Responsiveness and Minimal Important Change of the Mini- and Brief-Balance Evaluation Systems Tests in People with Incomplete Cervical Spinal Cord Injury: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Participants, and Ethics
2.2. Outcome Measures
2.3. Data Collection
2.4. Rehabilitation
2.5. Data Analysis
3. Results
3.1. Participant Characteristics
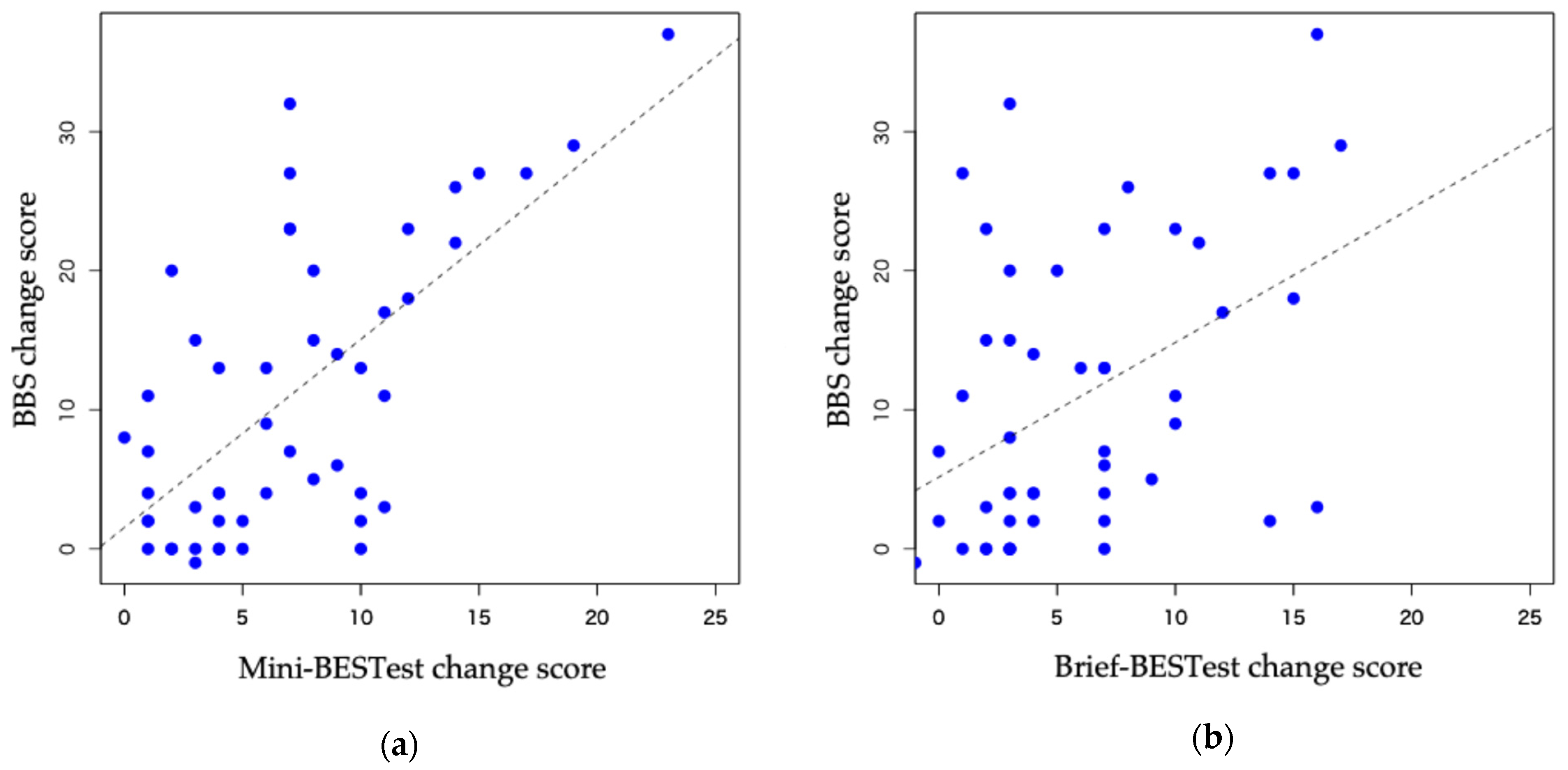
3.2. Responsiveness
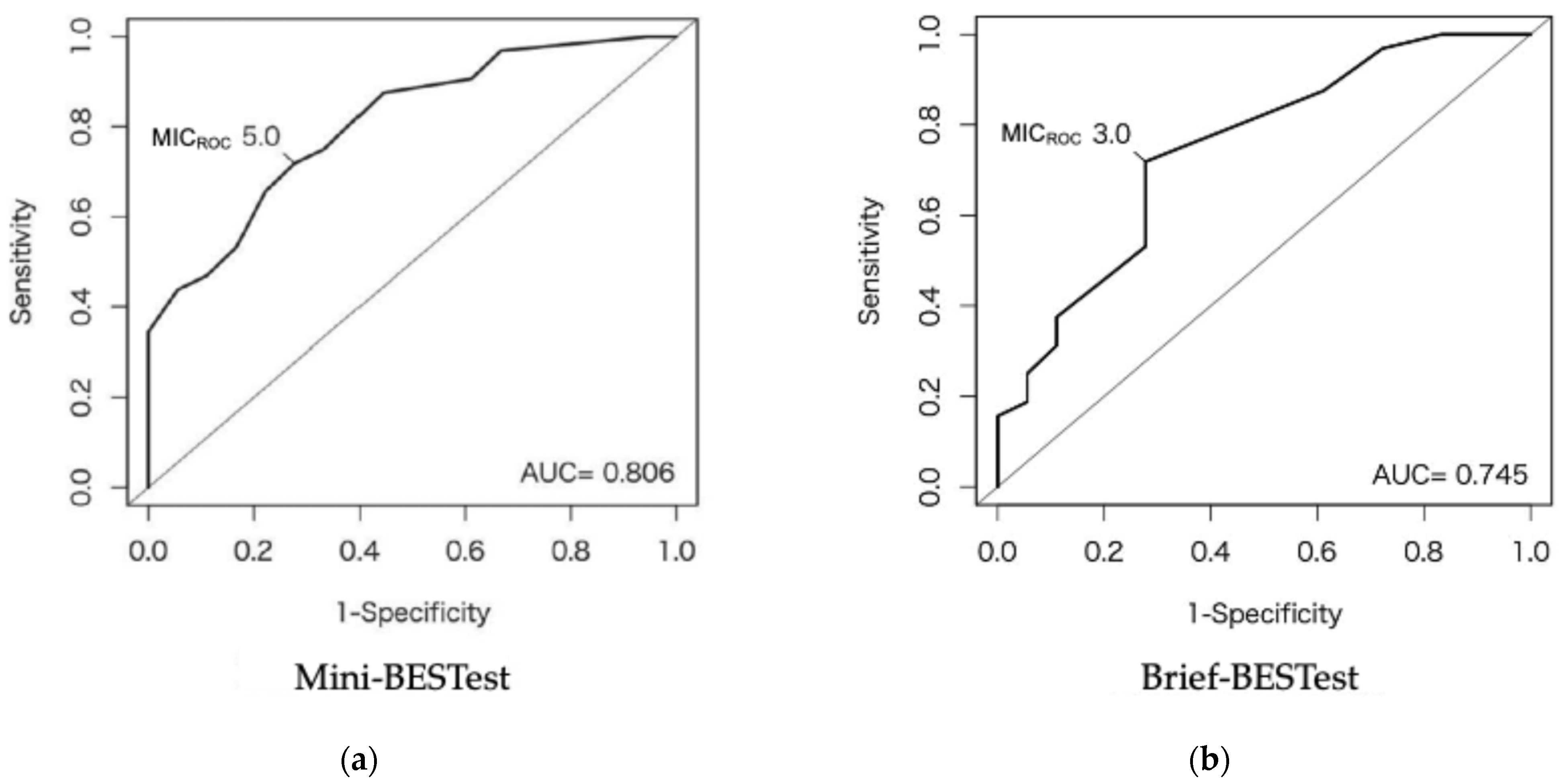
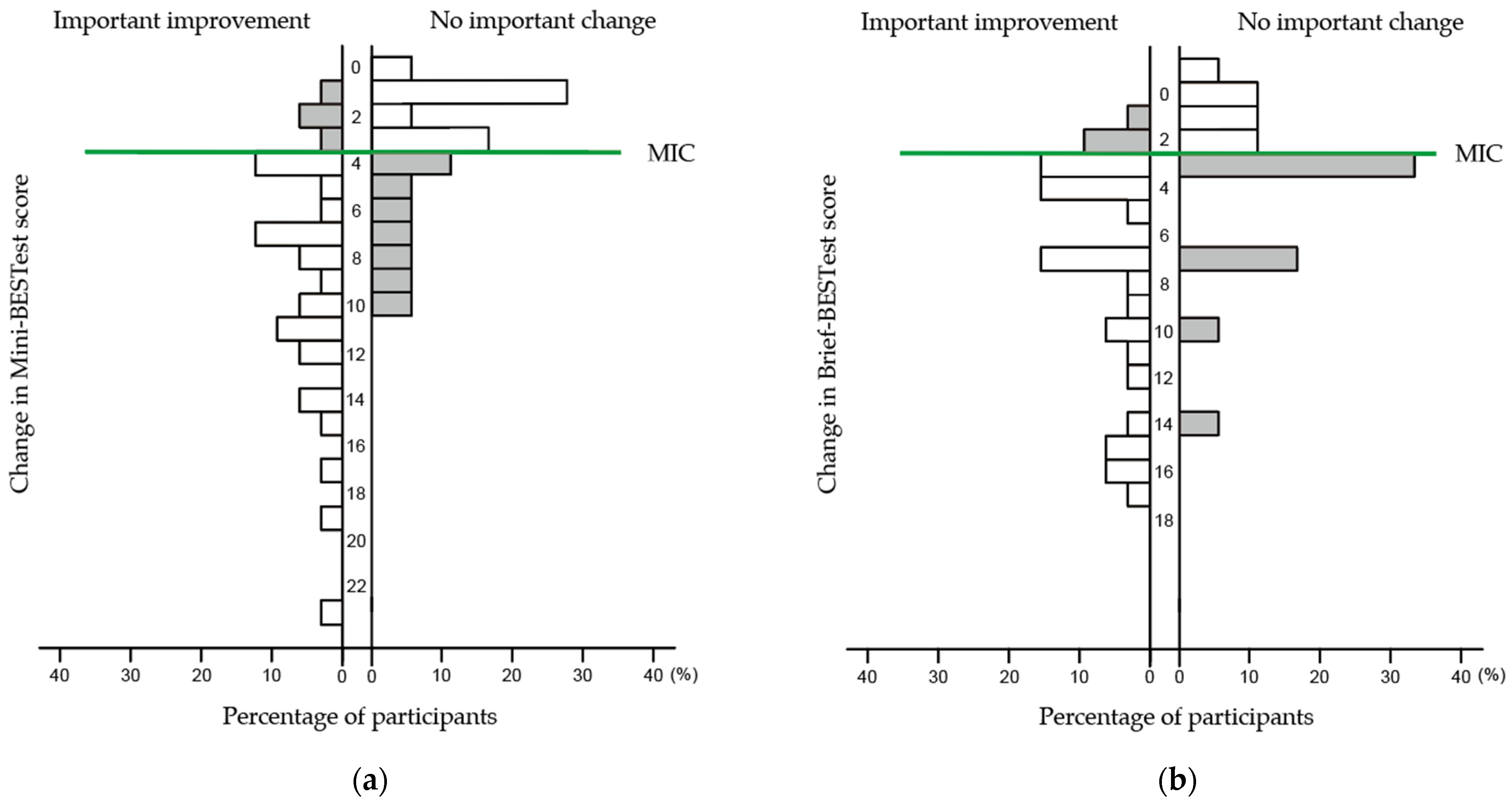
3.3. MIC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Miyakoshi, N.; Suda, K.; Kudo, D.; Sakai, H.; Nakagawa, Y.; Mikami, Y.; Suzuki, S.; Tokioka, T.; Tokuhiro, A.; Takei, H.; et al. A Nationwide Survey on the Incidence and Characteristics of Traumatic Spinal Cord Injury in Japan in 2018. Spinal Cord 2021, 59, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Scivoletto, G.; Tamburella, F.; Laurenza, L.; Torre, M.; Molinari, M. Who is Going to Walk? A Review of the Factors Influencing Walking Recovery after Spinal Cord Injury. Front. Hum. Neurosci. 2014, 8, 141. [Google Scholar] [CrossRef]
- Lemay, J.F.; Noamani, A.; Unger, J.; Houston, D.J.; Rouhani, H.; Musselmann, K.E. Using Wearable Sensors to Characterize Gait After Spinal Cord Injury: Evaluation of Test-Retest Reliability and Construct Validity. Spinal Cord 2021, 59, 675–683. [Google Scholar] [CrossRef]
- Lee, J.W.; Mauceri, S.; Chan, K.; Unger, J.; Musselman, K.E.; Masani, K. Stepping Responses for Reactive Balance for Individuals with Incomplete Spinal Cord Injury. J. Biomech. 2023, 151, 111519. [Google Scholar] [CrossRef] [PubMed]
- Noamani, A.; Lemay, J.F.; Musselman, K.E.; Rouhani, H. Characterization of Standing balance after incomplete Spinal Cord Injury: Alteration in Integration of Sensory Information in Ambulatory Individuals. Gait Posture 2021, 83, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.M.; Carpenter, M.G.; Liu-Ambrose, T.; Chisholm, A.E.; Lam, T. Attentional Requirements of Postural Control in People with Spinal Cord Injury: The Effect of Dual Task. Spinal Cord 2017, 55, 915–920. [Google Scholar] [CrossRef]
- Lemay, J.F.; Gagnon, D.; Duclos, C.; Grangeon, M.; Gauthier, C.; Nadeau, S. Influence of Visual Inputs on Quasi-Static Standing Postural Steadiness in Individuals With Spinal Cord Injury. Gait Posture 2013, 38, 357–360. [Google Scholar] [CrossRef]
- Arora, T.; Oates, A.; Lynd, K.; Musselman, K.E. Current State of Balance Assessment during Transferring, Sitting, Standing and Walking Activities for the Spinal Cord Injured Population: A Systematic Review. J. Spinal Cord Med. 2020, 43, 10–23. [Google Scholar] [CrossRef]
- Jørgensen, V.; Opheim, A.; Halvarsson, A.; Franzén, E.; Roaldsen, K.S. Comparison of the Berg Balance Scale and the Mini-BESTest for Assessing Balance in Ambulatory People with Spinal Cord Injury: Validation Study. Phys. Ther. 2017, 97, 677–687. [Google Scholar] [CrossRef]
- Franchignoni, F.; Horak, F.; Godi, M.; Nardone, A.; Giordano, A. Using Psychometric Techniques to Improve the Balance Evaluation Systems Test: The Mini-BESTest. J. Rehabil. Med. 2010, 42, 323–331. [Google Scholar] [CrossRef]
- Padgett, P.K.; Jacobs, J.V.; Kasser, S.L. Is the BESTest at its Best? A Suggested Brief Version Based on Interrater Reliability, Validity, Internal Consistency, and Theoretical Construct. Phys. Ther. 2012, 92, 1197–1207. [Google Scholar] [CrossRef]
- Paixão, C.; Rebelo, P.; Oliveira, A.; Jácome, C.; Cruz, J.; Martins, V.; Simão, P.; Marques, A. Responsiveness and Minimal Clinically Important Difference of the Brief-BESTest in People With COPD After Pulmonary Rehabilitation. Phys. Ther. 2021, 101, pzab209. [Google Scholar] [CrossRef] [PubMed]
- Godi, M.; Arcolin, I.; Giardini, M.; Corna, S.; Schieppati, M. Responsiveness and Minimal Clinically Important Difference of the Mini-BESTest in Patients with Parkinson’s Disease. Gait Posture 2020, 80, 14–19. [Google Scholar] [CrossRef]
- Winairuk, T.; Pang, M.Y.C.; Saengsirisuwan, V.; Horak, F.B.; Boonsinsukh, R. Comparison of Measurement Properties of Three Shortened Versions of the Balance Evaluation System Test (BESTest) in People with Subacute Stroke. J. Rehabil. Med. 2019, 51, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Godi, M.; Franchignoni, F.; Caligari, M.; Giordano, A.; Turcato, A.M.; Nardone, A. Comparison of Reliability, Validity, and Responsiveness of the Mini-BESTest and Berg Balance Scale in Patients with Balance Disorders. Phys. Ther. 2013, 93, 158–167. [Google Scholar] [CrossRef]
- Beauchamp, M.K.; Niebuhr, R.; Roche, P.; Kirkwood, R.; Sibley, K.M. A Prospective Study to Establish the Minimal Clinically Important Difference of the Mini-BESTest in Individuals with Stroke. Clin. Rehabil. 2021, 35, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C.; Pang, M.Y.; Ouyang, H.; Jehu, D.A. Minimal Clinically Important Difference of Four Commonly Used Balance Assessment Tools in Individuals after Total Knee Arthroplasty: A Prospective Cohort Study. PM R 2020, 12, 238–245. [Google Scholar] [CrossRef]
- Mokkink, L.; Terwee, C.; de Vet, H. Key Concepts in Clinical Epidemiology: Responsiveness, the Longitudinal Aspect of Validity. J. Clin. Epidemiol. 2021, 140, 159–162. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Curt, A.; Steeves, J.D.; Coleman, W.P.; Tuszynski, M.H.; Lammertse, D.; Bartlett, P.F.; Blight, A.R.; Dietz, V.; Ditunno, J.; et al. Guidelines for the Conduct of Clinical Trials for Spinal Cord Injury as Developed by the ICCP Panel: Spontaneous Recovery after Spinal Cord Injury and Statistical Power Needed for Therapeutic Clinical trials. Spinal Cord 2007, 45, 190–205. [Google Scholar] [CrossRef]
- Angst, F.; Aeschlimann, A.; Angst, J. The Minimal Clinically Important Difference Raised the Significance of Outcome Effects Above the Statistical Level, with Methodological Implications for Future Studies. J. Clin. Epidemiol. 2017, 82, 128–136. [Google Scholar] [CrossRef]
- Terluin, B.; Eekhout, I.; Terwee, C.B.; de Vet, H.C. Minimal Important Change (MIC) Based on a Predictive Modeling Approach was More Precise than MIC Based on ROC Analysis. J. Clin. Epidemiol. 2015, 68, 1388–1396. [Google Scholar] [CrossRef]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of Health Status. Ascertaining the Minimal Clinically important Difference. Control Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef]
- Elkins, M.R.; Pinto, R.Z.; Verhagen, A.; Grygorowicz, M.; Söderlund, A.; Guemann, M.; Gómez-Conesa, A.; Blanton, S.; Brismée, J.M.; Agarwal, S.; et al. Statistical Inference Through Estimation: Recommendations from the International Society of Physiotherapy Journal Editors. Braz. J. Phys. Ther. 2022, 26, 100387. [Google Scholar] [CrossRef]
- Revicki, D.; Hays, R.D.; Cella, D.; Sloan, J. Recommended Methods for Determining Responsiveness and Minimally Important Differences for Patient-Reported Outcomes. J. Clin. Epidemiol. 2008, 61, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, A.B.; Lopez, J.A.; Lopez, J.J.; Martinez, I. Psychometric properties of the Mini-Balance Evaluation Systems Test (Mini-BESTest) among multiple populations: A COSMIN systematic review and meta-analysis. Disabil. Rehabil. 2025. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.T.; Lin, C.Y.; Tsang, W.W.N.; Yan, C.H.; Wong, A.Y.L. Psychometric Properties of Brief-Balance Evaluation Systems Test Among Multiple Populations: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 155–175.e152. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Morooka, Y.; Takakura, Y.; Kunisawa, Y.; Okubo, Y.; Araki, S.; Obayashi, S. Reliability of the Mini-BESTest and Brief-BESTest for Assessing Patients with Incomplete Spinal Cord Injury. Spinal Cord 2024, 62, 676–682. [Google Scholar] [CrossRef]
- Berg, K. Measuring Balance in the Elderly: Preliminary Development of an Instrument. Physiother. Can. 1989, 41, 304–311. [Google Scholar] [CrossRef]
- Tamburella, F.; Scivoletto, G.; Iosa, M.; Molinari, M. Reliability, Validity, and Effectiveness of Center of Pressure Parameters in Assessing Stabilometric Platform in Subjects with Incomplete Spinal Cord Injury: A Serial Cross-sectional Study. J. Neuroeng. Rehabil. 2014, 11, 86. [Google Scholar] [CrossRef]
- Datta, S.; Lorenz, D.J.; Harkema, S.J. Dynamic Longitudinal Evaluation of the Utility of the Berg Balance Scale in Individuals with Motor Incomplete Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2012, 93, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Kamper, S.J.; Maher, C.G.; Mackay, G. Global Rating of Change Scales: A Review of Strengths and Weaknesses and Considerations for Design. J. Man. Manip. Ther. 2009, 17, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Franchignoni, F.; Ferriero, G.; Giordano, A.; Monticone, M.; Grioni, G.; Burger, H. The Minimal Clinically-important Difference of the Prosthesis Evaluation Questionnaire—Mobility Scale in Subjects Undergoing Lower Limb Prosthetic Rehabilitation Training. Eur. J. Phys. Rehabil. Med. 2020, 56, 82–87. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.W.; Knol, D.L.; Bouter, L.M.; de Vet, H.C. The COSMIN Checklist for Assessing the Methodological Quality of Studies on Measurement Properties of Health Status Measurement Instruments: An International Delphi Study. Qual. Life Res. 2010, 19, 539–549. [Google Scholar] [CrossRef]
- Terluin, B.; Eekhout, I.; Terwee, C.B. The Anchor-based Minimal important change, based on receiver operating Characteristic Analysis or Predictive Modeling, May Need to be Adjusted for the Proportion of Improved Patients. J. Clin. Epidemiol. 2017, 83, 90–100. [Google Scholar] [CrossRef]
- Terwee, C.B.; Peipert, J.D.; Chapman, R.; Lai, J.S.; Terluin, B.; Cella, D.; Griffiths, P.; Mokkink, L.B. Minimal Important Change (MIC): A Conceptual Clarification and Systematic Review of MIC Estimates of PROMIS Measures. Qual. Life Res. 2021, 30, 2729–2754. [Google Scholar] [CrossRef]
- Terwee, C.B.; Bot, S.D.; de Boer, M.R.; van der Windt, D.A.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C. Quality Criteria were Proposed for Measurement Properties of Health Status Questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef]
- de Vet, H.C.; Ostelo, R.W.; Terwee, C.B.; van der Roer, N.; Knol, D.L.; Beckerman, H.; Boers, M.; Bouter, L.M. Minimally Important Change Determined by a Visual Method Integrating an Anchor-based and a Distribution-based Approach. Qual. Life Res. 2007, 16, 131–142. [Google Scholar] [CrossRef]
- Amsterdam Public Health [Internet]. Amsterdam: Consensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN). Available online: http://www.cosmin.nl (accessed on 6 September 2024).
- Crosby, R.D.; Kolotkin, R.L.; Williams, G.R. Defining Clinically Meaningful Change in Health-related Quality of Life. J. Clin. Epidemiol. 2003, 56, 395–407. [Google Scholar] [CrossRef]
- Mizuochi, K. Rehabilitation Medicine in the Acute Care Setting in Japan. Japan Med. Assoc. J. 2012, 55, 246–252. [Google Scholar] [PubMed]


| Participants (n = 50) | Dropouts (n = 19) | |
|---|---|---|
| Sex (Males/Females) | 37/13 | 15/4 |
| Age (years) | 68.3 (13.4) | 69.6 (9.3) |
| AIS (A,B,C/D) | 0/50 | 0/19 |
| NLI | ||
| C4 | 9 (18.0) | 3 (15.8) |
| C5 | 13 (26.0) | 4 (21.1) |
| C6 | 5 (10.0) | 3 (15.8) |
| C7 | 5 (10.0) | 1 (5.3) |
| C8 | 18 (36.0) | 8 (42.1) |
| Causes of injury | ||
| Falls on level surface | 20 (40.0) | 6 (31.6) |
| Fall from a height | 16 (32.0) | 4 (21.1) |
| Traffic accident | 5 (10.0) | 2 (10.5) |
| Other | 9 (18.0) | 7 (36.4) |
| Time from injury to rehabilitation (days) | 3.2 (1.4) | 2.8 (1.3) |
| Time from injury to assessment (days) | 5.0 (1.5) | 4.6 (1.5) |
| Between assessment (days) | 15.3 (8.7) | – |
| UEMS (0–50) | 41.0 (9.7) | 43.8 (6.6) |
| LEMS (0–50) | 48.1 (3.0) | 48.3 (3.0) |
| Walking aid (Walker/None) | 23/27 | 4/15 |
| BBS (0–56) | 40.3 (15.1) | 46.3 (10.2) |
| Group | Baseline | Follow-Up | Change Score | p-Value | ||
|---|---|---|---|---|---|---|
| Baseline vs. Follow-Up | Improved vs. Unimproved | |||||
| Mini-BESTest | Improved (n = 32) | 13.5 (7.2) | 22.3 (5.5) | 8.8 (5.2) | <0.05 * | <0.05 † |
| Unimproved (n = 18) | 15.5 (7.6) | 19.3 (7.3) | 3.8 (3.1) | <0.05 * | ||
| Brief-BESTest | Improved (n = 32) | 8.2 (7.0) | 15.6 (6.1) | 7.4 (4.9) | <0.05 * | <0.05 ‡ |
| Unimproved (n = 18) | 8.6 (6.9) | 12.4 (8.2) | 3.8 (3.8) | <0.05 * | ||
| Mini-BESTest | Brief-BESTest | |
|---|---|---|
| MICROC | 5.0 | 3.0 |
| AUC (95% CI) | 0.81 (0.69–0.93) | 0.75 (0.60–0.89) |
| Sensitivity | 71.9 | 71.9 |
| Specificity | 72.2 | 72.2 |
| MICpredict | 4.1 | 2.6 |
| MICadjusted | 3.7 | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morooka, Y.; Kunisawa, Y.; Obayashi, S.; Takakura, Y. Responsiveness and Minimal Important Change of the Mini- and Brief-Balance Evaluation Systems Tests in People with Incomplete Cervical Spinal Cord Injury: A Prospective Cohort Study. Neurol. Int. 2025, 17, 43. https://doi.org/10.3390/neurolint17030043
Morooka Y, Kunisawa Y, Obayashi S, Takakura Y. Responsiveness and Minimal Important Change of the Mini- and Brief-Balance Evaluation Systems Tests in People with Incomplete Cervical Spinal Cord Injury: A Prospective Cohort Study. Neurology International. 2025; 17(3):43. https://doi.org/10.3390/neurolint17030043
Chicago/Turabian StyleMorooka, Yusuke, Yosuke Kunisawa, Shigeru Obayashi, and Yasuyuki Takakura. 2025. "Responsiveness and Minimal Important Change of the Mini- and Brief-Balance Evaluation Systems Tests in People with Incomplete Cervical Spinal Cord Injury: A Prospective Cohort Study" Neurology International 17, no. 3: 43. https://doi.org/10.3390/neurolint17030043
APA StyleMorooka, Y., Kunisawa, Y., Obayashi, S., & Takakura, Y. (2025). Responsiveness and Minimal Important Change of the Mini- and Brief-Balance Evaluation Systems Tests in People with Incomplete Cervical Spinal Cord Injury: A Prospective Cohort Study. Neurology International, 17(3), 43. https://doi.org/10.3390/neurolint17030043






