Abstract
Paired associative stimulation (PAS) is a non-invasive brain stimulation technique combining transcranial magnetic stimulation and peripheral nerve stimulation. PAS allows connections between cortical areas and peripheral nerves (C/P PAS) or between cortical regions (C/C PAS) to be strengthened or weakened by spike-timing-dependent neural plasticity mechanisms. Since PAS modulates both neurophysiological features and motor performance, there is growing interest in its application in neurorehabilitation. We aimed to synthesize evidence on the motor rehabilitation role of PAS in stroke patients. We performed a literature search following the PRISMA Extension for Scoping Reviews Framework. Eight studies were included: one investigated C/C PAS between the cerebellum and the affected primary motor area (M1), seven applied C/P PAS over the lesional, contralesional, or both M1. Seven studies evaluated the outcome on upper limb and one on lower limb motor recovery. Although several studies omit crucial methodological details, PAS highlighted effects mainly on corticospinal excitability, and, more rarely, an improvement in motor performance. However, most studies failed to prove a correlation between neurophysiological changes and motor improvement. Although current studies seem to suggest a role of PAS in post-stroke rehabilitation, their heterogeneity and limited number do not yet allow definitive conclusions to be drawn.
1. Introduction
Physiological reactions are frequent following stroke and aim to repair the damaged tissue. Plasticity refers to the ability of the brain to modify its structure and function in response to experience and environmental demand [1]. This enhanced plasticity following brain damage leads to new axon sprouting, new synapse formation, and the remapping of sensory–motor areas [2]. Several studies confirm a close relationship between neuroplasticity and functional recovery following stroke [3]. Changes in the activity and connection between neurons can be identified around the lesion up to remote areas or in the contralateral hemisphere, explaining spontaneous recovery after cerebral damage [4]. Post-stroke rehabilitation aims to improve functional recovery and promote neuroplasticity, supporting this dynamic process in rebuilding connections between neurons [5]. Non-invasive brain stimulation (NIBS) techniques are a promising adjuvant strategy for enhancing post-stroke recovery through the modulation of cortical excitability and neuronal plasticity [6]. The combination of NIBS and motor or behavioral intervention has gained substantial interest over the last years due to the promising potentiality that the combined approach offers [3]. Several studies on post-stroke patients combined NIBS and rehabilitative approaches such as intensive physiotherapy or occupational therapy [7,8], robot-assisted training [9,10,11], virtual reality rehabilitation [12,13,14,15], and task-oriented training [16] for promoting motor recovery. Between the available NIBS techniques, transcranial magnetic stimulation (TMS) has been used to investigate and induce plasticity in the human brain [17]. The combination of TMS and peripheral nerve electrical stimulation (PNS) is known as paired associative stimulation (PAS). PAS is an emerging NIBS approach, introduced by Stefan et al. [18], which uses the Cell Assembly Theory first formulated by Hebb in 1949 [19,20]. Hebb postulated that repeated activation of a presynaptic cell immediately before the activation of a postsynaptic cell induces synaptic strengthening, so-called long-term potentiation (LTP). Hebb did not propose an opposite activity-dependent reduction in synaptic strength or long-term depression (LTD). Indeed, later work described a heterosynaptic LTD, when a presynaptic cell repeatedly and persistently fails to excite the postsynaptic cell [21], and a homosynaptic mechanism based on low-frequency stimulation of the presynaptic element [22]. Studies on animal models have shown how PAS can influence motor cortex excitability, whereas TMS or PNS, commonly used in rehabilitation, showed no significant effect when used alone [23]. PAS’s effect on the human brain was first studied on healthy subjects, and the observed increase in Motor Evoked Potential (MEP), the response induced by a TMS pulse over the Primary Motor Cortex (M1), suggested the plasticity of brain structures [24,25]. Many single-session studies explored the effects of different PAS protocols in stroke patients [26,27,28]. Even though promising results were found on cortical excitability and motor performance, no results were found on repeated sessions of PAS, particularly when combined with rehabilitative treatment. Several mechanisms may justify the use of PAS-empowered rehabilitative approaches: First, the increase in corticomuscular excitability induced by PAS may favor the subsequent response to neurorehabilitation treatment [28,29]. Furthermore, PAS protocols act on circuits involved in use-dependent plasticity, reinforcing connections useful for performing a specific motor task during rehabilitation [30,31]. However, although the effectiveness of PAS in stroke rehabilitation is still unclear, the emerging interest in this NIBS technique makes it necessary to summarize the current evidence on PAS-empowered motor rehabilitation. Thus, this work aims to review the available literature on PAS for motor rehabilitation following stroke. Moreover, we aimed to provide information about parameters and sites of stimulation, as well as outcomes and patients who could benefit from PAS. Due to the heterogeneity of evidence in this field, we applied a scoping review approach following the Preferred Reporting Systems for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) Framework [32].
2. Materials and Methods
The protocol of this scoping review has been redacted following the PRISMA-ScR Framework and has been pre-registered on an Open Science Framework (OSF) with the following doi: https://doi.org/10.17605/OSF.IO/86UAC (accessed on 20 October 2022).
2.1. Search Strategy
The PICO framework was used to define the research question. Articles published in peer-reviewed journals and pre-peer-reviewed web publications were potentially eligible for inclusion. The literature search was performed in the following electronic bibliographic databases: PubMed, Web of Science, Science Direct, and Embase. The database search was completed on 21 September 2022 and frequently updated until 31 December 2023. The search strategy included a controlled vocabulary and keywords adapted to the characteristics of the single database. A comprehensive description of the search strategy is available as a Supplemental Material (see Supplementary Materials). All the studies carried out on post-stroke adult patients where a PAS treatment was applied for the rehabilitation of motor function were considered. Only studies applying more than a single session on consecutive days were included. No restrictions on rehabilitation settings were used.
2.2. Study Inclusion/Exclusion Criteria
The study population includes adult stroke patients, without regard to the type of lesion (ischemic or hemorrhagic), time from injury, and site of brain damage, who underwent PAS as a rehabilitation treatment, in combination or not with other rehabilitation techniques. We considered eligible multi-session clinical trials (RCT, nRCT, and pre–post studies) with or without a comparator. Inclusion criteria were (i) reference in English; (ii) study subjects and setting as described above; and (iii) studies that describe the application of PAS as a rehabilitative approach for upper or lower limb in stroke patients. Exclusion criteria were (i) studies regarding PAS in patients with different pathologies other than stroke; (ii) studies evaluating the effects of PAS on non-motor outcomes in stroke patients (i.e., dysphagia).
2.3. Study Selection
Duplicate articles were excluded. Two independent reviewers (A.A. and G.F.) screened the title and abstract, and disagreement between them was solved by a third reviewer (A.B.). A.A. and G.F. reviewed the full text of the selected studies, and discordance was solved by A.B. and/or S.S. (see Figure 1).
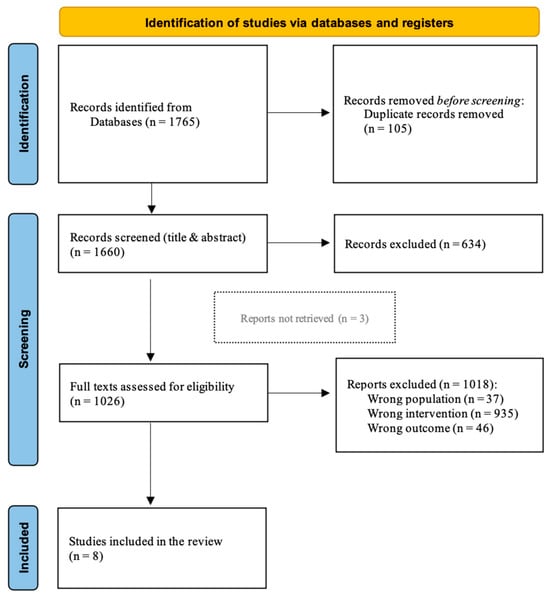
Figure 1.
PRISMA review flow-chart.
2.4. Data Extraction
Two authors (A.B. and A.A.) independently extracted data using a pre-defined framework. The data framework included a field for the author(s), year of publication, country of origin, study design, sample size, type of stroke, time from stroke, PAS parameters (type, points of application, intensity and frequency of stimulation, ISI, and time of application), associated treatments, comparator details, outcome measures, and possible adverse effects related to treatment. The critical appraisal of the included papers was performed using the Cochrane Risk of Bias Tool (RoB) for RCTs [33]; nRCT and pre–post studies were evaluated using the Joanna Briggs Institute (JBI) critical appraisal tool [34]. Due to the nature of the project and the heterogeneity of the included studies, a narrative collection of results was planned.
3. Results
Database searching identified 1765 records. After removing duplicates, 1660 records were screened for the title and abstract and 634 records were excluded. Of the 1026 remaining papers, 1018 did not meet inclusion criteria and were excluded from the collection: 37 studies applied PAS to a different population, 935 studies applied a different stimulation protocol, and 46 studies evaluated a different outcome. Among the eight remaining studies [35,36,37,38,39,40,41,42], three full texts were unavailable [39,40,41]; however, we decided to include them in the scoping review due to the poor literature on the topic (Figure 1). Seven included studies were RCTs [34,35,36,37,38,39,40], and only one was a case series study [42]. All the studies were published in the last 20 years with a wide geographic distribution: four in France and one each in China, Turkey, Australia, and Ukraine. Most of the studies involved patients with ischemic stroke [35,36,38,39], two studies involved stroke patients without distinction between hemorrhagic or ischemic etiology [37,42], and two studies did not specify stroke origin [40,41]. Two studies involved chronic stroke patients (>six months post-stroke) [35,42], five studies involved subacute patients (1 to 6 months post-stroke) [36,37,38,40,41], and one study did not specify the time from stroke onset [39]. A total of 288 subjects were recruited. The median number of patients involved in the studies was 27.5 patients (IQR 24.75–40.25); of them, 16 (IQR 13.5–20.0) were male (one study did not specify this data [34]). Using available data, a median number of 13 (IQR 11.5–15) patients received real stimulation with different PAS protocols (one study did not specify patients’ distribution among treatment groups [39]). A detailed description of the included studies is reported in Table 1 and Table 2.

Table 1.
Characteristics of included studies. Abbreviations: PAS = paired associative stimulation; TMS = transcranial magnetic stimulation; RCT = randomized controlled trial; RoB = risk of bias; * = conference abstract.

Table 2.
Clinical characteristics of the study populations, and PAS parameters and types of treatment. Abbreviations: PAS = paired associative stimulation; TMS = transcranial magnetic stimulation; PNS = peripheral nerve stimulation; ISI = Interstimulus Interval; C/C = Cortico-Cortical; C/P = Cortico-Peripheral; M1 = Primary Motor Cortex; RMT = Resting Motor Threshold; MEP = Motor Evoked Potential; ECR = Extensor Carpi Radialis; EDC = Extensor Digitorum Communis; NMES = Neuromuscular Electrical Stimulation; CS = Conditioning Stimulus; TS = Test Stimulus; FENS = Functional Electrical Stimulation; FMA-UE = Fugl-Meyer Assessment Upper Extremity; fMRI = functional Magnetic Resonance Imaging; MI = Motricity Index; BRS-UE = Brunnstrom Recovery Stages Upper Extremity; JHFT = Jebsen Hand Function Test; GS = Grip Strength; STEF = Simple Test for Evaluating and Function; BRS-H = Brunnstrom Recovery Stages Hand; MAS = Modified Ashworth Scale; MCAS = Motor Club Assessment Scale; BI = Barthel Index; M(BI) = Modified Barthel Index; MVC = Maximum voluntary contraction; ROM = Range of Motion; * = Conference abstract.
3.1. PAS Procedures
C/P stimulation was the PAS type most used in the included studies [36,37,38,39,40,41,42]. Only one study adopted a C/C protocol [35]. Most studies used the PAS protocol for upper limb treatment [35,36,37,38,39,40,41]. Only one of the C/P studies aimed to improve lower limb function and gait [42].
3.1.1. Cortico-Peripheral PAS
TMS was applied over the lesioned M1 in two of the included C/P studies [37,40]. One study stimulated the contralesional M1 [38]. One study stimulated both lesional and contralesional M1 in two different groups of treatment [36]. Three studies did not specify the TMS point of application [39,41,42]. Only two studies specified TMS intensity and stimulation frequency [36,38]. PNS was applied to the affected upper [37,41] or lower [42] extremity in three cases. One study used PNS in both hands in two different groups of treatment [36]. Two studies specified the site of stimulation but not the side [38,40]. One study did not specify the PNS point of application [39]. Only four studies defined both these parameters regarding the intensity and frequency of PNS [36,37,38,42]. Only four studies specified the ISI [36,37,40,42]: three of them applied the two stimuli with an ISI of 25 ms or 35 ms for an LTP effect [37,40,42]; and one used an ISI of 25 ms or 10 ms in two different groups of treatment to achieve LTP or LTD, respectively [36].
3.1.2. Cortico-Cortical PAS
The study that applied C/C PAS stimulated the contralesional cerebellum and the lesional M1, specifying intensity, the frequency of stimulation, and ISI [35].
3.2. Treatment Duration
A median number of 10 (IQR 5–19.5) sessions of PAS was applied in the included study, with a minimum of 5 [35,37,40,41] and a maximum of 28 [36,42]. Only two studies quantified PAS duration in 30 min [41,42].
3.3. Associated Treatments
Four studies combined PAS with motor rehabilitation [35,36,37,38]. Specifically, three studies applied PAS before rehabilitation treatment [35,36,38], whereas one did not specify the order of the combined treatment [37]. The motor rehabilitation consisted of active-assisted range of motion exercises combined with motor imagery, strength training against gravity, and task-specific training [35]; good limb placement, bed movement, transfer training, operation treatment, daily life activity training, and other comprehensive rehabilitation treatment [36]; and activities to improve strength, flexibility, transfers, posture, balance, coordination, and activities of daily living [38]. Four studies did not specify these data [39,40,41,42].
3.4. Comparators
The most used comparator treatment was sham stimulation [35,37,39,40]: two studies delivered it through a sham coil applied following the same procedures used for real stimulation [35,37]; and two studies applied sham stimulation without describing the sham procedure [39,40]. One study applied a not-specified placebo as a treatment comparator [41]. Two studies used only physical therapy for the patients assigned to the control group [36,38].
3.5. Outcome Measures
3.5.1. Neurophysiological Measures
MEP, Resting Motor Threshold (RMT), and functional Magnetic Resonance Imaging (fMRI) were used to assess the effects of PAS on corticospinal excitability. MEP was the most used outcome measure of PAS efficacy [35,36,37,39,40,41,42]. Six studies reported an increase in MEP amplitude [35,36,39,42] and/or surface area (the level of corticospinal projections excitability of the target muscle) [37,39,41] in the experimental group compared to the control; however, out of all these, only four studies reported quantitative results [35,37,41,42] but no statistically significative results in both within- and between-group comparisons. One study reported a significant increase in the MEP amplitude of groups who received PAS in different protocols without reporting quantitative data (only graphs available) [36]. RMT is the amount of TMS machine output necessary to produce an MEP that exceeds an established peak-to-peak amplitude (usually 50 μV) 50% of the time in a finite number of trials [43,44]. RMT was recorded in two studies [36,39]: one study reported a significant reduction in RMT of the lesioned side in the groups who received real stimulation, only through qualitative and graphical results [36]. Kuznietsova et al. described a reduction in RMT in the experimental group without reporting numerical data [39]. fMRI was recorded in two studies and revealed increased activation of the affected hemisphere without reaching statistical significance [35,38].
3.5.2. Clinical Measures
The efficacy of PAS on upper limb function was evaluated using the Fugl-Meyer Assessment for Upper Extremity (FMA-UE) [35,36,37,39,40], the Motricity Index (Upper Extremity section) (MI-UE) [38], and the upper extremity section of the Brunnstrom Recovery Stages (BRS-UE) [38]. One study reported a significant improvement in FMA-UE score in the experimental group compared to the control, reporting only qualitative data and graphical results [36]. All other studies evaluating upper limb function did not record significant differences between the experimental and the control group [37,38,40,41]. Changes in hand function following PAS were evaluated using the Jebsen Hand Function Test (JHFT) [35], the grip strength (GS) [35], the Simple Test for Evaluating Hand Function (STEF) [36], and the hand section of the Brunnstrom Recovery Stages (BRS-H) [38]. No significant changes were recorded between the study groups. No effects of PAS on muscle tone [38], cognitive, and emotional function [36] were documented. Two studies assessed the efficacy of PAS on the ability to perform activities of daily living [36,38]. Of these, one study reported a significant improvement in the Barthel Index score only in the group that received real stimulation [36]. The efficacy of PAS on lower limb function (maximum voluntary contraction and range of motion) and activities (walking) was evaluated, and no changes were found between pre- and post-treatment [42]. Table 3 summarizes these data.

Table 3.
Outcome measures and study results following the International Classification of Functioning (ICF) model. Abbreviations: PAS = paired associative stimulation; PT = physical therapy; TMS = transcranial magnetic stimulation; PNS = peripheral nerve stimulation; ISI = Interstimulus Interval; FMA-UE = Fugl-Meyer Assessment Upper Extremity; fMRI = functional Magnetic Resonance Imaging; FAr = Fractional anisotropy ratio; CST = Corticospinal tract; DTCT = Dentate-thalamo-cortical tracts; MI = Motricity Index; BRS-UE = Brunnstrom Recovery Stages Upper Extremity; JHFT = Jebsen Hand Function Test; GS = Grip strength; STEF = Simple Test for Evaluating and Function; BRS-H = Brunnstrom Recovery Stages Hand; MAS = Modified Ashworth Scale; MCAS = Motor Club Assessment Scale; BI = Barthel Index; M(BI) = Modified Barthel Index; MVC = Maximum Voluntary Contraction; ROM = Range of Motion; MEP-TA = Motor Evoked Potentials recorded from Tibialis Anterior muscle; MEP-PL = Motor Evoked Potentials recorded from Peroneus Longus; MVC-TA = Maximum Voluntary Contraction measured from Tibialis Anterior; MVC-PL = Maximum Voluntary Contraction measured from Peroneus Longus; MRC = Medical Research Council Scale; C/C = Cortico-Cortical; C/P = Cortico-Peripheral; M1 = Primary Motor Cortex; RMT = Resting Motor Threshold; MEP = Motor Evoked Potential; ECR = Extensor Carpi Radialis; EDC = Extensor Digitorum Communis; NMES = Neuromuscular Electrical Stimulation; CS = Conditioning Stimulus; TS = Test Stimulus; FENS = Functional Electrical Stimulation; D5 = Day five; D30 = Day thirty.
3.6. Adverse Effects
Possible adverse effects of PAS were recorded only in three of the included studies [35,37,38]. Two subjects showed temporary headaches after stimulation: both received C/C PAS and were allocated one in the real stimulation group and one in the control group [35]. One subject showed reflex syncope immediately after the end of the C/C sham PAS [35]. Two studies did not report adverse effects [37,38].
3.7. Quality Assessment
Considering the risk of bias evaluation of the included studies for which the full text was available [34,35,36,37,41], a heterogeneous methodological quality was noticed (Table 1). Particularly, among the RCTs involved, two studies showed an overall low risk of bias [35,38], while in the other two [36,37], the absence of explicit information on different methodological key points did not allow a precise estimation of the related methodological quality [32].
4. Discussion
Although recent studies have provided a better understanding of the neurophysiological mechanism underlying PAS, and supporting its contribution to stroke recovery, few studies have specifically investigated its role in rehabilitation. This lack may be due to their relatively recent introduction in the clinical setting, which makes further studies necessary to evaluate the applicability of this technique for patient recovery [45]. Moreover, the technologies required to implement PAS into practice are extremely expensive and require specific skills not always available outside the research settings [46]. However, considering their potential from a neurorehabilitation perspective, here, to the best of our knowledge, we have gathered evidence on PAS-empowered post-stroke motor rehabilitation. In our review, we aimed to synthesize the state-of-the-art of PAS as an adjuvant to stroke rehabilitation, identifying parameters, sites of stimulation, and patients who can benefit from this combined stimulation. The implications of our results will be discussed, considering the C/P and C/C PAS studies separately.
4.1. C/P PAS
M1 represents the most frequently stimulated area due to its relatively easy accessibility with NIBS techniques, as well as the possibility of measuring the effects of its modulation (e.g., RMT and MEPs recorded from target muscles) [43,44]. Moreover, M1 is a crucial part of a wide network responsible for the regulation of motor acts where sensory stimuli, exogenous and endogenous, play a key role [47].
Several studies have shown that PNS can inhibit the subsequent homotopic muscle response evoked by a TMS pulse on M1, leading to a decrease in MEP amplitude, depending on the specific temporal interval between the sensory and the motor stimulus [48]. This phenomenon is referred to as short-latency afferent inhibition and highlights a close coupling between sensory and motor networks, dependent on the modulation of inhibitory circuits exerted by excitatory cholinergic thalamocortical afferents [48]. Therefore, considering the importance of sensorimotor integration in motor control, it is unsurprising that PAS protocols target M1 in combination with accessible peripheral regions [28,49]. Consistently, most of the included studies stimulated the impaired hand, with a particular focus on the extensor muscles, frequently impaired after stroke. By contrast, few studies stimulated the median or, generically, the whole paretic hand [50]. Interestingly, only one study applied PAS stimulation to both hands, using an excitatory protocol on the paretic one and an inhibitory protocol on the healthy one [36]. This study design is present (when the information is available) in most of the other included studies. However, limited to the paretic hand, it is based on the model developed by Di Pino et al. on post-stroke interhemispheric disequilibrium: after stroke, the normal reciprocal inhibition between the two hemispheres is altered and the damaged hemisphere is no longer able to adequately counteract the healthy one, which therefore exerts a marked inhibition on the injured hemisphere hindering the recovery of impaired functions [51]. Although recent models have considered the role of other factors in addition to the mere distinction between the injured and healthy hemisphere, this interpretation has been widely used showing remarkable efficacy in the recovery of common symptoms after stroke [52,53]. Consistently, studies that exploited this interpretative model, like the one of Sui et al. [36], have demonstrated an improvement in neurophysiological parameters, i.e., an increase in MEPs’ amplitude and a decrease in the RMT of the damaged M1 (and changes in the opposite direction on healthy M1 when stimulated using an inhibitory protocol), and in motor and functional recovery.
However, it is crucial to note that statistically significant changes following PAS were observed only for the FMA score [36], showing a dissociation between neurophysiological and clinical measures. This phenomenon could reflect that functional changes observed in the subjects cannot be solely attributed to physiological modifications and clinical measures may be too coarse to detect these changes.
Regarding the quality of the reporting, some papers did not show their results except in the abstract or graphical form, making it complex to evaluate what was achieved [36,39]. In contrast, other studies used outcome measurement scales that are scarcely used internationally (e.g., Motor Club Assessment Scale, MCAS), making the generalization of the obtained results difficult [39]. Looking at the stroke timeframe, half of the studies evaluated patients in the subacute phase (between 1 and 6 months), three studies evaluated the chronic phase (>6 months), and one considered patients in the acute/subacute phase (<6 months). This choice may depend on the need to reconcile, on the one hand, the clinical stabilization of the patient (normally difficult to achieve in the acute phase) and, on the other, to exploit the interval of increased cortical plasticity that gradually decreases over time [54,55]. In this sense, the subacute phase seems to be the most suitable to reconcile these needs [56]. The study of Sui et al. showed significant changes in sub-acute patients following PAS [36], offering insights into applying this protocol to improve motor function even after the acute injury.
We cannot draw definitive conclusions about the number of treatment sessions due to the heterogeneity of the studies. Sui et al. found a functional improvement after 28 sessions of stimulation [36]; therefore, fewer sessions may not be sufficient to achieve this goal.
Only one study used PAS to improve lower limb function; the combined stimulation of M1 and the common peroneal nerve of the affected leg showed neurophysiological changes in the related brain areas and a functional improvement in gait [42]. The reason why the lower limb is much less investigated surely depends on its mesial area of cortical representation, less accessible with NIBS techniques. In addition, upper limb impairment most frequently afflicts stroke survivors’ daily autonomy, making the evaluation of lower limb recovery less frequent in the literature [57,58,59]. Functional improvements are observed, even in this case, in chronic patients, making the application of PAS of great interest in patients with gait impairment following stroke.
4.2. C/C PAS
PAS protocols aimed at improving connections between two or more brain areas (C/C PAS) have been more recently exploited to strengthen or weaken connections based on the timing of the stimulations [60]. Indeed, cortical areas are interconnected by extensive fiber bundles, both intra- and inter-hemispheric, and these reciprocal connections are crucial for the modulation of numerous activities and, in particular, motor actions [61,62]. Recent studies have shown that C/C PAS on areas involved in motor control induces significant changes, not only in neurophysiological parameters but also in motor actions [63,64], making its application of growing interest in the rehabilitation field. Consistently, several RCTs are underway to evaluate its potential in combination with various rehabilitative approaches like upper limb robot-assisted therapy [65].
Recently, several advanced PAS techniques involving combined trans-modality stimulation (e.g., between motor cortical areas and visual or acoustic ones) have been employed, remaining tied to research contexts despite promising results [66]. Therefore, although these techniques are likely to become part of stroke rehabilitation in the future, at the moment, we have limited our discussion to the study that we included in our review. Rosso et al. used a more explored “within” motor system protocol that exploited the long-range connections of the cerebellum and M1 [35], like the dentate nucleus–thalamus–cortical pathway [67]. Indeed, the contralesional cerebellum plays a significant role in the reorganization of the motor network and during the recovery process following stroke [68]. Notably, stroke patients often need to relearn basic motor strategies, a process actively governed by the cerebellum [69] that can be empowered by the simultaneous application of NIBS techniques [70].
Consistently, Rosso et al. found a significant improvement in hand function at one-month follow-up [35], leading to the hypothesis that cerebellar modulation influences motor output through morphological modifications and LTP mechanisms in the motor areas. These changes are possible in the presence of integral afferent and efferent circuits of M1 as necessary substrates for functional improvements [71]. Improvements in motor outcomes were achieved with only five days of stimulation in a group of patients who had suffered from stroke years earlier; therefore, C/C PAS seems to offer an attractive rehabilitative opportunity in chronic patients, even with a small number of sessions, probably due to its focus on the CNS, free from the influences of various peripheral factors that could reduce the effectiveness of the treatment [72].
In line with our findings, a promising role for PAS has been demonstrated in other neurological disorders, such as spinal cord injury (SCI). Neuromodulatory effects of PAS have been proposed to improve functional outcomes because of cortico-spinal and cortico-peripheral stimulation protocols [73,74]. C/P PAS, particularly, has demonstrated excellent results in terms of corticospinal transmission and functional outcome, reasonably exploiting the spike-timing-dependent plasticity principles similar to those described in this review [75,76]. However, while SCI affects areas of the nervous system that are remote from the higher brain centers, stroke damages a network of closely related and mutually influencing areas, making treatment outcomes more complex and difficult to predict [77,78]. Summarizing, although the low number of included studies makes it difficult to generalize the results, it is possible to highlight some crucial aspects. Firstly, clinical studies about PAS are highly heterogeneous in terms of stimulation protocol and parameters, stroke timeframe, session duration, number, comparators, and the motor and functional assessment scale. Moreover, PAS-empowered rehabilitation is widely used for upper limb recovery, compared to only one study using PAS for lower limb rehabilitation. Finally, the traditional C/P PAS paradigm, as described in the literature, is the most used for rehabilitative purposes, compared to the more recent C/C technique. A substantial number of studies omitted crucial information about the generalizability of the intervention, like the site and parameters of stimulation, or the specific site of the stroke lesion. Many studies evaluated the effects of PAS in terms of neurophysiological changes like MEPs and RMT, while only a few studies showed changes in clinical or functional scales used to evaluate the clinical correlation of neurophysiological aspects. Less than half of the studies reported no adverse effects or provided information to analyze their characteristics, which were in any case rare and generally minor and transient. Considering that the presence of adverse effects has not been evaluated in most of the included studies, the improvement in the reporting quality appears to be essential for a thorough analysis of the obtained results, and future work needs to adopt a methodology capable of addressing this issue.
Considering the above, it is necessary to underline that our scoping review aimed to synthesize evidence on PAS protocols in stroke rehabilitation, without considering the possible neurophysiological limitations of this technique, which, at present, require further investigation. Consequently, we were unable to quantitatively evaluate the precise interactions between PAS and rehabilitative intervention. Furthermore, it is worth noting that spinal cord circuitry gating, along with the functional status of polysynaptic descending pathways regulating the interaction between M1 and peripheral effectors, could potentially influence the outcomes of PAS protocols. However, no information is currently available on these fundamental issues. Understanding these crucial aspects could significantly impact the outcomes of PAS protocols, informing their use in neurorehabilitation.
5. Conclusions
The small number and the heterogeneity of the studies included in our review make it challenging to identify the role of PAS in motor rehabilitation following stroke. Despite the fact that several outcome measures have been used to quantify the efficacy of PAS in stroke rehabilitation, the study of neurophysiological modifications such as MEP and RMT is the most frequent. Our data may provide valuable information about the current use of PAS in neurorehabilitation, becoming a reference point for future studies on tailored PAS protocols identifying patients where combined stimulation can be added to motor rehabilitation to reach the best rehabilitative outcome.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/neurolint16030043/s1, Supplementary Materials: the details about the search strategy.
Author Contributions
Conceptualization, A.B., A.A., G.F. and S.S.; methodology, A.B., A.A., G.F. and S.S.; investigation, A.B., A.A., G.F. and S.S.; data curation, A.B., A.A., G.F. and S.S.; writing—original draft preparation, A.B. and A.A.; writing—review and editing, A.B., A.A., N.L., F.M., G.K., A.D. and S.S; supervision, N.L., F.M., G.K., A.D. and S.S; funding acquisition, F.M., A.D. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the 2010–2012 Research Programme of Emilia Romagna Region [grant number 1786/2012] and by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006)—A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022); Ricerca Finalizzata 2018—Giovani Ricercatori (GR-2018-12366027); EU project PRIMI (HORIZON-CL4-2022-DIGITAL-EMER- GING-02-101120727).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article. All the literature used for this review is listed in the bibliography.
Acknowledgments
We thank Matilde Piva and Alessandra Zanetti for their help during the screening process.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Freed, W.J.; de Medinaceli, L.; Wyatt, R.J. Promoting Functional Plasticity in the Damaged Nervous System. Science 1985, 227, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Joy, M.T.; Carmichael, S.T. Encouraging an Excitable Brain State: Mechanisms of Brain Repair in Stroke. Nat. Rev. Neurosci. 2021, 22, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Mustafaoglu, R.; Rossi, S.; Cavdar, F.A.; Agyenkwa, S.K.; Pang, M.Y.C.; Straudi, S. Non-Invasive Brain Stimulation Techniques for the Improvement of Upper Limb Motor Function and Performance in Activities of Daily Living After Stroke: A Systematic Review and Network Meta-Analysis. Arch. Phys. Med. Rehabil. 2023, 104, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Xu, W. Enhancing Brain Plasticity to Promote Stroke Recovery. Front. Neurol. 2020, 11, 554089. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, N.V. Molecular Mechanisms of Neuroplasticity: An Expanding Universe. Biochemistry 2017, 82, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Wessel, M.J.; Zimerman, M.; Hummel, F.C. Non-Invasive Brain Stimulation: An Interventional Tool for Enhancing Behavioral Training after Stroke. Front. Hum. Neurosci. 2015, 9, 265. [Google Scholar] [CrossRef]
- Bornheim, S.; Croisier, J.-L.; Maquet, P.; Kaux, J.-F. Transcranial Direct Current Stimulation Associated with Physical-Therapy in Acute Stroke Patients—A Randomized, Triple Blind, Sham-Controlled Study. Brain Stimul. 2020, 13, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Kwon, B.; Seo, H.; Park, J.; Paik, N.-J. Low-Frequency Repetitive Transcranial Magnetic Stimulation Over Contralesional Motor Cortex for Motor Recovery in Subacute Ischemic Stroke: A Randomized Sham-Controlled Trial. Neurorehabilit. Neural Repair 2020, 34, 856–867. [Google Scholar] [CrossRef]
- Bressi, F.; Cinnera, A.M.; Morone, G.; Campagnola, B.; Cricenti, L.; Santacaterina, F.; Miccinilli, S.; Zollo, L.; Paolucci, S.; Di Lazzaro, V.; et al. Combining Robot-Assisted Gait Training and Non-Invasive Brain Stimulation in Chronic Stroke Patients: A Systematic Review. Front. Neurol. 2022, 13, 795788. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Tran, V.-D.; Dario, P.; Posteraro, F. Effects of Transcranial Direct Current Stimulation (tDCS) Combined with Wrist Robot-Assisted Rehabilitation on Motor Recovery in Subacute Stroke Patients: A Randomized Controlled Trial. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1458–1466. [Google Scholar] [CrossRef]
- Straudi, S.; Fregni, F.; Martinuzzi, C.; Pavarelli, C.; Salvioli, S.; Basaglia, N. tDCS and Robotics on Upper Limb Stroke Rehabilitation: Effect Modification by Stroke Duration and Type of Stroke. BioMed Res. Int. 2016, 2016, 5068127. [Google Scholar] [CrossRef] [PubMed]
- Cassani, R.; Novak, G.S.; Falk, T.H.; Oliveira, A.A. Virtual Reality and Non-Invasive Brain Stimulation for Rehabilitation Applications: A Systematic Review. J. Neuroeng. Rehabil. 2020, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Chen, C.-L.; Huang, Y.-Z.; Chen, H.-C.; Chen, C.-Y.; Wu, C.-Y.; Lin, K. Augmented Efficacy of Intermittent Theta Burst Stimulation on the Virtual Reality-Based Cycling Training for Upper Limb Function in Patients with Stroke: A Double-Blinded, Randomized Controlled Trial. J. Neuroeng. Rehabil. 2021, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Yan, Z.; Gu, F.; Tao, X.; Xue, T.; Liu, D.; Wang, Z. Transcranial Direct Current Stimulation with Virtual Reality versus Virtual Reality Alone for Upper Extremity Rehabilitation in Stroke: A Meta-Analysis. Heliyon 2023, 9, e12695. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Cui, L.; Wang, J.; Feng, W.; Bao, Y.; Xie, Q. Effects of Transcranial Direct Current Stimulation with Virtual Reality on Upper Limb Function in Patients with Ischemic Stroke: A Randomized Controlled Trial. J. Neuroeng. Rehabil. 2020, 17, 73. [Google Scholar] [CrossRef]
- Baroni, A.; Magro, G.; Martinuzzi, C.; Brondi, L.; Masiero, S.; Milani, G.; Zani, G.; Bergonzoni, A.; Basaglia, N.; Straudi, S. Combined Effects of Cerebellar tDCS and Task-Oriented Circuit Training in People with Multiple Sclerosis: A Pilot Randomized Control Trial. Restor. Neurol. Neurosci. 2022, 40, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Pavon, J.C.; San Agustín, A.; Wang, M.C.; Veniero, D.; Pons, J.L. Can We Manipulate Brain Connectivity? A Systematic Review of Cortico-Cortical Paired Associative Stimulation Effects. Clin. Neurophysiol. 2023, 154, 169–193. [Google Scholar] [CrossRef]
- Stefan, K.; Kunesch, E.; Cohen, L.G.; Benecke, R.; Classen, J. Induction of Plasticity in the Human Motor Cortex by Paired Associative Stimulation. Brain 2000, 123 Pt 3, 572–584. [Google Scholar] [CrossRef]
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; Wiley: Oxford, UK, 1949; p. 335. [Google Scholar]
- Classen, J.; Wolters, A.; Stefan, K.; Wycislo, M.; Sandbrink, F.; Schmidt, A.; Kunesch, E. Paired Associative Stimulation. Suppl. Clin. Neurophysiol. 2004, 57, 563–569. [Google Scholar]
- Stent, G.S. A Physiological Mechanism for Hebb’s Postulate of Learning. Proc. Natl. Acad. Sci. USA 1973, 70, 997–1001. [Google Scholar] [CrossRef]
- Kirkwood, A.; Dudek, S.M.; Gold, J.T.; Aizenman, C.D.; Bear, M.F. Common Forms of Synaptic Plasticity in the Hippocampus and Neocortex in Vitro. Science 1993, 260, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sui, Y.; Guo, T.; Wang, S.; Hu, Y.; Lu, Y. Effect of Paired Associative Stimulation on Motor Cortex Excitability in Rats. Curr. Med. Sci. 2018, 38, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Leodori, G.; Cutrona, C.; Marchet, F.; De Bartolo, M.I.; Mancuso, M.; Belvisi, D.; Conte, A.; Berardelli, A.; Fabbrini, G. Motor Cortical Correlates of Paired Associative Stimulation Induced Plasticity: A TMS-EEG Study. Brain Sci. 2023, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.F.M.; Orekhov, Y.; Liu, Y.; Ziemann, U. Homeostatic Plasticity in Human Motor Cortex Demonstrated by Two Consecutive Sessions of Paired Associative Stimulation. Eur. J. Neurosci. 2007, 25, 3461–3468. [Google Scholar] [CrossRef] [PubMed]
- Castel-Lacanal, E.; Gerdelat-Mas, A.; Marque, P.; Loubinoux, I.; Simonetta-Moreau, M. Induction of Cortical Plastic Changes in Wrist Muscles by Paired Associative Stimulation in Healthy Subjects and Post-Stroke Patients. Exp. Brain Res. 2007, 180, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Castel-Lacanal, E.; Marque, P.; Tardy, J.; de Boissezon, X.; Guiraud, V.; Chollet, F.; Loubinoux, I.; Moreau, M.S. Induction of Cortical Plastic Changes in Wrist Muscles by Paired Associative Stimulation in the Recovery Phase of Stroke Patients. Neurorehabilit. Neural Repair 2009, 23, 366–372. [Google Scholar] [CrossRef]
- Palmer, J.A.; Wolf, S.L.; Borich, M.R. Paired Associative Stimulation Modulates Corticomotor Excitability in Chronic Stroke: A Preliminary Investigation. Restor. Neurol. Neurosci. 2018, 36, 183–194. [Google Scholar] [CrossRef]
- Tsuji, T.; Suzuki, K.; Masakado, Y.; Ota, T.; Kimura, A.; Liu, M.; Chino, N. Long-Lasting Effects of Paired Associative Stimulation in Hemiparetic Stroke Patients. Int. Congr. Ser. 2005, 1278, 280–283. [Google Scholar] [CrossRef]
- Koganemaru, S.; Fukuyama, H.; Mima, T. Two Is More Than One: How to Combine Brain Stimulation Rehabilitative Training for Functional Recovery? Front. Syst. Neurosci. 2015, 9, 154. [Google Scholar] [CrossRef]
- Bestmann, S.; Krakauer, J.W. The Uses and Interpretations of the Motor-Evoked Potential for Understanding Behaviour. Exp. Brain Res. 2015, 233, 679–689. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Joanna Briggs Institute. Checklist for Systematic Reviews and Research Syntheses; Joanna Briggs Institute: Adelaide, Australia, 2017. [Google Scholar]
- Rosso, C.; Moulton, E.J.; Kemlin, C.; Leder, S.; Corvol, J.-C.; Mehdi, S.; Obadia, M.A.; Obadia, M.; Yger, M.; Meseguer, E.; et al. Cerebello-Motor Paired Associative Stimulation and Motor Recovery in Stroke: A Randomized, Sham-Controlled, Double-Blind Pilot Trial. Neurotherapeutics 2022, 19, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.-F.; Tong, L.-Q.; Zhang, X.-Y.; Song, Z.-H.; Guo, T.-C. Effects of Paired Associated Stimulation with Different Stimulation Position on Motor Cortex Excitability and Upper Limb Motor Function in Patients with Cerebral Infarction. J. Clin. Neurosci. 2021, 90, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Tarri, M.; Brihmat, N.; Gasq, D.; Lepage, B.; Loubinoux, I.; De Boissezon, X.; Marque, P.; Castel-Lacanal, E. Five-Day Course of Paired Associative Stimulation Fails to Improve Motor Function in Stroke Patients. Ann. Phys. Rehabil. Med. 2018, 61, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Tosun, A.; Türe, S.; Askin, A.; Yardimci, E.U.; Demirdal, S.U.; Kurt Incesu, T.; Tosun, O.; Kocyigit, H.; Akhan, G.; Gelal, F.M. Effects of Low-Frequency Repetitive Transcranial Magnetic Stimulation and Neuromuscular Electrical Stimulation on Upper Extremity Motor Recovery in the Early Period after Stroke: A Preliminary Study. Top. Stroke Rehabil. 2017, 24, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kuznietsova, S.; Skachkova, N.; Semonova, O. ID 243—Enhancement of Cortical Excitability in Stroke Patients after Combined Repetitive Transcranial and Peripheral Magnetic Stimulation. Clin. Neurophysiol. 2016, 127, e121. [Google Scholar] [CrossRef]
- Tarri, M.; Simonetta-Moreau, M.; Loubinoux, I.; De Boissezon, X.; Gasq, D.; Marque, P.; Castel-Lacanal, E. Study of the Effects of a 5-Day Brain Stimulation with Paired Associative Stimulation (PAS) against Placebo in 28 Hemiplegic Patients. Ann. Phys. Rehabil. Med. 2015, 58, e2. [Google Scholar] [CrossRef][Green Version]
- Mohamed, T.; Marion, S.-M.M.; Isabelle, L.; Xavier, D.B.; David, G.; Phillipe, M.; Evelyne, C.-L. P 123. CIPASS: Trial of a Daily Program of Cerebral Stimulation by TMS Using a PAS Paradigm in the Recovery Phase of Stroke Patients. Clin. Neurophysiol. 2013, 124, e123. [Google Scholar] [CrossRef]
- Uy, J.; Ridding, M.C.; Hillier, S.; Thompson, P.D.; Miles, T.S. Does Induction of Plastic Change in Motor Cortex Improve Leg Function after Stroke? Neurology 2003, 61, 982–984. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and Recommendations for TMS Use in Healthy Subjects and Patient Populations, with Updates on Training, Ethical and Regulatory Issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Barker, A.T.; Berardelli, A.; Caramia, M.D.; Caruso, G.; Cracco, R.Q.; Dimitrijević, M.R.; Hallett, M.; Katayama, Y.; Lücking, C.H. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord and Roots: Basic Principles and Procedures for Routine Clinical Application. Report of an IFCN Committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, J.; Cortes, M.; Tsagaris, K.Z.; Climent, A.; Gerber, L.M.; Oromendia, C.; Fonzetti, P.; Ratan, R.R.; Kitago, T.; Iacoboni, M.; et al. Paired Associative Stimulation as a Tool to Assess Plasticity Enhancers in Chronic Stroke. Front. Neurosci. 2019, 13, 792. [Google Scholar] [CrossRef]
- Edwards, J.D.; Dominguez-Vargas, A.U.; Rosso, C.; Branscheidt, M.; Sheehy, L.; Quandt, F.; Zamora, S.A.; Fleming, M.K.; Azzollini, V.; Mooney, R.A.; et al. A Translational Roadmap for Transcranial Magnetic and Direct Current Stimulation in Stroke Rehabilitation: Consensus-Based Core Recommendations from the Third Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2023, 19, 145–157. [Google Scholar] [CrossRef]
- Strick, P.L.; Dum, R.P.; Rathelot, J.-A. The Cortical Motor Areas and the Emergence of Motor Skills: A Neuroanatomical Perspective. Annu. Rev. Neurosci. 2021, 44, 425–447. [Google Scholar] [CrossRef] [PubMed]
- Turco, C.V.; El-Sayes, J.; Savoie, M.J.; Fassett, H.J.; Locke, M.B.; Nelson, A.J. Short- and Long-Latency Afferent Inhibition; Uses, Mechanisms and Influencing Factors. Brain Stimul. 2018, 11, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.L.; King, E.M.; Buetefisch, C.M.; Borich, M.R. Putting the “Sensory” Into Sensorimotor Control: The Role of Sensorimotor Integration in Goal-Directed Hand Movements After Stroke. Front. Integr. Neurosci. 2019, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, P. The Nature of Hand Motor Impairment after Stroke and Its Treatment. Curr. Treat. Options Cardiovasc. Med. 2007, 9, 221–228. [Google Scholar] [CrossRef]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capone, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C.; et al. Modulation of Brain Plasticity in Stroke: A Novel Model for Neurorehabilitation. Nat. Rev. Neurol. 2014, 10, 597–608. [Google Scholar] [CrossRef]
- Koch, G.; Bonnì, S.; Giacobbe, V.; Bucchi, G.; Basile, B.; Lupo, F.; Versace, V.; Bozzali, M.; Caltagirone, C. θ-Burst Stimulation of the Left Hemisphere Accelerates Recovery of Hemispatial Neglect. Neurology 2012, 78, 24–30. [Google Scholar] [CrossRef]
- Motolese, F.; Capone, F.; Di Lazzaro, V. New Tools for Shaping Plasticity to Enhance Recovery after Stroke. Handb. Clin. Neurol. 2022, 184, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Dimyan, M.A.; Cohen, L.G. Neuroplasticity in the Context of Motor Rehabilitation after Stroke. Nat. Rev. Neurol. 2011, 7, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Morotti, A.; Poli, L.; Costa, P. Acute Stroke. Semin. Neurol. 2019, 39, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.B. Current Trends in Stroke Rehabilitation. A Review with Focus on Brain Plasticity. Acta Neurol. Scand. 2011, 123, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Anwer, S.; Waris, A.; Gilani, S.O.; Iqbal, J.; Shaikh, N.; Pujari, A.N.; Niazi, I.K. Rehabilitation of Upper Limb Motor Impairment in Stroke: A Narrative Review on the Prevalence, Risk Factors, and Economic Statistics of Stroke and State of the Art Therapies. Healthcare 2022, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H. The Recovery of Walking in Stroke Patients: A Review. Int. J. Rehabil. Res. 2010, 33, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.H. Modern Coordinates for the Motor Homunculus. J. Physiol. 2020, 598, 5305–5306. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Ponzo, V.; Di Lorenzo, F.; Caltagirone, C.; Veniero, D. Hebbian and Anti-Hebbian Spike-Timing-Dependent Plasticity of Human Cortico-Cortical Connections. J. Neurosci. 2013, 33, 9725–9733. [Google Scholar] [CrossRef]
- Ashe, J.; Lungu, O.V.; Basford, A.T.; Lu, X. Cortical Control of Motor Sequences. Curr. Opin. Neurobiol. 2006, 16, 213–221. [Google Scholar] [CrossRef]
- Mesulam, M. The Evolving Landscape of Human Cortical Connectivity: Facts and Inferences. Neuroimage 2012, 62, 2182–2189. [Google Scholar] [CrossRef]
- Casarotto, A.; Dolfini, E.; Cardellicchio, P.; Fadiga, L.; D’Ausilio, A.; Koch, G. Mechanisms of Hebbian-like Plasticity in the Ventral Premotor—Primary Motor Network. J. Physiol. 2023, 601, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Casarotto, A.; Dolfini, E.; Fadiga, L.; Koch, G.; D’Ausilio, A. Cortico-Cortical Paired Associative Stimulation Conditioning Superficial Ventral Premotor Cortex-Primary Motor Cortex Connectivity Influences Motor Cortical Activity during Precision Grip. J. Physiol. 2023, 601, 3945–3960. [Google Scholar] [CrossRef]
- Cinnera, A.M.; Bonnì, S.; D’Acunto, A.; Maiella, M.; Ferraresi, M.; Casula, E.P.; Pezzopane, V.; Tramontano, M.; Iosa, M.; Paolucci, S.; et al. Cortico-Cortical Stimulation and Robot-Assisted Therapy (CCS and RAT) for Upper Limb Recovery after Stroke: Study Protocol for a Randomised Controlled Trial. Trials 2023, 24, 823. [Google Scholar] [CrossRef]
- Guidali, G.; Roncoroni, C.; Bolognini, N. Paired Associative Stimulations: Novel Tools for Interacting with Sensory and Motor Cortical Plasticity. Behav. Brain Res. 2021, 414, 113484. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, D.A.; Block, H.J.; Celnik, P.A. Cerebellar-M1 Connectivity Changes Associated with Motor Learning Are Somatotopic Specific. J. Neurosci. 2017, 37, 2377–2386. [Google Scholar] [CrossRef]
- Rehme, A.K.; Eickhoff, S.B.; Rottschy, C.; Fink, G.R.; Grefkes, C. Activation Likelihood Estimation Meta-Analysis of Motor-Related Neural Activity after Stroke. Neuroimage 2012, 59, 2771–2782. [Google Scholar] [CrossRef]
- Celnik, P. Understanding and Modulating Motor Learning with Cerebellar Stimulation. Cerebellum 2015, 14, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Bonnì, S.; Casula, E.P.; Iosa, M.; Paolucci, S.; Pellicciari, M.C.; Cinnera, A.M.; Ponzo, V.; Maiella, M.; Picazio, S.; et al. Effect of Cerebellar Stimulation on Gait and Balance Recovery in Patients with Hemiparetic Stroke: A Randomized Clinical Trial. JAMA Neurol. 2018, 76, 170–178. [Google Scholar] [CrossRef]
- Krakauer, J.W.; Shadmehr, R. Consolidation of Motor Memory. Trends Neurosci. 2006, 29, 58–64. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord, Roots and Peripheral Nerves: Basic Principles and Procedures for Routine Clinical and Research Application. An Updated Report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Mazwi, N.L.; Adeletti, K.; Hirschberg, R.E. Traumatic Spinal Cord Injury: Recovery, Rehabilitation, and Prognosis. Curr. Trauma Rep. 2015, 1, 182–192. [Google Scholar] [CrossRef]
- Ling, Y.T.; Alam, M.; Zheng, Y.-P. Spinal Cord Injury: Lessons about Neuroplasticity from Paired Associative Stimulation. Neuroscientist 2020, 26, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Bunday, K.L.; Perez, M.A. Motor Recovery after Spinal Cord Injury Enhanced by Strengthening Corticospinal Synaptic Transmission. Curr. Biol. 2012, 22, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Debanne, D.; Inglebert, Y. Spike Timing-Dependent Plasticity and Memory. Curr. Opin. Neurobiol. 2023, 80, 102707. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.L.; Spronk, M.; Kulkarni, K.; Repovš, G.; Anticevic, A.; Cole, M.W. Mapping the Human Brain’s Cortical-Subcortical Functional Network Organization. Neuroimage 2019, 185, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Guggisberg, A.G.; Koch, P.J.; Hummel, F.C.; Buetefisch, C.M. Brain Networks and Their Relevance for Stroke Rehabilitation. Clin. Neurophysiol. 2019, 130, 1098–1124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).