Characterization of QuantiFERON-TB-Plus Results in Patients with Tuberculosis Infection and Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. QuantiFERON-TB-Plus (QFT-Plus)
2.3. Statistical Analysis
3. Results
3.1. Demographic and Epidemiological Characteristics of the Population
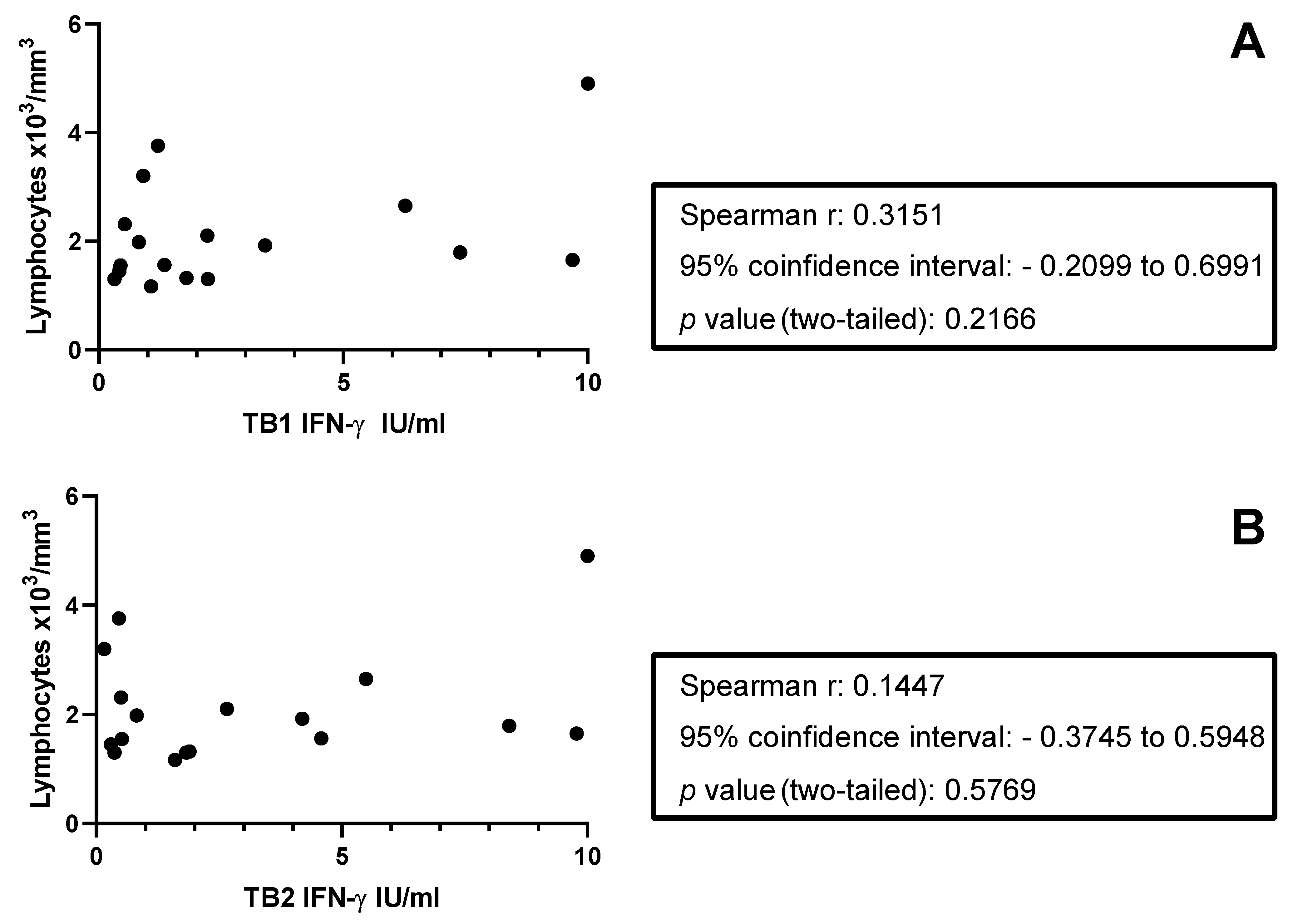
3.2. IFN-γ Response to QFT-Plus in TBI Subjects with or Without MS
3.3. Detailed Analysis of the Response to TB1 and TB2 in TBI with or Without MS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | Multiple scleroris |
| TB | tuberculosis |
| TBI | Tuberculosis infection |
| IMID | Immune mediated inflammatory disease |
| IGRA | Interferon gamma release assay |
| IFN | Interferon |
| TST | Tuberculin skin test |
References
- WHO. WHO Global Tuberculosis Report; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Houben, R.M.G.J.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef]
- Kontsevaya, I.; Cabibbe, A.M.; Cirillo, D.M.; DiNardo, A.R.; Frahm, N.; Gillespie, S.H.; Holtzman, D.; Meiwes, L.; Petruccioli, E.; Reimann, M.; et al. Update on the diagnosis of tuberculosis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2023, 30, 1115–1122. [Google Scholar] [CrossRef]
- Coussens, A.K.; Zaidi, S.M.A.; Allwood, B.W.; Dewan, P.K.; Gray, G.; Kohli, M.; Kredo, T.; Marais, B.J.; Marks, G.B.; Martinez, L.; et al. Classification of early tuberculosis states to guide research for improved care and prevention: An international Delphi consensus exercise. Lancet Respir. Med. 2024, 12, 484–498. [Google Scholar] [CrossRef]
- Richards, A.S.; Sossen, B.; Emery, J.C.; Horton, K.C.; Heinsohn, T.; Frascella, B.; Balzarini, F.; Oradini-Alacreu, A.; Häcker, B.; Odone, A.; et al. Quantifying progression and regression across the spectrum of pulmonary tuberculosis: A data synthesis study. Lancet Glob. Health 2023, 11, e684–e692. [Google Scholar] [CrossRef]
- Drain, P.K.; Bajema, K.L.; Dowdy, D.; Dheda, K.; Naidoo, K.; Schumacher, S.G.; Ma, S.; Meermeier, E.; Lewinsohn, D.M.; Sherman, D.R. Incipient and Subclinical Tuberculosis: A Clinical Review of Early Stages and Progression of Infection. Clin. Microbiol. Rev. 2018, 31, e00021-18. [Google Scholar] [CrossRef] [PubMed]
- Peddireddy, V.; Doddam, S.N.; Ahmed, N. Mycobacterial Dormancy Systems and Host Responses in Tuberculosis. Front. Immunol. 2017, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Gideon, H.P.; Phuah, J.; Myers, A.J.; Bryson, B.D.; Rodgers, M.A.; Coleman, M.T.; Maiello, P.; Rutledge, T.; Marino, S.; Fortune, S.M.; et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog. 2015, 11, e1004603. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Barreira-Silva, P.; Boyce, S.; Powers, J.; Cavallo, K.; Behar, S.M. CD4 T cell help prevents CD8 T cell exhaustion and promotes control of Mycobacterium tuberculosis infection. Cell Rep. 2021, 36, 109696. [Google Scholar] [CrossRef]
- Ogongo, P.; Tezera, L.B.; Ardain, A.; Nhamoyebonde, S.; Ramsuran, D.; Singh, A.; Ng’oepe, A.; Karim, F.; Naidoo, T.; Khan, K.; et al. Tissue-resident-like CD4+ T cells secreting IL-17 control Mycobacterium tuberculosis in the human lung. J. Clin. Investig. 2021, 131, e142014. [Google Scholar] [CrossRef]
- Brighenti, S.; Ordway, D.J. Regulation of Immunity to Tuberculosis. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Roy Chowdhury, R.; Vallania, F.; Yang, Q.; Lopez Angel, C.J.; Darboe, F.; Penn-Nicholson, A.; Rozot, V.; Nemes, E.; Malherbe, S.T.; Ronacher, K.; et al. A multi-cohort study of the immune factors associated with M. tuberculosis infection outcomes. Nature 2018, 560, 644–648. [Google Scholar] [CrossRef]
- Tiberi, S.; Carvalho, A.C.C.; Sulis, G.; Vaghela, D.; Rendon, A.; de Q Mello, F.C.; Rahman, A.; Matin, N.; Zumla, A.; Pontali, E. The cursed duet today: Tuberculosis and HIV-coinfection. Presse Medicale Paris Fr. 1983 2017, 46, e23–e39. [Google Scholar] [CrossRef] [PubMed]
- Lawn, S.D.; Myer, L.; Edwards, D.; Bekker, L.-G.; Wood, R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS Lond. Engl. 2009, 23, 1717–1725. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Aiello, A.; De Lorenzo, C.; Migliori, G.B.; Goletti, D. Management of tuberculosis risk, screening and preventive therapy in patients with chronic autoimmune arthritis undergoing biotechnological and targeted immunosuppressive agents. Front. Immunol. 2025, 16, 1494283. [Google Scholar] [CrossRef]
- Bai, W.; Ameyaw, E.K. Global, regional and national trends in tuberculosis incidence and main risk factors: A study using data from 2000 to 2021. BMC Public Health 2024, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Sundbaum, J.K.; Arkema, E.V.; Bruchfeld, J.; Jonsson, J.; Askling, J.; Baecklund, E. Tuberculosis in Biologic-naïve Patients With Rheumatoid Arthritis: Risk Factors and Tuberculosis Characteristics. J. Rheumatol. 2021, 48, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fan, W.; Yang, G.; Xu, Z.; Wang, J.; Cheng, Q.; Yu, M. Risk of tuberculosis in patients treated with TNF-α antagonists: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2017, 7, e012567. [Google Scholar] [CrossRef]
- Baronnet, L.; Barnetche, T.; Kahn, V.; Lacoin, C.; Richez, C.; Schaeverbeke, T. Incidence of tuberculosis in patients with rheumatoid arthritis. A systematic literature review. Jt. Bone Spine 2011, 78, 279–284. [Google Scholar] [CrossRef]
- Dobler, C.C. Biologic Agents and Tuberculosis. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Evangelatos, G.; Koulouri, V.; Iliopoulos, A.; Fragoulis, G.E. Tuberculosis and targeted synthetic or biologic DMARDs, beyond tumor necrosis factor inhibitors. Ther. Adv. Musculoskelet. Dis. 2020, 12. [Google Scholar] [CrossRef]
- Rath, E.; Bonelli, M.; Duftner, C.; Gruber, J.; Mandl, P.; Moazedi-Furst, F.; Pieringer, H.; Puchner, R.; Flick, H.; Salzer, H.J.F.; et al. National consensus statement by the Austrian Societies for Rheumatology, Pulmonology, Infectiology, Dermatology and Gastroenterology regarding the management of latent tuberculosis and the associated utilization of biologic and targeted synthetic disease modifying antirheumatic drugs (DMARDs). Wien. Klin. Wochenschr. 2022, 134, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Moiola, L.; Barcella, V.; Benatti, S.; Capobianco, M.; Capra, R.; Cinque, P.; Comi, G.; Fasolo, M.M.; Franzetti, F.; Galli, M.; et al. The risk of infection in patients with multiple sclerosis treated with disease-modifying therapies: A Delphi consensus statement. Mult. Scler. Houndmills Basingstoke Engl. 2021, 27, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Hirt, J.; Dembowska, K.; Woelfle, T.; Axfors, C.; Granziera, C.; Kuhle, J.; Kappos, L.; Hemkens, L.G.; Janiaud, P. Clinical trial evidence of quality-of-life effects of disease-modifying therapies for multiple sclerosis: A systematic analysis. J. Neurol. 2024, 271, 3131–3141. [Google Scholar] [CrossRef]
- DiMauro, K.A.; Swetlik, C.; Cohen, J.A. Management of multiple sclerosis in older adults: Review of current evidence and future perspectives. J. Neurol. 2024, 271, 3794–3805. [Google Scholar] [CrossRef]
- Winkelmann, A.; Loebermann, M.; Reisinger, E.C.; Hartung, H.-P.; Zettl, U.K. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat. Rev. Neurol. 2016, 12, 217–233. [Google Scholar] [CrossRef]
- Graf, J.; Leussink, V.I.; Dehmel, T.; Ringelstein, M.; Goebels, N.; Adams, O.; MacKenzie, C.R.; Warnke, C.; Feldt, T.; Lammerskitten, A.; et al. Infectious risk stratification in multiple sclerosis patients receiving immunotherapy. Ann. Clin. Transl. Neurol. 2017, 4, 909–914. [Google Scholar] [CrossRef]
- Gross, C.C.; Schulte-Mecklenbeck, A.; Klinsing, S.; Posevitz-Fejfár, A.; Wiendl, H.; Klotz, L. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2015, 3, e183. [Google Scholar] [CrossRef]
- Mazzola, M.A.; Raheja, R.; Murugaiyan, G.; Rajabi, H.; Kumar, D.; Pertel, T.; Regev, K.; Griffin, R.; Aly, L.; Kivisakk, P.; et al. Identification of a novel mechanism of action of fingolimod (FTY720) on human effector T cell function through TCF-1 upregulation. J. Neuroinflammation 2015, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Montes Diaz, G.; Fraussen, J.; Van Wijmeersch, B.; Hupperts, R.; Somers, V. Dimethyl fumarate induces a persistent change in the composition of the innate and adaptive immune system in multiple sclerosis patients. Sci. Rep. 2018, 8, 8194. [Google Scholar] [CrossRef]
- Benkert, T.F.; Dietz, L.; Hartmann, E.M.; Leich, E.; Rosenwald, A.; Serfling, E.; Buttmann, M.; Berberich-Siebelt, F. Natalizumab exerts direct signaling capacity and supports a pro-inflammatory phenotype in some patients with multiple sclerosis. PLoS ONE 2012, 7, e52208. [Google Scholar] [CrossRef]
- Nasa, P.; Jain, R.; Juneja, D. Delphi methodology in healthcare research: How to decide its appropriateness. World J. Methodol. 2021, 11, 116–129. [Google Scholar] [CrossRef]
- Navas, C.; Torres-Duque, C.A.; Munoz-Ceron, J.; Álvarez, C.; García, J.R.; Zarco, L.; Vélez, L.A.; Awad, C.; Castro, C.A. Diagnosis and treatment of latent tuberculosis in patients with multiple sclerosis, expert consensus. On behalf of the Colombian Association of Neurology, Committee of Multiple Sclerosis. Mult. Scler. J.-Exp. Transl. Clin. 2018, 4. [Google Scholar] [CrossRef]
- da Silva, D.A.A.; da Silva, M.V.; Barros, C.C.O.; Alexandre, P.B.D.; Timóteo, R.P.; Catarino, J.S.; Sales-Campos, H.; Machado, J.R.; Rodrigues, D.B.R.; Oliveira, C.J.; et al. TNF-α blockade impairs in vitro tuberculous granuloma formation and down modulate Th1, Th17 and Treg cytokines. PLoS ONE 2018, 13, e0194430. [Google Scholar] [CrossRef] [PubMed]
- Blandizzi, C.; Gionchetti, P.; Armuzzi, A.; Caporali, R.; Chimenti, S.; Cimaz, R.; Cimino, L.; Lapadula, G.; Lionetti, P.; Marchesoni, A.; et al. The role of tumour necrosis factor in the pathogenesis of immune-mediated diseases. Int. J. Immunopathol. Pharmacol. 2014, 27, 1–10. [Google Scholar] [CrossRef]
- Keane, J.; Gershon, S.; Wise, R.P.; Mirabile-Levens, E.; Kasznica, J.; Schwieterman, W.D.; Siegel, J.N.; Braun, M.M. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 2001, 345, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.K.; Coté, T.R.; Block, J.A.; Manadan, A.M.; Siegel, J.N.; Braun, M.M. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2004, 39, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Hsia, E.C.; Cush, J.J.; Matteson, E.L.; Beutler, A.; Doyle, M.K.; Hsu, B.; Xu, S.; Rahman, M.U. Comprehensive tuberculosis screening program in patients with inflammatory arthritides treated with golimumab, a human anti-tumor necrosis factor antibody, in Phase III clinical trials. Arthritis Care Res. 2013, 65, 309–313. [Google Scholar] [CrossRef]
- Gómez-Reino, J.J.; Carmona, L.; Valverde, V.R.; Mola, E.M.; Montero, M.D.; BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: A multicenter active-surveillance report. Arthritis Rheum. 2003, 48, 2122–2127. [Google Scholar] [CrossRef]
- Ai, J.-W.; Zhang, S.; Ruan, Q.-L.; Yu, Y.-Q.; Zhang, B.-Y.; Liu, Q.-H.; Zhang, W.-H. The Risk of Tuberculosis in Patients with Rheumatoid Arthritis Treated with Tumor Necrosis Factor-α Antagonist: A Metaanalysis of Both Randomized Controlled Trials and Registry/Cohort Studies. J. Rheumatol. 2015, 42, 2229–2237. [Google Scholar] [CrossRef]
- Fragoso, Y.D.; Adoni, T.; Anacleto, A.; Brooks, J.B.B.; de Jesus Carvalho, M.; Claudino, R.; Damasceno, A.; Ferreira, M.L.B.; da Gama, P.D.; Goncalves, M.V.M.; et al. How do we manage and treat a patient with multiple sclerosis at risk of tuberculosis? Expert Rev. Neurother. 2014, 14, 1251–1260. [Google Scholar] [CrossRef]
- Alonzi, T.; Petruccioli, E.; Aiello, A.; Repele, F.; Goletti, D. Diagnostic tests for tuberculosis infection and predictive indicators of disease progression: Utilizing host and pathogen biomarkers to enhance the TB elimination strategies. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2025, 155, 107880. [Google Scholar] [CrossRef]
- Buonsenso, D.; Seddon, J.A.; Esposito, S.; Barcellini, L. QuantiFERON-TB Gold Plus Performance in Children: A Narrative Review. Pediatr. Infect. Dis. J. 2023, 42, e158–e165. [Google Scholar] [CrossRef] [PubMed]
- Petruccioli, E.; Chiacchio, T.; Navarra, A.; Vanini, V.; Cuzzi, G.; Cimaglia, C.; Codecasa, L.R.; Pinnetti, C.; Riccardi, N.; Palmieri, F.; et al. Effect of HIV-infection on QuantiFERON-plus accuracy in patients with active tuberculosis and latent infection. J. Infect. 2020, 80, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Bua, A.; Ruggeri, M.; Zanetti, S.; Molicotti, P. Effect of teriflunomide on QuantiFERON-TB Gold results. Med. Microbiol. Immunol. 2017, 206, 73–75. [Google Scholar] [CrossRef]
- Chiacchio, T.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; Massafra, U.; Baldi, G.; Navarra, A.; Scrivo, R.; Mastroianni, C.; Sauzullo, I.; et al. Characterization of QuantiFERON-TB-Plus results in latent tuberculosis infected patients with or without immune-mediated inflammatory diseases. J. Infect. 2019, 79, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Petruccioli, E.; Petrone, L.; Najafi-Fard, S.; Navarra, A.; Vanini, V.; Cuzzi, G.; Cantini, F.; Gualano, G.; Palmieri, F.; Goletti, D. Alternative biomarkers of tuberculosis infection in patients with immune-mediated inflammatory diseases. Front. Med. 2023, 10, 1271632. [Google Scholar] [CrossRef]
- Uzorka, J.W.; Delfos, N.M.; Witte, A.M.C.; Scheper, H.; van Soolingen, D.; Arend, S.M. Tuberculosis after a borderline QuantiFERON result during screening before infliximab. Eur. Respir. J. 2018, 52, 1800913. [Google Scholar] [CrossRef]
- Bouley, A.J.; Baber, U.; Egnor, E.; Samaan, S.; Sloane, J.A. Prevalence of Latent Tuberculosis in the Multiple Sclerosis Clinic and Effect of Multiple Sclerosis Treatment on Tuberculosis Testing. Int. J. MS Care 2021, 23, 26–30. [Google Scholar] [CrossRef]
- Baldassari, L.E.; Feng, J.; Macaron, G.; Planchon, S.M.; Alshehri, E.; Moss, B.P.; Ontaneda, D.; Willis, M.A. Tuberculosis screening in multiple sclerosis: Effect of disease-modifying therapies and lymphopenia on the prevalence of indeterminate TB screening results in the clinical setting. Mult. Scler. J.-Exp. Transl. Clin. 2019, 5. [Google Scholar] [CrossRef]
- Uzorka, J.W.; Kroft, L.J.M.; Bakker, J.A.; van Zwet, E.W.; Huisman, E.; Knetsch-Prins, C.; van der Zwan, C.J.; Ottenhoff, T.H.M.; Arend, S.M. Proof of concept that most borderline Quantiferon results are true antigen-specific responses. Eur. Respir. J. 2017, 50, 1701630. [Google Scholar] [CrossRef]
- Pai, M.; Joshi, R.; Dogra, S.; Zwerling, A.A.; Gajalakshmi, D.; Goswami, K.; Reddy, M.V.R.; Kalantri, S.; Hill, P.C.; Menzies, D.; et al. T-cell assay conversions and reversions among household contacts of tuberculosis patients in rural India. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2009, 13, 84–92. [Google Scholar]
- Schablon, A.; Nienhaus, A.; Ringshausen, F.C.; Preisser, A.M.; Peters, C. Occupational screening for tuberculosis and the use of a borderline zone for interpretation of the IGRA in German healthcare workers. PLoS ONE 2014, 9, e115322. [Google Scholar] [CrossRef]
- van Zyl-Smit, R.N.; Lehloenya, R.J.; Meldau, R.; Dheda, K. Impact of correcting the lymphocyte count to improve the sensitivity of TB antigen-specific peripheral blood-based quantitative T cell assays (T-SPOT.(®)TB and QFT-GIT). J. Thorac. Dis. 2016, 8, 482–489. [Google Scholar] [CrossRef]
- Nemes, E.; Rozot, V.; Geldenhuys, H.; Bilek, N.; Mabwe, S.; Abrahams, D.; Makhethe, L.; Erasmus, M.; Keyser, A.; Toefy, A.; et al. Optimization and Interpretation of Serial QuantiFERON Testing to Measure Acquisition of Mycobacterium tuberculosis Infection. Am. J. Respir. Crit. Care Med. 2017, 196, 638–648. [Google Scholar] [CrossRef]
- Tagmouti, S.; Slater, M.; Benedetti, A.; Kik, S.V.; Banaei, N.; Cattamanchi, A.; Metcalfe, J.; Dowdy, D.; van Zyl Smit, R.; Dendukuri, N.; et al. Reproducibility of Interferon Gamma (IFN-γ) Release Assays. A Systematic Review. Ann. Am. Thorac. Soc. 2014, 11, 1267–1276. [Google Scholar] [CrossRef]
- Woo, K.-S.; Choi, J.-L.; Kim, B.-R.; Han, J.-Y.; Kim, J.-M.; Kim, K.-H. Repeatability of QuantiFERON-TB Gold In-Tube Assay Results Near Cut-Off Points. Ann. Lab. Med. 2016, 36, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, J.Z.; Cattamanchi, A.; McCulloch, C.E.; Lew, J.D.; Ha, N.P.; Graviss, E.A. Test Variability of the QuantiFERON-TB Gold In-Tube Assay in Clinical Practice. Am. J. Respir. Crit. Care Med. 2013, 187, 206–211. [Google Scholar] [CrossRef]
- Farroni, C.; Altera, A.M.G.; Salmi, A.; Vanini, V.; Cuzzi, G.; Lindestam Arlehamn, C.S.; Sette, A.; Delogu, G.; Palucci, I.; Sbarra, S.; et al. Specific immune response to M. tuberculosis and ability to in vitro control mycobacterial replication are not impaired in subjects with immune-mediated inflammatory disease and tuberculosis infection. Front. Immunol. 2025, 15, 1484143. [Google Scholar] [CrossRef] [PubMed]
- de Paus, R.A.; van Meijgaarden, K.E.; Prins, C.; Kamphorst, M.H.; Arend, S.M.; Ottenhoff, T.H.M.; Joosten, S.A. Immunological characterization of latent tuberculosis infection in a low endemic country. Tuberc. Edinb. Scotl. 2017, 106, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, S.C.; Mbandi, S.K.; Fiore-Gartland, A.; Penn-Nicholson, A.; Musvosvi, M.; Mulenga, H.; Fisher, M.; Hadley, K.; Erasmus, M.; Nombida, O.; et al. Prospective multicentre head-to-head validation of host blood transcriptomic biomarkers for pulmonary tuberculosis by real-time PCR. Commun. Med. 2022, 2, 26. [Google Scholar] [CrossRef]
- Hamada, Y.; Penn-Nicholson, A.; Krishnan, S.; Cirillo, D.M.; Matteelli, A.; Wyss, R.; Denkinger, C.M.; Rangaka, M.X.; Ruhwald, M.; Schumacher, S.G. Are mRNA based transcriptomic signatures ready for diagnosing tuberculosis in the clinic?-A review of evidence and the technological landscape. EBioMedicine 2022, 82, 104174. [Google Scholar] [CrossRef] [PubMed]
- Petruccioli, E.; Petrone, L.; Chiacchio, T.; Farroni, C.; Cuzzi, G.; Navarra, A.; Vanini, V.; Massafra, U.; Lo Pizzo, M.; Guggino, G.; et al. Mycobacterium tuberculosis Immune Response in Patients with Immune-Mediated Inflammatory Disease. Front. Immunol. 2021, 12, 716857. [Google Scholar] [CrossRef]
- Ogongo, P. A broader evaluation of vaccine-induced T cell immunity against tuberculosis. Front. Tuberc. 2024, 2. [Google Scholar] [CrossRef]
- Scriba, T.J.; Coussens, A.K.; Fletcher, H.A. Human Immunology of Tuberculosis. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Lloyd, T.; Steigler, P.; Mpande, C.A.M.; Rozot, V.; Mosito, B.; Schreuder, C.; Reid, T.D.; Hatherill, M.; Scriba, T.J.; Little, F.; et al. Multidimensional analysis of immune responses identified biomarkers of recent Mycobacterium tuberculosis infection. PLoS Comput. Biol. 2021, 17, e1009197. [Google Scholar] [CrossRef]
- Mpande, C.A.M.; Rozot, V.; Mosito, B.; Musvosvi, M.; Dintwe, O.B.; Bilek, N.; Hatherill, M.; Scriba, T.J.; Nemes, E.; ACS Study Team. Immune profiling of Mycobacterium tuberculosis-specific T cells in recent and remote infection. EBioMedicine 2021, 64, 103233. [Google Scholar] [CrossRef] [PubMed]
- Portevin, D.; Moukambi, F.; Clowes, P.; Bauer, A.; Chachage, M.; Ntinginya, N.E.; Mfinanga, E.; Said, K.; Haraka, F.; Rachow, A.; et al. Assessment of the novel T-cell activation marker-tuberculosis assay for diagnosis of active tuberculosis in children: A prospective proof-of-concept study. Lancet Infect. Dis. 2014, 14, 931–938. [Google Scholar] [CrossRef]
- Nogueira, B.M.F.; Krishnan, S.; Barreto-Duarte, B.; Araújo-Pereira, M.; Queiroz, A.T.L.; Ellner, J.J.; Salgame, P.; Scriba, T.J.; Sterling, T.R.; Gupta, A.; et al. Diagnostic biomarkers for active tuberculosis: Progress and challenges. EMBO Mol. Med. 2022, 14, e14088. [Google Scholar] [CrossRef]
- Warsinske, H.C.; Rao, A.M.; Moreira, F.M.F.; Santos, P.C.P.; Liu, A.B.; Scott, M.; Malherbe, S.T.; Ronacher, K.; Walzl, G.; Winter, J.; et al. Assessment of Validity of a Blood-Based 3-Gene Signature Score for Progression and Diagnosis of Tuberculosis, Disease Severity, and Treatment Response. JAMA Netw. Open 2018, 1, e183779. [Google Scholar] [CrossRef]

| MS-TBI N = 20 | NON-MS-TBI N = 106 | Total N = 126 | p | |
|---|---|---|---|---|
| Female, N (%) | 12 (60) | 56 (50) | 68 (54) | 0.97 * |
| Median age, IQR | 41 (35–57) | 41 (35–56) | 42 (28–55) | 0.37 # |
| Origin, N (%) | ||||
| Est Europe, N (%) | 5 (25) | 29 (27) | 34 (27) | na ** |
| West Europe, N (%) | 12 (60) | 55 (52) | 67 (53) | |
| South America, N (%) | 0 (0) | 3 (3) | 3 (2) | |
| Asia, N (%) | 1 (5) | 10 (9) | 11 (9) | |
| Africa, N (%) | 2 (10) | 9 (9) | 11 (9) | |
| TB exposure, N (%) | ||||
| Recent | 0 | 72 (68) | 72 (57) | p ≤ 0.0001 * |
| Remote | 20 (100) | 34 (32) | 54 (43) |
| MS-TBI N = 20 | |
|---|---|
| Under MS therapy N (%) | 9 (45) |
| Type of MS therapy | |
| Glatiramer acetate | 1 (11) |
| IFN-βN (%) | 5 (56) |
| Anti-CD20N (%) | 1 (11) |
| Dimethyl fumarateN (%) | 1 (11) |
| TeriflunomideN (%) | 1 (11) |
| Primary progressive MS (%) | 2 (10) |
| Relapsing–remitting MS (%) | 18 (93) |
| Expanded Disability Status Scale | |
| 0 | 2 (10) |
| 1–2 | 5 (25) |
| 3–4 | 9 (45) |
| 5–6.5 | 4 (20) |
| Lymphocytes × 103/mm3 | 1.8 (1.5–2.3) |
| QFT-Plus Tube | MS-TBI | NON-MS-TBI | p * | NON-MS-TBI Recent Exposure | p * | NON-MS-TBI Remote Exposure | p * | |
|---|---|---|---|---|---|---|---|---|
| Enrolled subjects N | 20 | 106 | 72 | 34 | ||||
| Number of responders N (%) | TB1 | 18 (90) | 101 (95) | 0.30 | 71 (99) | 0.12 | 30 (88) | >0.99 |
| TB2 | 18 (90) | 102 (96) | 0.24 | 69 (96) | 0.30 | 33 (97) | 0.55 | |
| Number of results falling in the grey zone 0.2–0.7 IU/mL N (%) | TB1 | 5 (25) | 12 (11) | 0.15 | 5 (7) | 0.04 | 7 (21) | 0.7 |
| TB2 | 6 (30) | 7 (7) | 0.006 | 4 (6) | 0.006 | 3 (9) | 0.06 |
| QFT-Plus Tube | MS-TBI Under MS Therapy N = 9 | MS-TBI NO-MS Therapy N = 11 | p * | |
|---|---|---|---|---|
| Number of results falling in the grey zone 0.2–0.7 IU/mL N (%) | TB1 | 3 (33) | 2 (18) | 0.6169 |
| TB2 | 3 (33) | 3 (27) | >0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruccioli, E.; Prosperini, L.; Ruggieri, S.; Vanini, V.; Salmi, A.; Cuzzi, G.; Galgani, S.; Haggiag, S.; Tortorella, C.; Parisi, G.; et al. Characterization of QuantiFERON-TB-Plus Results in Patients with Tuberculosis Infection and Multiple Sclerosis. Neurol. Int. 2025, 17, 119. https://doi.org/10.3390/neurolint17080119
Petruccioli E, Prosperini L, Ruggieri S, Vanini V, Salmi A, Cuzzi G, Galgani S, Haggiag S, Tortorella C, Parisi G, et al. Characterization of QuantiFERON-TB-Plus Results in Patients with Tuberculosis Infection and Multiple Sclerosis. Neurology International. 2025; 17(8):119. https://doi.org/10.3390/neurolint17080119
Chicago/Turabian StylePetruccioli, Elisa, Luca Prosperini, Serena Ruggieri, Valentina Vanini, Andrea Salmi, Gilda Cuzzi, Simonetta Galgani, Shalom Haggiag, Carla Tortorella, Gabriella Parisi, and et al. 2025. "Characterization of QuantiFERON-TB-Plus Results in Patients with Tuberculosis Infection and Multiple Sclerosis" Neurology International 17, no. 8: 119. https://doi.org/10.3390/neurolint17080119
APA StylePetruccioli, E., Prosperini, L., Ruggieri, S., Vanini, V., Salmi, A., Cuzzi, G., Galgani, S., Haggiag, S., Tortorella, C., Parisi, G., D’Agostino, A., Gualano, G., Palmieri, F., Gasperini, C., & Goletti, D. (2025). Characterization of QuantiFERON-TB-Plus Results in Patients with Tuberculosis Infection and Multiple Sclerosis. Neurology International, 17(8), 119. https://doi.org/10.3390/neurolint17080119









