Does CytoSorb Interfere with Immunosuppression? A Pharmacokinetic and Functional Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Human Whole Blood and Plasma
2.3. Determination of Protein Binding
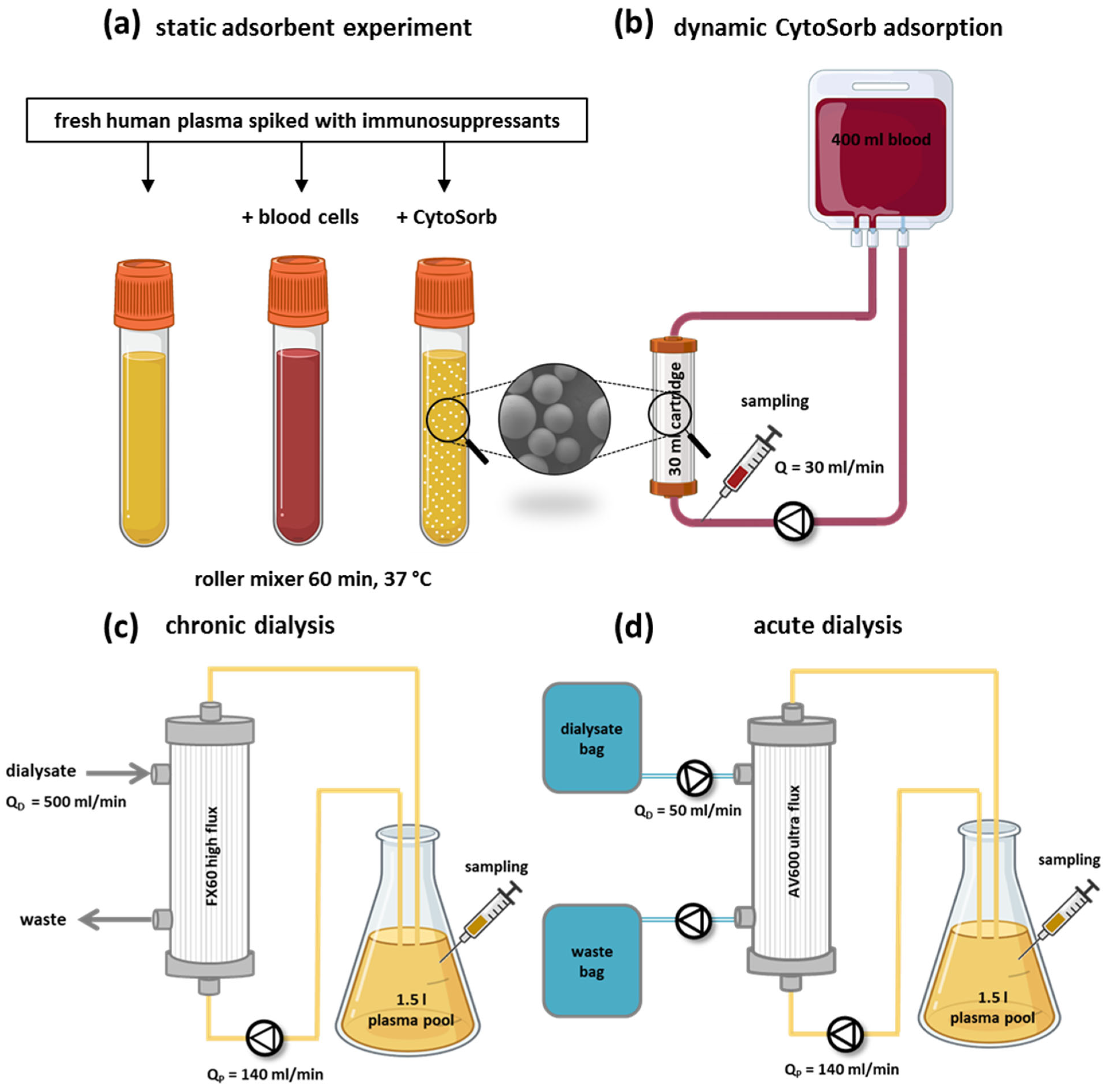
2.4. Static CytoSorb Adsorption Setup
2.5. Dynamic CytoSorb Adsorption Setup
2.6. Removal of Immunosuppressants by Dialysis
2.7. Clearance Calculation
2.8. Whole Blood Cell Model
2.9. Quantification of Immunosuppressants and Cytokines
2.10. Statistics
2.11. Use of AI-Assisted Editing
3. Results
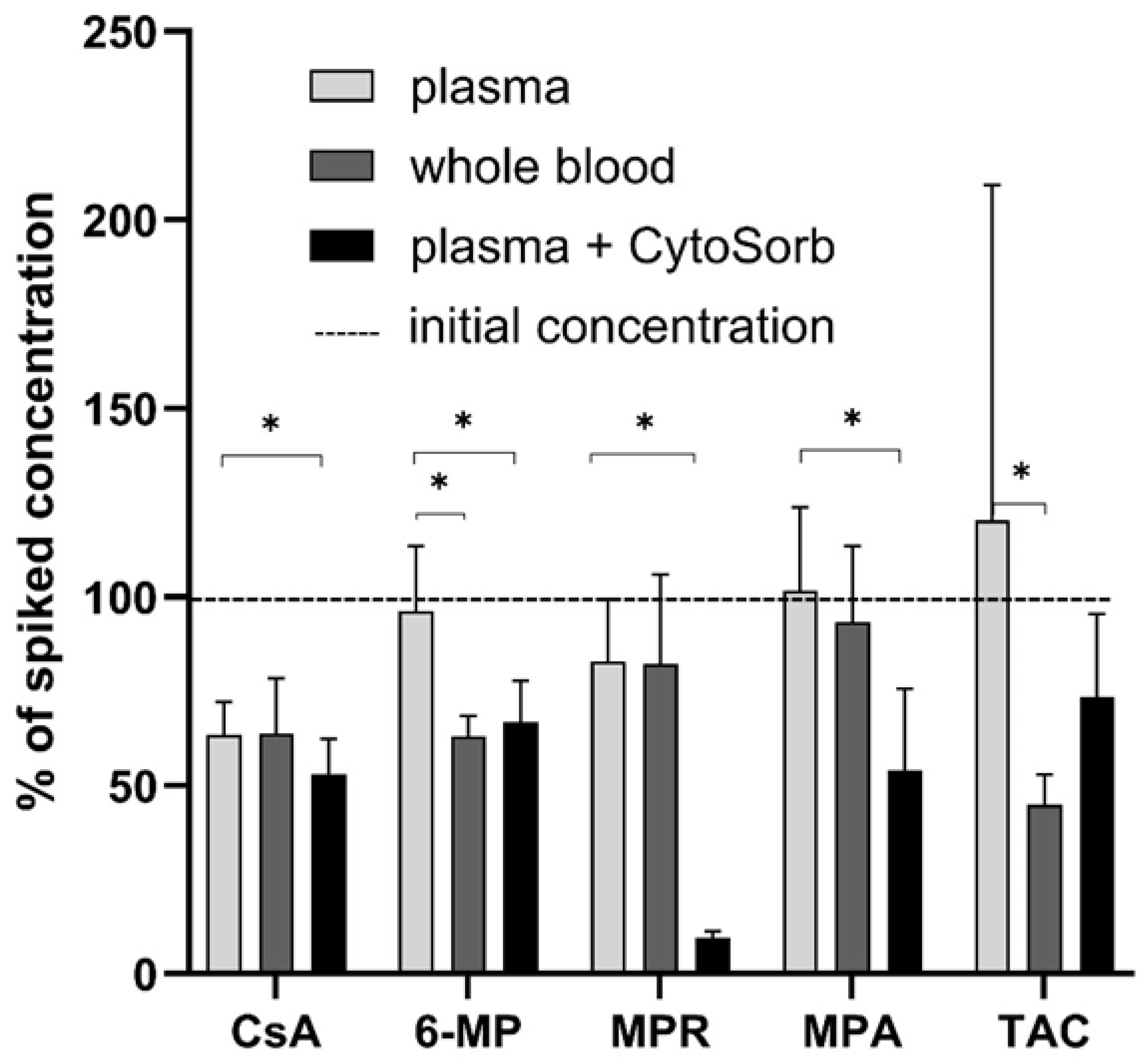
3.1. Static CytoSorb Adsorption
3.2. Protein Binding of Immunosuppressants
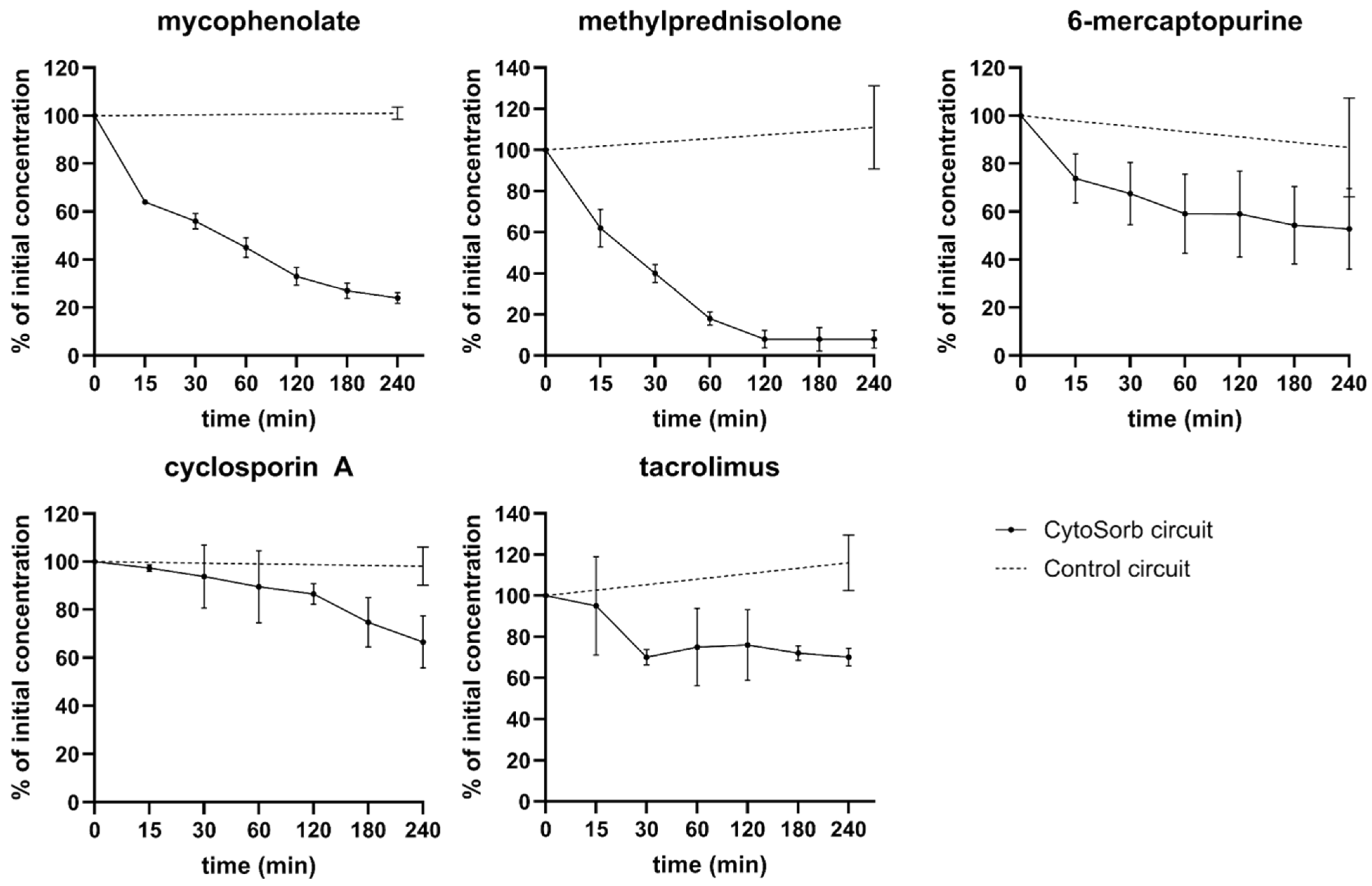
3.3. Dynamic CytoSorb Adsorption
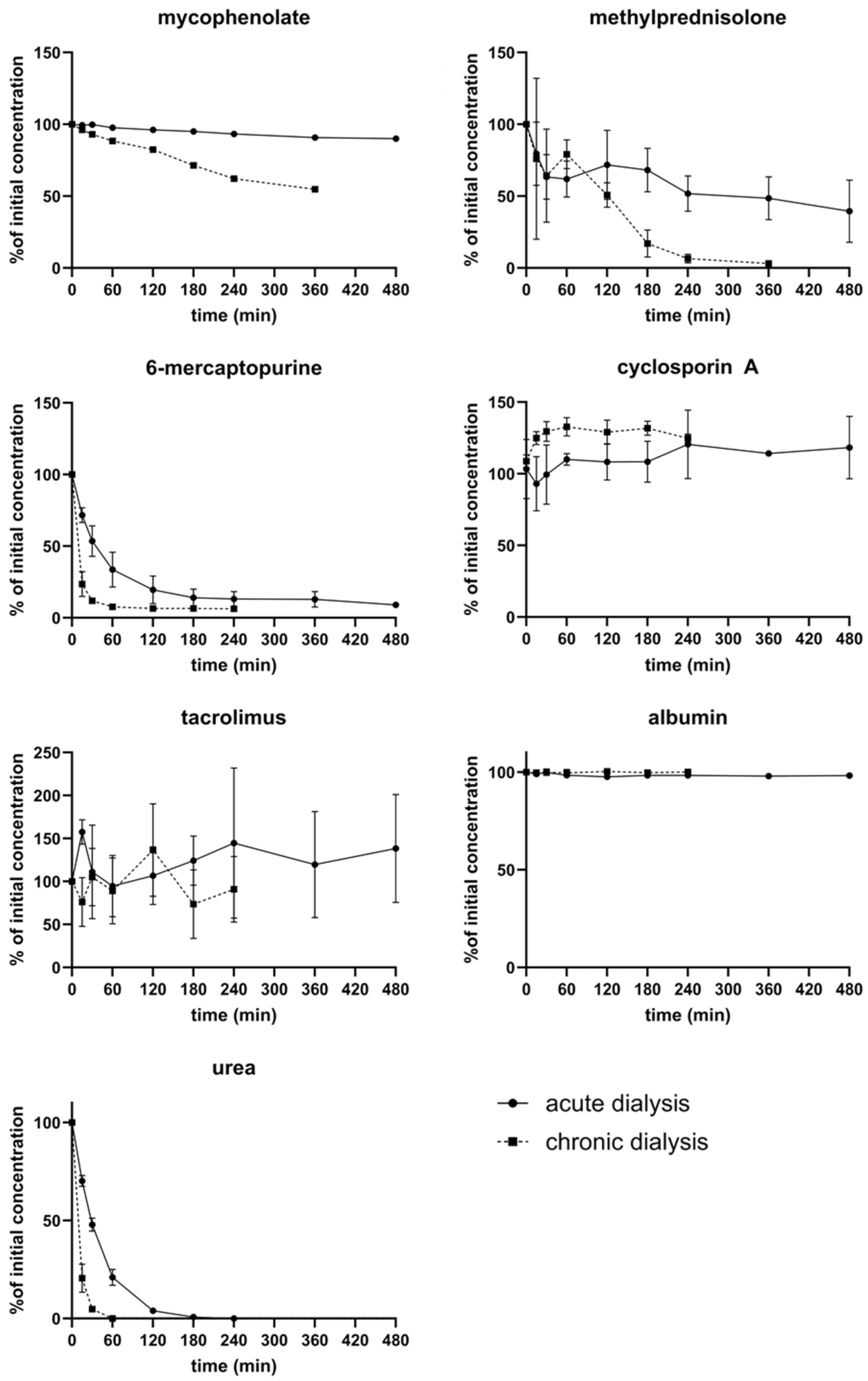
3.4. Removal of Immunosuppressants by Dialysis
3.5. Clearance of Immunosuppressants in Extracorporeal Circuits
3.6. Whole Blood Cell Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARDS | acute respiratory distress syndrome |
| LPS | lipopolysaccharide |
| ICU | intensive Care Unit |
| TAC | tacrolimus |
| MPR | methylprednisolone |
| MPA | mycophenolic Acid |
| 6-MP | 6-mercaptopurine |
| CVVHD | continuous veno-venous hemodialysis |
| ELISA | enzyme-linked immunosorbent assay |
| HPLC | high performance liquid chromatography |
| SD | standard deviation |
| VD | volume of distribution |
References
- Wadia, P.P.; Tambur, A.R. Yin and yang of cytokine regulation in solid organ graft rejection and tolerance. Clin. Lab. Med. 2008, 28, 469–479. [Google Scholar] [CrossRef]
- Leber, B.; Liebchen, U.; Rohrhofer, L.; Weber, J.; Klaus, T.; Scheier, J.; Sucher, R.; Stiegler, P. Pharmacokinetics of immunosuppressive agents during hemoperfusion in a sheep model. Front. Med. 2023, 10, 1258661. [Google Scholar] [CrossRef]
- Pirenne, J.; Pirenne-Noizat, F.; de Groote, D.; Vrindts, Y.; Lopez, M.; Gathy, R.; Jacquet, N.; Meurisse, M.; Honore, P.; Franchimont, P. Cytokines and organ transplantation. A review. Nucl. Med. Biol. 1994, 21, 545–555. [Google Scholar] [CrossRef]
- Harm, S.; Gabor, F.; Hartmann, J. Characterization of Adsorbents for Cytokine Removal from Blood in an In Vitro Model. J. Immunol. Res. 2015, 2015, 484736. [Google Scholar] [CrossRef]
- Harm, S.; Gruber, A.; Gabor, F.; Hartmann, J. Adsorption of Selected Antibiotics to Resins in Extracorporeal Blood Purification. Blood Purif. 2016, 41, 55–63. [Google Scholar] [CrossRef]
- Schadler, D.; Pausch, C.; Heise, D.; Meier-Hellmann, A.; Brederlau, J.; Weiler, N.; Marx, G.; Putensen, C.; Spies, C.; Jorres, A.; et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS ONE 2017, 12, e0187015. [Google Scholar] [CrossRef]
- Hawchar, F.; Laszlo, I.; Oveges, N.; Trasy, D.; Ondrik, Z.; Molnar, Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J. Crit. Care 2019, 49, 172–178. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Bagshaw, S.M.; Antonelli, M.; Foster, D.M.; Klein, D.J.; Marshall, J.C.; Palevsky, P.M.; Weisberg, L.S.; Schorr, C.A.; Trzeciak, S.; et al. Effect of Targeted Polymyxin B Hemoperfusion on 28-Day Mortality in Patients with Septic Shock and Elevated Endotoxin Level: The EUPHRATES Randomized Clinical Trial. JAMA 2018, 320, 1455–1463. [Google Scholar] [CrossRef]
- Payen, D.M.; Guilhot, J.; Launey, Y.; Lukaszewicz, A.C.; Kaaki, M.; Veber, B.; Pottecher, J.; Joannes-Boyau, O.; Martin-Lefevre, L.; Jabaudon, M.; et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: A multicenter randomized control trial. Intensive Care Med. 2015, 41, 975–984. [Google Scholar] [CrossRef]
- Supady, A.; Weber, E.; Rieder, M.; Lother, A.; Niklaus, T.; Zahn, T.; Frech, F.; Muller, S.; Kuhl, M.; Benk, C.; et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): A single centre, open-label, randomised, controlled trial. Lancet Respir. Med. 2021, 9, 755–762. [Google Scholar] [CrossRef]
- Supady, A.; Brodie, D.; Wengenmayer, T. Extracorporeal haemoadsorption: Does the evidence support its routine use in critical care? Lancet Respir. Med. 2022, 10, 307–312. [Google Scholar] [CrossRef]
- Brunkhorst, F.M.; Adamzik, M.; Axer, H.; Bauer, M.; Bode, C.; Bone, H.G.; Brenner, T.; Bucher, M.; David, S.; Dietrich, M.; et al. S3 guideline on sepsis-prevention, diagnosis, therapy, and follow-up care-update 2025. Med. Klin. Intensivmed. Notfmed. 2025. [Google Scholar] [CrossRef]
- Tomescu, D.R.; Olimpia Dima, S.; Ungureanu, D.; Popescu, M.; Tulbure, D.; Popescu, I. First report of cytokine removal using CytoSorb(R) in severe noninfectious inflammatory syndrome after liver transplantation. Int. J. Artif. Organs 2016, 39, 136–140. [Google Scholar] [CrossRef]
- Nemeth, E.; Kovacs, E.; Racz, K.; Soltesz, A.; Szigeti, S.; Kiss, N.; Csikos, G.; Koritsanszky, K.B.; Berzsenyi, V.; Trembickij, G.; et al. Impact of intraoperative cytokine adsorption on outcome of patients undergoing orthotopic heart transplantation-an observational study. Clin. Transplant. 2018, 32, e13211. [Google Scholar] [CrossRef] [PubMed]
- Boffini, M.; Cassoni, P.; Gambella, A.; Simonato, E.; Delsedime, L.; Marro, M.; Fanelli, V.; Costamagna, A.; Lausi, P.O.; Solidoro, P.; et al. Is there life on the airway tree? A pilot study of bronchial cell vitality and tissue morphology in the ex vivo lung perfusion (EVLP) era of lung transplantation. Artif. Organs 2022, 46, 2234–2243. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Hoff, M.; Nicholson, M.L. Treatment of transplant kidneys during machine perfusion. Transpl. Int. 2021, 34, 224–232. [Google Scholar] [CrossRef]
- Dervieux, T.; Boulieu, R. Simultaneous determination of 6-thioguanine and methyl 6-mercaptopurine nucleotides of azathioprine in red blood cells by HPLC. Clin. Chem. 1998, 44, 551–555. [Google Scholar] [CrossRef]
- Lennard, L. Assay of 6-thioinosinic acid and 6-thioguanine nucleotides, active metabolites of 6-mercaptopurine, in human red blood cells. J. Chromatogr. 1987, 423, 169–178. [Google Scholar] [CrossRef]
- Levy, J.; Brown, E.; Lawrence, A. Chapter 18 Drug dosing. In Oxford Handbook of Dialysis; Oxford University Press: Oxford, UK, 2016; Volume 4. [Google Scholar] [CrossRef]
- Horbett, T.A.; Weathersby, P.K. Adsorption of proteins from plasma to a series of hydrophilic-hydrophobic copolymers. I. Analysis with the in situ radioiodination technique. J. Biomed. Mater. Res. 1981, 15, 403–423. [Google Scholar] [CrossRef]
- Loo, T.L.; Luce, J.K.; Sullivan, M.P.; Frei, E., 3rd. Clinical pharmacologic observations on 6-mercaptopurine and 6-methylthiopurine ribonucleoside. Clin. Pharmacol. Ther. 1968, 9, 180–194. [Google Scholar] [CrossRef]
- Lemaire, M.; Tillement, J.P. Role of lipoproteins and erythrocytes in the in vitro binding and distribution of cyclosporin A in the blood. J. Pharm. Pharmacol. 1982, 34, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Szefler, S.J.; Ebling, W.F.; Georgitis, J.W.; Jusko, W.J. Methylprednisolone versus prednisolone pharmacokinetics in relation to dose in adults. Eur. J. Clin. Pharmacol. 1986, 30, 323–329. [Google Scholar] [CrossRef]
- de Winter, B.C.; van Gelder, T.; Sombogaard, F.; Shaw, L.M.; van Hest, R.M.; Mathot, R.A. Pharmacokinetic role of protein binding of mycophenolic acid and its glucuronide metabolite in renal transplant recipients. J. Pharmacokinet. Pharmacodyn. 2009, 36, 541–564. [Google Scholar] [CrossRef]
- Iwasaki, K.; Miyazaki, Y.; Teramura, Y.; Kawamura, A.; Tozuka, Z.; Hata, T.; Undre, N. Binding of tacrolimus (FK506) with human plasma proteins re-evaluation and effect of mycophenolic acid. Res. Commun. Mol. Pathol. Pharmacol. 1996, 94, 251–257. [Google Scholar]
- Brunner, H.; Mann, H.; Stiller, S.; Sieberth, H.G. Permeability for middle and higher molecular weight substances. Comparison between polysulfone and cuprophan dialyzers. Contrib. Nephrol. 1985, 46, 33–42. [Google Scholar]
- Sefer, S.D.; Degoricija, V. About Drug Dialyzability. Acta Clin. Croat. 2003, 42, 11. [Google Scholar]
- Ronco, C.; Reis, T.; Husain-Syed, F. Blood purification in intensive care: State of the art and perspectives. Intensive Care Med. 2021, 47, 1171–1186. [Google Scholar] [CrossRef]
- Matzke, G.R.; Aronoff, G.R.; Atkinson, A.J. Drug dosing consideration in patients receiving renal replacement therapy. Clin. Pharmacol. Ther. 2011, 90, 960–969. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Hartmann, J.; Harm, S. Removal of bile acids by extracorporeal therapies: An in vitro study. Int. J. Artif. Organs 2018, 41, 52–57. [Google Scholar] [CrossRef]
- Harm, S.; Schildbock, C.; Hartmann, J. Removal of stabilizers from human serum albumin by adsorbents and dialysis used in blood purification. PLoS ONE 2018, 13, e0191741. [Google Scholar] [CrossRef]
- Venkataramanan, R.; Shaw, L.M.; Sarkozi, L. Clinical pharmacokinetics of tacrolimus. Clin. Pharmacokinet. 1995, 29, 404–430. [Google Scholar] [CrossRef] [PubMed]
- Kogelmann, K.; Jarczak, D.; Scheller, M.; Druner, M. Hemoadsorption by CytoSorb in septic patients: A case series. Crit. Care 2017, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Ankawi, G.; Fan, W.; Pomarè Montin, D. A new series of sorbent devices for multiple indications: Current evidence and future directions. Blood Purif. 2019, 47, 94–100. [Google Scholar] [CrossRef]
- Kellum, J.A.; Song, M.; Venkataraman, R. Hemoadsorption removes circulating inflammatory mediators in septic patients. Nephrol. Dial. Transplant. 2018, 33, 756–764. [Google Scholar] [CrossRef]
- Harm, S.; Falkenhagen, D.; Hartmann, J. Pore size--a key property for selective toxin removal in blood purification. Int. J. Artif. Organs 2014, 37, 668–678. [Google Scholar] [CrossRef]
- Derijks, L.J.; Gilissen, L.P.; Hooymans, P.M. Review article: Pharmacokinetics of thiopurines in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2004, 19, 759–769. [Google Scholar] [CrossRef]
- Ankawi, G.; Xie, Y.; Yang, B.; Xie, Y.; Xie, P.; Ronco, C. What Have We Learned about the Use of Cytosorb Adsorption Columns? Blood Purif. 2019, 48, 196–202. [Google Scholar] [CrossRef]
- Bohler, J.; Donauer, J.; Keller, F. Pharmacokinetic principles during continuous renal replacement therapy: Drugs and dosage. Kidney Int Suppl 1999, S24–S28. [Google Scholar] [CrossRef]
- Derijks, L.J.; Gilissen, L.P.; Engels, L.G.; Bos, L.P.; Bus, P.J.; Lohman, J.J.; Curvers, W.L.; Van Deventer, S.J.; Hommes, D.W.; Hooymans, P.M. Pharmacokinetics of 6-mercaptopurine in patients with inflammatory bowel disease: Implications for therapy. Ther. Drug Monit. 2004, 26, 311–318. [Google Scholar] [CrossRef]
- Koefoed-Nielsen, P.B.; Karamperis, N.; Hojskov, C.; Poulsen, J.H.; Jorgensen, K.A. The calcineurin activity profiles of cyclosporin and tacrolimus are different in stable renal transplant patients. Transpl. Int. 2006, 19, 821–827. [Google Scholar] [CrossRef]
- Lennard, L. Therapeutic drug monitoring of cytotoxic drugs. Toxicology 2002, 181–182, 137–143. [Google Scholar] [CrossRef]
- Honore, P.M.; Jacobs, R.; Joannes-Boyau, O. Newly designed CRRT membranes for sepsis and SIRS—A pragmatic approach for bedside intensivists summarizing the more recent advances: A systematic structured review. Ann. Intensive Care 2019, 9, 22. [Google Scholar] [CrossRef]
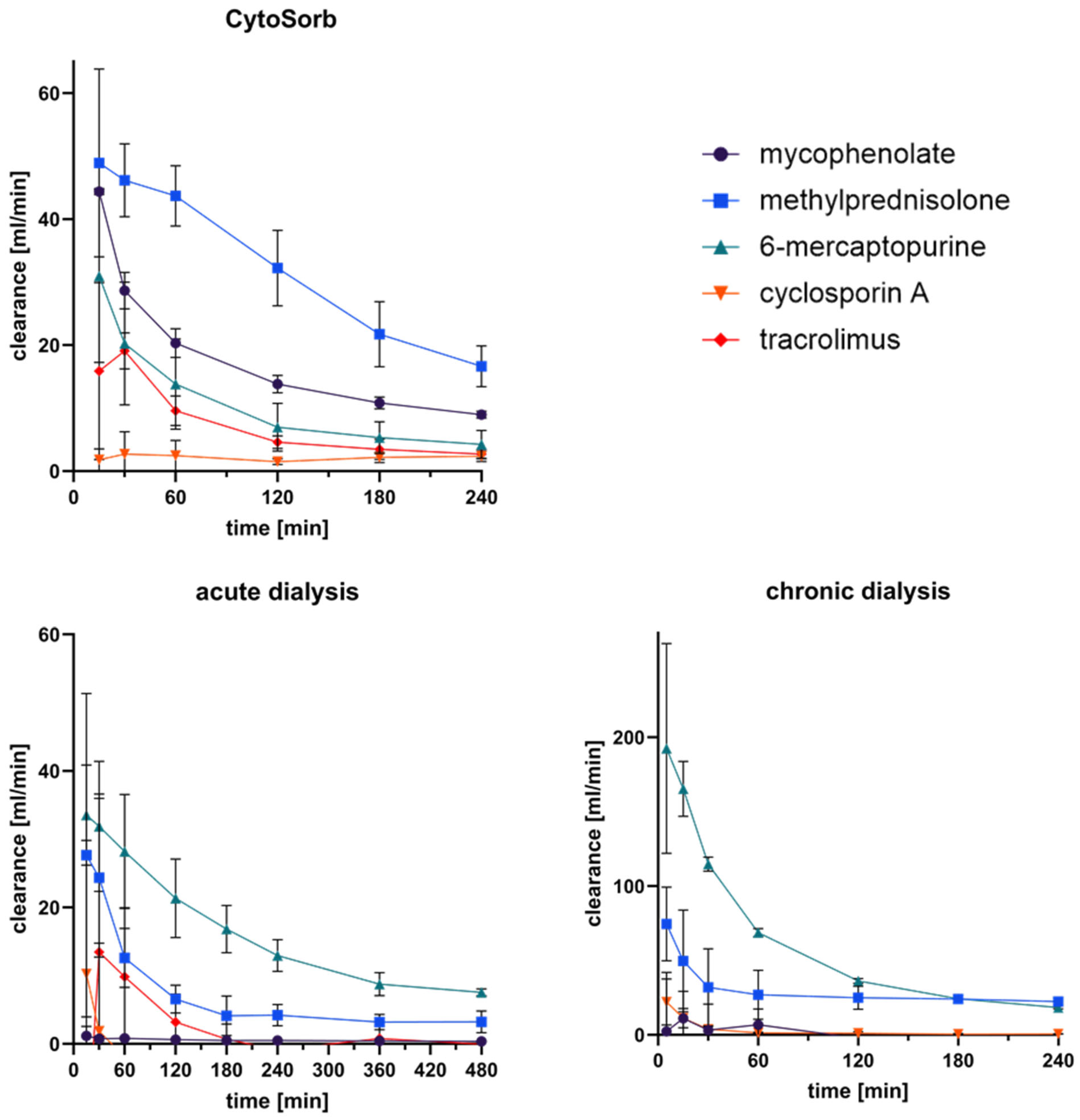
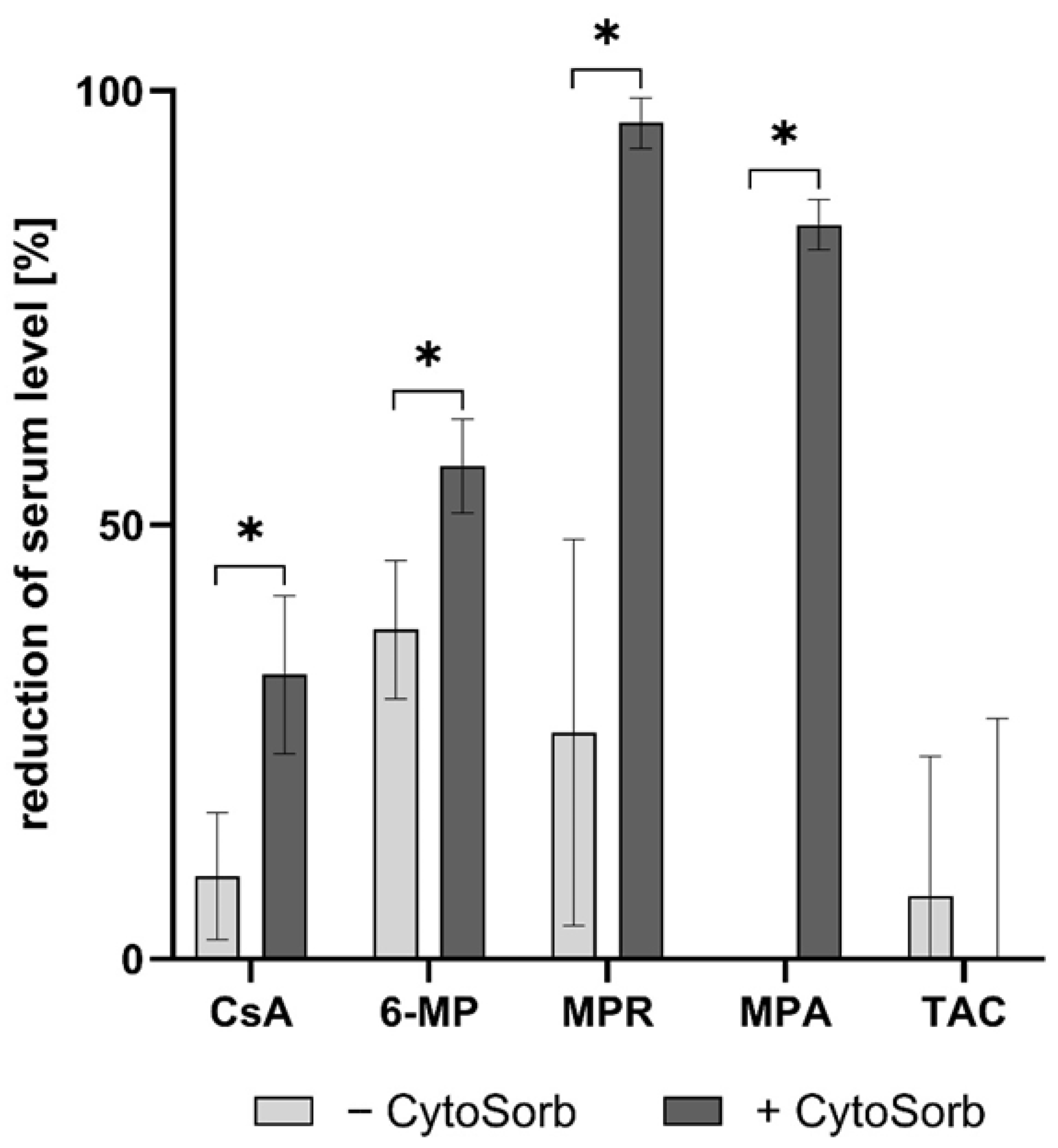
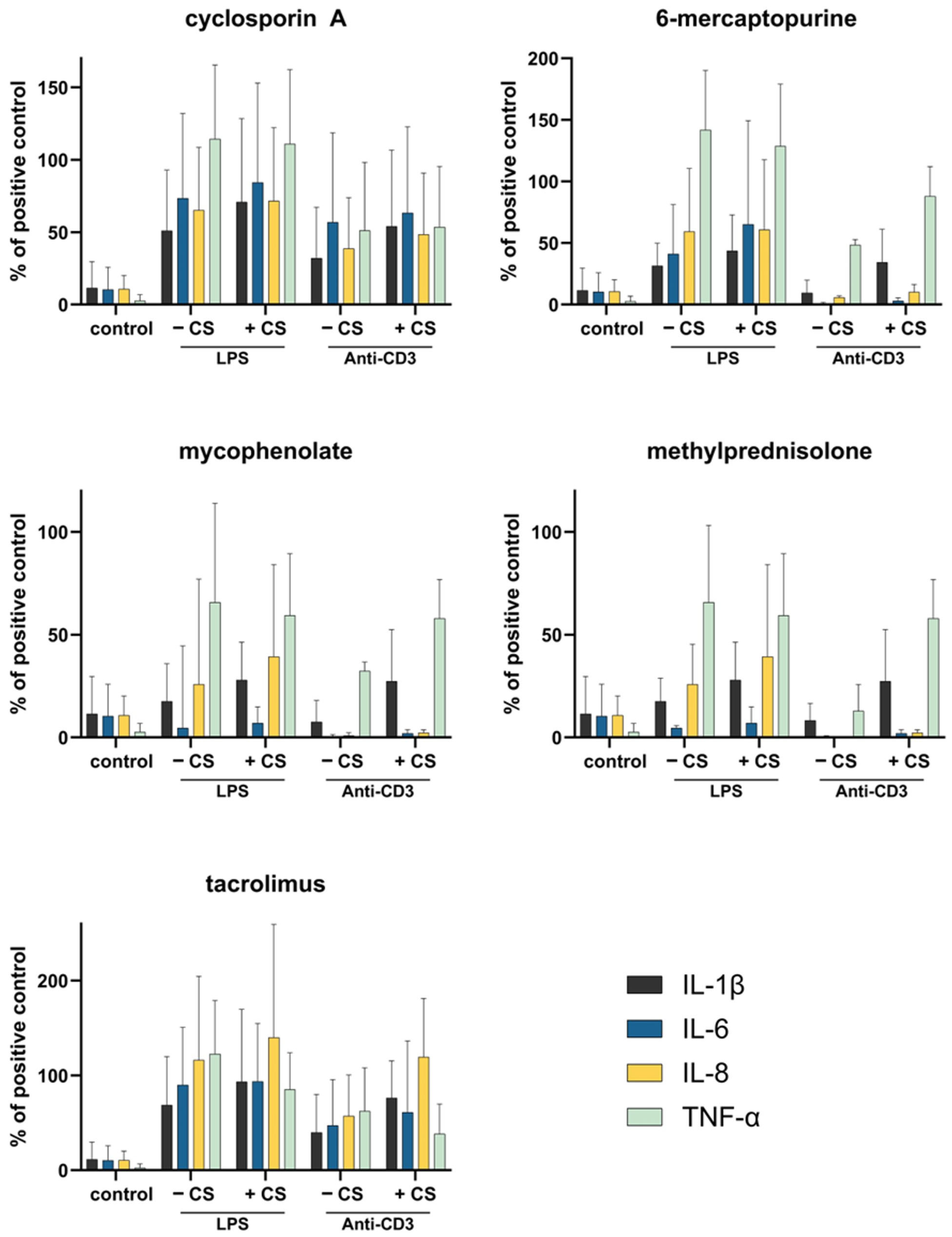
| Immunosuppressant | Plasma Spike-Concentration [ng/mL] | Immunosuppressive Action |
|---|---|---|
| tacrolimus (TAC) | 20 | TAC inhibits calcineurin and consequently IL-2 synthesis and thus T cell proliferation. |
| mycophenolate (MPA) | 4000 | MPA inhibits the synthesis of guanosine and thus the proliferation of T and B lymphocytes. |
| methylprednisolone (MPR) | 200 | MPR is a synthetic glucocorticoid and has anti-inflammatory and immunosuppressive effects. |
| cyclosporin (CsA) | 1200 | CsA inhibits calcineurin and consequently IL-2 synthesis and thus T cell proliferation. |
| 6-mercaptopurine (6-MP) | 1000 | 6-MP is an antimetabolite, which means that it is incorporated into the DNA instead of adenine and guanine during cell division. The resulting DNA thereby loses its function. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harm, S.; Schildböck, C.; Cont, D.; Weber, V.; Hartmann, J. Does CytoSorb Interfere with Immunosuppression? A Pharmacokinetic and Functional Evaluation. Pharmaceutics 2025, 17, 1468. https://doi.org/10.3390/pharmaceutics17111468
Harm S, Schildböck C, Cont D, Weber V, Hartmann J. Does CytoSorb Interfere with Immunosuppression? A Pharmacokinetic and Functional Evaluation. Pharmaceutics. 2025; 17(11):1468. https://doi.org/10.3390/pharmaceutics17111468
Chicago/Turabian StyleHarm, Stephan, Claudia Schildböck, Denisa Cont, Viktoria Weber, and Jens Hartmann. 2025. "Does CytoSorb Interfere with Immunosuppression? A Pharmacokinetic and Functional Evaluation" Pharmaceutics 17, no. 11: 1468. https://doi.org/10.3390/pharmaceutics17111468
APA StyleHarm, S., Schildböck, C., Cont, D., Weber, V., & Hartmann, J. (2025). Does CytoSorb Interfere with Immunosuppression? A Pharmacokinetic and Functional Evaluation. Pharmaceutics, 17(11), 1468. https://doi.org/10.3390/pharmaceutics17111468









