Abstract
Cefazolin is a widely used first-generation cephalosporin. While generally well tolerated, several case reports have described severe coagulopathy induced by intravenous (IV) cefazolin. This was seen particularly in patients with impaired renal function, where antibiotic choice is limited and may require specific dose adjustments. Altered renal handling of antibiotics and their metabolites may potentiate toxicity and side effects. We report a case of a 72-year-old Chinese man who had been treated for methicillin-sensitive staphylococcus aureus (MSSA, coagulase-positive) infective endocarditis with cefazolin and, consequently, developed significantly elevated international normalised ratio (INR) while on therapy. This resolved within 48 h after cessation of cefazolin and administration of oral vitamin K. Malnourished patients with pre-existing or acute kidney injury may be at an increased risk of cefazolin-related coagulopathy.
1. Introduction
Cefazolin is a widely used first-generation cephalosporin. While generally well tolerated, several case reports have described severe coagulopathy induced by intravenous (IV) cefazolin [1,2]. Particularly in patients with impaired renal function, antibiotic choice is limited and may require specific dose adjustments. Altered renal handling of antibiotics and their metabolites may potentiate toxicity and side effects [1,2,3].
We report a case of a 72-year-old Chinese man who had been treated for methicillin-sensitive staphylococcus aureus (MSSA, coagulase-positive) infective endocarditis with cefazolin and, consequently, developed significantly elevated international normalised ratio (INR) while on therapy.
2. Case Report
A 72-year-old Chinese man with hypertension, end-stage kidney disease, as well as a prior bioprosthetic aortic valve replacement for severe aortic stenosis had been admitted to the intensive care unit (ICU) with septic shock. Prior to admission, he had been on oral anti-hypertensive agents but had not been not on any antiplatelets or anticoagulation. There had been no new medications or newly initiated supplements. Three consecutive blood cultures revealed MSSA bacteraemia and transoesophageal echocardiography confirmed prosthetic valve infective endocarditis. The tunnelled dialysis catheter was removed, and IV cefazolin 1 g 12-hourly was started. His fever lysed, blood pressure stabilised, and repeat blood cultures at 72 h were negative for bacterial growth. The patient did not receive anticoagulation as an inpatient and was initiated on peritoneal dialysis during his hospital stay. Intravenous heparin was not administered during dialysis.
His total white cell count improved from 19.09 (×109/L) to 12.63 (×109/L) by day 14 of admission, while his haemoglobin concentration remained stable between 7–8 g/dL. His platelet count was low at presentation 14 (×109/L) but rose to 225 (×109/L) by day 14. His baseline INR was 0.93, which rose slightly to 1.39 on day 8, and then sharply to 8.24 on day 14 of his admission (Figure 1). While normal at presentation, his PT was 73.4 s, and activated partial thromboplastin time (aPTT) was 88.0 s on day 14. His fibrinogen (4.81 g/L) and d-dimer (8.0 μg/mL) were both elevated. There was no evidence of worsening sepsis, bleeding, liver failure, or elevated transaminases.
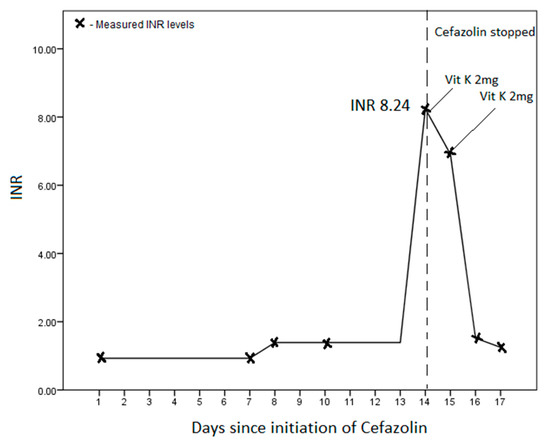
Figure 1.
International normalised ratio (INR) trend on initiation and subsequent cessation of IV cefazolin.
We did not measure serum cefazolin levels during therapy. However, in the absence of an alternative reason for the markedly elevated INR, cefazolin was implicated and stopped. A total of four milligrams of oral vitamin K was given over the next two days, which led to a rapid improvement in the INR (Figure 1). After three days of stopping the cefazolin, the patient had two episodes of melaena, which was managed conservatively with IV esomeprazole. He had persistent hypotension and was unable to continue with renal replacement therapy and, subsequently, transited to palliative care.
3. Discussion
In prior case reports, cefazolin use has been demonstrated to induce vitamin-K-deficient coagulopathy [1,2,3,4,5,6]. Although more commonly associated with ticarcillin and nitrofurantoin, this remained a less well-known complication of cefazolin [1]. While the underlying mechanism was not fully understood, several mechanisms have been proposed. It may be the result of altered intestinal flora due to the antimicrobial effect of cefazolin, which is the main source of vitamin K [2]. Alternatively, the cephalosporin itself may exert a direct effect in suppressing synthesis of vitamin K by the liver [2,3]. Structurally, cefazolin contains the methyl-thiadiazole side chain that appeared to exert similar effects to vitamin K epoxide reductase inhibitors. Other cephalosporins without this side chain do not exert similar effects [4]. The result is a patient who has prolonged PT, aPTT, and elevated INR with an increased bleeding risk.
Several factors may predispose patients to developing cefazolin-induced coagulopathy. First, a component of malnutrition, with a reduced oral intake of vitamin K, may be contributory. Of note, our patient had a low serum albumin of 17 g/dL, and hypoalbuminemia had been reported in several cases of cefazolin-induced coagulopathy [4,6]. Second, the effect appears to be dose-dependent. Patients with higher doses of cefazolin or with more prolonged courses of treatment tended to develop more severe coagulopathy (Table 1).

Table 1.
Summary of prior case reports on cefazolin-related deranged coagulopathy.
In addition to the above factors, abnormal metabolism and excretory function in patients with impaired renal function may also contribute to cefazolin toxicity [3,7]. True enough, this phenomenon appeared to be frequently reported in patients with acute kidney injury or end-stage kidney disease [1,2,4,5,7,8]. Patients with uraemia, in addition to being malnourished, may also demonstrate reduced renal clearance of the drug, which thereby potentiates its toxicity [3,7].
While on therapy, the onset of coagulopathy appeared to be variable, from as early as 2–4 days into therapy [2,6] in some patients, and up to 14–21 days in others. Upon cessation of cefazolin and commencement of vitamin K therapy, the coagulopathy consistently resolved within 48 h [1,2,5,6,7]. Although some patients did not experience any complications, others had bleeding manifestations, which may have been mild (bruises, haematuria) or more severe (gastrointestinal bleeding, intracranial haemorrhage) [5,6,7].
The prevalence of cefazolin-induced coagulopathy remains unknown [9], and it is not routine to monitor the coagulation profile while on cefazolin [10]. Cefazolin levels are also not routinely measured. While larger prospective studies are required to examine this association, we suggest that monitoring of the coagulation profile may be considered in patients on prolonged therapy, and in particular, patients who are malnourished or have renal impairment. Furthermore, in these “high-risk” patients, monitoring of cefazolin levels may also be useful to avoid toxicity [11].
Author Contributions
J.N.N., T.S.L., S.M.T., and T.P. were involved in the conception, data interpretation, literature review, and writing of the manuscript. R.A., J.Y., P.A.T., and L.H.W.L. were involved in the conception, writing, and critical review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and presented fully anonymised non-identifiable information of a single-patient case report that did not require prior approval by the Institutional Review Board.
Informed Consent Statement
Written informed consent was obtained from the patient involved in the study.
Data Availability Statement
Data may be made available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Ethics/Patient Consent
Written informed consent was obtained from the patient prior for this report.
References
- Chung, A.H.; Watson, K. Cefazolin-induced hypoprothrombinemia. Am. J. Health Syst. Pharm. 2008, 65, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Al Khowtair, J.; Al Sulaiti, G.; Nasser, A.; Ummunnisa, F.; Ahmed, Z.; Hassens, Y. Cefazolin and Coagulation Disorder: A case report and review of literature. Qatar. Med. J. 2013, 21, 54–55. [Google Scholar] [CrossRef]
- Lerner, P.I.; Lubin, A. Letter: Coagulopathy with cefazolin in uremia. N. Engl. J. Med. 1974, 290, 1324. [Google Scholar] [PubMed]
- Shearer, M.J.; Bechtold, H.; Andrassy, K.; Koderisch, J.; McCarthy, P.T.; Trenk, D.; Jahnchen, E.; Ritz, E. Mechanism of cephalosporin-induced hypoprothrombinemia: Relation to cephalosporin side chain, vitamin K metabolism, and vitamin K status. J. Clin. Pharmacol. 1988, 28, 88–95. [Google Scholar] [CrossRef]
- Kurz, R.W.; Wallner, M.; Graninger, W.; Tragl, R.H. Hypoprothrombinaemia and bleeding associated with cefazolin. J. Antimicrob. Chemother. 1986, 18, 772–773. [Google Scholar] [CrossRef]
- Shimada, K.; Matsuda, T.; Inamatsu, T.; Urayama, K. Bleeding secondary to vitamin K deficiency in patients receiving parenteral cephem antibiotics. J. Antimicrob. Chemother. 1984, 14 (Suppl. B), 325–330. [Google Scholar] [CrossRef]
- Peetermans, W.; Verbist, L. Stollingsstoornissen door cefalosporines met methyl-thio-tetrazole zijketen [Coagulation disorders caused by cephalosporins containing methylthiotetrazole side chains]. Acta Clin. Belg. 1990, 45, 327–333. [Google Scholar] [CrossRef]
- Kuypers, D.R.; Claes, K. Intracerebral haemorrhage caused by cefazoline induced hypoprothrombinaemia in a renal transplant recipient. Nephrol. Dial. Transplant. 2002, 17, 532–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agnelli, G.; Del Favero, A.; Parise, P.; Guerciolini, R.; Pastricci, B.; Nenci, G.G.; Ofosu, F. Cephalosporin-induced hypoprothrombinemia: Is the N-methylthiotetrazole side chain the culprit? Antimicrob. Agents Chemother. 1986, 29, 1108–1109. [Google Scholar] [CrossRef]
- Neu, H.C. The new beta-lactamase-stable cephalosporins. Ann. Intern. Med. 1982, 97, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of β-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).