Abstract
Background/Objectives: Cell-mediated and peptide-assisted delivery systems have emerged as powerful platforms at the intersection of chemistry, nanotechnology, and molecular medicine. By leveraging the intrinsic targeting, transport, and signaling capacities of living cells and bioinspired peptides, these systems facilitate the delivery of therapeutic agents across otherwise restrictive biological barriers such as the blood–brain barrier (BBB) and the tumor microenvironment. This review aims to summarize recent advances in engineered cell carriers, peptide vectors, and hybrid nanostructures designed for enhanced intracellular and tissue-specific delivery. Methods: We surveyed recent literature covering molecular design principles, mechanistic studies, and in vitro/in vivo evaluations of cell-mediated and peptide-enabled delivery platforms. Emphasis was placed on neuro-oncology, immunotherapy, and regenerative medicine, with particular focus on uptake pathways, endosomal escape mechanisms, and structure–function relationships. Results: Analysis of current strategies reveals significant progress in optimizing cell-based transport systems, peptide conjugates, and multifunctional nanostructures for the targeted delivery of drugs, nucleic acids, and immunomodulatory agents. Key innovations include improved BBB penetration, enhanced tumor homing, and more efficient cytosolic delivery enabled by advanced peptide designs and engineered cellular carriers. Several platforms have progressed toward clinical translation, underscoring their therapeutic potential. Conclusions: Cell-mediated and peptide-assisted delivery technologies represent a rapidly evolving frontier with broad relevance to next-generation therapeutics. Despite notable advances, challenges remain in scalability, manufacturing, safety, and regulatory approval. Continued integration of chemical design, molecular engineering, and translational research will be essential to fully realize the clinical impact of these delivery systems.
1. Introduction
Targeted intracellular delivery remains an important challenge in modern pharmacology and gene therapy. Classical nanocarriers, though versatile, often fail to replicate the precision, biocompatibility, and dynamic responsiveness of biological systems. The emergence of cell-mediated and peptide-based vectors has introduced a bioinspired paradigm, integrating molecular recognition, receptor-mediated transcytosis, and immune cell homing mechanisms into drug delivery design.
Cell-mediated systems—using leukocytes, macrophages, dendritic cells (DCs), or stem cells as biological shuttles—can actively navigate physiological barriers and deliver therapeutic cargos to otherwise inaccessible tissues. Similarly, cell-penetrating peptides (CPPs), receptor-targeting sequences, and self-assembling peptidomimetics have been developed to mimic or harness cellular communication pathways [1,2,3]. Cell-mediated delivery systems and CPPs represent two complementary strategies designed to overcome biological barriers and achieve efficient intracellular transport of therapeutic agents [4,5,6]. CPPs are short peptide sequences capable of translocating across cellular and other membranes, thereby facilitating the direct delivery of diverse cargos such as small molecules, nucleic acids, proteins, or nanoparticles into target cells and compartments within [7,8,9]. In contrast, cell-mediated delivery systems utilize living cells—such as immune cells, stem cells, or macrophages—as biological carriers that can naturally home to disease sites and transport therapeutic payloads in a physiological context [10,11,12,13]. The combination of these two approaches offers a synergistic advantage: CPPs can enhance the loading efficiency of therapeutic cargos into carrier cells and promote controlled intracellular release, while the inherent homing ability of carrier cells improves biodistribution and accumulation in the targeted tissues. Integrating CPPs into cell-mediated systems thus holds great promise for achieving precise, efficient, and safe therapeutic delivery in complex biological environments [14,15,16,17].
Immune-modulating peptides (IMPs) and cell-mediated delivery systems form a promising fusion in the development of next-generation immunotherapies [18,19]. IMPs are short peptide sequences that regulate immune responses by interacting with receptors or signaling pathways involved in inflammation, immune activation, or tolerance [20]. They can either stimulate immune functions—for example, enhancing antigen presentation or T-cell activation—or suppress pathological inflammation in autoimmune and inflammatory diseases [21]. Despite their high potency and selectivity, IMPs often hindered by limitations such as enzymatic degradation, short circulation half-life, and insufficient tissue specificity. Cell-mediated delivery systems, including immune cells, stem cells, and engineered exosomes, provide a solution contributing as living carriers that naturally migrate to immune-relevant sites [22,23]. Incorporating IMPs into such systems enhances targeted delivery, protects the peptides from degradation, and enables controlled or stimulus-responsive release in the microenvironment of immune activation [24,25,26]. Conversely, certain IMPs can modulate the phenotype or function of the carrier cells themselves, further optimizing therapeutic outcomes [27]. The integration of immune-modulating peptides with cell-mediated delivery platforms thus represents a synergistic approach that unites precise immunoregulation with biologically guided targeting and sustained therapeutic action [28,29].
RGD peptides, containing the arginine–glycine–aspartic acid (RGD) motif, are among the most studied targeting ligands due to their high affinity for integrin receptors, particularly αvβ3 and αvβ5, which are overexpressed on angiogenic endothelial cells (ECs) and various tumor types [30,31,32]. These peptides facilitate selective recognition and adhesion to integrin-rich tissues, enabling enhanced targeting in cancer therapy, tissue regeneration, and vascular repair [33,34,35,36]. Cell-mediated delivery systems—such as macrophages, mesenchymal stem cells, and immune cells—possess innate homing abilities that can be further optimized through RGD functionalization [37]. Conjugating or decorating carrier cells, nanoparticles, or their surface vesicles with RGD peptides can improve cell adhesion, transmigration across endothelial barriers, and accumulation at pathological sites characterized by integrin overexpression [38,39]. Conversely, RGD–integrin interactions can modulate the activation state and migration behavior of the carrier cells themselves, refining their therapeutic performance [40]. The integration of RGD peptides into cell-mediated delivery systems thus provides a dual advantage: enhanced biological targeting precision through receptor-mediated recognition and improved cellular navigation within complex tissue microenvironments [41,42]. This interplay holds significant potential for advancing targeted cancer therapies, regenerative medicine, and vascular tissue engineering [43,44,45].
Together, these systems redefine the concept of “smart” delivery: not merely passive transporters but active biological participants in therapeutic intervention.
2. Peptide-Based Delivery Systems
2.1. Cell-Penetrating Peptides and Gene Transfer
CPPs such as trans-activator of transcription (TAT), penetratin, or Ras-related protein (RALA) have long been recognized as potent translocation motifs. Recent advances have refined their selectivity and efficiency through modular design. Ding et al. developed neurokinin-1 receptor (NK1R) targeting vectors (SP-PEG4-K(C18)-(LLHH)3-R9) combining cell-penetrating, targeting, and endosomal escape domains to achieve specific transfection of glioma cells and successful blood-brain barrier (BBB) crossing in zebrafish models. The work exemplifies receptor-mediated uptake coupled with peptide self-assembly into gene-delivery nanocomplexes, achieving >30% higher transfection than the transfection reagent Lipofectamine in NK1R-positive cells [46].
Similarly, Jeyarajan et al. engineered a heregulin-α–NLS fusion protein targeting HER2/3-overexpressing breast cancer cells, enabling nucleus-specific plasmid DNA delivery [47]. By modularly combining a receptor ligand with a nuclear localization signal, the system attained selective uptake in MDA-MB-453 cells while avoiding non-target tissues.
In parallel, Neves et al. optimized the cationic RALA peptide/pDNA system for p53 delivery, demonstrating that the N/P ratio can fine-tune condensation, charge, and gene-expression efficiency, directly modulating apoptosis in cancer cells [48]. These studies collectively illustrate that fine molecular engineering—through charge balance, lipidation, and receptor ligands—translates into controllable intracellular trafficking and therapeutic output.
2.2. CPPs in Vaccine Development
CPPs have also redefined vaccine formulation by enabling cytosolic delivery of protein and peptide antigens. Hasannejad-Asl et al. comprehensively reviewed CPP-conjugated vaccines, highlighting their capacity to enhance cross-presentation, antigen processing, and T-cell activation [49]. Strategies to enhance CPP stability are presented in Figure 1.
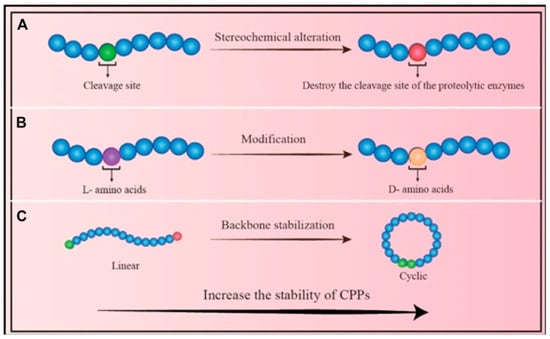
Figure 1.
Approaches to Enhance CPP Stability: (A) Stereochemical modification: Altering the chemical configuration of amino acid residues reduces the enzymatic susceptibility of CPPs. (B) Amino acid substitution: Replacing natural residues with alternative isomers can significantly improve CPP stability. (C) Backbone cyclization: Converting linear CPPs into cyclic forms enhances resistance to enzymatic degradation and increases overall structural stability [49].
Notably, Z12-conjugated (small nucleolar RNA) protein vaccines promoted multi-epitopic CD4+/CD8+ responses against brain tumors, while LAH4-adjuvanted protein vaccines induced strong CTL immunity via endosomal acidification and proteasomal processing [50]. Pep-1/E7 nanoparticles likewise elicited Th1-biased protective responses in HPV16 tumor models comparable to Freund’s adjuvant [51].
Collectively, these CPP-based vaccines merge efficient intracellular transport with immunomodulation, yielding self-adjuvating platforms that circumvent the limitations of traditional emulsions or liposomes.
2.3. Peptidomimetic and Self-Assembled Nanostructures
Beyond natural sequences, peptidomimetics offer enhanced stability and tunable self-assembly. In a recent review, Jabbari et al. summarized the peptidomimetic self-assembled nanoparticles (NPs) that integrate biological recognition motifs with structural robustness [52]. These NPs serve as scaffolds for sustained antigen presentation, tumor targeting, and gene delivery while resisting enzymatic degradation. Their self-assembly—driven by hydrophobic, hydrogen-bonding, and electrostatic interactions—provides an elegant route to multivalent nanostructures with programmable morphology and function.
Table 1 summarizes seven peptide-based delivery systems, all of which show generally strong in vitro efficacy and low toxicity across various cell types and models. Their scalability is usually feasible through solid-phase peptide synthesis, though most systems have not yet been evaluated at true industrial or GMP scale. Overall, the technologies remain at early preclinical stages, with most evidence limited to in-vitro studies and small animal experiments, and no peptide-based systems yet in clinical trials.

Table 1.
Summary of preclinical peptide-based delivery systems evaluated for efficacy, toxicity, scalability, and translational readiness.
3. Cell-Mediated Delivery Systems
3.1. Engineered Immune Cells as Therapeutic Couriers
Leveraging the intrinsic homing ability of immune cells has emerged as a powerful strategy for precision oncology. Wang et al. introduced monocyte-based photoactive nanoparticle delivery, where monocytes loaded with near-infrared (NIR) responsive polyacrylates (PAAs) achieved deep tumor penetration and dual photodynamic/photothermal ablation [53]. Metabolic surface glycoengineering with cyclic RGD peptides enhanced tumor migration and retention, enabling precise image-guided therapy.
Similarly, Liu et al. developed β-cyclodextrin/peptide-linked liposomes that release Matrix metalloproteinase-2 (MMP-2) inhibitors and photosensitizers sequentially within the tumor microenvironment [54]. This approach remodeled the tumor immune niche, increased NK-cell infiltration >100-fold, and yielded potent synergistic photo-immunotherapy—an exemplary demonstration of cell-microenvironment interplay for therapeutic benefit.
Mast cells, macrophages, and stem cells have also been investigated as carriers or trigger cells [55]. Their migratory behavior, cytokine release, and biocompatibility render them attractive vehicles for localized delivery of oncolytic, gene, or regenerative payloads.
3.2. Endothelial Cell-Mediated Vascular Repair
Zhou et al. introduced an elegant endothelial-cell-mediated gene delivery graft in which adhesive peptides with Arg–Glu–Asp–Val (REDV) motif capture ECs and trigger localized gene release through matrix metalloproteinase-cleavable linkers [56]. This responsive interface initiated selective adhesion, enzymatic activation, and in situ endothelialization of vascular grafts in vivo—illustrating how cellular microenvironment cues can be harnessed for regenerative biointerfaces.
Table 2 compares several endosomal-escape mechanisms—including proton-sponge buffering, direct membrane disruption, membrane fusion, and endosomal enzyme–responsive systems—highlighting their strengths, limitations, and current development status all of data with crucial importance for cell based delivery systems. Overall, while many strategies demonstrate strong mechanistic rationale and good in-vitro performance, most lack robust in-vivo validation and remain at early preclinical stages, with safety, specificity, and scalability posing the main challenges.

Table 2.
Summary of Mechanisms and Translational Readiness of Endosomal Escape Strategies for Cell-Based Delivery Systems.
4. Overcoming the Blood–Brain Barrier
4.1. Nanomaterial and Peptide Strategies
The BBB remains the principal obstacle in CNS therapeutics. As emphasized in a review by Patel and Xie, delivering strategies now extend beyond passive diffusion to receptor-mediated, adsorptive, and cell-mediated transcytosis [57,58]. Pathways of transport across the blood–brain barrier are shown in Figure 2. Nanocarriers equipped with CPPs or receptor-binding motifs (e.g., angiopep-2, transferrin) demonstrate promising BBB permeability, while cell-based transporters such as macrophages or stem cells exploit natural trafficking routes to deliver nanodrugs to diseased brain regions [59].
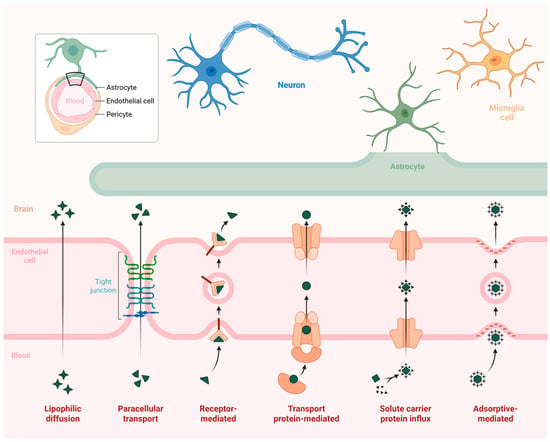
Figure 2.
Mechanisms of Blood–Brain Barrier Permeation.
Mathupala highlighted the potential of CPP-siRNA duplexes for non-invasive CNS delivery, proposing molecular constructs capable of crossing the BBB systemically [60]. Similarly, Man et al.’s PEG12-KL4 peptide enabled pulmonary delivery of EGFR/PD-L1-targeted siRNAs, with efficient knockdown and enhanced T-cell–mediated cytotoxicity—offering a blueprint for nucleic acid delivery through accessible epithelial interfaces [61].
4.2. Bioactive Nanoparticles for Neurodegenerative Diseases
Kshirsagar et al. reviewed bioactive compound-loaded nanoparticles addressing Alzheimer’s pathology (shown in Figure 3) through antioxidant and anti-inflammatory mechanisms [62]. Importantly, cell-mediated and transcytosis-based BBB transport emerged as critical determinants of efficacy, reinforcing the concept that combining nanotechnology with biological transport systems yields the most promising neurotherapeutic outcomes.
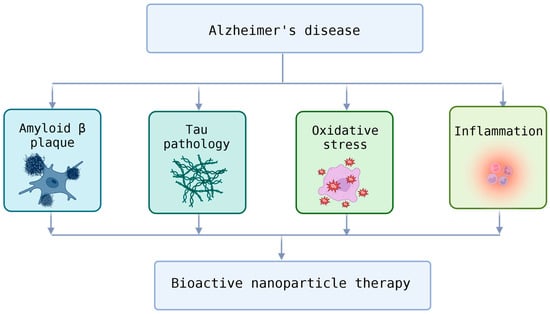
Figure 3.
Therapeutic Targets in Alzheimer’s Disease and the Potential of Bioactive Nanoparticle Therapy.
5. Immunotherapeutic Integration
Wang and Wang outlined the molecular rationale for intracellular peptide delivery into DCs to stimulate pattern-recognition receptor signaling and augment cytotoxic T-cell responses [63]. Kimura et al. expanded this principle using endosomal sorting complexes required for transport (ESCRT) mediated extracellular vesicles for efficient antigen loading and CTL activation—highlighting a convergence between cell-derived vesicles and engineered peptide vectors in vaccine design [64].
The synergy between photo-immunotherapy as in microenvironment-/light-responsive bio-nanosystems (MLRNs) and immune-cell engagement (e.g., NK or CD8+ recruitment) underscores a transformative shift from single-agent cytotoxicity toward immune-coordinated multimodal therapy.
Table 3 outlines multiple cell-uptake mechanisms—including clathrin-mediated endocytosis, caveolae-mediated uptake, macropinocytosis, phagocytosis, and direct translocation—highlighting their pathways, cargo preferences, and biological constraints. While each mechanism offers unique advantages for targeting specific cell types or achieving efficient internalization, they also come with limitations such as size restrictions, dependence on energy or receptor pathways, and variable reliability across different biological contexts. Overall, selecting the optimal uptake route requires balancing specificity, efficiency, and compatibility with the delivered cargo.

Table 3.
Summary of Mechanistic Comparison of Endocytic and Non-Endocytic Cellular Uptake Pathways of Peptide CMDS hybrid systems.
6. Ocular and Localized Delivery
Beyond systemic delivery, cell-mediated concepts extend to ocular surfaces, where the application of traditional eye drops is limited by poor retention. In a study by He et al. a soluble microneedle patch loaded with immunogenic PKHB1 peptide penetrated corneal barriers, released the peptide sustainably, and elicited CD8+ T-cell–mediated antiviral responses against HSV-1 keratitis [65]. The system exemplifies how microengineered matrices can recreate cell-mediated immunity in confined tissues.
7. Design Principles and Structure–Function Insights
7.1. Molecular Modularity
Across all platforms, the efficiency and precision of cell-mediated and peptide-based delivery systems depend on their modular molecular architecture, in which individual functional domains perform distinct yet complementary tasks to achieve targeted, controlled, and biocompatible therapeutic delivery [66].
Targeting domains (e.g., Substance P, heregulin, RGD, REDV) ensure receptor-specific recognition and guide the delivery system toward the intended tissue or cell type. Such motifs exploit overexpressed receptors or adhesion molecules—Substance P targets NK1Rs in glioma cells, heregulin interacts with HER2/3 in breast cancer, while RGD and REDV sequences mediate integrin or endothelial cell adhesion, respectively [67].
Cell-penetrating peptide (CPP) or fusogenic domains facilitate membrane translocation and endosomal escape, enabling intracellular delivery of nucleic acids, proteins, or small molecules (Figure 4). Classical CPPs like TAT, penetratin, or RALA promote uptake through electrostatic interactions, while pH-sensitive fusogenic sequences such as LAH4 or HA2 undergo conformational transitions that destabilize endosomal membranes and release the cargo into the cytosol. GraphCPP leverages graph neural networks to learn residue–residue interaction patterns within peptide sequences, enabling far more nuanced CPP prediction than traditional feature-based classifiers. In benchmark evaluations, GraphCPP improves prediction accuracy by roughly 8–15% over conventional SVM and random-forest models and shows markedly higher precision for borderline or atypical CPPs. Its graph-based embedding also enhances generalization, reducing false positives by capturing structural motifs missed by linear sequence descriptors [68,69].
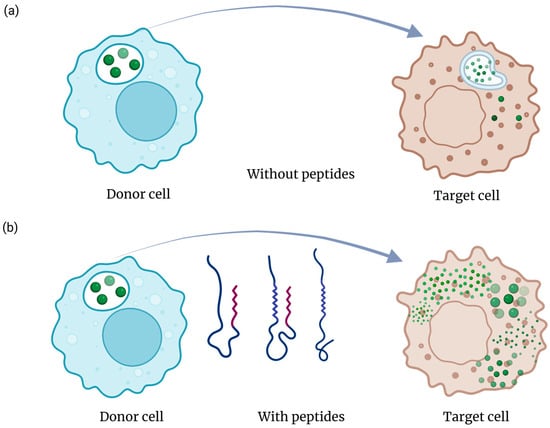
Figure 4.
Sematic representation of endosomal escape in the absence (a) and presence (b) of peptide sequences. Peptides can enhance the efficacy of CMDS by enhancing the endosomal escape of cargo molecules.
Responsive linkers—including pH-, MMP-, or redox-cleavable motifs—provide spatiotemporal control over activation and release. For instance, MMP-sensitive linkers allow for selective drug liberation within protease-rich tumor microenvironments, while disulfide-based redox linkers respond to intracellular glutathione gradients for cytosolic release of nucleic acids or peptides [70].
Hydrophobic anchors or PEG linkers enhance physicochemical stability and pharmacokinetic tunability. Hydrophobic tails such as stearic acid or cholesterol promote self-assembly into nanoparticles and strengthen membrane affinity, whereas PEGylation improves solubility, reduces immune recognition, and prolongs systemic circulation [71].
This modular design paradigm allows rational adaptation of vectors to distinct therapeutic contexts—in nucleic acid delivery, protein vaccination, or immune stimulation—by enabling the systematic optimization of each structural component. Through deliberate combination of targeting, translocation, responsiveness, and stabilization modules, these hybrid systems evolve from simple carriers into multifunctional, adaptive platforms capable of precise spatiotemporal control and enhanced therapeutic efficacy.
7.2. Cell Interactions and Microenvironment Sensitivity
Effective cell-mediated and peptide-based delivery systems must not only carry their therapeutic payloads but also interface intelligently with the biological microenvironment. The performance of these systems depends on their ability to recognize, respond to, and exploit the physicochemical and biochemical cues of target tissues. This sensitivity governs biodistribution, internalization, and controlled release, linking molecular design to cellular behavior and tissue specificity.
Microenvironment-responsive activation allows selective function at the disease site. Many tumors and inflamed tissues are characterized by acidic pH, elevated enzyme activity (e.g., matrix metalloproteinases, cathepsins), oxidative stress, or abnormal cytokine profiles. Delivery vectors incorporating pH-sensitive residues or MMP-cleavable peptide linkers exploit these pathological conditions to trigger cargo release or surface activation specifically within the diseased milieu. For example, MMP-2-responsive nanoconjugates enable site-specific release of photosensitizers and immunomodulators in tumor tissue, enhancing therapeutic precision while minimizing systemic toxicity [72].
Receptor-mediated uptake and cell selectivity arise from the interplay between targeting ligands and overexpressed membrane receptors. Peptide ligands such as RGD, REDV, Substance P, or heregulin interact with integrins, endothelial adhesion receptors, or growth factor receptors, enabling selective internalization by cancer, endothelial, or immune cells. The specificity of these interactions underpins the success of strategies such as NK1R-mediated glioma targeting and HER2/3-driven gene delivery in breast cancer [73].
Cellular cooperation and transport mechanisms expand the reach of therapeutic systems beyond passive diffusion. Engineered immune or endothelial cells can actively ferry nanoparticles and peptide–drug conjugates across biological barriers such as the blood–brain barrier (BBB) or tumor stroma (Figure 5). Intranasal drug delivery provides a noninvasive route to bypass the BBB, enabling rapid and targeted delivery to the brain. While it offers reduced systemic side effects, its efficiency can be limited by mucociliary clearance and enzymatic degradation. Monocyte- or macrophage-based carriers, for instance, migrate to inflammatory or hypoxic regions, while endothelial cells can serve as living transfection platforms that promote localized angiogenesis or regeneration [74].
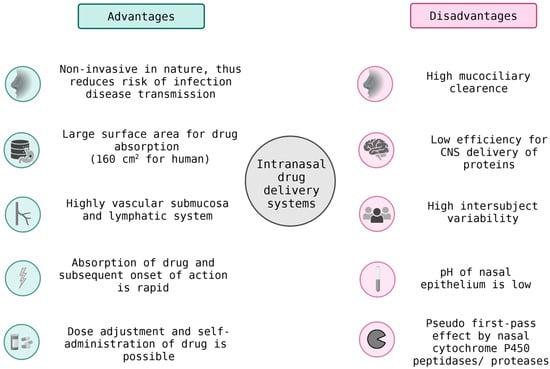
Figure 5.
Intranasal Drug Delivery Systems: Benefits and Limitations in BBB Transport.
Dynamic biophysical interactions—including electrostatic attraction, hydrophobic partitioning, and membrane curvature effects—govern the initial contact between delivery systems and cell membranes. These interactions dictate internalization routes such as clathrin-mediated endocytosis, macropinocytosis, or lipid-raft–assisted uptake. Understanding and tuning these parameters allows rational modulation of uptake efficiency and intracellular trafficking pathways [75].
Immune compatibility and biological feedback are critical determinants of long-term efficacy. Peptide vectors and hybrid nanoparticles are necessary to avoid unwanted immune activation while engaging beneficial immune pathways when desired—for example, in vaccine delivery or cancer immunotherapy. Materials engineered with zwitterionic or PEGylated surfaces minimize the risk of nonspecific opsonization, whereas immunogenic peptides or adjuvant motifs can be incorporated deliberately to enhance antigen presentation and T-cell activation [76].
Overall, microenvironmental responsiveness transforms delivery systems from inert carriers into biointeractive entities capable of sensing and adapting to their surroundings. By integrating physicochemical responsiveness, receptor specificity, and cellular cooperation, such systems achieve precise control over localization, uptake, and release kinetics. This responsiveness is a defining feature of next-generation therapeutic platforms—where chemistry, materials science, and cell biology converge to achieve targeted and context-dependent drug delivery [77].
8. Translational and Regulatory Considerations
The transition of cell-mediated and peptide-based delivery systems from laboratory discovery to clinical application remains a formidable challenge. Despite impressive preclinical advances demonstrating potent therapeutic efficacy in vitro and in vivo, translation is often hindered by manufacturing complexity, product heterogeneity, and regulatory uncertainty. These systems, by their very nature—combining synthetic peptides, nanomaterials, and living cells—occupy a grey zone at the intersection of biologics, advanced therapy medicinal products (ATMPs), and nanomedicines, demanding specialized oversight frameworks and rigorous quality control [78,79].
Manufacturing and scalability are the main obstacles. The production of multifunctional peptide–nanoparticle conjugates or hybrid cell-based systems requires precise control over sequence fidelity, assembly conditions, and surface functionalization. Conventional batch synthesis can introduce variability in particle size, peptide density, or biological activity, complicating reproducibility and regulatory approval. Recent progress in continuous-flow solid-phase peptide synthesis (CF-SPPS) and automated purification offers a solution scalable and reproducible manufacturing under Good Manufacturing Practice (GMP) standards. Flow-based synthesis not only minimizes solvent use and reaction time but also allows for precise in-line monitoring of product quality—aligning with the green chemistry and quality-by-design (QbD) principles are favored by regulatory agencies. Using these technique, the process mass intensity value of the SPPS decreases to value of ca. 300, of which is in the order of magnitude of regular small-organic drug molecules. Importantly, by scale-up these values remains basically the same [80,81].
Characterization and standardization are critical for ensuring clinical reliability. Comprehensive physicochemical and biological profiling—including size distribution, charge, purity, stability, and in vitro bioactivity—must be supported by validated analytical assays. For hybrid systems, additional characterization of biodistribution, persistence, and immunogenicity is required to predict long-term safety. The integration of high-resolution imaging, quantitative mass spectrometry, and in vivo pharmacokinetic modeling aids in establishing robust comparability between production batches [82].
Immunological and biosafety evaluation remains a cornerstone of translational assessment. While peptide-based carriers generally exhibit low inherent immunogenicity, in combination with adjuvants, targeting ligands, or cellular components can lead to unpredictable immune responses. Regulatory agencies therefore demand extensive toxicological testing, cytokine-release profiling, and long-term immunosurveillance before first-in-human trials. In the context of cell-mediated delivery, additional attention must be given to cell persistence, tumorigenicity risk, and vector integration stability, particularly for engineered immune or stem cells [83].
Digital and AI-assisted optimization is emerging as a transformative tool for translation. Machine-learning algorithms can predict optimal peptide sequences, linker chemistries, and assembly parameters, reduce experimental cycles and help identifying lead candidates with more favorable manufacturing profiles. Combined with automated flow synthesis, such computational tools enable rapid, data-driven optimization for process control and traceability in accordance with regulatory expectations. GraphCPP leverages graph neural networks to learn residue–residue interaction patterns within peptide sequences, enabling far more nuanced CPP prediction than traditional feature-based classifiers. In benchmark evaluations, GraphCPP improves prediction accuracy by roughly 8–15% over conventional SVM and random-forest models and shows markedly higher precision for borderline or atypical CPPs. Its graph-based embedding also enhances generalization, reducing false positives by capturing structural motifs missed by linear sequence descriptors [69,84].
Clinical Translation and Regulatory Landscape of Cell–Peptide Hybrids have been examined in a limited but growing number of clinical trials, which have already demonstrated the translational potential of cell- and peptide-based delivery systems, particularly in oncology and immunotherapy. Early first-in-human studies with the tumor-targeting cell-penetrating peptide p28 (NSC745104) in adults and children with advanced solid or CNS tumors established acceptable safety and preliminary antitumor activity, illustrating that CPP-based agents can meet contemporary clinical and regulatory safety thresholds [85,86]. Dendritic cell-based vaccines and exosome formulations—often loaded with tumor peptides or antigens—have progressed to phase II and III trials, including IFN-γ–matured dendritic cell-derived exosomes (IFN-γ–Dex) in non-small cell lung cancer and DCVax-L in glioblastoma, highlighting the feasibility of complex cell-based products that integrate peptide antigens or peptide-decorated vesicles in a GMP-compliant manner [87,88,89]. In parallel, tumor-penetrating peptides such as certepetide (LSTA1) have entered later-stage trials in combination with chemotherapy, where they act as peptide-based enhancers of intratumoral drug penetration [90]. Selected examples are summarized in Table 4.

Table 4.
Overview of Peptide-Enabled and Cell-based Cancer Therapies in Ongoing Clinical Trials.
Table 4 describes key clinical-stage peptide- and dendritic-cell-based therapeutic modalities currently evaluated across oncology indications. It highlights five representative products—from tumor-penetrating and tumor-targeting cell-penetrating peptides (CPPs) to autologous dendritic-cell vaccines and exosome-based immunotherapies—covering phases I to III. Each entry outlines the cancer indication, ClinicalTrials.gov identifier, development phase, and a short note describing the mechanism or distinguishing features (e.g., p53-stabilizing CPP p28, antigen-loaded DC exosomes, tumor-lysate–pulsed DC vaccines, MSI-targeted immunotherapy, and the tumor-penetrating peptide LSTA1 that improves chemotherapeutic delivery). The table below provides an overview of advanced peptide-enabled and peptide-augmented clinical strategies in cancer therapy.
From a regulatory standpoint, cell–peptide hybrids sit at the interface between biologics, advanced therapy medicinal products (ATMPs), and nanomedicines, complicating classification and CMC (chemistry, manufacturing and controls). In the EU, many peptide–cell constructs will fall under the ATMP framework (Regulation (EC) No 1394/2007) as somatic cell therapy or combined ATMPs when cells are substantially manipulated or used for a non-homologous function [41,91,92]. This implies stringent centralized authorization, risk-based evaluation, and ATMP-specific GMP requirements, including full traceability, donor eligibility documentation, and long-term post-authorization follow-up. In the US, autologous carriers and ex vivo–modified cells are regulated as human cells, tissues, and cellular and tissue-based products (HCT/Ps) with additional cGTP/cGMP obligations under 21 CFR Part 1271 [93,94,95].
For cell–peptide hybrids in particular, several specific hurdles arise: (i) GMP for autologous products, where patient-specific manufacturing, donor screening, and chain-of-identity/chain-of-custody must be tightly controlled; (ii) potency assays that remain valid after peptide modification or repeated peptide loading—e.g., assays for antigen presentation, cytokine release, or target killing must be qualified and shown to correlate with clinical effect; and (iii) product heterogeneity and batch variability, since peptide decoration densities, cell activation status, or exosome cargo composition can change with starting material or process drift. Regulators increasingly expect a risk-based, ATMP-style control strategy with well-defined critical quality attributes (CQAs), in-process controls, and stability data tailored to these hybrid constructs [91,92,95]. Addressing these aspects early in development is essential to move cell-mediated and peptide-based delivery systems from promising preclinical tools to licensable therapies.
Regulatory convergence and framework adaptation are equally essential. The hybrid nature of these therapies calls for harmonization between biologics, device, and nanomedicine guidelines. Collaborative efforts between academia, industry, and medicines regulatory (EMA, FDA) and other authorities (e.g., ICH) are beginning to define specific standards for advanced combination products, including documentation of critical quality attributes (CQAs), batch release criteria, and post-market surveillance requirements [96].
In summary, while manufacturing reproducibility, immunological safety, and regulatory classification remain primary translational obstacles, emerging technologies such as continuous-flow peptide synthesis, automated analytics, and AI-driven optimization may offer viable solutions. Together with GMP-compliant cell engineering platforms, these innovations are paving the way toward scalable, standardized, and clinically translatable cell-mediated and peptide-based therapeutics, bridging the gap between experimental achievements and regulatory approval.
9. Outlook and Future Perspectives
The convergence of cell biology, peptide chemistry, and nanotechnology is resulting in the emergence of a new generation of adaptive therapeutic systems capable of dynamic interaction with their biological environment. These next-generation delivery platforms transcend the traditional concept of passive carriers, evolving instead into integrated, feedback-responsive therapeutic networks. Their development is expected to transform precision medicine, immunotherapy, and regenerative strategies through several interrelated innovations:
Hybrid bioartificial carriers integrating living cells with synthetic scaffolds will enable unparalleled precision in drug delivery and biological feedback control. By combining the navigational and homing properties of immune or stem cells with the stability and modularity of engineered nanomaterials, such systems can respond dynamically to local biochemical signals. Engineered macrophages, endothelial cells, or exosomes functionalized with peptide–polymer conjugates exemplify this hybrid approach, allowing sustained, on-demand release of therapeutic payloads and real-time adaptation to pathological changes [97].
Organelle-targeting peptides and multi-responsive linkers will extend the precision of delivery to the subcellular level. By incorporating sequences that direct cargos to mitochondria, lysosomes, or nuclei, researchers can achieve spatiotemporally controlled pharmacological effects. When combined with multi-stimuli–responsive linkers—triggered by pH, redox processes, enzymes, or light—these designs will enable hierarchical activation within specific organelles, improving therapeutic selectivity by minimizing off-target effects [98].
AI-driven sequence design and predictive modeling are poised to revolutionize the discovery and optimization of functional peptides. Machine learning (ML) algorithms and molecular dynamics (MD) simulations can correlate sequence features with membrane translocation, receptor binding, and toxicity profiles, thereby generating optimized CPPs or targeting ligands with enhanced selectivity and reduced immunogenicity. Integrating computational prediction with continuous-flow synthesis and high-throughput screening (HTS) will accelerate the translation of in silico designs into clinically viable candidates [99].
Multicellular therapeutic ecosystems, in which immune cells, endothelial cells, and synthetic vectors act cooperatively, may redefine drug delivery as a systems-level biological process. Instead of isolated carrier entities, future therapeutics could function as coordinated networks where different cellular and synthetic components perform complementary roles—such as targeting, immune modulation, and tissue repair—within a controlled, self-regulating framework. This cooperative paradigm mirrors natural tissue organization, promising improved therapeutic durability and safety [100].
Ultimately, cell-mediated and peptide-based delivery systems exemplify the evolution of drug delivery science from passive encapsulation toward interactive and intelligent therapeutics. By uniting the programmability of synthetic chemistry with the adaptability of living systems, these platforms hold the potential to deliver personalized, context-sensitive interventions for complex diseases—including cancer, neurodegeneration, and chronic inflammation—while advancing the broader vision of responsive, regenerative, and precision medicine.
Several rapidly developing modalities are expanding the conceptual and technological landscape of cell-mediated delivery, and brief acknowledgment of these advances further strengthens the novelty and completeness of the CMDS framework. Neutrophil-mediated delivery (“neutrophil hitchhiking”) has emerged as a powerful strategy in which nanoparticles leverage neutrophils’ natural recruitment to inflammatory and tumor microenvironments to achieve targeted transport across vascular and stromal barriers [101,102]. Cell-backpack systems, involving polymeric or hydrogel micro-patches affixed to leukocytes without internalization, offer long-lived and programmable delivery vehicles that preserve cellular motility and enable sustained release or immunomodulation in vivo [103,104]. On the peptide–nanocarrier side, advanced amphiphilic CPP-based nanocage systems, such as the PepFect and NickFect families, have demonstrated high efficiency in delivering DNA, siRNA, mRNA, and Cas9 RNPs through self-assembled, endosomolytic nanostructures [105]. Finally, complementary carrier-free protein delivery technologies such as iTOP (induced transduction by osmocytosis and propanebetaine) further expand intracellular delivery capabilities, enabling efficient cytosolic entry of recombinant proteins and genome-editing complexes without conventional vectors [106]. Together, these emerging approaches illustrate the accelerating diversification of CMDS technologies and highlight future directions where biological carriers, engineered peptides, and synthetic nanostructures converge into increasingly adaptive and clinically scalable therapeutic systems.
Author Contributions
Conceptualization, E.E., I.M.M. and B.B.; methodology, E.E. and R.D.; software, B.B.; resources, I.M.M.; data curation, E.E. and R.D.; writing—original draft preparation, E.E.; writing—review and editing, I.M.M.; visualization, E.E.; supervision, I.M.M.; funding acquisition, I.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful to the Hungarian Research Foundation (OTKA ANN 139484). The financial support of the National Research, Development and Innovation Office (TKP2021-EGA-31) is acknowledged. Project no. RRF-2.3.1-21-2022-00015 has been implemented with support provided by the European Union.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ATMPs | Advanced Therapy Medicinal Products |
| AI | Artificial Intelligence |
| BBB | Blood-Brain-Barrier |
| CF-SPPS | Continuous-Flow Solid-Phase Peptide Synthesis |
| CPPs | Cell-penetrating peptides |
| CQAs | critical quality attributes |
| DCs | Dendritic Cells |
| ECs | Endothelial Cells |
| ESCRT | Endosomal Sorting Complexes Required for Transport |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| GMP | Good Manufacturing Practices |
| HTS | High-Throughput Screening |
| ICH | International Council for Harmonisation |
| IMPs | Immune-Modulating Peptides |
| MD | Molecular Dynamics |
| ML | Machine Learning |
| MMP | Matrix Metallo Proteinase |
| NIR | Near-Infra Red |
| NK1R | Neurokinin-1 Receptor |
| RGD | Arginine–Glycine–Aspartic acid motif |
| REDV | Arg–Glu–Asp–Val motif |
| QbD | Quality-by-Design |
| PAAs | Polyacrylates |
| RALA | Ras-Related Protein |
| TAT | Trans-Activator of Transcription |
References
- Gori, A.; Lodigiani, G.; Colombarolli, S.G.; Bergamaschi, G.; Vitali, A. Cell Penetrating Peptides: Classification, Mechanisms, Methods of Study, and Applications. ChemMedChem 2023, 18, e202300236. [Google Scholar] [CrossRef]
- Milletti, F. Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discov. Today 2012, 17, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.; Melikov, K.; Vives, E.; Ramos, C.; Verbeure, B.; Gait, M.; Chernomordik, L.; Lebleu, B. Cell-penetrating peptides—A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 2003, 278, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Khan, A.R.; Fu, M.; Wang, R.; Ji, J.; Zhai, G. Cell-penetrating peptide: A means of breaking through the physiological barriers of different tissues and organs. J. Control. Release 2019, 309, 106–124. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.; Adams, D.; Alewood, P. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.; Ma, Z.; Lu, P.; Kebebe, D.; Liu, Z. Combination of cell-penetrating peptides with nanomaterials for the potential therapeutics of central nervous system disorders: A review. J. Nanobiotechnol. 2021, 19, 255. [Google Scholar] [CrossRef]
- Liu, J.; Cabral, H.; Mi, P. Nanocarriers address intracellular barriers for efficient drug delivery, overcoming drug resistance, subcellular targeting and controlled release. Adv. Drug Deliv. Rev. 2024, 207, 115239. [Google Scholar] [CrossRef]
- Desale, K.; Kuche, K.; Jain, S. Cell-penetrating peptides (CPPs): An overview of applications for improving the potential of nanotherapeutics. Biomater. Sci. 2021, 9, 1153–1188. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kon, E.; Sharma, P.; Peer, D. Endosomal escape: A bottleneck for LNP- mediated therapeutics. Proc. Natl. Acad. Sci. USA 2024, 121, e2307800120. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.; Shen, W. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef]
- Dai, J.; Ashrafizadeh, M.; Aref, A.; Sethi, G.; Ertas, Y. Peptide-functionalized, -assembled and—Loaded nanoparticles in cancer therapy. Drug Discov. Today 2024, 29, 103981. [Google Scholar] [CrossRef]
- Zorko, M.; Jones, S.; Langel, Ü. Cell-penetrating peptides in protein mimicry and cancer therapeutics. Adv. Drug Deliv. Rev. 2022, 180, 114044. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Kanasty, R.; Dorkin, J.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef]
- Khan, M.M.; Filipczak, N.; Torchilin, V.P. Cell penetrating peptides: A versatile vector for co-delivery of drug and genes in cancer. J. Control. Release 2021, 330, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; Fatima, F.; Zaidi, S.; Zhou, D.; Deng, W.; Liu, S. Engineering siRNA therapeutics: Challenges and strategies. J. Nanobiotechnol. 2023, 21, 381. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lei, L.; Cai, X.; Wei, H.; Yu, C.-Y. Immunomodulatory Peptides for Tumor Treatment. Adv. Healthc. Mater. 2025, 14, 2400512. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Alharbi, S. Anti-inflammatory role of glucagon-like peptide 1 receptor agonists and its clinical implications. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222367. [Google Scholar] [CrossRef]
- Bendotti, G.; Montefusco, L.; Lunati, M.; Usuelli, V.; Pastore, I.; Lazzaroni, E.; Assi, E.; Seelam, A.; El Essawy, B.; Jang, J.; et al. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022, 182, 106320. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, E216–E230. [Google Scholar] [CrossRef]
- Hancock, R.; Sahl, H. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Slominski, R.; Raman, C.; Chen, J.; Slominski, A. How cancer hijacks the body?s homeostasis through the neuroendocrine system. Trends Neurosci. 2023, 46, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Van Hul, M.; Cani, P. The gut microbiota in obesity and weight management: Microbes as friends or foe? Nat. Rev. Endocrinol. 2023, 19, 258–271. [Google Scholar] [CrossRef]
- Powers, J.; Hancock, R. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Anderson, M.; Haagsman, H.; Davidson, D. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Pang, G.; Chen, P.; Cao, X.; Yu, H.; Zhang, L.W.; Zhao, J.; Wang, F.-J. Improving combination cancer immunotherapy by manipulating dual immunomodulatory signals with enzyme-triggered, cell-penetrating peptide-mediated biomodulators. Biomater. Sci. 2024, 12, 776–789. [Google Scholar] [CrossRef]
- Yin, L.; Li, X.; Wang, R.; Zeng, Y.; Zeng, Z.; Xie, T. Recent Research Progress of RGD Peptide–Modified Nanodrug Delivery Systems in Tumor Therapy. Int. J. Pept. Res. Ther. 2023, 29, 53. [Google Scholar] [CrossRef]
- Battistini, L.; Bugatti, K.; Sartori, A.; Curti, C.; Zanardi, F. RGD Peptide-Drug Conjugates as Effective Dual Targeting Platforms: Recent Advances. Eur. J. Org. Chem. 2021, 2021, 2506–2528. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Kim, M.; Sahu, A.; Kim, G.B.; Nam, G.H.; Um, W.; Shin, S.J.; Jeong, Y.Y.; Kim, I.-S.; Kim, K.; Kwon, I.C.; et al. Comparison of in vivo targeting ability between cRGD and collagen-targeting peptide conjugated nano-carriers for atherosclerosis. J. Control. Release 2018, 269, 337–346. [Google Scholar] [CrossRef]
- Bai, Y.; Zheng, X.; Zhong, X.; Cui, Q.; Zhang, S.; Wen, X.; Heng, B.; He, S.; Shen, Y.; Zhang, J.; et al. Manipulation of Heterogeneous Surface Electric Potential Promotes Osteogenesis by Strengthening RGD Peptide Binding and Cellular Mechanosensing. Adv. Mater. 2023, 35, e2209769. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Javid, H.; Oryani, M.; Rezagholinejad, N.; Esparham, A.; Tajaldini, M.; Karimi-Shahri, M. RGD peptide in cancer targeting: Benefits, challenges, solutions, and possible integrin-RGD interactions. Cancer Med. 2024, 13, e6800. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.; Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, Y.; Zhao, H.; Niu, J.; Ling, X.; Zhu, C.; Wang, L.; Yang, H.; Yang, Z.; Pan, G.; et al. Bio-clickable mussel-inspired peptides improve titanium-based material osseointegration synergistically with immunopolarization-regulation. Bioact. Mater. 2022, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Liang, R.; Erel-Akbaba, G.; Saad, L.; Obeid, P.; Gao, J.; Chiocca, E.; Weissleder, R.; Tannous, B. Immune Checkpoint Inhibition in GBM Primed with Radiation by Engineered Extracellular Vesicles. ACS Nano 2022, 16, 1940–1953. [Google Scholar] [CrossRef]
- Zhang, C.; Shang, Y.; Chen, X.; Midgley, A.C.; Wang, Z.; Zhu, D.; Wu, J.; Chen, P.; Wu, L.; Wang, X.; et al. Supramolecular Nanofibers Containing Arginine-Glycine-Aspartate (RGD) Peptides Boost Therapeutic Efficacy of Extracellular Vesicles in Kidney Repair. ACS Nano 2020, 14, 12133–12147. [Google Scholar] [CrossRef]
- Sugahara, K.; Teesalu, T.; Karmali, P.; Kotamraju, V.; Agemy, L.; Girard, O.; Hanahan, D.; Mattrey, R.; Ruoslahti, E. Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef]
- Wei, M.; Lu, T.; Nong, Z.; Li, G.; Pan, X.; Wei, Y.; Yang, Y.; Wu, N.; Huang, J.; Pan, M.; et al. Reductive response and RGD targeting nano-graphene oxide drug delivery system. J. Drug Deliv. Sci. Technol. 2019, 53, 101202. [Google Scholar] [CrossRef]
- Qin, J.; Xue, L.; Gong, N.; Zhang, H.; Shepherd, S.J.; Haley, R.M.; Swingle, K.L.; Mitchell, M.J. RGD peptide-based lipids for targeted mRNA delivery and gene editing applications. RSC Adv. 2022, 12, 25397–25404. [Google Scholar] [CrossRef] [PubMed]
- Galindo, J.M.; Merino, S.; Herrero, M.A. Advanced Hydrogels: Enhancing Tissue Bioengineering with RGD Peptides and Carbon Nanomaterials. ChemMedChem 2025, 20, e202400587. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.; Kohane, D.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef]
- Ding, G.; Wang, T.; Han, Z.; Tian, L.; Cheng, Q.; Luo, L.; Zhao, B.; Wang, C.; Feng, S.; Wang, L.; et al. Substance P containing peptide gene delivery vectors for specifically transfecting glioma cells mediated by a neurokinin-1 receptor. J. Mater. Chem. B 2021, 9, 6347–6356. [Google Scholar] [CrossRef]
- Jeyarajan, S.; Xavier, J.; Rao, N.M.; Gopal, V. Plasmid DNA delivery into MDA-MB-453 cells mediated by recombinant Her-NLS fusion protein. Int. J. Nanomed. 2010, 5, 725–733. [Google Scholar] [CrossRef]
- Neves, A.R.; Sousa, A.; Faria, R.; Albuquerque, T.; Queiroz, J.A.; Costa, D. Cancer gene therapy mediated by RALA/plasmid DNA vectors: Nitrogen to phosphate groups ratio (N/P) as a tool for tunable transfection efficiency and apoptosis. Colloids Surf. B Biointerfaces 2020, 185, 110610. [Google Scholar] [CrossRef]
- Hasannejad-Asl, B.; Pooresmaeil, F.; Takamoli, S.; Dabiri, M.; Bolhassani, A. Cell penetrating peptide: A potent delivery system in vaccine development. Front. Pharmacol. 2022, 13, 1072685. [Google Scholar] [CrossRef]
- Zhang, T.T.; Kang, T.H.; Ma, B.; Xu, Y.; Hung, C.F.; Wu, T.C. LAH4 enhances CD8+ T cell immunity of protein/peptide-based vaccines. Vaccine 2012, 30, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Mardani, G.; Bolhassani, A.; Agi, E.; Shahbazi, S.; Mehdi Sadat, S. Protein vaccination with HPV16 E7/Pep-1 nanoparticles elicits a protective T-helper cell-mediated immune response. IUBMB Life 2016, 68, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, E. Targeted delivery with peptidomimetic conjugated self-assembled nanoparticles. Pharm. Res. 2009, 26, 612–630. [Google Scholar] [CrossRef]
- Wang, P.; Wu, W.; Gao, R.; Zhu, H.; Wang, J.; Du, R.; Li, X.; Zhang, C.; Cao, S.; Xiang, R. Engineered Cell-Assisted Photoactive Nanoparticle Delivery for Image-Guided Synergistic Photodynamic/Photothermal Therapy of Cancer. ACS Appl. Mater. Interfaces 2019, 11, 13935–13944. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lei, D.; Li, J.; Xin, J.; Zhang, L.; Fu, L.; Wang, J.; Zeng, W.; Yao, C.; Zhang, Z.; et al. MMP-2 Inhibitor-Mediated Tumor Microenvironment Regulation Using a Sequentially Released Bio-Nanosystem for Enhanced Cancer Photo-Immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 41834–41850. [Google Scholar] [CrossRef]
- Park, K. Mast cells for cell-mediated therapy. J. Control. Release 2015, 202, 118. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Wei, T.; Bai, L.; Zhao, J.; Wang, K.; Feng, Y. Endothelial Cell-Mediated Gene Delivery for In Situ Accelerated Endothelialization of a Vascular Graft. ACS Appl. Mater. Interfaces 2021, 13, 16097–16105. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.M.; Patel, B. Crossing the Blood–Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 2017, 31, 109–133. [Google Scholar] [CrossRef]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, C.L.; Liu, C.M. Drug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of glioma. Drug Des. Devel Ther. 2015, 9, 2089–2100. [Google Scholar] [CrossRef]
- Mathupala, S.P. Delivery of small-interfering RNA (siRNA) to the brain. Expert Opin. Ther. Pat. 2009, 19, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Man, R.C.H.; Qiu, Y.; Leung, S.W.S.; Fruhwirth, G.O.; Lam, J.K.W. Co-delivery of PD-L1- and EGFR-targeting siRNAs by synthetic PEG12-KL4 peptide to the lungs as potential strategy against non-small cell lung cancer. Eur. J. Pharm. Biopharm. 2024, 195, 114177. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, N.; Patil, A.; Suryawanshi, M. Bioactive compound nanoparticles for Alzheimer’s disease. Inflammopharmacology 2025, 33, 2963–2976. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, R.F. Enhancing cancer immunotherapy by intracellular delivery of cell-penetrating peptides and stimulation of pattern-recognition receptor signaling. Adv. Immunol. 2012, 114, 151–176. [Google Scholar] [CrossRef]
- Kimura, S.; Maeda, K.; Nagashima, R.; Miura, K.; Arakawa, M.; Ebina, H.; Tanaka, N.; Morita, E. Efficient immunogenic peptide antigen delivery to dendritic cells using an ESCRT-mediated extracellular vesicle formation method. Vaccine 2021, 39, 2976–2982. [Google Scholar] [CrossRef]
- He, Y.; Zhang, D.; Liu, J.; Ouyang, J.; Zhang, C.; Tan, Q.; Jiang, J.; Hu, K. Ocular Soluble Microneedle Patch Loaded with Immunogenic Cell Death Inducer PKHB1 Peptide for Alleviating Herpes Simplex Keratitis. ACS Appl. Mater. Interfaces 2025, 17, 42824–42837. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Reissmann, S.; Filatova, M.P. New generation of cell-penetrating peptides: Functionality and potential clinical application. J. Pept. Sci. 2021, 27, e3300. [Google Scholar] [CrossRef]
- Imre, A.; Balogh, B.; Mándity, I. GraphCPP: The new state-of-the-art method for cell-penetrating peptide prediction via graph neural networks. Br. J. Pharmacol. 2025, 182, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Kundu, D.; Gogoi, M.; Shrestha, A.K.; Karanth, N.G.; Patra, S. Enzyme-Responsive and Enzyme Immobilized Nanoplatforms for Therapeutic Delivery: An Overview of Research Innovations and Biomedical Applications. In Nanopharmaceuticals: Principles and Applications Vol. 3; Yata, V.K., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 165–200. [Google Scholar]
- Guo, C.; Yuan, H.; Wang, Y.; Feng, Y.; Zhang, Y.; Yin, T.; He, H.; Gou, J.; Tang, X. The interplay between PEGylated nanoparticles and blood immune system. Adv. Drug Deliv. Rev. 2023, 200, 115044. [Google Scholar] [CrossRef] [PubMed]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-Responsive Materials for Tissue Engineering and Drug Delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef]
- Ruoslahti, E. Peptides as Targeting Elements and Tissue Penetration Devices for Nanoparticles. Adv. Mater. 2012, 24, 3747–3756. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Cell-mediated delivery of nanoparticles: Taking advantage of circulatory cells to target nanoparticles. J. Control. Release 2014, 190, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Hanson, M.C.; Rakhra, K.; Tokatlian, T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem. Rev. 2015, 115, 11109–11146. [Google Scholar] [CrossRef] [PubMed]
- Erdei, E.; Marcos, J.T.; Varró, N.; Horváti, K.; Bacsa, B.; Mándity, I.M. Spatiotemporal control in biomedicine: Photoswitchable peptides and foldamers. Br. J. Pharmacol. 2025, 182, 4458–4465. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Erdei, E.; Mándoki, A.; Deák, A.; Balogh, B.; Molnár, L.; Mándity, I.M. BioGoldNCDB: A Database of Gold Nanoclusters and Related Nanoparticles with Biomedical Activity. Molecules 2025, 30, 3310. [Google Scholar] [CrossRef]
- Hartrampf, N.; Saebi, A.; Poskus, M.; Gates, Z.P.; Callahan, A.J.; Cowfer, A.E.; Hanna, S.; Antilla, S.; Schissel, C.K.; Quartararo, A.J.; et al. Synthesis of proteins by automated flow chemistry. Science 2020, 368, 980–987. [Google Scholar] [CrossRef]
- Varró, N.; Mándityné Huszka, B.; Erdei, E.; Mándoki, A.; Mándity, I.M. Upscaled Synthesis of α- and β-Peptides in a Continuous-Flow Reactor Using Propylene Carbonate as an Eco-Friendly Solvent. Chem. Methods 2025, 5, e202500010. [Google Scholar] [CrossRef]
- Tinkle, S.; McNeil, S.E.; Mühlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef]
- Park, K. Facing the Truth about Nanotechnology in Drug Delivery. ACS Nano 2013, 7, 7442–7447. [Google Scholar] [CrossRef]
- Zhavoronkov, A.; Ivanenkov, Y.A.; Aliper, A.; Veselov, M.S.; Aladinskiy, V.A.; Aladinskaya, A.V.; Terentiev, V.A.; Polykovskiy, D.A.; Kuznetsov, M.D.; Asadulaev, A.; et al. Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat. Biotechnol. 2019, 37, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.N.; Mehta, R.R.; Yamada, T.; Lekmine, F.; Christov, K.; Chakrabarty, A.M.; Bratescu, L.; Green, A.; Bratescu, E. Non-toxic azurin peptide (p28) induces p53-mediated apoptosis in breast cancer cells. Mol. Cancer Ther. 2009, 8, 2947–2958. [Google Scholar] [CrossRef]
- Lulla, R.R.; Goldman, S.; Yamada, T.; Beattie, C.W.; Bressler, L.R.; Pacini, M.; Pollack, I.F.; Fisher, P.G.; Packer, R.J.; Dunkel, I.J.; et al. Phase I trial of p28 (NSC745104), a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive central nervous system tumors: A Pediatric Brain Tumor Consortium Study. Neuro-Oncology 2016, 18, 1319–1325. [Google Scholar] [CrossRef]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Hsu, D.S.; Riedlinger, G.; et al. A Phase I study of dexosome immunotherapy in patients with advanced non–small cell lung cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef]
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartowski, A.; Giroux-Leprieur, E.; André, F.; et al. Dendritic cell–derived exosomes as maintenance immunotherapy after first-line chemotherapy in NSCLC. J. Clin. Oncol. 2016, 34, 4133–4142. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.E.; Taylor, S.; D’Andre, S.D.; et al. First results on survival from a large Phase III clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef]
- Kloor, M.; Reuschenbach, M.; Ebert, M.P.; Drayton, R.M.; Schneider, M.; Nachbichler, S.B.; Grabe, N.; Mascaux, C.; Taillefer, A.; Ligtenberg, M.J.; et al. Vaccination of MSI-H colorectal cancer patients with frameshift peptide antigens: A phase I/IIa clinical trial. J. Clin. Oncol. 2015, 33, 0320. [Google Scholar] [CrossRef]
- Dean, A.; Gill, S.; McGregor, M.; Broadbridge, V.; Jarvelainen, H.A.; Price, T.J. Phase I trial of the first-in-class agent CEND-1 in combination with gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer. Ann. Oncol. 2020, 31, S941. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Regulation (EC) No 1394/2007 on Advanced Therapy Medicinal Products (ATMPs). Off. J. Eur. Union 2007, L324. [Google Scholar]
- EMA Committee for Advanced Therapies (CAT). Reflection Paper on Classification of Advanced Therapy Medicinal Products; EMA/CAT/600280/2010; European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps): 21 CFR Part 1271; Federal Register: Washington, DC, USA, 2001. [Google Scholar]
- European Medicines Agency (EMA). Guideline on Human Cell-Based Medicinal Products; EMA/CHMP/410869/2006; European Medicines Agency: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Bowman, D.M.; Gatof, J. Reviewing the Regulatory Barriers for Nanomedicine: Global Questions and Challenges. Nanomedicine 2015, 10, 3275–3286. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, R.; Guo, J.; Wang, X. The role and future prospects of artificial intelligence algorithms in peptide drug development. Biomed. Pharmacother. 2024, 175, 116709. [Google Scholar] [CrossRef]
- Irvine, D.J.; Swartz, M.A.; Szeto, G.L. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013, 12, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, H.; Zhang, Q.; Tao, H. Research Progress of Neutrophil-Mediated Drug Delivery Strategies for Inflammation-Related Disease. Pharmaceutics 2023, 15, 1881. [Google Scholar] [CrossRef]
- Luo, Z.; Lu, Y.; Shi, Y.; Jiang, M.; Shan, X.; Li, X.; Zhang, J.; Qin, B.; Liu, X.; Guo, X.; et al. Neutrophil hitchhiking for drug delivery to the bone marrow. Nat. Nanotechnol. 2023, 18, 647–656. [Google Scholar] [CrossRef]
- Doshi, N.; Swiston, A.J.; Gilbert, J.B.; Alcaraz, M.L.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Cell-based drug delivery devices using phagocytosis-resistant backpacks. Adv. Mater. 2011, 23, H105–H109. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.W., IV; Evans, M.A.; Wang, L.L.-W.; Lau, L.; Angelini, T.E.; Horner, P.J.; Mitragotri, S.; López, G.P. Cellular backpacks for macrophage immunotherapy. Sci. Adv. 2020, 6, eaaz6579. [Google Scholar] [CrossRef]
- Freimann, K.; Arukuusk, P.; Kurrikoff, K.; Hansen, M.; Zuchner, T.; Langel, Ü. Formulation of stable and homogeneous cell-penetrating peptide NF55 nanoparticles for efficient gene delivery in vivo. Mol. Ther. Nucleic Acids 2018, 10, 28–35. [Google Scholar] [CrossRef] [PubMed]
- D’Astolfo, D.S.; Pagliero, R.J.; Pras, A.; Karyala, S.V.; Malik, P.; Wen, B.; Gabriel, R.; DeAngelo, S.L.; Hoffmann, T.; Becker, C.; et al. Efficient intracellular delivery of native proteins. Cell 2015, 161, 674–690. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).