Abstract
Depression is a common mental disorder with high economic burden, characterized by high disability and mortality rates. The etiology of depression remains unclear to date, and there are various hypotheses regarding the pathogenesis of depression in clinical practice, including the monoamine neurotransmitter hypothesis, the hypothalamic–pituitary–adrenal (HPA) axis dysregulation hypothesis, the inflammatory cytokine hypothesis, and the neurotrophic factor hypothesis. These theories offer specific directional aid in the clinical management of individuals suffering from depression. Medicinal intervention stands as a critical approach within the spectrum of depression treatments, and this article reviews the specific mechanisms of different hypotheses on the pathogenesis of depression in recent years, as well as the research progress on related therapeutic drugs.
1. Introduction
Depression is characterized by persistent sadness, sleep/appetite disturbances, anhedonia, and social anxiety. Severe cases involve delusions or suicidal ideation [1]. Post-COVID-19, global anxiety/depression prevalence rose by 27.6% [2]. Over 350 million people are affected worldwide; China’s incidence is 6%, affecting ~95 million. By 2030, depression is projected to be the leading global disease burden [3]. It increases comorbidity risk (e.g., hypertension, diabetes, CVD) and mortality [4,5], impacting public health and productivity (e.g., ~6 lost work hours/week, higher unemployment) [6,7].
Despite neuropsychiatric advances, the precise pathogenesis remains elusive [8]. Current research implicates monoaminergic dysfunction [9], HPA axis impairment [10], reduced brain derived neurotrophic factor (BDNF) [11], altered neuroplasticity [12], inflammation [13], and gut microbiome imbalance [14]. Treatment response typically takes ≥4 weeks, often accompanied by side effects (sexual dysfunction, GI issues, anxiety) [15]. Investigating mechanisms and developing novel, effective, well-tolerated antidepressants is crucial. This review analyzes depression pathogenesis hypotheses and summarizes current therapeutics. Therefore, it is essential to further investigate the underlying mechanisms of depression and explore novel medications that exhibit exceptional efficacy, excellent tolerability, minimum side reactions, and broad applicability. This review aims to provide a comprehensive analysis of the various hypotheses explaining the causes of depression. Additionally, we present a summary of the existing medications that have demonstrated therapeutic effects on depression. The objective is to offer references for the early detection, prevention, and advancement of new antidepressant treatments.
2. Methods of Literature Search
2.1. Search Strategy
A systematic literature search was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The search encompassed electronic databases including Web of Science, PubMed and Embase from 1 January 2010 to 1 June 2025. Key search terms and their combinations included: Depression pathogenesis; Monoamine hypothesis; HPA axis; Neuroinflammation; Oxidative stress; Neuroplasticity; Gut–brain axis; Antidepressants; Herbal antidepressants; Novel therapies. Boolean operators (AND/OR) were used to refine the search. Additional articles were identified through manual screening of reference lists from relevant reviews and primary studies.
Boolean Search String:
(“Depression” OR “Major Depressive Disorder” OR “Depressive Disorder”) AND (“Pathogenesis” OR “Etiology” OR “Mechanism” OR “Monoamine Hypothesis” OR “HPA axis” OR “Hypothalamic–Pituitary–Adrenal Axis” OR “Neuroinflammation” OR “Inflammatory Cytokines” OR “Oxidative Stress” OR “Neuroplasticity” OR “Neurotrophic Factors” OR “BDNF” OR “Gut–Brain Axis” OR “Gut Microbiota”) AND (“Drug Therapy” OR “Antidepressants” OR “Pharmacotherapy” OR “Monoamine Oxidase Inhibitors” OR “Tricyclic Antidepressants” OR “Selective Serotonin Reuptake Inhibitors” OR “Serotonin-Norepinephrine Reuptake Inhibitors” OR “Atypical Antidepressants” OR “Herbal Medicine” OR “Novel Therapies”) NOT (“Animals” OR “Animal Models” NOT “Humans”).
2.2. Inclusion and Exclusion Criteria
Inclusion criteria: Original research articles; Studies focusing on depression pathogenesis mechanisms or drug therapies; Articles published in English. Exclusion criteria: Case reports, non-peer-reviewed publications, and conference abstracts; Studies on comorbidities not directly related to depression; Articles with insufficient methodological detail or irrelevance to core themes; Articles with insufficient methodological detail or irrelevance to core themes.
2.3. Study Selection Process
All identified records underwent a three-stage screening process: Initial screening: Titles and abstracts were assessed for relevance. Full-text review: Potentially eligible articles were evaluated for methodological rigor and alignment with review objectives. Final inclusion: Data from selected studies were synthesized into thematic sections (pathogenesis hypotheses, drug therapies). The screening workflow is summarized in the PRISMA flowchart below (Figure 1).
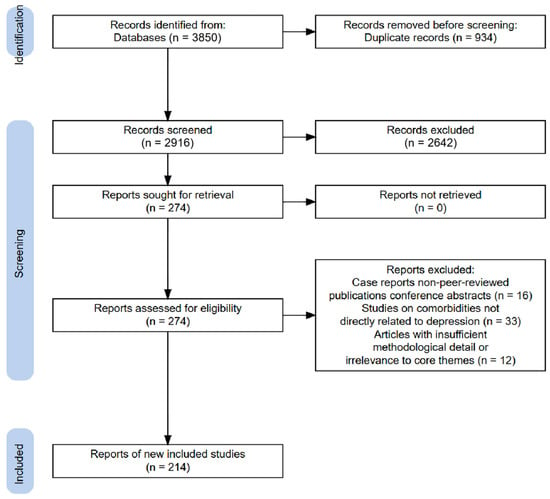
Figure 1.
PRISMA flow diagram of the included studies (created de novo).
2.4. Data Extraction, Synthesis and Quality Assessment
Data from included studies were extracted using a standardized template, capturing: Study characteristics, pathophysiological mechanisms, treatment outcomes and risk of bias indicators (randomization, blinding, attrition). Findings were thematically organized into sections addressing pathogenesis hypotheses (Section 3) and therapeutic strategies (Section 4).
To ensure methodological rigor and minimize bias, a structured quality assessment was performed using standardized tools tailored to study design: Randomized Controlled Trials (RCTs) were evaluated using the *Cochrane Risk-of-Bias Tool, assessing randomization processes, deviations from intended interventions, missing outcome data and outcome measurement; Observational Studies underwent assessment via the Newcastle-Ottawa Scale (NOS); Mechanistic Studies were scrutinized using a “custom 9-item checklist” evaluating critical parameters including: Pathway-specific modulation approaches, Dose–response relationships, Reproducibility metrics and Appropriateness of statistical methods.
3. Pathogenesis of Depression
3.1. Hypothesis of Monoamine Neurotransmitters
Proposed in 1972 [16], this hypothesis links depression to reduced availability/dysfunction of monoamines (Norepinephrine (NE/NA), Serotonin (5-HT), Dopamine (DA)) in the synaptic cleft, disrupting mood/cognitive signaling [17,18]. NE modulates prefrontal function (attention/behavior) [19]; 5-HT regulates pain/neuroendocrine functions; low levels cause anxiety [20]; DA affects motor/reward systems [21]. Enzymes governing monoamine synthesis/metabolism/transport are key. Monoamine oxidase (MAO) degrades monoamines, reducing transmission [22]. Stress impairs tryptophan hydroxylase (TPH) function, lowering brain 5-HT [23]. Dysfunctional neurotransmitter transporters are implicated [24,25,26,27]. Receptor abnormalities (e.g., reduced 5-HT receptors [28], presynaptic α2-adrenoceptor hypersensitivity [29]) also contribute and offer treatment targets [30] (Figure 2).
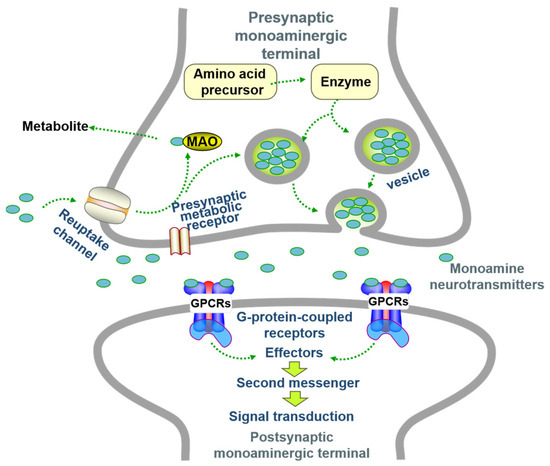
Figure 2.
The steps of monoamine neurotransmitter transmission at the synapse (adapted from the reference [26]).
3.2. Impairment of the HPA Axis
The HPA axis regulates stress responses. Stress triggers hypothalamic corticotropin-releasing hormone (CRH) release, stimulating pituitary adrenocorticotropic hormone (ACTH), leading to adrenal glucocorticoids (GCs) secretion [31]. Chronic stress causes HPA dysfunction in 40–60% of patients, featuring hypercortisolemia, impaired GC feedback, and receptor signaling defects [32,33] (Figure 3). Depressed patients show elevated cerebrospinal fluid CRH and reduced frontal CRH receptors [34,35,36]. GCs are vital for stress response and homeostasis [37]. Chronic stress reduces GR activity, suppresses feedback, and alters behavior [28]. Elevated cortisol in depression causes neuronal degeneration (e.g., hippocampus, Prefrontal cortex) [38].
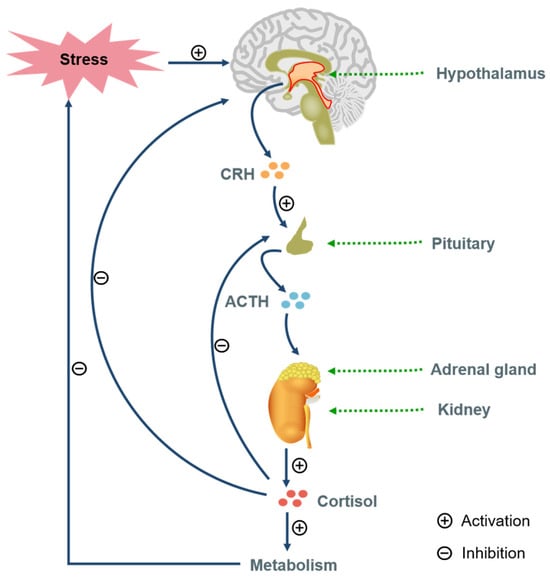
Figure 3.
The regulation of stress response by the hypothalamic–pituitary–adrenal axis (adapted from Reference [35]).
3.3. The Role of Inflammatory Cytokines and Oxidative Stress
Inflammation and oxidative stress are key contributors to depression pathophysiology. Pro-inflammatory cytokines (e.g., IL-6, TNF-α, IL-1β) dominate over anti-inflammatory counterparts, disrupting neuroendocrine/immune responses [39,40,41]. These cytokines activate pathways like indoleamine 2,3-dioxygenase (IDO), diverting tryptophan metabolism from serotonin synthesis toward neurotoxic kynurenine metabolites [42,43,44,45,46,47,48]. NLRP3 inflammasome activation promotes Caspase-1-mediated IL-1β/IL-18 release, exacerbating neuroinflammation [49,50,51,52,53]. TLR4 signaling (triggered by endogenous ligands like HMGB1) amplifies cytokine release via NF-κB [54,55,56,57] (Figure 4).
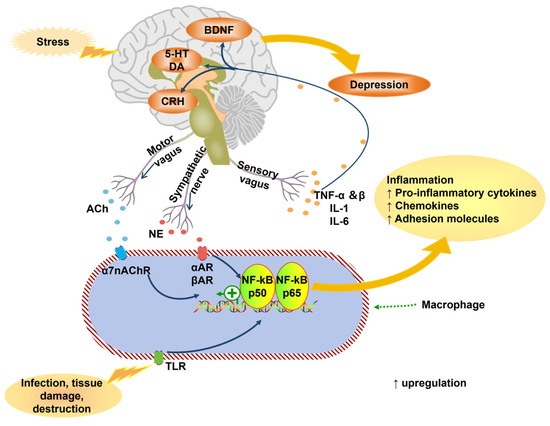
Figure 4.
The connection between inflammatory cytokines and depression (adapted from the reference [41]). ACh, acetylcholine; TNF, tumor necrosis factor; IL, Interleukin; nAChR, nicotinic acetylcholine receptor; AR, adrenergic receptor; NF-kB, nuclear factor-kB; TLR, toll-like receptors.
Oxidative stress interacts bidirectionally with inflammation. Reactive oxygen species (ROS) activate inflammatory pathways (NF-κB, MAPK) and cause mitochondrial dysfunction, impairing energy metabolism and promoting neuronal apoptosis [58,59,60]. Depressed patients show elevated oxidative markers (MDA, 8-OHdG) and reduced antioxidants (SOD, catalase, glutathione) [61,62]. This vicious cycle contributes to neuronal dysfunction and treatment resistance, suggesting antioxidants may augment antidepressant efficacy [63].
3.4. Hypothesis of Neuroplasticity and Neurotrophic
3.4.1. Neuroplasticity Hypothesis
Neuroplasticity encompasses the brain’s structural and functional adaptability in response to experience, stress, and disease, crucial for neuronal development and morphology [64]. In depression, impaired neuroplasticity disrupts neural circuits governing emotion, cognition, and stress response [65]. Depression involves reduced hippocampal volume/neurogenesis, correlating with symptom severity [66]. Synaptic plasticity (LTP/LTD) affects neurotransmitter function [19]. Stress (via HPA axis), neuroinflammation, oxidative stress, and GABAergic dysfunction impair neuroplasticity, forming a complex basis for depression [67,68,69]. This hypothesis underscores neuroplasticity’s central role in depression pathophysiology and its potential as a therapeutic target.
3.4.2. Neurotrophic Hypothesis
BDNF is central, supporting neuron survival, learning, memory, and mood via TrkB receptor signaling (MAPK, PLCγ, PI3K pathways) [70,71,72] (Figure 5). proBDNF (binding p75NTR) promotes apoptosis; mBDNF (binding TrkB) enhances plasticity and is neuroprotective. Reduced BDNF impairs hippocampal neurogenesis/synaptic plasticity, mood regulation, and stress coping [73,74,75,76,77,78]. Antidepressants increase BDNF expression [79,80,81], making it a potential biomarker.
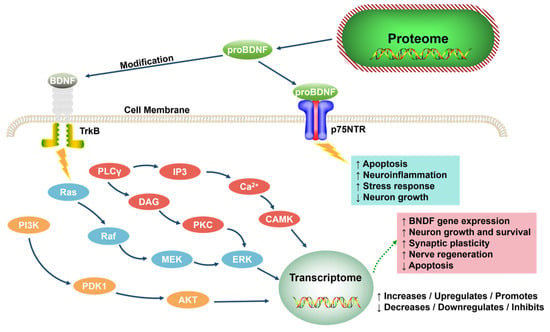
Figure 5.
The role of neurotrophic factors in the function of the nervous system (adapted from the reference [72]). proBDNF, the precursor form of BDNF; TrkB, tropomyosin receptor kinase B; NTR, neurotrophin receptor; PLCγ, phospholipase Cγ; IP3, inositol 1,4,5-trisphosphate; DAG, diacylglycerol; PKC, protein kinase C; CAMK, calcium/calmodulin-dependent protein kinase; Ras, rat sarcoma; Raf, rapidly accelerated fibrosarcoma; MEK, MAPK/ERK kinase; ERK, extracellular signal-regulated kinase; PI3K, phosphoinositide 3-kinase; PDK1, phosphoinositide-dependent kinase 1; AKT, serine/threonine-protein kinase B.
In summary, BDNF plays multiple roles in the pathogenesis of depression, including influencing neurogenesis, synaptic plasticity, neuroprotection, emotional regulation, and the ability to cope with stress. Therefore, BDNF is not only crucial for understanding the pathophysiology of depression but may also be a key target for the development of new therapeutic strategies.
3.5. Dysfunction of Gut Microbiota
The gut microbiota, essential for health, is often dysregulated in depression, showing reduced diversity and shifts towards pro-inflammatory bacterial profiles [82,83,84,85,86]. Fecal transplants from patients induce depressive-like behaviors in rodents [87]. The microbiota-gut–brain (MGB) axis communicates via autonomic, immune, and endocrine pathways [88,89] (Figure 6).
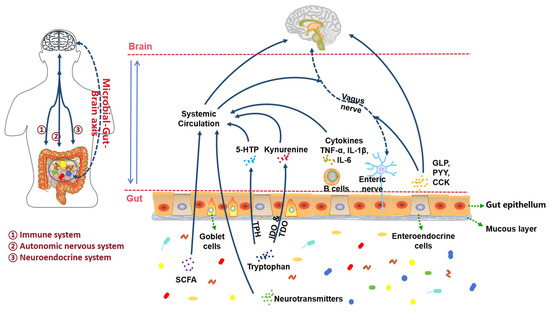
Figure 6.
The pathways of microbiota-gut–brain axis (adapted from the reference [89]). SCFA, short chain fatty acid; TPH, tryptophan hydroxylase; IDO, indoleamine 2,3-dioxygenase; TDO, tryptophan 2,3-dioxygenase; 5-HTP, 5-hydroxytryptophan; TNF, tumor necrosis factor; IL, interleukin; GLP, glucagon-like peptide; PYY, peptide YY; CCK, cholecystokinin.
3.5.1. Autonomic Nervous System Pathway
Gut microbiota metabolites (short-chain fatty acids (SCFAs), neurotransmitters, amino acid derivatives) signal via the enteric nervous system and vagus nerve to the central nervous system (CNS) [89,90,91]. Microbiota regulate central processes including neurogenesis, neuronal activity, and glial function [92]. Microbes regulate SCFA production, enhancing colonic 5-HT synthesis via TPH1 in Enterochromaffin Cells [23,93,94]. Stress-induced Lactobacillus reduction lowers IDO inhibition, increasing kynurenine and depressive behaviors [95,96]. SCFAs modulate ENS activity and microglial maturation [97,98,99]; exogenous SCFAs improve depression-related parameters [100,101].
3.5.2. Immune System Pathways
Increased gut permeability allows bacterial translocation (e.g., Lipopolysaccharide LPS), activating immune cells and microglia, causing neuroinflammation and blood–brain barrier (BBB) damage [102,103]. Probiotics (e.g., B. infantis CCFM687, L. reuteri NK33, B. adolescentis NK98) reverse HPA overactivation and inflammation [104,105,106]. Stress increases permeability and bacterial translocation linked to MAPK p38/Nrf2 pathways [107,108]. LPS also activates IDO, reducing 5-HT [109,110,111,112].
3.5.3. Endocrine System Pathways
Stress alters microbiota, affecting HPA axis function [89,113]. Early-life/maternal stress increases cortisol and pro-inflammatory cytokines, dysregulating HPA feedback [114,115]. Depressive rats show microbiota changes and increased hypothalamic CRH, mirroring CRH injection effects [116]. Gut hormones (e.g., Peptide YY, PYY) act as signaling molecules between microbiota and brain [117,118,119].
Despite extensive research and various hypotheses proposed in recent years, the etiology and pathogenesis of depression are still not fully understood. However, researchers from different perspectives raise questions that are inevitably interconnected and complementary to each other, which will help in better understanding depression.
4. Drug Therapy for Depression
Treatment depends on severity. Mild cases may use psychotherapy/lifestyle changes [120]. Moderate-severe depression requires pharmacotherapy, evolving from monoamine oxidase inhibitors/tricyclic antidepressants (MAOIs/TCAs) to selective serotonin reuptake inhibitors (SSRIs), serotonin and NE reuptake inhibitors (SNRIs), and atypicals. These pharmaceutical compounds exert their effects through a variety of mechanisms, from modulating monoaminergic neurotransmission to targeting neurotrophic signaling cascades [121]. Herbal medicines show promise via multi-target actions [122,123].
4.1. Monoamine Oxidase Inhibitor and Tricyclic Antidepressants
In the 1950s, the first generation of antidepressants, including MAOIs and tricyclic antidepressants, emerged. These drugs work by inhibiting monoamine oxidase activity and blocking the reuptake of 5-HT and NE, respectively, thereby increasing their concentrations in the synaptic cleft, which clinically results in the improvement of depressive symptoms [124,125].
MAOIs (e.g., Phenelzine, Isocarboxazid) inhibit monoamine oxidase, increasing synaptic monoamine concentrations (e.g., 5-HT, DA) to alleviate depression [126]. They show efficacy in atypical depression and some treatment-resistant cases [127]. However, MAOIs require strict dietary tyramine avoidance due to hypertensive crisis risk and cause side effects (dizziness, insomnia) [128]. A recent study demonstrated the efficacy of MAOIs in treatment-resistant depression (TRD) unresponsive to multiple antidepressant classes, including SSRIs, SNRIs, and atypical antidepressants. Patients treated with either phenelzine or tranylcypromine for 6 months exhibited significant symptomatic improvement, manifested as enhanced mood, sleep quality, cognitive function, and social engagement. Concurrently, substantial reductions exceeding 58% were observed in both Hamilton Depression Rating Scale (HDRS) and Beck Depression Inventory (BDI) scores. Mild adverse effects—including insomnia, headaches, and orthostatic hypotension—were reported in all subjects, though these remained manageable with supportive care [129].
TCAs (e.g., Amitriptyline, Imipramine) primarily inhibit 5-HT/NE reuptake, elevating synaptic neurotransmitter levels [130]. They are cost-effective and prevent relapse but cause significant anticholinergic effects (dry mouth, constipation, blurred vision), cardiotoxicity, and cognitive impairment [124,125,131]. Tertiary amine TCAs (e.g., amitriptyline) exhibit greater toxicity than secondary amines, with overdose mortality risks (e.g., dothiepin: 53.3 deaths/million prescriptions) [132].
4.2. Selective Serotonin Reuptake Inhibitors
SSRIs (e.g., Fluoxetine, Sertraline) block presynaptic 5-HT reuptake, increasing synaptic serotonin and promoting neuroplasticity [133]. They are first-line for mild-moderate depression due to better safety and tolerability vs. TCAs [134]. Common limitations include sexual dysfunction (e.g., reduced libido) [135] and withdrawal symptoms (dizziness, dysesthesia) upon discontinuation. Additionally, abrupt cessation of SSRIs can result in withdrawal symptoms such as dizziness and dysesthesia. A meta-analysis of 14 studies (n = 4459) reported a pooled incidence of antidepressant withdrawal symptoms at 53.6% (95% CI: 43.2–63.7%), with the highest incidence for paroxetine (78.2%; 95% CI: 65.1–87.2%) and lowest for fluoxetine (43.5%; 95% CI: 31.2–56.7%) [136]. Since different patients may respond differently to various SSRIs, their use should be under the guidance of a professional doctor.
4.3. Serotonin-Norepinephrine Reuptake Inhibitors
SNRIs (e.g., Venlafaxine, Duloxetine) inhibit both 5-HT and NE reuptake, offering broader efficacy than SSRIs and potentially faster onset for some symptoms [137,138]. They minimally inhibit cytochrome P450 but risk serotonin syndrome with MAOIs [139]. Adverse effects include hypertension (13.1% (95% CI: 9.8–17.3%) at >300 mg/day venlafaxine), nausea (41.2%, (95% CI: 34.5–48.3%)), urinary dysfunction (10.4% (95% CI: 7.1–15.0%) with milnacipran), and hyperhidrosis [140,141]. Withdrawal syndromes occur, especially with short-half-life agents [142].
4.4. Atypical Antidepressants
This heterogeneous class acts via diverse mechanisms (e.g., 5-HT receptor modulation, NE/DA enhancement) distinct from conventional antidepressants [143]. Some agents (e.g., Bupropion, Agomelatine) exhibit rapid onset, fewer anticholinergic effects than TCAs, and efficacy beyond depression (e.g., anxiety, sleep improvement) [144,145]. However, they may cause sedation, weight gain, or activation [146]. Trazodone improves sleep but may increase suicide risk in insomnia [147]; agomelatine commonly causes headache (10.3% (n = 339, single-study data without CI)) and rhinitis (6.7%) [148]. Withdrawal reactions remain a concern [149].
4.5. Herbal and Emerging Agents
Traditional herbal medicines and early-stage compounds offer multi-target mechanisms but require further validation. Key examples are summarized in Table 1.

Table 1.
Overview of antidepressants (classical and emerging agents).
4.6. Novel Drug Treatment Strategies
(R,S)-Ketamine, an NMDA receptor antagonist, rapidly alleviates depressive symptoms via its metabolite (2R,6R)-hydroxynorketamine, which activates AMPA receptors [163,164]. Its S-(+)-isomer (esketamine) is approved for treatment-resistant depression (TRD) and acute suicidality [165]. AXS-05 (dextromethorphan/bupropion), targeting NMDA, showed clinical efficacy in 2023 [166]. In addition, another NMDARs also showed meaningful antidepressant activity in clinical phase III trials [167]. Ketamine’s GABA interneuron inhibition is crucial for its effects [165], and GABAA targeting agents (brexanolone, zuranolone) are FDA-approved [166].
Psilocybin, a 5-HT2A receptor agonist, received FDA “breakthrough therapy” designation for TRD [168]. It enhances glutamate release and promotes long-term neural plasticity in cortico-limbic circuits [172], spurring research into 5-HT2A agonists [170]. Cariprazine (D2/D3 partial agonist), initially approved for schizophrenia/bipolar disorder, shows adjunctive potential for bipolar depression and TRD [171,172,173]. In addition to the above drugs, recent clinical studies have also focused on novel antidepressants, such as Ezogabine (a KCNQ channel-acting drug) [174] and LY341495 (an mGlu2/3 receptor antagonist) [175]. Exploration of highly active compounds continues to drive novel target discovery [176]. Therefore, in-depth exploration of highly active antidepressants and systematic elucidation of their mechanisms of action are key directions for advancing antidepressant drug development.
5. Limitations and Future Directions
5.1. Critical Appraisal
Despite advances in understanding depression pathogenesis, significant limitations persist in translating findings to clinical practice: (1) Heterogeneity in biomarker data (e.g., cytokines, oxidative stress markers, microbiome profiles) due to methodological variations, clinical diversity (depression subtypes, comorbidities), and demographic confounders, limits diagnostic utility; (2) Preclinical success of IDO inhibitors has not translated to human trials due to compensatory pathway activation (e.g., TDO upregulation), poor blood–brain barrier penetration, and lack of validated biomarkers for patient stratification; (3) Microbiome findings exhibit poor reproducibility across studies, influenced by geography, diet, sequencing methods, and medication confounders; human causality remains correlational despite suggestive fecal transplant data; (4) Target engagement evidence for novel agents (e.g., NLRP3 inhibitors, kynurenine modulators) is predominantly derived from animal models, with limited human mechanistic validation. Table 2 synthesizes key evidentiary gaps and quality assessments using the GRADE framework.

Table 2.
Critical appraisal of evidence on depression pathogenesis and treatment (GRADE: High [H], Moderate [M], Low [L], Very Low [VL]; CI: Confidence Interval).
5.2. Future Priorities
Define depression endotypes via multi-omics. Develop brain-penetrant IDO inhibitors with predictive biomarkers. Establish standardized microbiome protocols. Implement longitudinal biomarker studies during treatment.
6. Conclusions
The pathogenesis of depression is complex, involving multiple factors such as biochemistry, environment, and psychosocial aspects. To date, it has not been possible to isolate a single pathological process that determines the development and progression of the disease. With in-depth research, various hypotheses about the pathogenesis of depression have been supported, and people have begun to recognize that depression is the result of the combined effects of multiple factors, including the neuroendocrine, immune system, and gut microbiota. However, each pathogenic mechanism still needs to be further clarified. At present, treatment methods for depression include psychotherapy, pharmacotherapy, electroconvulsive therapy, and other approaches, and it is usually necessary to consider the patient’s specific symptoms, the severity of the condition, and potential drug interactions when choosing the treatment method. For patients with mild to moderate depression, the most common treatment method is pharmacotherapy. While traditional antidepressants (such as TCAs, MAOIs, and SSRIs) remain dominant in clinical treatment, their adverse effects, issues of drug resistance, and significant withdrawal reactions continue to pose significant challenges in clinical application. Over the past decade, global enthusiasm for the development of new antidepressant drugs has continued to grow, with a large number of candidate molecules targeting novel targets emerging, and their clinical trial data demonstrating breakthrough therapeutic potential. Meanwhile, traditional herbal medicines, due to their multi-component synergistic mechanisms of action (such as salidroside regulating the HPA axis and psilocybin/psilocin regulating 5-HT2A), are increasingly gaining attention in the field of depression treatment, offering new directions for developing low-side-effect, multi-pathway intervention strategies.
Although traditional medicine has provided various treatment options for depression, we still need to achieve greater breakthroughs in combating this disease. Notably, in-depth research into existing highly active antidepressants has brought many new drugs (e.g., (R, S)-Ketamine and psilocybin). Traditional herbal medicines are increasingly gaining attention due to their unique mechanisms of action and lower side effects. Systematically exploring their active components and mechanisms of action not only holds promise for providing new options for pharmacological treatment of depression but may also reveal entirely new therapeutic targets. Additionally, in-depth analysis of the molecular mechanisms of depression, development of more rigorous clinical trials collectively constitutes the key pathways to advancing depression treatment toward a more scientific and effective direction.
Author Contributions
M.X.: Writing—original draft. Z.Z. (Zhiyu Zhang): Writing—original draft. Z.Z. (Zhoudong Zhang): Writing—original draft. Y.S.: Writing—review and editing. D.L.: searched and analyzed the literature. W.W.: assisted with manuscript revision and production of figures. T.S.: Writing—review and editing, Funding acquisition. H.L.: designed and supervised this manuscript. C.T.: Writing—review and editing, Funding acquisition. H.Y.: Writing—review and editing. B.Y.: searched and analyzed the literature. Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the financial support by the Program for National Key Research and Development Program of China (Grant No. 2024YFA0917800); Program of National Natural Science Foundation of China (Grant No. 22208155).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The all authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results. Author C.T. was employed by the company Jiangsu Institute of Industrial Biotechnology. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Chen, S.; Wang, K.; Wang, H.; Gao, Y.; Nie, K.; Jiang, X.; Su, H.; Tang, Y.; Lu, F.; Dong, H.; et al. The therapeutic effects of saikosaponins on depression through the modulation of neuroplasticity: From molecular mechanisms to potential clinical applications. Pharmacol. Res. 2024, 201, 107090. [Google Scholar] [CrossRef]
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Wang, H.; Liu, Z.; Yu, X.; Yan, J.; Yu, Y.; Kou, C.; Xu, X.; Lu, J.; et al. Prevalence of mental disorders in China: A cross-sectional epidemiological study. Lancet Psychiatry 2019, 6, 211–224. [Google Scholar] [CrossRef]
- Walker, J.; Hansen, C.H.; Martin, P.; Symeonides, S.; Ramessur, R.; Murray, G.; Sharpe, M. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: A cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry 2014, 1, 343–350. [Google Scholar] [CrossRef]
- Scott, K.M.; Bruffaerts, R.; Tsang, A.; Ormel, J.; Alonso, J.; Angermeyer, M.C.; Benjet, C.; Bromet, E.; de Girolamo, G.; de Graaf, R.; et al. Depression–anxiety relationships with chronic physical conditions: Results from the World Mental Health surveys. J. Affect. Disord. 2007, 103, 113–120. [Google Scholar] [CrossRef]
- Stewart, W.F.; Ricci, J.A.; Chee, E.; Hahn, S.R.; Morganstein, D. Cost of Lost Productive Work Time Among US Workers With Depression. JAMA 2003, 289, 3135. [Google Scholar] [CrossRef]
- Lerner, D.; Adler, D.A.; Chang, H.; Lapitsky, L.; Hood, M.Y.; Perissinotto, C.; Reed, J.; McLaughlin, T.J.; Berndt, E.R.; Rogers, W.H. Unemployment, Job Retention, and Productivity Loss Among Employees With Depression. Psychiatr. Serv. 2004, 55, 1371–1378. [Google Scholar] [CrossRef]
- Wen, J.; Fu, C.H.Y.; Tosun, D.; Veturi, Y.; Yang, Z.; Abdulkadir, A.; Mamourian, E.; Srinivasan, D.; Skampardoni, I.; Singh, A.; et al. Characterizing Heterogeneity in Neuroimaging, Cognition, Clinical Symptoms, and Genetics Among Patients With Late-Life Depression. JAMA Psychiatry 2022, 79, 464–474. [Google Scholar] [CrossRef]
- Maletic, V.; Eramo, A.; Gwin, K.; Offord, S.J.; Duffy, R.A. The Role of Norepinephrine and Its α-Adrenergic Receptors in the Pathophysiology and Treatment of Major Depressive Disorder and Schizophrenia: A Systematic Review. Front. Psychiatry 2017, 8, 42. [Google Scholar] [CrossRef]
- Juruena, M.F.; Bocharova, M.; Agustini, B.; Young, A.H. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. J. Affect. Disord. 2018, 233, 45–67. [Google Scholar] [CrossRef]
- Lapolla, T.; Saltiel, P.F.; Silvershein, D. Major depressive disorder: Mechanism-based prescribing for personalized medicine. Neuropsychiatr. Dis. Treat. 2015, 11, 875–888. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef]
- Feng, X.; Ma, X.; Li, J.; Zhou, Q.; Liu, Y.; Song, J.; Liu, J.; Situ, Q.; Wang, L.; Zhang, J.; et al. Inflammatory Pathogenesis of Post-stroke Depression. Aging Dis. 2024, 16, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Martínez, S.; Segura-Real, L.; Gómez-García, A.P.; Tesoro-Cruz, E.; Constantino-Jonapa, L.A.; Amedei, A.; Aguirre-García, M.M. Neuroinflammation, Microbiota-Gut-Brain Axis, and Depression: The Vicious Circle. J. Integr. Neurosci. 2023, 22, 65. [Google Scholar] [CrossRef]
- Marwaha, S.; Palmer, E.; Suppes, T.; Cons, E.; Young, A.H.; Upthegrove, R. Novel and emerging treatments for major depression. Lancet 2023, 401, 141–153. [Google Scholar] [CrossRef]
- Schildkraut, J.J.; Draskoczy, P.R.; Gershon, E.S.; Reich, P.; Grab, E.L. Catecholamine metabolism in affective disorders —IV. Preliminary studies of norepinephrine metabolism in depressed patients treated with amitriptyline. J. Psychiatr. Res. 1972, 9, 173–185. [Google Scholar] [CrossRef]
- Nemeroff, C.B. The Role of Corticotropin-Releasing Factor in the Pathogenesis of Major Depression. Pharmacopsychiatry 1988, 21, 76–82. [Google Scholar] [CrossRef]
- Lesch, K.P.; Wolozin, B.L.; Murphy, D.L.; Riederer, P. Primary Structure of the Human Platelet Serotonin Uptake Site: Identity with the Brain Serotonin Transporter. J. Neurochem. 1993, 60, 2319–2322. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.W.; Ball, S.G.; Martinez, J.; Robinson, M.J.; Yang, C.R.; Russell, J.M.; Shekhar, A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress. Anxiety 2010, 27, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Ma, L.; Wang, Y.-G.; Ye, F.; Wang, C.; Zhou, W.-H.; Zhao, X. Genistein, a dietary soy isoflavone, exerts antidepressant-like effects in mice: Involvement of serotonergic system. Neurochem. Int. 2017, 108, 426–435. [Google Scholar] [CrossRef]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Basic psychopharmacology of antidepressants, part 1: Antidepressants have seven distinct mechanisms of action. J. Clin. Psychiatry 1998, 59, 5–14. [Google Scholar]
- Chen, Y.; Xu, H.; Zhu, M.; Liu, K.; Lin, B.; Luo, R.; Chen, C.; Li, M. Stress inhibits tryptophan hydroxylase expression in a rat model of depression. Oncotarget 2017, 8, 63247. [Google Scholar] [CrossRef]
- Ayala-Lopez, N.; Watts, S.W. Physiology and Pharmacology of Neurotransmitter Transporters. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2021; pp. 2279–2295. [Google Scholar] [CrossRef]
- Bröer, S.; Gether, U. The solute carrier 6 family of transporters. Br. J. Pharmacol. 2012, 167, 256–278. [Google Scholar] [CrossRef] [PubMed]
- Brigitta, B. Pathophysiology of depression and mechanisms of treatment. Dialog- Clin. Neurosci. 2002, 4, 7–20. [Google Scholar] [CrossRef]
- Ananth, M.R.; DeLorenzo, C.; Yang, J.; Mann, J.J.; Parsey, R.V. Decreased Pretreatment Amygdalae Serotonin Transporter Binding in Unipolar Depression Remitters: A Prospective PET Study. J. Nucl. Med. 2018, 59, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Boas, G.R.V.; de Lacerda, R.B.; Paes, M.M.; Gubert, P.; Almeida, W.L.d.C.; Rescia, V.C.; de Carvalho, P.M.G.; de Carvalho, A.A.V.; Oesterreich, S.A. Molecular aspects of depression: A review from neurobiology to treatment. Eur. J. Pharmacol. 2019, 851, 99–121. [Google Scholar] [CrossRef]
- Kaufman, J.; DeLorenzo, C.; Choudhury, S.; Parsey, R.V. The 5-HT1A receptor in Major Depressive Disorder. Eur. Neuropsychopharmacol. 2016, 26, 397–410. [Google Scholar] [CrossRef]
- Jacobsen, J.P.; Krystal, A.D.; Krishnan, K.R.R.; Caron, M.G. Adjunctive 5-Hydroxytryptophan Slow-Release for Treatment-Resistant Depression: Clinical and Preclinical Rationale. Trends Pharmacol. Sci. 2016, 37, 933–944. [Google Scholar] [CrossRef]
- Du, X.; Pang, T.Y. Is Dysregulation of the HPA-Axis a Core Pathophysiology Mediating Co-Morbid Depression in Neurodegenerative Diseases? Front. Psychiatry 2015, 6, 32. [Google Scholar] [CrossRef]
- Holsboer, F. The Corticosteroid Receptor Hypothesis of Depression. Neuropsychopharmacology 2000, 23, 477–501. [Google Scholar] [CrossRef]
- Juruena, M.F. Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav. 2014, 38, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Cullinan, W.E. Neurocircuitry of stress: Central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci. 1997, 20, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, J.J.; Inda, C.; Refojo, D.; Holsboer, F.; Arzt, E.; Silberstein, S. The Corticotropin-Releasing Hormone Network and the Hypothalamic-Pituitary-Adrenal Axis: Molecular and Cellular Mechanisms Involved. Neuroendocrinology 2011, 94, 12–20. [Google Scholar] [CrossRef]
- Austin, M.C.; Janosky, J.E.; Murphy, H.A. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol. Psychiatry 2003, 8, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Nikkheslat, N.; Pariante, C.M.; Zunszain, P.A. Neuroendocrine Abnormalities in Major Depression: An Insight Into Glucocorticoids, Cytokines, and the Kynurenine Pathway. In Inflammation and Immunity in Depression; Academic Press: Cambridge, MA, USA, 2018; pp. 45–60. [Google Scholar] [CrossRef]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef]
- Bouças, A.P.; Rheinheimer, J.; Lagopoulos, J. Why Severe COVID-19 Patients Are at Greater Risk of Developing Depression: A Molecular Perspective. Neurosci. 2020, 28, 11–19. [Google Scholar] [CrossRef]
- Machado, M.O.; Oriolo, G.; Bortolato, B.; Köhler, C.A.; Maes, M.; Solmi, M.; Grande, I.; Martín-Santos, R.; Vieta, E.; Carvalho, A.F. Biological mechanisms of depression following treatment with interferon for chronic hepatitis C: A critical systematic review. J. Affect. Disord. 2017, 209, 235–245. [Google Scholar] [CrossRef]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef]
- Han, K.-M.; Ham, B.-J. How Inflammation Affects the Brain in Depression: A Review of Functional and Structural MRI Studies. J. Clin. Neurol. 2021, 17, 503–515. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Klabnik, J.; Donnell, J.O. Novel Therapeutic Targets in Depression and Anxiety: Antioxidants as a Candidate Treatment. Curr. Neuropharmacol. 2014, 12, 108–119. [Google Scholar] [CrossRef]
- Palta, P.; Samuel, L.J.; Miller, E.R.; Szanton, S.L. Depression and Oxidative Stress. Psychosom. Med. 2014, 76, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Tobe, E.H. Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr. Dis. Treat. 2013, 9, 567–573. [Google Scholar] [CrossRef]
- Chen, L.-M.; Bao, C.-H.; Wu, Y.; Liang, S.-H.; Wang, D.; Wu, L.-Y.; Huang, Y.; Liu, H.-R.; Wu, H.-G. Tryptophan-kynurenine metabolism: A link between the gut and brain for depression in inflammatory bowel disease. J. Neuroinflammation 2021, 18, 135. [Google Scholar] [CrossRef]
- Maes, M.; Leonard, B.; Myint, A.; Kubera, M.; Verkerk, R. The new ‘5-HT’ hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chen, L.; Lim, G.; Sung, B.; Wang, S.; McCabe, M.F.; Rusanescu, G.; Yang, L.; Tian, Y.; Mao, J. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J. Clin. Investig. 2012, 122, 2940–2954. [Google Scholar] [CrossRef] [PubMed]
- Usui-Kawanishi, F.; Kani, K.; Karasawa, T.; Honda, H.; Takayama, N.; Takahashi, M.; Takatsu, K.; Nagai, Y. Isoliquiritigenin inhibits NLRP3 inflammasome activation with CAPS mutations by suppressing caspase-1 activation and mutated NLRP3 aggregation. Genes Cells 2024, 29, 423–431. [Google Scholar] [CrossRef]
- Chai, Y.; Cai, Y.; Fu, Y.; Wang, Y.; Zhang, Y.; Zhang, X.; Zhu, L.; Miao, M.; Yan, T. Salidroside Ameliorates Depression by Suppressing NLRP3-Mediated Pyroptosis via P2X7/NF-κB/NLRP3 Signaling Pathway. Front. Pharmacol. 2022, 13, 812362. [Google Scholar] [CrossRef]
- Xia, C.-Y.; Guo, Y.-X.; Lian, W.-W.; Yan, Y.; Ma, B.-Z.; Cheng, Y.-C.; Xu, J.-K.; He, J.; Zhang, W.-K. The NLRP3 inflammasome in depression: Potential mechanisms and therapies. Pharmacol. Res. 2023, 187, 106625. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Liu, Y.-Z.; Shen, X.-L.; Wu, T.-Y.; Zhang, T.; Wang, W.; Wang, Y.-X.; Jiang, C.-L. NLRP3 Inflammasome Mediates Chronic Mild Stress-Induced Depression in Mice via Neuroinflammation. Int. J. Neuropsychopharmacol. 2015, 18, pyv006. [Google Scholar] [CrossRef]
- Han, C.; Pei, H.; Shen, H.; Zhai, L.; Yang, Y.; Li, W.; Wang, J. Antcin K targets NLRP3 to suppress neuroinflammation and improve the neurological behaviors of mice with depression. Int. Immunopharmacol. 2023, 117, 109908. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.; Liu, Z.; Li, X.; Liu, M.; Wang, Y.; Zhang, K.; Sun, N. Association of the TLR4 gene with depressive symptoms and antidepressant efficacy in major depressive disorder. Neurosci. Lett. 2020, 736, 135292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Wang, C.; He, C.; Ma, Q.; Li, J.; Wang, W.; Xu, Y.-T.; Wang, T. Qingwenzhike Prescription Alleviates Acute Lung Injury Induced by LPS via Inhibiting TLR4/NF-kB Pathway and NLRP3 Inflammasome Activation. Front. Pharmacol. 2021, 12, 790072. [Google Scholar] [CrossRef]
- Kéri, S.; Szabó, C.; Kelemen, O. Expression of Toll-Like Receptors in peripheral blood mononuclear cells and response to cognitive-behavioral therapy in major depressive disorder. Brain, Behav. Immun. 2014, 40, 235–243. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Liu, L.; Zhang, W.; Zhang, Y.; Liu, Y.-Z.; Shen, X.-L.; Gong, H.; Yang, Y.-Y.; Bi, X.-Y.; Jiang, C.-L.; et al. High-mobility group box-1 was released actively and involved in LPS induced depressive-like behavior. J. Psychiatr. Res. 2015, 64, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Moylan, S.; Berk, M.; Dean, O.M.; Samuni, Y.; Williams, L.J.; O’nEil, A.; Hayley, A.C.; Pasco, J.A.; Anderson, G.; Jacka, F.N.; et al. Oxidative & nitrosative stress in depression: Why so much stress? Neurosci. Biobehav. Rev. 2014, 45, 46–62. [Google Scholar] [CrossRef]
- Wigner, P.; Czarny, P.; Galecki, P.; Su, K.-P.; Sliwinski, T. The molecular aspects of oxidative & nitrosative stress and the tryptophan catabolites pathway (TRYCATs) as potential causes of depression. Psychiatry Res. 2018, 262, 566–574. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Fındıklı, E.; İzci, F.; Kurutaş, E.B.; Tuman, T.C. Evaluation of malondialdehyde, superoxide dismutase and catalase activity and their diagnostic value in drug naïve, first episode, non-smoker major depression patients and healthy controls. Psychiatry Res. 2016, 238, 81–85. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yao, J.K. Oxidative stress and therapeutic implications in psychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 197–199. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Duman, R.S.; Malberg, J.; Thome, J. Neural plasticity to stress and antidepressant treatment. Biol. Psychiatry 1999, 46, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Pilar-Cuéllar, F.; Vidal, R.; Díaz, A.; Castro, E.; dos Anjos, S.; Pascual-Brazo, J.; Linge, R.; Vargas, V.; Blanco, H.; Martínez-Villayandre, B.; et al. Neural Plasticity and Proliferation in the Generation of Antidepressant Effects: Hippocampal Implication. Neural Plast. 2013, 2013, 537265. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.L.; Voss, G.T.; Rodrigues, K.d.C.; Pinz, M.P.; Biondi, J.V.; Becker, N.P.; Blodorn, E.; Domingues, W.B.; Larroza, A.; Campos, V.F.; et al. Prospecting for a quinoline containing selenium for comorbidities depression and memory impairment induced by restriction stress in mice. Psychopharmacology 2022, 239, 59–81. [Google Scholar] [CrossRef]
- Ruiz, N.A.L.; del Ángel, D.S.; Olguín, H.J.; Silva, M.L. Neuroprogression: The hidden mechanism of depression. Neuropsychiatr. Dis. Treat. 2018, 14, 2837–2845. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef]
- Zelada, M.I.; Garrido, V.; Liberona, A.; Jones, N.; Zúñiga, K.; Silva, H.; Nieto, R.R. Brain-Derived Neurotrophic Factor (BDNF) as a Predictor of Treatment Response in Major Depressive Disorder (MDD): A Systematic Review. Int. J. Mol. Sci. 2023, 24, 14810. [Google Scholar] [CrossRef]
- Notaras, M.; van den Buuse, M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry 2020, 25, 2251–2274. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Srivastava, P.; Bungau, S. Unfolding the Role of BDNF as a Biomarker for Treatment of Depression. J. Mol. Neurosci. 2021, 71, 2008–2021. [Google Scholar] [CrossRef]
- Sambataro, F.; Murty, V.P.; Lemaitre, H.S.; Reed, J.D.; Das, S.; Goldberg, T.E.; Callicott, J.H.; Weinberger, D.R.; Mattay, V.S. BNDF modulates normal human hippocampal ageing. Mol. Psychiatry 2010, 15, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A Neurotrophic Model for Stress-Related Mood Disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Han, R.; Liu, Z.; Sun, N.; Liu, S.; Li, L.; Shen, Y.; Xiu, J.; Xu, Q. BDNF Alleviates Neuroinflammation in the Hippocampus of Type 1 Diabetic Mice via Blocking the Aberrant HMGB1/RAGE/NF-κB Pathway. Aging Dis. 2019, 10, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-W.; Dranovsky, A.; Hen, R. The when and where of BDNF and the antidepressant response. Biol. Psychiatry 2008, 63, 640–641. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.O.; Martins, L.F.; Tahiri, E.; Duarte, C.B. Brain-derived neurotrophic factor-induced regulation of RNA metabolism in neuronal development and synaptic plasticity. Wiley Interdiscip. Rev. RNA 2022, 13, e1713. [Google Scholar] [CrossRef]
- Ambrus, L.; Lindqvist, D.; Träskman-Bendz, L.; Westrin, Å. Hypothalamic–pituitary–adrenal axis hyperactivity is associated with decreased brain-derived neurotrophic factor in female suicide attempters. Nord. J. Psychiatry 2016, 70, 575–581. [Google Scholar] [CrossRef]
- Lepack, A.E.; Fuchikami, M.; Dwyer, J.M.; Banasr, M.; Duman, R.S. BDNF Release Is Required for the Behavioral Actions of Ketamine. Int. J. Neuropsychopharmacol. 2014, 18, pyu033. [Google Scholar] [CrossRef]
- Song, X.; Liu, B.; Cui, L.; Zhou, B.; Liu, W.; Xu, F.; Hayashi, T.; Hattori, S.; Ushiki-Kaku, Y.; Tashiro, S.-I.; et al. Silibinin ameliorates anxiety/depression-like behaviors in amyloid β-treated rats by upregulating BDNF/TrkB pathway and attenuating autophagy in hippocampus. Physiol. Behav. 2017, 179, 487–493. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Liu, J.; Cai, E.; Zhao, Y.; Li, H.; Zhang, L.; Li, P.; Gao, Y. Sesquiterpenoids from the Root of Panax ginseng Attenuates Lipopolysaccharide-Induced Depressive-Like Behavior through the Brain-Derived Neurotrophic Factor/Tropomyosin-Related Kinase B and Sirtuin Type 1/Nuclear Factor-κB Signaling Pathways. J. Agric. Food Chem. 2017, 66, 265–271. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, J.; Zheng, J.; Li, X.; Zhao, F. Deterministic transition of enterotypes shapes the infant gut microbiome at an early age. Genome Biol. 2021, 22, 243. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar Fecal Microbiota Signatures in Patients With Diarrhea-Predominant Irritable Bowel Syndrome and Patients With Depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611.e5. [Google Scholar] [CrossRef]
- Zheng, P.; Yang, J.; Li, Y.; Wu, J.; Liang, W.; Yin, B.; Tan, X.; Huang, Y.; Chai, T.; Zhang, H.; et al. Gut Microbial Signatures Can Discriminate Unipolar from Bipolar Depression. Adv. Sci. 2020, 7, 1902862. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. EBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Microbiota and neuroimmune signalling—Metchnikoff to microglia. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Fischbach, M.A. Small molecules from the human microbiota. Science 2015, 349, 1254766. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Maes, M. The Microbiota-Gut-Immune-Glia (MGIG) Axis in Major Depression. Mol. Neurobiol. 2020, 57, 4269–4295. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2014, 29, 1395–1403. [Google Scholar] [CrossRef]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A link between stress and depression: Shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 2009, 11, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.A.; Goertz, J.E.; Ren, T.; Rich, S.S.; Onengut-Gumuscu, S.; Farber, E.; Wu, M.; Overall, C.C.; Kipnis, J.; Gaultier, A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017, 7, srep43859. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. Gut Microbiota-Dependent Modulation of Energy Metabolism. J. Innate Immun. 2018, 10, 163–171. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Schneeberger, M.; Matheis, F.; Wang, P.; Kerner, Z.; Ilanges, A.; Pellegrino, K.; del Mármol, J.; Castro, T.B.R.; Furuichi, M.; et al. Microbiota modulate sympathetic neurons via a gut–brain circuit. Nature 2020, 583, 441–446. [Google Scholar] [CrossRef]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell 2019, 6, 454–481. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Wilson, S.J.; Bailey, M.L.; Andridge, R.; Peng, J.; Jaremka, L.M.; Fagundes, C.P.; Malarkey, W.B.; Laskowski, B.; Belury, M.A. Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 2018, 98, 52–60. [Google Scholar] [CrossRef]
- Sarkar, S.R.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef]
- Tian, P.; Zou, R.; Song, L.; Zhang, X.; Jiang, B.; Wang, G.; Lee, Y.-K.; Zhao, J.; Zhang, H.; Chen, W. Ingestion of Bifidobacterium longum subspecies infantis strain CCFM687 regulated emotional behavior and the central BDNF pathway in chronic stress-induced depressive mice through reshaping the gut microbiota. Food Funct. 2019, 10, 7588–7598. [Google Scholar] [CrossRef]
- Winther, G.; Jørgensen, B.M.P.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sørensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176. [Google Scholar] [CrossRef]
- Jang, H.-M.; Lee, K.-E.; Kim, D.-H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients 2019, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.-L.; Inserra, A.; Lewis, M.D.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 2016, 21, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, D.; Caso, J.R.; Bris, Á.G.; Maus, S.R.; Madrigal, J.L.M.; García-Bueno, B.; MacDowell, K.S.; Alou, L.; Gómez-Lus, M.L.; Leza, J.C. Bacterial translocation affects intracellular neuroinflammatory pathways in a depression-like model in rats. Neuropharmacology 2016, 103, 122–133. [Google Scholar] [CrossRef]
- Bested, A.C.; Logan, A.C.; Selhub, E.M. Intestinal microbiota, probiotics and mental health: From Metchnikoff to modern advances: Part II–contemporary contextual research. Gut Pathog. 2013, 5, 3. [Google Scholar] [CrossRef]
- Bhandari, S.; Larson, M.E.; Kumar, N.; Stein, D. Association of Inflammatory Bowel Disease (IBD) with Depressive Symptoms in the United States Population and Independent Predictors of Depressive Symptoms in an IBD Population: A NHANES Study. Gut Liver 2017, 11, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Nishioku, T.; Matsumoto, J.; Dohgu, S.; Sumi, N.; Miyao, K.; Takata, F.; Shuto, H.; Yamauchi, A.; Kataoka, Y. Tumor Necrosis Factor-α Mediates the Blood–Brain Barrier Dysfunction Induced by Activated Microglia in Mouse Brain Microvascular Endothelial Cells. J. Pharmacol. Sci. 2010, 112, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kubera, M.; Leunis, J.-C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuroendocrinol. Lett. 2008, 29, 117–124. [Google Scholar]
- Spencer, R.L.; Deak, T. A users guide to HPA axis research. Physiol. Behav. 2017, 178, 43–65. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Park, A.J.; Collins, J.; Blennerhassett, P.A.; Ghia, J.E.; Verdu, E.F.; Bercik, P.; Collins, S.M. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil. 2013, 25, 733-e575. [Google Scholar] [CrossRef]
- Ichimura, A.; Hirasawa, A.; Hara, T.; Tsujimoto, G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 2009, 89, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Ihara, S.; Katayama, N.; Nogami, W.; Amano, M.; Noda, S.; Kurata, C.; Kobayashi, Y.; Sasaki, Y.; Mitsuda, D.; Ozawa, M.; et al. Comparison of changes in stress coping strategies between cognitive behavioral therapy and pharmacotherapy. Front. Psychiatry 2024, 15, 1343637. [Google Scholar] [CrossRef]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef]
- Zhao, J.; Jia, Y.; Zhao, W.; Chen, H.; Zhang, X.; Ngo, F.Y.; Luo, D.; Song, Y.; Lao, L.; Rong, J.; et al. Botanical Drug Puerarin Ameliorates Liposaccharide-Induced Depressive Behaviors in Mice via Inhibiting RagA/mTOR/p70S6K Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 7716201. [Google Scholar] [CrossRef]
- Gao, L.-N.; Yan, M.; Zhou, L.; Wang, J.; Sai, C.; Fu, Y.; Liu, Y.; Ding, L. Puerarin Alleviates Depression-Like Behavior Induced by High-Fat Diet Combined With Chronic Unpredictable Mild Stress via Repairing TLR4-Induced Inflammatory Damages and Phospholipid Metabolism Disorders. Front. Pharmacol. 2021, 12, 767333. [Google Scholar] [CrossRef]
- Kuhn, R. The treatment of depressive states with G 22355 (imipramine hydrochloride). Am. J. Psychiatry 1958, 115, 459–464. [Google Scholar] [CrossRef]
- Boyce, P.; Judd, F. The Place for the Tricyclic Antidepressants in the Treatment of Depression. Aust. N. Z. J. Psychiatry 1999, 33, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Shulman, K.I.; Herrmann, N.; Walker, S.E. Current Place of Monoamine Oxidase Inhibitors in the Treatment of Depression. CNS Drugs 2013, 27, 789–797. [Google Scholar] [CrossRef]
- Henkel, V.; Mergl, R.; Allgaier, A.-K.; Kohnen, R.; Möller, H.-J.; Hegerl, U. Treatment of depression with atypical features: A meta-analytic approach. Psychiatry Res. 2006, 141, 89–101. [Google Scholar] [CrossRef]
- Orsolini, L.; Vellante, F.; Valchera, A.; Fornaro, M.; Carano, A.; Pompili, M.; Perna, G.; Serafini, G.; Di Nicola, M.; Martinotti, G.; et al. Atypical Antipsychotics in Major Depressive Disorder. In Understanding Depression; Springer: Singapore, 2018; pp. 257–268. [Google Scholar] [CrossRef]
- de Filippis, R.; Foysal, A.A. Case Report: The Role of Monoamine Oxidase Inhibitors in Treating Resistant Depression. Open Access Libr. J. 2024, 11, 1–12. [Google Scholar] [CrossRef]
- Feighner, J.P. Mechanism of action of antidepressant medications. J. Clin. Psychiatry 1999, 60, 4–13. [Google Scholar] [PubMed]
- Pannu, A.; Goyal, R.K. From Evidence to Practice: A Comprehensive Analysis of Side Effects in Synthetic Anti-Depressant Therapy. Curr. Drug Saf. 2025, 20, 120–147. [Google Scholar] [CrossRef]
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef]
- E Murphy, S.; Capitão, L.P.; Giles, S.L.C.; Cowen, P.J.; Stringaris, A.; Harmer, C.J. The knowns and unknowns of SSRI treatment in young people with depression and anxiety: Efficacy, predictors, and mechanisms of action. Lancet Psychiatry 2021, 8, 824–835. [Google Scholar] [CrossRef]
- Den Boer, J.A.; Bosker, F.J.; Slaap, B.R. Serotonergic drugs in the treatment of depressive and anxiety disorders. Hum. Psychopharmacol. Clin. Exp. 2000, 15, 315–336. [Google Scholar] [CrossRef]
- Lurati, A.R. Management of Antidepressant Therapy–Induced Sexual Dysfunction in Women. J. Nurse Pract. 2022, 18, 522–524. [Google Scholar] [CrossRef]
- Horowitz, M.A.; Taylor, D. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry 2019, 6, 538–546. [Google Scholar] [CrossRef]
- Lambert, O.; Bourin, M. SNRIs: Mechanism of action and clinical features. Expert Rev. Neurother. 2014, 2, 849–858. [Google Scholar] [CrossRef]
- Li, J.; Lu, C.; Gao, Z.; Feng, Y.; Luo, H.; Lu, T.; Sun, X.; Hu, J.; Luo, Y. SNRIs achieve faster antidepressant effects than SSRIs by elevating the concentrations of dopamine in the forebrain. Neuropharmacology 2020, 177, 108237. [Google Scholar] [CrossRef] [PubMed]
- Zemanova, N.; Anzenbacher, P.; Anzenbacherova, E. The role of cytochromes P450 in the metabolism of selected antidepressants and anxiolytics under psychological stress. Biomed. Pap. 2022, 166, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Prost, J.F.; Solles, A.; Briley, M. Efficacy and tolerability of milnacipran: An overview. Int. Clin. Psychopharmacol. 1996, 11, 47–51. [Google Scholar] [CrossRef]
- Puech, A.; Montgomery, S.A.; Prost, J.F.; Solles, A.; Briley, M. Milnacipran, a new serotonin and noradrenaline reuptake inhibitor: An overview of its antidepressant activity and clinical tolerability. Int. Clin. Psychopharmacol. 1997, 12, 99–108. [Google Scholar] [CrossRef]
- Sobieraj, D.M.; Martinez, B.K.; Hernandez, A.V.; Coleman, C.I.; Ross, J.S.; Berg, K.M.; Steffens, D.C.; Baker, W.L. Adverse Effects of Pharmacologic Treatments of Major Depression in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Grinchii, D.; Dremencov, E. Mechanism of Action of Atypical Antipsychotic Drugs in Mood Disorders. Int. J. Mol. Sci. 2020, 21, 9532. [Google Scholar] [CrossRef]
- Morais, M.; Patrício, P.; Mateus-Pinheiro, A.; Alves, N.D.; Machado-Santos, A.R.; Correia, J.S.; Pereira, J.; Pinto, L.; Sousa, N.; Bessa, J.M. The modulation of adult neuroplasticity is involved in the mood-improving actions of atypical antipsychotics in an animal model of depression. Transl. Psychiatry 2017, 7, e1146. [Google Scholar] [CrossRef]
- Fasipe, O.J. The emergence of new antidepressants for clinical use: Agomelatine paradox versus other novel agents. IBRO Rep. 2019, 6, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Pruckner, N.; Holthoff-Detto, V. Antidepressant pharmacotherapy in old-age depression—A review and clinical approach. Eur. J. Clin. Pharmacol. 2017, 73, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Carboni, L.; Rullo, L.; Caputi, F.F.; Stamatakos, S.; Candeletti, S.; Romualdi, P. Chronic Trazodone and Citalopram Treatments Increase Trophic Factor and Circadian Rhythm Gene Expression in Rat Brain Regions Relevant for Antidepressant Efficacy. Int. J. Mol. Sci. 2022, 23, 14041. [Google Scholar] [CrossRef]
- Howland, R. Critical appraisal and update on the clinical utility of agomelatine, a melatonergic agonist, for the treatment of major depressive disease in adults. Neuropsychiatr. Dis. Treat. 2009, 5, 563–576. [Google Scholar] [CrossRef]
- Jha, M.K.; Mathew, S.J. Pharmacotherapies for Treatment-Resistant Depression: How Antipsychotics Fit in the Rapidly Evolving Therapeutic Landscape. Am. J. Psychiatry 2023, 180, 190–199. [Google Scholar] [CrossRef]
- Panocka, I.; Perfumi, M.; Angeletti, S.; Ciccocioppo, R.; Massi, M. Effects of Hypericum perforatum Extract on Ethanol Intake, and on Behavioral Despair: A Search for the Neurochemical Systems Involved. Pharmacol. Biochem. Behav. 2000, 66, 105–111. [Google Scholar] [CrossRef]
- Brattström, A. Long-term effects of St. John’s wort (Hypericum perforatum) treatment: A 1-year safety study in mild to moderate depression. Phytomedicine 2009, 16, 277–283. [Google Scholar] [CrossRef]
- Seifritz, E.; Hatzinger, M.; Holsboer-Trachsler, E. Efficacy of Hypericum extract WS® 5570 compared with paroxetine in patients with a moderate major depressive episode—A subgroup analysis. Int. J. Psychiatry Clin. Pract. 2016, 20, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Vance, K.M.; Ribnicky, D.M.; Hermann, G.E.; Rogers, R.C. St. John’s Wort enhances the synaptic activity of the nucleus of the solitary tract. Nutrition 2014, 30, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- van Diermen, D.; Marston, A.; Bravo, J.; Reist, M.; Carrupt, P.-A.; Hostettmann, K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J. Ethnopharmacol. 2009, 122, 397–401. [Google Scholar] [CrossRef]
- Yang, S.-J.; Yu, H.-Y.; Kang, D.-Y.; Ma, Z.-Q.; Qu, R.; Fu, Q.; Ma, S.-P. Antidepressant-like effects of salidroside on olfactory bulbectomy-induced pro-inflammatory cytokine production and hyperactivity of HPA axis in rats. Pharmacol. Biochem. Behav. 2014, 124, 451–457. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, T.; Gao, J.; Chang, X.; He, H.; Miao, M.; Yan, T. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci. Lett. 2015, 606, 1–6. [Google Scholar] [CrossRef]
- Mao, J.J.; Xie, S.X.; Zee, J.; Soeller, I.; Li, Q.S.; Rockwell, K.; Amsterdam, J.D. Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial. Phytomedicine 2015, 22, 394–399. [Google Scholar] [CrossRef]
- Jin, Y.; Cui, R.; Zhao, L.; Fan, J.; Li, B. Mechanisms of Panax ginseng action as an antidepressant. Cell Prolif. 2019, 52, e12696. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Yang, L.; Wang, Y.; Lu, P.; Li, W.; Miao, J.; Gong, Y.; Zhang, B.; Yin, Y. Ginseng total saponins rescue susceptibility to adult depression-like behaviors in mice induced by early-life stress via regulating CREB/BDNF/TrkB signaling. CyTA J. Food 2023, 21, 701–710. [Google Scholar] [CrossRef]
- Chen, L.; Dai, J.; Wang, Z.; Zhang, H.; Huang, Y.; Zhao, Y.; Rahman, K. Ginseng Total Saponins Reverse Corticosterone-Induced Changes in Depression-Like Behavior and Hippocampal Plasticity-Related Proteins by Interfering with GSK-3β-CREB Signaling Pathway. Evid.-Based Complement. Altern. Med. 2014, 2014, 506735. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Wang, J.-J.; Cheng, P.; Chen, L.-X.; Hu, J.-M.; Zhu, G.-Q. Ginsenoside Rg1 in neurological diseases: From bench to bedside. Acta Pharmacol. Sin. 2022, 44, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-X.; Qi, Z.; Shao, Z.-J.; Li, S.-S.; Qi, Y.-L.; Gao, K.; Liu, S.-X.; Li, Z.; Sun, Y.-S.; Li, P.-Y. Study on Antidepressant Activity of Pseudo-Ginsenoside HQ on Depression-Like Behavior in Mice. Molecules 2019, 24, 870. [Google Scholar] [CrossRef]
- Chen, T.; Cheng, L.; Ma, J.; Yuan, J.; Pi, C.; Xiong, L.; Chen, J.; Liu, H.; Tang, J.; Zhong, Y.; et al. Molecular mechanisms of rapid-acting antidepressants: New perspectives for developing antidepressants. Pharmacol. Res. 2023, 194, 106837. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef]
- A Cristea, I.; Naudet, F. US Food and Drug Administration approval of esketamine and brexanolone. Lancet Psychiatry 2019, 6, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Akbar, D.; Rhee, T.G.; Ceban, F.; Ho, R.; Teopiz, K.M.; Cao, B.; Subramaniapillai, M.; Kwan, A.T.H.; Rosenblat, J.D.; McIntyre, R.S. Dextromethorphan-Bupropion for the Treatment of Depression: A Systematic Review of Efficacy and Safety in Clinical Trials. CNS Drugs 2023, 37, 867–881. [Google Scholar] [CrossRef]
- Fava, M.; Stahl, S.M.; Pani, L.; De Martin, S.; Cutler, A.J.; Maletic, V.; Gorodetzky, C.W.; Vocci, F.J.; Sapienza, F.L.; Kosten, T.R.; et al. Efficacy and safety of esmethadone (REL-1017) in patients with major depressive disorder and inadequate response to standard antidepressants: A phase 3 randomized controlled trial. J. Clin. Psychiatry 2024, 85, 24m15265. [Google Scholar] [CrossRef] [PubMed]
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M. Psychedelics and Psychedelic-Assisted Psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef]
- Cameron, L.P.; Tombari, R.J.; Lu, J.; Pell, A.J.; Hurley, Z.Q.; Ehinger, Y.; Vargas, M.V.; McCarroll, M.N.; Taylor, J.C.; Myers-Turnbull, D.; et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 2021, 589, 474–479. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Cariprazine: First Global Approval. Drugs 2015, 75, 2035–2043. [Google Scholar] [CrossRef]
- Ali, E.; Latif, F.; Mashkoor, Y.; Sheikh, A.; Iqbal, A.; Owais, R.; Ahmed, J.; Naveed, S.; Moeed, A.; Ullah, I.; et al. Role of Adjunctive Cariprazine for Treatment-Resistant Depression in patients with Major Depressive Disorder: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Asian J. Psychiatry 2024, 95, 104005. [Google Scholar] [CrossRef]
- Papp, M.; Gruca, P.; Litwa, E.; Lason, M.; Newman-Tancredi, A.; Depoortère, R. The 5-HT1A receptor biased agonists, NLX-204 and NLX-101, like ketamine, elicit rapid-acting antidepressant activity in the rat chronic mild stress model via cortical mechanisms. J. Psychopharmacol. 2024, 38, 661–671. [Google Scholar] [CrossRef]
- Efthimiou, O.; Taipale, H.; Radua, J.; Schneider-Thoma, J.; Pinzón-Espinosa, J.; Ortuño, M.; Vinkers, C.H.; Mittendorfer-Rutz, E.; Cardoner, N.; Tanskanen, A.; et al. Efficacy and effectiveness of antipsychotics in schizophrenia: Network meta-analyses combining evidence from randomised controlled trials and real-world data. Lancet Psychiatry 2024, 11, 102–111. [Google Scholar] [CrossRef]
- Chowdhury, A.; Boukezzi, S.; Costi, S.; Hameed, S.; Jacob, Y.; Salas, R.; Iosifescu, D.V.; Han, M.-H.; Swann, A.; Mathew, S.J.; et al. Effects of the KCNQ (Kv7) Channel Opener Ezogabine on Resting-State Functional Connectivity of Striatal Brain Reward Regions, Depression, and Anhedonia in Major Depressive Disorder: Results From a Randomized Controlled Trial. Biol. Psychiatry 2025. [Google Scholar] [CrossRef] [PubMed]
- Arcella, A.; Alborghetti, M.; Traficante, A.; Oliva, M.A.; Staffieri, S.; Russo, V.; Caridi, M.; Battaglia, G. Pharmacological Blockade of Group II Metabotropic Glutamate Receptors Reduces the Incidence of Brain Tumors Induced by Prenatal Exposure to N-ethyl-N-nitrosourea in Rats. Curr. Neuropharmacol. 2024, 23, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Zelek-Molik, A.; Litwa, E. Trends in research on novel antidepressant treatments. Front. Pharmacol. 2025, 16, 1544795. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).