PET and SPECT Tracer Development via Copper-Mediated Radiohalogenation of Divergent and Stable Aryl-Boronic Esters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Information
2.3. General Procedure for the Synthesis of B(Epin) and B(Ppin) Compounds Starting from the Corresponding Boronic Acids
2.4. General Procedure for the Preparation of Radiolabeled Arenes via CMRF
2.5. General Procedure for the Preparation of Radiolabeled Arenes via CMRI
3. Results
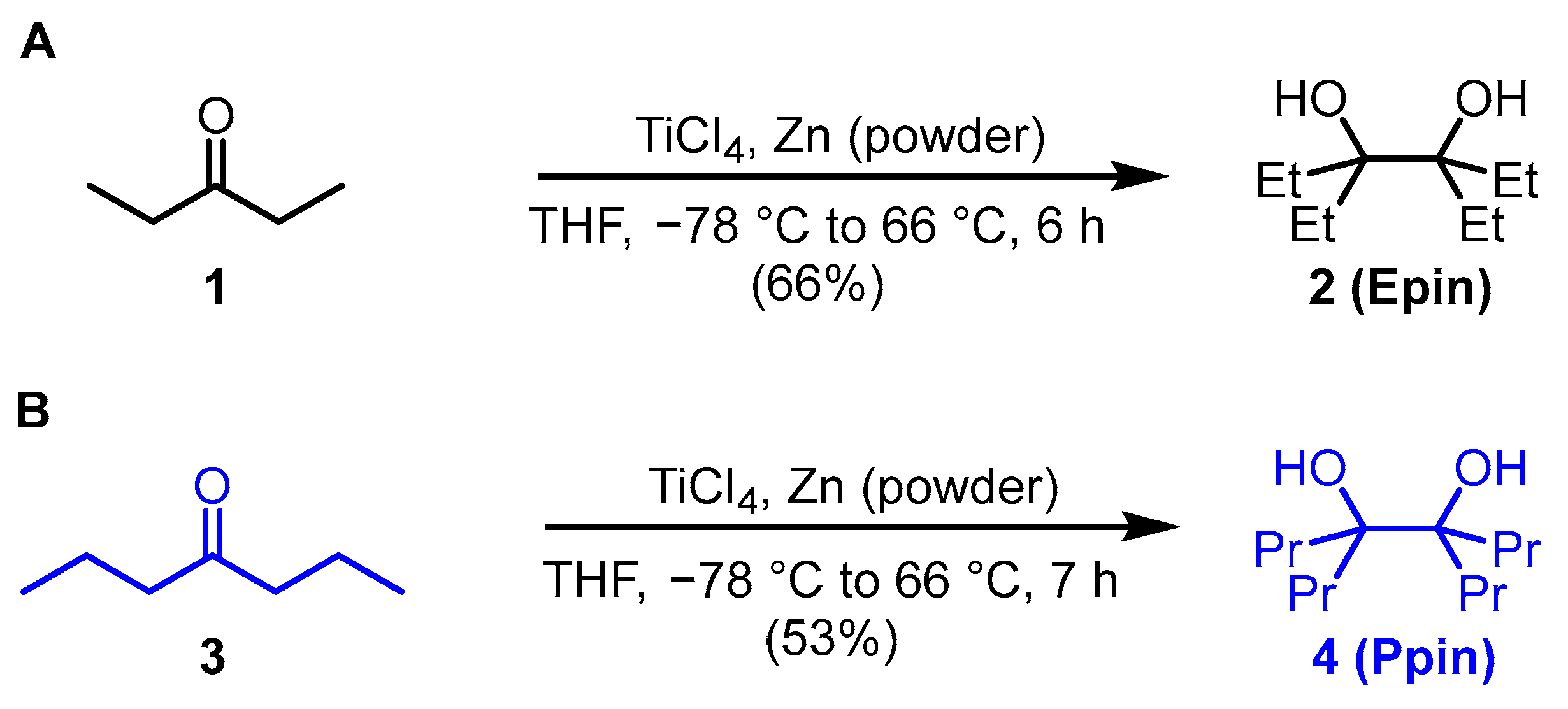
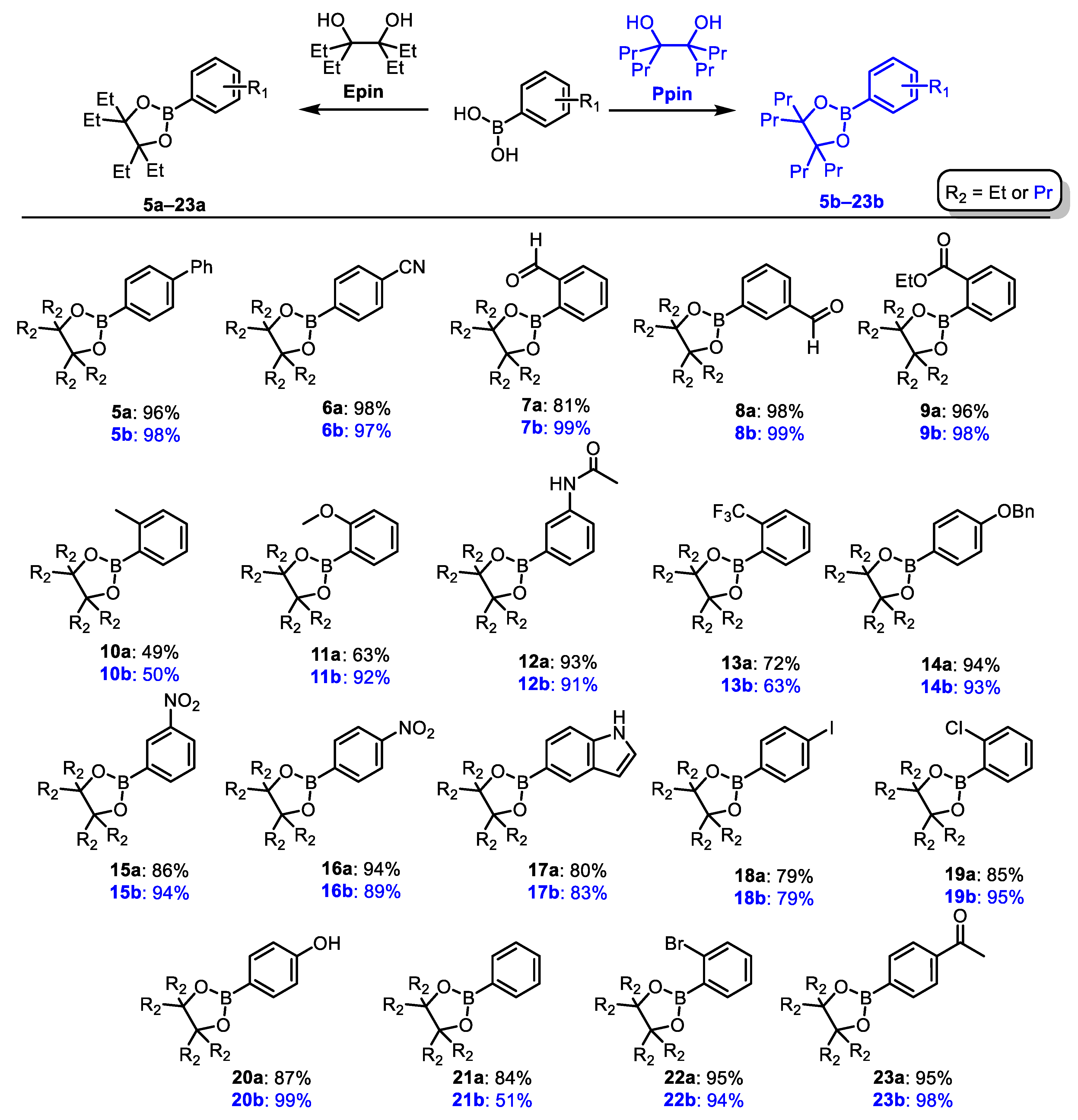
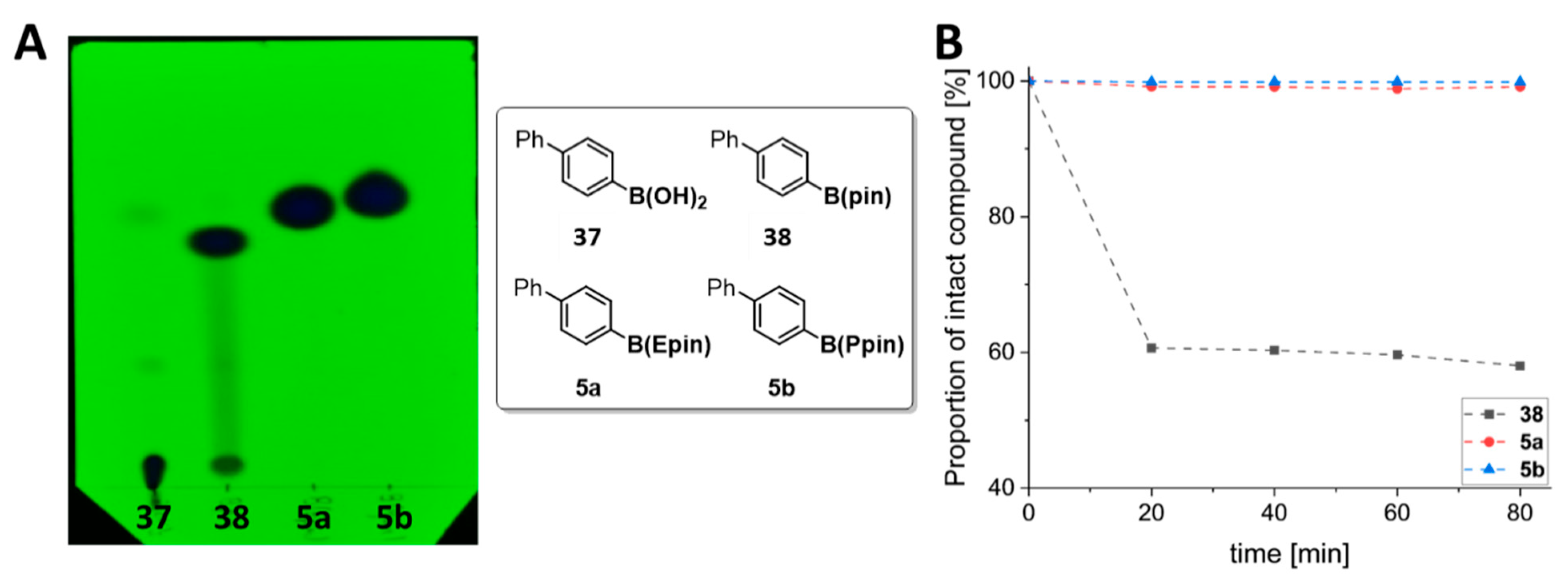
3.1. Organic Synthesis of Radiolabeling Precursors and Reference Compounds
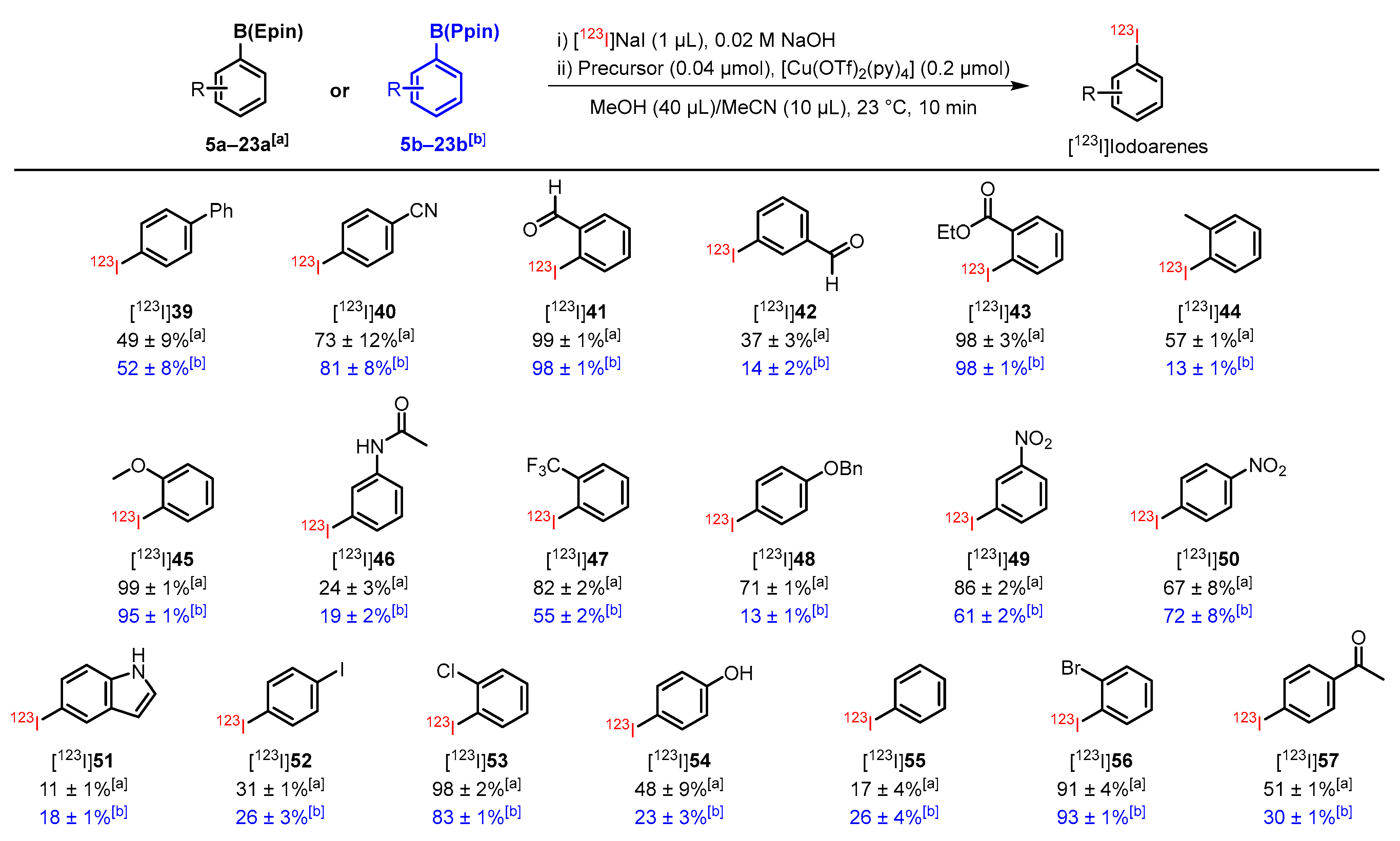
3.2. Substrate Scope of CMRI
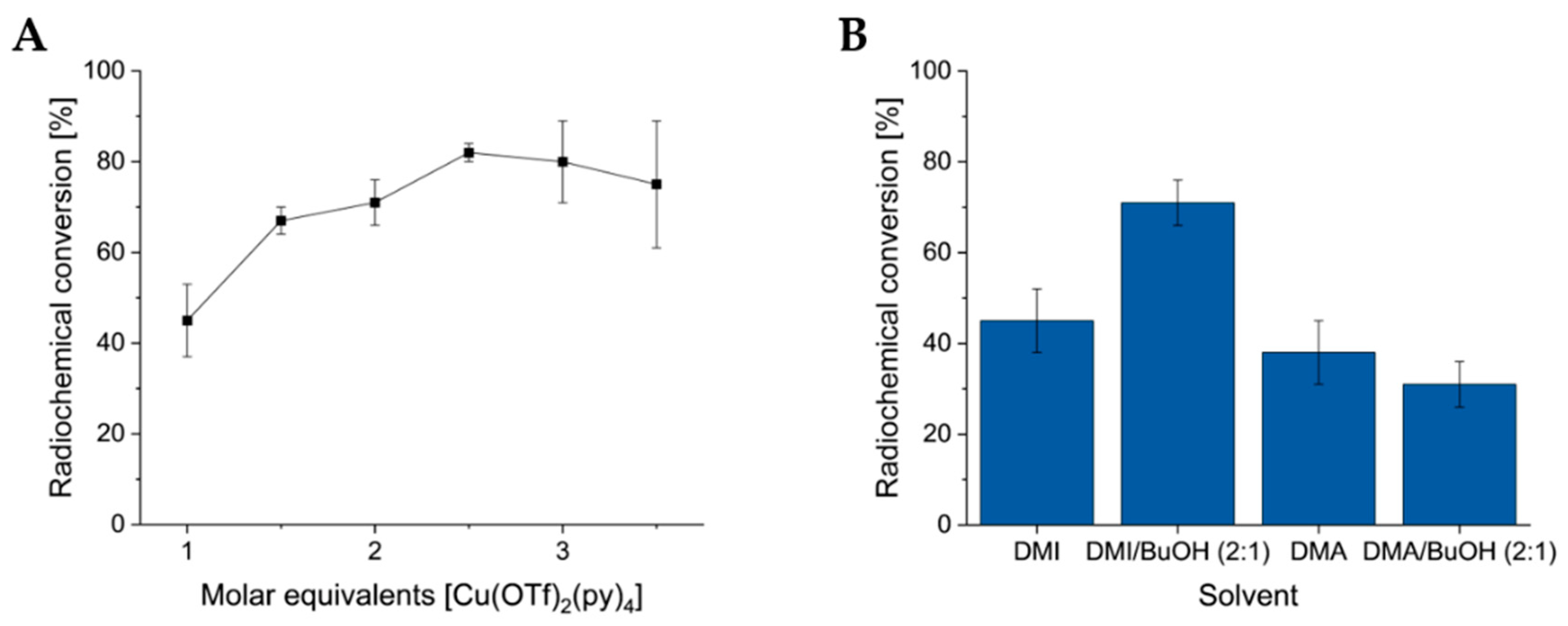
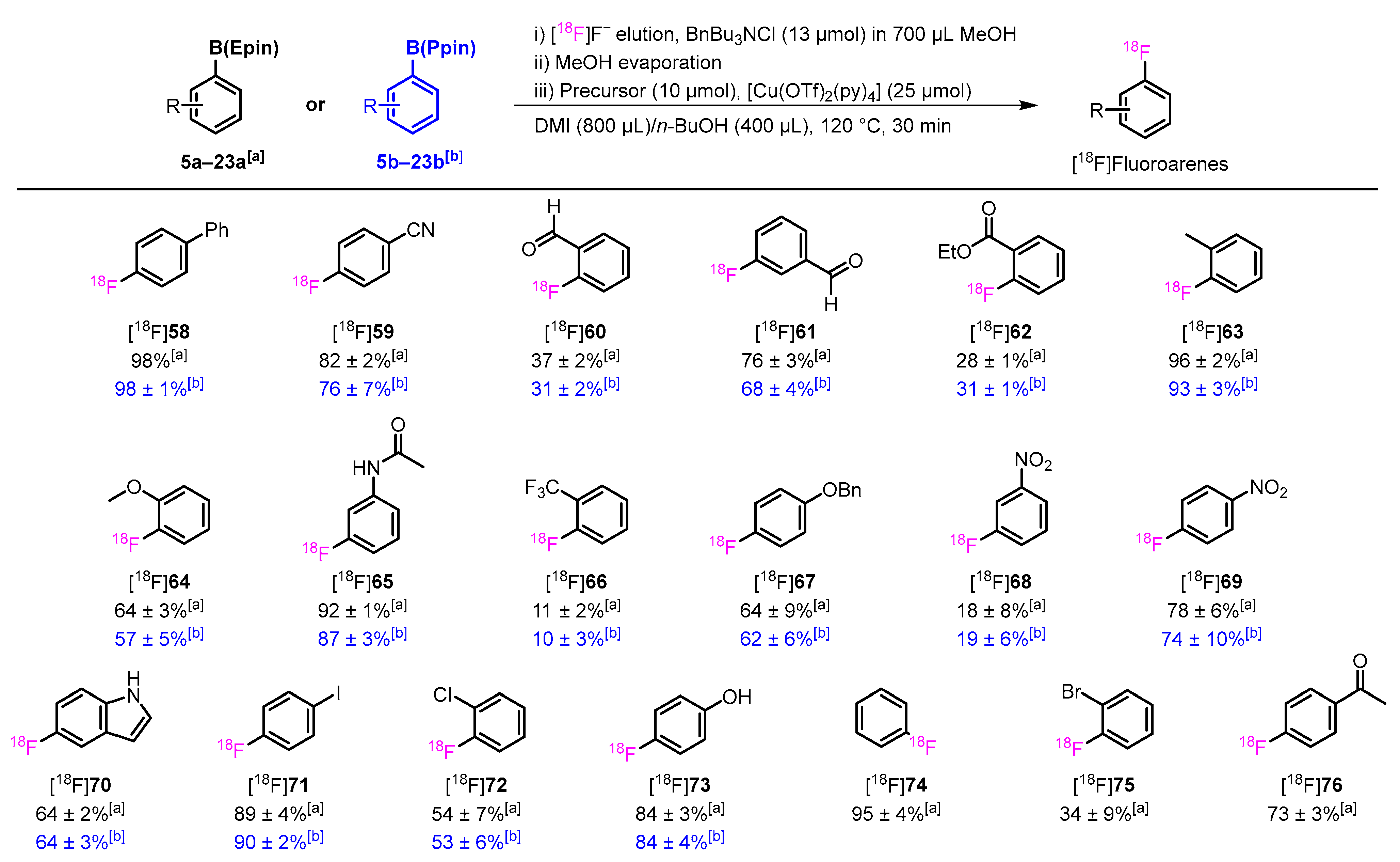
3.3. Optimization of CMRF and Substrate Scope
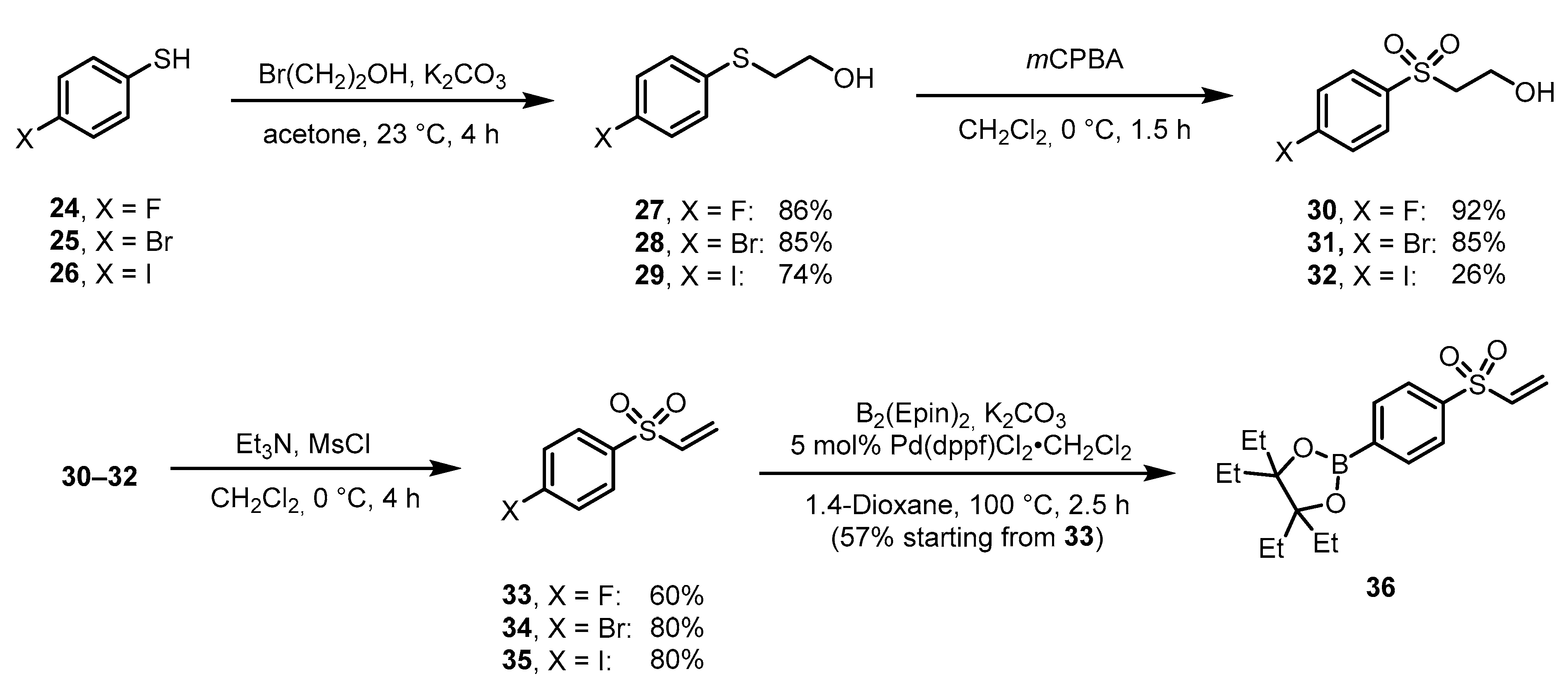
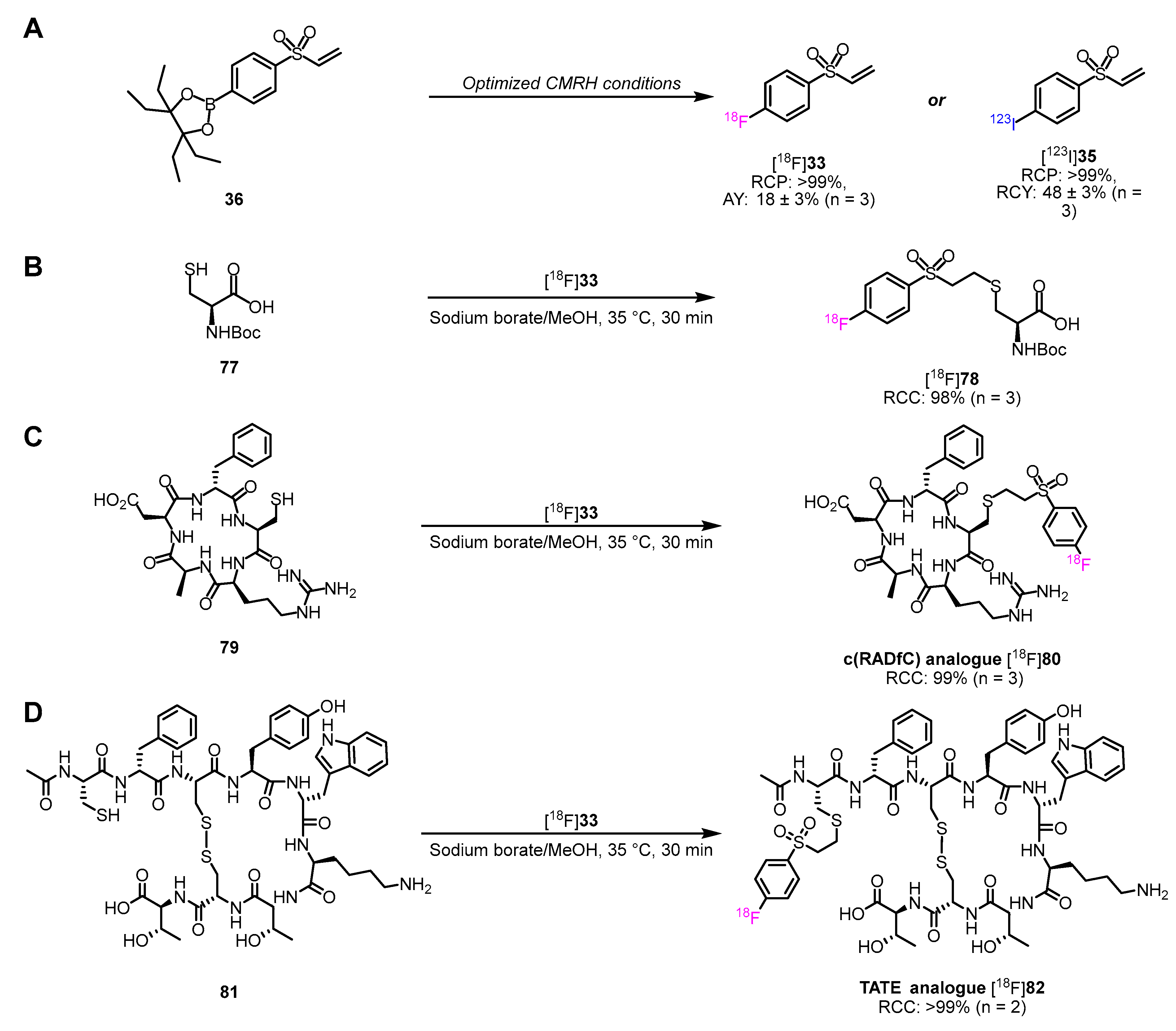
3.4. Preparation of Radiohalogenated Prosthetic Groups Towards Peptide Radiolabeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alavi, A.; Werner, T.J.; Stępień, E.Ł.; Moskal, P. Unparalleled and Revolutionary Impact of PET Imaging on Research and Day to Day Practice of Medicine. Bio-Algorithms Med-Syst. 2022, 17, 203–212. [Google Scholar] [CrossRef]
- Alavi, A.; Basu, S. Planar and SPECT Imaging in the Era of PET and PET–CT: Can It Survive the Test of Time? Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Israel, O.; Pellet, O.; Biassoni, L.; De Palma, D.; Estrada-Lobato, E.; Gnanasegaran, G.; Kuwert, T.; La Fougère, C.; Mariani, G.; Massalha, S.; et al. Two Decades of SPECT/CT—The Coming of Age of a Technology: An Updated Review of Literature Evidence. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1990–2012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Lee, D.; Shim, H. Metabolic Positron Emission Tomography Imaging in Cancer Detection and Therapy Response. Semin. Oncol. 2011, 38, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Phelps, M.E. Positron Emission Tomography Provides Molecular Imaging of Biological Processes. Proc. Natl. Acad. Sci. USA. 2000, 97, 9226–9233. [Google Scholar] [CrossRef]
- Ametamey, S.M.; Honer, M.; Schubiger, P.A. Molecular Imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [Google Scholar] [CrossRef]
- Donnelly, D.J. Small Molecule PET Tracers in Drug Discovery. Semin. Nucl. Med. 2017, 47, 454–460. [Google Scholar] [CrossRef]
- Donnelly, D.J. PET Imaging in Drug Discovery and Development. In Handbook of Radiopharmaceuticals; Scott, P., Kilbourn, M., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 703–725. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Kunos, C.A.; Mankoff, D.A.; Schultz, M.K.; Graves, S.A.; Pryma, D.A. Radiopharmaceutical Chemistry and Drug Development—What’s Changed? Semin. Radiat. Oncol. 2021, 31, 3–11. [Google Scholar] [CrossRef]
- Craig, A.; Kogler, J.; Laube, M.; Ullrich, M.; Donat, C.K.; Wodtke, R.; Kopka, K.; Stadlbauer, S. Preparation of 18F-Labeled Tracers Targeting Fibroblast Activation Protein via Sulfur [18F]Fluoride Exchange Reaction. Pharmaceutics 2023, 15, 2749. [Google Scholar] [CrossRef]
- FDA-Approved PET Radiopharmaceuticals. Available online: http://www.radiopharmaceuticals.info/pet-radiopharmaceuticals.html (accessed on 4 August 2024).
- Clarke, B.N. PET Radiopharmaceuticals: What’s New, What’s Reimbursed, and What’s Next? J. Nucl. Med. Technol. 2018, 46, 12–16. [Google Scholar] [CrossRef]
- Coenen, H.H.; Elsinga, P.H.; Iwata, R.; Kilbourn, M.R.; Pillai, M.R.A.; Rajan, M.G.R.; Wagner, H.N.; Zaknun, J.J. Fluorine-18 Radiopharmaceuticals Beyond [18F]FDG for Use in Oncology and Neurosciences. Nucl. Med. Biol. 2010, 37, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Bowden, G.D.; Scott, P.J.H.; Boros, E. Radiochemistry: A Hot Field with Opportunities for Cool Chemistry. ACS Cent. Sci. 2023, 9, 2183–2195. [Google Scholar] [CrossRef]
- Park, H.-M. 123I: Almost a Designer Radioiodine for Thyroid Scanning. J. Nucl. Med. 2002, 43, 77–78. [Google Scholar] [PubMed]
- Snay, E.R.; Treves, S.T.; Fahey, F.H. Improved Quality of Pediatric 123I-MIBG Images with Medium-Energy Collimators. J. Nucl. Med. Technol. 2011, 39, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.; Gross, M.D. Radiochemistry, Biochemistry, and Kinetics of 131I-metaiodobenzylguanidine (MIBG) and 123I-MIBG: Clinical Implications of the Use of 123I-MIBG. Med. Pediatr. Oncol. 1987, 15, 170–177. [Google Scholar] [CrossRef]
- Nuvoli, S.; Spanu, A.; Piras, M.R.; Nieddu, A.; Mulas, A.; Rocchitta, G.; Galleri, G.; Serra, P.A.; Madeddu, G. 123I-Ioflupane Brain SPECT and 123I-MIBG Cardiac Planar Scintigraphy Combined Use in Uncertain Parkinsonian Disorders. Medicine 2017, 96, e6967. [Google Scholar] [CrossRef]
- Chan, C.Y.; Chen, Z.; Guibbal, F.; Dias, G.; Destro, G.; O’Neill, E.; Veal, M.; Lau, D.; Mosley, M.; Wilson, T.C.; et al. [123I]CC1: A PARP-Targeting, Auger Electron–Emitting Radiopharmaceutical for Radionuclide Therapy of Cancer. J. Nucl. Med. 2023, 64, 1965–1971. [Google Scholar] [CrossRef]
- Koehler, L.; Gagnon, K.; McQuarrie, S.; Wuest, F. Iodine-124: A Promising Positron Emitter for Organic PET Chemistry. Molecules 2010, 15, 2686–2718. [Google Scholar] [CrossRef]
- Adam, M.J.; Wilbur, D.S. Radiohalogens for Imaging and Therapy. Chem. Soc. Rev. 2005, 34, 153–163. [Google Scholar] [CrossRef]
- Ehrhardt, J.D., Jr.; Güleç, S. A Review of the History of Radioactive Iodine Theranostics: The Origin of Nuclear Ontology. Mol. Imaging Radionucl. Ther. 2020, 29, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, E.B. Radioiodine: The Classic Theranostic Agent. Semin. Nucl. Med. 2012, 42, 164–170. [Google Scholar] [CrossRef]
- Anger, H.O. Scintillation Camera. Rev. Sci. Instrum. 1958, 29, 27–33. [Google Scholar] [CrossRef]
- FDA-Approved Radiopharmaceuticals. Available online: http://www.radiopharmaceuticals.info/radiopharmaceuticals.html (accessed on 4 August 2024).
- Antuganov, D.; Zykov, M.; Timofeev, V.; Timofeeva, K.; Antuganova, Y.; Orlovskaya, V.; Fedorova, O.; Krasikova, R. Copper-Mediated Radiofluorination of Aryl Pinacolboronate Esters: A Straightforward Protocol by Using Pyridinium Sulfonates. Eur. J. Org. Chem. 2019, 2019, 918–922. [Google Scholar] [CrossRef]
- Jeon, M.H.; Kwon, Y.-D.; Kim, M.P.; Torres, G.B.; Seo, J.K.; Son, J.; Ryu, Y.H.; Hong, S.Y.; Chun, J.-H. Late-Stage 18F/19F Isotopic Exchange for the Synthesis of 18 F-Labeled Sulfamoyl Fluorides. Org. Lett. 2021, 23, 2766–2771. [Google Scholar] [CrossRef] [PubMed]
- Orlovskaya, V.V.; Craig, A.S.; Fedorova, O.S.; Kuznetsova, O.F.; Neumaier, B.; Krasikova, R.N.; Zlatopolskiy, B.D. Production of 6-l-[18F]Fluoro-m-Tyrosine in an Automated Synthesis Module for 11C-Labeling. Molecules 2021, 26, 5550. [Google Scholar] [CrossRef]
- Lee, E.; Kamlet, A.S.; Powers, D.C.; Neumann, C.N.; Boursalian, G.B.; Furuya, T.; Choi, D.C.; Hooker, J.M.; Ritter, T. A Fluoride-Derived Electrophilic Late-Stage Fluorination Reagent for PET Imaging. Science 2011, 334, 639–642. [Google Scholar] [CrossRef]
- Rotstein, B.H.; Stephenson, N.A.; Vasdev, N.; Liang, S.H. Spirocyclic Hypervalent Iodine(III)-Mediated Radiofluorination of Non-Activated and Hindered Aromatics. Nat. Commun. 2014, 5, 4365. [Google Scholar] [CrossRef]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjugate Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef]
- Dubost, E.; McErlain, H.; Babin, V.; Sutherland, A.; Cailly, T. Recent Advances in Synthetic Methods for Radioiodination. J. Org. Chem. 2020, 85, 8300–8310. [Google Scholar] [CrossRef]
- Ambrosini, V.; Zanoni, L.; Filice, A.; Lamberti, G.; Argalia, G.; Fortunati, E.; Campana, D.; Versari, A.; Fanti, S. Radiolabeled Somatostatin Analogues for Diagnosis and Treatment of Neuroendocrine Tumors. Cancers 2022, 14, 1055. [Google Scholar] [CrossRef] [PubMed]
- Okarvi, S.M. Peptide-based Radiopharmaceuticals: Future Tools for Diagnostic Imaging of Cancers and Other Diseases. Med. Res. Rev. 2004, 24, 357–397. [Google Scholar] [CrossRef]
- Neels, O.C.; Kopka, K.; Liolios, C.; Afshar-Oromieh, A. Radiolabeled PSMA Inhibitors. Cancers 2021, 13, 6255. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Maecke, H.R.; Okarvi, S.M. Radiolabeled Peptides: Valuable Tools for the Detection and Treatment of Cancer. Theranostics 2012, 2, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Gröner, B.; Willmann, M.; Donnerstag, L.; Urusova, E.A.; Neumaier, F.; Humpert, S.; Endepols, H.; Neumaier, B.; Zlatopolskiy, B.D. 7-[18F]Fluoro-8-Azaisatoic Anhydrides: Versatile Prosthetic Groups for the Preparation of PET Tracers. J. Med. Chem. 2023, 66, 12629–12644. [Google Scholar] [CrossRef]
- Marik, J.; Sutcliffe, J.L. Click for PET: Rapid Preparation of [18F]Fluoropeptides Using CuI Catalyzed 1,3-Dipolar Cycloaddition. Tetrahedron Lett. 2006, 47, 6681–6684. [Google Scholar] [CrossRef]
- Glaser, M.; Årstad, E. “Click Labeling” with 2-[18F]Fluoroethylazide for Positron Emission Tomography. Bioconjugate Chem. 2007, 18, 989–993. [Google Scholar] [CrossRef]
- Richter, S.; Wuest, F. 18F-Labeled Peptides: The Future Is Bright. Molecules 2014, 19, 20536–20556. [Google Scholar] [CrossRef]
- Schirrmacher, R.; Wängler, B.; Bailey, J.; Bernard-Gauthier, V.; Schirrmacher, E.; Wängler, C. Small Prosthetic Groups in 18F-Radiochemistry: Useful Auxiliaries for the Design of 18F-PET Tracers. Semin. Nucl. Med. 2017, 47, 474–492. [Google Scholar] [CrossRef]
- Chalker, J.M.; Bernardes, G.J.L.; Lin, Y.A.; Davis, B.G. Chemical Modification of Proteins at Cysteine: Opportunities in Chemistry and Biology. Chem. Asian J. 2009, 4, 630–640. [Google Scholar] [CrossRef]
- Gundam, S.R.; Callstrom, M.R.; Pandey, M.K. Synthesis and Application of 1-[18F]Fluoro-4-Isothiocyanatobenzene for Radiofluorination of Peptides in Aqueous Medium. J. Org. Chem. 2025, 90, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Davis, B.G. Selective Chemical Protein Modification. Nat. Commun. 2014, 5, 4740. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.M.; Akula, M.R.; Kabalka, G.W. Fluorine-18 Radiochemistry: A Novel Thiol-Reactive Prosthetic Group, [18F]FBAMPy. Nat. Sci. 2016, 8, 1–7. [Google Scholar] [CrossRef][Green Version]
- Ma, G.; McDaniel, J.W.; Murphy, J.M. One-Step Synthesis of [18F]Fluoro-4-(Vinylsulfonyl)Benzene: A Thiol Reactive Synthon for Selective Radiofluorination of Peptides. Org. Lett. 2021, 23, 530–534. [Google Scholar] [CrossRef]
- Failla, M.; Floresta, G.; Abbate, V. Peptide-Based Positron Emission Tomography Probes: Current Strategies for Synthesis and Radiolabelling. RSC Med. Chem. 2023, 14, 592–623. [Google Scholar] [CrossRef]
- Kniess, T.; Kuchar, M.; Pietzsch, J. Automated Radiosynthesis of the Thiol-Reactive Labeling Agent N-[6-(4-[18F]Fluorobenzylidene)Aminooxyhexyl]Maleimide ([18F]FBAM). Appl. Radiat. Isot. 2011, 69, 1226–1230. [Google Scholar] [CrossRef]
- Ichiishi, N.; Brooks, A.F.; Topczewski, J.J.; Rodnick, M.E.; Sanford, M.S.; Scott, P.J.H. Copper-Catalyzed [18F]Fluorination of (Mesityl)(Aryl)Iodonium Salts. Org. Lett. 2014, 16, 3224–3227. [Google Scholar] [CrossRef]
- Mossine, A.V.; Brooks, A.F.; Makaravage, K.J.; Miller, J.M.; Ichiishi, N.; Sanford, M.S.; Scott, P.J.H. Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids. Org. Lett. 2015, 17, 5780–5783. [Google Scholar] [CrossRef]
- Makaravage, K.J.; Brooks, A.F.; Mossine, A.V.; Sanford, M.S.; Scott, P.J.H. Copper-Mediated Radiofluorination of Arylstannanes with [18F]KF. Org. Lett. 2016, 18, 5440–5443. [Google Scholar] [CrossRef]
- Lee, S.J.; Makaravage, K.J.; Brooks, A.F.; Scott, P.J.H.; Sanford, M.S. Copper-Mediated Aminoquinoline-Directed Radiofluorination of Aromatic C−H Bonds with K 18F. Angew. Chem. Int. Ed. 2019, 58, 3119–3122. [Google Scholar] [CrossRef]
- Craig, A.; Kolks, N.; Urusova, E.A.; Zischler, J.; Brugger, M.; Endepols, H.; Neumaier, B.; Zlatopolskiy, B.D. Preparation of Labeled Aromatic Amino Acids via Late-Stage 18F-Fluorination of Chiral Nickel and Copper Complexes. Chem. Commun. 2020, 56, 9505–9508. [Google Scholar] [CrossRef] [PubMed]
- Zischler, J.; Kolks, N.; Modemann, D.; Neumaier, B.; Zlatopolskiy, B.D. Alcohol-Enhanced Cu-Mediated Radiofluorination. Chemistry 2017, 23, 3251–3256. [Google Scholar] [CrossRef]
- Bolik, K.-V.; Hellmann, J.; Maschauer, S.; Neu, E.; Einsiedel, J.; Riss, P.; Vogg, N.; König, J.; Fromm, M.F.; Hübner, H.; et al. Heteroaryl Derivatives of Suvorexant as OX1R Selective PET Ligand Candidates: Cu-Mediated 18F-Fluorination of Boroxines, In Vitro and Initial In Vivo Evaluation. EJNMMI Res. 2024, 14, 80. [Google Scholar] [CrossRef]
- Tredwell, M.; Preshlock, S.M.; Taylor, N.J.; Gruber, S.; Huiban, M.; Passchier, J.; Mercier, J.; Génicot, C.; Gouverneur, V. A General Copper-Mediated Nucleophilic 18F Fluorination of Arenes. Angew. Chem. Int. Ed. 2014, 53, 7751–7755. [Google Scholar] [CrossRef] [PubMed]
- Mixdorf, J.C.; Hoffman, S.L.V.; Aluicio-Sarduy, E.; Barnhart, T.E.; Engle, J.W.; Ellison, P.A. Copper-Mediated Radiobromination of (Hetero)Aryl Boronic Pinacol Esters. J. Org. Chem. 2023, 88, 2089–2094. [Google Scholar] [CrossRef]
- Reilly, S.W.; Makvandi, M.; Xu, K.; Mach, R.H. Rapid Cu-Catalyzed [211At]Astatination and [125I]Iodination of Boronic Esters at Room Temperature. Org. Lett. 2018, 20, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.C.; McSweeney, G.; Preshlock, S.; Verhoog, S.; Tredwell, M.; Cailly, T.; Gouverneur, V. Radiosynthesis of SPECT Tracers via a Copper Mediated 123I Iodination of (Hetero)Aryl Boron Reagents. Chem. Commun. 2016, 52, 13277–13280. [Google Scholar] [CrossRef]
- Oka, N.; Yamada, T.; Sajiki, H.; Akai, S.; Ikawa, T. Aryl Boronic Esters Are Stable on Silica Gel and Reactive Under Suzuki–Miyaura Coupling Conditions. Org. Lett. 2022, 24, 3510–3514. [Google Scholar] [CrossRef]
- Gaube, G.; Miller, D.; McCallum, R.; Pipaon Fernandez, N.; Leitch, D. Base-Free Palladium-Catalyzed Borylation of Enol Carboxylates and Further Reactivity Toward Deboronation and Cross-Coupling. Tetrahedron 2025, 182, 134682. [Google Scholar] [CrossRef]
- Geaneotes, P.J.; Janosko, C.P.; Afeke, C.; Deiters, A.; Floreancig, P.E. Potent and Selective Oxidatively Labile Ether-Based Prodrugs through Late-Stage Boronate Incorporation. Angew. Chem. Int. Ed. 2024, 63, e202409229. [Google Scholar] [CrossRef]
- Kreller, M.; Pietzsch, H.; Walther, M.; Tietze, H.; Kaever, P.; Knieß, T.; Füchtner, F.; Steinbach, J.; Preusche, S. Introduction of the New Center for Radiopharmaceutical Cancer Research at Helmholtz-Zentrum Dresden-Rossendorf. Instruments 2019, 3, 9. [Google Scholar] [CrossRef]
- Laube, M.; Brandt, F.; Kopka, K.; Pietzsch, H.-J.; Pietzsch, J.; Loeser, R.; Wodtke, R. Development of 123I-Labelled Acrylamides as Radiotracer Candidates for Transglutaminase 2. Nucl. Med. Biol. 2021, 96–97, S79–S80. [Google Scholar] [CrossRef]
- Rubio-Presa, R.; Suárez-Pantiga, S.; Pedrosa, M.R.; Sanz, R. Molybdenum-Catalyzed Sustainable Friedländer Synthesis of Quinolines. Adv. Synth. Catal. 2018, 360, 2216–2220. [Google Scholar] [CrossRef]
- Kanagasundaram, T.; Laube, M.; Wodtke, J.; Kramer, C.S.; Stadlbauer, S.; Pietzsch, J.; Kopka, K. Radiolabeled Silicon-Rhodamines as Bimodal PET/SPECT-NIR Imaging Agents. Pharmaceuticals 2021, 14, 1155. [Google Scholar] [CrossRef]
- Murphy, C.L.; Hall, A.; Roberts, E.J.; Ryan, M.D.; Clary, J.W.; Singaram, B. Preparation and Reactions of 4-Iodobutyl Pinacolborate. Synthesis of Substituted Alkyl and Aryl Pinacolboronates via 4-Iodobutyl Pinacolborate Utilizing Tetrahydrofuran as the Leaving Group. Tetrahedron Lett. 2015, 56, 3032–3033. [Google Scholar] [CrossRef]
- Cai, H.; Conti, P.S. RGD-Based PET Tracers for Imaging Receptor Integrin αvβ3 Expression. Label. Comp. Radiopharm. 2013, 56, 264–279. [Google Scholar] [CrossRef]
- Liu, S. Radiolabeled Cyclic RGD Peptides as Integrin αvβ3-Targeted Radiotracers: Maximizing Binding Affinity via Bivalency. Bioconjugate Chem. 2009, 20, 2199–2213. [Google Scholar] [CrossRef]
- Beyer, L.; Gosewisch, A.; Lindner, S.; Völter, F.; Mittlmeier, L.M.; Tiling, R.; Brendel, M.; Cyran, C.C.; Unterrainer, M.; Rübenthaler, J.; et al. Dosimetry and Optimal Scan Time of [18F]SiTATE-PET/CT in Patients with Neuroendocrine Tumours. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3571–3581. [Google Scholar] [CrossRef]
- Pauwels, E.; Cleeren, F.; Tshibangu, T.; Koole, M.; Serdons, K.; Boeckxstaens, L.; Dekervel, J.; Vandamme, T.; Lybaert, W.; Den Broeck, B.V.; et al. 18F-AlF-NOTA-Octreotide Outperforms 68Ga-DOTATATE/NOC PET in Neuroendocrine Tumor Patients: Results from a Prospective, Multicenter Study. J. Nucl. Med. 2023, 64, 632–638. [Google Scholar] [CrossRef]
- Taylor, N.J.; Emer, E.; Preshlock, S.; Schedler, M.; Tredwell, M.; Verhoog, S.; Mercier, J.; Genicot, C.; Gouverneur, V. Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc. 2017, 139, 8267–8276. [Google Scholar] [CrossRef]
- Wright, J.S.; Sharninghausen, L.S.; Preshlock, S.; Brooks, A.F.; Sanford, M.S.; Scott, P.J.H. Sequential Ir/Cu-Mediated Method for the Meta-Selective C–H Radiofluorination of (Hetero)Arenes. J. Am. Chem. Soc. 2021, 143, 6915–6921. [Google Scholar] [CrossRef] [PubMed]
- Hitosugi, S.; Tanimoto, D.; Nakanishi, W.; Isobe, H. A Facile Chromatographic Method for Purification of Pinacol Boronic Esters. Chem. Lett. 2012, 41, 972–973. [Google Scholar] [CrossRef]
- Bowden, G.D.; Müller, M.; Herth, M.M.; Sanford, M.S.; Scott, P.J.H. Copper-Mediated Radiochemistry: Historical Impact, Current Trends, and Future Possibilities. npj Imaging 2025, 3, 25. [Google Scholar] [CrossRef]
- Kondo, Y.; Kimura, H.; Tanaka, M.; Hattori, Y.; Kawashima, H.; Takahashi, K.; Yasui, H. Mechanistic Insights into the Effect of Sodium Iodide on Copper-Mediated Iododeboronation. Chem. Eur. J. 2024, 30, e202403303. [Google Scholar] [CrossRef] [PubMed]
- Ellison, P.A.; Olson, A.P.; Barnhart, T.E.; Hoffman, S.L.V.; Reilly, S.W.; Makvandi, M.; Bartels, J.L.; Murali, D.; DeJesus, O.T.; Lapi, S.E.; et al. Improved Production of 76Br, 77Br and 80mBr via CoSe Cyclotron Targets and Vertical Dry Distillation. Nucl. Med. Biol. 2020, 80–81, 32–36. [Google Scholar] [CrossRef]
- Makvandi, M.; Samanta, M.; Martorano, P.; Lee, H.; Gitto, S.B.; Patel, K.; Groff, D.; Pogoriler, J.; Martinez, D.; Riad, A.; et al. Pre-Clinical Investigation of Astatine-211-Parthanatine for High-Risk Neuroblastoma. Commun. Biol. 2022, 5, 1260. [Google Scholar] [CrossRef]
- Klootwyk, B.M.; Ryan, A.E.; Lopez, A.; McCloskey, M.J.R.; Janosko, C.P.; Deiters, A.; Floreancig, P.E. Peroxide-Mediated Release of Organophosphates from Boron-Containing Phosphotriesters: A New Class of Organophosphate Prodrugs. Org. Lett. 2023, 25, 5530–5535. [Google Scholar] [CrossRef]
- Janaagal, A.; Kushwaha, A.; Jhaldiyal, P.; Dhilip Kumar, T.J.; Gupta, I. Photoredox Catalysis by 21-Thiaporphyrins: A Green and Efficient Approach for C−N Borylation and C−H Arylation. Chem. A Eur. J. 2024, 30, e202401623. [Google Scholar] [CrossRef]
- Bryce, M.R.; Wang, C.; Batsanov, A.S.; Sage, I. An Improved Synthesis and Structural Characterisation of 2-(4-Acetylthiophenylethynyl)-4-nitro-5-phenylethynylaniline: The Molecule Showing High Negative Differential Resistance (NDR). Synthesis 2003, 13, 2089–2095. [Google Scholar] [CrossRef]
- Jiang, S.; Ge, Y.; Zhang, A.; Hu, S.; Jin, T.; Chen, J.; Zhang, H.; Qin, Y.; Ma, X.; Tang, J.; et al. Cuprate Ion-Pair Catalyzed Conjugate Borylation of Vinyl Sulfones in a Biphasic System. Org. Biomol. Chem. 2025, 23, 3330–3335. [Google Scholar] [CrossRef]
- Wang, X.; Xie, G.; Zhao, Y.; Zheng, K.; Fang, Y.; Wang, X. Facile Pinacol Coupling of Aliphatic Ketones by Brook Rearrangement in the Presence of Samarium Species. Tetrahedron Lett. 2021, 72, 153069. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craig, A.; Sachse, F.J.; Laube, M.; Brandt, F.; Kopka, K.; Stadlbauer, S. PET and SPECT Tracer Development via Copper-Mediated Radiohalogenation of Divergent and Stable Aryl-Boronic Esters. Pharmaceutics 2025, 17, 837. https://doi.org/10.3390/pharmaceutics17070837
Craig A, Sachse FJ, Laube M, Brandt F, Kopka K, Stadlbauer S. PET and SPECT Tracer Development via Copper-Mediated Radiohalogenation of Divergent and Stable Aryl-Boronic Esters. Pharmaceutics. 2025; 17(7):837. https://doi.org/10.3390/pharmaceutics17070837
Chicago/Turabian StyleCraig, Austin, Frederik J. Sachse, Markus Laube, Florian Brandt, Klaus Kopka, and Sven Stadlbauer. 2025. "PET and SPECT Tracer Development via Copper-Mediated Radiohalogenation of Divergent and Stable Aryl-Boronic Esters" Pharmaceutics 17, no. 7: 837. https://doi.org/10.3390/pharmaceutics17070837
APA StyleCraig, A., Sachse, F. J., Laube, M., Brandt, F., Kopka, K., & Stadlbauer, S. (2025). PET and SPECT Tracer Development via Copper-Mediated Radiohalogenation of Divergent and Stable Aryl-Boronic Esters. Pharmaceutics, 17(7), 837. https://doi.org/10.3390/pharmaceutics17070837







