Abstract
The microbiota associated with Vanilla planifolia grown in three production systems in Puebla, México, was evaluated: shade cloth, cocuite, and acahual. Rhizosphere and soil samples were analyzed, from which bacteria, fungi, yeasts, and actinomycetes were isolated. The bacterial and actinomycete isolates were characterized morphologically and biochemically, and their potential as growth promoters was evaluated. Morphological and microscopic characteristics identified the fungi. In parallel, agronomic variables were measured in five plants per system, and the data were analyzed using ANOVA and Tukey’s test (p ≤ 0.05). The results showed that the shade cloth favored a greater number of internodes, total leaves, and biomass, although with a higher incidence of diseased leaves. The cocuite presented intermediate values, while the acahual had lower leaf density but fewer leaf health problems. Microbial composition varied across systems, with potentially beneficial bacteria and actinomycetes, as well as both beneficial and pathogenic fungi, being prominent. These findings demonstrate the influence of the management system on the microbiota and health of V. planifolia, providing a basis for more sustainable production strategies for vanilla cultivation in Mexico.
1. Introduction
Vanilla planifolia, commonly known as vanilla, is an orchid native to the tropical forests of Mexico and Central America. This crop has global economic value, especially for its production of vanillin, essential in the food and cosmetics industries, appreciated for its characteristic aroma and flavor [1]. Although its center of origin is Mexico, this species is cultivated in various parts of the world and faces several challenges, such as its dependence on manual pollination, its low growth rate, water stress, and, especially, its susceptibility to various pests and diseases, which can significantly affect its production and yield [2,3].
In Mexico, particularly in the areas of Puebla and Veracruz, vanilla is cultivated using three central production systems: under acahual, under cocuite, and under shade cloth. Each system generates distinct microenvironments that significantly influence the composition of the plant’s associated microorganisms. The undergrowth system, which corresponds to areas undergoing forest regeneration without fertilization or soil movement, harbors greater biodiversity, which may include beneficial microorganisms. The cocuite, characterized by the presence of the Gliricidia sepium tree as shade, provides a more stable microclimate and additional organic matter to the soil, complemented by mulch made of banana leaves [4]. Finally, the shade mesh system controls solar radiation and the microclimate more artificially, which can modify the dynamics of the microorganisms present in the soil [5,6].
The interaction of the vanilla plant with the microorganisms present in the soil and its rhizosphere is a key factor in improving its nutrition, disease resistance, and, consequently, production [7]. Beneficial microorganisms, such as nitrogen-fixing bacteria and actinomycetes, phosphate solubilizers, and mycorrhizal fungi, contribute to the plant’s health. In contrast, the presence of pathogens, such as certain bacteria or fungi, represents a significant risk to its development, production, and survival [8]. Likewise, fungi of the genus Trichoderma, present in the rhizosphere or as endophytes, promote plant development by producing phytohormones such as auxins and gibberellins, and synthesize organic acids that lower soil pH, favoring the solubilization of essential nutrients such as phosphates, magnesium, iron and manganese [9,10]. Furthermore, these fungi exert biological control against phytopathogens through competition for nutrients and space, mycoparasitism, antibiosis, promotion of plant growth and induction of defensive responses [11,12,13]. During mycoparasitism, they secrete enzymes such as proteases, chitinases, and glucanases that degrade the cell wall of their host fungi, affecting their plasma membrane, cytoplasm, spore germination, and germ tube elongation [14,15,16]. Their proliferation is favored by organic matter and compounds from parasitized fungi [17,18]. Studying the interaction of Trichoderma and other microorganisms with Vanilla planifolia under different production systems allows us to understand how the microbiota influences crop productivity and health, providing a basis for sustainable microbiological management strategies.
The isolation and characterization of the microbial communities associated with V. planifolia allow for the development of biological control strategies based on beneficial microorganisms, such as microbial antagonists, that can improve nutrient uptake and reduce disease incidence [19]. In turn, identifying pathogens present in each system can help develop more precise and sustainable control strategies, minimizing the use of agrochemicals that can affect both the beneficial soil microbiota and the final pod quality [20].
The objective of this study was to isolate the microorganisms present in vanilla crops grown under acahual, cocuite, and shade cloth production systems, in order to identify their microbial diversity, both beneficial and phytopathogenic. This will expand our knowledge of the ability of these microorganisms to improve nutrient uptake and pathogen resistance under different growing conditions. It also seeks to determine which production system is most sustainable and productive, leveraging microorganisms to maximize plant yield and reduce disease incidence, while adapting to the specific characteristics of each growing system.
2. Materials and Methods
2.1. Description of the Study Area and Sample Collection
Samples were collected from vanilla plantations under the acahual, cocuite, and shade cloth production systems located in Tenampulco, Puebla, Mexico (20°11′52.23″ N, 97°22′06.09″ W), at an altitude of 240 m a.s.l. This region is part of the eastern slope of the Sierra Nororiental de Puebla, with an altitudinal range of 80 to 360 m a.s.l. at its highest point (Cerro de la Campana). A warm-humid climate with abundant summer rainfall characterizes it. The soils of the Tenampulco region, Puebla, are predominantly young and have little-evolved profiles, such as regosols, fluvisols, and andosols [21].
In the municipality of Tenampulco, Puebla, Mexico, vanilla is grown under three production systems with contrasting characteristics. (1) The acahual system is the traditional vanilla cultivation system, practiced since the 9th and 20th centuries. It is characterized by minimal management, limited to periodic weeding, using native trees (oaks (Quercus sp.) and pepper (Pimenta dioca)) as tutors; its soils are sandy loam, rich in organic matter and with good drainage capacity, which provides an environment closer to the natural habitat of the species. (2) The cocuite system, based on living tutors such as Gliricidia sepium, Bauhinia variegata and plantain (Musa sp.), takes advantage of nitrogen fixation and the contribution of organic matter, well-aerated soils with moderate humidity, suitable for root development. (3) The shade mesh system offers intensive management, with continuous cleaning and moderate cover of leaf litter, under structures that reduce solar radiation by 50%, with high humidity and microbial diversity, which favors water retention but limits aeration.
Sample collection was carried out in the three production systems: acahual, shade cloth, and cocuite. Five Vanilla planifolia plants were randomly selected from each system. For each plant, rhizosphere samples (roots with soil attachment) and surrounding soil were obtained. Rhizosphere sampling was carried out by carefully extracting active root fragments at a depth of approximately 10–15 cm, removing excess non-adherent soil, and placing the material in sterile bags. Rhizosphere soil was collected using sterile augers within a 10 cm radius of the root, taking three to five subsamples that were subsequently homogenized to form a composite sample per plant. All samples were stored under refrigerated conditions (4 °C) and processed in the laboratory within a period of no more than 48 h. The procedure followed the recommendations of the soil sampling guide for microbiological analysis by [22].
2.2. Isolation of Microorganisms Associated with Vanilla planifolia
Serial dilutions were made from the soil and rhizosphere samples obtained to obtain microbial isolates. Ten g of soil were weighed and poured into a flask containing 100 mL of saline solution. Dilutions were made up to 10−9 (NOM, 110-SSA1.1994). For bacterial isolation, 0.1 mL of the 10−7, 10−8, and 10−9 dilutions were plated on sterilized trypticase soy agar (TSA) plates and prepared according to the manufacturer’s instructions. Potato dextrose agar (PDA) was used for the isolation of fungi. Bacteria and fungi plates were incubated for 48 h to 72 h at 28 °C ± 2 °C.
2.3. Colonial and Microscopic Characterization of Microorganisms Associated with Vanilla planifolia
The morphological characteristics of the isolated microorganisms were analyzed on TSA and PDA using pure cultures of the bacterial isolates, incubated for 24 h at 28 ± 2 °C. Colony morphology, including shape, size, pigmentation, and color, was documented after incubation, and Gram staining [23] was also performed on the bacterial and fungal isolates. In addition, biochemical characterization tests for the bacterial and actinomycete isolates, such as Voges-Proskauer oxidase, nitrate reduction, catalase, indole, citrate, and starch hydrolysis, were performed using the standardized procedure described by [24]. For fungi, lactophenol blue staining was performed, observing them under a microscope and identifying their morphological structures, such as hyphae, conidiophores, and spores. In addition, their texture, surface, front and back color, and diffusible pigment content were evaluated in the Petri dish [25].
2.4. Identification of Growth-Promoting Traits of Microorganisms Associated with Vanilla planifolia
For the evaluation of PGPR (Plant Growth Promoting Rhizospheric Bacteria) tests, phosphate solubilization was started. For this purpose, a point was taken and placed in triplicate on Pikovskaya medium (5 g of tricalcium phosphate (Ca3(PO3)2), 10 g of glucose (C3H2O3), 0.002 g of manganese sulfate (MnSO3 H2O), 0.2 g of sodium chloride (NaCl), 0.2 g of potassium chloride (KCl), 0.1 g of magnesium sulfate (MgSO3), 0.5 g of ammonium sulfate ((NH3)2SO3), 0.5 g of potassium chloride (KCl), 0.1 g of magnesium sulfate (MgSO3), 0.5 g of potassium chloride (KCl), 0.1 g of yeast extract, 15 g of agar, and 1000 mL of sterile distilled water at pH 7.0). The medium was sterilized and allowed to cool before seeding. Rhizobacteria were inoculated into the center of the Petri dishes and incubated at 28 ± 1 °C for 4–5 days. The appearance of a clear zone around the bacterial colony indicated a positive result.
Regarding the evaluation of pathogen inhibition through confrontations, 9 different confrontations were carried out between 3 pathogenic fungi and 3 beneficial fungi. The pathogens confronted were: SC1 = Phytophthora, SM1 = Cladiosporum, and CT9 = Fusarium. The beneficial fungi are three strains of Trichoderma, designated SM2 = Trichoderma 1, SM7 = Trichoderma 2, and SC2 = Trichoderma 3.
Trichoderma suspensions were prepared in 0.1% Tween 80 to a concentration of 106 spores per milliliter. The same was performed with the pathogenic fungi (SC1 = Phytophthora, SM1 = Cladiosporum, and CT9 = Fusarium).
Seeding was performed in Petri dishes with potato dextrose agar (PDA), placing 10 µL of solution in the center and incubating at 28 °C for 24 h. Subsequently, 1 µL of different Trichoderma strains was inoculated in each quadrant at a distance of 4 cm from the pathogen, and incubated at 28 °C for 5–7 days. Controls for pathogens and beneficial microorganisms were included, with three replicates. After incubation, the percentage of radial growth inhibition (%RIG) was evaluated by comparing the growth of the pathogen in the challenged environment with that of a control.
where
R1: is the radial growth of the pathogen in the control.
R2: is the radial growth of the pathogen in the presence of Trichoderma.
The degree of antagonism was also determined using the Bell scale [26] to classify the degree between the different Trichoderma strains and the pathogens. This scale establishes 5 categories, which are presented in the following table (Table 1).

Table 1.
Bell scale to determine the degree of antagonism of Trichoderma spp.
The evaluation using this scale was carried out visually, observing the interactions between the three strains of Thichoderma and the pathogens. This evaluation seeks to complement the quantitative data from PICR and thus determine the effectiveness of Trichoderma as a biological control agent.
2.5. Evaluation of Phenological Characteristics of Vanilla planifolia Grown in Three Production Systems
Finally, Vanilla planifolia plants grown in three production systems were evaluated: shade cloth, cocuite, and acahual, selecting five representative plants per system. Eight agronomic variables were measured for each plant: sample length (SL), stem diameter (SD), number of internodes (N), total number of leaves (TL), number of diseased leaves (DL), leaf length (LL), leaf width (LW), fresh weight (FW), and dry weight (DW). Two-meter-long sections were taken from each system for the measurements. In each system, 2 m-long stem sections were taken for measurements. Stem diameter was recorded with a caliper, leaf dimensions with a ruler, and internode and leaf counts were performed manually. Fresh weights were determined immediately, and dry weights were determined after drying in an oven to a constant weight.
The data obtained were analyzed using analysis of variance (ANOVA) to identify significant differences between production systems, and means were compared using Tukey’s test at a 5% significance level (p ≤ 0.05). Additionally, the coefficient of variation (CV, %) and the least significant difference (LSD) were calculated for each variable evaluated. The results are presented in a comparative table and accompanied by representative images of the plants in the three production systems, allowing the morphological differences observed to be visualized.
3. Results
3.1. Identification of Bacteria in Roots
After isolating and purifying bacterial colonies, several genera were identified with the potential to promote plant growth in three cultivation systems: cocuite, shade cloth, and acahual. In the cocuite system, 14 colonies were isolated, of which 7 (C3, C5, C6, C9, C12, C13, and C14) showed positive results in at least three plant growth promotion tests. These results suggest the presence of bacteria from genera such as Paenibacillus and Bacillus, known for their ability to improve soil health and promote plant growth (Figure 1). Despite the predominance of beneficial bacteria, a colony of negative cocci was also identified that could belong to the genus Neisseria, posing a potential pathogenicity risk (Figure 1).
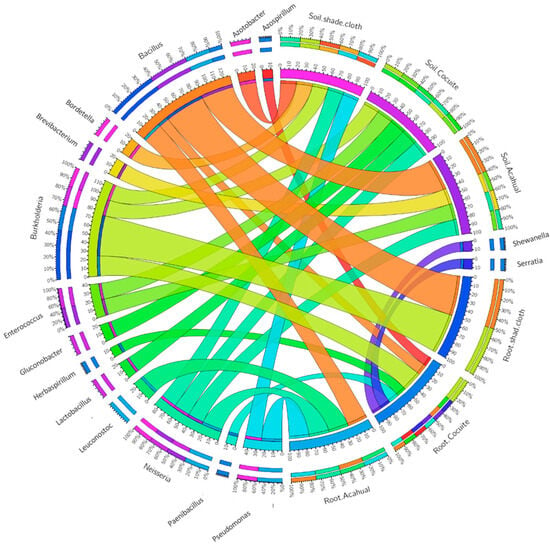
Figure 1.
Bacteria were identified in the soil and roots of three different Vanilla planifolia production systems.
In the shadow box system, seven colonies were located, of which only two (M2 and M6) showed favorable characteristics. One of these colonies was identified as Bacillus, a genus widely recognized for its properties in promoting plant growth. However, the low number of colonies with promoting activity suggests that this system could have a less diverse microbiome or less potential to improve plant health.
Finally, in the acahual system, 10 bacterial colonies were isolated, of which 4 (A3, A6, A7 and A8) were selected. These colonies belong to genera such as Bacillus and Pseudomonas, which are known for their biocontrol properties and improved plant nutrition. Similarly to the cocciitis system, a colony of negative cocci was identified that could correspond to Neisseria, which highlights the need for precaution in its management due to its pathogenic potential (Figure 1).
Regarding the bacteria found in the soil, the cocuite system contained 9 bacterial colonies, of which 5 (C1, C2, C7, C8 and C10) were selected. Among them, the C7 and C8 colonies, which were negative cocci, could belong to genera such as Neisseria or Azotobacter, which have the potential to be pathogens. Furthermore, beneficial genera such as Bacillus and Pseudomonas have been identified, known for their ability to promote soil health and biocontrol of pathogens. In the acahual system, five colonies were selected (A1, A3, A4, A5, A6), with a possible pathogen identified among the colonies of negative cocci, in addition to beneficial bacteria such as Enterococcus and Bacillus. In the shadow mall system, six colonies were located, of which five were selected (M2, M3, M4, M5 and M6). Of these, two colonies were negative cocci, which could indicate the presence of Neisseria or Azotobacter, along with other genera such as Bordetella and Pseudomonas (Figure 1).
These findings indicate considerable bacterial diversity in the three cultivation systems evaluated, with a prevalence of genera with the potential to promote plant growth, such as Bacillus and Pseudomonas. However, bacteria with pathogenic characteristics have also been identified, such as Neisseria and Azotobacter, which underlines the importance of a more detailed analysis to avoid risks to plants. The results show that the coculate and acahual systems have a more diverse microbiome and with greater capacity to promote plant growth, in comparison with the shadow system, which had a smaller number of beneficial colonies.
3.2. Diversity of Fungi Associated with the Vanilla planifolia Root
Different types of fungis were identified in the three production systems. Of the microorganisms identified, it was observed that in the acahual system, the roots present in the root and the soil are mostly decomposed and saprophytes, such as Penicillium, Aspergillus and Cladosporium, which suggests that this system has a high microbiological activity that favors the decomposition of organic matter, promoting a healthy soil for the cultivation of vanilla. In the under-shadow system, even though some of the same beneficial genera will be found, this system has a greater prevalence of pathogens such as Phytophthora and Fusarium at the root, which suggests that low-under-shadow management could be creating more favorable conditions for the proliferation of pathogens; however, the presence of Trichoderma is a positive point, as it could act as a biocontroller in this system (Figure 2).
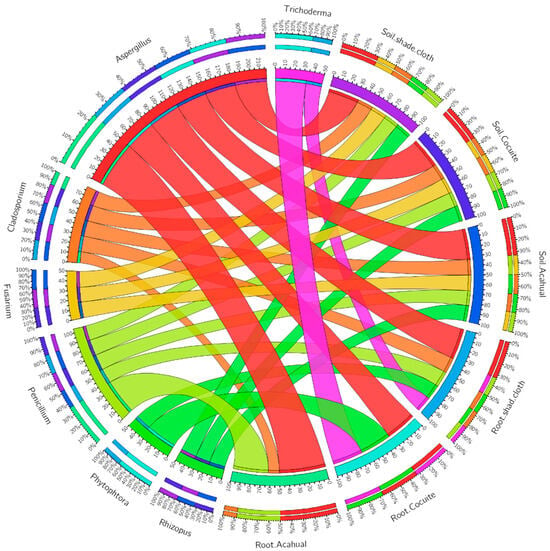
Figure 2.
Fungi were identified in the soil and roots of 3 different Vanilla planifolia production systems.
On the other hand, an interesting combination of beneficial genera and pathogens is found in the cocuite system. Trichoderma is present at the root, suggesting an effective biocontrol potential in this system. However, the presence of Phytophthora at the root is a sign of risk, which indicates the need for more careful monitoring to avoid damage to the plants.
3.3. Diversity of Fungi Associated with Vanilla planifolia Soil
In the results of three agricultural production systems (acahual, cocuite and malla shadow), key fungi that fulfill both beneficial and pathogenic roles were identified (Figure 2). One of the most prominent genera is Aspergillus, which is present in all systems. This fungi has wide and smooth conidiophores, with globose and black conidia, which indicates its ability to adapt to different environmental conditions. Aspergillus is known for its ability to decompose organic matter, releasing essential nutrients that plants reuse. Even under certain circumstances, such as excessively humid conditions, it can also behave as an opportunistic pathogen.
Another thing widely present in the bones of these systems is Penicillium, identified by its wide conidiophores that end in globose vesicles, with conidia in wide chains. This type is fundamental for the degradation of plant matter and organic residues in the soil, helping to maintain an environment rich in nutrients for the cultivation of vanilla. Its presence in the three production systems reflects its adaptability to different soil conditions, as well as its beneficial role in nutrient mineralization. However, some species of Penicillium can produce mycotoxins, which could represent a risk if soil conditions are not adequate.
On the other hand, fungi such as Fusarium and Rhizopus are also identified in all systems, but their presence is more worrying due to their pathogenic potential. Fusarium, with its large and septate macroconidia, is known for causing root and stem diseases, such as vascular disease. Rhizopus, although it is mainly a disruptor, can become a pathogen under certain conditions, especially in damp or poorly managed soils. The appearance of these diseases in the three systems suggests the need for constant monitoring to prevent their populations from growing and negatively affecting the health of vanilla cultivation, particularly in systems such as shade and cocuite, where moisture conditions can favor their proliferation.
3.4. Dual Confrontations of Pathogenic Fungi Against Beneficial Fungi
Six controls were established to observe the development of each microorganism in the soil: 3 strains of Trichoderma as beneficial fungi and Fusarium, Cladosporium and Phytophthora as pathogenic fungi, as well as 9 different comparisons. When evaluating the percentage of inhibition of radial growth (%PICR), it can be observed that the best inhibited by the strains of Trichoderma was CT9 (Fusarium) with an average of 87.38% of inhibition of growth, while the least inhibited was Phytophthora with an average of inhibition of 51.60%. Regarding Trichoderma strains, the best strain in all comparisons was strain 3 (SC2), followed by strain 2 (SM7).
In addition to the percentage of PICR, the level of in vitro antagonism of Trichoderma against different pathogens was evaluated using the in vitro antagonism scale for Trichoderma [26] and the degree of growth of the different strains. In this case, it was possible to confirm that the best strain with a lower mean of antagonism was the SC2 Trichoderma 3 strain, while, for pathogens, with a class 1 mean, it faced confrontations with Fusarium (Table 2).

Table 2.
Evaluation of the percentage and class of inhibition of radial growth.
3.5. Evaluation of Agronomic Variables in Vanilla Plants from Three Production Systems
Five plants of V. planifolia were taken from three vanilla production systems (malla shadow, cocuite, and acahual), where eight agronomic variables were evaluated to compare the morphological characteristics of these plants.
In each system, 2 m-long samples were taken. The mean diameter of the height of the 3 systems was statistically similar. However, the number of children, total number and sick days were statistically different between the 3 systems, where the highest averages were slightly lower, followed by cocuite and acahual, respectively. It is observed that, although there are more young children in the shade, there are also more sick children. In contrast, there are fewer children overall, but a smaller number of sick children. For the plant variables, there is no statistically significant difference between the systems. However, the current system’s plants were shorter on average, which is statistically different from the other systems. Finally, for the fresh and dry weight variables, these were significantly different in the three systems, obtaining the highest averages in low shade, followed by cocuite and acahual (Table 3).

Table 3.
Analysis of variance of the agronomic variables of the three V. planifolia production systems. abc: Means with the same letter in each column are not significantly different from the Tukey test (p ≤ 0.05). LM: Sample length. DT: Tallo diameter. NE: Number of entrenudos. HT: Total hojas. HE: Hojas infirmas. LH: Longitude of today. AH: Ancho de hoja. PF: Fresh weight. PS: Dry weight. CV (%): Coefficient of variation. DMS: Minimum Significant Difference.
4. Discussion
The results obtained in this study demonstrate a clear differentiation in the microbiota present in the three production systems of Vanilla planifolia: acahual, cocuite, and malla Shadow. Each of the systems presents a particular microbial diversity, influenced by environmental conditions, soil management and the amount of organic matter, which directly affects the presence of fungi, bacteria, actinomycete yeasts and their respective functions in the rhizospheric ecosystem.
The interaction between the microbiota associated with V. planifolia and the agronomic variables evaluated in the three production systems confirms that the health and yield of the crop are strictly conditioned by the microbial composition of the soil and the rhizosphere.
In the shaded system, the plants showed a greater number of seeds, total leaves and biomass, which coincides with the abundance of plant growth-promoting bacteria (Bacillus, Pseudomonas). However, this same system recorded the highest incidence of sick people, which is explained by the proliferation of pathogens such as Fusarium and Phytophthora, favored by high humidity and low air content. This suggests that, even though the beneficial microbiota contributes to greater vegetative growth, pathogenic pressure compromises the health of the crop, forcing the implementation of biocontrol strategies.
In the low-shadow system, a greater diversity of plant growth-promoting bacteria (PGPRs) and actinomycetes was observed, as well as a significant presence of pathogenic fungi. This system, characterized by the regulation of solar radiation and greater moisture retention [27], favors the proliferation of bacterial genera such as Bacillus, Pseudomonas and Azospirillum, which perform critical functions such as potassium fixation, siderophore production, and phosphorus solubilization, all of them essential to improve plant nutrition. These genera are recognized for their ability to promote plant growth by improving the availability of nutrients and inducing resistance to pathogens. Further, [28] concluded in his investigation that the rhizosphere of under-shade vanilla plants contains a microbial community with high potential to increase the availability of nutrients for commercial plantations of this crop. In addition to that, [20] mentions that the under-shade vanilla production system increases the number of communities’ microbial growth in the roots and soil of Vanilla planifolia.
Several studies on biocontrol of pathogens in vanilla, such as [29], have identified rhizobacteria associated with this species, including Staphylococcus, Paenibacillus, and Stenotrophomonas, which exhibit antagonistic capacity against pathogens in vitro. Furthermore, a notable abundance of actinomycetes was found in this system, which stands out for its ability to fix potassium and solubilize phosphorus. However, the biggest problem in this system is the presence of pathogens such as Phytophthora and Fusarium, which represent a serious threat to the health of vanilla cultivation.
The competition between beneficial microorganisms and pathogens in this system reduces the need to implement more intensive biological control strategies, such as the use of Trichoderma. This long antagonist is highly effective in inhibiting these pathogens. Ref. [20] found a wide diversity of bacterial colonies in the production of vanilla under the shade, with different microorganisms under the covers, but with common genera such as Bacillus, Enterobacter and Bradyrhizobium, as well as Halomonas. The Bacillus are known for favoring the growth of plants through various mechanisms, direct and indirect; and the majority of these carry out solubilization and mineralization of nutrients such as phosphorus and potassium, the fixation of nitrogen, the production of phytohormones and siderophores, as well as the stimulation of human defenses [30,31].
In contrast, the acahual system presents the smallest number of entrants and leaves, as well as the lowest biomass, which reflects a lower diversity of PGPRs and the absence of actinomycetes that facilitate the assimilation of nutrients. However, this system leads to a lower incidence of sick leaves, which is associated with a microbial community dominated by saprophytic fungi (Aspergillus, Cladosporium) that maintain the ecological balance and reduce the proliferation of pathogens. Therefore, even though plant vigor is limited in terms of growth, their plant health is more stable, which makes it a resilient but less productive system.
The acahual system, a natural regenerative ecosystem with agronomic management, showed a lower diversity of PGPR but also an absence of actinomycetes, which can be achieved under more natural and less controlled soil conditions, with a lower availability of specific ecological niches for these microorganisms. However, the microbiota observed in this system includes bacterial colonies such as Rhizobium and Paenibacillus, which have important capabilities such as nitrogen fixation and phosphorus solubilization, contributing to long-term soil sustainability. These bacterial communities differed from those in other production systems. However, we must consider that the characteristics and composition of the soil can play an important role in the configuration of the development of bacteria associated with plants [32].
It has been found that, in the substrates commonly used as tree cuttings and leaf litter, for the establishment of vanilla crops, the phosphorus content is very low and can be a limiting factor for the nutrition and growth of these plants [33]. In this system, the absence of Penicillium is notable, distinguishing it from other systems. This is a key point, as Ref. [28] identified the microorganisms most effective in increasing P concentration in Penicillium griseofulvum, which was isolated from the rhizosphere of vanilla plants. However, the lower incidence of pathogens in this system suggests a more favorable microbial balance, with effective competition between saprophytic and pathogenic microorganisms, which reduces the risk of infections in the crop. This system seems to favor a more sustainable and resilient approach, where natural soil regeneration and microbial biodiversity promote a balanced ecosystem.
The cocuite system, on the other hand, showed intermediate values in the number of leaves, cells, and biomass, which is related to a microbiota that combines bacteria such as Paenibacillus and Enterobacter, capable of fixing nitrogen and solubilizing phosphorus, together with long endophytes and Trichoderma that provide biocontrol functions. This balance allowed for adequate development of plants and a moderate incidence of sick leaves, suggesting that the microclimate generated by Gliricidia sepium and the continued incorporation of organic matter favor a more balanced system between nutrition and plant health.
The cocuite system, which uses Gliricidia sepium to provide shade and support for vanilla plants, showed an intermediate microbial composition, with a lower diversity of PGPRs and actinomycetes compared to shade mesh, as well as the presence of yeasts of the genus Saccharomyces only in this system, but with a favorable balance between beneficial and pathogenic fungi. The predominant bacterial genera were Enterobacter and Serratia, which exhibit capabilities such as nitrogen fixation and phosphorus solubilization, essential functions in plant nutrition [34].
Although microbial diversity in this system is more limited, the identified genera play key roles in improving soil fertility. Among them, a considerable number of endophyte fungi were obtained, as noted by [35], who mentioned that these fungi in vanilla play an important role in protecting the plant and developing the organoleptic characteristics of the fruit. Furthermore, some studies, such as those by [34,36] show that when colonizing the roots, these roots contribute to a certain point in the capture and transfer of nutrients to the plant, favoring its growth. Therefore, the lower presence of pathogens compared to the shade suggests that the cocuite system could offer less favorable conditions for the development of fungal diseases, possibly due to a more stable microclimate and the constant drop in organic matter of Gliricidia [37,38].
A typical pattern in the three systems was the presence of saprophytic fungi such as Penicillium, Aspergillus and Cladosporium. These fungi are widely known for their ability to produce large amounts of extracellular enzymes [39]. Furthermore, these genera are abundant in the leaf litter, which can explain their presence in the system of small shadow and cocuite, where the plant is covered.
Even though these people contribute to the general health of the soil, favoring the availability of essential nutrients for the cultivation of vanilla, the prevalence of Fusarium and Phytophthora in under shade was observed, in contrast to their lower incidence in acahual and cocuite, which indicates that more humid and shaded environmental conditions favor the pathogens in this system, which implies a greater risk of diseases in the cultivation of vanilla under low shade. In general, studies such as those by [40] highlight that interpreting the interaction of biotic and abiotic factors that participate is complex, but that we do not know the exact functional response of endophyte dogs—Also, [20] use different accessions of vanilla and obtained, along with other studies such as [41,42] that the bacterial profile of the soil and roots in the soil depends on the plant and the substrate, but not on the plant genotype.
In comparisons between strains of Trichoderma spp. against strong pathogens, significant results were obtained for the management of diseases in Vanilla planifolia production systems. Trichoderma is a species widely recognized for its ability to act as a biocontroller, inhibiting the growth of pathogens through various mechanisms, such as nutrient competition, the production of antimicrobial secondary metabolites, and direct mycoparasitism, where Trichoderma spp. Degrades and uses the pathogen’s mycelium as a source of nutrients. Furthermore, considering that some authors such as [29] have demonstrated that there is no correlation between antagonism in vitro conditions and in winter conditions, this ability can occur in the field and by applying an exact dose, attacking and controlling pathogens of the species.
The results of both tests to evaluate competence showed that Trichoderma spp. It was highly effective in inhibiting the growth of three pathogens, especially in Fusarium spp. and Phytophthora, with inhibition rates that exceeded 90%. Authors such as [43,44] reported inhibition from 52% to 76% of Trichoderma strains in pathogens such as Fusarium oxysporum. This confirms the ability of Trichoderma spp. to reduce the development of these pathogens significantly. Furthermore, visibly, Trichoderma spp. demonstrated a high capacity to compete for space and media with pathogens, limiting their access to essential resources for growth. The latter was observed in different investigations as reported by [39,43], where strains of Trichoderma spp and Trichoderma harzianum were used.
In systems such as shade, where humidity and shade conditions favor the proliferation of pathogenic fungi, the introduction of Trichoderma spp. can be beneficial. It can be particularly effective in preventing the colonization and spread of pathogens by occupying available ecological niches. It was also observed that strains of Trichoderma spp. Increase the production of volatile compounds and antifungal secondary metabolites, such as harzianine and gliotoxin, which contribute to the remote inhibition of the growth of pathogens, preventing their expansion to areas not colonized by the antagonistic fungi. It has been demonstrated that Trichoderma harzianum has important antagonistic characteristics such as nutrient, space and antibiotic competence which can help in the control of these pathogens [44,45].
The results show that strains of Trichoderma spp. It was especially effective against Fusarium spp., the primary pathogen in vanilla, known for causing diseases such as vascular disease. In the case of Phytophthora, control was considerable, even though it was observed that this pathogen requires a higher concentration of Trichoderma spp. to achieve optimal levels of inhibition, as suggested by [35], which means that in the field, it would be necessary to adjust the application dosage of Trichoderma spp. to maximize its effectiveness.
5. Conclusions
The results of this study demonstrate that different production systems have a significant impact on the microbial composition of the soil and rhizosphere of Vanilla planifolia, which in turn affects crop health and yield. The shade cloth system stood out for its high diversity of PGPR, suggesting a favorable environment for promoting plant growth. However, it also presented a higher incidence of pathogens such as Fusarium and Phytophthora, underscoring the need for proper management to mitigate disease risk. The acahual system, with its natural regeneration approach, showed a more favorable balance between beneficial and pathogenic microorganisms, making it a more sustainable option in the long term, although with a lower diversity of growth-promoting bacteria. Meanwhile, the cocuite system proved to be an intermediate option, with a good balance between nutrient availability and a lower incidence of pathogens, using Gliricidia sepium as a shade plant.
Based on these findings, it is concluded that the microbial diversity and functional characteristics of fungi, bacteria, and actinomycetes are profoundly influenced by the environmental conditions of each production system. The shade cloth system, although it has greater potential to promote plant growth, requires intensive biological management strategies, such as the application of Trichoderma or other biocontrol agents, to mitigate disease risk. On the other hand, the acahual and cocuite systems, with their reduced intervention and more agroecological approach, offer a more balanced and sustainable environment, although with the need to optimize soil fertility through management practices that favor the development of plant growth-promoting bacteria.
A critical comparison of the systems shows that the growth and health of V. planifolia depend on the balance between beneficial and pathogenic microorganisms. The shade cloth promotes greater vegetative growth thanks to the presence of PGPRs and actinomycetes, but its high incidence of disease limits its sustainability without intensive biological management. Acahual, in contrast, produces plants with less vigor but significantly better health, the result of a more balanced microbial ecosystem less susceptible to pathogens. Cocuite is positioned as an intermediate alternative, capable of sustaining adequate growth and a moderate incidence of disease, thanks to the coexistence of microorganisms with complementary functions. These findings highlight the importance of integrating microbiota management with the monitoring of agronomic variables, prioritizing not only plant growth but also reducing phytosanitary risks. In this sense, the targeted application of biocontrol agents such as Trichoderma and the strengthening of Bacillus and Pseudomonas populations represent key tools for optimizing the balance between productivity and health in the various vanilla production systems.
Regarding the confrontations and biological control between the three Trichoderma strains and the pathogenic fungi Fusarium, Cladosporium, and Phytophthora, this study notably demonstrates the great potential of Trichoderma as a biocontrol agent in Vanilla planifolia cultivation, especially in production systems such as shade nets where various pathogens are present. Trichoderma was shown to be capable of significantly inhibiting the growth of these pathogens through mechanisms of mycoparasitism, competition for the environment, and the potential production of antifungal metabolites. This demonstrates this fungus as an effective tool for reducing the use of chemical fungicides and promoting more sustainable crop management, making it a key element in integrated pest management programs.
However, for effective biocontrol in the field, Trichoderma application rates and methods must be adjusted according to the production system and environmental conditions. In systems such as shade cloth, where conditions favor the proliferation of pathogens, the use of Trichoderma can help prevent and control diseases. In contrast, in less-intervened systems such as acahual, more careful management may be required to ensure that Trichoderma competes effectively with the soil microbiota. These results demonstrate the importance of an integrated approach that combines Trichoderma with other agricultural practices to ensure crop health and the sustainability of the production system.
Author Contributions
D.F.G.-F. and L.J.G.-G. Conceptualization, D.F.G.-F. and L.J.G.-G. writing—original draft preparation, D.F.G.-F., L.J.G.-G., D.R.-L., C.H.A.-A., R.I.A.-G., C.H.-D., F.L.-M. and J.M.R.-G. writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cribb, P.; Soto, M. A new infrageneric classification and synopsis of the genus Vanilla Plum. ex-Mill. (Orchidaceae: Vanillinae). Lankesteriana 2010, 9, 355–398. [Google Scholar]
- Soto, M.; Dressler, R. A revision of the Mexican and Central American species of Vanilla Plumier ex Miller with a characterization of their its region of the nuclear ribosomal DNA. Lankesteriana 2010, 9, 285–354. [Google Scholar]
- Reyes, D.; Flórez, A.; Huerta, M.; Kelso, H.; Avendaño, C.; Lobato, R.; Aragón, A.; López, J. Variación morfométrica de fruto y semillas en cuatro especies del género Vanilla. Ecosistemas Recur. Agropecu. 2014, 1, 205–218. [Google Scholar]
- Del Carmen Orozco-Mosqueda, M.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, Á.F.; López, D.R.; García, D.J.; Arenas, O.R.; Tapia, J.A.R.; Lara, M.H.; Silva, A.P. Diversidad de Vanilla spp. (Orchidaceae) y sus perfiles bioclimáticos en México. Rev. Biol. Trop. 2017, 65, 975–987. [Google Scholar] [CrossRef]
- Reyes López, D.; González Arnao, M.T.; Menchaca García, R.A.; Cruz Palacios, M.I.; Tovar Soto, A.; Kelso Bucio, H.A.; Barcenas Graniel, J. Rescate, conservación, investigación y utilización de la biodiversidad de la vainilla en México. In I Seminario Internacional de Vainilla; Instituto de Investigación y Servicios Forestales, Universidad Nacional: Heredia, Costa Rica, 2013. [Google Scholar]
- Hamdache, A.; Ezziyyani, M.; Lamarti, A. Evaluation of Strawberry Seed Treatments with Biological Control Agents Bacillus amyloliquefaciens. In Advanced Intelligent Systems for Sustainable Development (AI2SD’2019). AI2SD 2019. Advances in Intelligent Systems and Computing; Ezziyyani, M., Ed.; Springer: Cham, Switzerland, 2020; Volume 1103. [Google Scholar] [CrossRef]
- Gamboa-Gaitán, M.A. Colombian vanilla and its microbiota. I. First report of Fusarium taxa from both wild and cultivated species. Acta Bot. Hung. 2013, 55, 239–245. [Google Scholar] [CrossRef]
- Torres-De la Cruz, M.; Ortiz-García, C.F.; Bautista-Muñoz, C.; Ramírez-Pool, J.A.; Ávalos-Contreras, N.; Cappello-García, S.; De la Cruz-Pérez, A. Diversidad de Trichoderma en el agroecosistema cacao del estado de Tabasco, México. Rev. Mex. Biodivers. 2015, 86, 947–961. [Google Scholar] [CrossRef]
- Sharma, V.; Salwan, R.; Sharma, P. The comparative mechanistic aspects of Trichoderma and Probiotics: Scope for future research. Physiol. Mol. Plant Pathol. 2017, 100, 84–96. [Google Scholar] [CrossRef]
- De Aguiar, R.A.; da Cunha, M.G.; Junior, M.L. Management of white mold in processing tomatoes by Trichoderma spp. and chemical fungicides applied by drip irrigation. Biol. Control 2014, 74, 1–5. [Google Scholar] [CrossRef]
- Sandle, T. Trichoderma. In Encyclopedia of Food Microbiology; Batt, C.A., Tortorello, M.-L., Eds.; Academic Press: London, UK, 2014; pp. 644–646. [Google Scholar]
- Vargas-Hoyos, H.A.; Gilchrist-Ramelli, E. Producción de enzimas hidrolíticas y actividad antagónica de Trichoderma asperellum sobre dos cepas de Fusarium aisladas de cultivos de tomate (Solanum lycopersicum). Rev. Mex. Micol. 2015, 42, 9–16. [Google Scholar]
- Marcello, C.M.; Steindorff, A.S.; da Silva, S.P.; Silva, R.D.N.; Bataus, L.A.M.; Ulhoa, C.J. Expression analysis of the exo-β-1,3-glucanase from the mycoparasitic fungus Trichoderma asperellum. Microbiol. Res. 2010, 165, 75–81. [Google Scholar] [CrossRef]
- García-Espejo, C.N.; Mamani-Mamani, M.M.; Chávez-Lizárraga, G.A.; Álvarez-Aliaga, M.T. Evaluación de la actividad enzimática del Trichoderma inhamatum (BOL-12 QD) como posible biocontrolador. J. Selva Andin. Res. Soc. 2016, 7, 20–32. [Google Scholar] [CrossRef]
- Romero-Cortes, T.; López-Pérez, P.A.; Ramírez-Lepe, M.; Cuervo-Parra, J.A. Modelado cinético del micoparasitismo por Trichoderma harzianum contra Cladosporium cladosporioides aislado de frutos de cacao (Theobroma cacao). Chil. J. Agric. Anim. Sci. 2016, 31, 32–45. [Google Scholar] [CrossRef]
- Argumedo-Delira, R.; Alarcón, A.; Ferrera-Cerrato, R.; Peña-Cabriales, J.J. El género fúngico Trichoderma y su relación con contaminantes orgánicos e inorgánicos. Rev. Int. Contam. Ambient. 2009, 25, 257–269. [Google Scholar]
- Ramos, E.Y.A.; Navarro, R.I.Z.; Zumaqué, L.E.O.; Violeth, J.L.B. Evaluación de sustratos y procesos de fermentación sólida para la producción de esporas de Trichoderma sp. Rev. Colomb. Biotecnol. 2008, 10, 23–34. [Google Scholar]
- Banerjee, G.; Chattopadhyay, P. Vanillin biotechnology: The perspectives and future. J. Sci. Food Agric. 2018, 99, 499–506. [Google Scholar] [CrossRef]
- Mahadeo, K.; Taïbi, A.; Meile, J.-C.; Côme, B.; Gauvin-Bialecki, A.; Boubakri, H.; Herrera-Belaroussi, A.; Kodja, H. Exploring endophytic bacteria communities of Vanilla planifolia. BMC Microbiol. 2024, 24, 1–15. [Google Scholar] [CrossRef]
- INEGI. Anuario Estadístico Federativa 2006; PAOT. Available online: http://centro.paot.org.mx/documentos/inegi/anuario_esta_federativa_2006.pdf (accessed on 1 March 2025).
- Pardo, I.M.G.; Rodríguez, J.M.M.; Díaz, A.M.S. Guía de Muestreo de Suelo Para Análisis Microbiológico; Corporación Colombiana de Investigación Agropecuaria (AGROSAVIA): Bogotá, Colombia, 2020. [CrossRef]
- Gram, C. Sobre la coloración aislada de esquizomicetos en preparaciones de corte y secas. Fortschritte Med. 1884, 2, 185–189. [Google Scholar]
- Cowan, S.T.; Steel, K.J. Manual for the Identification of Medical Bacteria, 2nd ed.; Cambridge University Press: Cambridge, UK, 1965. [Google Scholar]
- Corrales Ramírez, L.C.; Caycedo Lozano, L. Principios Fisicoquímicos de los Colorantes Utilzados en Microbiología; NOVA Publicación Científica Cienc: Biomédicas, Spain, 2020. [Google Scholar]
- Bell, D.K.; Wells, H.D.; Markham, C.R. In vitro antagonism of Trichoderma spp. against six fungal plant pathogens. Phytopathology 1982, 72, 379–382. [Google Scholar] [CrossRef]
- Quiroz-Martínez, B.; Álvarez, F.; Espinosa, H.; Salgado-Maldonado, G. Concordant Biogeographic Patterns among Multiple Taxonomic Groups in the Mexican Freshwater Biota. PLoS ONE 2014, 9, e105510. [Google Scholar] [CrossRef] [PubMed]
- Álvarez López, C.L. Identificación Y Caracterización Bioquímica, Morfológica Y Molecular de Microorganismos Cultivables Asociados a la Rizosfera Y Al Sustrato de Plantas de Vainilla. Ph.D. Thesis, Facultad de Ciencias, Universidad Nacional de Colombia, Sede Medellín, Medellín, Colombia, 2012. [Google Scholar]
- Adame-García, J.; Luna-Rodríguez, M.; Iglesias-Andreu, L.G. Vanilla Rhizobacteria as Antagonists against Fusarium oxysporum f. sp. vanillae. Int. J. Agric. Biol. 2015, 18, 23–30. [Google Scholar] [CrossRef]
- Sansinenea, E. Bacillus spp.: As plant growth-promoting bacteria. In Secondary Metabolites of Plant Growth-Promoting Rhizomicroorganisms: Discovery and Applications; Singh, H.B., Keswani, C., Reddy, M.S., Sansinenea, E., García-Estrada, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 225–237. [Google Scholar]
- Lalanne-Tisné, G.; Barral, B.; Taibi, A.; Coulibaly, Z.K.; Burguet, P.; Rasoarahona, F.; Quinton, L.; Meile, J.-C.; Boubakri, H.; Kodja, H. Exploring the Phytobeneficial and Biocontrol Capacities of Endophytic Bacteria Isolated from Hybrid Vanilla Pods. Microorganisms 2023, 11, 1754. [Google Scholar] [CrossRef]
- Bakker, P.A.; Berendsen, R.L.; Van Pelt, J.A.; Vismans, G.; Yu, K.; Li, E.; Van Bentum, S.; Poppeliers, S.W.; Gil, J.J.S.; Zhang, H.; et al. The Soil-Borne Identity and Microbiome-Assisted Agriculture: Looking Back to the Future. Mol. Plant 2020, 13, 1394–1401. [Google Scholar] [CrossRef]
- Osorio, A.; Gómez, N.; Arango, D.; Moreno, F.; Díez, M.; Osorio, W. Establecimiento y manejo del cultivo de vainilla. In Cultivo de Vainilla: Contribuciones Para el Desarrollo de su Cadena Productiva en Colombia; Moreno, F., Díez, M.C., Eds.; Universidad Nacional de Colombia: Bogotá, Colombia, 2011; pp. 45–58. [Google Scholar]
- Romero-Cortes, T.; España, V.H.P.; Pérez, P.A.L.; Rodríguez-Jimenes, G.D.C.; Robles-Olvera, V.J.; Burgos, J.E.A.; Cuervo-Parra, J.A. Antifungal activity of vanilla juice and vanillin against Alternaria alternata. CyTA-J. Food 2019, 17, 375–383. [Google Scholar] [CrossRef]
- Koyyappurath, S.; Conéjéro, G.; Dijoux, J.B.; Lapeyre-Montès, F.; Jade, K.; Chiroleu, F.; Gatineau, F.; Verdeil, J.L.; Besse, P.; Grisoni, M. Differential Responses of Vanilla Accessions to Root Rot and Colonization by Fusarium oxysporum f. sp. radicis-vanillae. Front. Plant Sci. 2015, 6, 1125. [Google Scholar] [CrossRef]
- Carbajal-Valenzuela, I.A.; Muñoz-Sanchez, A.H.; Hernández-Hernández, J.; Barona-Gómez, F.; Truong, C.; Cibrián-Jaramillo, A. Microbial Diversity in Cultivated and Feral Vanilla Vanilla planifolia Orchids Affected by Stem and Rot Disease. Microb. Ecol. 2021, 84, 821–833. [Google Scholar] [CrossRef]
- Murphy, B.R.; Jadwiszczak, M.J.; Soldi, E.; Hodkinson, T.R. Endophytes from the crop wild relative Hordeum secalinum L. improve agronomic traits in unstressed and salt-stressed barley. Cogent Food Agric. 2018, 4, 1549195. [Google Scholar] [CrossRef]
- Scott, M.; Rani, M.; Samsatly, J.; Charron, J.-B.; Jabaji, S. Endófitos de cultivares de cáñamo industrial (Cannabis sativa L.): Identificación de bacterias y hongos cultivables en hojas, pecíolos y semillas. Can. J. Microbiol. 2018, 64, 664–680. [Google Scholar] [CrossRef]
- Webster, J.; Weber, R. Introduction to Fungi; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Franco-Galindo, L.S.; Mosquera-Espinosa, A.T. Biocontrol de Fusarium spp. en el cultivo de vainilla: Un nuevo modelo de estudio. Temas Agrar. 2023, 28, 95–114. [Google Scholar] [CrossRef]
- Meliani, A.; Bensoltane, A.; Mederbel, K. Microbial Diversity and Abundance in Soil: Related to Plant and Soil Type. Am. J. Plant Nutr. Fertil. Technol. 2012, 2, 10–18. [Google Scholar] [CrossRef][Green Version]
- Micallef, S.A.; Channer, S.; Shiaris, M.P.; Colón-Carmona, A. Plant age and genotype impact the progression of bacterial community succession in the Arabidopsis rhizosphere. Plant Signal. Behav. 2009, 4, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Suseela Bhai, R.; Remya, B.; Danesh, J.; Eapen, J.S. In vitro and in planta assays for biological control of Fusarium root rot disease of vanilla. J. Biol. Control 2009, 23, 83–86. [Google Scholar]
- Jayasekhar, M.; Manonmani, K.; Justin, C.; Gailce, L. Development of integrated biocontrol strategy for the management of stem rot disease (Fusarium oxysporum f. sp. vanillae) of vanilla. Agric. Sci. Dig. 2008, 28, 109–111. Available online: https://arccarticles.s3.amazonaws.com/webArticle/articles/asd282008.pdf (accessed on 11 September 2025).
- Radjacommare, A.; Sengoda-Gounder, V.; Ramasamy, S. Control biológico de hongos fitopatógenos de vainilla mediante la acción lítica de especies de Trichoderma y Pseudomonas fluorescens. Arch. Phytopathol. Plant Prot. 2010, 43, 1–17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).