Targeting SIRT-1/AMPK/Nrf2 Signaling Pathway by Tenofovir Protected Against Cyclophosphamide-Induced Nephrotoxicity and Cardiotoxicity in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Chemicals Utilized
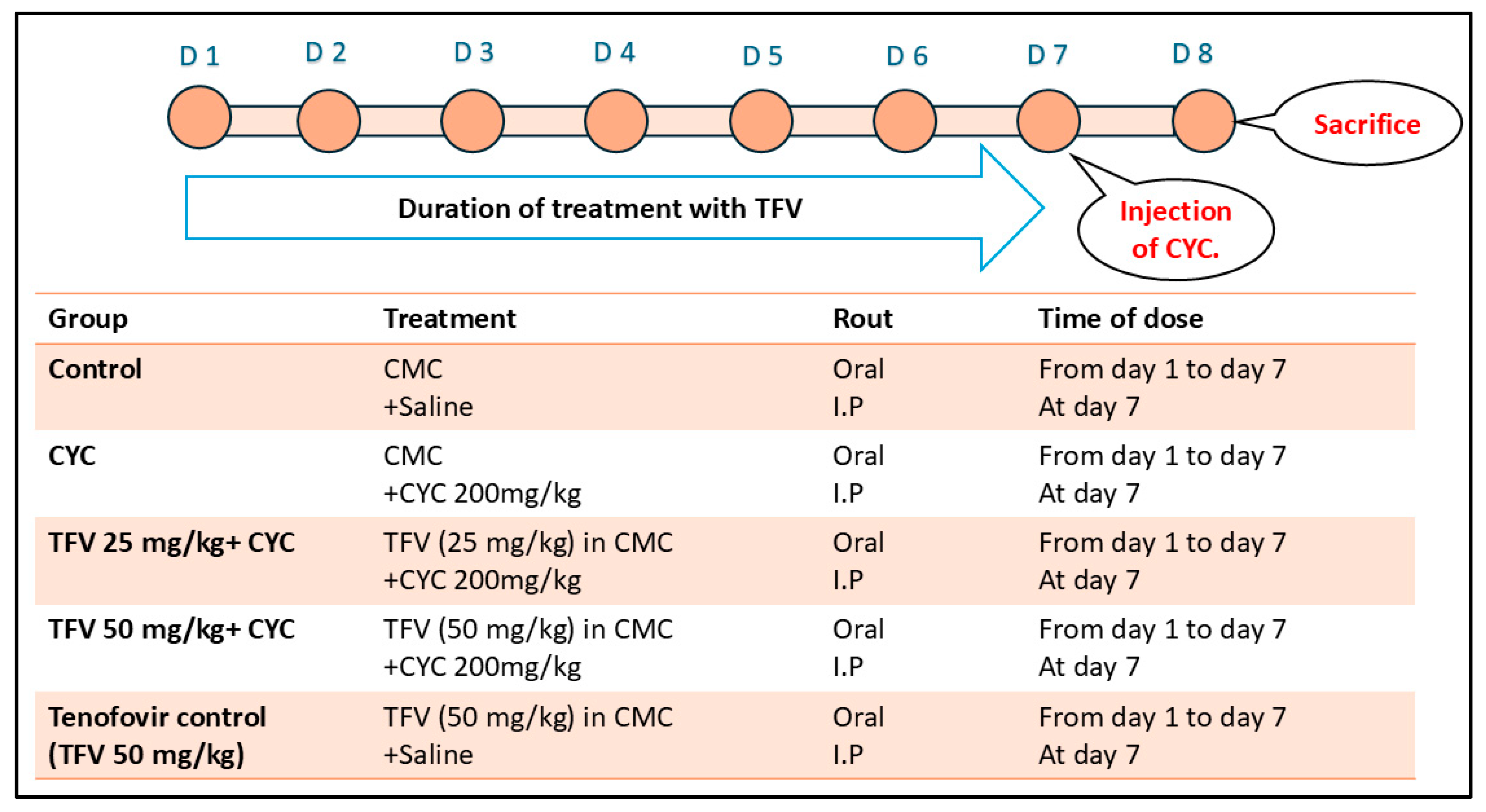
2.3. Experimental Design
2.4. Samples Collection
2.4.1. Urine Gathering
2.4.2. Blood and Tissue Collection
2.5. Assessments
2.5.1. Assessment of Serum Lactate Dehydrogenase (LDH), Creatine Kinase-Myocardial Band (CK-MB), Creatinine (Cr), and Blood Urea Nitrogen (BUN) Altered via CYC Injection
2.5.2. Assessment of Urine Total Protein (TP) and Creatinine (Cr) Altered via CYC Injection
2.5.3. Assessment of Renal and Cardiac Malondialdehyde (MDA) Content and Total Antioxidant Capacity (TAC)
2.5.4. Enzyme-Linked Immunosorbent Assay (ELISA) of Renal and Cardiac AMP-Activated Protein Kinase (AMPK), Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2), Heme Oxygenase-1 (Ho-1), B-Cell Lymphoma-2 (Bcl-2), and Silent Information Regulator Sirtuin 1 (Sirt1) Levels
2.6. Histopathological Examination of Renal and Cardiac Tissues
2.7. Immunohistochemical Assessment of Renal and Cardiac Expression of Interleukin-1B (IL-1B) and Microtubule-Associated Protein 1A/1B-Light Chain 3 (LC3)
2.8. Statistical Analysis
3. Results
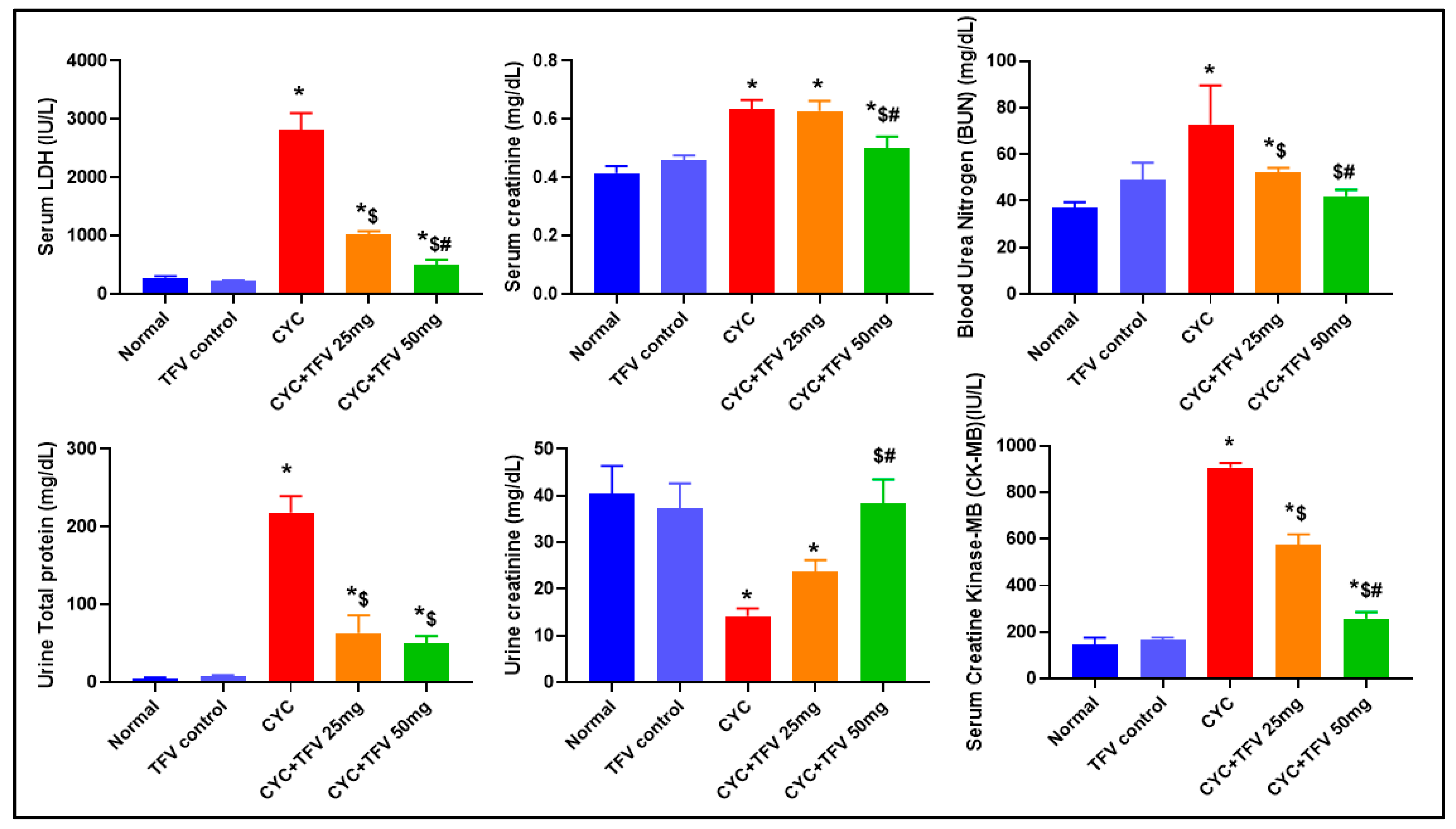
3.1. The Impact of TFV (TFV 25 and 50 mg/kg) on Serum LDH, CK-MB, Cr, and BUN Altered via CYC Injection
3.2. The Impact of TFV (TFV 25 and 50 mg/kg) on Urine TP and Cr Altered via CYC Injection
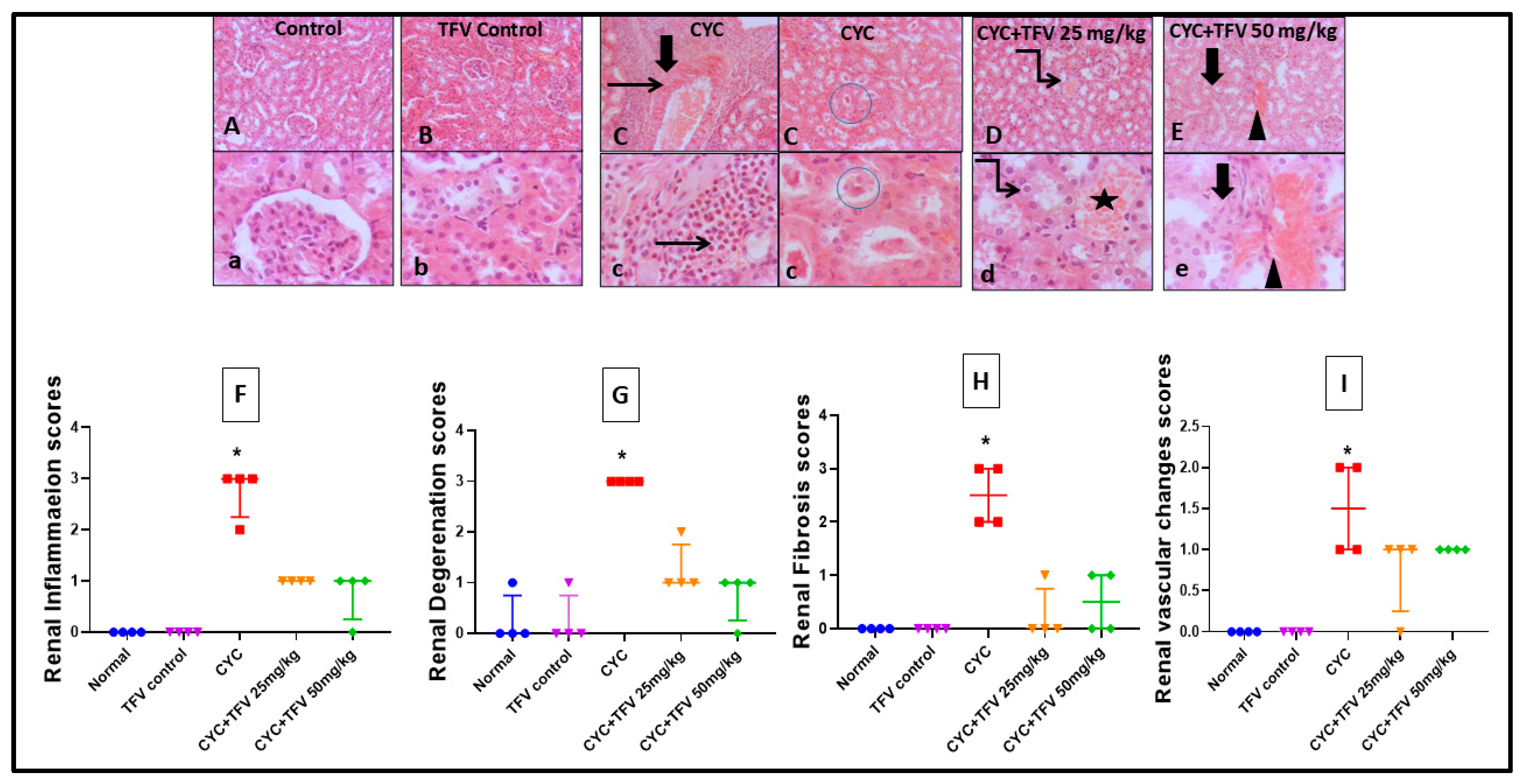
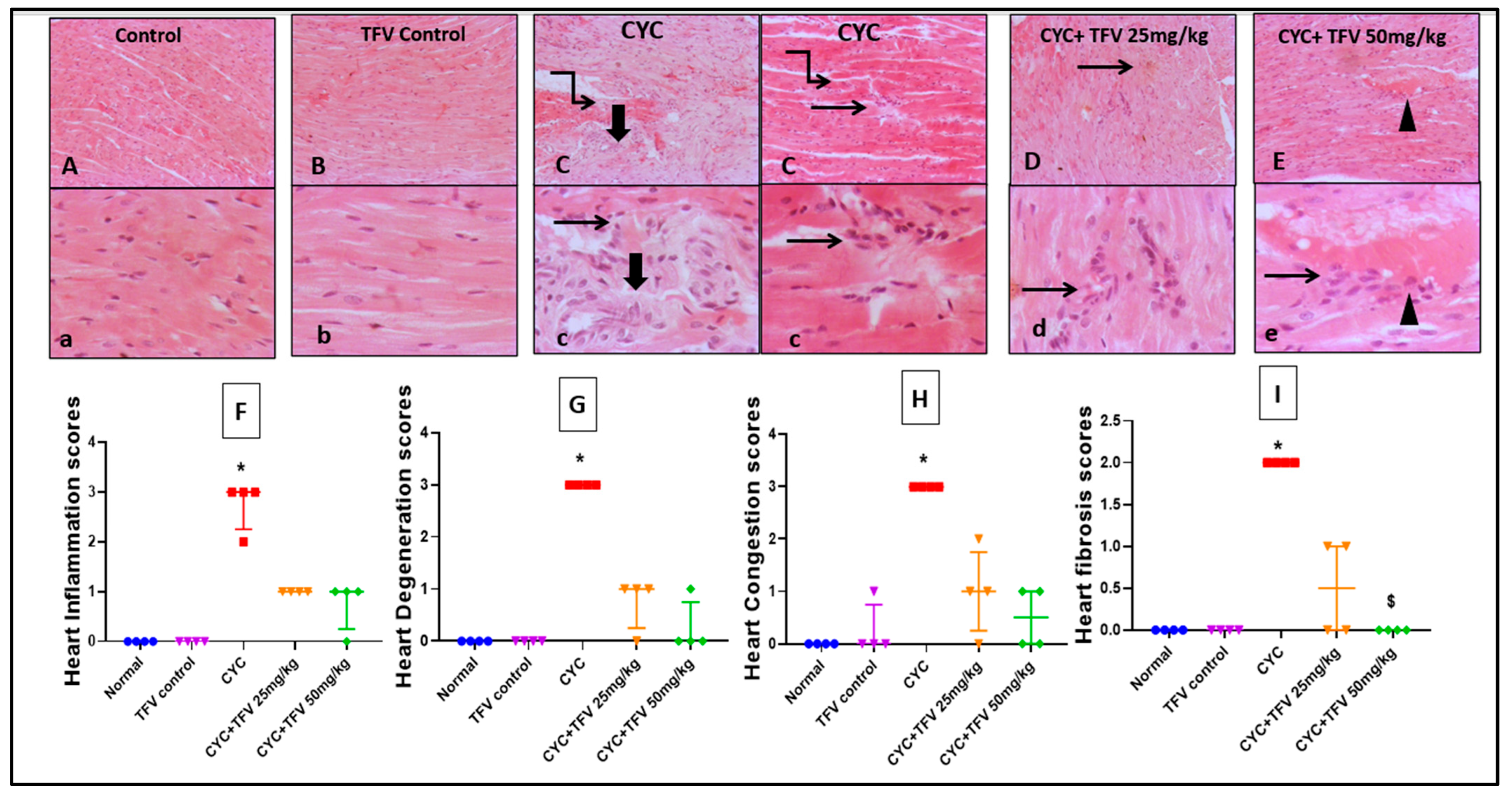
3.3. The Impact of TFV (TFV 25 and 50 mg/kg) on Renal and Cardiac Histopathology Alteration Caused by CYC
3.3.1. Renal Tissue
3.3.2. Cardiac Tissue
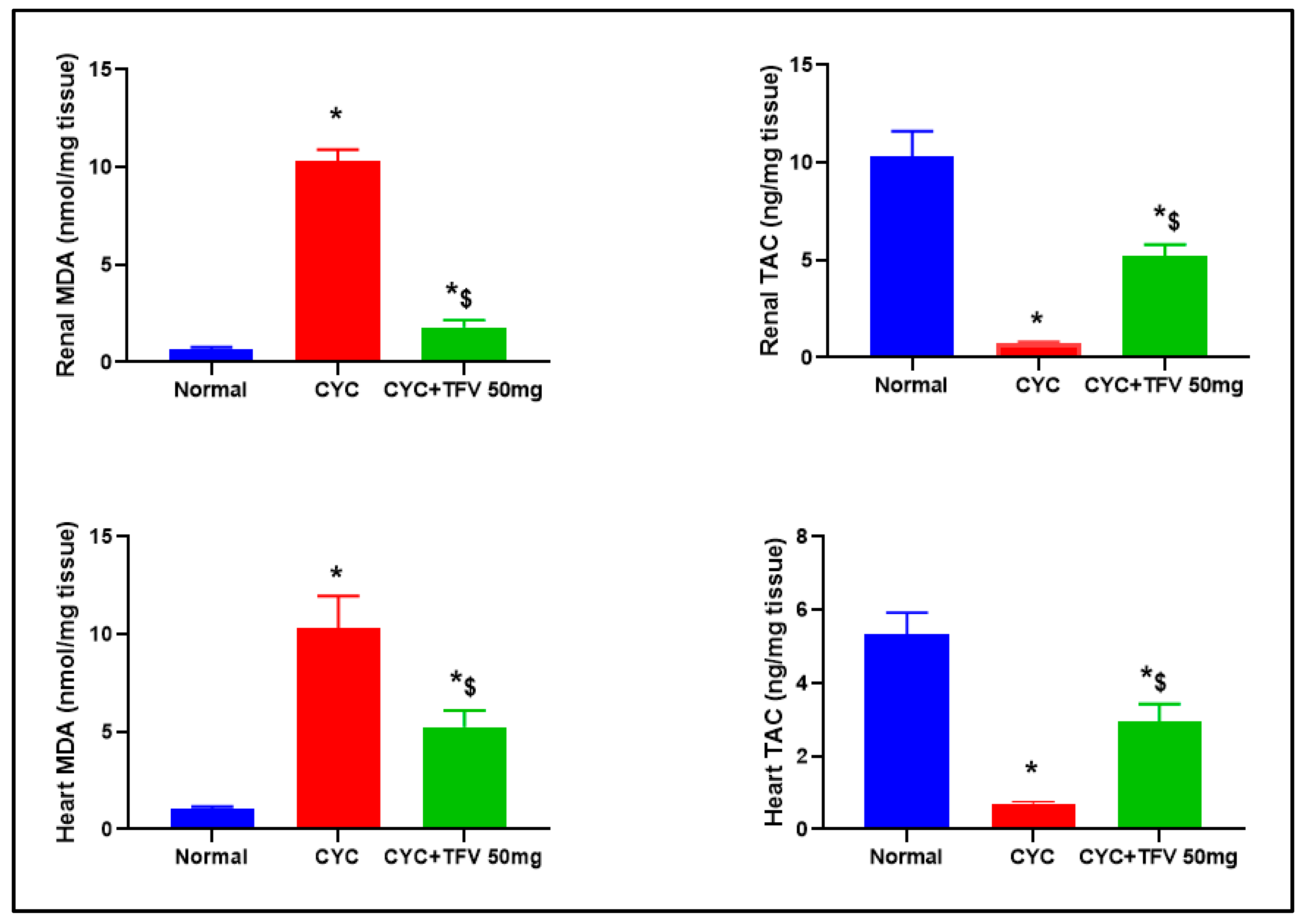
3.4. The Impact of TFV (50 mg/kg) on Renal and Cardiac MDA and TAC Altered via CYC Injection
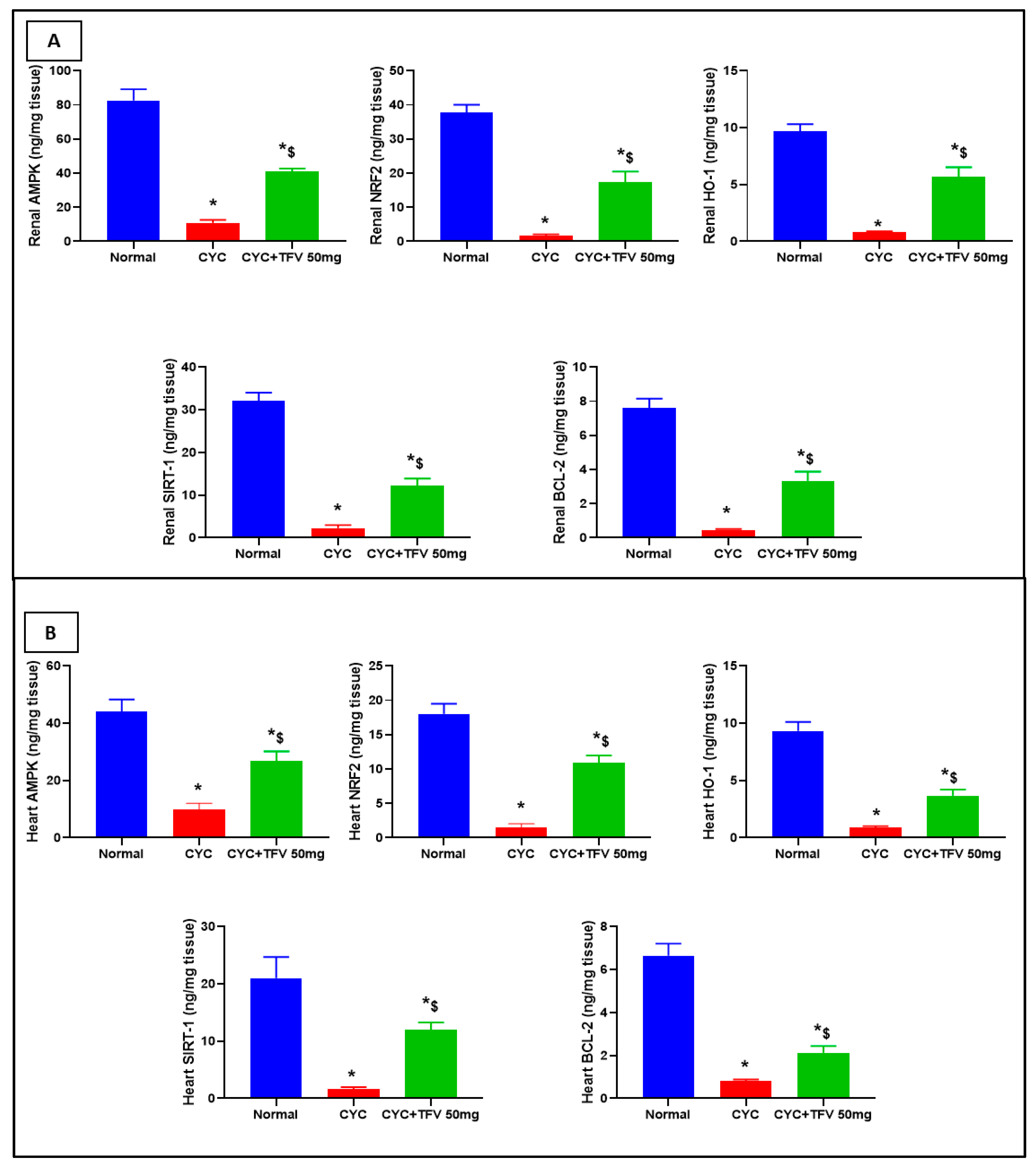
3.5. The Impact of TFV (50 mg/kg) on Renal and Cardiac AMPK, Nrf2, HO-1, Bcl-2, and SIRT1 Altered via CYC Injection
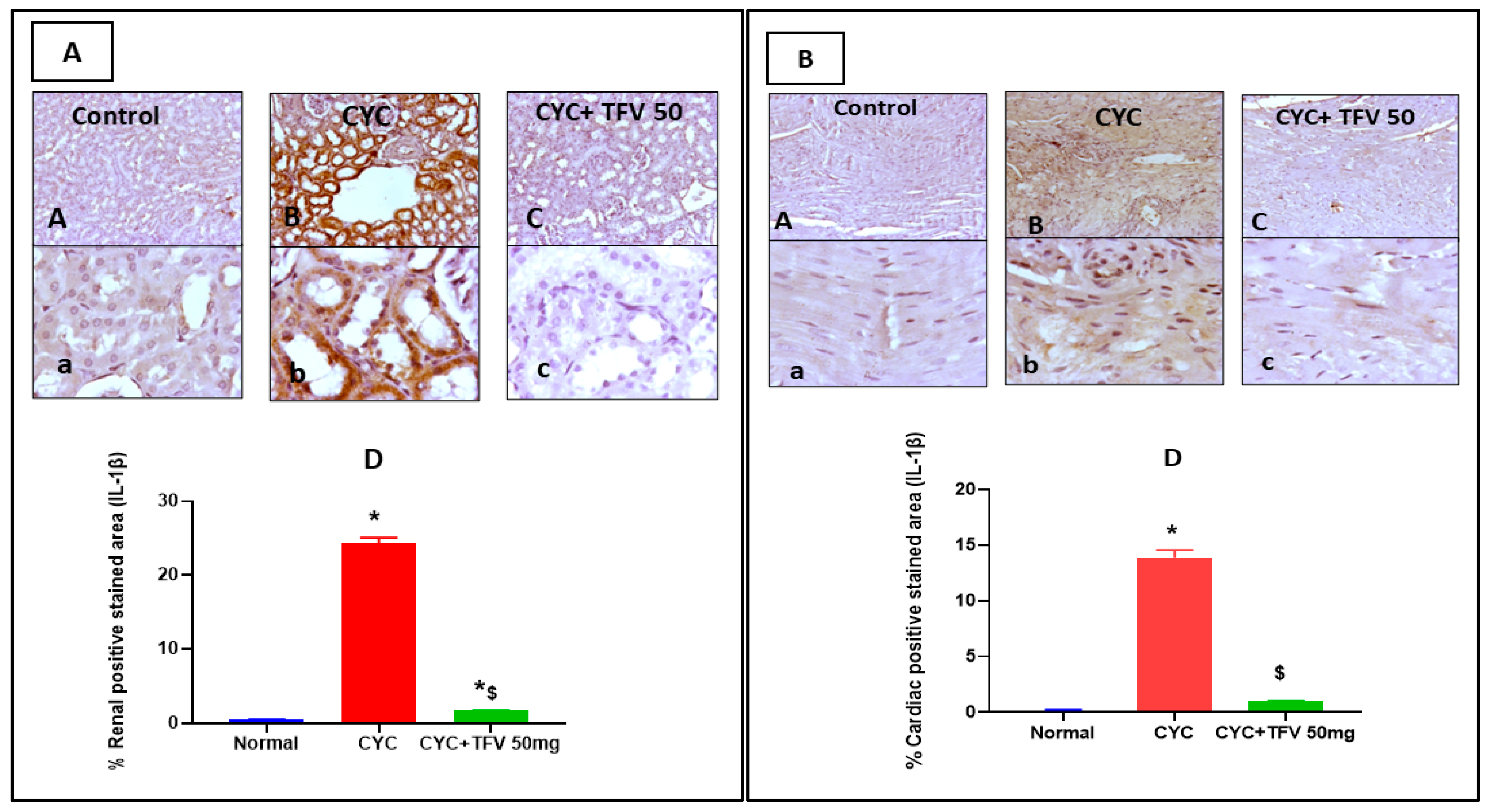
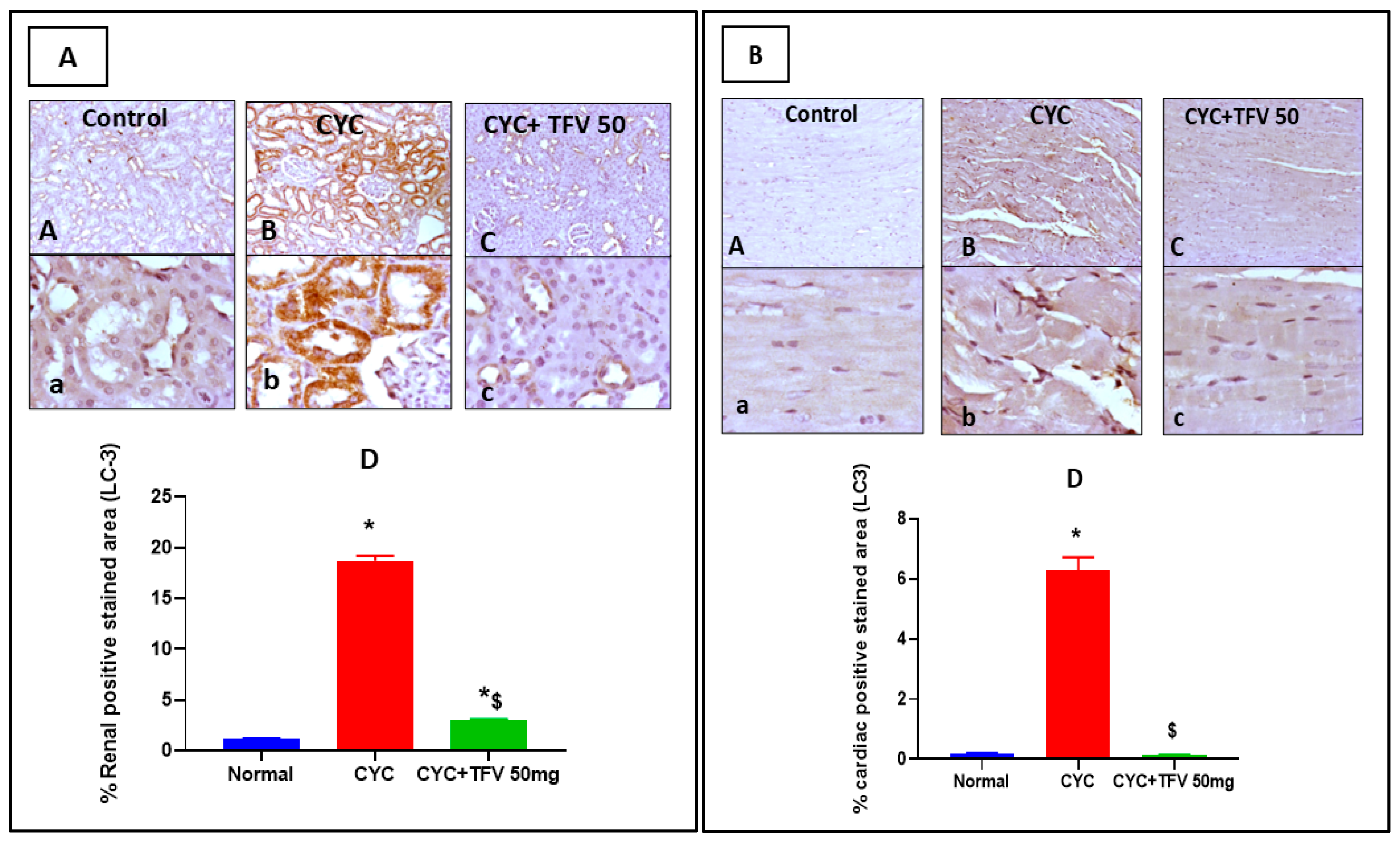
3.6. The Impact of TFV (50 mg/kg) on Renal and Cardiac IL-1B and LC3 Alteration Induced by CYC and Immunohistochemically Analyzed
4. Discussion
Limitations and Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| ACUC | Animal Care and Use Committee |
| AMPK | AMP-Activated Protein Kinase |
| BCL-2 | B-Cell Lymphoma-2 |
| BUN | Blood Urea Nitrogen |
| CMC | Carboxy Methyl Cellulose |
| CK-MB | Creatine Kinase-Myocardial Band |
| CYC | Cyclophosphamide |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HBV | Chronic Hepatitis B Virus |
| HIV | Human Immunodeficiency Virus |
| HO-1 | Heme Oxygenase-1 |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| IL-10 | Interleukin-10 |
| IL-1B | Interleukin-1B |
| IL-8 | Interleukin-8 |
| LC3 | Microtubule-Associated Protein 1A/1B-Light Chain 3 |
| LDH | Lactate Dehydrogenase |
| MDA | Malondialdehyde |
| NRF2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| SEM | Standard Error of the Mean |
| SIRT-1 | Silent Information Regulator Sirtuin 1 |
| TAC | Total Antioxidant Capacity |
| TFV | Tenofovir |
| TNF-α | Tumor Necrosis Factor Alpha |
| TP | Total Protein |
| VCAM-1 | Vascular Cell Adhesion Protein-1 |
References
- Brianna; Lee, S.H. Chemotherapy: How to reduce its adverse effects while maintaining the potency? Med. Oncol. 2023, 40, 88. [Google Scholar] [CrossRef]
- Fraiser, L.H.; Kanekal, S.; Kehrer, J.P. Cyclophosphamide Toxicity. Drugs 1991, 42, 781–795. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Ogino, M.H.; Tadi, P. Cyclophosphamide. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553087/ (accessed on 3 July 2023).
- Campochiarro, C.; Allanore, Y.; Braun-Moscovici, Y.; Matucci-Cerinic, M.; Balbir-Gurman, A. Is cyclophosphamide still the gold standard in early severe rapidly progressive systemic sclerosis? Autoimmun. Rev. 2024, 23, 103439. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.; Kalthur, S.G.; Padmashali, S.; Monappa, V. Toxic effects of different doses of cyclophosphamide on liver and kidney tissue in Swiss albino mice: A histopathological study. Ethiop. J. Health Sci. 2018, 28, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, E.; Moloudizargari, M.; Asghari, M.H.; Abdollahi, M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Expert Opin. Drug Metab. Toxicol. 2017, 13, 525–536. [Google Scholar] [CrossRef]
- Malik, S.W.; Myers, J.L.; DeRemee, R.A.; Specks, U. Lung toxicity associated with cyclophosphamide use. Two distinct patterns. Am. J. Respir. Crit. Care Med. 1996, 154, 1851–1856. [Google Scholar] [CrossRef]
- McDonald, G.B.; Slattery, J.T.; Bouvier, M.E.; Ren, S.; Batchelder, A.L.; Kalhorn, T.F.; Schoch, H.G.; Anasetti, C.; Gooley, T. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood J. Am. Soc. Hematol. 2003, 101, 2043–2048. [Google Scholar] [CrossRef]
- Ayash, L.J.; Wright, J.E.; Tretyakov, O.; Gonin, R.; Elias, A.; Wheeler, C.; Eder, J.P.; Rosowsky, A.; Antman, K.; Frei, E., III. Cyclophosphamide pharmacokinetics: Correlation with cardiac toxicity and tumor response. J. Clin. Oncol. 1992, 10, 995–1000. [Google Scholar] [CrossRef]
- Abukhalil, M.H.; Althunibat, O.Y.; Althobaiti, N.A.; Alaryani, F.S.; Albalawi, A.E.; Alhasani, R.H.; Felemban, S.G.; Al-Zayadneh, A.J.; Al-Shara, B.; Alwardat, S. Cyclophosphamide-induced pulmonary toxicity involves oxidative stress, inflammation, apoptosis, and fibrosis with impaired Nrf2/HO-1 Signaling: Protective role of rosmarinic acid. Food Chem. Toxicol. 2025, 202, 115552. [Google Scholar] [CrossRef] [PubMed]
- Ince, S.; Demirel, H.H.; Demirkapi, E.N.; Kucukkurt, I.; Eryavuz, A.; Arslan-Acaroz, D.; Acaroz, U.; Tureyen, A. Magnolin alleviates cyclophosphamide-induced oxidative stress, inflammation, and apoptosis via Nrf2/HO-1 signaling pathway. Toxicol. Res. 2024, 13, tfae129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cengiz, M.; Kutlu, H.M.; Cengiz, B.P.; Ayhancı, A. Escin attenuates oxidative damage, apoptosis and lipid peroxidation in a model of cyclophosphamide-induced liver damage. Drug Chem. Toxicol. 2022, 45, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Krishnan, A.; Zahiruddin, S.; Ahmad, S.; Vohora, D. Amelioration of cyclophosphamide-induced DNA damage, oxidative stress, and hepato- and neurotoxicity by Piper longum extract in rats: The role of γH2AX and 8-OHdG. Front. Pharmacol. 2023, 14, 1147823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iqubal, A.; Najmi, A.K.; Md, S.; Alkreathy, H.M.; Ali, J.; Syed, M.A.; Haque, S.E. Oral delivery of nerolidol alleviates cyclophosphamide-induced renal inflammation, apoptosis, and fibrosis via modulation of NF-κB/cleaved caspase-3/TGF-β signaling molecules. Drug Deliv. 2023, 30, 2241661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kearney, B.P.; Flaherty, J.F.; Shah, J. Tenofovir disoproxil fumarate: Clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 2004, 43, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Funderburg, N.T.; McComsey, G.A.; Kulkarni, M.; Bannerman, T.; Mantini, J.; Thornton, B.; Liu, H.C.; Zhang, Y.; Song, Q.; Fang, L.; et al. Equivalent decline in inflammation markers with tenofovir disoproxil fumarate vs. tenofovir alafenamide. EBioMedicine 2016, 13, 321–327. [Google Scholar] [CrossRef]
- Calza, L.; Magistrelli, E.; Danese, I.; Colangeli, V.; Borderi, M.; Bon, I.; Re, M.C.; Mancini, R.; Conti, M.; Motta, R.; et al. Changes in serum markers of inflammation and endothelial activation in HIV-infected antiretroviral naive patients starting a treatment with Abacavir-lamivudine or Tenofovir-Emtricitabine plus Efavirenz. Curr. HIV Res. 2016, 14, 61–70. [Google Scholar] [CrossRef]
- Dysangco, A.; Liu, Z.; Stein, J.H.; Dubé, M.P.; Gupta, S.K. HIV infection, antiretroviral therapy, and measures of endothelial function, inflammation, metabolism, and oxidative stress. PLoS ONE 2017, 12, e0183511. [Google Scholar] [CrossRef]
- Lawal, S.; Olojede, S.O.; Sulaiman, S.O.; Aladeyelu, O.S.; Moodley, R.; Naidu, E.C.S.; Rennie, C.O.; Azu, O.O. Tenofovir-silver nanoparticles conjugate ameliorates neurocognitive disorders and protects ultrastructural and cytoarchitectonic properties of the prefrontal cortex in diabetic rats. Bosn. J. Basic Med. Sci. 2022, 22, 569. [Google Scholar] [CrossRef]
- Tveden-Nyborg, P.; Bergmann, T.K.; Lykkesfeldt, J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin. Pharmacol. Toxicol. 2018, 123, 233–235. [Google Scholar] [CrossRef]
- Taslimi, P.; Kandemir, F.M.; Demir, Y.; İleritürk, M.; Temel, Y.; Caglayan, C.; Gulçin, I. The antidiabetic and anticholinergic effects of chrysin on cyclophosphamide-induced multiple organ toxicity in rats: Pharmacological evaluation of some metabolic enzyme activities. J. Biochem. Mol. Toxicol. 2019, 33, e22313. [Google Scholar]
- Abouelezz, H.M.; El-Kashef, D.H.; Abdеlaziz, R.R.; Nader, M.A. Tenofovir alone or combined with doxorubicin abrogates DMBA-induced mammary cell carcinoma: An insight into its modulatory impact on oxidative/Notch/apoptotic signaling. Life Sci. 2023, 326, 121798. [Google Scholar] [PubMed]
- Meyerholz, D.K.; Beck, A.P. Fundamental Concepts for Semiquantitative Tissue Scoring in Translational Research. ILAR J. 2018, 59, 13–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsu, S.M.; Raine, L. Protein A, avidin, and biotin in immunohistochemistry. J. Histochem. Cytochem. 1981, 29, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Helps, S.C.; Thornton, E.; Kleinig, T.J.M.; Manavis, J.B.; Vink, R. Automatic nonsubjective estimation of antigen content visualized by immunohistochemistry using color deconvolution. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 82–90. [Google Scholar] [CrossRef]
- Ayza, M.A.; Zewdie, K.A.; Yigzaw, E.F.; Ayele, S.G.; Tesfaye, B.A.; Tafere, G.G.; Abrha, M.G. Potential Protective Effects of Antioxidants against Cyclophosphamide-Induced Nephrotoxicity. Int. J. Nephrol. 2022, 2022, 5096825. [Google Scholar] [CrossRef]
- Vennier, A.; Canet, E.; Guardiolle, V.; Reizine, F.; Trochu, J.-N.; Le Tourneau, T.; Touzeau, C.; Houot, R.; Seguin, A.; Reignier, J.; et al. Clinical features and outcomes of patients admitted to the ICU for Cyclophosphamide-associated cardiac toxicity: A retrospective cohort. Support. Care Cancer 2023, 31, 474. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Devaki, T.; Manohar, B.M.; Babu, M.S. Effect of squalene on cyclophosphamide-induced toxicity. Clin. Chim. Acta 2006, 364, 335–342. [Google Scholar] [CrossRef]
- Chang, T.K.; Waxman, D.J. Cyclophosphamide modulates rat hepatic cytochrome P450 2C11 and steroid 5α-reductase activity and messenger RNA levels through the combined action of acrolein and phosphoramide mustard. Cancer Res. 1993, 53, 2490–2497. [Google Scholar]
- Golmohammadi, M.G.; Banaei, S.; Timar, M.; Abedi, A. Saponin protects against cyclophosphamide-induced kidney and liver damage via antioxidant and anti-inflammatory actions. Physiol. Int. 2023, 110, 108–120. [Google Scholar] [CrossRef]
- Abd-ElRaouf, A.; Nada, A.S.; Mohammed, N.E.-D.A.; Amer, H.A.; Abd-ElRahman, S.S.; Abdelsalam, R.M.; Salem, H.A. Low dose gamma irradiation attenuates cyclophosphamide-induced cardiotoxicity in rats: Role of NF-κB signaling pathway. Int. J. Radiat. Biol. 2021, 97, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Sahinturk, V.; Uslu, S.; Ayhanci, A.; Kacar, S.; Uyar, R. Protective effects of selenium on cyclophosphamide-induced oxidative stress and kidney injury. Biol. Trace Elem. Res. 2018, 185, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ayhanci, A.; Heybeli, N.; Sahin, İ.K.; Cengiz, M. Myelosuppression and oxidative stress induced by cyclophosphamide in rats: The protective role of selenium. Adıyaman Univ. J. Sci. 2019, 9, 252–265. [Google Scholar]
- Mansour, H.H.; Hasan, H.F. Protective effect of N-acetylcysteine on cyclophosphamide-induced cardiotoxicity in rats. Environ. Toxicol. Pharmacol. 2015, 40, 417–422. [Google Scholar] [CrossRef]
- Goudarzi, M.; Khodayar, M.J.; Tabatabaei, S.M.T.; Ghaznavi, H.; Fatemi, I.; Mehrzadi, S. Pretreatment with melatonin protects against cyclophosphamide-induced oxidative stress and renal damage in mice. Fundam. Clin. Pharmacol. 2017, 31, 625–635. [Google Scholar] [CrossRef]
- Blankenberg, F.G.; Naumovski, L.; Tait, J.F.; Post, A.M.; Strauss, H.W. Imaging cyclophosphamide-induced intramedullary apoptosis in rats using 99mTc-radiolabeled annexin V. J. Nucl. Med. 2001, 42, 309–316. [Google Scholar]
- Saghir, S.A.M.; Alharbi, S.A.; Al-Garadi, M.A.; Al-Gabri, N.; Rady, H.Y.; Olama, N.K.; Abdulghani, M.A.M.; Al Hroob, A.M.; Almaiman, A.A.; Bin-Jumah, M.; et al. Curcumin prevents cyclophosphamide-induced lung injury in rats by suppressing oxidative stress and apoptosis. Processes 2020, 8, 127. [Google Scholar] [CrossRef]
- Sherif, I.O. The effect of natural antioxidants in cyclophosphamide-induced hepatotoxicity: Role of Nrf2/HO-1 pathway. Int. Immunopharmacol. 2018, 61, 29–36. [Google Scholar] [CrossRef]
- Al-Amarat, W.; Abukhalil, M.H.; Alruhaimi, R.S.; Alqhtani, H.A.; Aldawood, N.; Alfwuaires, M.A.; Althunibat, O.Y.; Aladaileh, S.H.; Algefare, A.I.; Alanezi, A.A.; et al. Upregulation of Nrf2/HO-1 Signaling and Attenuation of Oxidative Stress, Inflammation, and Cell Death Mediate the Protective Effect of Apigenin against Cyclophosphamide Hepatotoxicity. Metabolites 2022, 12, 648. [Google Scholar] [CrossRef]
- Alruhaimi, R.S. Protective effect of arbutin against cyclophosphamide-induced oxidative stress, inflammation, and hepatotoxicity via Nrf2/HO-1 pathway in rats. Environ. Sci. Pollut. Res. 2023, 30, 68101–68110. [Google Scholar] [CrossRef]
- Hassanein, E.H.; Kamel, E.O.; Gad-Elrab, W.M.; Ahmed, M.A.; Mohammedsaleh, Z.M.; Ali, F.E. Nrf2/HO-1 as a therapeutic target in renal fibrosis. Life Sci. 2023, 334, 122209. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Abukhalil, M.H.; Jghef, M.M.; Alfwuaires, M.A.; Algefare, A.I.; Alsuwayt, B.; Alazragi, R.; Abourehab, M.A.S.; Almuqati, A.F.; Karimulla, S. Hepatoprotective effect of taxifolin on cyclophosphamide-induced oxidative stress, inflammation, and apoptosis in mice: Involvement of Nrf2/HO-1 signaling. Biomol. Biomed. 2023, 23, 649. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.; Kamel, E.O.; Gad-Elrab, W.M.; Ahmed, M.A.; Mohammedsaleh, Z.M.; Ali, F.E. Lansoprazole attenuates cyclophosphamide-induced cardiopulmonary injury by modulating redox-sensitive pathways and inflammation. Mol. Cell. Biochem. 2023, 478, 2319–2335. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.G.; Mohamed, E.A.; Abd Ellah, M.F.; Ali, A.A. Molecular mechanisms underlying cyclophosphamide-induced cognitive impairment and strategies for neuroprotection in preclinical models. Mol. Cell. Biochem. 2024, 479, 1873–1893. [Google Scholar] [CrossRef] [PubMed]
- Sherif, D.A.; Makled, M.N.; Suddek, G.M. The HIV reverse transcriptase Inhibitor Tenofovir suppressed DMH/HFD-induced colorectal cancer in Wistar rats. Fundam. Clin. Pharmacol. 2021, 35, 940–954. [Google Scholar] [CrossRef]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwivedi, U.N.; Kakkar, P. AMP-activated protein kinase: An energy sensor and survival mechanism in the reinstatement of metabolic homeostasis. Exp. Cell Res. 2023, 428, 113614. [Google Scholar] [CrossRef]
- Park, J.-M.; Lee, D.-H.; Kim, D.-H. Redefining the role of AMPK in autophagy and the energy stress response. Nat. Commun. 2023, 14, 2994. [Google Scholar] [CrossRef]
- Liu, T.F.; McCall, C.E. Deacetylation by SIRT1 reprograms inflammation and cancer. Genes Cancer 2013, 4, 135–147. [Google Scholar] [CrossRef]
- Santos, L.; Benitez-Rosendo, A.; Bresque, M.; Camacho-Pereira, J.; Calliari, A.; Escande, C. Sirtuins: The NAD+-dependent multifaceted modulators of inflammation. Antioxid. Redox Signal. 2023, 39, 1185–1208. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.; Rong, K.; Liang, J.; Wang, Z.; Zhao, J.; Zhang, P.; Li, Y.; Wang, L.; Ma, H.; et al. Eupatilin attenuates the senescence of nucleus pulposus cells and mitigates intervertebral disc degeneration via inhibition of the MAPK/NF-κB signaling pathway. Front. Pharmacol. 2022, 13, 940475. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, H.; Yang, D. Eupatilin suppresses osteoclastogenesis and periodontal bone loss by inhibiting the MAPKs/Siglec-15 pathway. Int. Immunopharmacol. 2024, 139, 112720. [Google Scholar] [CrossRef]
- Mosaoa, R.M.; Al-Rabia, M.W.; Asfour, H.Z.; Alhakamy, N.A.; Mansouri, R.A.; El-Agamy, D.S.; Abdulaal, W.H.; Mohamed, G.A.; Ibrahim, S.R.; Elshal, M. Targeting SIRT1/AMPK/Nrf2/NF-κB by sitagliptin protects against oxidative stress-mediated ER stress and inflammation during ANIT-induced cholestatic liver injury. Toxicology 2024, 507, 153889. [Google Scholar] [CrossRef]
- Liang, H.-Y.; Peng, F.; Pan, M.-J.; Liao, S.-L.; Wei, C.; Wei, G.-Y.; Xie, X.; Xue, K.-Y.; Chen, M.-K.; Yang, J.-K. Exosomes derived from BMSCs ameliorate cyclophosphamide-induced testosterone deficiency by enhancing the autophagy of Leydig cells via the AMPK-mTOR signaling pathway. Asian J. Androl. 2023, 25, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lin, X.; Li, H.; Yuan, J.; Peng, Y.; Dong, L.; Dai, S. Paeoniflorin ameliorates renal function in cyclophosphamide-induced mice via AMPK suppressed inflammation and apoptosis. Biomed. Pharmacother. 2016, 84, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Abdelzaher, W.Y.; AboBakr Ali, A.H.S.; El-Tahawy, N.F.G. Mast cell stabilizer modulates Sirt1/Nrf2/TNF pathway and inhibits oxidative stress, inflammation, and apoptosis in rat model of cyclophosphamide hepatotoxicity. Immunopharmacol. Immunotoxicol. 2020, 42, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Alherz, F.A.; Saleh, A.; Alsheikh, M.Y.; Borg, H.M.; Kabel, A.M.; Abd Elmaaboud, M.A. Shikonin mitigates cyclophosphamide-induced cardiotoxicity in mice: The role of sirtuin-1, NLRP3 inflammasome, autophagy, and apoptosis. J. Pharm. Pharmacol. 2024, 76, 1482–1496. [Google Scholar] [CrossRef]
- Haggagy, M.G.; Ahmed, L.A.; Sharaky, M.; Elhefnawi, M.M.; Omran, M.M. SIRT1 as a potential key regulator for mediating apoptosis in oropharyngeal cancer using cyclophosphamide and all-trans retinoic acid. Sci. Rep. 2024, 14, 41. [Google Scholar] [CrossRef]
- Xiu, Z.; Tang, S.; Kong, P.; Yan, M.; Tong, X.; Liu, X.; Liang, X.; Li, R.; Duan, Y. Zigui-Yichong-Fang protects against cyclophosphamide-induced premature ovarian insufficiency via the SIRT1/Foxo3a pathway. J. Ethnopharmacol. 2023, 314, 116608. [Google Scholar] [CrossRef]
- Elrashidy, R.A.; Hasan, R.A. Cilostazol preconditioning alleviates cyclophosphamide-induced cardiotoxicity in male rats: Mechanistic insights into SIRT1 signaling pathway. Life Sci. 2021, 266, 118822. [Google Scholar] [CrossRef]
- Pahwa, P.; Vyas, A.K.; Sevak, J.K.; Singh, R.; Maras, J.S.; Patra, S.; Sarin, S.K.; Trehanpati, N. Modulation of CD8+T cells, NK cells and Th1cytokines by metabolic milieu in decline of HBV-viremia in pregnant women treated with tenofovir-disoproxil from second trimester of pregnancy. J. Reprod. Immunol. 2024, 162, 104208. [Google Scholar] [CrossRef]
- Zimmermann, K.; Baldinger, J.; Mayerhofer, B.; Atanasov, A.G.; Dirsch, V.M.; Heiss, E.H. Activated AMPK boosts the Nrf2/HO-1 signaling axis—A role for the unfolded protein response. Free. Radic. Biol. Med. 2015, 88, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lowry, S.F.; Guarente, L.; Haimovich, B. Roles of SIRT1 in the acute and restorative phases following induction of inflammation. J. Biol. Chem. 2010, 285, 41391–41401. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ji, F.; Hao, J.; Zhong, X.; Wang, D.; Ren, H.; Hu, Z. SIRT1 Inhibits the catabolic effect of IL-1b through TLR2/SIRT1/NF-kB pathway in human degenerative nucleus pulposus cells. Pain Physician 2016, 19, E215. [Google Scholar] [PubMed]
- Xue, F.; Huang, J.-W.; Ding, P.-Y.; Zang, H.-G.; Kou, Z.-J.; Li, T.; Fan, J.; Peng, Z.-W.; Yan, W.-J. Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav. Brain Res. 2016, 309, 1–8. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Uzun-Goren, D.; Uz, Y.H. Preventive effects of quercetin against inflammation and apoptosis in cyclophosphamide-induced testicular damage. Iran. J. Basic Med. Sci. 2024, 27, 647. [Google Scholar]
- Xie, Q.; Liao, Q.; Wang, L.; Zhang, Y.; Chen, J.; Bai, H.; Li, K.; Ai, J. The dominant mechanism of cyclophosphamide-induced damage to ovarian reserve: Premature activation or apoptosis of primordial follicles? Reprod. Sci. 2024, 31, 30–44. [Google Scholar] [CrossRef]
- El kiki, S.M.; Omran, M.M.; Mansour, H.H.; Hasan, H.F. Metformin and/or low dose radiation reduces cardiotoxicity and apoptosis induced by cyclophosphamide through SIRT-1/SOD and BAX/Bcl-2 pathways in rats. Mol. Biol. Rep. 2020, 47, 5115–5126. [Google Scholar] [CrossRef]
- Ijaz, M.U.; Mustafa, S.; Batool, R.; Naz, H.; Ahmed, H.; Anwar, H. Ameliorative effect of herbacetin against cyclophosphamide-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Hum. Exp. Toxicol. 2022, 41, 09603271221132140. [Google Scholar] [CrossRef]
- Lin, F.; Zhu, Y.-T.; Qin, Z.-H. Biomarkers of autophagy. In Autophagy: Biology and Diseases: Technology and Methodology; Xie, Z., Ed.; Springer: Singapore, 2021; pp. 265–287. [Google Scholar]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720. [Google Scholar] [CrossRef]
- Tanida, I.; Minematsu-Ikeguchi, N.; Ueno, T.; Kominami, E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 2005, 1, 84–91. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A.; Shah, S.V. Autophagy function and regulation in kidney disease. Biomolecules 2020, 10, 100. [Google Scholar] [CrossRef]
- Caglayan, C.; Temel, Y.; Kandemir, F.M.; Yildirim, S.; Kucukler, S. Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ. Sci. Pollut. Res. 2018, 25, 20968–20984. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alresheedi, Y.S.; Nour, O.A.; Nader, M.A.; Zaghloul, M.S. Targeting SIRT-1/AMPK/Nrf2 Signaling Pathway by Tenofovir Protected Against Cyclophosphamide-Induced Nephrotoxicity and Cardiotoxicity in Rats. Pharmaceutics 2025, 17, 1467. https://doi.org/10.3390/pharmaceutics17111467
Alresheedi YS, Nour OA, Nader MA, Zaghloul MS. Targeting SIRT-1/AMPK/Nrf2 Signaling Pathway by Tenofovir Protected Against Cyclophosphamide-Induced Nephrotoxicity and Cardiotoxicity in Rats. Pharmaceutics. 2025; 17(11):1467. https://doi.org/10.3390/pharmaceutics17111467
Chicago/Turabian StyleAlresheedi, Yousef S., Omnia A. Nour, Manar A. Nader, and Marwa S. Zaghloul. 2025. "Targeting SIRT-1/AMPK/Nrf2 Signaling Pathway by Tenofovir Protected Against Cyclophosphamide-Induced Nephrotoxicity and Cardiotoxicity in Rats" Pharmaceutics 17, no. 11: 1467. https://doi.org/10.3390/pharmaceutics17111467
APA StyleAlresheedi, Y. S., Nour, O. A., Nader, M. A., & Zaghloul, M. S. (2025). Targeting SIRT-1/AMPK/Nrf2 Signaling Pathway by Tenofovir Protected Against Cyclophosphamide-Induced Nephrotoxicity and Cardiotoxicity in Rats. Pharmaceutics, 17(11), 1467. https://doi.org/10.3390/pharmaceutics17111467






