Contributions
Conceptualization, S.Y.Y. and A.P.-L.; methodology, S.Y.Y.; writing—original draft preparation, S.Y.Y.; writing—review and editing, S.Y.Y.; visualization, S.Y.Y.; supervision, A.P.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable.
Acknowledgments
We would like to express our sincere gratitude to Hildaura E. Acosta de Patiño, Director of the Center for Research and Information on Medicines and Toxics (CIIMET), for always granting us access to specimens and laboratory facilities for the development of our research. We are also grateful for her continuous support, guidance, and for providing all the necessary information and resources.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| WHO | World Health Organization |
| NGS | Next-Generation Sequencing |
| PCR | Polymerase Chain Reaction |
| rRNA | ribosomal Ribonucleic Acid |
| ASVs | Amplicon Sequence Variants |
| OTUs | Operational Taxonomic Units |
| PacBio | Pacific Biosciences |
| IDSA | Infectious Diseases Society of America |
| AMPs | Antimicrobial Peptides |
| BGCs | Biosynthetic Gene Clusters |
References
- Valencia, B.M.; Zavaleta, A. La medicina complementaria en el tratamiento de las enfermedades tropicales desatendidas: Accidentes ofídicos. Rev. Peru. Med. Integr. 2017, 2, 58–67. [Google Scholar] [CrossRef][Green Version]
- Ahmed, S.; Koudou, G.B.; Bagot, M.; Drabo, F.; Bougma, W.R.; Pulford, C.; Bockarie, M.; Harrison, R.A. Health and economic burden estimates of snakebite management upon health facilities in three regions of southern Burkina Faso. PLoS Negl. Trop. Dis. 2021, 15, e0009464. [Google Scholar] [CrossRef]
- Kaulgud, R.S.; Hasan, T.; Vanti, G.L.; Kurjogi, M.M.; Astagimath, M.; Veeresh, S.; Belur, S. Nucleotidase as a Clinical Prognostic Marker in snakebites: A prospective study. Indian J. Crit. Care Med. 2025, 29, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.S.; A Wijesinghe, C.; Jayamanne, S.F.; A Buckley, N.; Dawson, A.H.; Lalloo, D.G.; De Silva, H.J. Delayed psychological morbidity associated with snakebite envenoming. PLoS Negl. Trop. Dis. 2011, 5, e1255. [Google Scholar] [CrossRef]
- Martín, G.; Erinjery, J.J.; Ediriweera, D.; de Silva, H.J.; Lalloo, D.G.; Iwamura, T.; Murray, K.A. A mechanistic model of snakebite as a zoonosis: Envenoming incidence is driven by snake ecology, socioeconomics and its impacts on snakes. PLoS Negl. Trop. Dis. 2022, 16, e0009867. [Google Scholar] [CrossRef]
- Martín, G.; Erinjery, J.; Ediriweera, D.; de Silva, H.J.; Lalloo, D.G.; Iwamura, T.; Murray, K.A. Redefining snakebite envenoming as a zoonosis: Disease incidence is driven by snake ecology, socioeconomics and anthropogenic impacts. bioRxiv 2021. [Google Scholar] [CrossRef]
- Chippaux, J.P.; Massougbodji, A.; Habib, A.G. The WHO strategy for prevention and control of snakebite envenoming: A sub-Saharan Africa plan. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190083. [Google Scholar] [CrossRef]
- Aglanu, L.M.; Amuasi, J.H.; Schut, B.A.; Steinhorst, J.; Beyuo, A.; Dari, C.D.; Agbogbatey, M.K.; Blankson, E.S.; Punguyire, D.; Lalloo, D.G.; et al. What the snake leaves in its wake: Functional limitations and disabilities among snakebite victims in Ghanaian communities. PLoS Negl. Trop. Dis. 2022, 16, e0010322. [Google Scholar] [CrossRef]
- Essafti, M.; Fajri, M.; Rahmani, C.; Abdelaziz, S.; Mouaffak, Y.; Younous, S. Snakebite envenomation in children: An ongoing burden in Morocco. Ann. Med. Surg. 2022, 77, 103574. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.W.; Wang, J.D.; Huang, J.A.; Hu, S.Y.; Wang, L.M.; Tsan, Y.T. Wound infections secondary to snakebite in central Taiwan. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 272–276. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; Bonilla-Aldana, J.L.; Ulloque-Badaracco, J.R.; Al-Kassab-Córdova, A.; Hernandez-Bustamante, E.A.; Alarcon-Braga, E.A.; Siddiq, A.; Benites-Zapata, V.A.; Rodriguez-Morales, A.J.; Luna, C.; et al. Snakebite-associated infections: A systematic review and meta-analysis. Am. J. Trop. Med. Hyg. 2024, 110, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, A.; Dalhat, M.M.; Joseph, B.O.; Ahmed, A.; Nguku, P.; Poggensee, G.; Adeiza, M.; Yahya, G.I.; Hamza, M.; Habib, Z.G.; et al. Predictors of depression among patients receiving treatment for snakebite in General Hospital, Kaltungo, Gombe State, Nigeria: August 2015. Int. J. Ment. Health Syst. 2017, 11, 26. [Google Scholar] [CrossRef]
- Wagener, M.; Naidoo, M.; Aldous, C. Wound infection secondary to snakebite. S. Afr. Med. J. 2017, 107, 315–319. [Google Scholar] [CrossRef]
- Maduwage, K.; Isbister, G.K. Current treatment for venom-induced consumption coagulopathy resulting from snakebite. PLoS Negl. Trop. Dis. 2014, 8, e3220. [Google Scholar] [CrossRef]
- Ahannach, S.; Delanghe, L.; Spacova, I.; Wittouck, S.; Van Beeck, W.; De Boeck, I.; Lebeer, S. Microbial enrichment and storage for metagenomics of vaginal, skin, and saliva samples. iScience 2021, 24, 103306. [Google Scholar] [CrossRef] [PubMed]
- Houttu, V.; Boulund, U.; Nicolaou, M.; Holleboom, A.G.; Grefhorst, A.; Galenkamp, H.; Born, B.-J.v.D.; Zwinderman, K.; Nieuwdorp, M. Physical activity and dietary composition relate to differences in gut microbial patterns in a multi-ethnic cohort-the HELIUS study. Metabolites 2021, 11, 858. [Google Scholar] [CrossRef]
- Gulyás, G.; Kakuk, B.; Dörmő, Á.; Járay, T.; Prazsák, I.; Csabai, Z.; Henkrich, M.M.; Boldogkői, Z.; Tombácz, D. Cross-comparison of gut metagenomic profiling strategies. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Abuabara-Franco, E.; Rico-Fontalvo, J.E.; Leal-Martínez, V.; Pájaro-Galvis, N.; Bohórquez-Rivero, J.; Barrios, N.d.J.; Ortega-Gaibao, M.F.; Figueroa-Quintero, M. Lesión renal aguda secundaria a mordedurade serpiente del génerobothrops: A propósitode un caso. Rev. Colomb. Nefrol. 2022, 9, e536. [Google Scholar] [CrossRef]
- Maguiña-Vargas, C.; Chincha-Lino, O.; Vilcapoma-Balbín, P.; Morante, D. Actualización en clínica y terapia de mordedura de serpiente (ofidismo). Rev. Med. Hered. 2020, 31, 48–55. [Google Scholar] [CrossRef]
- Miralda Méndez, S.T. Caracterización clínica del paciente pediátrico atendido por mordedura de serpiente, Hospital Escuela, Tegucigalpa, 2015–2019. Rev. Med. Hondureña 2021, 89, 24–28. [Google Scholar] [CrossRef]
- Quiroga-Centeno, A.C.; Hoyos-Rizo, K.; Chaparro-Zaraza, A.F.; Pinilla-Merchán, P.F.; Chávez, M.C.P.; Serrano-Pastrana, J.P.; Ochoa, S.A.G. Infección temprana de la malla quirúrgica en herniorrafia incisional. Incidencia, factores de riesgo y desenlaces en más de 60.000 pacientes. Rev. Colomb. Cir. 2022, 37, 194–205. [Google Scholar] [CrossRef]
- Paul, A.; Joseph, J.J.; Saijan, S.; Sebastian, S.; Tom, A.A.; Iqbal, T. Unveiling the potential threat of bacterial oral flora of snake in snake bite envenomation: A case report. Infect. Dis. Clin. Pract. 2021, 29, e184–e185. [Google Scholar] [CrossRef]
- Chuang, P.C.; Lin, W.H.; Chen, Y.C.; Chien, C.C.; Chiu, I.M.; Tsai, T.S. Oral bacteria and their antibiotic susceptibilities in Taiwanese venomous snakes. Microorganisms 2022, 10, 951. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chen, Y.-C.; Goh, Z.N.L.; Seak, C.-K.; Seak, J.C.-Y.; Shi-Ying, G.; Seak, C.-J.; SPOT Investigators. Wound infections of snakebites from the venomous Protobothrops mucrosquamatus and Viridovipera stejnegeri in Taiwan: Bacteriology, antibiotic susceptibility, and predicting the need for antibiotics—A BITE study. Toxins 2020, 12, 575. [Google Scholar] [CrossRef]
- Resiere, D.; Mehdaoui, H.; Névière, R.; Olive, C.; Severyns, M.; Beaudoin, A.; Florentin, J.; Brouste, Y.; Banydeen, R.; Cabié, A.; et al. Infectious complications following snakebite by Bothrops lanceolatus in Martinique: A case series. Am. J. Trop. Med. Hyg. 2020, 102, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-C.; Huang, S.-T.; Lai, W.-C.; Yang, C.-C.; Hung, D.-Z.; Liu, P.-Y.; Hung, Y.-M. Bacteriology of Naja atra snakebite wound and its implications for antibiotic therapy. Am. J. Trop. Med. Hyg. 2016, 94, 1129–1135. [Google Scholar] [CrossRef]
- Dehghani, R.; Sharif, M.R.; Moniri, R.; Sharif, A.; Kashani, H.H. The identification of bacterial flora in oral cavity of snakes. Comp. Clin. Pathol. 2016, 25, 279–283. [Google Scholar] [CrossRef]
- Brenes-Chacon, H.; Gutiérrez, J.M.; Avila-Aguero, M.L. Use of antibiotics following snakebite in the era of antimicrobial stewardship. Toxins 2024, 16, 37. [Google Scholar] [CrossRef]
- Amador Ahumada, C.; Luna Rondón, J.M.; Puello Alcocer, E.C. Comportamiento de la intoxicación moderada y severa por Ofidiotoxicosis Bothrópica en Córdoba- Colombia. Rev. Av. Salud 2017, 2, 7–15. [Google Scholar] [CrossRef]
- Zapata, J.; Carvallo, A. Complicaciones Asociadas a Tratamiento Biológico en Pacientes con Artritis Reumatoide: Consideraciones en relación a un Caso Clínico. Rev. Chil. Reumatol. 2023, 35, 55–58. [Google Scholar] [CrossRef]
- Santisteban, R.R.; Muñoz-Rodríguez, L.C.; Díaz Nieto, J.; Pachón Londoño, V.; Curiel Peña, J. Seroprevalencia del virus de inmunodeficiencia felina (VIF) y el virus de la leucemia felina (ViLeF) en gatos del centro de Risaralda, Colombia. Rev. Investig. Vet. Peru. 2021, 32, e18901. [Google Scholar] [CrossRef]
- Sierra, Y.D.; Vence, N.; Herrera, P.; Cañate, A.S.; Vanegas, J. Parásitos gastrointestinales en mamíferos silvestres cautivos en el Centro de Fauna de San Emigdio, Palmira (Colombia). Rev. Fac. Med. Vet. Zootec. 2021, 67, 230–238. [Google Scholar] [CrossRef]
- Mao, Y.-C.; Chuang, H.-N.; Shih, C.-H.; Hsieh, H.-H.; Jiang, Y.-H.; Chiang, L.-C.; Lin, W.-L.; Hsiao, T.-H.; Liu, P.-Y. An investigation of conventional microbial culture for the Naja atra bite wound, and the comparison between culture-based 16S Sanger sequencing and 16S metagenomics of the snake oropharyngeal bacterial microbiota. PLoS Negl. Trop. Dis. 2021, 15, e0009331. [Google Scholar] [CrossRef]
- Martinez-Montalvo, C.M.; Cortes, C.; Arévalo-Romero, A. Una neumonía complicada por un germen inusual: Reporte de caso. Infectio 2020, 24, 255–258. [Google Scholar] [CrossRef]
- Loza Sánchez, E.H. INFECCIÓN DE PIEL Y PARTES BLANDAS, TRATAMIENTO EN EDAD PEDIÁTRICA. Enfermería Investig. 2024, 9, 53–63. [Google Scholar] [CrossRef]
- Lin, J.H.; Sung, W.C.; Mu, H.W.; Hung, D.Z. Local cytotoxic effects in cobra envenoming: A pilot study. Toxins 2022, 14, 122. [Google Scholar] [CrossRef]
- Sachett, J.A.G.; Da Silva, I.M.; Alves, E.C.; Oliveira, S.S.; Sampaio, V.S.; Vale, F.F.D.; Romero, G.A.S.; Dos Santos, M.C.; Marques, H.O.; Colombini, M.; et al. Poor efficacy of preemptive amoxicillin clavulanate for preventing secondary infection from Bothrops snakebites in the Brazilian Amazon: A randomized controlled clinical trial. PLoS Negl. Trop. Dis. 2017, 11, e0005745. [Google Scholar] [CrossRef] [PubMed]
- Artavia-León, A.; Romero-Guerrero, A.; Sancho-Blanco, C.; Rojas, N.; Umaña-Castro, R. Diversity of aerobic bacteria isolated from oral and cloacal cavities from free-living snakes species in Costa Rica Rainforest. Int. Sch. Res. Not. 2017, 2017, 8934285. [Google Scholar] [CrossRef] [PubMed]
- Résière, D.; Olive, C.; Kallel, H.; Cabié, A.; Névière, R.; Mégarbane, B.; Gutiérrez, J.M.; Mehdaoui, H. Oral Microbiota of the snake Bothrops lanceolatus in Martinique. Int. J. Environ. Res. Public Health 2018, 15, 2122. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.K.d.G.; Pereira, H.d.S.; Elias, I.C.; Soares, G.S.; Santos, M.; Talhari, C.; Cordeiro-Santos, M.; Monteiro, W.M.; Sachett, J.d.A.G. Secondary infection profile after snakebite treated at a tertiary referral center in the Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2022, 55, e0244. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Vardanega, J.; Smith, S.; White, J.; Little, M.; Hanson, J. The incidence of infection complicating snakebites in tropical Australia: Implications for clinical management and antimicrobial prophylaxis. J. Trop. Med. 2023, 2023, 5812766. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.H.; Tsai, T.S. Comparisons of the oral Microbiota from seven species of wild venomous snakes in Taiwan using the high-throughput amplicon sequencing of the full-length 16S rRNA gene. Biology 2023, 12, 1206. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.; Gao, S.Y.; Lin, C.C. Wound infections from Taiwan cobra (Naja atra) bites: Determining bacteriology, antibiotic susceptibility, and the use of antibiotics—A cobra BITE study. Toxins 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.; Gao, S.Y.; Lin, C.C. Wound Infection of Snakebite from Venomous Protobothrops mucrosquamatus, Viridovipera stejnegeri and Naja atra in Taiwan: Validation of BITE and Cobra BITE Scoring Systems and their Bacteriological Differences in Wound Cultures. Toxins 2023, 15, 78. [Google Scholar] [CrossRef]
- Mao, Y.C.; Liu, P.Y.; Lai, K.L.; Luo, Y.; Chen, K.T.; Lai, C.S. Clinical characteristics of snakebite envenomings in Taiwan. Toxins 2024, 17, 14. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Cui, L.; Huang, S. Metagenomic and metatranscriptomic insight into oral biofilms in periodontitis and related systemic diseases. Front. Microbiol. 2021, 12, 728585. [Google Scholar] [CrossRef]
- Hu, S.; Lou, Z.; Shen, Y.; Tu, M. Bacteriological studies of venomous snakebite wounds in Hangzhou, southeast China. Am. J. Trop. Med. Hyg. 2022, 107, 925–929. [Google Scholar] [CrossRef]
- Houcke, S.; Resiere, D.; Lontsingoula, G.R.; Cook, F.; Lafouasse, P.; Pujo, J.M.; Demar, M.; Matheus, S.; Hommel, D.; Kallel, H. Characteristics of snakebite-related infection in french Guiana. Toxins 2022, 14, 89. [Google Scholar] [CrossRef]
- Chiang, L.-C.; Tsai, W.-J.; Liu, P.-Y.; Ho, C.-H.; Su, H.-Y.; Lai, C.-S.; Lai, K.-L.; Lin, W.-L.; Lee, C.-H.; Yang, Y.-Y.; et al. Envenomation by Trimeresurus stejnegeri stejnegeri: Clinical manifestations, treatment and associated factors for wound necrosis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20200043. [Google Scholar] [CrossRef]
- Schurer, J.M.; Admasu, M.T.; Bonaventure, M.; Hakizimana, D.; Murara, E.; MacDonald, L.E.; Rafferty, E. “I sold my towel and shoes to pay the traditional healer”: Care-seeking costs and productivity losses among snakebite victims in Eastern Province, Rwanda. PLoS Negl. Trop. Dis. 2023, 17, e0011768. [Google Scholar] [CrossRef]
- Steinhorst, J.; Aglanu, L.M.; Ravensbergen, S.J.; Dari, C.D.; Abass, K.M.; Mireku, S.O.; Poku, J.K.A.; Enuameh, Y.A.K.; Blessmann, J.; Harrison, R.A.; et al. “The medicine is not for sale”: Practices of traditional healers in snakebite envenoming in Ghana. PLoS Negl. Trop. Dis. 2021, 15, e0009298. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Yang, C.-H.O.; Hsu, C.-P.; Liu, C.-C.; Yu, J.-S.; Lo, C.-H.; Fann, W.-C.; Chen, Y.-C.; Lin, C.C. Taiwan cobra envenoming: Serum venom concentration before and after specific treatment and relationship with debridement of necrotic wound tissue. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 29, e20220027. [Google Scholar] [CrossRef]
- Jackson, C.R.; Randolph, K.C.; Osborn, S.L.; Tyler, H.L. Culture dependent and independent analysis of bacterial communities associated with commercial salad leaf vegetables. BMC Microbiol. 2013, 13, 274. [Google Scholar] [CrossRef]
- Moon, J.-H.; Lee, J.-H.; Lee, J.-Y. Subgingival microbiome in smokers and non-smokers in Korean chronic periodontitis patients. Mol. Oral Microbiol. 2015, 30, 227–241. [Google Scholar] [CrossRef]
- Viana, T.F.C.; Campelo, A.P.S.; Baldani, J.I.; Fernandes-Júnior, P.I.; Baldani, V.L.D.; Silva, W.M.; Paggi, G.M.; Brasil, M.S. Cultivable bacterial diversity associated with bromeliad roots from ironstone outcrops in central Brazil. Braz. J. Biol. 2020, 80, 872–880. [Google Scholar] [CrossRef]
- Tedjo, D.I.; Jonkers, D.M.A.E.; Savelkoul, P.H.; Masclee, A.A.; van Best, N.; Pierik, M.J.; Penders, J. The effect of sampling and storage on the fecal microbiota composition in healthy and diseased subjects. PLoS ONE 2015, 10, e0126685. [Google Scholar] [CrossRef]
- Salter, S.J.; Cox, M.J.; Turek, E.M.; Calus, S.T.; Cookson, W.O.; Moffatt, M.F.; Turner, P.; Parkhill, J.; Loman, N.J.; Walker, A.W. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014, 12, 87. [Google Scholar] [CrossRef]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- McNab, E.; Benedetto, D.; Hsiang, T. The creeping bentgrass microbiome: Traditional culturing and sequencing results compared with metagenomic techniques. Int. Turfgrass Soc. Res. J. 2022, 14, 911–915. [Google Scholar] [CrossRef]
- Ngo, C.T.; Aujoulat, F.; Veas, F.; Jumas-Bilak, E.; Manguin, S. Bacterial diversity associated with wild caught Anopheles mosquitoes from Dak Nong Province, Vietnam using culture and DNA fingerprint. PLoS ONE 2015, 10, e0118634. [Google Scholar] [CrossRef] [PubMed]
- Demirci, T.; Oraç, A.; Aktaş, K.; Dertli, E.; Akyol, I.; Akın, N. Comparison of culture-dependent and culture-independent techniques in the detection of lactic acid bacteria biodiversity and dynamics throughout the ripening process: The case of Turkish artisanal Tulum cheese produced in the Anamur region. J. Dairy Res. 2021, 88, 445–451. [Google Scholar] [CrossRef]
- Yashiro, E.; Spear, R.N.; McManus, P.S. Culture-dependent and culture-independent assessment of bacteria in the apple phyllosphere: Apple phyllosphere bacteria. J. Appl. Microbiol. 2011, 110, 1284–1296. [Google Scholar] [CrossRef]
- Shin, J.; Lee, S.; Go, M.-J.; Lee, S.Y.; Kim, S.C.; Lee, C.-H.; Cho, B.-K. Analysis of the mouse gut microbiome using full-length 16S rRNA amplicon sequencing. Sci. Rep. 2016, 6, 29681. [Google Scholar] [CrossRef]
- Graf, J.; Ledala, N.; Caimano, M.J.; Jackson, E.; Gratalo, D.; Fasulo, D.; Driscoll, M.D.; Coleman, S.; Matson, A.P. High-resolution differentiation of Enteric bacteria in premature infant fecal microbiomes using a novel rRNA amplicon. mBio 2021, 12, e03656-20. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.-Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Callahan, B.J.; Wong, J.; Heiner, C.; Oh, S.; Theriot, C.M.; Gulati, A.S.; McGill, S.K.; Dougherty, M.K. High-throughput amplicon sequencing of the full-length 16S rRNA gene with single-nucleotide resolution. Nucleic Acids Res. 2019, 47, e103. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Zhang, B.; Yao, J.; Li, M.D. MultiTax-human: An extensive and high-resolution human-related full-length 16S rRNA reference database and taxonomy. Microbiol. Spectr. 2025, 13, e0131224. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, M.; McCauley, M.; Villéger, S.; Jackson, C.R. Ranking the biases: The choice of OTUs vs. ASVs in 16S rRNA amplicon data analysis has stronger effects on diversity measures than rarefaction and OTU identity threshold. PLoS ONE 2022, 17, e0264443. [Google Scholar] [CrossRef] [PubMed]
- Murovec, B.; Deutsch, L.; Stres, B. General Unified Microbiome Profiling Pipeline (GUMPP) for large scale, streamlined and reproducible analysis of bacterial 16S rRNA data to predicted microbial metagenomes, enzymatic reactions and metabolic pathways. Metabolites 2021, 11, 336. [Google Scholar] [CrossRef]
- Grützke, J.; Malorny, B.; Hammerl, J.A.; Busch, A.; Tausch, S.H.; Tomaso, H.; Deneke, C. Fishing in the soup—Pathogen detection in food safety using metabarcoding and metagenomic sequencing. Front. Microbiol. 2019, 10, 1805. [Google Scholar] [CrossRef]
- Schloss, P.D.; Handelsman, J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 2005, 71, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Komiya, S.; Matsuo, Y.; Nakagawa, S.; Morimoto, Y.; Kryukov, K.; Okada, H.; Hirota, K. MinION, a portable long-read sequencer, enables rapid vaginal microbiota analysis in a clinical setting. BMC Med. Genom. 2022, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Cuscó, A.; Catozzi, C.; Viñes, J.; Sanchez, A.; Francino, O. Microbiota profiling with long amplicons using Nanopore sequencing: Full-length 16S rRNA gene and the 16S-ITS-23S of the rrn operon. F1000Research 2019, 7, 1755. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, A.B.; Tunsjø, H.S.; Meisal, R.; Charnock, C. A preliminary study on the potential of Nanopore MinION and Illumina MiSeq 16S rRNA gene sequencing to characterize building-dust microbiomes. Sci. Rep. 2020, 10, 3209. [Google Scholar] [CrossRef]
- Cong, X.; Liu, X.; Zhou, D.; Xu, Y.; Liu, J.; Tong, F. Characterization and comparison of the fecal bacterial microbiota in Red Back Pine Root Snake (Oligodon formosanus) and Chinese Slug-Eating Snake (Pareas chinensis). Front. Microbiol. 2025, 16, 1575405. [Google Scholar] [CrossRef]
- Smith, S.N.; Colston, T.J.; Siler, C.D. Venomous snakes reveal ecological and phylogenetic factors influencing variation in gut and oral microbiomes. Front. Microbiol. 2021, 12, 657754. [Google Scholar] [CrossRef]
- Bell, S.E.; Nash, A.K.; Zanghi, B.M.; Otto, C.M.; Perry, E.B. An assessment of the stability of the canine oral Microbiota after probiotic administration in healthy dogs over time. Front. Vet. Sci. 2020, 7, 616. [Google Scholar] [CrossRef]
- Hu, X.; Yang, L.; Zhang, Y.; Yang, M.; Li, J.; Fan, Y.; Guo, P.; Tian, Z. Fecal and oral microbiome analysis of snakes from China reveals a novel natural emerging disease reservoir. Front. Microbiol. 2023, 14, 1339188. [Google Scholar] [CrossRef]
- Du, Y.; Chen, J.-Q.; Liu, Q.; Fu, J.-C.; Lin, C.-X.; Lin, L.-H.; Li, H.; Qu, Y.-F.; Ji, X. Dietary correlates of oral and gut Microbiota in the water monitor lizard, Varanus salvator (Laurenti, 1768). Front. Microbiol. 2021, 12, 771527. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Walker, A.W.; Berry, S.H.; Duncan, S.H.; Farquarson, F.M.; Louis, P.; Thomson, J.M. The impact of different DNA extraction kits and laboratories upon the assessment of human gut microbiota composition by 16S rRNA gene sequencing. PLoS ONE 2014, 9, e88982. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.; Nadeau, K.; Xiong, X.; Wang, P.W.; Copeland, J.K.; Lee, J.Y.; Pierre, J.S.; Ty, M.; Taj, B.; Brumell, J.H.; et al. Systematic profiling of the chicken gut microbiome reveals dietary supplementation with antibiotics alters expression of multiple microbial pathways with minimal impact on community structure. Microbiome 2022, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A microbial signature for Crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef]
- Anderson, A.C.; von Ohle, C.; Frese, C.; Boutin, S.; Bridson, C.; Schoilew, K.; Peikert, S.A.; Hellwig, E.; Pelz, K.; Wittmer, A.; et al. The oral microbiota is a reservoir for antimicrobial resistance: Resistome and phenotypic resistance characteristics of oral biofilm in health, caries, and periodontitis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.C.; Kodaira, K.; Motta, C.d.C.B.; Barberato-Filho, S.; Silva, M.T.; Guimarães, C.C.; Martins, C.C.; Lopes, L.C. Antimicrobial resistance of microorganisms present in periodontal diseases: A systematic review and meta-analysis. Front. Microbiol. 2022, 13, 961986. [Google Scholar] [CrossRef]
- Pinart, M.; Nimptsch, K.; Forslund, S.K.; Schlicht, K.; Gueimonde, M.; Brigidi, P.; Turroni, S.; Ahrens, W.; Hebestreit, A.; Wolters, M.; et al. Identification and characterization of human observational studies in nutritional epidemiology on gut microbiomics for joint data analysis. Nutrients 2021, 13, 3292. [Google Scholar] [CrossRef]
- Srila, W.; Sripilai, K.; Binlateh, T.; Thammanichanon, P.; Tiskratok, W.; Noisa, P.; Jitprasertwong, P. Relationship between the salivary microbiome and oral malodor metabolites in older Thai individuals with periodontitis and the cytotoxic effects of malodor compounds on human oral squamous carcinoma (HSC-4) cells. Dent. J. 2025, 13, 36. [Google Scholar] [CrossRef]
- Thu, M.S.; Sawaswong, V.; Chanchaem, P.; Klomkliew, P.; Campbell, B.J.; Hirankarn, N.; Fothergill, J.L.; Payungporn, S. Optimization of a DNA extraction protocol for improving bacterial and fungal classification based on Nanopore sequencing. Access Microbiol. 2024, 6, 000754-v3. [Google Scholar] [CrossRef]
- You, K.; Yang, L.; Su, Z.; Shen, J.; Fan, X.; Guo, Y.; Yuan, Z.; Lu, H. Butyric acid modulates gut Microbiota to alleviate inflammation and secondary bone loss in ankylosing spondylitis. Biomedicines 2024, 13, 9. [Google Scholar] [CrossRef]
- Koregol, A.C.; Kalburgi, N.B.; Puttarevanna, T.; Patil, R.S.; Singh, P.; Sulakod, K. Antimicrobial efficacy of grape seed extract in terminating the ramifications of plaque microorganisms: A randomized control study. Med. Pharm. Rep. 2022, 95, 185–190. [Google Scholar] [CrossRef]
- Tong, Z.; Zhou, X.; Chu, Y.; Zhang, T.; Zhang, J.; Zhao, X.; Wang, Z.; Ding, R.; Meng, Q.; Yu, J.; et al. Implications of oral streptococcal bacteriophages in autism spectrum disorder. npj Biofilms Microbiomes 2022, 8, 91. [Google Scholar] [CrossRef]
- Krishnankutty, S.P.; Muraleedharan, M.; Perumal, R.C.; Michael, S.; Benny, J.; Balan, B.; Kumar, P.; Manazhi, J.; Kumar, B.D.; Santhosh, S.; et al. Next-generation sequencing analysis reveals high bacterial diversity in wild venomous and non-venomous snakes from India. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 41. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, S.; Guo, D.; Zhu, J.; Chen, H.; Bai, L.; Luo, X.; Yin, Y. Comparative analysis of intestinal bacteria among venom secretion and non-secrection snakes. Sci. Rep. 2019, 9, 6335. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, G.; Shi, Q.; Yang, S.; Ma, T.; Mishra, S.K.; Wen, A.; Xu, H.; Wang, Q.; Jiang, Y.; et al. Characterizing the microbiota in gastrointestinal tract segments of Rhabdophis subminiatus: Dynamic changes and functional predictions. Microbiologyopen 2019, 8, e00789. [Google Scholar] [CrossRef]
- Colston, T.J.; Noonan, B.P.; Jackson, C.R. Phylogenetic analysis of bacterial communities in different regions of the gastrointestinal tract of Agkistrodon piscivorus, the cottonmouth snake. PLoS ONE 2015, 10, e0128793. [Google Scholar] [CrossRef] [PubMed]
- Batut, B.; Gravouil, K.; Defois, C.; Hiltemann, S.; Brugère, J.-F.; Peyretaillade, E.; Peyret, P. ASaiM: A Galaxy-based framework to analyze microbiota data. GigaScience 2018, 7, giy057. [Google Scholar] [CrossRef]
- Munoz-Benavent, M.; Hartkopf, F.; Van Den Bossche, T.; Piro, V.C.; García-Ferris, C.; Latorre, A.; Renard, B.Y.; Muth, T. gNOMO: A multi-omics pipeline for integrated host and microbiome analysis of non-model organisms. Res. Sq. 2019. [Google Scholar] [CrossRef]
- Onate, F.P.; Batto, J.-M.; Juste, C.; Fadlallah, J.; Fougeroux, C.; Gouas, D.; Pons, N.; Kennedy, S.; Levenez, F.; Dore, J.; et al. Quality control of microbiota metagenomics by k-mer analysis. BMC Genom. 2015, 16, 183. [Google Scholar] [CrossRef]
- Meslier, V.; Menozzi, E.; David, A.; Morabito, C.; Del Pozo, S.L.; Famechon, A.; North, J.; Quinquis, B.; Koletsi, S.; Macnaughtan, J.; et al. Evaluation of an adapted semi-automated DNA extraction for human salivary shotgun metagenomics. Biomolecules 2023, 13, 1505. [Google Scholar] [CrossRef]
- Idris, A.; Hasnain, S.Z.; Huat, L.Z.; Koh, D. Human diseases, immunity and the oral microbiota—Insights gained from metagenomic studies. Oral Sci. Int. 2017, 14, 27–32. [Google Scholar] [CrossRef]
- Zelasko, S.; Swaney, M.H.; Suh, W.S.; Sandstrom, S.; Carlson, C.; Cagnazzo, J.; Golfinos, A.; Fossen, J.; Andes, D.; Kalan, L.R.; et al. Altered oral microbiota of drug-resistant organism carriers exhibit impaired gram-negative pathogen inhibition. bioRxiv 2024. [Google Scholar] [CrossRef]
- Pochon, Z.; Bergfeldt, N.; Kırdök, E.; Vicente, M.; Naidoo, T.; van der Valk, T.; Altınışık, N.E.; Krzewińska, M.; Dalén, L.; Götherström, A.; et al. aMeta: An accurate and memory-efficient ancient metagenomic profiling workflow. Genome Biol. 2023, 24, 242. [Google Scholar] [CrossRef] [PubMed]
- Utter, D.R.; Borisy, G.G.; Eren, A.M.; Cavanaugh, C.M.; Mark Welch, J.L. Metapangenomics of the oral microbiome provides insights into habitat adaptation and cultivar diversity. Genome Biol. 2020, 21, 293. [Google Scholar] [CrossRef]
- Fleming, E.; Pabst, V.; Scholar, Z.; Xiong, R.; Voigt, A.Y.; Zhou, W.; Hoyt, A.; Hardy, R.; Peterson, A.; Beach, R.; et al. Cultivation of common bacterial species and strains from human skin, oral, and gut microbiota. BMC Microbiol. 2021, 21, 278. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Gao, Z.; Wang, H.; Zou, H. Comparison of the intestinal bacterial communities between captive and semi-free-range red-crowned cranes (Grus japonensis) before reintroduction in Zhalong National Nature Reserve, China. Animals 2023, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Song, P.; Liu, D.; Zhang, J.; Qin, W.; Wang, H.; Liang, C.; Gao, H.; Zhang, T. Marked variations in gut microbial diversity, functions, and disease risk between wild and captive alpine musk deer. Appl. Microbiol. Biotechnol. 2023, 107, 5517–5529. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; You, Y.; Hong, Q.; Liu, Q.; Xu, C.; Chen, W.; Zhu, Y.; Du, X.; Fan, P. The gut microbiota of gibbons across host genus and captive site in China. Am. J. Primatol. 2022, 84, e23360. [Google Scholar] [CrossRef]
- Jiang, F.; Song, P.; Wang, H.; Zhang, J.; Liu, D.; Cai, Z.; Gao, H.; Chi, X.; Zhang, T. Comparative analysis of gut microbial composition and potential functions in captive forest and alpine musk deer. Appl. Microbiol. Biotechnol. 2022, 106, 1325–1339. [Google Scholar] [CrossRef]
- Alberdi, A.; Martin Bideguren, G.; Aizpurua, O. Diversity and compositional changes in the gut microbiota of wild and captive vertebrates: A meta-analysis. Sci. Rep. 2021, 11, 22660. [Google Scholar] [CrossRef]
- Reese, A.T.; Chadaideh, K.S.; E Diggins, C.; Schell, L.D.; Beckel, M.; Callahan, P.; Ryan, R.; Thompson, M.E.; Carmody, R.N. Effects of domestication on the gut microbiota parallel those of human industrialization. eLife 2021, 10, e60197. [Google Scholar] [CrossRef]
- Bornbusch, S.L.; Greene, L.K.; Rahobilalaina, S.; Calkins, S.; Rothman, R.S.; Clarke, T.A.; LaFleur, M.; Drea, C.M. Gut microbiota of ring-tailed lemurs (Lemur catta) vary across natural and captive populations and correlate with environmental microbiota. Anim. Microbiome 2022, 4, 29. [Google Scholar] [CrossRef]
- Liu, X.; Yu, J.; Huan, Z.; Xu, M.; Song, T.; Yang, R.; Zhu, W.; Jiang, J. Comparing the gut microbiota of Sichuan golden monkeys across multiple captive and wild settings: Roles of anthropogenic activities and host factors. BMC Genom. 2024, 25, 148. [Google Scholar] [CrossRef]
- Alam, I.; Ojha, R.; Quasimi, H.; Alam, A. Therapeutic potential of snake venoms as antimicrobial agents. Front. Drug Chem. Clin. Res. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Espín-Angulo, J.; Vela, D. Exploring the Venom Gland Transcriptome of Bothrops asper and Bothrops jararaca: De Novo Assembly and Analysis of Novel Toxic Proteins. Toxins 2024, 16, 511. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Aguilar, A.; Cortés-Gómez, A.M.; Ruiz-Agudelo, C.A. Ecosystem services provided by amphibians and reptiles in Neotropical ecosystems. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2013, 9, 257–272. [Google Scholar] [CrossRef]
- Esmaeilishirazifard, E.; Usher, L.; Trim, C.; Denise, H.; Sangal, V.; Tyson, G.H.; Barlow, A.; Redway, K.F.; Taylor, J.D.; Kremyda-Vlachou, M.; et al. Microbial adaptation to venom is common in snakes and spiders. bioRxiv 2018. [Google Scholar] [CrossRef]
- Wei, L.; Gao, J.; Zhang, S.; Wu, S.; Xie, Z.; Ling, G.; Kuang, Y.-Q.; Yang, Y.; Yu, H.; Wang, Y. Identification and characterization of the first cathelicidin from sea snakes with potent antimicrobial and anti-inflammatory activity and special mechanism. J. Biol. Chem. 2015, 290, 16633–16652. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Chen, L.; Guang, H.; Li, Z.; Yang, H.; Li, J.; You, D.; Yu, H.; Lai, R. Cathelicidin-BF, a snake cathelicidin-derived antimicrobial peptide, could be an excellent therapeutic agent for acne vulgaris. PLoS ONE 2011, 6, e22120. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Stiles, B.G.; Girish, K.S.; Swamy, S.N.; Hemshekhar, M.; Tan, K.S.; Rowan, E.G.; Sethi, G.; Chow, V.T. Snake venom phospholipases A2: A novel tool against bacterial diseases. Curr. Med. Chem. 2012, 19, 6150–6162. [Google Scholar] [CrossRef]
- Allane, D.; Oussedik-Oumehdi, H.; Harrat, Z.; Seve, M.; Laraba-Djebari, F. Isolation and characterization of an anti-leishmanial disintegrin from Cerastes cerastes venom. J. Biochem. Mol. Toxicol. 2018, 32, e22018. [Google Scholar] [CrossRef] [PubMed]
- Barros, G.A.C.; Pereira, A.V.; Barros, L.C.; Lourenço, A., Jr.; Calvi, S.A.; Santos, L.D.; Barraviera, B.; Ferreira, R.S. In vitro activity of phospholipase A2 and of peptides from Crotalus durissus terrificus venom against amastigote and promastigote forms of Leishmania (L.) infantum chagasi. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 48. [Google Scholar] [CrossRef]
- Okumu, M.O.; Eyaan, K.L.; Bett, L.K.; Gitahi, N. Antibacterial Activity of Venom from the Puff Adder (Bitis arietans), Egyptian Cobra (Naja haje), and Red Spitting Cobra (Naja pallida). Int. J. Microbiol. 2023, 2023, 7924853. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, B.; Kleinschmit, A.J.; Santibáñez-López, C.E.; Graham, M.R. Microbiota discovered in scorpion venom. bioRxiv 2025. [Google Scholar] [CrossRef]
- Yacoub, T.; Rima, M.; Karam, M.; Fajloun, J.Z. Antimicrobials from venomous animals: An overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- Teodoro, A.; Gonçalves, F.J.M.; Oliveira, H.; Marques, S. Venom of Viperidae: A perspective of its antibacterial and antitumor potential. Curr. Drug Targets 2022, 23, 126–144. [Google Scholar] [CrossRef]
- Almeida, J.R.; Palacios, A.L.V.; Patiño, R.S.P.; Mendes, B.; Teixeira, C.A.S.; Gomes, P.; da Silva, S.L. Harnessing snake venom phospholipases A2 to novel approaches for overcoming antibiotic resistance. Drug Dev. Res. 2019, 80, 68–85. [Google Scholar] [CrossRef]
- Schneider, R.; Primon-Barros, M.; Von Borowski, R.G.; Chat, S.; Nonin-Lecomte, S.; Gillet, R.; Macedo, A.J. Pseudonajide peptide derived from snake venom alters cell envelope integrity interfering on biofilm formation in Staphylococcus epidermidis. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Fadum, J.M.; Borton, M.A.; Daly, R.A.; Wrighton, K.C.; Hall, E.K. Dominant nitrogen metabolisms of a warm, seasonally anoxic freshwater ecosystem revealed using genome resolved metatranscriptomics. mSystems 2024, 9, e0105923. [Google Scholar] [CrossRef]
- Paula, C.C.P.D.; Sirová, D.; Sarmento, H.; Fernandes, C.C.; Kishi, L.T.; Bichuette, M.E.; Seleghim, M.H.R. First report of halobacteria dominance in a tropical cave microbiome. bioRxiv 2021. [Google Scholar] [CrossRef]
- Martínez-Mota, R.; Vásquez-Aguilar, A.A.; Hernández-Rodríguez, D.; Suárez-Domínguez, E.A.; Krömer, T. Close neighbors, not intruders: Investigating the role of tank bromeliads in shaping faunal microbiomes. PeerJ 2025, 13, e19376. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, T.; Roberts, S.; Silva-Sanchez, C.; Sutton, L.; Laventure, K. The use of serum protein analysis in the diagnosis of fatal envenomation via Crotalus horridus (timber rattlesnake). J. Forensic Sci. 2023, 68, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Safika, S.; Indrawati, A.; Afiff, U.; Hastuti, Y.T.; Zureni, Z.; Jati, A.P. First Study on profiling of gut microbiome in wild and captive Sumatran orangutans (Pongo abelii). Vet. World 2023, 16, 717–727. [Google Scholar] [CrossRef]
- Fernández-Pato, A.; Sinha, T.; Gacesa, R.; Andreu-Sánchez, S.; Gois, M.F.B.; Gelderloos-Arends, J.; Jansen, D.B.H.; Kruk, M.; Jaeger, M.; Joosten, L.A.B.; et al. Choice of DNA extraction method affects stool microbiome recovery and subsequent phenotypic association analyses. Sci. Rep. 2024, 14, 3911. [Google Scholar] [CrossRef]
- Deikumah, J.P.; Biney, R.P.; Awoonor-Williams, J.K.; Gyakobo, M.K. Compendium of medically important snakes, venom activity and clinical presentations in Ghana. PLoS Negl. Trop. Dis. 2023, 17, e0011050. [Google Scholar] [CrossRef]
- Kanika, N.H.; Liaqat, N.; Chen, H.; Ke, J.; Lu, G.; Wang, J.; Wang, C. Fish gut microbiome and its application in aquaculture and biological conservation. Front. Microbiol. 2024, 15, 1521048. [Google Scholar] [CrossRef]
- Ostria-Hernández, M.L.; Hernández-Zulueta, J.; Vargas-Ponce, O.; Díaz-Pérez, L.; Araya, R.; Rodríguez-Troncoso, A.P.; Ríos-Jara, E.; Rodríguez-Zaragoza, F.A. Core microbiome of corals Pocillopora damicornis and Pocillopora verrucosa in the northeastern tropical Pacific. Mar. Ecol. 2022, 43, e12729. [Google Scholar] [CrossRef]
- Quek, J.J.W.; Wong, J.L.; Tan, J.L.; Yeo, C.C.; Saw, S.H. Integrating metagenomic and culture-based techniques to detect foodborne pathogens and antimicrobial resistance genes in Malaysian produce. Foods 2025, 14, 352. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chiang, P.W.; Rogozin, D.Y.; Degermendzhy, A.G.; Chiu, H.H.; Tang, S.L. Salvaging complete and high-quality genomes of novel microbial species from a meromictic lake using a workflow combining long- and short-read sequencing platforms. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lertsakulbunlue, S.; Suebtuam, R.; Eamchotchawalit, T.; Chantkran, W.; Chaisakul, J. Clinical profile and pharmacological management of snakebites in community care units: A retrospective study using two military hospital databases in South Thailand. Trop. Med. Infect. Dis. 2023, 8, 346. [Google Scholar] [CrossRef]
- Esmaeilishirazifard, E.; Usher, L.; Trim, C.; Denise, H.; Sangal, V.; Tyson, G.H.; Barlow, A.; Redway, K.F.; Taylor, J.D.; Kremyda-Vlachou, M.; et al. Bacterial adaptation to venom in snakes and Arachnida. Microbiol. Spectr. 2022, 10, e0240821. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, S.; Neila-Ibáñez, C.; Hennig-Pauka, I.; Guan, X.; Dunkelberger, J.; de Oliveira, I.F.; Ferrando, M.L.; Correa-Fiz, F.; Aragon, V.; Boekhorst, J.; et al. Streptococcus suis infection on European farms is associated with an altered tonsil microbiome and resistome. bioRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.A.; Tavares, C.O.; Moreira, G.C.; Mendes, C.A.C. Secondary infection with Aeromonas hydrophila and death of two patients with probable Bothrops envenomation. Rev. Soc. Bras. Med. Trop. 2025, 58, e04302024. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.R.S.; de Souza de Azevedo, P.O.; Converti, A.; de Souza Oliveira, R.P. Cultivation of lactic acid bacteria and evaluation of the antimicrobial potential of partially purified bacteriocin-like inhibitory substances against cariogenic and food pathogens. Fermentation 2022, 8, 400. [Google Scholar] [CrossRef]
- Andrade-Oliveira, A.L.; Rossi, C.C.; Souza-Silva, T.; Giambiagi-deMarval, M. Staphylococcus nepalensis, a commensal of the oral microbiota of domestic cats, is a reservoir of transferrable antimicrobial resistance. Microbiology 2020, 166, 727–734. [Google Scholar] [CrossRef]
- Augimeri, G.; Caparello, G.; Caputo, I.; Reda, R.; Testarelli, L.; Bonofiglio, D. Mediterranean diet: A potential player in the link between oral microbiome and oral diseases. J. Oral Microbiol. 2024, 16, 2329474. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).