Abstract
Non-alcoholic fatty liver disease (NAFLD) is the main cause of chronic liver disease globally. NAFLD is a complex pathology, considered to be the hepatic expression of metabolic syndrome (MetS). It is supposed to become the main indication for liver transplantation in the coming years and is estimated to affect 57.5–74.0% of obese people, 22.5% of children and 52.8% of obese children, with 50% of individuals with type 2 diabetes being diagnosed with NAFLD. Recent research has proved that an increase in adipose tissue insulin resistance index is an important marker of liver injury in patients with NAFLD. Despite being the main underlying cause of incidental liver damage and a growing worldwide health problem, NAFLD is mostly under-appreciated. Currently, NAFLD is considered a multifactorial disease, with various factors contributing to its pathogenesis, associated with insulin resistance and diabetes mellitus, but also with cardiovascular, kidney and endocrine disorders (polycystic ovary syndrome, hypothyroidism, growth hormone deficiency). Hepatitis B and hepatitis C, sleep apnea, inflammatory bowel diseases, cystic fibrosis, viral infections, autoimmune liver diseases and malnutrition are some other conditions in which NAFLD can be found. The aim of this review is to emphasize that, from the clinician’s perspective, NAFLD is an actual and valuable key diagnosis factor for multiple conditions; thus, efforts need to be made in order to increase recognition of the disease and its consequences. Although there is no global consensus, physicians should consider screening people who are at risk of NAFLD. A large dissemination of current concepts on NAFLD and an extensive collaboration between physicians, such as gastroenterologists, internists, cardiologists, diabetologists, nutritionists and endocrinologists, is equally needed to ensure we have the knowledge and resources to address this public health challenge.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, affecting approximately 25% of the general population worldwide and thus representing an important burden on the health system [1,2].
NAFLD is characterized by a deposit of fat in at least 5% of hepatocytes, in the absence of secondary causes of chronic liver disease and without notable alcohol consumption (<20 g/day in women and <30 g/day in men) [3,4]. NAFLD is a heterogeneous condition, initially developing as simple steatosis and progressing to non-alcoholic steatohepatitis (NASH). Currently, it is estimated that up to 25% of patients with NAFLD will develop NASH, histologically expressed by inflammation, hepatocellular ballooning and different stages of fibrosis [5]. Without adequate treatment, NASH can lead to liver cirrhosis, followed by liver failure and then hepatocellular carcinoma (HCC). Notably, some patients with NAFLD may develop HCC without underlying fibrosis or cirrhosis [6]. Patients with NAFLD present a higher risk not only of liver complications but also of increased incidence of cardiovascular mortality [7,8].
According to European guidelines, imaging investigations, such as ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI), are the first-line tests in NAFLD diagnosis. Abdominal ultrasound is preferred due to its greater accessibility and lower costs, although it has limited sensitivity in detecting steatosis when it is below 20% or in people with a high body mass index (BMI) (>40 kg/m2) [3]. The first elastographic method used to quantify the degree of fibrosis was one-dimensional transient elastography (TE), followed by the emergence of elastographic methods integrated into conventional ultrasound devices, such as point shear wave elastography (pSWE) or two-dimensional elastography (2D-SWE). TE and MRI elastography can provide additional information in subjects with NAFLD, allowing the quantification, in addition to fibrosis, of steatosis by using the controlled attenuation parameter for TE, also known as CAP [9], as well as fat-proton density by using the proton density fat fraction (PDFF) mode for MRI elastography [10]. MRI-PDFF is able to detect any degree of steatosis with a high accuracy [11]. MRI-PDFF is the most accurate method but appears to be more suitable for the evaluation and selected follow-up of patients in clinical trials, while conventional ultrasound and CAP could be used as triage in large, unselected populations [12].
The gold standard for assessing the severity of liver disease is liver biopsy, an invasive and expensive method; for this reason, non-invasive methods of assessing liver fibrosis in NAFLD have been developed over time, namely scores based on clinical and laboratory parameters. According to current clinical guidelines, scores such as NAFLD fibrosis score (NFS) and FIB-4 (Table 1) are considered accessible tools for an initial assessment of fibrosis in NAFLD patients. When such scores cannot exclude an advanced fibrosis, an elastography-based method is adopted in the risk stratification algorithm, FibroScan being the most validated and widely available tool. In the decision to use one test or another in clinical practice, the availability, accessibility and cost must be carefully considered, and, given their rapid development, noninvasive methods have almost completely replaced invasive tests in practice. Therefore, only when noninvasive methods cannot rule out significant fibrosis should liver biopsy be considered [3,13].

Table 1.
Examples of evaluation scores in predicting NAFLD severity.
2. New Considerations in Terminology
NAFLD is a bidirectional link to metabolic diseases, from the perspective of both pathogenesis and epidemiologically. Currently, NAFLD is considered a multifactorial disease, with many factors contributing to its pathogenesis, such as genetic predisposition (PNPLA3, TM6SF2 and MBOAT7 genes), diet (intake of saturated fatty acids and fructose), sedentary lifestyle, obesity, insulin resistance and changes in the intestinal microbiome [14,15]. Therefore, NAFLD has a high prevalence among populations with obesity (50–90%), type 2 diabetes (T2DM) (43–72%) or dyslipidemia (20–80%) compared to the general population (25%) [16].
Similarly, NAFLD patients have a high prevalence of metabolic conditions, such as obesity, dyslipidemia, insulin resistance (IR) and T2DM. In this context, NAFLD is frequently recognized as the hepatic expression of metabolic syndrome (MetS), a perspective that led, in 2020, to the modification of the nomenclature and diagnostic criteria. The definition of NAFLD, proposed in 2020, was metabolic dysfunction-associated fatty liver disease (MAFLD) [17].
Thus, MAFLD describes a condition characterized by hepatic steatosis (diagnosed by imaging techniques, blood biomarkers or histological examination) and one of the following metabolic criteria: overweight/obesity, T2DM or a metabolic disorder, defined by the presence of a minimum of two metabolic anomalies, including an increased abdominal circumference, arterial hypertension, dyslipidemia, prediabetes, increased values of homeostasis model assessment-insulin resistance (HOMA-IR) and elevated serum levels of C-reactive protein (CRP) (Table 2) [18].

Table 2.
Metabolic dysregulation criteria in MAFLD diagnosis.
However, the term MAFLD was not universally accepted in the medical scientific community. Although several studies used its definition and highlighted its accuracy, there were opinions that this definition could be improved upon; for instance, nutritionists considered it not useful to include in the diagnostic criteria both abdominal circumference and BMI and disputed the specificity of BMI in evaluating overweight/obesity. Furthermore, it has been pointed out that MAFLD could not exclude other etiologies of liver injury such as alcohol, viruses or drugs [19].
All these controversies determined that, in June 2023, hepatic steatosis was given a new name. The new nomenclature aimed to eliminate the terms “non-alcoholic” and “fatty”; therefore, “MASLD” replaced the term NAFLD. Its diagnosis implies the presence of a minimum of one of the five cardiometabolic risk factors presented in Table 3. The term MetALD was also defined to describe patients with MASLD who consumed higher amounts of alcohol (140 g/week to 350 g/week for female and 210 g/week to 420 g/week for male) [20].

Table 3.
Cardiometabolic criteria diagnosis of MASLD.
Steatotic liver disease (SLD) encompasses certain types of steatosis other than MASLD and MetALD, namely, alcohol-associated liver disease (ALD), SLD with specific etiology—drug-induced liver damage (DILI), monogenic diseases (lysosomal acid lipase deficiency, hypobeta-lipoproteinemia, inborn errors of metabolism, Wilson’s disease) and others (hepatitis C virus, malnutrition, celiac disease) [20], as well as cryptogenetic SLD, defined in patients without cardiometabolic risk factors or any other known steatosis etiology [21]. In accordance with the current global consensus, going forward, we will use the new universally accepted terminology in our paper.
3. MASLD, a Common Link Through Various Pathologies
3.1. T2DM, Consequence of MASLD?
Complex interactions between the hepatic fat deposit, perivisceral adiposity [22] and IR make it challenging to assess the mechanisms whereby MASLD increases the risk of T2DM [5].
In MASLD, IR occurs in skeletal muscle, liver and adipose tissue. Therefore, the hepatic glucose synthesis and the lipolysis of adipose tissue will be barely suppressed by insulin, consequently with an increased basal glucose and plasma-free fatty acid (FFA) concentration. IR also affects glucose tolerance; therefore, pancreatic β cells are overstimulated, leading to T2DM appearance [23].
Endogenous hepatic glucose synthesis is regulated by insulin and glucagon, which maintain glycemic values in an optimal range. In MASLD patients, postprandial glucose synthesis by gluconeogenesis is increased, despite a high level of insulin, causing a permanent hyperglycemic status [23].
Patients with both MASLD and T2DM have hepatic IR (Figure 1). The excessive gluconeogenetic substrate is used in novo glycerol synthesis, through glyceroneogenesis, part of triglyceride synthesis. De novo lipogenesis is characterized by the synthesis of fatty acids from acetyl-CoA units derived mainly from dietary carbohydrates. A diet rich in carbohydrates, together with hyperinsulinemia, stimulates liver lipogenesis, FFA synthesis and very low-density lipoproteins (VLDL) synthesis. The lipotoxicity of saturated fatty acids exerts both on liver cells, pancreatic β cells, endothelial cells, skeletal muscle and cardiomyocytes [24]. Therefore, the accumulation of ectopic adipose tissue in patients with MASLD occurs not only in the liver and skeletal muscles but also intrapancreatically, which causes β-cell dysfunction. A study conducted by Gastaldelli A et al. proved that reducing liver and pancreatic adipose tissue improves both glycemic control and β-cell function in T2DM patients [25].
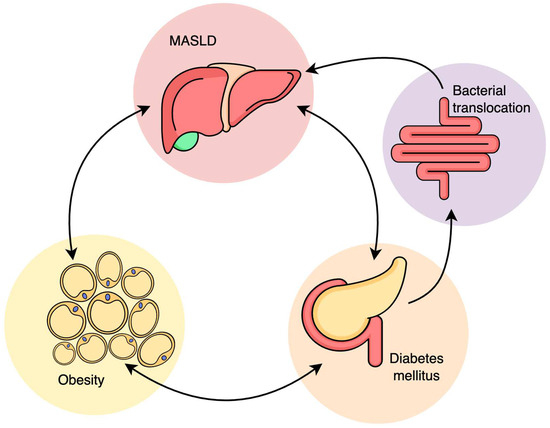
Figure 1.
Relationship between MASLD, obesity, insulin resistance and diabetes mellitus.
Excess adipose tissue is a key to inflammation, releasing the pro-inflammatory cytokines involved in IR and T2DM. Given that the liver is the main organ where ectopic accumulation of adipose tissue occurs, hepatic FFA will exacerbate IR, with lysosomal instability and increased pro-inflammatory cytokine release. Compensatory, hepatocytes increase the beta-oxidation of fatty acids, and lipid overload will alter mitochondrial antioxidant capacity [25].
These processes lead to oxidative stress and intensify IR. Thus, the interaction between hepatic lipid tissue accumulation and IR creates a vicious cycle that develops and maintains both MASLD and T2DM.
3.2. MASLD, Consequence of T2DM?
T2DM plays a significant role in the evolution of simple steatosis to NASH, liver cirrhosis and HCC. T2DM is a major factor in disease progression. Younossi ZM et al. showed, in a study conducted in 20 countries, an alarming prevalence (55%) of MASLD among T2DM individuals [26]. Accordingly, an increased incidence of MASLD and liver fibrosis was described in patients with T2DM based on eight studies from 2020 and 2021, conducted by Stefan N Cusi et al., using TE and/or MRI-based techniques as screening diagnostics for MASLD, NASH and liver fibrosis [27]. Current data estimates that 20 to 30% of patients with MASLD develop NASH, a condition that precedes advanced fibrosis. Kabarra K et al. showed that T2DM increases the risk of disease progression by two to three times [28].
A strong connection between steatohepatitis and T2DM does not prove causality, but it does demonstrate the impact of diabetes on the liver [25]. Patrick Wainwright and Christopher D Byrne highlighted, in a study published in 2019, that IR increases the release of fatty acids from adipose tissue, which are subsequently transported to hepatocytes. Initially, the hepatic accumulation of lipids represents an adaptive mechanism to metabolic stress. Later, however, the continuous flow of FFA in the liver activates pro-inflammatory pathways, leading to an intracellular increase of triglycerides [29].
Targher G and Byrne CD proved that lipotoxicity is associated with peripheral IR, hepatic glucose synthesis and gluconeogenesis, all these mechanisms leading to hyperglycemia, pancreatic cell dysfunction and altered insulin secretion [30,31]. The excess of intracellular fatty acids promotes mitochondrial dysfunction and oxidative stress, which are trigger factors for cellular apoptosis. Moreover, in diabetic patients, the release of pro-inflammatory mediators from the adipose tissue, as well as the presence of intestinal microbiota endotoxins resulted by bacterial translocation, activate Kupffer liver cells and interleukin secretion (IL-1b, IL-6 and αTNF). Secondary hepatocellular injury leads to the activation of stellate cells and the development of collagen deposits [31].
Recent and comprehensive meta-analyses have aimed to assess the bidirectional connection between MASLD and T2DM (Table 4).

Table 4.
A summary of the most recent systematic reviews and meta-analyses aimed at evaluating the relationship between T2DM and MASLD.
3.3. MASLD, a Link to Obesity
Obesity is reflected as a major risk factor in MASLD development; therefore, body mass index (BMI) and waist circumference are intensely correlated with both MASLD and the disease [40]. MASLD patients over 40 years of age are most likely to be obese. In fact, every range of obesity is correlated with MASLD, from overweight to moderate and severe obesity [41], and more than 95% of patients with severe obesity undergoing bariatric surgery may develop MASLD [42], thus highlighting the close pathogenic link between the two conditions [43].
Lipotoxicity and glucotoxicity are key factors in the initiation of simple steatosis and progression to NASH. Fatty liver deposition promoted by a high-fat, high-carbohydrate diet occurs through various mechanisms, as mentioned, such as mitochondrial and endoplasmic reticulum defects and oxidative stress [44]. Polyzos et al. focused, in their study, on the activation of immune liver cells and the release of cytokines with further inflammation promotion, also contributing to fibrosis [45]. Erica Vetrano et al. highlighted the relationship between obesity and MASLD/HCC, noting that obesity causes liver synthesis of adipokines and hormones, which may contribute to the progression of MASLD to NASH, cirrhosis and HCC [46], adipocytes being particularly secretory active substances. The most studied adipokines are adiponectin, leptin, resistin, visfatin, omentin and adipsin, the latter also having an immunological role in the alternative mechanism of complement activation, as highlighted by Margarete Milek et al. in 2022 [47].
Visfatin has metabolic actions similar to insulin, stimulating the uptake of glucose by adipose and muscle cells, at the same time inhibiting the release of glucose from hepatocytes. The role of visfatin and omentin in obesity-induced IR has been described in a recent study published in 2022 by Mona Mohamed et al. [48].
Resistin is an adipokine secreted by both adipocytes and macrophages. Adriana Nieva Vazquez et al. highlighted, in a study in 2014, the pro-inflammatory role of resistin in promoting IR in sepsis, mainly due to hepatic-releasing glucose [49].
Milan Obradovic et al. studied, in 2021, the role of leptin, a protein encoded by the obesity gene and secreted by adipocytes, which inhibits appetite and also contributes to metabolism. Leptin activity is mediated by specific receptors of target cells [50]. Obesity is characterized by an increased concentration of leptin, though hyperleptinemia in obesity and T2DM is rather associated with leptin resistance, as revealed by Lahlou et al. in a study on leptin resistance [50], similar to insulin resistance [51].
Liping Luo’s study showed that adiponectin is secreted almost exclusively by adipocytes, which circulate in high concentrations in the blood and promote insulin sensitivity [52]. Also, adiponectin accelerates the metabolism of fatty acids and reduces the expression of endothelial adhesion molecules (ICAM-1 and VCAM-1), improving endothelial function. This effect occurs due to the inhibition of nuclear factor κB (nuclear factors κB-NFκB), consequently decreasing the production of IL-6 synthesis. Sheetal Parida et al., as well as Nikolaos Perakakis, in their studies concluded that a low concentration of adiponectin was correlated with an increased risk of IR, T2DM and hypertension, as well as coronary heart disease [53,54].
Many researchers have focused on these interacting conditions, as shown in Table 5.

Table 5.
A summary of the most recent systematic reviews and meta-analyses aimed at evaluating the relationship between obesity and MASLD.
3.4. MASLD, a Link to Cardiovascular Disease (CVD)
Many epidemiological studies support the hypothesis that MASLD patients have an increased risk of cardiovascular events. However, the real prevalence of cardiovascular events in patients with MASLD is not completely accomplished and is assumed to be even underestimated, since MASLD diagnosis is often missed, as many patients with this condition have normal liver enzymes and the usual imaging methods of MASLD screening (abdominal ultrasound) do not detect steatosis when fat liver infiltration is below 30%. Generally, people with MASLD have increased cardio-metabolic risk, even in the absence of MetS components [58].
A 2015 report of the Framingham Heart Study showed that hepatic steatosis was strongly correlated with subclinical CVD events, independent of other metabolic risk factors [59]. A meta-analysis carried out by Morrison et al. in 2019 concluded that MASLD is accompanied by a thickening of the vascular intima, with endothelial and cardiac dysfunction [35].
A comprehensive meta-analysis of 10,576,383 people from 24 countries, regarding non-obese MASLD individuals and aiming to eliminate obesity as a main risk factor, highlighted an increased incidence of new-onset CVD in lean MASLD patients [60]. Another study published in 2020 showed that additional cardiac complications, such as cardiomyopathy, heart valve regurgitations and cardiac arrhythmias, were also more common in people with MASLD [61].
Many studies associate MASLD with an increased incidence of coronary atherosclerosis, favored by increased lipolysis and VLDL secretion, increased fibrinogen and CRP [62].
Recent meta-analyses focused on MASLD as an independent risk factor for developing CVD after excluding other risk factors, with related nonfatal and fatal CV events; moreover, this risk increases with MASLD progression to NASH [36,63].
Christopher D Byrne et al. concluded, in their study, that IR-associated MASLD and the resulting hyperglycemic status induce a series of vascular changes that include endothelial dysfunction, cell proliferation and changes in the LDL-cholesterol receptor of the extracellular matrix, with a decrease in LDL clearance. Its fraction, sdLDL, has increased affinity for the proteoglycans of the vascular intima, which causes the penetration of more LDL particles into the vascular wall [64]. Therefore, MASLD is commonly associated with dyslipidemia (high triglycerides level, low HDL level, increased VLDL) and elevated levels of proinflammatory cytokines that promote the development of CVD [65]. Yoo HJ highlighted, in a study published in 2015, that hepatokines (fibroblast growth factor 21, fetuin-A and selenoprotein P) may have a role in CVD development [66]. Sheila Gato et al. concluded, in a recent study, that a potential link mechanism between MASLD and CVD may have its origin in the expansion and inflammation of visceral adipose tissue [67]. CRP accelerates atherosclerosis by the increased expression of plasminogen activator inhibitor 1 (I1Pl) and adhesion receptors on endothelial cells and inhibits the formation of nitric oxide, increasing the uptake of LDL cholesterol by macrophages [67].
The liver has a dual role: it is the target of systemic inflammatory changes and, at the same time, the source of proatherogenic factors. MASLD and NASH contribute to CVD through inflammatory cytokines produced in the liver, but released systemically, and oxidative stress mediators incriminated in the NASH pathogenesis. Jingjing Cai et al. noted, in their study, the contribution of MASLD to the onset of CV-associated events driven by IR and dyslipidemia [67].
Increased cardiovascular morbidity and mortality [68] is probably the most important clinical feature associated with MASLD. To date, growing evidence has shown that CVD represents an important mortality risk factor in patients with MASLD, as highlighted by Giovanni Targher et al. [61].
Although large population studies are still needed for confirmation, this hypothesis draws attention to the possibility that MASLD, especially in its severe form (NASH), may be considered not only as a marker of cardiovascular disease but also the first site of the atherosclerosis process [61,69,70].
The association between CVD and MASLD is highlighted in various systematic reviews and meta-analysis, as illustrated in Table 6.

Table 6.
A summary of the most recent systematic reviews and meta-analyses aimed at evaluating the relationship between CVD and MASLD.
3.5. The Relationship Between MASLD and Cancer
Consequently, due to a higher prevalence of obesity and T2DM, the prevalence of HCC-related MASLD is increasing. Thus, MASLD is estimated to become the main cause of HCC globally, exceeding hepatitis B and C virus infection. The risk of HCC is amplified by genetic predisposition, such as PNPLA3 or transmembrane superfamily member 2 -6, in the presence of obesity or T2DM [79].
There is growing evidence for the correlation between MASLD and HCC, which provides the pathophysiologic hypothesis that T2DM is associated with a two-fold increased risk of HCC [80,81], but with a lower association with extrahepatic cancers, especially colorectal cancer [82,83,84]. Therefore, even if extrahepatic cancer incidence is not alarming, the growing incidence of MASLD-HCC requires the need to improve strategies for cancer screening in these individuals [85].
A nationwide cohort study focused on the correlation between the risk of young-onset digestive tract cancers and MASLD, recently published by Joo-Hyun Park, showed that MASLD was associated with an increased risk of overall digestive tract cancers, such as stomach, colorectal, liver, pancreatic, biliary tract and gallbladder, suggesting that MASLD may be an independent, modifiable risk factor for an increasing incidence of young-onset digestive tract cancers [86]. Additionally, MASLD increases the development and recurrence of colorectal cancer liver metastasis [87,88].
3.6. MASLD, a Link to Endocrine Disorders
Endocrine disruptors (EDCs) are currently considered an important health and environmental concept. Growing evidence indicates that in utero exposure to bisphenol A is associated with hepatic steatosis in adults, with EDCs altering β-oxidation hepatic capacity, most probably by epigenetic mechanisms [89,90].
EDCs (dioxins, phthalates, bisphenol A, as well as organic pollutants) can lead to IR by inducing oxidative stress or by modifying gene transcription [91]. Further studies are needed to specify the role of EDCs in MASLD pathogenesis [92].
Obesity, IR and T2DM seem to be the underlying key factors associated with MASLD in several endocrine disorders, justifying active screening for MASLD in this population; conditions such as polycystic ovary syndrome (PCOS), hypothyroidism, growth hormone (GH) deficiency, primary hyperaldosteronism (PA) and hypogonadism are the most studied.
3.6.1. MASLD and Polycystic Ovary Syndrome
Women diagnosed with PCOS have a higher risk of developing T2DM and MASLD [93]. The mechanisms underlying the development of MASLD in PCOS are multifactorial; however, Falzarano C. Lofton et al. support the role of IR as a key factor in MASLD occurrence [93]. Moreover, women with PCOS and hyperandrogenism have a much higher risk of MASLD, strongly associated with severe IR [94]. Wu J Yao et al. showed, in a meta-analysis, that normo-androgenic women with PCOS do not appear to have an increased prevalence of MASLD compared to control groups [95]. The relation hyperandrogenism—MASLD can be explained by the down-regulation of LDL receptors, the elongation of VLDL and LDL half-life, the induction of fat accumulation in the hepatocytes and finally the development of MASLD [96]. For these reasons, screening for MASLD in women with PCOS and associated MetS or IR is vital [97,98]. Recent studies have focused on the link between PCOS and MASLD prevalence, as shown in Table 7.

Table 7.
A summary of the most recent systematic reviews and meta-analyses aimed at evaluating the relationship between PCOS and MASLD.
3.6.2. MASLD and Hypothyroidism
Hypothyroidism appears to be associated with hepatic steatosis in various studies [103,104]. Mantovani et al. published a meta-analysis in 2018, which included a total of 15 studies with 44,140 participants, that suggested that hypothyroidism is strongly associated with both the presence and the severity of MASLD [105].
The most significant characteristics of patients with untreated hypothyroidism are the appearance of lipid metabolism disorders, such as increased serum cholesterol and triglycerides, and the accumulation of fat in the hepatocytes [106,107]. Moslehi A et al. highlighted, in a study published in 2018, the role of transcription factors in lipid synthesis, including the sterol regulatory element binding protein (SREBP) and the liver X receptor (LXR) [108]. SREBP controls lipid synthesis, notably influenced by thyroid hormone levels. The effect of thyroid hormones on SREBP-2, an isoform of SREBP, namely, decreasing LDL receptor expression, leads to an increase in serum cholesterol levels. The influence of thyroid hormones on lipid metabolism and liver function, together with the control molecular mechanisms, have been extensively studied [109].
Therefore, the interconnection between MASLD and hypothyroidism has been a topic for multiple studies, sometimes having contradictory results [110,111]. Liu et al. showed a positive association between free triiodothyronine (FT3), thyroid-stimulating hormone (TSH) levels and the incidence of MASLD in euthyroid people [112]. In a recently published meta-analysis, Guo et al. highlighted that TSH levels are positively correlated with MASLD, increasing with MASLD progression [113], an association also noted by Borges-Canha et al. in patients with morbid obesity [114]. Jaruvongvanich, V. et al. disproved these associations [115]. Therefore, it is reasonable to recognize the existence of non-thyroid etiologies of MASLD in patients with hypothyroidism [116]. However, Bano et al. highlighted the relationship between hypothyroidism and MASLD, independent of the presence of other metabolic risk factors [117]. A series of meta-analysis suggest the correlation between MASLD and hypothyroidism, as illustrated in Table 8.

Table 8.
A summary of the most recent systematic reviews and meta-analyses aimed at evaluating the relationship between hypothyroidism and MASLD.
3.6.3. MASLD and GH Deficiency
Due to the extensive effects of GH on glucose metabolism, GH deficiency was commonly associated with MASLD [120]. MASLD usually develops shortly after the diagnosis of adult GH deficiency (GHDA) and can rapidly progress to NASH and/or advanced fibrosis [121]. Huang Z et al. highlighted that GH deficiency is defined by an imbalance between low levels of GH and elevated insulin levels [122], and Meienberg F et al. similarly concluded that MASLD was increasingly accepted as part of the metabolic complications associated with GHDA [123]. Furthermore, small studies using liver biopsy for diagnosis have shown a higher incidence of NASH in patients with GHDA [124], liver fibrosis and cirrhosis, even claiming liver transplantation as the cause. Furthermore, in patients with biopsy-diagnosed MASLD, a reduced GH has been correlated with advanced steatosis, while low IGF-1 and IGF-1/IGFBP-3 levels have been associated with liver fibrosis and/or NASH [125].
GH effects are mainly mediated by IGF-1, synthesized in hepatocytes. IGF-1 circulates are associated mainly with IGFBP-3 (secreted by Kupffer cells). Clemmons DR et al. noted, in a study, that IGFBP increases IGF-1 half-life, directing it to distinct tissues, whereas IGF-1 promotes cell growth and affects carbohydrate, protein and lipid metabolism [126]. The main effect of GH on adipose tissue is lipolysis, with increased serum levels of FFA and glycerol [127]. Sharma R et al. highlighted, in a recent study, that although increased circulating FFA is correlated with a higher risk of MASLD, this does not occur under physiological circumstances because GH induces FFA uptake by increasing lipoprotein lipase activity in skeletal muscle [127], and JAK2-STAT (Janus kinase (JAK)2 activator transcription) signaling suppresses lipid uptake and de novo lipogenesis in the liver [104,105].
However, data on the efficacy of GH therapy in patients with MASLD are limited and controversial [123,128,129]. Treatment initiation, impact on glucose metabolism and T2DM incidence, as well as the long-term effects of GH therapy and risk of HCC, particularly in patients with NASH and advanced fibrosis, should also be carefully evaluated [130,131,132].
3.6.4. MASLD and Acromegaly
Excessive GH and IGF-1 characterize acromegaly, the most common cause of GH-secreting pituitary adenoma. Petrossians, P et al. showed, in their study, that increased GH levels are correlated with an increased lipolysis and a proper body composition, with increased lean body mass and decreased adipose tissue, both visceral and subcutaneous [133]. It is known that acromegaly promotes IR, and consequently hyperglycemia, increased insulin and triglyceride levels, and an increased risk of T2DM [134].
While Winhofer, Y et al., in their study, showed that intrahepatic lipids, measured by magnetic resonance spectroscopy, are rather low in patients with active acromegaly compared to healthy subjects [135], Koutsou-Tassopoulou, A et al. concluded that hepatic steatosis is commonly found in acromegaly, suggesting that lipotoxicity and IR may overcome the direct effects of GH on liver cells [136].
3.6.5. MASLD and Panhypopituitarism
Panhypopituitarism, characterized by concomitant pituitary deficiencies, has also been associated with MASLD [137]. Ritter et al. noted the association between hypothyroidism and MASLD, due to the multiple consequences of thyroid hormones on hepatic lipid metabolism [104], and Sarkar et al. associated low free testosterone in men with a notably higher incidence of biopsy-diagnosed NASH and advanced liver fibrosis [138]. Zhang et al. concluded that a low prolactin level is a risk factor for MASLD occurrence, directly correlated with steatosis severity [139]. Additionally, MASLD is a common finding in hypogonadism, independent of gender and etiology [140].
3.6.6. MASLD and Estrogen Deficiency
Over time, numerous studies have been published attesting that conditions associated with estrogen deficiency, both physiologically in postmenopausal women and in women with hypogonadism, are associated with an increased prevalence of MASLD, NASH and advanced fibrosis [141].
Based on a consistent line of evidence, postmenopausal status is considered an independent risk factor for MASLD development and progression [142]. Klair et al. reported, in a study, that premature menopause is associated with a higher risk of advanced liver fibrosis [141]. Although adiposity disposal (subcutaneous adiposity and visceral adiposity) is closely linked with sex hormones [142], it has been proven that hypogonadism, in both men and women, is strongly associated with MASLD, by affecting glucose tolerance, IR and by the association of arterial hypertension and atherogenic dyslipidemia [143].
3.6.7. MASLD and Primary Hyperaldosteronism
PA is the most frequent endocrine cause of secondary hypertension, responsible for almost 10% of all cases. [144]. PA can cause not only hypertension but also IR and dyslipidemia [145]. Srinivasa S et al. reported that activation of the mineralocorticoid receptor by aldosterone leads to the impairment of insulin sensitivity in skeletal muscles and adipocytes by stimulating the pathogenic proinflammatory pathways and the metabolic pathways of oxidative stress, endothelial dysfunction, cell proliferation and inflammation, increasing the risk of MetS and MASLD [146,147].
3.6.8. MASLD and Hyperprolactinemia
Prolactin is a hormone derived from the pituitary gland and is involved in reproduction and lactation. Because the prolactin receptor is also present in the liver, it can play an important role in hepatic metabolic regulation [146]. A negative correlation between plasma prolactin levels and BMI, IR and MASLD development has been observed in recent observational studies [139,147].
Despite the favorable impact of prolactin on metabolic homeostasis, Serri O et al. reported that a significant increase in prolactin levels was commonly correlated with metabolic disorders, such as overweight, obesity and hyperinsulinemia, all of which are proposed crucial factors in MASLD pathogenesis [148]. Although prolactin is thought to reduce hepatic fat content, Zhang P et al. concluded that it is probable that a chronically increased prolactin level is involved in MASLD occurrence and progression [139].
3.7. MASLD and Vitamin D Deficiency
Vitamin D is currently recognized for its role in phospho-calcium metabolism. However, its receptors are ubiquitously present; thus, vitamin D exerts multiple effects in different organs and systems [149].
Recent studies associate vitamin D deficiency with several metabolic disorders, including MASLD [150,151]. Abramovitch S et al. showed, in their study, vitamin D’s effects in the liver, with an anti-inflammatory and anti-fibrotic result [152]. This protective effect may be due to the capacity of stellate cells to inhibit fibrogenesis [153]; meanwhile, several epidemiological studies point to an association between decreased vitamin D levels and MASLD, although no causal connection has been found [154,155]. A recent study by Borges-Canha et al. showed that vitamin D deficiency was associated with an increased risk of hepatic steatosis in morbidly obese patients [156]. In contrast, Wang et al. did not find a causal correlation between MASLD vitamin D deficiency in a Chinese population of over 9000 participants [157], and Barchetta et al., in a randomized trial, concluded that 24 weeks of high-dose oral vitamin D supplementation did not improve hepatic steatosis in patients with MASLD and T2DM [158]. For these reasons, future extensive studies are needed to focus on the role of vitamin D and its supplementation in MASLD.
Figure 2 summarizes the bidirectional connection between MASLD and the most commonly associated conditions.
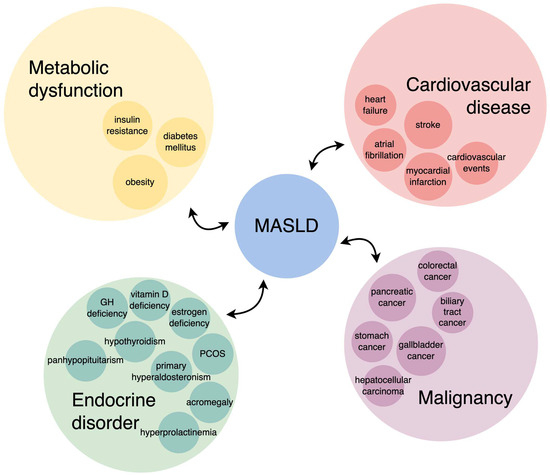
Figure 2.
MASLD and the most commonly associated conditions—a bidirectional connection.
4. MASLD Management—Actual and Future Perspectives
The ideal goal of the treatment is the resolution of histological lesions, to diminish the risk of evolution towards liver cirrhosis, with the objectives of the treatment therefore being the improvement of biochemical and histological liver parameters, as well as the control of all features of the metabolic syndrome associated with hepatic steatosis: weight, glycemic and lipid metabolism or blood pressure control. The treatment methods include lifestyle optimization, obesity pharmacotherapy and hypoglycemic therapy.
EASL recommends an energy restriction of 500–1000 kcal per week, with a target weight loss of 7–10% for obese/overweight patients, a low-fat, moderate-to-high-carbohydrate diet or a low-carb or high-protein ketogenic diet, such as the Mediterranean diet [159]. Alcohol is an aggravating factor, and AASLD recommends that people with MASLD or NASH should avoid alcohol. EASL allows alcohol consumption below 30 g/day for men and 20g/day for women. The role of coffee consumption for the treatment of MASLD is unclear, although some studies indicate that regular coffee consumption may have protective effects.
Pharmacological treatment is indicated for patients with fibrosis stage ≥ 2, and those with stages 0 or 1 have a high risk for the progression of fibrosis (elderly, diabetes, metabolic syndrome, elevated ALT and high necroinflammatory activity) [159].
4.1. Antioxidants
Vitamin E reduces reactive oxygen species level and prevents oxidative damage to cells by different mechanisms, alleviating senescence and cell apoptosis. These properties can slow the progression of liver damage and even facilitate the reversibility of liver fibrosis. The PIVENS (Pioglitazone, Vitamin E, or Placebo for Non-alcoholic Steatohepatitis) trial demonstrated an improvement in steatosis and a significant decrease in hepatocyte ballooning and inflammation [160]. Although vitamin D deficiency is common in MASLD/NASH, data on the efficacy of vitamin D supplementation have not been conclusive. Some studies have suggested that vitamin D may induce antifibrotic effects by suppressing the proliferation of stellate cells [161,162]. Other antioxidants, such as S-adenosyl methionine. (SAM) and betaine, are supplements with cytoprotective, antiapoptotic and antisteatotic effects and can also decrease IR [163]. N-acetyl cysteine (NAC), a glutathione precursor, increases glutathione levels in the hepatocyte and diminishes reactive oxygen species that cause hepatocyte damage; therefore, NAC supplementation can protect cellular structures against oxidative stress [164]. A pilot study showed that the administration of 300 mg/day glutathione for 4 months can decrease ALT and improves steatosis in patients without severe fibrosis [165].
4.2. Antidiabetic Drugs
Given that IR is one of the causes of non-alcoholic fatty liver disease, it is self-evident that the use of antidiabetic drugs in liver disease is an interesting issue. Drugs such as metformin, thiazolidinediones, dipeptidyl peptidase-4 inhibitors (iDPP-4), glucagon-like peptide-1 (GLP-1) agonists or sodium-glucose cotransporter 2 inhibitors (iSGLT2) have been shown to improve liver function in addition to lowering blood glucose.
GLP1 agonists are the most promising class, reducing hepatic steatosis and elevated liver enzymes in patients with diabetes and MASLD, but further studies are needed to assess their effect on progression to liver cirrhosis [166,167].
A therapeutic combination of a dual analogue of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) tirzepatide seems to have a favorable evolution on MASLD [168].
The promising results in improving the metabolic parameters of these new therapies led to the investigation of other more advanced molecules, such as triple analogs of GLP-1/GIP/glucagon. Retatrutide is one such triple analog used in clinical trials that appears promising in the management of MASLD, improving mitochondrial oxidative stress, with consequently possible antifibrotic effects [169].
Pioglitazone—a peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist—contributes to a decrease in lipotoxicity, increasing hepatic lipogenesis and insulin sensitivity, adiponectin accumulation and improving necroinflammation, therefore delaying fibrosis progression [170]. The dual PPAR-α/γ agonist saroglitazar reduces steatosis, decreases inflammation and improves IR by increasing fatty acid oxidation and decreasing lipolysis [171]. The dual PPAR-α/δ agonist Elafibranor improves steatosis and reduces inflammation and fibrosis [172]. Pan-PPAR agonists, antifibrotics that activate all three types of PPAR receptors—alpha, gamma and delta—appear to improve insulin sensitivity and macrophage activation, reducing liver fibrosis and inflammation [173].
Analysis of the clinical trials on SGLT-2 inhibitors (canagliflozin and luseogliflozin) showed a decrease in alanine aminotransferase (ALT) and consequently a resolution of steatosis, inflammation and ballooning of hepatocytes [174].
DDP-4 inhibitors are often prescribed to diabetic MASLD patients due to their beneficial effects in decreasing ALT and AST, as well as glycated hemoglobin (HbA1c) [175].
Metformin decreases insulin resistance by reducing hepatic gluconeogenesis and fatty acid oxidation, increases peripheral and hepatic sensitivity to insulin and decreases intestinal glucose absorption and serum lipid concentration. Metformin has no significant effects on liver enzymes and histology in NASH/MASLD but is associated with reduced incidence of hepatocellular carcinoma [176].
4.3. Other Therapies
Hypolipemiant therapy has an important role in the therapeutic scheme of diabetic patients with MASLD because it decreases the cardiovascular risk. Statins administration is associated with the inhibition of liver inflammation, improving liver fibrosis and reduction carcinogenesis risk. Furthermore, the cholesterol absorption inhibitor ezetimibe may decrease liver enzymes and improve steatosis, but histological efficacy remains uncertain [177].
Angiotensin II receptor blockers reduce fibroblast activity and liver fibrosis by inhibiting the activation of stellate cells that express angiotensin receptors [178,179].
Farnesoid X receptor ligand obeticholic acid (AOC) represents the synthetic version of natural chenodeoxycholic bile acid, with a role in reducing hepatic gluconeogenesis, lipogenesis and steatosis [180].
Resmetirom, thyroid hormone receptor β, is the predominant hepatic receptor for thyroxine, which increases cholesterol metabolism and mediates its secretion through bile. The molecule is a highly selective agonist and was developed to address dyslipidemia but has been observed to also reduce hepatic steatosis [181].
Qualitative and quantitative changes in gut microbiome composition and disturbances in the gut–liver axis favoring the translocation of endotoxins into the bloodstream appears to be independently associated with the development of MASLD and the progression to NASH and HCC. In MASLD patients, a significantly increased Firmicutes/Bacteroidetes ratio has been revealed in recent studies, as well as reduced levels of Akkermansia and L. murinus [182,183]. Recent studies have suggested that the ingestion of L. acidophilus, L. fermentum, L. paracasei and L. plantarum significantly decreases serum triglyceride and total cholesterol levels and improves MASLD disease progression. Anaerobutyricum soehngenii (Eubacterium hallii) seems to improve insulin resistance and glycemic profiles in subjects with metabolic syndrome, representing a therapeutic potential in MASLD [184].
Concerning all these features, we emphasize that only through a concerted effort by the medical community can MASLD be successfully managed. Through education and awareness, early diagnosis, lifestyle change and appropriate therapeutic management, steatosis can regress and progressive forms of the disease to cirrhosis and HCC can be avoided, leading to an improved quality of life.
5. Conclusions
The exponential increase in the prevalence of metabolic diseases and obesity, primarily diabetes and cardiovascular diseases, justifies an active screening for MASLD in this context, given the strong correlation and common etiopathogenesis of these conditions.
MASLD is a highly heterogeneous metabolic disorder; therefore, recognizing clinical pathologic associations is crucial to implementing an individualized, personalized approach to our patients.
Based on its definition and pathophysiology, the monitoring of patients with MASLD must be multidisciplinary. The contribution of gastroenterologists, diabetologists, endocrinologists, nutritionists, internists and cardiologists must be managed within an integrated assessment, ideally requiring a multidisciplinary team. A large dissemination of current knowledge on MASLD and an extensive collaboration between physicians is equally needed to ensure we have the ability and resources to address this public health challenge.
Author Contributions
Conceptualization, C.M.M. and M.P.; methodology, C.M.M., S.M.C. and M.S.P.; software, G.A.I. and I.C.M.; validation, C.M.M., D.P. and M.P.; formal analysis, I.C.M. and G.A.I.; investigation, C.M.M., D.P., S.M.C., M.S.P. and M.P.; resources, C.M.M.; data curation, C.M.M., D.P., S.M.C., M.S.P., G.A.I., I.C.M. and M.P.; writing—original draft preparation, C.M.M., D.P., I.C.M., M.S.P. and M.P.; writing—review and editing, C.M.M., I.C.M. and D.P.; visualization, I.C.M. and G.A.I.; supervision, M.P.; project administration, C.M.M.; funding acquisition, C.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ALD | alcohol-associated liver disease |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| CAP | controlled attenuation parameter |
| CRP | C-reactive protein |
| CT | computer tomography |
| CVD | cardiovascular disease |
| DILI | drug-induced liver damage |
| EDCs | endocrine disruptors |
| FFA | free fatty acids |
| FT3 | free triiodothyronine |
| GGT | gamma-glutamyl transpeptidase |
| GH | growth hormone |
| GHDA | adult GH deficiency |
| GIP | glucose-dependent insulinotropic polypeptide |
| GLP-1 | glucagon-like peptide-1 |
| HbA1c | glycated hemoglobin |
| HOMA-IR | Homeostasis Model Assessment-Insulin Resistance |
| I1Pl | plasminogen activator inhibitor 1 |
| iDPP-4 | dipeptidyl peptidase-4 inhibitor |
| IR | insulin resistance |
| iSGLT2 | sodium-glucose cotransporter 2 inhibitor |
| JAK | Janus kinase |
| LDL | low-density lipoproteins |
| LXR | liver X receptor |
| MAFLD | metabolic dysfunction-associated fatty liver disease |
| MASLD | metabolic dysfunction–associated steatotic liver disease |
| MetS | metabolic syndrome |
| MRI | magnetic resonance imaging |
| NAC | N-acetyl cysteine |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| NFS | NAFLD Fibrosis Score |
| OR | odds ratio |
| PA | primary hyperaldosteronism |
| PCOS | polycystic ovary syndrome |
| PDFF | proton density fat fraction |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| pSWE | point shear wave elastography |
| SAM | S-adenosyl methionine |
| SLD | steatotic liver disease |
| SREBP | sterol regulatory element binding protein |
| T2DM | type 2 diabetes |
| TE | transient elastography |
| TSH | thyroid-stimulating hormone |
| VLDL | very low-density lipoproteins |
References
- Kaya, E.; Yilmaz, Y. Metabolic-Associated Fatty Liver Disease (MAFLD): A Multi-Systemic Disease Beyond the Liver. J. Clin. Transl. Hepatol. 2022, 10, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Sangro, P.; de la Torre Aláez, M.; Sangro, B.; D’Avola, D. Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): An Update of the Recent Advances in Pharmacological Treatment. J. Physiol. Biochem. 2023, 79, 869–879. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Yilmaz, Y.; Yu, M.-L.; Wai-Sun Wong, V.; Fernandez, M.C.; Isakov, V.A.; Duseja, A.K.; Mendez-Sanchez, N.; Eguchi, Y.; Bugianesi, E.; et al. Clinical and Patient-Reported Outcomes from Patients with Nonalcoholic Fatty Liver Disease Across the World: Data from the Global Non-Alcoholic Steatohepatitis (NASH)/Non-Alcoholic Fatty Liver Disease (NAFLD) Registry. Clin. Gastroenterol. Hepatol. 2022, 20, 2296–2306.e6. [Google Scholar] [CrossRef]
- Dajani, A.I.; Abuhammour, A. Agents for the Treatment of Fatty Liver Disease: Focus on Essential Phospholipids. Drugs Ther. Perspect. 2021, 37, 249–264. [Google Scholar] [CrossRef]
- Zhang, S.; Mak, L.-Y.; Yuen, M.-F.; Seto, W.-K. Screening Strategy for Non-Alcoholic Fatty Liver Disease. Clin. Mol. Hepatol. 2023, 29, S103–S122. [Google Scholar] [CrossRef]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.-G.; Mi, Y.-Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.-H.; Cardoso, A.C.; et al. Individual Patient Data Meta-Analysis of Controlled Attenuation Parameter (CAP) Technology for Assessing Steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef]
- Park, C.C.; Nguyen, P.; Hernandez, C.; Bettencourt, R.; Ramirez, K.; Fortney, L.; Hooker, J.; Sy, E.; Savides, M.T.; Alquiraish, M.H.; et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 598–607.e2. [Google Scholar] [CrossRef]
- Imajo, K.; Toyoda, H.; Yasuda, S.; Suzuki, Y.; Sugimoto, K.; Kuroda, H.; Akita, T.; Tanaka, J.; Yasui, Y.; Tamaki, N.; et al. Utility of Ultrasound-Guided Attenuation Parameter for Grading Steatosis with Reference to MRI-PDFF in a Large Cohort. Clin. Gastroenterol. Hepatol. 2022, 20, 2533–2541.e7. [Google Scholar] [CrossRef] [PubMed]
- Schaapman, J.J.; Tushuizen, M.E.; Coenraad, M.J.; Lamb, H.J. Multiparametric MRI in Patients with Nonalcoholic Fatty Liver Disease. J. Magn. Reson. Imaging 2021, 53, 1623–1631. [Google Scholar] [CrossRef]
- Arab, J.P.; Dirchwolf, M.; Álvares-da-Silva, M.R.; Barrera, F.; Benítez, C.; Castellanos-Fernandez, M.; Castro-Narro, G.; Chavez-Tapia, N.; Chiodi, D.; Cotrim, H.; et al. Latin American Association for the Study of the Liver (ALEH) Practice Guidance for the Diagnosis and Treatment of Non-Alcoholic Fatty Liver Disease. Ann. Hepatol. 2020, 19, 674–690. [Google Scholar] [CrossRef]
- Dallio, M.; Masarone, M.; Romeo, M.; Tuccillo, C.; Morisco, F.; Persico, M.; Loguercio, C.; Federico, A. PNPLA3, TM6SF2, and MBOAT7 Influence on Nutraceutical Therapy Response for Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Front. Med. 2021, 8, 734847. [Google Scholar] [CrossRef]
- Zarghamravanbakhsh, P.; Frenkel, M.; Poretsky, L. Metabolic Causes and Consequences of Nonalcoholic Fatty Liver Disease (NAFLD). Metab. Open 2021, 12, 100149. [Google Scholar] [CrossRef] [PubMed]
- Hartleb, M.; Mastalerz-Migas, A.; Kowalski, P.; Okopień, B.; Popovic, B.; Proga, K.; Cywińska-Durczak, B. Healthcare Practitioners’ Diagnostic and Treatment Practice Patterns of Nonalcoholic Fatty Liver Disease in Poland: A Cross-Sectional Survey. Eur. J. Gastroenterol. Hepatol. 2022, 34, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Pipitone, R.M.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Tulone, A.; Pennisi, G.; Grimaudo, S.; Petta, S. MAFLD: A Multisystem Disease. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188221145549. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.-F.; Schattenberg, J.M.; et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Grabherr, F.; Grander, C.; Effenberger, M.; Schwärzler, J.; Tilg, H. MAFLD: What 2 Years of the Redefinition of Fatty Liver Disease Has Taught Us. Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221139101. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Mubarak, M. Changes in the Terminology and Diagnostic Criteria of Non-Alcoholic Fatty Liver Disease: Implications and Opportunities. World J. Gastrointest. Pathophysiol. 2024, 15, 92864. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Hurley, D.L.; Garvey, W.T. Adiposity-Based Chronic Disease as a New Diagnostic Term: The American Association of Clinical Endocrinologists and American College of Endocrinology Position Statement. Endocr. Pract. 2017, 23, 372–378. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and Diabetes Mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Tilg, H.; Byrne, C.D. Non-Alcoholic Fatty Liver Disease: A Multisystem Disease Requiring a Multidisciplinary and Holistic Approach. Lancet Gastroenterol. Hepatol. 2021, 6, 578–588. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K. From NASH to Diabetes and from Diabetes to NASH: Mechanisms and Treatment Options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The Global Epidemiology of NAFLD and NASH in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Stefan, N.; Cusi, K. A Global View of the Interplay between Non-Alcoholic Fatty Liver Disease and Diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef]
- Kabarra, K.; Golabi, P.; Younossi, Z.M. Nonalcoholic Steatohepatitis: Global Impact and Clinical Consequences. Endocr. Connect. 2021, 10, R240–R247. [Google Scholar] [CrossRef]
- Wainwright, P.; Byrne, C.D. Bidirectional Relationships and Disconnects between NAFLD and Features of the Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 367. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D. A Perspective on Metabolic Syndrome and Nonalcoholic Fatty Liver Disease. Metab. Syndr. Relat. Disord. 2015, 13, 235–238. [Google Scholar] [CrossRef]
- Xia, M.-F.; Bian, H.; Gao, X. NAFLD and Diabetes: Two Sides of the Same Coin? Rationale for Gene-Based Personalized NAFLD Treatment. Front. Pharmacol. 2019, 10, 877. [Google Scholar] [CrossRef]
- Cao, L.; An, Y.; Liu, H.; Jiang, J.; Liu, W.; Zhou, Y.; Shi, M.; Dai, W.; Lv, Y.; Zhao, Y.; et al. Global Epidemiology of Type 2 Diabetes in Patients with NAFLD or MAFLD: A Systematic Review and Meta-Analysis. BMC Med. 2024, 22, 101. [Google Scholar] [CrossRef] [PubMed]
- En Li Cho, E.; Ang, C.Z.; Quek, J.; Fu, C.E.; Lim, L.K.E.; Heng, Z.E.Q.; Tan, D.J.H.; Lim, W.H.; Yong, J.N.; Zeng, R.; et al. Global Prevalence of Non-Alcoholic Fatty Liver Disease in Type 2 Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis. Gut 2023, 72, 2138–2148. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Byrne, C.D.; Bonora, E.; Targher, G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-Analysis. Diabetes Care 2018, 41, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.E.; Zaccardi, F.; Khunti, K.; Davies, M.J. Causality between Non-Alcoholic Fatty Liver Disease and Risk of Cardiovascular Disease and Type 2 Diabetes: A Meta-Analysis with Bias Analysis. Liver Int. 2019, 39, 557–567. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Tilg, H.; Byrne, C.D.; Targher, G. Non-Alcoholic Fatty Liver Disease and Risk of Incident Diabetes Mellitus: An Updated Meta-Analysis of 501 022 Adult Individuals. Gut 2021, 70, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic Fatty Liver Disease Is Associated with an Almost Twofold Increased Risk of Incident Type 2 Diabetes and Metabolic Syndrome. Evidence from a Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef]
- Alenezi, Y.M.; Harris, R.; Morling, J.; Card, T. Prevalence of Non-Alcoholic Fatty Liver Disease (NAFLD) in Saudi Arabia: Systematic Review and Meta-Analysis. Cureus 2024, 15, e40308. [Google Scholar] [CrossRef]
- Jarvis, H.; Craig, D.; Barker, R.; Spiers, G.; Stow, D.; Anstee, Q.M.; Hanratty, B. Metabolic Risk Factors and Incident Advanced Liver Disease in Non-Alcoholic Fatty Liver Disease (NAFLD): A Systematic Review and Meta-Analysis of Population-Based Observational Studies. PLoS Med. 2020, 17, e1003100. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Rinella, M.; Charlton, M. The Globalization of Nonalcoholic Fatty Liver Disease: Prevalence and Impact on World Health. Hepatology 2016, 64, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, Regional and National Prevalence of Overweight and Obesity in Children and Adults 1980–2013: A Systematic Analysis. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Subichin, M.; Clanton, J.; Makuszewski, M.; Bohon, A.; Zografakis, J.G.; Dan, A. Liver Disease in the Morbidly Obese: A Review of 1000 Consecutive Patients Undergoing Weight Loss Surgery. Surg. Obes. Relat. Dis. 2015, 11, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular Mechanisms of Lipotoxicity and Glucotoxicity in Nonalcoholic Fatty Liver Disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipokines in Nonalcoholic Fatty Liver Disease. Metabolism 2016, 65, 1062–1079. [Google Scholar] [CrossRef]
- Vetrano, E.; Rinaldi, L.; Mormone, A.; Giorgione, C.; Galiero, R.; Caturano, A.; Nevola, R.; Marfella, R.; Sasso, F.C. Non-Alcoholic Fatty Liver Disease (NAFLD), Type 2 Diabetes, and Non-Viral Hepatocarcinoma: Pathophysiological Mechanisms and New Therapeutic Strategies. Biomedicines 2023, 11, 468. [Google Scholar] [CrossRef]
- Milek, M.; Moulla, Y.; Kern, M.; Stroh, C.; Dietrich, A.; Schön, M.R.; Gärtner, D.; Lohmann, T.; Dressler, M.; Kovacs, P.; et al. Adipsin Serum Concentrations and Adipose Tissue Expression in People with Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 2222. [Google Scholar] [CrossRef]
- Abdalla, M.M.I. Role of Visfatin in Obesity-Induced Insulin Resistance. World J. Clin. Cases 2022, 10, 10840–10851. [Google Scholar] [CrossRef]
- Nieva-Vazquez, A.; Pérez-Fuentes, R.; Torres-Rasgado, E.; López-López, J.G.; Romero, J.R. Serum Resistin Levels Are Associated with Adiposity and Insulin Sensitivity in Obese Hispanic Subjects. Metab. Syndr. Relat. Disord. 2014, 12, 143–148. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Lahlou, N.; Clement, K.; Carel, J.C.; Vaisse, C.; Lotton, C.; Le Bihan, Y.; Basdevant, A.; Lebouc, Y.; Froguel, P.; Roger, M.; et al. Soluble Leptin Receptor in Serum of Subjects with Complete Resistance to Leptin: Relation to Fat Mass. Diabetes 2000, 49, 1347–1352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, L.; Liu, M. Adiponectin: Friend or Foe in Obesity and Inflammation. Med. Rev. 2022, 2, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Siddharth, S.; Sharma, D. Adiponectin, Obesity, and Cancer: Clash of the Bigwigs in Health and Disease. Int. J. Mol. Sci. 2019, 20, 2519. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Farr, O.M.; Mantzoros, C.S. Leptin in Leanness and Obesity: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Quek, J.; Chan, K.E.; Wong, Z.Y.; Tan, C.; Tan, B.; Lim, W.H.; Tan, D.J.H.; Tang, A.S.P.; Tay, P.; Xiao, J.; et al. Global Prevalence of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis in the Overweight and Obese Population: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 20–30. [Google Scholar] [CrossRef]
- Le, M.H.; Le, D.M.; Baez, T.C.; Wu, Y.; Ito, T.; Lee, E.Y.; Lee, K.; Stave, C.D.; Henry, L.; Barnett, S.D.; et al. Global Incidence of Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of 63 Studies and 1,201,807 Persons. J. Hepatol. 2023, 79, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.; Hosiny, B.E.; Ismaiel, M.; Leucuta, D.-C.; Popa, S.-L.; Catana, C.S.; Dumitrascu, D.L. Waist to Height Ratio in Nonalcoholic Fatty Liver Disease—Systematic Review and Meta-Analysis. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102160. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Zoppini, G.; Day, C.P. Risk of All-Cause and Cardiovascular Mortality in Patients with Chronic Liver Disease. Gut 2011, 60, 1602–1603, author reply 1603–1604. [Google Scholar] [CrossRef]
- Mellinger, J.L.; Pencina, K.M.; Massaro, J.M.; Hoffmann, U.; Seshadri, S.; Fox, C.S.; O’Donnell, C.J.; Speliotes, E.K. Hepatic Steatosis and Cardiovascular Disease Outcomes: An Analysis of the Framingham Heart Study. J. Hepatol. 2015, 63, 470–476. [Google Scholar] [CrossRef]
- Ye, Q.; Zou, B.; Yeo, Y.H.; Li, J.; Huang, D.Q.; Wu, Y.; Yang, H.; Liu, C.; Kam, L.Y.; Tan, X.X.E.; et al. Global Prevalence, Incidence, and Outcomes of Non-Obese or Lean Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 739–752. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. NAFLD and Increased Risk of Cardiovascular Disease: Clinical Associations, Pathophysiological Mechanisms and Pharmacological Implications. Gut 2020, 69, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- Bonapace, S.; Perseghin, G.; Molon, G.; Canali, G.; Bertolini, L.; Zoppini, G.; Barbieri, E.; Targher, G. Nonalcoholic Fatty Liver Disease Is Associated with Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. Diabetes Care 2012, 35, 389–395. [Google Scholar] [CrossRef]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stål, P.; Kechagias, S.; Hultcrantz, R. Fibrosis Stage Is the Strongest Predictor for Disease-Specific Mortality in NAFLD after up to 33 Years of Follow-Up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. Non-Alcoholic Fatty Liver Disease-Related Risk of Cardiovascular Disease and Other Cardiac Complications. Diabetes Obes. Metab. 2022, 24 (Suppl. 2), 28–43. [Google Scholar] [CrossRef] [PubMed]
- Verrijken, A.; Francque, S.; Mertens, I.; Prawitt, J.; Caron, S.; Hubens, G.; Van Marck, E.; Staels, B.; Michielsen, P.; Van Gaal, L. Prothrombotic Factors in Histologically Proven Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2014, 59, 121–129. [Google Scholar] [CrossRef]
- Yoo, H.J.; Choi, K.M. Hepatokines as a Link between Obesity and Cardiovascular Diseases. Diabetes Metab. J. 2015, 39, 10–15. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, X.-J.; Ji, Y.-X.; Zhang, P.; She, Z.-G.; Li, H. Nonalcoholic Fatty Liver Disease Pandemic Fuels the Upsurge in Cardiovascular Diseases. Circ. Res. 2020, 126, 679–704. [Google Scholar] [CrossRef]
- Pîrșcoveanu, D.; Albu, C.; Târtea, E.-A.; Mărginean, I.; Iacob, G.; Pinoșanu, E.; Țucă, A.; Mărginean, C.; Sandu, R.; Pîrșcoveanu, M. Updating Data on Cognitive Impairment in Stroke Patients. J. Mind Med. Sci. 2024, 11, 49–61. [Google Scholar] [CrossRef]
- Gavril, R.S.; Mitu, O.; Zota, I.M.; Constantin, M.M.L.; Mastaleru, A.; Gavril, O.I.; Vasilcu, T.; Drugescu, A.; Arhire, L.I.; Mihalache, L.; et al. Sciendo. Intern. Med. 2021, 18, 21–27. [Google Scholar] [CrossRef]
- Niederseer, D.; Wernly, B.; Aigner, E.; Stickel, F.; Datz, C. NAFLD and Cardiovascular Diseases: Epidemiological, Mechanistic and Therapeutic Considerations. J. Clin. Med. 2021, 10, 467. [Google Scholar] [CrossRef]
- Zhou, B.-G.; Ju, S.-Y.; Mei, Y.-Z.; Jiang, X.; Wang, M.; Zheng, A.-J.; Ding, Y.-B. A Systematic Review and Meta-Analysis of Cohort Studies on the Potential Association between NAFLD/MAFLD and Risk of Incident Atrial Fibrillation. Front. Endocrinol. 2023, 14, 1160532. [Google Scholar] [CrossRef]
- Mantovani, A.; Csermely, A.; Petracca, G.; Beatrice, G.; Corey, K.E.; Simon, T.G.; Byrne, C.D.; Targher, G. Non-Alcoholic Fatty Liver Disease and Risk of Fatal and Non-Fatal Cardiovascular Events: An Updated Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-Alcoholic Fatty Liver Disease and Risk of Incident Cardiovascular Disease: A Meta-Analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef]
- Bisaccia, G.; Ricci, F.; Khanji, M.Y.; Sorella, A.; Melchiorre, E.; Iannetti, G.; Galanti, K.; Mantini, C.; Pizzi, A.D.; Tana, C.; et al. Cardiovascular Morbidity and Mortality Related to Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101643. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Ang, S.P.; Huang, H.; Momi, N.K.; Hameed, M.; Naz, S.; Batra, N.; Ishak, A.; Doshi, N.; Gera, A.; et al. Association between Nonalcoholic Fatty Liver Disease and Atrial Fibrillation and Other Clinical Outcomes: A Meta-Analysis. J. Investig. Med. 2023, 71, 591–602. [Google Scholar] [CrossRef]
- Mantovani, A.; Dauriz, M.; Sandri, D.; Bonapace, S.; Zoppini, G.; Tilg, H.; Byrne, C.D.; Targher, G. Association between Non-Alcoholic Fatty Liver Disease and Risk of Atrial Fibrillation in Adult Individuals: An Updated Meta-Analysis. Liver Int. 2019, 39, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Mahfood Haddad, T.; Hamdeh, S.; Kanmanthareddy, A.; Alla, V.M. Nonalcoholic Fatty Liver Disease and the Risk of Clinical Cardiovascular Events: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. 2017, 11 (Suppl. 1), S209–S216. [Google Scholar] [CrossRef]
- Jamalinia, M.; Zare, F.; Noorizadeh, K.; Bagheri Lankarani, K. Systematic Review with Meta-Analysis: Steatosis Severity and Subclinical Atherosclerosis in Metabolic Dysfunction-Associated Steatotic Liver Disease. Aliment. Pharmacol. Ther. 2024, 59, 445–458. [Google Scholar] [CrossRef]
- Gellert-Kristensen, H.; Richardson, T.G.; Davey Smith, G.; Nordestgaard, B.G.; Tybjaerg-Hansen, A.; Stender, S. Combined Effect of PNPLA3, TM6SF2, and HSD17B13 Variants on Risk of Cirrhosis and Hepatocellular Carcinoma in the General Population. Hepatology 2020, 72, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Doycheva, I.; Zhang, T.; Amjad, W.; Thuluvath, P.J. Diabetes and Hepatocellular Carcinoma: Incidence Trends and Impact of Liver Disease Etiology. J. Clin. Exp. Hepatol. 2020, 10, 296–303. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin. Gastroenterol. Hepatol. 2019, 17, 748–755.e3. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Sharma, R.; Khalili, H.; Hagström, H.; Ludvigsson, J.F. Cancer Risk in Patients with Biopsy-Confirmed Nonalcoholic Fatty Liver Disease: A Population-Based Cohort Study. Hepatology 2021, 74, 2410–2423. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dauriz, M.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Bonora, E.; Targher, G. Association between Nonalcoholic Fatty Liver Disease and Colorectal Tumours in Asymptomatic Adults Undergoing Screening Colonoscopy: A Systematic Review and Meta-Analysis. Metabolism 2018, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.E.; Pîrvu, D.C.; Mărginean, C.M.; Dijmărescu, A.L.; Muñoz-Groza, A.E.; Meşină, C.; Bălşeanu, T.A.; Băleanu, V.D.; Ţenea-Cojan, T.Ş.; Ciobanu, D. Molecular Prognostic Factors in Colorectal Cancer: 5-Year Follow-Up. Rom. J. Morphol. Embryol. 2023, 64, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Hong, J.Y.; Shen, J.J.; Han, K.; Park, J.O.; Park, Y.S.; Lim, H.Y. Increased Risk of Young-Onset Digestive Tract Cancers Among Young Adults Age 20–39 Years With Nonalcoholic Fatty Liver Disease: A Nationwide Cohort Study. J. Clin. Oncol. 2023, 41, 3363–3373. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Wang, Z.; Yang, Y.M.; Billet, S.; Tu, W.; Pimienta, M.; Cassel, S.L.; Pandol, S.J.; Lu, S.C.; Sutterwala, F.S.; et al. NOD-like Receptor C4 Inflammasome Regulates the Growth of Colon Cancer Liver Metastasis in NAFLD. Hepatology 2019, 70, 1582–1599. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Liu, Z.; Chang, Z.; Sun, Z.; Zhao, L. Concomitant NAFLD Facilitates Liver Metastases and PD-1-Refractory by Recruiting MDSCs via CXCL5/CXCR2 in Colorectal Cancer. Cell. Mol. Gastroenterol. Hepatol. 2024, 18, 101351. [Google Scholar] [CrossRef]
- Wei, J.; Sun, X.; Chen, Y.; Li, Y.; Song, L.; Zhou, Z.; Xu, B.; Lin, Y.; Xu, S. Perinatal Exposure to Bisphenol A Exacerbates Nonalcoholic Steatohepatitis-like Phenotype in Male Rat Offspring Fed on a High-Fat Diet. J. Endocrinol. 2014, 222, 313–325. [Google Scholar] [CrossRef]
- Strakovsky, R.S.; Wang, H.; Engeseth, N.J.; Flaws, J.A.; Helferich, W.G.; Pan, Y.-X.; Lezmi, S. Developmental Bisphenol A (BPA) Exposure Leads to Sex-Specific Modification of Hepatic Gene Expression and Epigenome at Birth That May Exacerbate High-Fat Diet-Induced Hepatic Steatosis. Toxicol. Appl. Pharmacol. 2015, 284, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, L.; Zhang, H.; Wei, W.; Jia, L. Perinatal BPA Exposure Induces Hyperglycemia, Oxidative Stress and Decreased Adiponectin Production in Later Life of Male Rat Offspring. Int. J. Environ. Res. Public Health 2014, 11, 3728–3742. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Deretzi, G.; Zavos, C.; Mantzoros, C.S. The Emerging Role of Endocrine Disruptors in Pathogenesis of Insulin Resistance: A Concept Implicating Nonalcoholic Fatty Liver Disease. Curr. Mol. Med. 2012, 12, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Falzarano, C.; Lofton, T.; Osei-Ntansah, A.; Oliver, T.; Southward, T.; Stewart, S.; Andrisse, S. Nonalcoholic Fatty Liver Disease in Women and Girls with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2022, 107, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Vassilatou, E.; Vassiliadi, D.A.; Salambasis, K.; Lazaridou, H.; Koutsomitopoulos, N.; Kelekis, N.; Kassanos, D.; Hadjidakis, D.; Dimitriadis, G. Increased Prevalence of Polycystic Ovary Syndrome in Premenopausal Women with Nonalcoholic Fatty Liver Disease. Eur. J. Endocrinol. 2015, 173, 739–747. [Google Scholar] [CrossRef]
- Wu, J.; Yao, X.-Y.; Shi, R.-X.; Liu, S.-F.; Wang, X.-Y. A Potential Link between Polycystic Ovary Syndrome and Non-Alcoholic Fatty Liver Disease: An Update Meta-Analysis. Reprod. Health 2018, 15, 77. [Google Scholar] [CrossRef]
- Baranova, A.; Tran, T.P.; Afendy, A.; Wang, L.; Shamsaddini, A.; Mehta, R.; Chandhoke, V.; Birerdinc, A.; Younossi, Z.M. Molecular Signature of Adipose Tissue in Patients with Both Non-Alcoholic Fatty Liver Disease (NAFLD) and Polycystic Ovarian Syndrome (PCOS). J. Transl. Med. 2013, 11, 133. [Google Scholar] [CrossRef]
- Kelley, C.E.; Brown, A.J.; Diehl, A.M.; Setji, T.L. Review of Nonalcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome. World J. Gastroenterol. 2014, 20, 14172–14184. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Endocrine Society Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef]
- Manzano-Nunez, R.; Santana-Dominguez, M.; Rivera-Esteban, J.; Sabiote, C.; Sena, E.; Bañares, J.; Tacke, F.; Pericàs, J.M. Non-Alcoholic Fatty Liver Disease in Patients with Polycystic Ovary Syndrome: A Systematic Review, Meta-Analysis, and Meta-Regression. J. Clin. Med. 2023, 12, 856. [Google Scholar] [CrossRef]
- Rocha, A.L.L.; Faria, L.C.; Guimarães, T.C.M.; Moreira, G.V.; Cândido, A.L.; Couto, C.A.; Reis, F.M. Non-Alcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. J. Endocrinol. Investig. 2017, 40, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Shengir, M.; Chen, T.; Guadagno, E.; Ramanakumar, A.V.; Ghali, P.; Deschenes, M.; Wong, P.; Krishnamurthy, S.; Sebastiani, G. Non-alcoholic Fatty Liver Disease in Premenopausal Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis. JGH Open 2021, 5, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Zheng, H.; Peng, H. Association between Polycystic Ovary Syndrome and Risk of Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. Endokrynol. Pol. 2023, 74, 520–527. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Mantovani, A.; Nascimbeni, F.; Lugari, S.; Targher, G. Pathogenesis of Hypothyroidism-Induced NAFLD: Evidence for a Distinct Disease Entity? Dig. Liver Dis. 2019, 51, 462–470. [Google Scholar] [CrossRef]
- Ritter, M.J.; Amano, I.; Hollenberg, A.N. Thyroid Hormone Signaling and the Liver. Hepatology 2020, 72, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Nascimbeni, F.; Lonardo, A.; Zoppini, G.; Bonora, E.; Mantzoros, C.S.; Targher, G. Association Between Primary Hypothyroidism and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Thyroid 2018, 28, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Peng, D. Update on Dyslipidemia in Hypothyroidism: The Mechanism of Dyslipidemia in Hypothyroidism. Endocr. Connect. 2022, 11, e210002. [Google Scholar] [CrossRef] [PubMed]
- Eshraghian, A.; Hamidian Jahromi, A. Non-Alcoholic Fatty Liver Disease and Thyroid Dysfunction: A Systematic Review. World J. Gastroenterol. 2014, 20, 8102–8109. [Google Scholar] [CrossRef]
- Moslehi, A.; Hamidi-Zad, Z. Role of SREBPs in Liver Diseases: A Mini-Review. J. Clin. Transl. Hepatol. 2018, 6, 332–338. [Google Scholar] [CrossRef]
- Mavromati, M.; Jornayvaz, F.R. Hypothyroidism-Associated Dyslipidemia: Potential Molecular Mechanisms Leading to NAFLD. Int. J. Mol. Sci. 2021, 22, 12797. [Google Scholar] [CrossRef]
- He, W.; An, X.; Li, L.; Shao, X.; Li, Q.; Yao, Q.; Zhang, J. Relationship between Hypothyroidism and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2017, 8, 335. [Google Scholar] [CrossRef]
- Loosen, S.H.; Demir, M.; Kostev, K.; Luedde, T.; Roderburg, C. Incidences of Hypothyroidism and Autoimmune Thyroiditis Are Increased in Patients with Nonalcoholic Fatty Liver Disease. Eur. J. Gastroenterol. Hepatol. 2021, 33, e1008–e1012. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.; Yu, X.; Qi, X. Thyroid Function and Risk of Non-Alcoholic Fatty Liver Disease in Euthyroid Subjects. Ann. Hepatol. 2018, 17, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, M.; Han, B.; Qi, X. Association of Non-Alcoholic Fatty Liver Disease with Thyroid Function: A Systematic Review and Meta-Analysis. Dig. Liver Dis. 2018, 50, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Borges-Canha, M.; Neves, J.S.; Mendonça, F.; Silva, M.M.; Costa, C.; Cabral, P.M.; Guerreiro, V.; Lourenço, R.; Meira, P.; Salazar, D.; et al. Thyroid Function and the Risk of Non-Alcoholic Fatty Liver Disease in Morbid Obesity. Front. Endocrinol. 2020, 11, 572128. [Google Scholar] [CrossRef]
- Jaruvongvanich, V.; Sanguankeo, A.; Upala, S. Nonalcoholic Fatty Liver Disease Is Not Associated with Thyroid Hormone Levels and Hypothyroidism: A Systematic Review and Meta-Analysis. Eur. Thyroid. J. 2017, 6, 208–215. [Google Scholar] [CrossRef]
- Biciuşcă, V.; Popescu, M.; Petrescu, I.O.; Stan, I.S.; Durand, P.; Petrescu, M.; Velea, R.; Traşcă, D.M.; Popescu, I.A.S.; Udriştoiu, I.; et al. Hepatic Pathological Features in Naïve Patients with Chronic Hepatitis C Who Have Developed Thyroid Disorder. Rom. J. Morphol. Embryol. 2020, 61, 1085–1097. [Google Scholar] [CrossRef]
- Bano, A.; Chaker, L.; Plompen, E.P.C.; Hofman, A.; Dehghan, A.; Franco, O.H.; Janssen, H.L.A.; Darwish Murad, S.; Peeters, R.P. Thyroid Function and the Risk of Nonalcoholic Fatty Liver Disease: The Rotterdam Study. J. Clin. Endocrinol. Metab. 2016, 101, 3204–3211. [Google Scholar] [CrossRef]
- Mantovani, A.; Csermely, A.; Bilson, J.; Borella, N.; Enrico, S.; Pecoraro, B.; Shtembari, E.; Morandin, R.; Polyzos, S.; Valenti, L.; et al. Association between Primary Hypothyroidism and Metabolic Dysfunction-Associated Steatotic Liver Disease: An Updated Meta-Analysis. Gut 2024, 73, 1554–1561. [Google Scholar] [CrossRef]
- Zeng, X.; Li, B.; Zou, Y. The Relationship between Non-Alcoholic Fatty Liver Disease and Hypothyroidism: A Systematic Review and Meta-Analysis. Medicine 2021, 100, e25738. [Google Scholar] [CrossRef]
- Adamek, A.; Kasprzak, A. Insulin-Like Growth Factor (IGF) System in Liver Diseases. Int. J. Mol. Sci. 2018, 19, 1308. [Google Scholar] [CrossRef]
- Doycheva, I.; Erickson, D.; Watt, K.D. Growth Hormone Deficiency and NAFLD: An Overlooked and Underrecognized Link. Hepatol. Commun. 2022, 6, 2227–2237. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, L.; Waters, M.J.; Chen, C. Insulin and Growth Hormone Balance: Implications for Obesity. Trends Endocrinol. Metab. 2020, 31, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Meienberg, F.; Yee, M.; Johnston, D.; Cox, J.; Robinson, S.; Bell, J.D.; Thomas, E.L.; Taylor-Robinson, S.D.; Godsland, I. Liver Fat in Adults with GH Deficiency: Comparison to Matched Controls and the Effect of GH Replacement. Clin. Endocrinol. 2016, 85, 76–84. [Google Scholar] [CrossRef]
- Nishizawa, H.; Iguchi, G.; Murawaki, A.; Fukuoka, H.; Hayashi, Y.; Kaji, H.; Yamamoto, M.; Suda, K.; Takahashi, M.; Seo, Y.; et al. Nonalcoholic Fatty Liver Disease in Adult Hypopituitary Patients with GH Deficiency and the Impact of GH Replacement Therapy. Eur. J. Endocrinol. 2012, 167, 67–74. [Google Scholar] [CrossRef]
- Dichtel, L.E.; Corey, K.E.; Misdraji, J.; Bredella, M.A.; Schorr, M.; Osganian, S.A.; Young, B.J.; Sung, J.C.; Miller, K.K. The Association Between IGF-1 Levels and the Histologic Severity of Nonalcoholic Fatty Liver Disease. Clin. Transl. Gastroenterol. 2017, 8, e217. [Google Scholar] [CrossRef]
- Clemmons, D.R. Metabolic Actions of Insulin-like Growth Factor-I in Normal Physiology and Diabetes. Endocrinol. Metab. Clin. North Am. 2012, 41, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kopchick, J.J.; Puri, V.; Sharma, V.M. Effect of Growth Hormone on Insulin Signaling. Mol. Cell Endocrinol. 2020, 518, 111038. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Furtado, A.C.L.; Carvalho-Louro, D.M.; Regattieri, N.A.T.; Rodrigues, M.P.; Montenegro, M.L.R.N.; Ferro, A.M.; Pirangi, P.S.; Naves, L.A. Transient Elastography and Controlled Attenuation Parameter (CAP) in the Assessment of Liver Steatosis in Severe Adult Growth Hormone Deficiency. Front. Endocrinol. 2019, 10, 364. [Google Scholar] [CrossRef]
- Matsumoto, R.; Fukuoka, H.; Iguchi, G.; Nishizawa, H.; Bando, H.; Suda, K.; Takahashi, M.; Takahashi, Y. Long-Term Effects of Growth Hormone Replacement Therapy on Liver Function in Adult Patients with Growth Hormone Deficiency. Growth Horm. IGF Res. 2014, 24, 174–179. [Google Scholar] [CrossRef]
- Cianfarani, S. Risk of Cancer in Patients Treated with Recombinant Human Growth Hormone in Childhood. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 92–98. [Google Scholar] [CrossRef]
- Kaltenecker, D.; Themanns, M.; Mueller, K.M.; Spirk, K.; Suske, T.; Merkel, O.; Kenner, L.; Luís, A.; Kozlov, A.; Haybaeck, J.; et al. Hepatic Growth Hormone—JAK2—STAT5 Signalling: Metabolic Function, Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma Progression. Cytokine 2019, 124, 154569. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sun, L.; Zhang, S.; Wang, Y.; Wang, G. Effect of Long-Term Growth Hormone Replacement on Glucose Metabolism in Adults with Growth Hormone Deficiency: A Systematic Review and Meta-Analysis. Pituitary 2021, 24, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Petrossians, P.; Daly, A.F.; Natchev, E.; Maione, L.; Blijdorp, K.; Sahnoun-Fathallah, M.; Auriemma, R.; Diallo, A.M.; Hulting, A.-L.; Ferone, D.; et al. Acromegaly at Diagnosis in 3173 Patients from the Liège Acromegaly Survey (LAS) Database. Endocr. Relat. Cancer 2017, 24, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Møller, N.; Jørgensen, J.O.L. Effects of Growth Hormone on Glucose, Lipid, and Protein Metabolism in Human Subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef]
- Winhofer, Y.; Wolf, P.; Krssak, M.; Wolfsberger, S.; Tura, A.; Pacini, G.; Gessl, A.; Raber, W.; Just, I.; Kautzky-Willer, A.; et al. No Evidence of Ectopic Lipid Accumulation in the Pathophysiology of the Acromegalic Cardiomyopathy. J. Clin. Endocrinol. Metab. 2014, 99, jc20142242. [Google Scholar] [CrossRef] [PubMed]
- Koutsou-Tassopoulou, A.; Papapostoli-Sklavounou, I.; Krawczyk, M.; Friesenhahn-Ochs, B.; Weber, S.N.; Lammert, F.; Stokes, C.S. Hepatic Steatosis in Patients with Acromegaly. Endocrinol. Diabetes Metab. 2019, 2, e00090. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yonei, Y.; Tanaka, S.; Mori, K.; Kanemasa, K.; Imai, S.; Taketani, H.; Hara, T.; Seko, Y.; Ishiba, H.; et al. Lower Levels of Insulin-like Growth Factor-1 Standard Deviation Score Are Associated with Histological Severity of Non-Alcoholic Fatty Liver Disease. Hepatol. Res. 2015, 45, 771–781. [Google Scholar] [CrossRef]
- Sarkar, M.; Yates, K.; Suzuki, A.; Lavine, J.; Gill, R.; Ziegler, T.; Terrault, N.; Dhindsa, S. Low Testosterone Is Associated with Nonalcoholic Steatohepatitis and Fibrosis Severity in Men. Clin. Gastroenterol. Hepatol. 2021, 19, 400–402.e2. [Google Scholar] [CrossRef]
- Zhang, P.; Ge, Z.; Wang, H.; Feng, W.; Sun, X.; Chu, X.; Jiang, C.; Wang, Y.; Zhu, D.; Bi, Y. Prolactin Improves Hepatic Steatosis via CD36 Pathway. J. Hepatol. 2018, 68, 1247–1255. [Google Scholar] [CrossRef]
- Seo, N.K.; Koo, H.S.; Haam, J.-H.; Kim, H.Y.; Kim, M.J.; Park, K.-C.; Park, K.-S.; Kim, Y.-S. Prediction of Prevalent but Not Incident Non-Alcoholic Fatty Liver Disease by Levels of Serum Testosterone. J. Gastroenterol. Hepatol. 2015, 30, 1211–1216. [Google Scholar] [CrossRef]
- Klair, J.S.; Yang, J.D.; Abdelmalek, M.F.; Guy, C.D.; Gill, R.M.; Yates, K.; Unalp-Arida, A.; Lavine, J.E.; Clark, J.M.; Diehl, A.M.; et al. A Longer Duration of Estrogen Deficiency Increases Fibrosis Risk among Postmenopausal Women with Nonalcoholic Fatty Liver Disease. Hepatology 2016, 64, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and Menopause Impact Severity of Fibrosis among Patients with Nonalcoholic Steatohepatitis. Hepatology 2014, 59, 1406–1414. [Google Scholar] [CrossRef]
- Hannemann, A.; Wallaschofski, H. Prevalence of Primary Aldosteronism in Patient’s Cohorts and in Population-Based Studies--a Review of the Current Literature. Horm. Metab. Res. 2012, 44, 157–162. [Google Scholar] [CrossRef]
- Giacchetti, G.; Ronconi, V.; Turchi, F.; Agostinelli, L.; Mantero, F.; Rilli, S.; Boscaro, M. Aldosterone as a Key Mediator of the Cardiometabolic Syndrome in Primary Aldosteronism: An Observational Study. J. Hypertens. 2007, 25, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, X.; de Los Ríos, E.A.; Díaz, J.M.; Lerma-Alvarado, R.M.; Martínez de la Escalera, L.; López-Barrera, F.; Lemini, M.; Arnold, E.; Martínez de la Escalera, G.; Clapp, C.; et al. Prolactin Promotes Adipose Tissue Fitness and Insulin Sensitivity in Obese Males. Endocrinology 2017, 158, 56–68. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, W.; Chu, X.; Sun, X.; Zhu, D.; Bi, Y. A Newly Noninvasive Model for Prediction of Non-Alcoholic Fatty Liver Disease: Utility of Serum Prolactin Levels. BMC Gastroenterol. 2019, 19, 202. [Google Scholar] [CrossRef]
- Serri, O.; Li, L.; Mamputu, J.-C.; Beauchamp, M.-C.; Maingrette, F.; Renier, G. The Influences of Hyperprolactinemia and Obesity on Cardiovascular Risk Markers: Effects of Cabergoline Therapy. Clin. Endocrinol. 2006, 64, 366–370. [Google Scholar] [CrossRef]
- Alshahrani, F.; Aljohani, N. Vitamin D: Deficiency, Sufficiency and Toxicity. Nutrients 2013, 5, 3605–3616. [Google Scholar] [CrossRef]
- Cimini, F.A.; Barchetta, I.; Carotti, S.; Bertoccini, L.; Baroni, M.G.; Vespasiani-Gentilucci, U.; Cavallo, M.-G.; Morini, S. Relationship between Adipose Tissue Dysfunction, Vitamin D Deficiency and the Pathogenesis of Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2017, 23, 3407–3417. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Associations of Vitamin D with Insulin Resistance, Obesity, Type 2 Diabetes, and Metabolic Syndrome. J. Steroid Biochem. Mol. Biol. 2018, 175, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Abramovitch, S.; Dahan-Bachar, L.; Sharvit, E.; Weisman, Y.; Ben Tov, A.; Brazowski, E.; Reif, S. Vitamin D Inhibits Proliferation and Profibrotic Marker Expression in Hepatic Stellate Cells and Decreases Thioacetamide-Induced Liver Fibrosis in Rats. Gut 2011, 60, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Beilfuss, A.; Sowa, J.-P.; Sydor, S.; Beste, M.; Bechmann, L.P.; Schlattjan, M.; Syn, W.-K.; Wedemeyer, I.; Mathé, Z.; Jochum, C.; et al. Vitamin D Counteracts Fibrogenic TGF-β Signalling in Human Hepatic Stellate Cells Both Receptor-Dependently and Independently. Gut 2015, 64, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Eliades, M.; Spyrou, E. Vitamin D: A New Player in Non-Alcoholic Fatty Liver Disease? World J. Gastroenterol. 2015, 21, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Eliades, M.; Spyrou, E.; Agrawal, N.; Lazo, M.; Brancati, F.L.; Potter, J.J.; Koteish, A.A.; Clark, J.M.; Guallar, E.; Hernaez, R. Meta-Analysis: Vitamin D and Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2013, 38, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Borges-Canha, M.; Neves, J.S.; Mendonça, F.; Silva, M.M.; Costa, C.; Cabral, P.M.; Guerreiro, V.; Lourenço, R.; Meira, P.; Salazar, D.; et al. The Impact of Vitamin D in Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in Patients with Morbid Obesity. Diabetes Metab. Syndr. Obes. 2021, 14, 487–495. [Google Scholar] [CrossRef]
- Wang, N.; Chen, C.; Zhao, L.; Chen, Y.; Han, B.; Xia, F.; Cheng, J.; Li, Q.; Lu, Y. Vitamin D and Nonalcoholic Fatty Liver Disease: Bi-Directional Mendelian Randomization Analysis. EBioMedicine 2018, 28, 187–193. [Google Scholar] [CrossRef]
- Barchetta, I.; Del Ben, M.; Angelico, F.; Di Martino, M.; Fraioli, A.; La Torre, G.; Saulle, R.; Perri, L.; Morini, S.; Tiberti, C.; et al. No Effects of Oral Vitamin D Supplementation on Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. BMC Med. 2016, 14, 92. [Google Scholar] [CrossRef]
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Vitamin D Supplementation and Non-Alcoholic Fatty Liver Disease: Present and Future. Nutrients 2017, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Sakpal, M.; Satsangi, S.; Mehta, M.; Duseja, A.; Bhadada, S.; Das, A.; Dhiman, R.K.; Chawla, Y.K. Vitamin D Supplementation in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. JGH Open 2017, 1, 62–67. [Google Scholar] [CrossRef]
- Purohit, V.; Abdelmalek, M.F.; Barve, S.; Benevenga, N.J.; Halsted, C.H.; Kaplowitz, N.; Kharbanda, K.K.; Liu, Q.-Y.; Lu, S.C.; McClain, C.J.; et al. Role of S-Adenosylmethionine, Folate, and Betaine in the Treatment of Alcoholic Liver Disease: Summary of a Symposium23. Am. J. Clin. Nutr. 2007, 86, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Hang, W.; Shu, H.; Wen, Z.; Liu, J.; Jin, Z.; Shi, Z.; Chen, C.; Wang, D.W. N-Acetyl Cysteine Ameliorates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease and Intracellular Triglyceride Accumulation by Preserving Mitochondrial Function. Front. Pharmacol. 2021, 12, 636204. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Kessoku, T.; Sumida, Y.; Kobayashi, T.; Kato, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Fujita, K.; Yoneda, M.; et al. Efficacy of Glutathione for the Treatment of Nonalcoholic Fatty Liver Disease: An Open-Label, Single-Arm, Multicenter, Pilot Study. BMC Gastroenterol. 2017, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Kongmalai, T.; Srinonprasert, V.; Anothaisintawee, T.; Kongmalai, P.; McKay, G.; Attia, J.; Thakkinstian, A. New Anti-Diabetic Agents for the Treatment of Non-Alcoholic Fatty Liver Disease: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2023, 14, 1182037. [Google Scholar] [CrossRef]
- Song, T.; Jia, Y.; Li, Z.; Wang, F.; Ren, L.; Chen, S. Effects of Liraglutide on Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Ther. 2021, 12, 1735–1749. [Google Scholar] [CrossRef]
- Loomba, R.; Hartman, M.L.; Lawitz, E.J.; Vuppalanchi, R.; Boursier, J.; Bugianesi, E.; Yoneda, M.; Behling, C.; Cummings, O.W.; Tang, Y.; et al. Tirzepatide for Metabolic Dysfunction–Associated Steatohepatitis with Liver Fibrosis. N. Engl. J. Med. 2024, 391, 299–310. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Kaplan, L.M.; Frias, J.P.; Brouwers, B.; Wu, Q.; Thomas, M.K.; Harris, C.; Schloot, N.C.; Du, Y.; Mather, K.J.; et al. Triple Hormone Receptor Agonist Retatrutide for Metabolic Dysfunction-Associated Steatotic Liver Disease: A Randomized Phase 2a Trial. Nat. Med. 2024, 30, 2037–2048. [Google Scholar] [CrossRef]
- Pepa, G.D.; Russo, M.; Vitale, M.; Carli, F.; Vetrani, C.; Masulli, M.; Riccardi, G.; Vaccaro, O.; Gastaldelli, A.; Rivellese, A.A.; et al. Pioglitazone Even at Low Dosage Improves NAFLD in Type 2 Diabetes: Clinical and Pathophysiological Insights from a Subgroup of the TOSCA.IT Randomised Trial. Diabetes Res. Clin. Pract. 2021, 178, 108984. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Samajdar, S.S.; Das, S. Effects of Saroglitazar in the Treatment of Non-Alcoholic Fatty Liver Disease or Non-Alcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102174. [Google Scholar] [CrossRef] [PubMed]
- Westerouen Van Meeteren, M.J.; Drenth, J.P.H.; Tjwa, E.T.T.L. Elafibranor: A Potential Drug for the Treatment of Nonalcoholic Steatohepatitis (NASH). Expert Opin. Investig. Drugs 2020, 29, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Kobayashi, T.; Asako, N.; Iwaki, M.; Saito, S.; Nakajima, A. Pan-Peroxisome Proliferator-Activated Receptor Agonist Lanifibranor as a Dominant Candidate Pharmacological Therapy for Nonalcoholic Fatty Liver Disease. Hepatobiliary Surg. Nutr. 2022, 11, 43335–43435. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yoneda, M.; Tokushige, K.; Kawanaka, M.; Fujii, H.; Yoneda, M.; Imajo, K.; Takahashi, H.; Ono, M.; Nozaki, Y.; et al. Hepatoprotective Effect of SGLT2 Inhibitor on Nonalcoholic Fatty Liver Disease. Diabetes Res. Open Access 2020, 2, 17. [Google Scholar]
- dos Santos, L.R.; Duarte, M.L.; Peccin, M.S.; Gagliardi, A.R.d.T.; Melnik, T. Dipeptidyl Peptidase IV Inhibitors for Nonalcoholic Fatty Liver Disease—Systematic Review and Metanalysis. Curr. Diabetes Rev. 2021, 17, 9–20. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Yan, C.; Li, C.; Zhang, L.; Zhang, L.; Liang, E.; Liu, T.; Mao, J. Effect of Metformin on Nonalcoholic Fatty Liver Based on Meta-Analysis and Network Pharmacology. Medicine 2022, 101, e31437. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Rhee, H.; Kim, Y.; Lee, M.; Lee, B.-W.; Kang, E.S.; Cha, B.-S.; Choi, J.-Y.; Lee, Y. Ezetimibe Combination Therapy with Statin for Non-Alcoholic Fatty Liver Disease: An Open-Label Randomized Controlled Trial (ESSENTIAL Study). BMC Med. 2022, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Wilkinson, N.; Tiniakos, D.; Wilkinson, J.; Burt, A.D.; McColl, E.; Stocken, D.D.; Steen, N.; Barnes, J.; Goudie, N.; et al. A Randomised Controlled Trial of Losartan as an Anti-Fibrotic Agent in Non-Alcoholic Steatohepatitis. PLoS ONE 2017, 12, e0175717. [Google Scholar] [CrossRef]
- Hirata, T.; Tomita, K.; Kawai, T.; Yokoyama, H.; Shimada, A.; Kikuchi, M.; Hirose, H.; Ebinuma, H.; Irie, J.; Ojiro, K.; et al. Effect of Telmisartan or Losartan for Treatment of Nonalcoholic Fatty Liver Disease: Fatty Liver Protection Trial by Telmisartan or Losartan Study (FANTASY). Int. J. Endocrinol. 2013, 2013, 587140. [Google Scholar] [CrossRef]
- Tang, Y.; Fan, Y.; Wang, Y.; Wang, D.; Huang, Q.; Chen, T.; Cao, X.; Wen, C.; Shen, X.; Li, J.; et al. A Current Understanding of FXR in NAFLD: The Multifaceted Regulatory Role of FXR and Novel Lead Discovery for Drug Development. Biomed. Pharmacother. 2024, 175, 116658. [Google Scholar] [CrossRef]
- Harrison, S.A.; Taub, R.; Neff, G.W.; Lucas, K.J.; Labriola, D.; Moussa, S.E.; Alkhouri, N.; Bashir, M.R. Resmetirom for Nonalcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial. Nat. Med. 2023, 29, 2919–2928. [Google Scholar] [CrossRef]
- Yuan, H.; Wu, X.; Wang, X.; Zhou, J.-Y.; Park, S. Microbial Dysbiosis Linked to Metabolic Dysfunction-Associated Fatty Liver Disease in Asians: Prevotella Copri Promotes Lipopolysaccharide Biosynthesis and Network Instability in the Prevotella Enterotype. Int. J. Mol. Sci. 2024, 25, 2183. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.G.; Kim, S.M.; Caussy, C.; Fu, T.; Guo, J.; Bassirian, S.; Singh, S.; Madamba, E.V.; Bettencourt, R.; Richards, L.; et al. A Universal Gut-Microbiome-Derived Signature Predicts Cirrhosis. Cell Metab. 2020, 32, 878–888.e6. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yu, C.-H.; Li, X.-J.; Yao, J.-M.; Fang, Z.-Y.; Yoon, S.-H.; Yu, W.-Y. Gut Dysbiosis in Nonalcoholic Fatty Liver Disease: Pathogenesis, Diagnosis, and Therapeutic Implications. Front. Cell. Infect. Microbiol. 2022, 12, 997018. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).