Abstract
The genus Diaporthe consists of saprobes, endophytes, and important plant pathogens. Members of this genus are widely distributed and have a broad host range, including grapevines. This study aimed to establish a baseline survey to assess the diversity of Diaporthe species infecting propagation material and to explore their dynamics in disease development. Initially, a survey was conducted in a nursery field, and isolations were carried out from 2-month-old symptomatic grafted vines of cv. Agiorgitiko grafted onto rootstock Richter 110. The initial molecular identification of the isolated mycobiome at the genus level was carried out by sequencing the universal internal transcribed spacer (ITS) locus, while subsequent species-level identification of the Diaporthe isolates was performed through phylogenetic approaches coupled with morphological characterization. Based on the combined analysis, five phylogenetically distinct Diaporthe spp. were identified in this study, taxonomically assigned to D. ampelina, D. eres, D. foeniculina, D. serafiniae, and D. novem. Pathogenicity trials demonstrated that the most aggressive species were D. ampelina followed by D. eres, while the remaining species were classified as opportunistic or weak pathogens of grapevine. Overall, accurate identification and monitoring of Diaporthe species involved in propagation material infections are important in order to develop species-specific effective management strategies in grapevine nurseries.
1. Introduction
The genus Diaporthe Nitschke (anamorph: Phomopsis (Sacc.) Bubák, family: Diaporthaceae), comprises taxonomically diverse species, among which are important phytopathogens, non-pathogenic endophytes, and saprobic species [1,2,3]. More than 800 species have been described in the genus [4], with a wide geographical distribution and a broad host range, including mammals and humans [5,6,7]. Moreover, many of them have been reported to be responsible for several losses in economically important crops, causing significant diseases with different symptomatology, like fruit rots, stem cankers, stem blights, leaf spots, and diebacks [8,9,10,11]. Several studies have shown that more than one Diaporthe species can co-occur in a single symptomatic host plant, hypothesizing that diseases are caused by a complex of Diaporthe fungi rather than from a single species [11,12,13]. A glaring example showcasing this phenomenon is the association of several Diaporthe species with different soybean diseases [14,15] as well as a stem canker disease in sunflowers caused by numerous Diaporthe species, which was identified in recent studies [16,17].
In grapevines, Phomopsis cane and leaf spot (PCLS) has been a very well-known disease since the late 1950s, which has mainly been attributed to Phomopsis viticola [18]. Since the recommendation by [19] to use the generic name Diaporthe over Phomopsis, several Diaporthe species associated with canker diseases in grapevines in Europe and other countries have been documented [11,20,21]. A number of studies conducted in Europe, South Australia, and Western North America state that D. ampelina (former P. viticola) is the predominant and most virulent species, with high isolation frequencies [11,22,23]. Other species like the type species D. eres, along with D. ambigua, D. neotheicola [23], D. hispaniae, D. hungariae, D. celeris [11], D. nebulae, D. novem, D. ceranoidis, D. serafiniae, and D. foeniculina [21], can co-occur with D. ampelina as latent or weak pathogens and can cause significant symptoms as well. Moreover, a recent study by [24] revealed that D. eres and D. foeniculina, along with D. ampelina, are associated with PCLS. In the same study, it was stated that the etiology of the disease should be reconsidered, since both species were found to be aggressive to grapevines as well. On the other hand, D. ampelina has not been associated with canker diseases in grapevines in surveys conducted in Chinese vineyards, whilst D. eres was the most common pathogen isolated from grapevine wood, among others [20,25]. The suggestion of the addition of Diaporthe ampelina to the pathogens associated with the grapevine trunk disease (GTD) complex by [23] intensified research regarding the role of other Diaporthe species in GTDs. Regarding the occurrence of Diaporthe dieback in grapevine nurseries, and dissemination of the species related to the disease via propagation material, only a few studies are available. More specifically, surveys conducted in Spain and Uruguay showed that different Diaporthe species could be isolated from nursery-produced vines [26,27], while a microbiome analysis based on a high throughput amplicon sequencing approach from [28] revealed the presence of Diaporthe spp. in propagation material, among other grapevine trunk disease (GTD)-associated taxa. This particular study explored the relative abundances of the most important GTD-related fungal genera after a hot-water treatment (HWT) in nurseries in two countries (Czech Republic and Spain). Based on their findings, the abundance of Diaporthe representatives was higher in plants that were hot-water treated at 53 °C compared to the control plants, indicating their ability to survive and potentially spread through the propagation material production process.
Historically, identification of Diaporthe fungi at the species level was mainly based on morphological and cultural characteristics, together with assumptions of host-specificity [2]. Species identification by means of morphology is nowadays considered insufficient due to the intra- and inter-specific variability present in the genus. According to recent findings, which support the hypothesis that more than one species can co-occur in a single host or that a single species can infect several hosts, the inclusion of host-specificity studies in the proper species delineation may not be reliable [11,29]. Furthermore, morphological characteristics are not always informative, and molecular approaches such as DNA-sequence-based multi-locus phylogenies are required for species identification or novel species description [30]. Various genetic loci and genes have been used in several taxonomic studies, which include the internal transcribed spacer (ITS), the beta-tubulin 2 gene (TUB2), the translation elongation factor 1-a (TEF1), the histone 3 gene (HIS3) and the calmodulin gene (CAL) [11,20,23,25,31].
The main objective of this study was to investigate the diversity of Diaporthe species that are associated with grapevine propagation material infections, with the aim of elucidating the role of each species in the development of Diaporthe dieback, an emerging grapevine trunk disease.
2. Materials and Methods
2.1. Sampling of Grafted Vines and Fungal Isolation Procedure
Twenty (20) two-month-old rooted grafted vines (cv. Agiorgitiko grafted onto rootstock Richter 110) showing decline symptoms, including wilting (Figure S1a,b), leaf chlorosis, necrosis, reduced vigor (Figure S1c,d), and internal diffused brown wood discoloration (Figure S1e), were uprooted from a nursery field located in Nemea, Korinthos province, and transferred to the Laboratory of Plant Pathology at the Agricultural University of Athens, Greece. The sampling strategy was intentionally targeted, selecting vines that expressed disease symptoms within a nursery plot, with the aim of maximizing the likelihood of isolating the agents potentially associated with GTDs. The whole root system was discarded, and vines were surface sterilized by wiping with 70% EtOH, washed with sterile distilled H2O, and left under a laminar flow hood to dry. Afterwards, vines were debarked using a sterile scalpel, and the first layers of plant tissue were removed until the brown wood discoloration appeared. Isolations were conducted from the discolored tissues, and segments were taken from three sections: the basal end (crown), the first and second internode, and the grafting union. Small fragments (approx. 2 × 2 mm) of symptomatic tissues were placed on Malt Extract Agar (MEA, Condalab, Madrid, Spain) medium supplemented with 50 μg/mL streptomycin sulfate to inhibit the growth of secondary microorganisms. For each vine, three MEA plates were used (one per vine section), and five fragments per section were placed in the medium. Plates were incubated at 25 °C for 5 to 10 days until the emergence of the fungal colonies. The developing fungal cultures were transferred to freshly made Potato Dextrose Agar (PDA, Condalab, Madrid, Spain) plates, hyphal tipped, and incubated at 25 °C.
2.2. Fungal DNA Isolation and PCR Amplification
All fungal isolates were grown in Malt Extract Broth (MEB, Condalab, Madrid, Spain) medium for several days, depending on the growth rate of each isolate, at 25 °C in the dark. The mycelia from each isolate were harvested, lyophilized in a freeze-dryer, and ground to a fine powder using a mortal and pestle under the presence of liquid nitrogen. Total DNA was isolated from all isolates according to the CTAB-based protocol described by [32] or a slightly modified protocol from [33]. In this modification, a Tris-EDTA buffer containing 10 μg/mL of the enzyme RNAse A for RNA degradation was used for the resuspension of the pellet in the final extraction step. The concentration and the integrity of the isolated genomic DNA was assessed using a NanoDrop 2000c Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and by electrophoresis in 1% agarose gel.
PCR amplifications were carried out using the KAPA Taq PCR Kit (Kit code—KK1014, KAPA Biosystems, Cape Town, South Africa) and were performed according to the manufacturer’s instructions. Briefly, all PCRs were performed at a final volume of 50 μL using 0.2 μM of dNTPs, 0.4 μM of each primer, and 0.5 Units of Taq DNA polymerase per reaction. In addition, DNA concentration isolated from each fungus was adjusted to 100 ng/μL, and a 1 μL template was used in each assay. For the initial molecular identification of the isolates at the genus level, the rRNA ITS locus containing the 5.8S region was amplified using the primers designed by [34]. Identification of Diaporthe isolates at the species level was performed using additional appropriate molecular markers based on the available literature for phylogeny in ascomycetes and Diaporthe species, and included the beta-tubulin 2, the histone 3, and the translation elongation factor 1 alpha genes [35,36]. The primer sets used, the sequence of each primer, and the PCR conditions for the amplification of each genetic locus are shown in Table S1. PCR products were analyzed by electrophoresis in 1% agarose gel, stained for 15 min in 0.1% EtBr solution and the results were visualized under UV light.
2.3. Sequencing
PCR products were precipitated using an Ammonium Acetate (NH4CH3CO2)-based purification protocol. Briefly, NH4CH3CO2 (0.5 volumes of the PCR product) was added to the product, and the mixture was homogenized. Subsequently the mixture was centrifuged for 30 min at 13,000 rpm and 4 °C, and the supernatant was transferred to a new 1.5 mL Eppendorf tube. Then, 2.5 volumes of 100% EtOH were added, and the mixture was homogenized. After homogenization, a 30 min centrifugation in 13,000 rpm at 4 °C was carried out, and the pellet was washed twice with 70% EtOH. Finally, the pellet was resuspended in TE Buffer (Tris-HCl and EDTA, pH = 8) and stored at −20 °C.
Both strands of the PCR products were subjected to sequencing, and sequences were visually inspected using the FinchTV (version 1.4.0) software. Afterwards, sequences were imported in the Benchling software (https://www.benchling.com/, accessed on 10 May 2024) and the first 20–25 nucleotides from the 5′ and 3′ sequences were manually trimmed. After sequence curation, the resulting chromatographs were inspected again with the FinchTV software to ensure accurate base-calling. The two strands were aligned using the mafft algorithm [37], and the consensus sequence was used for further analysis. The obtained sequences for each genetic locus were blasted against the GenBank nucleotide database (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 10 May 2024) to identify the taxonomically closest species to be included in the downstream phylogenetic analyses.
2.4. Phylogenetic Analysis of Diaporthe Isolates
The MEGA X software [38] was used throughout the entire phylogenetic analysis. The sequences of each genetic locus obtained from this study along with sequences retrieved from the GenBank database (Table S2) were aligned using the ClustalW method [39], and subsequently they were concatenated. The optimum nucleotide substitution models for each single locus and the concatenated sequences were determined. The model with the lowest BIC (Bayesian Information Criterion) score was selected and the General Time Reversible model [40] was utilized for further analysis. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.5448), while the rate variation model allowed for some sites to be evolutionarily invariable ([+I], 28.73% sites). The initial tree for the heuristic search was constructed automatically under the Maximum Parsimony method, and for the generation of the phylogenetic tree the Maximum-Likelihood statistical method (ML) was utilized with 1000 bootstrap replications.
To confirm the topology of the ML tree, a Bayesian inference analysis was conducted in BEAST v1.10.4 software [41] using the same substitution model mentioned above (GTR + G + I). The Markov Chains Monte Carlo (MCMC) sampling method was selected, starting with a random tree topology. The number of generations run by the MCMC algorithm was set at 10,000,000, and trees were sampled every 100 generations. The resulting consensus phylogenetic tree was rooted to Diaporthella corylina CBS121124 (isolated from Corylus sp., China), which was used as an outgroup. Phylogenetic trees were visualized and edited using the FigTree software (version 1.4.4, https://tree.bio.ed.ac.uk/).
2.5. Morphological Characterization
One isolate from each Diaporthe species was chosen for morphological characterization. Four characteristics were considered, which included the growth rate (cm/day), the size of alpha- and beta-conidia, and the size of pycnidial conidiomata. Growth rates were calculated by placing a 3 mm agar plug taken from the margins of the active mycelium on the center of Potato Dextrose Agar (PDA), Malt Extract Agar (MEA), and Oatmeal Agar (OA) plates and incubating them at 25 °C for 7 days in the dark, and measurements were taken every day. Three replicates per medium per species were used to calculate the mean growth rates. For the induction of pycnidia development, a 3 mm plug from each species was placed on Pine Needle Agar (PNA) medium [42], and plates were incubated for 10 days at 25 °C and under a 12 h photoperiod. Three PNA plates were used for the induction of pycnidia development. The mean size (length and width) of pycnidial conidiomata, alpha- and beta-conidia, were calculated by measuring 10 and 20 of each, respectively, per Diaporthe species per Petri dish in a Zeiss PrimoStar microscope, and images were captured using the Axiocam ERC 5s.
2.6. Pathogenicity Trials
Isolates used for the pathogenicity trials included two isolates of Diaporthe ampelina (V42B and V118B) isolated from different plants, two isolates of D. eres (V101M and V55M), also isolated from different grafted vines, and isolates V44M (D. foeniculina), V71G (D. serafiniae), and V92M (D. novem). The virulence dynamics of the different Diaporthe spp. was carried out by pathogenicity trials on 1-year-old lignified grapevine canes of cv. Roditis, harvested from a 25-year-old commercial vineyard located in Fokis prefecture, Greece, from apparently asymptomatic mature vines.
Canes were cut into pieces approximately 12 cm in length, each containing two nodes. A surface-disinfection was conducted by wiping the canes with 70% EtOH followed by air-drying under a flow-hood laminar. Afterwards, a 3 × 2 mm segment was discarded from the internode of each cane using a cork borer and a 3 × 2 mm plug from the edge of an actively growing colony on PDA was removed and inserted into the wound. Then, the wounds were covered with Parafilm (Amcor, Switzerland, Zurich), while the upper node was covered with melted paraffin wax to maintain humidity and avoid secondary contaminations. Inoculated canes were placed upright in 500 mL glass beakers with the node on the underside of the canes submerged in tap water to maintain hydration. The water was replaced every two days, and the canes remained under greenhouse conditions for 21 days at 25 °C ± 1 °C and a 12 h photoperiod. At the end of the incubation period, canes were surface sterilized and left to dry as described above, debarked, and the necrotic lesion length was measured above and below the inoculation site. To fulfill Koch’s postulates, isolations were conducted on MEA supplemented with streptomycin sulfate (100 μg/mL) from both regions flanking the inoculation site for every isolate used in the pathogenicity experiment. The experiment was conducted three times, and a total of 15 plants were used for each isolate. Furthermore, 15 plants served as negative controls, inoculated with a plain PDA plug.
2.7. Statistical Analyses
Data from the growth rate experiments were log-transformed and subjected to normality and homogeneity of variances using the Shapiro–Wilk and the Anderson–Darling statistical goodness-to-fit tests. As those conditions were met, subsequent analysis was performed using one-way ANOVA, followed by Tukey’s HSD test at α = 0.05. Data from the pathogenicity trials were also subjected to a normality test and homogeneity of variances using the Shapiro–Wilk and Levene’s test, respectively. However, since these data did not follow a normal distribution and variances were unequal, the non-parametric Kruskal–Wallis test was selected to determine if statistical differences occurred between the independent treatments. When significant, subsequent pairwise comparisons were performed using the Dunn’s test with Bonferroni adjustment at α = 0.01.
3. Results
3.1. Fungal Isolations
In total, 81 fungal isolates were recovered from the symptomatic grafted vines sampled from the nursery field. Based on the preliminary rRNA ITS region sequencing, the most prevalent fungal genus was Fusarium, accounting for 46 isolates (56.79%). Of those, two were isolated from the crown, sixteen from the internodes and twenty-eight from the grafting union. Moreover, seventeen (17) isolates were assigned to the genus Diaporthe (20.98%). Those Diaporthe isolates were obtained from all vine sections analyzed. More specifically, seven out of seventeen (41.18%) were isolated from the basal end, eight (47.06%) from the first and the second internodes and five (29.41%) from the grafting union. In addition, four isolates were assigned to the genus Trichoderma (4.94%), three to the genus Clonostachys (3.70%), five to the genus Dactylonectria (6.17%), and one to the genus Aspergillus (1.23%). Finally, five isolates (6.17%) were unidentified (Figure 1).

Figure 1.
Stacked bar chart showing the number of different fungal genera recovered from 2-month-old rooted grafted vines per vine section, as identified by the preliminary ITS region sequencing.
3.2. Phylogeny of Diaporthe
The phylogenetic trees constructed using both Maximum Likelihood and Bayesian inference analyses produced nearly identical topologies. The analysis comprised 90 taxa, including the outgroup species Diapothella corylina CBS121124 (isolated from Corylus sp., China). The final concatenated dataset of sequences from the four loci (ITS, TUB2, HIS3, and TEF1) consisted of 4180 nucleotide positions (ITS: 1318 bp, TUB2: 1443 bp, TEF1: 726 bp, and HIS3: 501 bp), including gaps. The resulting phylogenetic tree revealed that fungi of the genus Diaporthe isolated in this study were grouped into five well supported distinct clades. Six (6) isolates (V43B, UΝΚ, V118B, V42B, V45B and V41B) were placed within the D. ampelina species complex [43] and clustered with the closely related D. ampelina reference strains (Figure 2). The generation of the clade was supported by a high bootstrap value and posterior probability (99 and 0.98, respectively), confirming their identity as D. ampelina. Four isolates, V53M, V55M, V101M, and V91M, were grouped within the D. eres species complex and clustered with the reference strains AR5193 (isolated from Ulmus sp. in Germany) and DLR12 (isolated from Vitis vinifera in France), as well as with other synonymous-to D. eres strains, like D. cotoneastri, D. vacuae, D. celeris, and D. helicis. All the isolates in this group fell within D. eres (A) and were distinct from the D. eres (B) clade previously known as Diaporthe cf. nobilis/Phomopsis fukushii complex. This clustering was supported by a bootstrap value of 80 and posterior probability of 0.92. Isolates V71G, V73G, and V78G fell into the D. leucospermi species complex and were most closely related to the species D. serafiniae, although with a lower bootstrap value and posterior probabilities (70 and 0.80, respectively), which may suggest a lower resolution at this node. One isolate (V44M) belonged to the species D. foeniculina (bootstrap value of 99 and probability of 1), while isolates V94M and V92M were clustered with the species D. novem and fell into the D. arctii species complex, supported by a bootstrap value of 99 and a posterior probability of 1 (Figure 2).
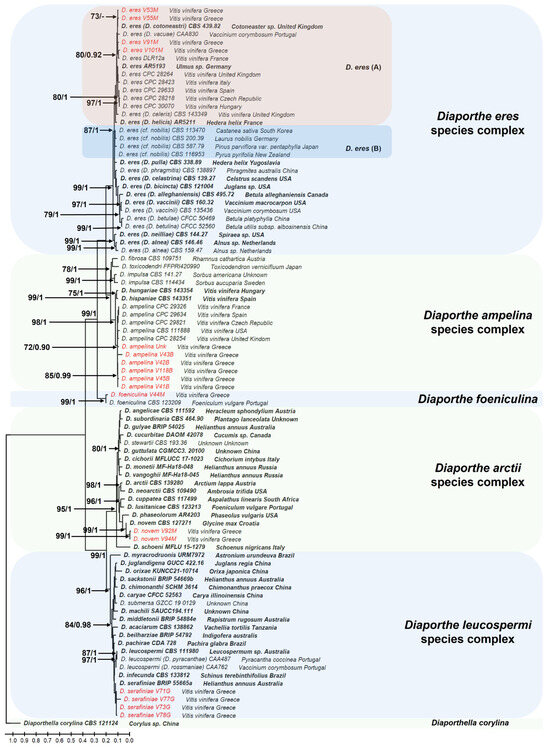
Figure 2.
The Diaporthe phylogenetic tree, inferred using the Maximum Likelihood method and General Time Reversible model. The tree with the highest log likelihood (−27,478.73) is shown. The percentage of trees in which the associated taxa are clustered together is shown next to the branches, as well as the posterior probabilities calculated with the Bayesian Inference analysis. Only bootstrap values > 70 and probabilities > 0.9 are shown in the branches. The initial tree for the heuristic search was obtained automatically by applying the Maximum Parsimony method. The tree is drawn to scale, with branch lengths measured in the number of nucleotide substitutions per site. This analysis involved 90 nucleotide sequences with the outgroup species designated to Diaporthella corylina CBS121124. Taxa in red represent the fungal species isolated in this study, while taxa in bold represent the ex-type cultures. There were a total of 4180 positions in the final dataset, including gaps.
3.3. Morphological Characterization
Growth rates of the five Diaporthe species were evaluated on three artificial media, PDA, MEA, and OA. Among the species, D. serafiniae exhibited the highest growth rate, particularly on PDA (2.20 ± 0.34 cm/day), a feature that distinguishes this species from the rest of the isolated taxa, especially from the slow-growing fungus D. ampelina. The latter exhibited the slowest growth, especially on OA medium (0.95 ± 0.09 cm/day), making this feature a useful characteristic for initial identification. D. foeniculina displayed the most variable growth, with high rates on OA (1.78 ± 0.02 cm/day) but limited growth on MEA (0.57 ± 0.06 cm/day). D. novem and D. eres demonstrated moderate to consistent growth across all media (Figure 3), a pattern that distinguishes these species from the rapid growth profiles of D. serafiniae and D. foeniculina. Moreover, D. eres and D. novem had the smallest alpha conidia, while D. ampelina and D. serafiniae had the largest, allowing the separation of these species based on alpha conidia size. The sizes of the beta conidia were approximately the same across all examined species, ranging from 22.64 ± 0.96 in D. novem to 25.80 ± 0.72 in D. foeniculina. Beta conidia were absent in D. serafiniae. The sizes of the alpha and beta conidia, as well as the size of the pycnidial conidiomata, are summarized in Table 1. Figure 4 depicts the morphological characteristics of D. ampelina, while representative morphologies of D. eres, D. foeniculina, D. serafiniae, and D. novem are shown in Supplementary Figures S2, S3, S4 and S5, respectively.

Figure 3.
Grouped histogram depicting the growth rates (cm/day) of the five Diaporthe species across the three media tested (PDA, MEA and OA). The bars above each histogram represent the standard error of the mean. The different letters indicate that there is a statistically significant difference between the different groups at α = 0.05 according to Tukey’s HSD test. PDA: potato dextrose agar, MEA: malt extract agar, OA: oatmeal agar.

Table 1.
Morphological characterization of Diaporthe species isolated in this study in terms of alpha and beta conidia size and the size of the pycnidial conidiomata. Values next to the ± symbol represent the standard error of the mean.
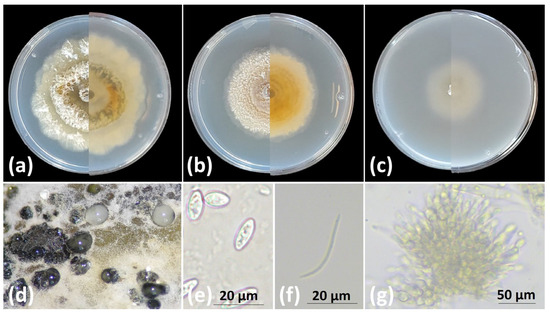
Figure 4.
Diaporthe ampelina cultures in plates with (a) PDA, (b) MEA, (c) OA, (left half: front view, right half: rear view), (d) oozing pycnidia in PDA, (e) alpha conidia, (f) beta conidia, (g) conidiomata. Scale bars: (e) and (f) 20 μm and (g) 100 μm.
3.4. Pathogenicity Trials
The pathogenic potential of the five Diaporthe species was assessed by inoculating detached lignified grapevine canes and measuring the resulting necrotic lesion lengths 21 days post-inoculation. Statistical analysis according to the Kruskal–Wallis Rank Sum test indicated a significant difference among groups at α = 0.01 (Table 2). Among the species tested, only D. ampelina and D. eres induced significant necrosis. In contrast, D. foeniculina, D. serafiniae, and D. novem caused either no symptoms or mild, localized lesions which only occurred in close proximity to the inoculation site. D. ampelina demonstrated the highest virulence, with mean lesion lengths of 42.1 mm for strain V42B, and 60.4 mm for strain V118B; both significantly longer compared to the mock-treated plants (p-value < 0.01). The necrosis induced by the two strains did not differ significantly from each other (p-value > 0.01). D. eres strains V55M and V101M also produced considerable necrotic lesions, with mean lengths of 26.8 mm and 19.6 mm, respectively. These values were lower than those caused by D. ampelina but still distinct from the control plants (p-value < 0.01). In contrast, strains of D. novem, D. foeniculina, and D. serafiniae were considered mildly pathogenic to grapevines under the conditions tested, producing average lesion lengths of 11.75 mm, 5.2 mm, and 3.7 mm, respectively (Figure 5a,c). A more detailed assessment of the symptoms revealed that unlike D. ampelina and D. eres, lesions caused by D. novem were limited to the phloem and were not observed in the xylem tissues, as inspections at the xylem level did not show any discoloration. The lesion lengths induced by the different species are summarized in Table 3.

Table 2.
Kruskal–Wallis Rank Sum test statistics for the pathogenicity assays, showing significant differences among groups (p-value < 0.01).

Figure 5.
(a) Box and Whiskers plot showing the necrotic lesion length range induced by the different Diaporthe species above and below the inoculation sites, (b) re-isolation rates of the different species one week post-incubation on MEA plates at 25 °C in the dark, fulfilling Koch’s postulates. (c) images captured 21 days post-inoculation of the different species, representing the data from sub-figure (a). In the sub-figures the discoloration levels and the recovery rates from the highest to the lowest are depicted. Different letters indicate the statistical difference between the group medians at α = 0.01 according to the non-parametric Dunn’s test. V42B and V118B: D. ampelina, V55M and V101M: D. eres, V94M: D. novem, V44M: D. foeniculina and V71G: D. serafiniae.

Table 3.
Pathogenicity assessment of seven Diaporthe isolates on detached grapevine canes. Mean lesion lengths (±SE) were measured 21 days post-inoculation, and recovery percentages reflect successful re-isolation of the inoculated fungi. Strains of D. ampelina and D. eres induced significantly longer necrotic lesions and showed higher recovery rates, indicating strong pathogenic potential. In contrast, D. novem, D. foeniculina, and D. serafiniae exhibited limited virulence and lower re-isolation frequencies, consistent with their classification as moderate or opportunistic pathogens. Different letters denote statistical groupings based on Dunn’s test in α ≤ 0.01).
Koch’s postulates were fulfilled for all species by re-isolation of the fungi and subsequent identification using morphological traits compared to the original isolate. Re-isolation rates for D. ampelina ranged from 59.26% (V42B) to 77.78% (V118B), and for D. eres from 25.93% (V101M) to 55.56% (V55M). Recovery of D. serafiniae and D. foeniculina occurred at lower frequencies; 3.7% and 22.22%, respectively, while D. novem was re-isolated from 25.93% of the inoculated canes (Figure 5b). No fungus was recovered from the mock-treated plants. Taking into consideration the low to negligible virulence and their recovery primarily from asymptomatic tissues, D. serafiniae and D. foeniculina were classified as opportunistic pathogens rather than primary pathogens of grapevines. On the other hand, D. novem, which induced longer necrotic lesions compared to the other two species, was classified as moderately pathogenic to grapevines.
4. Discussion
Grapevine trunk diseases (GTDs) are considered one of the major plagues in viticulture, affecting the grapevine industry and the wine sector worldwide [44,45]. In the last two decades, several trunk diseases have been extensively studied and described, including the well-known esca proper syndrome (Phaeomoniella chlamydospora, Phaeoacremonium minimum, Fomitiporia mediterranea), Botryosphaeria (Botryosphaeriaceae species), and Eutypa dieback (Eutypa lata), affecting mature vines, and Petri disease (Pa. chlamydospora, Pm. minimum and Cadophora luteo-olivacea) along with black foot disease (Ilyonectria, Dactylonectria) associated with young grapevine decline syndrome and propagation material infections [44]. Since 2013, Diaporthe ampelina (former Phomopsis viticola), a fungus implicated in Phomopsis cane and leaf spot disease in grapevines, was introduced into the trunk diseases complex due to its ability to cause cankers, necrotic lesions, discoloration, and dieback of cordons and canes [23]. As a result, a number of surveys have been conducted associated with the Diaporthe species responsible for grapevine trunk diseases in a few parts of the world, including China [20,25], a big part of Europe [11], South Africa [21] and North America [46]. In the current study, a baseline survey carried out in a grapevine nursery located in Nemea, the main viticultural region of Greece, revealed that grafted vines grown for two months in the nursery field were infected by more than one Diaporthe species simultaneously. Based on multi-locus sequencing, followed by an extensive phylogenetic analysis combining the maximum likelihood and the Bayesian inference, we identified five phylogenetically distinct Diaporthe species, namely D. ampelina, D. eres, D. foeniculina, D. serafiniae, and D. novem, all isolated from symptomatic young nursery vines, along with other endophytic fungi. Among the Diaporthe species isolated in this study, D. eres and D. foeniculina have been reported to infect peach trees [47] and citrus/mango trees in Greece [48,49], respectively. However, this is their first record in grapevines in Greece. In addition to that, and to the best of our knowledge, this study represents the first record of D. serafiniae and D. novem associated with grapevines in Greece. These findings expand the known geographic distribution of these species, which have been previously reported in several viticultural regions. Although in the present study the number of isolates obtained was limited, the analysis showed that D. ampelina was the most frequently isolated species, an observation that is in accordance with the available literature [23].
Due to the fact that the use of single loci for Diaporthe species identification may provide limited resolution in certain species complexes, multi-locus approaches remain the standard practice for species delimitation in addition to morphological characteristics [11]. In alignment with this practice, in this study we utilized four genetic loci to characterize the different species, enhancing the phylogenetic robustness. Indeed, the proper identification of the isolated species was not feasible by analyzing each locus separately (ITS, TEF1, TUB2, and HIS3), as closely related species share a high degree of homology in the conserved genes or regions analyzed. Furthermore, the size of the alpha and beta conidia, as well as the size of the pycnidial conidiomata are not always informative in species identification and description, as these characteristics overlap even between highly distinct species [29]. In our study, the size of both conidia types in D. eres, D. novem, and D. foeniculina were very similar, hence, these morphological traits should always be combined with a phylogeny-based approach when identifying Diaporthe spp. [50]. This is in accordance with other studies, which emphasize the importance of the proper molecular characterization of Diaporthe species in order to better understand their epidemiology and to develop efficient control strategies for different pathogens in grapevines [10,11,20,25,29,30]. As described by [43] in their recent study regarding the species boundaries and the species complexes in the genus Diaporthe, only taxa from the appropriate Diaporthe section or species complex should be included in the phylogenetic analysis. In our analysis, these suggestions were applied for accurate identification at the species level. Indeed, the identification of the majority of the species in this study was supported by high bootstrap values and posterior probabilities. In the case of D. serafiniae, this node was supported by somewhat lower bootstrap values compared to the other species, and this may reflect the low resolution of certain loci or incomplete lineage sorting within closely related Diaporthe species. This observation highlights the difficulty in resolving species boundaries in Diaporthe species complexes, where divergence is limited. Additionally, more informative molecular markers could be incorporated in the future to improve resolution, leading to a greater clarity in the phylogeny of this particular species.
This study also aimed to explore the dynamics of each species in symptom expression and disease development in grapevines. Greenhouse pathogenicity trials carried out in detached one-year-old lignified canes revealed that among the five species tested, only D. ampelina and D. eres exhibited high virulence. For each of the two species, two different isolates from different individuals were tested. In the case of D. ampelina, canes inoculated with isolate V118B exhibited necrotic lesions of 60.4 mm on average, while isolate V42B induced necrosis of 42.1 in length. Although not significantly different, isolate V42B caused lower necrosis compared to isolated V118B. This difference might be attributed to an intra-specific variation in virulence, an assumption also made by other researchers where different D. ampelina isolates displayed varying pathogenicity [24,51,52]. Of course, these observations need to be further validated by testing a large number of isolates, since genetic characteristics of each strain or the genotype of the host plant may play a role in this variation. Nonetheless, we report here that D. ampelina emerged as the most aggressive species, strengthening the findings of earlier data [21,53]. In addition, our work confirmed the pathogenic potential of D. eres in grapevines, an observation that corroborates the findings of previous research [20,24,27]. The two isolates tested induced significant necrosis, but to a lower extent compared to D. ampelina, which is in agreement with the available literature [27,46]. Within the constraints of our experimentation, the abovementioned species emerged as the most aggressive ones, consistent with reports from previous research in other viticultural regions [23,25,46].
Although earlier studies have identified D. novem as a potential grapevine pathogen [21], its role in disease development is not clear. In this study, canes artificially inoculated with D. novem displayed minor discoloration, which was limited to the phloem tissues. The observed phloem-limited infection may enable the pathogen to persist in living tissues without directly disrupting the xylem tissues, facilitating latent infections and gradual disease progression under certain circumstances. The lesion length induced by this species was not significantly different from that of isolate V55M of D. eres, but it also did not differ significantly from the control plants. Nevertheless, D. novem was re-isolated from 25.93% of the inoculated canes, supporting its mild virulence in grapevines. These findings align with those of [21] who also assessed the pathogenicity of D. novem in grapevines, among other species. In their work, they showed that although D. novem formed moderate lesions, it was re-isolated in a significant percentage of samples in both experiments they conducted. According to this, they concluded that D. novem can be considered a pathogen of grapevines [21]. Furthermore, and to the best of our knowledge, D. serafiniae has been found once in South Africa [21]. This study assessed the virulence of three different isolates, and observed a variation in pathogenicity, probably due to the different lignin content of the plant material used. As a result, they concluded that some isolates of D. serafiniae might act as potential pathogens of grapevines, and some were considered to be weak pathogens [21]. In our study, D. serafiniae induced the shortest necrotic lesions (3.7 mm), thus it was classified as a weak pathogen. Similar results were obtained in canes inoculated with D. foeniculina, where the necrotic lesions did not differ significantly from the control plants. Other than their low virulence, both fungi were successfully re-isolated from the inoculated canes, which means that they are able to colonize grapevines. Based on that, our study suggests that, similarly to D. serafiniae, D. foeniculina acts as a weak pathogen, but this classification is tentative, since additional data on host response to pathogen infection or histopathology are required to fully confirm their pathogenic profile. Our observation that D. foeniculina caused minimal lesions, mirroring results obtained in Croatia [54], California [23], and Uruguay [27], reinforcing its likely role as a weak pathogen in grapevines. Recently, this species was isolated in Cyprus from cordons and spurs, displaying severe dieback symptoms and internal wood discoloration [55]. While in this study the fungus was able to induce necrosis in rooted cuttings, this occurred seven months post inoculation, which might indicate that longer incubation periods might produce different outcomes. As a result, the classification of D. foeniculina and D. serafiniae as weak pathogens needs to be interpreted with caution. Their ability to colonize grapevine tissues without causing significant symptoms raises the possibility of endophytism, or potential latent infections that could be expressed under specific conditions.
The role of D. novem, D. foeniculina, and D. serafiniae in disease expression in the field remains uncertain, as they exhibited negligible virulence in grapevines under our experimental conditions. Still, it is not well understood what the contributing factors are that drive the shift in a fungus from an endophytic to a pathogenic phase. It is believed that the nursery practices associated with the production of propagation material induce stress in plants, making them vulnerable to latent pathogen attacks [56]. Also, climatic conditions, such as the increased temperatures in mid-summer documented in Greece, may lead to water deficiency in the nursery field, and as a result, to increased susceptibility of the grafted vines to wood-invading pathogens. These abiotic stresses may alter the plants’ biochemical, physiological, and ecological procedures and suppress the plants’ immune systems, making them vulnerable to opportunistic pathogens. Also, they facilitate the generation of new, more virulent, and better adapted pathogen strains [57,58]. Furthermore, the elevated temperatures may decrease pathogens’ overwintering and incubation period, leading to increased inoculum in the field over a growing season, and thus higher disease pressure [57]. Another factor that might play a role in plants’ stress is the microbial imbalances in the propagation material. In this study, approximately 56% and 6% of the isolated fungi from grafted vines were assigned to the genus Fusarium and Dactylonectria. Recent studies have shown that the abundance of the Fusarium genus representatives is higher in symptomatic vines compared to asymptomatic ones [59]. In this particular study, it was stated that co-inoculation of vines with Fusarium spp. and Dactylonectria macrodidyma resulted in higher disease severity, compared to the single inoculations. This might also be the case here, where the microbial imbalances in rooted grafted vines could influence the biotic stress of the plants and promote subsequent opportunistic infections by Diaporthe species. Given the potential influence of abiotic and biotic stressors on disease expression and development, nursery practices such as careful monitoring of mother plants, precise harvesting of the initial material, and minimization of mechanical or water stress during the propagation process may help reduce the risk of latent infections and improve plant health.
5. Conclusions
Our study highlights the complexity of the Diaporthe communities present in grapevine propagation material and highlights the importance of additional vigilant surveys in nurseries. Although D. ampelina and D. eres showed clear pathogenic potential, the role of the remaining species remains unsolved. Addressing these uncertainties through multi-year and multi-site surveys will be critical for designing effective control strategies in Greek nurseries and beyond. While our findings cannot be extrapolated to national prevalence, they establish a reference point for subsequent large scale studies and stimulate further research into the development of effective management approaches to mitigate infections caused by these emerging pathogens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres16110224/s1, Figure S1. Symptomatic 2-months old, rooted grafted vines in a nursery field located in Nemea, Greece; Figure S2. Diaporthe eres culture in plates with (a) PDA, (b) MEA, (c) OA, (left half: front view, right half: rear view); (d) pycnidia on pine needles induced in PNA medium, (e) alpha- (small, hyaline) and beta- (long, curved) conidia, (f) conidiomata. Scale bars: (e) 20 μm and (f) 50 μm; Figure S3. Diaporthe foeniculina culture in plaes with (a) PDA, (b) MEA, (c) OA, (left half: front view, right half: rear view); (d) pycnidia on pine needles induced in PNA medium, (e) alpha- (small, hyaline) and beta- (long, curved) conidia, (f) conidiomata. Scale bars: (e) 20 μm and (f) 50 μm; Figure S4. Diaporthe serafiniae cultures in plates with (a) PDA, (b) MEA, (c) OA, (left half: front view, right half: rear view); (d) pycnidia on pine needle induced in PNA medium, (e) alpha- conidia. Scale bars: (e) 20 μm; Figure S5. Diaporthe novem culture in plates with (a) PDA, (b) MEA, (c) OA, (left half: front view, right half: rear view); (d) pycnidia on pine needle induced in PNA medium, (e) alpha- conidia, (f) beta- conidia and (g) conidiomata. Scale bars: (e) 20 μm, (g) 50 μm; Table S1. Primers sets used for PCR amplification, their sequence and the respective PCR conditions; Table S2. Accession numbers of ITS, TUB2, HIS3 and TEF1 sequences retrieved from the GenBank database (normal font), as well as from the sequences obtained in this study, denoted in italicized bold. The table indicates the host from which each strain was isolated as well as the country of origin. Isolates in non-italicized bold indicate the ex-type cultures.
Author Contributions
Conceptualization: C.T., G.S. and E.P.; methodology: C.T., G.S. and E.P.; software: C.T.; validation: C.T., G.S. and E.P.; formal analysis: C.T.; investigation: C.T. and G.S.; writing—original draft preparation: C.T. and G.S.; writing—review and editing: C.T., G.S. and E.P. Visualization: C.T.; supervision: E.P.; funding acquisition: C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hellenic Foundation for Research and Innovation (HFRI) under the 3d Call for HFRI PhD Fellowships (Fellowship Number: 6200).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the Vine Nurseries Bakasietas (VNB) for kindly providing the grafted vines used for the isolations of fungal pathogens. All authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Udayanga, D.; Liu, X.; McKenzie, E.H.C.; Chukeatirote, E.; Bahkali, A.H.A.; Hyde, K.D. The genus Phomopsis: Biology, applications, species concepts and names of common phytopathogens. Fungal Divers. 2011, 50, 189–225. [Google Scholar] [CrossRef]
- Gomes, R.R.; Glienke, C.; Videira, S.I.R.; Lombard, L.; Groenewald, J.Z.; Crous, P.W. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Persoonia-Mol. Phylogeny Evol. Fungi 2013, 31, 1–41. [Google Scholar]
- Chepkirui, C.; Stadler, M. The genus Diaporthe: A rich source of diverse and bioactive metabolites. Mycol. Prog. 2017, 16, 477–494. [Google Scholar] [CrossRef]
- Tanney, J.B.; McMullin, D.R.; Green, B.D.; Miller, J.D.; Seifert, K.A. Production of antifungal and antiinsectan metabolites by the Picea endophyte Diaporthe maritima Sp. Nov. Fungal Biol. 2016, 120, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Iriart, X.; Binois, R.; Fior, A.; Blanchet, D.; Berry, A.; Cassaing, S.; Amazan, E.; Papot, E.; Carme, B.; Aznar, C.; et al. Eumycetoma caused by Diaporthe phaseolorum (Phomopsis phaseoli): A case report and a mini-review of Diaporthe/Phomopsis Spp invasive infections in humans. Clin. Microbiol. Infect. 2011, 17, 1492–1494. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, A.J.; Phillips, A.J.L.; Hyde, K.D.; Yan, J.Y.; Li, X.H. The Current status of species in Diaporthe. Mycosphere 2017, 8, 1106–1156. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, F.; Duan, W.; Crous, P.W.; Cai, L. Diaporthe is paraphyletic. IMA Fungus 2017, 8, 153–187. [Google Scholar] [CrossRef]
- Santos, J.M.; Vrandečić, K.; Ćosić, J.; Duvnjak, T.; Phillips, A.J.L. Resolving the Diaporthe species occurring on soybean in Croatia. Persoonia-Mol. Phylogeny Evol. Fungi 2011, 27, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Díaz, G.A.; Latorre, B.A.; Lolas, M.; Ferrada, E.; Naranjo, P.; Zoffoli, J.P. Identification and characterization of Diaporthe ambigua, D. australafricana, D. novem, and D. rudis causing a postharvest fruit rot in kiwifruit. Plant Dis. 2017, 101, 1402–1410. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Crous, P.W. Emerging citrus diseases in Europe caused by species of Diaporthe. IMA Fungus 2017, 8, 317–334. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Groenewald, J.Z.; Woodhall, J.; Armengol, J.; Cinelli, T.; Eichmeier, A.; Ezra, D.; Fontaine, F.; Gramaje, D.; Gutierrez-Aguirregabiria, A. Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe. Persoonia-Mol. Phylogeny Evol. Fungi 2018, 40, 135–153. [Google Scholar]
- Santos, J.M.; Phillips, A.J.L. Resolving the Complex of Diaporthe (Phomopsis) species occurring on Foeniculum vulgare in Portugal. Fungal Divers. 2009, 34, 111–125. [Google Scholar]
- Mena, E.; Stewart, S.; Montesano, M.; Ponce de León, I. Current understanding of the Diaporthe/Phomopsis complex causing soybean stem canker: A focus on molecular aspects of the interaction. Plant Pathol. 2023, 73, 31–46. [Google Scholar] [CrossRef]
- Hosseini, B.; El-Hasan, A.; Link, T.; Voegele, R.T. Analysis of the species spectrum of the Diaporthe/Phomopsis complex in European soybean seeds. Mycol. Prog. 2020, 19, 455–469. [Google Scholar] [CrossRef]
- Floyd, C.M.; Malvick, D.K. Diaporthe species associated with symptomatic and asymptomatic infection of soybean stems in Minnesota: Identity, virulence, and growth characteristics. Can. J. Plant Pathol. 2022, 44, 858–873. [Google Scholar] [CrossRef]
- Thompson, S.M.; Tan, Y.P.; Young, A.J.; Neate, S.M.; Aitken, E.A.B.; Shivas, R.G. Stem cankers on sunflower (Helianthus annuus) in Australia reveal a complex of pathogenic Diaporthe (Phomopsis) species. Persoonia-Mol. Phylogeny Evol. Fungi 2011, 27, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, A.; Mancebo, M.F.; Bazzalo, M.E.; Reid, R.J.; Sanchez, M.C.; Kontz, B.J.; Mathew, F.M. Six species of Diaporthe associated with phomopsis stem canker of sunflower in southern Pampean region of Argentina. Plant Health Prog. 2021, 22, 136–142. [Google Scholar] [CrossRef]
- Pine, T.S. Development of the Grape dead-arm disease. Phytopathology 1959, 49, 738–743. [Google Scholar]
- Rossman, A.Y.; Adams, G.C.; Cannon, P.F.; Castlebury, L.A.; Crous, P.W.; Gryzenhout, M.; Jaklitsch, W.M.; Mejia, L.C.; Stoykov, D.; Udayanga, D.; et al. Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus 2015, 6, 145–154. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Liu, M.; Zhang, W.; Chen, Z.; Udayanga, D.; Chukeatirote, E.; Li, X.; Yan, J.; Hyde, K.D. Morphological and molecular characterisation of Diaporthe species associated with grapevine trunk disease in China. Fungal Biol. 2015, 119, 283–294. [Google Scholar] [CrossRef]
- Lesuthu, P.; Mostert, L.; Spies, C.F.J.; Moyo, P.; Regnier, T.; Halleen, F. Diaporthe nebulae sp. nov. and first report of D. cynaroidis, D. novem, and D. serafiniae on grapevines in South Africa. Plant Dis. 2019, 103, 808–817. [Google Scholar] [CrossRef]
- Scheper, R.W.A.; Crane, D.C.; Whisson, D.L.; Scott, E.S. The Diaporthe teleomorph of Phomopsis Taxon 1 on grapevine. Mycol. Res. 2000, 104, 226–231. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Peduto, F.; Smith, R.J.; Gubler, W. Phomopsis dieback: A grapevine trunk disease caused by Phomopsis viticola in California. Plant Dis. 2013, 97, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Fedele, G.; Armengol, J.; Caffi, T.; Languasco, L.; Latinovic, N.; Latinovic, J.; León, M.; Marchi, G.; Mugnai, L.; Rossi, V. Diaporthe foeniculina and D. eres, in addition to D. ampelina, may cause Phomopsis cane and leaf spot disease in grapevine. Front. Plant Sci. 2024, 15, 1446663. [Google Scholar] [CrossRef]
- Manawasinghe, I.S.; Dissanayake, A.J.; Li, X.; Liu, M.; Wanasinghe, D.N.; Xu, J.; Zhao, W.; Zhang, W.; Zhou, Y.; Hyde, K.D. High genetic diversity and species complexity of Diaporthe associated with grapevine dieback in China. Front. Microbiol. 2019, 10, 1936. [Google Scholar] [CrossRef]
- Pintos, C.; Redondo, V.; Costas, D.; Aguín, O.; Mansilla, P. Fungi associated with grapevine trunk diseases in nursery-produced Vitis vinifera plants. Phytopathol. Mediterr. 2018, 57, 407–424. [Google Scholar]
- Carbone, M.J.; Reyna, R.; Moreira, V.; González-Barrios, P.; Mondino, P.; Alaniz, S. Four Diaporthe species associated with grapevine nursery plants and commercial vineyards in Uruguay. Plant Pathol. 2024, 74, 519–535. [Google Scholar] [CrossRef]
- Eichmeier, A.; Pečenka, J.; Peňázová, E.; Baránek, M.; Català-García, S.; León, M.; Armengol, J.; Gramaje, D. High-throughput amplicon sequencing-based analysis of active fungal communities inhabiting grapevine after hot-water treatments reveals unexpectedly high fungal diversity. Fungal Ecol. 2018, 36, 26–38. [Google Scholar] [CrossRef]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Chukeatirote, E.; Hyde, K.D. Insights into the Genus Diaporthe: Phylogenetic species delimitation in the D. eres species complex. Fungal Divers. 2014, 67, 203–229. [Google Scholar] [CrossRef]
- Santos, L.; Alves, A.; Alves, R. Evaluating multi-locus phylogenies for species boundaries determination in the genus Diaporthe. PeerJ 2017, 5, e3120. [Google Scholar] [CrossRef]
- Chaisiri, C.; Liu, X.; Lin, Y.; Fu, Y.; Zhu, F.; Luo, C. Phylogenetic and haplotype network analyses of Diaporthe eres species in China based on sequences of multiple loci. Biology 2021, 10, 179. [Google Scholar] [CrossRef]
- Karthikeyan, V.; Patharajan, S.; Palani, P.; Spadaro, D. Modified simple protocol for efficient fungal DNA extraction highly suitable for PCR based molecular methods. Glob. J. Mol. Sci. 2010, 5, 37–42. [Google Scholar]
- Oñate-Sánchez, L.; Vicente-Carbajosa, J. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res. Notes 2008, 1, 93. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In PCR Protocols; Academic Press, Inc: Cambridge, MA, USA, 1990; Volume 31, pp. 315–322. [Google Scholar]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A Method for Designing primer sets for speciation studies in filamentous Ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on Fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 2, 2.3.1–2.3.22. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000; ISBN 0195350510. [Google Scholar]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef]
- Su, Y.-Y.; Qi, Y.-L.; Cai, L. Induction of sporulation in plant pathogenic Fungi. Mycology 2012, 3, 195–200. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Zhu, J.-T.; Chen, Y.-Y.; Maharachchikumbura, S.S.N.; Hyde, K.D.; Liu, J.-K. A re-evaluation of Diaporthe: Refining the boundaries of species and species complexes. Fungal Divers. 2024, 126, 1–125. [Google Scholar] [CrossRef]
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef]
- Christopoulou, M.; Tsoukas, C.; Gkizi, D.; Triantafyllopoulou, A.; Tzima, A.K.; Paplomatas, E.J. Development of a molecular diagnostic to discriminate between Fomitiporia species and advancements in detection of the main grapevine decline-related pathogens in propagating material and mature vines. Plant Pathol. 2024, 73, 326–341. [Google Scholar] [CrossRef]
- Baumgartner, K.; Fujiyoshi, P.T.; Travadon, R.; Castlebury, L.A.; Wilcox, W.F.; Rolshausen, P.E. Characterization of species of Diaporthe from wood cankers of grape in Eastern North American vineyards. Plant Dis. 2013, 97, 912–920. [Google Scholar]
- Thomidis, T.; Michailides, T.J. Studies on Diaporthe eres as a new pathogen of peach trees in Greece. Plant Dis. 2009, 93, 1293–1297. [Google Scholar] [CrossRef]
- Vakalounakis, D.J.; Ntougias, S.; Kavroulakis, N.; Protopapadakis, E. Neofusicoccum parvum and Diaporthe foeniculina associated with twig and shoot blight and branch canker of citrus in Greece. J. Phytopathol. 2019, 167, 527–537. [Google Scholar] [CrossRef]
- Mathioudakis, M.M.; Tziros, G.T.; Kavroulakis, N. First report of Diaporthe foeniculina associated with branch canker of avocado in Greece. Plant Dis. 2020, 104, 3057. [Google Scholar] [CrossRef]
- Udayanga, D.; Liu, X.; Crous, P.W.; McKenzie, E.H.C.; Chukeatirote, E.; Hyde, K.D. A multi-locus phylogenetic evaluation of Diaporthe (Phomopsis). Fungal Divers. 2012, 56, 157–171. [Google Scholar] [CrossRef]
- Schilder, A.M.C.; Erincik, O.; Castlebury, L.; Rossman, A.; Ellis, M.A. Characterization of Phomopsis spp. infecting grapevines in the Great Lakes region of North America. Plant Dis. 2005, 89, 755–762. [Google Scholar] [CrossRef]
- Akgül, D.S.; Awan, Q.N. Characterization of Diaporthe ampelina isolates and their sensitivity to Hot-Water Treatments and fungicides in in vitro. Kahramanmaraş Sütçü İmam Üniv. Tarım Doğa Derg. 2022, 25, 1378–1389. [Google Scholar]
- Lawrence, D.P.; Travadon, R.; Baumgartner, K. Diversity of Diaporthe species associated with wood cankers of fruit and nut crops in Northern California. Mycologia 2015, 107, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Kaliterna, J.; Milicevic, T.; Cvjetkovic, B. Grapevine Trunk Diseases associated with fungi from the Diaporthaceae family in Croatian vineyards. Arh. Hig. Rada Toksikol. 2012, 63, 471. [Google Scholar] [CrossRef]
- Makris, G.; Solonos, S.; Christodoulou, M.; Kanetis, L.I. First report of Diaporthe foeniculina associated with grapevine trunk diseases on Vitis vinifera in Cyprus. Plant Dis. 2021, 106, 1294. [Google Scholar] [CrossRef]
- Waite, H.; M, W.-W.; Torley, P. Grapevine propagation: Principles and methods for the production of high-quality grapevine planting material. N. Z. J. Crop Hortic. Sci. 2015, 43, 144–161. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Mohammed, T.; Laasli, S.-E.; Gachara, G.; Ezzouggari, R.; Belabess, Z.; Aberkani, K.; Assougeum, A.; Meddich, A.; El Jarroudi, M.; et al. Effects of climate change on plant pathogens and host-pathogen interactions. Crop Environ. 2024, 3, 159–170. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, W.; Zhang, J.; Wang, H.; Peng, J.; Wang, X.; Yan, J. Belowground microbiota analysis indicates that Fusarium spp. exacerbate grapevine trunk disease. Environ. Microbiome 2023, 18, 29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).