Abstract
Severe gastrointestinal symptoms (GIS) and food hypersensitivity are tightly associated in young individuals with autism spectrum disorders (ASD). Here, we explored the relationship of GIS (gastrointestinal severity index, ROMA IV criteria, Bristol scale), ASD-like behaviors (Childhood Autism Rating Scale), and certain sociodemographic/clinical traits (epidemiological survey) with serum immunoreactivity (IgG, IgA, IgE titers) towards bovine milk caseins (BMC; by ELISA) and subfractions (by immunoblotting) in thirty-one pediatric patients (~3–15 y, 77% male) with mild-to-severe GIS and ASD-like behaviors. In total, 42%, 25%, and 23% of all participants exhibited no (IgG−/IgA−), mono (IgG+/IgA−), or dual (IgG+/IgA+) immunoreactivity to BMC, respectively; the trend was significantly associated with the severity of the GIS and ASD-like behaviors, regurgitations, and self-reported allergies (OR: 1 → (1.9–3.1) → 13.5–16.0)]. No IgE+ response to BMC was found. Dual responders were α > κ > β-casein, though nonspecific reactivity to other protein fractions was also observed. The IgA+ > IgG+ but not IgE+ response to BMC (mainly α-casein) seems to be related to the severity of GIS and ASD-like behaviors, although a larger number of ASD patients are needed to draw a causal association.
1. Introduction
Food hypersensitivity and severe gastrointestinal symptoms (GIS) are highly prevalent in young people with autism spectrum disorder (ASD). The global prevalence of ASD is currently estimated at 98/10,000 inhabitants (95% CI, 81–118), and it is associated with a high rate (~49%, CI95% 44–54) of GIS, such as bloating, functional dyspepsia, constipation, diarrhea, abdominal pain and distension, vomiting, and gastroesophageal reflux [1,2,3]. In addition, conservative figures indicate that one third of ASD patients will eventually develop food protein hypersensitivity, with dairy/wheat proteins the most frequently reported [3,4]. The convergence of a reduced luminal proteolytic capacity with an increased intestinal permeability to food proteins in ASD, causes a lymphocytic proinflammatory response [Th1 (TNFα, IFNγ), Th2 (IL-3, IL-4, IL-5, IL-10, IL-13), TH17/Treg (IL-10, IL-17, TGFβ), B (↑ IL-6, TNF-α, ↓ IL-10)] at intestinal and systemic levels [5,6]. This proinflammatory response is often accompanied by a functional deficit in other cell-mediated responses of the innate immune system [7].
The aforementioned scenario ultimately leads to Type I (IgE+, Th2 → B-cell activation), II (IgG+/IgM+; Th1 → B-cell activation), IV (IgG+/IgM+ > IgA+), and mixed food hypersensitivities [8,9]. While type I hypersensitivity has been extensively studied in ASD-affected individuals, type II/IV food hypersensitivities such as protein-induced enterocolitis syndrome, celiac disease, and allergic proctocolitis, often go unrecognized because their GIS resemble those produced by food intolerances (non-immune) [8,9,10]. Although their pathophysiology is not completely known, they seem to dysregulate the activity of major histocompatibility (MHC) complex and Treg cells and increase the number of naïve CD4+/CD8+ cells and proinflammatory cytokines [9,10,11] and the stepwise fluctuation in mucosal immunoglobulins, all of them observed in ASD patients fed ad libitum or restricted in bovine milk (BM) and wheat proteins [12,13].
Bovine milk caseins (BMC; ~81% of all milk proteins) are a heterogeneous mixture of proteins αs1(~31%), αs2 (~8%), β (~31%), κ (~10%), γ (~1%)-casein (CN) from here onwards (wherever needed). Under normal physiological conditions, the in vitro/in vivo digestion of BMC renders small peptides (≤6 amino acids) very low immunogenic potential [14]. The “fragile gut” of children with ASD is not only characterized by a deterioration in the intestinal barrier but also poor intraluminal proteolytic activity; so, it is not surprising that there is a more pronounced immunogenic response to BMC in children with ASD compared to neurotypical children [13,15]. It is noteworthy that certain neurotrophic peptides derived from the incomplete digestion of BMC [e.g., β-casomorphin-7 (BCM-7; Y-P-F-P-G-P-I)] have been linked to ASD-like behaviors; yet, their immunogenic potential has not been studied in depth [16]. Moreover, to our knowledge, the immunogenic potential of BMC fractions other than β-CN in ASD patients has not been reported previously. Thus, the purpose of this study was to evaluate the specific IgA/IgG/IgE titers against whole/fractionated BMC in thirty-one subjects with diagnosed ASD, exploring their relationship with the severity of GIS and autism-like behaviors and some other ecological/clinical traits.
2. Materials and Methods
2.1. Reagents
Polyclonal goat anti-human IgG, IgA, and IgE conjugated with horseradish peroxidase (HRP) were purchased either from Sigma-Aldrich Inc. (St. Louis, MO, USA) or Abcam (Cambridge, CB2 OAX, UK). Bio-Rad’s Precision Plus Protein™ Standard (10–250 kDa) and polyvinylidene difluoride (PVDF) membrane (C1620174, 0.45 μm pore size) were obtained from Bio-Rad (Marnes-La-Coquette, France). Bovine serum albumin (BSA; A2934), α- (C6780, 90% pure) and β- (C6905, 90% pure) bovine milk caseins (BMC), 3,3′,5,5′-tetramethylbenzidine (TMB), 3,3-diaminobenzidine (DAB), polysorbate (TWEEN® 20), phenol red and analytical/molecular biology-grade salts [NaCl, NaHCO3, KH2PO4, sodium dodecyl sulfate (SDS)], and acids (H2SO4), were purchased from Sigma-Aldrich. Polyacrylamide (PA) was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
2.2. Study Design, Setting, and Participants
Thirty-one young ASD patients (3–15 years, 77% men) were included in this case-series analysis and were recruited from January 2023 to April 2024 in Ciudad Juarez, Chihuahua, Mexico (31°44′42″ N, 106°29′06″ W). All participants were previously diagnosed with ASD by certified health professionals (pediatric neurologists and clinical psychiatrists), based on the Diagnostic and Statistical Manual of Mental Illnesses (DSM-V-TR, 5th Ed.) criteria [13,17]. The exclusion criteria included being ≥18 years old, having genetic disorders other than ASD, acute immunocompromised conditions, and chronic gastrointestinal disorders (e.g., achalasia, inflammatory bowel disease, and celiac disease). Parents, caregivers, or legal guardians gave informed written consent for their child to participate. The study’s protocol was reviewed and approved by the Bioethics Committee of the Autonomous University of Ciudad Juarez (Authorizations: CIEB-2020-1-20, CEI-2022-2-743) and was conducted according to the Declaration of Helsinki and Mexican regulations for clinical studies and biological waste handling and disposal. This case series partially complies with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology; https://www.strobe-statement.org) (accessed on 28 April 2024) standards [18].
2.3. Survey Questionnaire
The survey was administered through an in-depth interview with parents or caregivers. This brought together various previously validated instruments and included sociodemographic information, perinatal health, clinical history, consumption of medications and dietary supplements, eating problems (behavioral and functional), SGI evaluated by the Bristol scale, ROME IV criteria, gastrointestinal severity index (GSI) [19], and ASD-like behaviors [Childhood Autism Rating Scale (CARS)] [20]; internal consistency (Cronbach α = 0.87) convergent validity with DSM-V (Rho = 0.62, kappa = 0.33)]. Unadjusted associations between the immunoreactive to BMC groups [IgG−/IgA− (none; reference), IgG+/IgA− (mono), and IgG+/IgA+ (dual)] were expressed as odds ratios with a 95% confidence interval (CI95%).
2.4. Biological Samples
Peripheral (antecubital vein) blood samples were collected after an overnight fast (8–12 h) in 10 mL-BD Vacutainer® SSTTM tubes, centrifuged at 3000× g for 10 min after clotting for 30 min. Sera was then aliquoted (1 mL) in Eppendorf tubes containing 1% v.v−1 NaN3 (1 M) and stored at −20 °C until use (within a 7-day timeframe).
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
A direct ELISA [21] was performed to evaluate individual IgA, IgG, and IgE titers against BMC. Briefly, high-binding 96-well flat bottom microplates were coated with 100 μL of a recently prepared BMC mix [α-CN (Sigma C6780) + β-CN (Sigma C6905), 1:1 w.w−1] solution (5 µg.mL−1) prepared in coating buffer (0.1 M NaHCO3, pH 9.8, 1% phenol red) and left overnight. The next day, unbounded proteins were washed-out five times with TWEEN® 20 (0.2% v.v−1) + phosphate saline buffer (PBST; 15 mM KH2PO4, 0.15 M NaCl, 0.2%, 1% phenol red, pH 7.5), blocked (100 μL per well) with BSA in phosphate saline buffer (1%, w.v−1) for 2 h at 20 °C, washed-out five times with PBST, and incubated with serum samples (primary antibody) at ranging concentrations (1:40–10, 240) for 2 h at 20 °C. After, the microplates were washed-out with PBST, the HRP-conjugated secondary antibody [goat anti-human, IgG, IgA or IgE, diluted 1:2000 dilution] was added to each cell (100 μL) and incubated for 2 h at 20 °C, and the unbound conjugates were then washed-out with PBST, as explained above. The reaction was developed with TMB for 5 min, stopped with 1 M H2SO4, and the absorbance read at 450 nm in a microplate reader (SPECTROstar Omega, BMG LABTECH, Ortenberg, Germany). Negative (no plasma sample, no BMC) and positive (adult hypersensitized to bovine milk) controls were included on each plate. Lastly, scattergrams were constructed with the raw absorbance values from cohort subjects (n = 31) and the positive control (n = 1) and later used to discriminate non-reactive [control mean absorbance (CMA) for IgA and IgG) from reactive [IgG+ (CMA + 3 standard deviations, SD) IgA+ or IgE+ (CMA + 2.5 SD)] subjects, following the recommendations from Vojdani et al. (2014) [22].
2.6. Immunoblotting
After separating all the protein subfractions (BMC mix) and pre-stained molecular weight marker (10–250 kDa) using Laemmli’s SDS-PAGE [23], under the following conditions: 15% (w.v−1) polyacrylamide small gel, 65 V/30 min–90 V/120 min] in a vertical Bio-Rad Mini-Protean® III cell, they were electro-transferred (conditions: semidry blotting, 1.2 mA cm2, 80 mA, 1.5 h) to PVDF membranes (0.45 μm) using a blotting system (Thermo ScientificTM OwlTM HEP-1 series). The blotted membranes were firstly activated (absolute methanol, 30 seg) then blocked with TBST [0.05 M Tris, 0.15 M NaCl, 2% Tween-20 (pH 7.42)] for two minutes}, then with less-detergent (Tween-20, 0.05% v.v−1) TBST, and further cut into smaller vertical strips (1 line per strip). The immunoreactivity of each BMC fraction was further unveiled using immunoblotting [24] as follows: each strip was incubated overnight at 4 °C with each serum sample (primary antibody, 1:50 v.v−1 in TBST), washed-out (five times) with TBST, incubated with secondary antibodies [Goat anti-human (IgG or IgA or IgE)-HRP; 1:1000 in TBST], and washed-out again; the HRP reaction was started with DAB, stopped with phosphate saline buffer, washed with distilled water, and dried on a filter paper.
2.7. Statistical Analysis
Categorical and continuous variables were expressed as proportions (%) and mean ± standard deviation (X ± SD), after being tested for normality (Shapiro–Wilk test). Inter-group comparisons [reference (IgG−/IgA−), mono (IgG+/IgA−), or dual (IgG+/IgA+) immunoreactivity groups] for binary and/or multivariate response variables were evaluated using the Kruskal–Wallis test with Dunn’s Multiple comparison tests or the Chi-square test (Pearson uncorrected). Non-adjusted multivariate logistic regression analysis [odds ratio (OR, CI95%)] was used for predictive variables associated with immunoreactivity status [single or dual vs. reference group (no BMC reactants)]. Statistical analyses were performed using NCSS software (vs. 2023, LLC. Kaysville, UT, USA; https://www.ncss.com/software/ncss/), and statistical significance was considered when p < 0.05.
3. Results
3.1. Hypersensitivity to Bovine Milk Caseins (BMC Mix) and Associated Determinants
Thirty-one young (8.6 ± 5.9 y, 77% male) participants diagnosed with ASD (mild–severe) in the last 3–4 years, with moderate access (69–82%) access to health services (Table 1), participated in this study. In total, 42% (n = 13), 25% (n = 11), and 23% (n = 7) of all participants exhibited no (IgG−/IgA−), mono (IgG+/IgA−), or dual (IgG+/IgA+) immunoreactivity to BMC (Table 1; Figure S1), though none of them exhibited type 1 hypersensitivity (IgE+).

Table 1.
Characteristics of participants stratified by level of immunoreactivity to BMC 1−3.
Sociodemographic and clinical determinants associated with incremental immunoreactivity (none → mono → dual) to BMC included self-reported regurgitations, food allergies (Table 1), and severe GIS and ASD-like symptoms, as documented with both GSI [19] and CARS questionnaires [20], respectively (Figure 1). On the other hand, non-trending, yet statistically significant, determinants were self-perceived good digestive health (marginal significance, p = 0.047), postnatal diarrhea, and polypharmacy (>three medications a day; Table 1), while non-statistical differences (p > 0.05) between groups were documented for other determinants, such as perinatal/obstetric health status, current clinical history, postnatal lactation practices and current eating behaviors, Bristol scale, and GIS other than postnatal diarrhea and regurgitations (Tables S1 and S2).
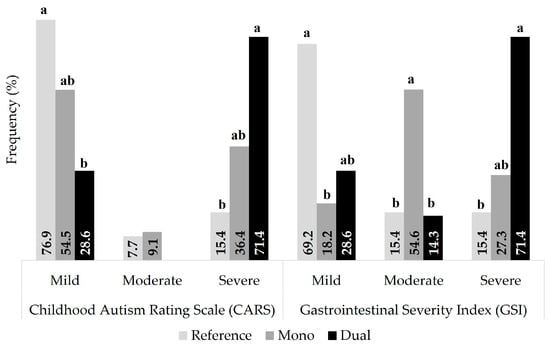
Figure 1.
Frequency of gastrointestinal symptoms and ASD-like behaviors stratified by immunoreactivity to BMC. Different superscript letters within the same severity level, documented using CARS [20] and GSI [19] questionnaires, mean significant differences (p < 0.05).
Multiple regression analysis subsequently showed that most statistically significant determinants (reported in Table 1) were accentuated particularly in the dual (IgG+/IgA+) immunoreactive group (Table 2).

Table 2.
Multivariate logistic regression analysis of clinical determinants associated with the level of immunoreactivity to BMC 1,2.
Severe CARS and GSI scores and the presence (self-reported) of regurgitations and food allergy were ~12.5–15 times more frequent [OR 13.5–16.0] in the dual group (IgG+/IgA+) than in the reference group (IgG−/IgA−), though the parents or caregivers reported (anecdotally) that the participant did not suffer from diarrhea in the postnatal stage [OR (CI95%) = 0.1 (0–1.1)]. Interestingly, the proportion of subjects with polypharmacy (>three prescribed medications) was mono> dual> reference groups, but the OR for all other parameters reported in Table 2 were not significantly different (p > 0.05) between the reference and mono-reactive (IgG+/IgA−) groups.
3.2. Differential Recognition of BMC Protein Fractions by IgG+/IgA+ Subjects (Dual Group)
Laemmli’s SDS-PAGE of BMC protein fractions (left) and their immunorecognition by the dual group (serum IgG+/IgA+, n = 6; right) are shown in Figure 2. Bands corresponding to αS1, αS2, β, and κ-CN were observed at ~32, ~30, ~29, and ~27 kDa, and two additional bands >65 kDa were tentatively identified as milk immunoglobulin (Ig) and BSA. In total, 83, 33, and 67% of all six participants were IgA+ to α-casein (CN), κ-CN and BSA, while 67%, 33%, 33%, and 33% were IgG+ to α-CN, κ-CN, BSA, and Ig, respectively.
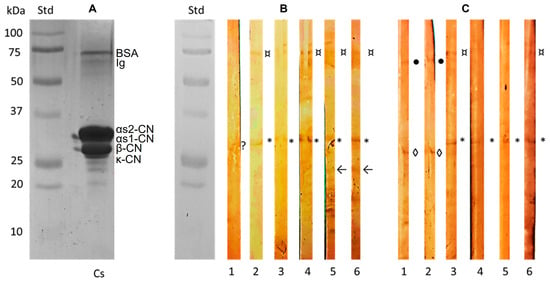
Figure 2.
Bovine milk caseins (BMC) subfractions and their immunodetection in participants with dual immunoreactivity (IgG+/IgA+) to BMC. Laemmli’s SDS-PAGE of mixed BMC (A) and corresponding IgA (B) or IgG (C) immunoblots identifying αS1/S2 (*), β (◊), κ (arrow) caseins (CN), milk immunoglobulin (Ig, ●), bovine serum albumin (BSA, ¤), negative signal (?).
4. Discussion
ASD comprises complex and lifelong neurodevelopmental disorders primarily mediated by the central nervous system (CNS). However, the enteric nervous system (ENS) is wired with the CNS in the so-called gut–brain axis, where both intestinal microbiota and mucosa-associated lymphoid tissue (MALT) are major components of the now called gut–brain connectome [25,26]. A wide range of immunological and microstructural dearrangements in the gut are causally linked with ASD-like behaviors, severe GIS, and increased immunoreactivity (innate/adaptative) to food components, particularly dietary proteins [8,9,10,11,12,13]. The evidence suggests that the chronicity of GIS accentuates the severity of ASD-like behaviors, such as irritability and hyperactivity [25,27,28]. In addition, certain cross-sectional studies suggest that food > respiratory > skin allergies are much more prevalent in children with ASD, compared to neurotypical children [1,3,29,30]. Although the overall burden of gastrointestinal disorders and food allergies in the ASD population is not known with certainty, parents with NT children suffering from multiple food allergies report that allergy to bovine milk is the one with the highest financial, social, and emotional burden [31]. However, the extent to which specific protein fractions from bovine milk (e.g., BMC) exert their immunogenic potential and how this correlates with both the prevalence and number of GIS in ASD has not been sufficiently explored [22,27]. In this study, using a stepwise immunochemical assessment, we report that the basal immunogenic status (not assessed by oral challenge with dietary proteins) of most participants, resembles a mixed or type IV (IgG+/IgM+ > IgA+) rather than type I (IgE-mediated) food hypersensitivity that was strongly associated with the severity of GIS and ASD-like behaviors in a progressive immunogenic manner [IgG−/IgA− (reference) < IgG+/IgA− (mono) < IgG−/IgA− (dual)].
In the non-probabilistic sample (case-series) of ASD patients reported here, a high rate (58%) of type IV [IgG+ with (23%) or without (25%) IgA+) but not type I hypersensitivity (IgE-mediated) to BMC was documented. Most research on food hypersensitivity in ASD individuals have been focused on type I hypersensitivity [1,3,8,11,29,30], as non-IgE-mediated hypersensitivities (and their associated GIS) often go unnoticed not only by caregivers but even by clinical pediatricians [8,9,29]. For instance, a cow’s milk protein allergy is probably the most common type IV food hypersensitivity in ASD children affected by food protein-induced enterocolitis syndrome (FPIES; known as “autistic enterocolitis”) [32,33]. Although the clinical diagnosis (including serologic testing) of both types of hypersensitivities is feasible in neurotypical children with sufficient verbal communication capacity, in those with ASD, it can be challenging and, therefore, could be underestimated, particularly type IV hypersensitivities [9,10,33], such as those detected here. Lastly, the dual humoral response (IgA+/IgG+) against BMC observed in 23% of all ASD patients studied here, suggests a strong MALT activation in the Lamina propia due to an increased mucosal permeability (known as leaky gut) [34,35]. MALT is the body’s first immunological barrier, and its deficient humoral response (also known as primary humoral immunodeficiency) leads to severe neurobehavioral disorders including ASD, psychotic disorders, and suicidal behaviors [36].
Severe GIS and ASD-like symptoms and frequent regurgitations and food allergies followed a quasi-incremental immunoreactive trend [reference (IgG−/IgA−) → mono (IgG+/IgA−) → dual (IgG+/IgA+)]; other distal determinants, such as self-perceived good digestive health, postnatal diarrhea, and polypharmacy, did not follow this trend. Non-IgE-mediated food hypersensitivities typically manifest as moderate-to-severe GIS, including profuse emesis (here possibly interpreted by the caregiver as regurgitation) but could also manifest as contact dermatitis, dermatitis herpetiformis, pulmonary hemosiderosis, failure to thrive, and even chronic malnutrition in severe cases [8]. Currently, there are few studies exploring the relationship between type IV hypersensitivities and GIS in children with ASD.
- El-Alameey et al. [37] found that forty children (6–12 y, 60% males) with ASD (62.5% moderate–severe) and severe GIS had a significantly higher immunoreactivity to BMC than neurotypical children, a fact associated with food selectivity, recurrent abdominal pain, and reduced serum dipeptidyl peptidase-IV (DPP-IV) activity, an enzyme that, together with milk-derived proline/glutamine-rich opioid peptides, plays a decisive role in the pathophysiology of ASD [38,39].
- Anatolyevna et al. [27] observed that ~80% and ~19% of fifty-one Russian children with ASD were IgG+ to BMC and wheat gliadins, respectively, but recognized the need to clarify the nature of immunoreactivity or intolerance when choosing optimal diet therapies.
- de Magistris et al. [13] showed that non-celiac ASD children fed unrestricted diets (milk and wheat consumers; n = 129) exhibited a higher apparent intestinal permeability [lactulose/mannitol test; OR = 14.8 (IC95% 2.0–111.6)], partially associated with a mild IgE+ but not IgA+ nor IgG+ immunoreactivity to BMC than those observed in neurotypical children (n = 44); the authors concluded that such discrepancies between the observed low immunoreactivity (for bovine milk but not wheat proteins) [22] and the high intestinal permeability could be related to “different pathways of intestinal damage” apparently associated with a high ASD clinical heterogeneity.
Although the logistic regression reported here provides partial evidence of a progressive and concurrent worsening in ASD-like behaviors and GIS with a quasi-incremental immunoreactive trend [reference (IgG−/IgA−) → mono (IgG+/IgA−) → dual (IgG+/IgA+)], the clinical trajectory of the sensitization could be different in each individual due to multiple factors, including a differential humoral response to different BMC protein subfractions. For instance, BCM-7 (derived from A1 variant of β-CN) binds to μ-opioid receptors located along the gut, the immune system, and CNS, but its threshold of exposure has not been established, and thus its dose-dependent neurotrophic action may vary among ASD individuals [17]. A complete overview on the metabolic fate and physiological consequences of BMC-derived peptides in both neurotypical and ASD children has been recently reported by Bjørklund et al. [29].
The non-IgE mediated immunogenic potential of BMC fractions other than β-CN in ASD patients, has rarely been reported. Here, SDS-PAGE [23] and immunoblotting [24] of the BMC mix [α-CN (Sigma C6780) + β-CN (Sigma C6905), >90% pure] revealed quite interesting yet unexpected results:
- First, the frequency of immunoreactivity toward BMC subfractions by IgG+/IgA+-BMC-sensitized individuals was αS2-CN> αS1-CN> κ-CN, β-CN> other minor fractions (e.g., BSA/Ig). Research on the specific immunoreactivity to β-CN and its harmful peptide (BMC-7) has been perhaps the most studied, such that its dietary elimination has allowed the differentiation of A1/A2 milk (β-CN variants), which differs in the presence of BMC-7 [40]. In addition, αS1/αS1-CN play a major role in Type 1 hypersensitivity to bovine milk (BM) in an age-specific manner [41]. However, enzymatically digested raw BM may also unveil T-cell but not IgE-reactive epitopes that may induce long-term immune tolerance (possibly IgG-mediated) in subjects with cow’s milk allergy [42].
- Second, although it might be obvious that the relative density of protein subfractions in the BMC mix used here (αS1-CN, αS2-CN > β-CN > κ-CN, BSA > Ig; Figure 2) differs from their natural occurrence in raw bovine milk (αS1-CN, αS2-CN > β-CN > κ-CN, β-lactoglobulin > α-lactalbumin > BSA > lactoferrin/Ig) [43], the presence of additional BSA and IgG bands (>50 kDa) and other minor proteins (<24 kDa) was evident. This issue has been reported by others when using the same pure standards, either alone or mixed [44,45,46].
- Third, the BSA immunoreactivity observed in patient-specific immunoblots could be due to the ELISA assay, given that its binding to the microplate (ELISA) or to PVDF membrane strips could be strong (e.g., covalent forces), though the BSA concentration in the BMC mix is considerably low compared to α, β-CN. Furthermore, the Ag-Ab reaction with BSA from the blocking solution (ELISA assay) is negligible since, under these conditions, BSA exhibits quite low capacity to adhere to the microplate (weak bounding forces) or to create stable conjugates that can be eliminated in the washing stages [47,48].
The evidence presented here indicates that the severity of GIS and ASD-like behaviors seems to be associated with a selective humoral response to BMC in susceptible young Mexicans living with ASD. Such sensitization seemed to be type IV rather than type I hypersensitivity, mainly targeting αS2-CN; several factors (alone or in combination) could be playing a role in this fact, including the following: (A) the natural switching to a long-term adaptative humoral response (mediated by IgA and IgG but not IgE), (B) that immunoreactivity was tested passively (basal serum titers) and not actively (e.g., after protein oral challenge), or (C) that the true allergenic response to bovine milk proteins was probably to non-casein proteins. Lastly, various parameters reported as predictors of systemic immunogenicity (Tables S1 and S2) could have reached statistical significance if the number of patients analyzed was higher and, preferably, sorted into paired groups. The inclusion of clinical tests demonstrating loss of intestinal integrity (high permeability to dietary antigens) and concurrent inflammatory processes could have helped to establish a stronger causal association between BMC immunoreactivity and the severity of gastrointestinal symptoms and aberrant behaviors.
5. Conclusions
IgA+ > IgG+ but not IgE+ response to BMC (mainly α-casein) seems to be related to the severity of GIS and ASD-like behaviors, although a larger number of ASD patients are needed to disentangle such the direction of causality of this phenomenon (worsening of ASD/GIS symptoms ↔ humoral response/immunoreactivity grading).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/gastroent15030039/s1: Figure S1: Scattergrams (absorbance at 450 nm) to identify subjects (n = 31) with IgA and IgG immunoreactivity to bovine milk caseins; Table S1: Early-life events in participants with ASD stratified by immunoreactivity to BMC; Table S2: Eating behaviors and gastrointestinal disturbances in ASD participants stratified by immunoreactivity to BMC.
Author Contributions
Conceptualization, Á.F.V.-Z. and A.W.-M.; methodology, Á.F.V.-Z., A.W.-M., and J.A.L.-D.; validation, A.W.-M., J.A.L.-D., and R.C.-V.; formal analysis, Á.F.V.-Z. and A.W.-M.; investigation, Á.F.V.-Z. and R.C.-V.; resources, A.W.-M. and J.A.L.-D.; data curation, A.W.-M. and J.A.L.-D.; writing—original draft preparation, Á.F.V.-Z. and R.C.-V.; writing—review and editing, Á.F.V.-Z. and A.W.-M.; visualization, Á.F.V.-Z. and A.W.-M.; supervision, J.A.L.-D. and R.C.-V.; project administration, A.W.-M.; funding acquisition, J.A.L.-D. and A.W.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki (DoH; https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/, accessed on 1 March 2023), and approved by the Institutional Bioethics Committee of the Autonomous University of Ciudad Juarez (Authorization CIEB-2020-1-20).
Informed Consent Statement
Informed consent was obtained from all anonymized subjects involved in the study.
Data Availability Statement
All surveyed/clinical data used and/or analyzed while conducting the study are included in both the manuscript and Supplementary Materials. Other participants’ data are unavailable due to privacy or ethical restrictions.
Acknowledgments
A.F.V.Z. wishes to thank the National Council of Humanities, Sciences, and Technologies (CONAHCyT) for the granted doctoral scholarship, and all authors are indebted to the Autonomous University of Ciudad Juarez (UACJ, Department of Chemical-Biological Sciences) for the institutional support received.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, H.; Liu, H.; Chen, X.; Zhang, J.; Tong, G.; Sun, Y. Association of food hypersensitivity in children with the risk of autism spectrum disorder: A meta-analysis. Eur. J. Pediatr. 2021, 180, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, B.; Wang, J.; Zhang, Z.; Chen, O. Global prevalence of autism spectrum disorder and its gastrointestinal symptoms: A systematic review and meta-analysis. Front. Psychiatry 2022, 13, 963102. [Google Scholar] [CrossRef]
- Xu, G.; Snetselaar, L.G.; Jing, J.; Liu, B.; Strathearn, L.; Bao, W. Association of food allergy and other allergic conditions with autism spectrum disorder in children. JAMA Netw. Open. 2018, 1, e180279. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Zoheir, K.M.A.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Al-Ayadhi, L.Y.; Alzahrani, M.Z.; Al-Shabanah, O.A.; Al-Harbi, M.M.; Attia, S.M. Dysregulation of Th1, Th2, Th17, and T regulatory cell-related transcription factor signaling in children with autism. Mol. Neurobiol. 2017, 54, 4390–4400. [Google Scholar] [CrossRef] [PubMed]
- Moaaz, M.; Youssry, S.; Elfatatry, A.; Abd El Rahman, M. Th17/Treg cells imbalance and their related cytokines (IL-17, IL-10 and TGF-β) in children with autism spectrum disorder. J. Neuroinmunol. 2019, 337, 577071. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Al-Ayadhi, L.Y.; Sarawi, W.; Attia, S.M.; Bakheet, S.A.; Alqarni, S.A.; Ali, N.; AsSobeai, H.M. Imbalance in pro-inflammatory and anti-inflammatory cytokines milieu in B cells of children with autism. Mol. Immunol. 2022, 141, 297–304. [Google Scholar] [CrossRef] [PubMed]
- TTheoharides, T.C.; Angelidou, A.; Alysandratos, K.-D.; Zhang, B.; Asadi, S.; Francis, K.; Toniato, E.; Kalogeromitros, D. Mast cell activation and autism. Biochim. Biophys. Acta 2012, 1822, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Al-Iede, M.; Sarhan, L.; Alshrouf, M.A.; Sai, Y. Perspectives on non-IgE-mediated gastrointestinal food allergy in pediatrics: A review of current evidence and guidelines. J. Asthma Allergy 2023, 16, 279–291. [Google Scholar] [CrossRef]
- Connors, L.; O’Keefe, A.; Rosenfield, L.; Kim, H. Non-IgE-mediated food hypersensitivity. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. S2), 56. [Google Scholar] [CrossRef]
- Ozen, A.; Gulcan, E.M.; Ercan Saricoban, H.; Ozkan, F.; Cengizlier, R. Food protein-induced non-immunoglobulin E-mediated allergic colitis in infants and older Children: What cytokines are involved? Int. Arch. Allergy Immunol. 2015, 168, 61–68. [Google Scholar] [CrossRef]
- Tedner, S.G.; Asarnoj, A.; Thulin, H.; Westman, M.; Konradsen, J.R.; Nilsson, C. Food allergy and hypersensitivity reactions in children and adults—A review. J. Intern. Med. 2022, 291, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Erbescu, A.; Papuc, S.M.; Budisteanu, M.; Arghir, A.; Neagu, M. Re-emerging concepts of immune dysregulation in autism spectrum disorders. Front. Psychiatr. 2022, 13, 1006612. [Google Scholar] [CrossRef]
- de Magistris, L.; Picardi, A.; Siniscalco, D.; Riccio, M.P.; Sapone, A.; Cariello, R.; Abbadessa, S.; Medici, N.; Lammers, K.M.; Schiraldi, C.; et al. Antibodies against food antigens in patients with autistic spectrum disorders. BioMed Res. Int. 2013, 1, 729349. [Google Scholar] [CrossRef]
- Kopf-Bolanz, K.A.; Schwander, F.; Gijs, M.; Vergères, G.; Portmann, R.; Egger, L. Validation of an in vitro digestive system for studying macronutrient decomposition in humans. J. Nutr. 2012, 142, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Sanctuary, M.R.; Kain, J.N.; Angkustsiri, K.; German, J.B. Dietary considerations in autism spectrum disorders: The potential role of protein digestion and microbial putrefaction in the gut-brain axis. Front. Nutr. 2018, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcelos, M.L.; Oliveira, L.M.F.; Hill, J.P.; Vidal, A.M.C. Difficulties in establishing the adverse effects of β-Casomorphin-7 released from β-Casein variants—A review. Foods 2023, 12, 3151. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM-V.: Washington, DC, USA, 2013. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Thulasi, V.; Steer, R.A.; Monteiro, I.M.; Ming, X. Overall severities of gastrointestinal symptoms in pediatric outpatients with and without autism spectrum disorder. Autism 2019, 23, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Flores-Rodríguez, Y.; Ceballos, O.R.; Albores-Gallo, L. Assessing autism with DSM-IV and DSM-5 criteria using the childhood autism rating scale (CARS). Salud Ment. 2022, 45, 3–10. [Google Scholar] [CrossRef]
- Hornbeck, P.V. Enzyme-linked immunosorbent assays. Curr. Prot. Immunol. 2015, 110, 2.1.1–2.1.23. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D.; Mukherjee, P.S. The prevalence of antibodies against wheat and milk proteins in blood donors and their contribution to neuroimmune reactivities. Nutrients 2014, 6, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Xu, P.; Gallagher, S. Immunoblotting and immunodetection. Curr. Prot. Cell Biol. 2017, 74, 6.2.1–6.2.37. [Google Scholar] [CrossRef]
- Hung, L.Y.; Margolis, K.G. Autism spectrum disorders and the gastrointestinal tract: Insights into mechanisms and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 142–163. [Google Scholar] [CrossRef]
- Masanetz, R.K.; Winkler, J.; Winner, B.; Günther, C.; Süß, P. The gut–immune–brain axis: An important route for neuropsychiatric morbidity in inflammatory bowel disease. Int. J. Mol. Sci. 2022, 23, 11111. [Google Scholar] [CrossRef]
- Anatolyevna, B.I.; Ivanovich, P.V.; Alekseevich, Z.A.; Vadimovich, B.D. Frequency of detection of markers of casein and gluten intolerance in children with autism spectrum disorders. Vopr. Pitan. (Nutr. Issues) 2019, 88, 41–47. [Google Scholar] [CrossRef]
- Leader, G.; Abberton, C.; Cunningham, S.; Gilmartin, K.; Grudzien, M.; Higgins, E.; Joshi, L.; Whelan, S.; Mannion, A. Gastrointestinal symptoms in autism spectrum disorder: A systematic review. Nutrients 2022, 14, 1471. [Google Scholar] [CrossRef]
- Thompson, K.; Wallisch, A.; Nowell, S.; Meredith, J.; Boyd, B. The role of oral hypersensitivity in feeding behaviors of young autistic children. Autism 2023, 27, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, W.; Yao, H.; Zheng, R.; Chen, W.; Zhang, W. Association between autism spectrum disorder and food allergy: A systematic review and meta-analysis. Autism Res. 2021, 14, 220–230. [Google Scholar] [CrossRef]
- Abrams, E.M.; Kim, H.; Gerdts, J.; Protudjer, J.L.P. Milk allergy most burdensome in multi-food allergic children. Pediatr. Allergy Immunol. 2020, 31, 827–834. [Google Scholar] [CrossRef]
- Martin-Martinez, B. Management of CMPA in infancy: Current approaches and future perspective. J. Immunol. Res. Innov. 2023, 1, 1–7. [Google Scholar] [CrossRef]
- Wasilewska, J.; Klukowski, M. Gastrointestinal symptoms and autism spectrum disorder: Links and risks—A possible new overlap syndrome. Ped. Health Med. Ther. 2015, 6, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Jarmołowska, B.; Bukało, M.; Fiedorowicz, E.; Cieślińska, A.; Kordulewska, N.K.; Moszyńska, M.; Świątecki, A.; Kostyra, E. Role of milk-derived opioid peptides and proline dipeptidyl peptidase-4 in autism spectrum disorders. Nutrients 2019, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Vita, A.A.; Zwickey, H.; Bradley, R. Associations between food-specific IgG antibodies and intestinal permeability biomarkers. Front. Nutr. 2022, 9, 962093. [Google Scholar] [CrossRef] [PubMed]
- Isung, J.; Williams, K.; Isomura, K.; Gromark, C.; Hesselmark, E.; Lichtenstein, P.; Larsson, H.; de la Cruz, L.F.; Sidorchuk, A.; Mataix-Cols, D. Association of primary humoral immunodeficiencies with psychiatric disorders and suicidal behavior and the role of autoimmune diseases. JAMA Psychiatry 2020, 77, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- El-Alameey, I.R.; Ahmed, H.H.; Eid, I.M.; El-Dory, G.H.A.D.A.; Gameel, M.A.N.A.L. Relationship of serum dipeptidyl peptidase-iv activity and anti-casein antibodies to gastrointestinal symptoms among children with autism spectrum disorder: An Egyptian study. Asian J. Pharm. Clin. Res. 2018, 11, 370–374. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Role of the intestinal immune system in health. In Crohn’s Disease and Ulcerative Colitis; Baumgart, D., Ed.; Springer: Cham, Switzerland, 2017; pp. 23–56. [Google Scholar] [CrossRef]
- Bjørklund, G.; Meguid, N.A.; Hemimi, M.; Sahakyan, E.; Fereshetyan, K.; Yenkoyan, K. The role of dietary peptides gluten and casein in the development of autism spectrum disorder: Biochemical perspectives. Mol. Neurobiol. 2024, 1–12. [Google Scholar] [CrossRef]
- Borş, A.; Borş, S.I.; Floriștean, V.C. Health-related outcomes and molecular methods for the characterization of A1 and A2 cow’s milk: Review and update. Vet. Sci. 2024, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Archila, L.D.; Khan, F.S.; Bhatnagar, N.; Robinson, D.; Farrington, M.L.; Kwok, W.W. αS1-Casein elucidate major T-cell responses in cow’s milk allergy. J. Allergy Clin. Immunol. 2017, 140, 854–857. [Google Scholar] [CrossRef]
- Huang, M.; Tan, H.; Xiong, Z.; Hu, W.; Wu, Y.; Meng, X.; Chen, H.; Li, X. Allergenicity evaluation of peptides from milk and yogurt after gastrointestinal digestion based on epitopes. Food Funct. 2022, 13, 10769–10789. [Google Scholar] [CrossRef]
- Costa, F.F.; Brito, M.A.V.P.; Furtado, M.A.M.; Martins, M.F.; de Oliveira, M.A.L.; de Castro Barra, P.M.; Garrido, L.A.; dos Santos, A.S.d.O. Microfluidic chip electrophoresis investigation of major milk proteins: Study of buffer effects and quantitative approaching. Anal. Methods 2014, 6, 1666–1673. [Google Scholar] [CrossRef]
- Verhegghe, M.; Rasschaert, G.; Herman, L.; Goossens, K.; Vandaele, L.; De Bleecker, K.; Vlaemynck, G.; Heyndrickx, M.; De Block, J. Reduction of Mycobacterium avium ssp. paratuberculosis in colostrum: Development and validation of 2 methods, one based on curdling and one based on centrifugation. J. Dairy Sci. 2017, 100, 3497–3512. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Auty, M.A.; Crowley, S.V.; Kelly, A.L.; O’Mahony, J.A.; Brodkorb, A. Self-association of bovine β-casein as influenced by calcium chloride, buffer type and temperature. Food Hydrocoll. 2019, 88, 190–198. [Google Scholar] [CrossRef]
- Loi, M.; Quintieri, L.; Fanelli, F.; Caputo, L.; Mulè, G. Application of a recombinant laccase-chlorogenic acid system in protein crosslink and antioxidant properties of the curd. Food Res. Int. 2018, 106, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, R.; Bariar, S.; Balakrishnan, A.; Nahar, P. BSA blocking in enzyme-linked immunosorbent assays is a non-mandatory step: A perspective study on mechanism of BSA blocking in common ELISA protocols. RSC Adv. 2015, 5, 100077–100083. [Google Scholar] [CrossRef]
- Xiao, Y.; Isaacs, S.N. Enzyme-linked immunosorbent assay (ELISA) and blocking with bovine serum albumin (BSA)—Not all BSAs are alike. J. Immunol. Methods 2012, 384, 148–151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).