Deranged Coagulation Profile Secondary to Cefazolin Use: Case Report
Abstract
1. Introduction
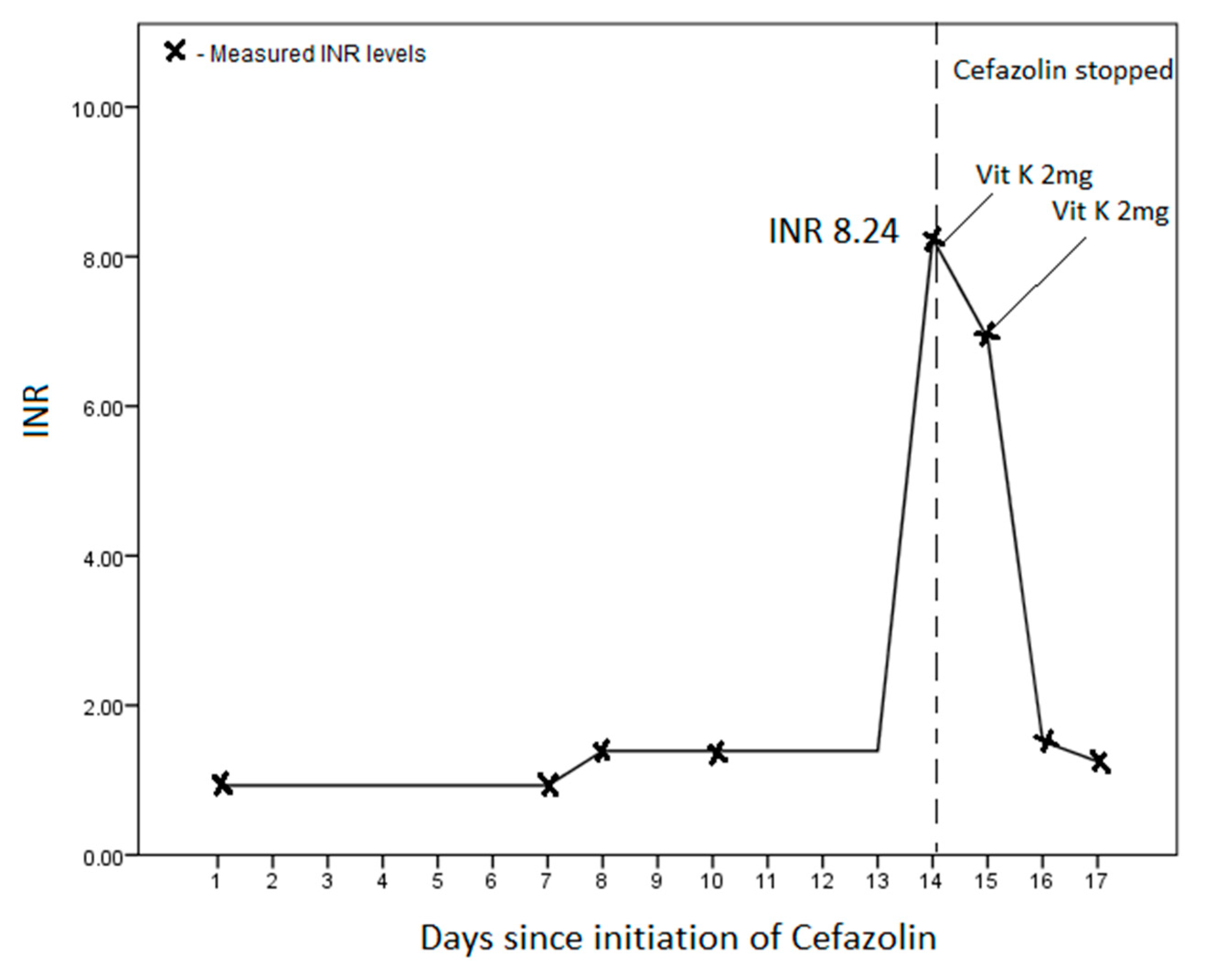
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics/Patient Consent
References
- Chung, A.H.; Watson, K. Cefazolin-induced hypoprothrombinemia. Am. J. Health Syst. Pharm. 2008, 65, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Al Khowtair, J.; Al Sulaiti, G.; Nasser, A.; Ummunnisa, F.; Ahmed, Z.; Hassens, Y. Cefazolin and Coagulation Disorder: A case report and review of literature. Qatar. Med. J. 2013, 21, 54–55. [Google Scholar] [CrossRef]
- Lerner, P.I.; Lubin, A. Letter: Coagulopathy with cefazolin in uremia. N. Engl. J. Med. 1974, 290, 1324. [Google Scholar] [PubMed]
- Shearer, M.J.; Bechtold, H.; Andrassy, K.; Koderisch, J.; McCarthy, P.T.; Trenk, D.; Jahnchen, E.; Ritz, E. Mechanism of cephalosporin-induced hypoprothrombinemia: Relation to cephalosporin side chain, vitamin K metabolism, and vitamin K status. J. Clin. Pharmacol. 1988, 28, 88–95. [Google Scholar] [CrossRef]
- Kurz, R.W.; Wallner, M.; Graninger, W.; Tragl, R.H. Hypoprothrombinaemia and bleeding associated with cefazolin. J. Antimicrob. Chemother. 1986, 18, 772–773. [Google Scholar] [CrossRef]
- Shimada, K.; Matsuda, T.; Inamatsu, T.; Urayama, K. Bleeding secondary to vitamin K deficiency in patients receiving parenteral cephem antibiotics. J. Antimicrob. Chemother. 1984, 14 (Suppl. B), 325–330. [Google Scholar] [CrossRef]
- Peetermans, W.; Verbist, L. Stollingsstoornissen door cefalosporines met methyl-thio-tetrazole zijketen [Coagulation disorders caused by cephalosporins containing methylthiotetrazole side chains]. Acta Clin. Belg. 1990, 45, 327–333. [Google Scholar] [CrossRef]
- Kuypers, D.R.; Claes, K. Intracerebral haemorrhage caused by cefazoline induced hypoprothrombinaemia in a renal transplant recipient. Nephrol. Dial. Transplant. 2002, 17, 532–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agnelli, G.; Del Favero, A.; Parise, P.; Guerciolini, R.; Pastricci, B.; Nenci, G.G.; Ofosu, F. Cephalosporin-induced hypoprothrombinemia: Is the N-methylthiotetrazole side chain the culprit? Antimicrob. Agents Chemother. 1986, 29, 1108–1109. [Google Scholar] [CrossRef]
- Neu, H.C. The new beta-lactamase-stable cephalosporins. Ann. Intern. Med. 1982, 97, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of β-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
| Study and Year | Age/ Sex | Comorbidities | Treatment Indication | Dose of IV Cefazolin | Duration of Treatment to Onset of Coagulopathy | Peak Deranged Coagulation | Time to Resolution After Cessation of Drug | Complications |
|---|---|---|---|---|---|---|---|---|
| Our study | 72/M | End-stage renal failure | S. aureus infective endocarditis | 1 g 12 h | 14 days | INR 8.29 | 2 days | Gastrointestinal bleeding |
| Chung et al., 2008 [1] | 50/F | Acute renal failure | E. coli bacteraemia | 1 g q24 h | 7 days | INR 4.0 | 2 days | None |
| Shaikh et al., 2013 [2] | 63/M | End-stage renal failure, hypertension, diabetes mellitus | Surgical prophylaxis | - | 4 days | INR 4.2 | 2 days | None |
| Kurz et al., 1986 [4] | 26/M | Acute renal failure following rhabdomyolysis | Surgical prophylaxis | 2 g 12 h | 12 days | Normotest 17% | 2 days | Surgical wound bleeding |
| Shimada et al., 1984 [5] | 79/F 87/F 71/F 87/F 74/M | - - - - - | Pneumonia Colitis Pyelonephritis Pneumonia, UTI Pneumonia | 3 g daily 2 g daily 2 g daily 3 g daily 2 g daily | 21 days 2 days 20 days 15 days 2 days | PT 65.4 s PT 15.0 s PT 21.3 s PT 27.7 s PT 28.2 s | All cases: 1–2 days | Bruising, Corneal bleeding None Haematuria Haematuria Haematemesis/Haemoptysis |
| Kuypers et al., 2002 [6] | 45 | End-stage renal failure | Surgical prophylaxis for cadaveric renal transplant | 2 g for 3 doses Then 6 g daily for 3 days | 5 days | PT 74.6s, Normotest <10% | Intracranial bleeding |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngiam, J.N.; Liong, T.S.; Tham, S.M.; Pramotedham, T.; AlAgha, R.; Yong, J.; Tambyah, P.A.; Lum, L.H.W. Deranged Coagulation Profile Secondary to Cefazolin Use: Case Report. Infect. Dis. Rep. 2021, 13, 187-190. https://doi.org/10.3390/idr13010021
Ngiam JN, Liong TS, Tham SM, Pramotedham T, AlAgha R, Yong J, Tambyah PA, Lum LHW. Deranged Coagulation Profile Secondary to Cefazolin Use: Case Report. Infectious Disease Reports. 2021; 13(1):187-190. https://doi.org/10.3390/idr13010021
Chicago/Turabian StyleNgiam, Jinghao Nicholas, Tze Sian Liong, Sai Meng Tham, Thanawin Pramotedham, Rawan AlAgha, Joy Yong, Paul Anantharajah Tambyah, and Lionel Hon Wai Lum. 2021. "Deranged Coagulation Profile Secondary to Cefazolin Use: Case Report" Infectious Disease Reports 13, no. 1: 187-190. https://doi.org/10.3390/idr13010021
APA StyleNgiam, J. N., Liong, T. S., Tham, S. M., Pramotedham, T., AlAgha, R., Yong, J., Tambyah, P. A., & Lum, L. H. W. (2021). Deranged Coagulation Profile Secondary to Cefazolin Use: Case Report. Infectious Disease Reports, 13(1), 187-190. https://doi.org/10.3390/idr13010021






