Abstract
Background: Transarterial chemoembolisation (TACE) and radioembolisation (TARE) can lead to the deterioration of liver function, especially in cases of a high tumour burden, potentially lessening the benefits of subsequent systemic treatments. We aimed to verify whether a high number of previous transarterial treatments modified the outcomes of patients who received sorafenib as a frontline systemic treatment. Methods: A retrospective analysis of a large multicenter dataset containing prospectively collected data of sorafenib-treated patients was conducted. Results: Data from 696 patients were analysed, with 139 patients having received >two transarterial procedures before starting sorafenib. A propensity score matched 139 identified pairs of patients. Having received >two locoregional treatments was independently associated with a shorter survival (hazard ratio 1.325, 95% confidence interval 1.018–1.725, p = 0.039). This pattern was confirmed amongst responders to sorafenib, but not in progressors. A trend toward a higher rate of the permanent discontinuation of sorafenib due to liver failure (18.7 vs. 10.8%, p = 0.089) and a lower rate of eligibility for second-line treatments (24.5 vs. 17.3%, p = 0.184) was observed in patients who had received >two transarterial procedures. Conclusions: Repeated endovascular treatments negatively impacted the survival of HCC patients, especially sorafenib-responders. An early switch to systemic therapies should be considered in cases that are unlikely to respond.
1. Introduction
Hepatocellular carcinoma (HCC) is a relevant global health problem causing more than 800,000 deaths worldwide every year [1,2]. HCC can be treated with different modalities, including surgical, percutaneous and systemic therapies, according to the stage of the disease.
While early stages of the disease are preferably treated with surgical or percutaneous therapies, patients in more advanced stages are usually candidates for a combination of different treatments according to their Barcelona Clinic for Liver Cancer (BCLC) stage. Patients in the intermediate stage (BCLC B) are usually treated with transarterial procedures, while patients in the advanced stage (BCLC C) with preserved liver function and an Eastern Cooperative Group Performance Status (ECOG-PS) are better suited for systemic therapies [3].
Among transarterial treatments, transarterial chemoembolisation (TACE) is the most popular option. However, the risk of liver function impairment following TACE is well known [4,5]. Transarterial radioembolisation (TARE), also known as selective internal radiotherapy, is another transarterial procedure which gained popularity following promising preliminary results [6]. Different from TACE, the efficacy of TARE is based on delivering the cytotoxic radioisotope Yttrium-90 to HCC rather than relying on the ischemic effect of embolisation. Therefore, it has the potential to treat larger tumours, including in neoplastic portal vein invasion [7], which is instead a contraindication to TACE.
TARE, however, is not recommended for locally advanced HCC following the failure of three different Phase 3 trials [8,9,10], but it has been used before these results and is currently used in selected cases in the context of a multidisciplinary discussion [11]. The potential benefit of TARE should be checked against the risk of radiation-induced liver damage, a condition which could lead to irreversible liver failure [8,9,10]
In 2022, the BCLC group updated its recommendations regarding two critical concepts: treatment stage migration and untreatable progression [12]. The first concept consists of selecting a treatment reserved for a more advanced stage for patients unsuitable for other recommended therapy. Untreatable progression applies to patients who either do not respond or progress after receiving the initial recommended treatment, but they still fit into the initial BCLC stage: in this scenario, the recommendations warrant considering a therapy destinated initially to a more advanced stage [13].
The low adherence to the guideline recommendations is a known phenomenon in HCC [14]. Due to the heterogeneity of the HCC population, multidisciplinary teams might recommend treatments diverging from the guidelines in case of comorbidities, borderline liver functional reserve, local expertise, or patients’ preferences. Consequently, patients within the early and intermediate stages might receive multiple locoregional treatments (especially when the tumour burden is relatively low) before a condition of untreatable progression is declared by the tumour boards, leading to the start of systemic treatment. Despite experts considering that delaying the decision to start a systemic therapy in intermediate HCC might have negative prognostic correlates, data supporting this approach are scarce. Therefore, we aimed to verify whether having received repeated transarterial procedures (both TACE and TARE) affected the overall survival (OS) of patients who had received sorafenib as a first-line systemic treatment.
2. Materials and Methods
2.1. Study Design
A retrospective analysis of prospectively collected data from the ARchives of Patients with hEpatocellular carcinoma treated with Sorafenib (ARPES) database was conducted. The ARPES database contains data from different Italian Centres (IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna; Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori IRCCS, Meldola; Degli Infermi Faenza Hospital; Ospedale Cardarelli, Napoli; Ospedale Papa Giovanni XXIII, Bergamo; Azienda Ospedaliero-Universitaria Pisana, Pisa; Centro Clinico e Ricerche Humanitas, Milano). This study included consecutive patients who received the first dose of sorafenib between January 2010 and December 2019. Data were collected individually from every centre. Starting from January 2010, co-investigators from every centre updated a shared electronic registry every 3 to 6 months. To guarantee an adequate observation time after the start of the systemic treatment, the time for the last follow-up visit was fixed on 31 July 2022. We consider the data analysis to be retrospective in nature because we analyzed the number of transarterial procedures that the patients received before starting sorafenib, while the observation of each patient began with the first administration of sorafenib.
2.2. Sorafenib Management
Sorafenib was prescribed for HCC in the advanced (BCLC-C) or intermediate stage (BCLC-B) when not amenable to locoregional treatments. The starting dose was 800 mg (two 200 mg tablets twice a day) according to the manufacturer’s instructions. Downdosing (including temporary suspension, interruption or dose reductions) was admitted to deal with non-tolerable adverse effects.
Sorafenib was continued until (i) clinical and radiologic progression, according to the modified Response Evaluation Criteria in Solid Tumours (mRECIST) [15]; patients stopped sorafenib in case of radiological progression; (ii) the non-tolerable toxicity from sorafenib; and (iii) the worsening of hepatic function, which excluded the possibility of receiving sorafenib safely.
2.3. Number of Previous Transarterial Procedures
Data regarding previous locoregional treatments were obtained from the ARPES database (including specific procedures and the number of treatments). Transarterial procedures included both TACE and TARE. Patients were categorised as having received zero to two or >two transarterial procedures before receiving sorafenib. An indication for performing transarterial procedures had been provided after an assessment from local multidisciplinary tumour boards, according to the comorbidities, performance status, liver function and tumor load As a rule of thumb, patients received an indication for TACE when all the following conditions were satisfied: (1) unresectable disease, not amenable to other curative procedures, including percutaneous treatments; (2) acceptable residual liver function (up to Child-Pugh B7); and (3) fairly high likelihood of achieving a complete response on all treated nodules without inducing irreversible liver failure preventing subsequent systemic treatments. Generally speaking, patients with more than six nodules or with nodules >6 cm were not considered candidates for TACE. At the same time, no more than three nodules were treated during each procedure. All TACE procedures were performed using superselective techniques. TARE was considered a therapeutic option for patients with a disease not amenable to curative procedures, a unilobar extension and a maximum tumour diameter up to 8 cm. Macrovascular invasion was generally considered as a contraindication to TACE, while portal vein VP1-VP2-VP3 thrombosis (i.e., up to the right/left portal branch homolateral to the lesions) was permitted for TARE. Extrahepatic disease or tumour thrombus in the main portal trunk or in the portal branch contralateral to the lesions were considered contraindications to transarterial treatments.
2.4. Transarterial Procedures Performed after the Start of Sorafenib
The policies of the participating centres did not contemplate locoregional treatments following the start of sorafenib as a routine practice. The choice to start sorafenib was in fact taken for patients deemed not amenable to these treatments.
Locoregional treatments while receiving sorafenib were performed only in selected cases, and this choice was always based upon a multidisciplinary evaluation. For example, these cases included patients with a deep and prolonged response (to achieve a complete response) or with a slow progression on a limited number of lesions, with the main bulk of HCC stable for a long time.
These situations accounted for seven patients in the whole study population, two and five of which belonging the groups who had received >two and ≤two transarterial procedures, respectively. Considering the small number of these cases and the limited impact on the prognosis, these cases maintained their original allocation to the respective study group.
2.5. Subgroup Analyses
A specific subgroup analysis was designed, distinguishing patients whose imaging response was progressive disease from those who achieved disease control (defined as either complete response, partial response or stable disease) according to the mRECIST criteria. This choice was made to better capture the potential effects of receiving multiple TACE procedures in the long term, as non-responders were far more likely to die from a rapidly progressing HCC than from liver failure. Similar subgroup analyses have been used to investigate the survival correlates of the improved management of sorafenib over time and other effects that might impact the long-term survival of sorafenib-treated patients [16,17].
2.6. Ethics
All patients gave their written informed consent for their data to be included in the prospective observational registry, which had been approved by the local ethics committee (protocol no. 098/2014/OSS).
The study was conducted according to the ethical guidelines of the latest Declaration of Helsinki.
2.7. Statistical Analyses
Continuous variables are expressed as median and interquartile ranges, and categorical variables are expressed as frequencies. The Mann–Whitney test was used to compare different groups, and the two-tails Fischer test was used for categorical variables.
OS was determined from the first received dose of sorafenib to either death, the last visit or the end of the follow-up period. Survival analysis was performed using the Kaplan–Meier method.
Predictors of survival were identified using a Cox regression analysis. Variables associated with survival at the univariate analysis with a p < 0.10 were included in the multivariate model. p-values < 0.05 were considered to be statistically significant.
Univariable and multivariable analyses were perfomed before and after performing propensity score matching to evaluate the initial differences between treatment groups and better assess the role of potential confounders.
The propensity score is the conditional probability of being treated given a set of observed potential confounders. This way, all the information from a group of potential confounders is summarised into a single balancing score variable. The propensity score assures that the distribution of measured baseline covariates is maintained unchanged between different groups. Propensity scores for having received >two transarterial treatments were calculated by performing non-parsimonious multivariate logistic regression models that included all measured potential predictors for the outcome and transarterial treatments. A comparison between groups (patients who had received ≤two transarterial procedures vs. patients who had received >two treatments) was performed using a propensity score matching.
Statistical analyses were performed using SPSS Statistic for Windows (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY, USA: IBM Corp.) and STATA/SE (StataCorp. Version 14.1. College Station, TX, USA: StataCorp LLC.).
3. Results
3.1. Study Population
We identified 696 patients from the ARPES database. This population consisted prevalently of males (84.6%), with a median age of 68 years (range 28–86). The main etiologies included viral infections (HCV 50.1%, HBV 22.2%) and non-viral causes (27.7%). The median duration of sorafenib treatment was 4.7 months (IQR: 2.3–10.3 months), with a median sorafenib dose of 474 mg daily.
One hundred and thirty-nine patients (20.0%) had received more than two intra-arterial treatments before starting sorafenib. Amongst the remaining population, 119 patients had received two different transarterial procedures, 147 patients had received one treatment and 291 did not receive any treatment before starting sorafenib (Table 1).

Table 1.
Detailed report of locoregional therapies performed in the whole study population (n = 696) prior to sorafenib initiation. Patients have been categorised according to the number of transarterial chemoembolisation (TACE) and radioembolisation (TARE) procedures received. Variables are reported as frequencies (percentages).
Patients who had received >two transarterial procedures were significantly different from the other group in terms of the disease duration and the prevalence of macrovascular invasion. A trend toward a higher age and prevalence of extrahepatic spread was also observed (Table 2).

Table 2.
Comparison of the characteristics of patients who received up to two and more than two transarterial procedures before starting sorafenib.
Independent predictors of OS in the whole study included the performance status, macrovascular invasion, AFP > 400 ng/mL, Child-Pugh B status and dermatological adverse events (evaluated as a time-dependent variable) (Table 3).

Table 3.
Univariable and multivariable Cox regression of overall survival in the whole study population (n = 696).
3.2. Propensity Score Analysis
The propensity score matching identified 139 pairs of patients. The post-matching comparison of the baseline characteristics of the study groups revealed no significant differences in the key variables (Table 4). The prevalence of patients who achieved disease control was similar across the study groups (48.2 vs. 49.6%, p = 0.905). The total number of locoregional procedures is reported in Figure 1.

Table 4.
Post-matching comparison of study groups (n = 278).
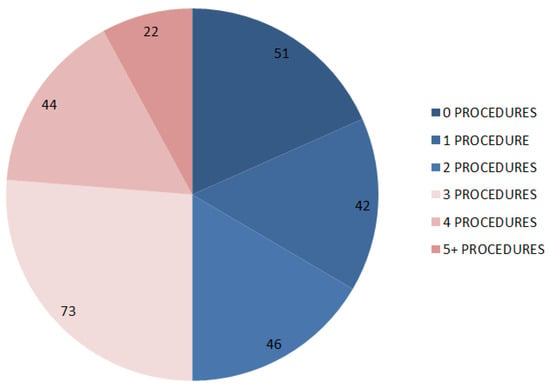
Figure 1.
Categorisation of patients included in the matched analysis according to the number of previous transarterial procedures.
The multivariable analysis performed in the matched population showed that Child-Pugh B status, AFP >400 ng/mL, macrovascular invasion, extrahepatic spread and >two transarterial procedures (hazard ratio 1.325, 95% confidence interval 1.018–1.725, p = 0.039) were independent prognostic factors (Table 4 and Table 5).

Table 5.
Univariable and multivariable Cox regression of overall survival in the matched study population (n = 278).
A high number of previous transarterial procedures was confirmed as an adverse prognostic factor in the group of patients who had achieved disease control with sorafenib (n = 136, hazard ratio 1.493, 95% confidence interval 1.034–2.156, p = 0.033) but not in patients who had progressive disease as their best radiological response (n = 142, hazard ratio 1.211, 95% confidence interval 0.836–1.753, p = 0.311). The survival curves of patients stratified according to the number of previous transarterial procedures are reported in Figure 2.
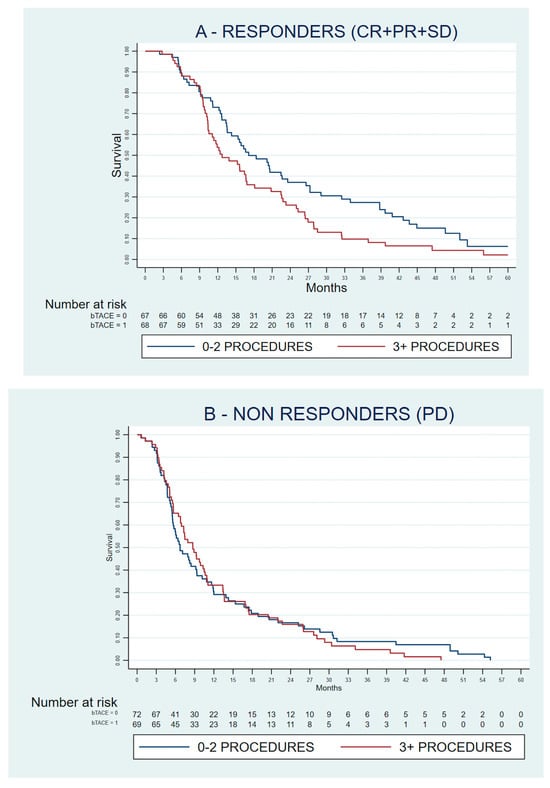
Figure 2.
Kaplan–Meyer curves of the overall survival of patients included in the matched analysis, stratified according to their best response to sorafenib as per the Response Evaluation Criteria in Solid Tumours version 1.1 (RECIST 1.1). (A) Responders; (B) Non-responders. CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease.
When analysing the causes of these differences, we found a trend toward a higher rate of the permanent discontinuation of sorafenib due to liver failure (18.7 vs. 10.8%, p = 0.089) and a lower rate of theoretical eligibility for second-line treatments (defined as ECOG-PS < 2 and Child-Pugh A status at the time of permanent sorafenib discontinuation—24.5 vs. 17.3%, p = 0.184) in patients who had received more than two transarterial procedures compared with the other group. Patients eligible for second-line treatments upon sorafenib failure experienced a longer OS compared to patients who had received sorafenib as the only drug [17.1 (13.5–20.7) vs. 8.9 (7.5–10.3) months, p < 0.001].
4. Discussion
We analysed a large dataset of sorafenib-treated patients to investigate whether a high number of previous transarterial procedures was related with an increased risk of liver function deterioration and a shorter survival. Only a minority of patients had received more than two transarterial procedures before starting sorafenib. A relatively low prevalence of heavily pretreated patients was expected, as the enrolling centres had long-term experience treating HCC. Their multidisciplinary teams were well aware of the disadvantages of performing multiple transarterial procedures in non-responding patients in terms of low efficacy and the risk of worsening hepatic function [18,19,20].
We found differences between patients when categorised according to the number of previous transarterial procedures. Patients who had received >two transarterial procedures had a longer disease duration and a lower prevalence of macrovascular invasion (a relative contraindication to TACE but not TARE). Due to this imbalance, a propensity score matching was necessary to compare the two groups properly.
In the matched population, the independent prognosticators were largely confirmed. Additionally, a higher number of previous locoregional procedures was associated with worse survival. This difference in OS was driven by responders, while patients progressing to sorafenib did not see their survival altered by the number of previous procedures (probably as their risk of death was determined by a rapid neoplastic growth rather than liver function deterioration). In this regard, the trend toward a higher rate of patients permanently discontinuing sorafenib for liver failure in the absence of progression found in highly pretreated patients provides a likely explanation for the OS difference. These results are consistent with our initial hypothesis that multiple transarterial treatments can hamper hepatic function in the long term, potentially compromising the survival benefit warranted by the systemic treatment.
Still, our results should be interpreted with caution due to the limitations of this study. First, our analysis involved only patients that received a systemic treatment during their history of HCC. Consequently, our database can not capture the possible benefit of repeated TACE in patients who will not meet the criteria for untreatable progression (for instance, patients achieving a complete response). On the other hand, patients who developed an irreversible worsening of their liver function after TACE but before starting a systemic treatment are not included in our database equally. At the same time, we were unable to assess whether patients had experienced reversible liver function deterioration after the first TACE, an event with potential prognostic relevance as it suggests a more fragile liver functional reserve [21] Second, patients who underwent conventional TACE or Drug-eluting Beads-TACE were both included. Therefore, a slight heterogeneity due to different centre-specific procedures has to be accounted for [22]. Third, even if collected prospectively, our data have been analysed retrospectively; consequently, the risk of bias is reduced sensibly but not eliminated. Finally, the multicenter approach adopted for this study certainly added elements of heterogeneity derived from a possibly different propensity of the single centres to perform transarterial procedures.
Despite these limitations, our study can lead to some speculations. For example, our results were obtained by exploring patients who received sorafenib in a timeframe in which second-line treatments were mainly available in the setting of randomised clinical trials. Different drug combinations have shown superiority over sorafenib in recent times, both in terms of the OS and response rate. These combinations include atezolizumab–bevacizumab [23] and tremelimumab–durvalumab [24]. It is likely that the benefit of preserving liver function in the long term will be even more significant in this new setting. Lenvatinib did not demonstrate a superiority to sorafenib in terms of OS in its registrative trial [25]. Still, its superior response rate [25] paired with very recent data showing an impressive median OS of 19.0 months for lenvatinib monotherapy [26] suggests that our previous consideration might also apply to lenvatinib. Therapeutic sequences, including regorafenib [27] and cabozantinib [28], represent another factor in increasing life expectancy and highlight the importance of preserving liver function. Unresectable HCC patients who could reach a third-line treatment as they maintained a good performance status and liver function had a median cumulative OS of 36 months from the sequence sorafenib–regorafenib–cabozantinib [29].
5. Conclusions
We found that a high number of previous locoregional treatments was an independent risk factor for mortality in patients who received sorafenib as a whole and in responses in particular. This finding should not be interpreted as a contraindication to performing transarterial procedures but only as a warning to switch patients to systemic therapy early in cases where they are unlikely to respond to these treatments (e.g., hypovascular HCC, large lesions, extreme multinodular disease) or have a borderline liver functional reserve.
Author Contributions
Conceptualisation, F.T. and M.P.; methodology, B.S. and L.I.; validation, V.S. and A.G.; formal analysis, F.T.; investigation, B.S.; resources, A.G.; data curation, A.C.-G., R.T., L.L., T.P., R.S., M.R., G.M., F.G.F. and F.P.; writing—original draft preparation, F.T.; writing—review and editing, A.G.; supervision, F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Azienda Ospedaliero-Univeristaria di Bologna (protocol code protocol no. 098/2014/OSS, approved on 26 February 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available on request due to restrictions (e.g., privacy, legal or ethical reasons).
Conflicts of Interest
F.T. has served as a consultant for Roche, Bayer, Ipsen and Eisai and as an advisory board member for Laforce. A.C.-G. is an advisor for AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, GSK and MSD and received grants and personal fees from Bayer, Eisai and MSD. F.T. is an advisor and a consultant for Bayer and an advisory board member for Sirtex, Alfasigma and Bristol-Myers Squibb. F.P. received honoraria from AstraZeneca, Bayer AG, Bracco, EISAI, Esaote, Exact Sciences, Ipsen, MSD, Roche, SamsungGE and Siemens Healthineers for participating in advisory boards or sponsored symposia. A.G. has served as a consultant for Bayer. All remaining authors have declared no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [CrossRef]
- EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [CrossRef] [PubMed]
- Forner, A.; Gilabert, M.; Bruix, J.; Raoul, J.-L. Treatment of Intermediate-Stage Hepatocellular Carcinoma. Nat. Rev. Clin. Oncol. 2014, 11, 525–535. [Google Scholar] [CrossRef]
- Hsin, I.-F.; Hsu, C.-Y.; Huang, H.-C.; Huang, Y.-H.; Lin, H.-C.; Lee, R.-C.; Chiang, J.-H.; Lee, F.-Y.; Huo, T.-I.; Lee, S.-D. Liver Failure after Transarterial Chemoembolization for Patients with Hepatocellular Carcinoma and Ascites: Incidence, Risk Factors, and Prognostic Prediction. J. Clin. Gastroenterol. 2011, 45, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef]
- Golfieri, R.; Bilbao, J.I.; Carpanese, L.; Cianni, R.; Gasparini, D.; Ezziddin, S.; Paprottka, P.M.; Fiore, F.; Cappelli, A.; Rodriguez, M.; et al. Comparison of the Survival and Tolerability of Radioembolization in Elderly vs. Younger Patients with Unresectable Hepatocellular Carcinoma. J. Hepatol. 2013, 59, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.-P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and Safety of Selective Internal Radiotherapy with Yttrium-90 Resin Microspheres Compared with Sorafenib in Locally Advanced and Inoperable Hepatocellular Carcinoma (SARAH): An Open-Label Randomised Controlled Phase 3 Trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.K.H.; Gandhi, M.; Tan, S.-B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients with Hepatocellular Carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Ricke, J.; Klümpen, H.J.; Amthauer, H.; Bargellini, I.; Bartenstein, P.; de Toni, E.N.; Gasbarrini, A.; Pech, M.; Peck-Radosavljevic, M.; Popovič, P.; et al. Impact of Combined Selective Internal Radiation Therapy and Sorafenib on Survival in Advanced Hepatocellular Carcinoma. J. Hepatol. 2019, 71, 1164–1174. [Google Scholar] [CrossRef]
- Reeves, H.L.; Reicher, J.; Priona, G.; Manas, D.M.; Littler, P. Selective Internal Radiation Therapy (SIRT) for Hepatocellular Carcinoma (HCC): Informing Clinical Practice for Multidisciplinary Teams in England. Frontline Gastroenterol. 2023, 14, 45–51. [Google Scholar] [CrossRef]
- Reig, M.; Darnell, A.; Forner, A.; Rimola, J.; Ayuso, C.; Bruix, J. Systemic Therapy for Hepatocellular Carcinoma: The Issue of Treatment Stage Migration and Registration of Progression Using the BCLC-Refined RECIST. Semin. Liver Dis. 2014, 34, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Leoni, S.; Piscaglia, F.; Serio, I.; Terzi, E.; Pettinari, I.; Croci, L.; Marinelli, S.; Benevento, F.; Golfieri, R.; Bolondi, L. Adherence to AASLD Guidelines for the Treatment of Hepatocellular Carcinoma in Clinical Practice: Experience of the Bologna Liver Oncology Group. Dig. Liver Dis. 2014, 46, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Raoul, J.-L.; Adhoute, X.; Penaranda, G.; Perrier, H.; Castellani, P.; Oules, V.; Bourlière, M. Sorafenib: Experience and Better Manage-Ment of Side Effects Improve Overall Survival in Hepatocellular Carcinoma Patients: A Real-Life Retrospective Analysis. Liver Cancer 2019, 8, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Tovoli, F.; Ielasi, L.; Casadei-Gardini, A.; Granito, A.; Foschi, F.G.; Rovesti, G.; Negrini, G.; Orsi, G.; Renzulli, M.; Piscaglia, F. Management of Adverse Events with Tailored Sorafenib Dosing Prolongs Survival of Hepatocellular Carcinoma Patients. J. Hepatol. 2019, 71, 1175–1183. [Google Scholar] [CrossRef]
- Miksad, R.A.; Ogasawara, S.; Xia, F.; Fellous, M.; Piscaglia, F. Liver Function Changes after Transarterial Chemoembolization in US Hepatocellular Carcinoma Patients: The LiverT Study. BMC Cancer 2019, 19, 795. [Google Scholar] [CrossRef]
- Hucke, F.; Sieghart, W.; Pinter, M.; Graziadei, I.; Vogel, W.; Müller, C.; Heinzl, H.; Waneck, F.; Trauner, M.; Peck-Radosavljevic, M. The ART-Strategy: Sequential Assessment of the ART Score Predicts Outcome of Patients with Hepatocellular Carcinoma Re-Treated with TACE. J. Hepatol. 2014, 60, 118–126. [Google Scholar] [CrossRef]
- Adhoute, X.; Penaranda, G.; Naude, S.; Raoul, J.L.; Perrier, H.; Bayle, O.; Monnet, O.; Beaurain, P.; Bazin, C.; Pol, B.; et al. Retreatment with TACE: The ABCR SCORE, an Aid to the Decision-Making Process. J. Hepatol. 2015, 62, 855–862. [Google Scholar] [CrossRef]
- D’Avola, D.; Granito, A.; de la Torre-Aláez, M.; Piscaglia, F. The Importance of Liver Functional Reserve in the Non-Surgical Treatment of Hepatocellular Carcinoma. J. Hepatol. 2022, 76, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- D’Avola, D.; Bilbao, J.I.; Sangro, B. Hepatocellular Carcinoma: Essentials Interventional Radiologists Need to Know. Cardiovasc. Interv. Radiol. 2019, 42, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van, D.T.; De, T.E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Merck and Eisai Provide Update on Phase 3 LEAP-002 Trial Evaluating KEYTRUDA® (Pembrolizumab) Plus LENVIMA® (Lenvatinib) versus LENVIMA Monotherapy in Patients with Unresectable Hepatocellular Carcinoma. Available online: https://www.merck.com/news/merck-and-eisai-provide-update-on-phase-3-leap-002-trial-evaluating-keytruda-pembrolizumab-plus-lenvima-lenvatinib-versus-lenvima-monotherapy-in-patients-with-unresectable-hepatocellul/ (accessed on 23 August 2022).
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Tovoli, F.; Dadduzio, V.; De Lorenzo, S.; Rimassa, L.; Masi, G.; Iavarone, M.; Marra, F.; Garajova, I.; Brizzi, M.P.; Daniele, B. Real-Life Clinical Data of Cabozantinib for Unresectable Hepatocellular Carcinoma. Liver Cancer 2021, 10, 370–379. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).