Abstract
Biodegradable polymers such as Polycaprolactone (PCL), Polylactic acid (PLA), and Poly(lactic-co-glycolic acid) (PLGA) are attracting attention as key platforms for anticancer drug delivery systems due to their excellent biocompatibility and controllable degradation rates. These polymers can overcome limitations of existing chemotherapeutics, such as low bioavailability, systemic toxicity, and nonspecific cell damage, and contribute to the development of precision medicine approaches and long-acting therapeutics. This paper discusses the chemical and physicochemical properties of these three polymers, their synthetic strategies, and the controlled drug release technology through surface functionalization and stimuli-responsive design. Furthermore, we highlight their potential for use in various formulations, including micelles, nanoparticles, hydrogels, and microspheres, enabling enhanced drug solubility, sustained release, and tumor targeting. Preclinical and clinical applications demonstrate that these polymer-based DDSs represent a promising approach for implementing next-generation precision anticancer treatment strategies, with further potential for clinical translation and widespread adoption.
1. Introduction
Cancer remains a serious global health problem, and existing chemotherapy regimens have clear limitations due to nonspecific drug distribution and systemic toxicity. Furthermore, many anticancer drugs have low water solubility and a narrow therapeutic index, resulting in limited efficacy and serious side effects. Therefore, drug delivery systems (DDSs) have emerged as a key strategy for improving drug solubility and stability, precisely controlling the rate and location of drug release, and selectively delivering drugs to target tissues [1]. Polymer-based DDSs have been developed in various forms, including nanoparticles, micelles, hydrogels, microspheres, and drug-polymer conjugates, and can maintain drug concentrations in the body for extended periods, minimizing damage to normal tissues, and maximizing tumor accumulation [2]. In particular, polymer nanoparticles can be surface-modified to enhance active targeting and in vivo stability, and their size and charge characteristics can be tailored to exploit the vascular permeability and retention (EPR) effect of tumor tissue. These properties lead to precision medicine approaches that increase treatment efficacy and reduce side effects compared to conventional chemotherapy [3].
Among these, biodegradable polymers ensure long-term safety because they are broken down and excreted into harmless metabolites in the body. Furthermore, they do not require surgical removal, making them ideal materials for implementing DDS [1]. Representative synthetic aliphatic polyesters include polycaprolactone (PCL), polylactic acid (PLA), and poly(lactic-co-glycolic acid) (PLGA). All of these polymers exhibit excellent biocompatibility and differ in physical and chemical properties and degradation rates, allowing them to be tailored to various therapeutic strategies [4]. For example, PCL is suitable for extended-release formulations lasting several months or longer due to its high hydrophobicity and slow degradation rate [5]. PLA offers a controlled release period ranging from several days to several months, depending on its molecular weight and crystallinity, and has been studied in various parenteral formulations [6]. On the other hand, PLGA, with its lactic acid:glycolic acid (LA:GA) ratio, allows for precise control of degradation rate and hydrophilicity, making it one of the most widely utilized polymers for finely designing drug release patterns [7].
These polymer-based DDSs play a crucial role in overcoming fundamental limitations of existing anticancer chemotherapy. Encapsulating anticancer drugs in polymer matrices extends their in vivo stability and half-life, enabling selective and sustained release at the tumor site [8]. This allows for maintaining intratumoral drug concentrations while minimizing exposure to normal tissues and alleviating drug resistance [9]. Indeed, PLA/PLGA-based formulations have significantly improved the solubility of poorly soluble drugs such as paclitaxel and doxorubicin, progressing to clinical trials and approval stages. PCL-based formulations are also showing promise in therapeutic strategies requiring ultra-long-acting drug release [10]. Despite this, existing review articles often focus on specific polymers (PCL or PLGA) or specific formulations, or provide an overview of DDSs without sufficiently addressing their connection to anticancer therapy. This review addresses these limitations by examining the three polymers, PCL, PLA, and PLGA, from a comparative and integrated perspective. By comprehensively covering the chemical and physical properties, synthetic strategies, functionalization techniques, drug release mechanisms, formulation types, and preclinical and clinical applications of the three polymers, it aims to provide readers with systematic criteria for selecting a suitable polymer for a specific therapeutic goal [11,12]. Furthermore, by analyzing recent research achievements and FDA approval cases, it differentiates itself from previous reviews by going beyond a simple technological overview to highlight their potential for clinical translation and their significance as precision anticancer treatment platforms [13].
Therefore, by comprehensively comparing these three representative biodegradable polymers, this review aims to provide in-depth insights into the design principles and clinical applicability of polymer-based anticancer DDSs. This aims to provide a basis for researchers and clinicians to select and design optimal polymer carriers and to contribute to the development of precision medicine anticancer treatment strategies in the future.
2. Structural and Physicochemical Properties of PCL, PLA, and PLGA
2.1. Chemical Composition and Biodegradability
Polycaprolactone (PCL), polylactic acid (PLA), and poly(lactic-co-glycolic acid) (PLGA) are all aliphatic polyester polymers containing ester bonds, exhibiting unique biodegradation behaviors depending on their chemical composition and microstructure (Figure 1).
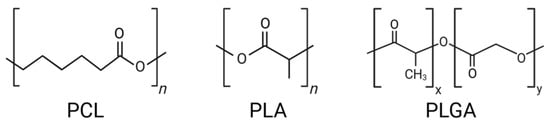
Figure 1.
Chemical structures of PCL, PLA, and PLGA.
PCL is a semi-crystalline polymer synthesized by ring-opening polymerization of ε-caprolactone monomers. Due to its high crystallinity and hydrophobicity, PCL exhibits a slow rate of pure hydrolysis, and complete biodegradation can take years. Degradation occurs gradually, primarily through the action of microbial enzymes or chemical hydrolysis in the presence of moisture [14] (Table 1).
PLA is composed of lactic acid derivatives, and its crystallinity and physical properties vary significantly depending on the ratio of the D- and L-isomers. Low D-isomer content exhibits high crystallinity, while high D-isomer content results in an amorphous structure. In the presence of moisture, PLA degrades into lactic acid monomers through hydrolysis of ester bonds, which are then completely converted to carbon dioxide and water through the body’s metabolic pathways [15] (Table 1).
PLGA is a copolymer of PLA and PGA, and its degradation rate and physical stability are controlled by the molar ratio of lactic acid (LA) to glycolic acid (GA). Typically, a 50:50 LA:GA ratio results in the fastest degradation, and both the generated lactic acid and glycolic acid are ultimately excreted harmlessly through the body’s metabolic pathways [16] (Table 1).
2.2. Thermal and Mechanical Properties
The thermal and mechanical properties of these polymers directly influence not only processability but also drug release rates and stability.
PCL has a relatively low melting point (approximately 58–61 °C) and a very low glass transition temperature (T_g ≈ −60 °C). The degree of crystallinity varies within a range of approximately 20–33% depending on processing conditions. Higher crystallinity increases the heat deflection temperature and elastic modulus, leading to increased strength. However, excessive injection flow rates can reduce crystallinity and thus thermal resistance [17].
PLA has a relatively high T_g of approximately 60 °C and melting point of 150–160 °C, and exhibits robust properties with a tensile strength of 50–70 MPa and an elastic modulus of 3–4 GPa. However, prolonged exposure to high temperatures and shear during melt processing leads to chain scission, which reduces molecular weight and consequently degrades thermal stability and mechanical properties. Insufficient crystallinity can lead to reduced strength and stiffness, leading to material brittleness [18].
The thermal properties of PLGA are controlled by the LA/GA ratio and molecular weight, with a T_g typically ranging from 40 to 60 °C. Higher lactide content improves T_g and structural stability, but higher Glycolide content increases hydrophilicity, leading to accelerated degradation and loss of mechanical integrity. Furthermore, processing processes involving heat and shear, such as hot melt extrusion or 3D printing, can result in a decrease in molecular weight and deterioration of physical properties [19].
2.3. Degradation Mechanisms and Their Impact on Drug Release Kinetics
Chemical composition and thermomechanical properties are closely linked to degradation behavior in the biological environment, which directly influences the rate and pattern of drug release. All three polymers degrade through hydrolysis of ester bonds, but exhibit different rates and patterns of degradation due to differences in crystallinity, hydrophobicity, and copolymerization ratio.
PCL exhibits a very slow degradation rate due to its high crystallinity and hydrophobicity, which limits water penetration. Its long-term structural stability suppresses initial burst release and enables consistent sustained release [20] (Figure 2).
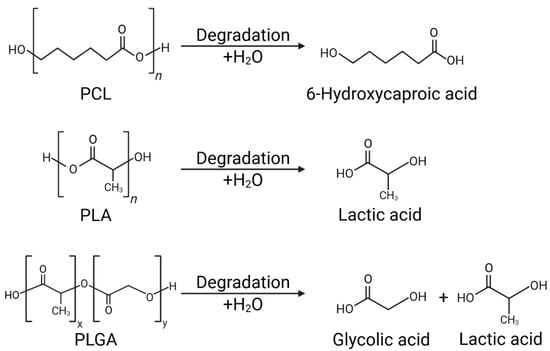
Figure 2.
Hydrolysis mechanisms of biodegradable polymers: PCL, PLA, and PLGA.
PLA primarily degrades via non-enzymatic hydrolysis, and its degradation rate varies depending on crystallinity, molecular weight, and processing history. Highly crystalline and high molecular weight PLA can persist in the body for months or longer, whereas amorphous PLA can completely degrade within weeks. The release period can be controlled from days to months through composition and processing conditions [21] (Figure 2). PLGA’s hydrophilicity and degradation rate are precisely controlled by varying the LA:GA ratio. Higher glycolide content leads to faster degradation, while higher lactide content favors sustained release. Drug hydrophilicity and hydrophobicity also significantly influence the release pattern. Hydrophilic drugs induce rapid dissolution and accelerated polymer degradation, whereas hydrophobic drugs retain the matrix for extended periods and exhibit gradual release [12] (Figure 2).
Thus, the degradation characteristics of PCL, PLA, and PLGA serve as key variables in the design and performance optimization of drug delivery systems. Process strategies, including formulation design, surface modification, and functional group introduction, must be implemented in tandem to ensure optimal therapeutic duration and release profiles.
A summary of the key findings described in Section 2.1, Section 2.2 and Section 2.3 is presented in Table 1.

Table 1.
Comparison of PCL, PLA, and PLGA Properties.
Table 1.
Comparison of PCL, PLA, and PLGA Properties.
| Polymer | PCL | PLA | PLGA |
|---|---|---|---|
| Chemical Composition | Semi-crystalline aliphatic polyester; synthesized by ROP of ε-caprolactone. | Aliphatic polyester; derived from L-lactide and D-lactide (chiral isomers). | Copolymer of lactic acid (LA) and glycolic acid (GA); tunable LA:GA ratio. |
| Crystallinity | 20–33% (high crystallinity). | Varies by D/L isomer ratio; low D = crystalline, high D = amorphous. | Amorphous to semi-crystalline depending on LA:GA ratio. |
| Melting Point | 58–61 °C | 150–160 °C | Not well-defined; varies by LA:GA ratio, typically amorphous (no sharp Tm). |
| Glass Transition | ≈−60 °C | ≈60 °C | 40–60 °C (higher LA → higher Tg). |
| Mechanical Properties | Flexible; low tensile strength; strength increases with crystallinity. | Tensile strength: 50–70 MPa; Elastic modulus: 3–4 GPa; brittle if low crystallinity. | Properties depend on LA:GA; higher LA = more rigid, higher GA = more hydrophilic and weaker. |
| Biodegradation Behavior | Very slow hydrolytic degradation; complete biodegradation may take years. | Hydrolytic degradation into lactic acid → metabolized to CO2 + H2O. | Hydrolytic degradation; fastest at 50:50 LA:GA ratio; metabolites excreted safely. |
| Degradation Rate | Slowest (months–years). | Intermediate (weeks–months depending on crystallinity/MW). | Tunable (days–months depending on LA:GA ratio, 50:50 degrades fastest). |
| Drug Release Characteristics | Minimal burst release; stable zero-order release; good for long-term implants. | Three-stage release profile; tunable from days to months. | Highly tunable; hydrophilic drugs accelerate degradation, hydrophobic drugs prolong release. |
3. Synthesis and Functionalization of PCL, PLA, and PLGA for Drug Delivery
3.1. Ring-Opening Polymerization and Copolymerization Strategies
Ring-opening polymerization (ROP) is widely used to synthesize high-molecular-weight biodegradable polymers [22]. ROP is a polymerization method that induces chain growth through ring-opening of cyclic monomers, and has various reaction pathways, including radical, anionic, cationic, enzyme-mediated, and coordination-based [23]. In particular, PLA is synthesized through ring-opening polymerization of lactide, typically using a tin oxide (Sn(Oct)2)-based catalyst [24]. The polymerization yield and final polymer properties are determined by the monomer-to-catalyst ratio, reaction temperature and time, and catalyst type. Side reactions, such as backbiting and transesterification, can lead to non-uniform molecular weight and molecular weight distribution [25,26]. To minimize these side reactions and achieve controlled polymerization, the use of high-purity monomers, removal of moisture from the reaction system (e.g., through reduced-pressure distillation), and the appropriate combination of initiator and catalyst are essential [27]. In particular, the combination of alcohol initiators and metal catalysts has been evaluated as a method for stably producing polymers with desired molecular weights [28]. Recently, the use of organic catalysts or low-toxicity metal catalysts has been attracting attention due to biocompatibility and residual toxicity, which are advantageous for the design of high-purity, functional polymers for drug delivery [29].
3.2. Surface Modifications and Functional Group Incorporation
Despite their biodegradability, polymer nanoparticles based on PLA, PLGA, and PCL have limitations such as rapid clearance from the body, induction of immune responses, and nonspecific distribution [30]. To overcome these limitations, strategies for enhancing biocompatibility and imparting targeting properties through surface modification are actively being studied. For example, PEG coating extends blood circulation time and imparts stealth by inhibiting acid binding, while chitosan- and lectin-based coatings promote mucosal adhesion and receptor-mediated uptake [31,32].
Furthermore, the introduction of functional groups such as -COOH, -NH2, and -SH provides covalent binding sites for biomolecules, enabling the stable immobilization of targeting ligands such as antibodies, peptides, and aptamers [33,34]. Various surface functionalization techniques, such as EDC/NHS coupling, dopamine self-polymerization, and tannic acid-based self-assembly, are being utilized for this purpose. The physicochemical properties of nanoparticles, such as surface charge, hydrophobicity/hydrophilicity, and mucosal permeability, directly impact cellular uptake and drug delivery efficiency, making precise surface engineering essential [35]. In particular, PCL’s high hydrophobicity limits the introduction of surface functional groups, necessitating surface activation or hydrophilic film formation [36]. In contrast, PLGA and PLA are readily amenable to the introduction of reactive functional groups, making them advantageous for bioactive molecule immobilization and targeting [34,37].
3.3. Smart Polymer Design for Controlled Drug Release
Smart polymers, or stimuli-responsive polymers, are polymers that can change their physical and chemical properties, such as solubility, hydrophilicity, structure, and degradability, in response to external stimuli such as temperature, pH, redox state, and enzyme activity. This allows for precise control of the timing and rate of drug release, drawing attention in drug delivery technology [38,39].
Smart polymers can be manufactured from synthetic polymers such as PEG, PLA, PLGA, and PCL, as well as natural polymers such as chitosan, gelatin, and albumin. They offer advantages such as high stability, ease of mass production, and the potential for diverse biocompatibility modifications [40]. Smart polymers actively control drug release by sensing environmental stimuli. Specifically, in anticancer treatment, they respond to specific conditions (pH, enzymes, redox, etc.) of the tumor microenvironment, selectively releasing drugs, thereby enhancing therapeutic efficacy and reducing systemic toxicity [38]. Furthermore, not only single-stimulus but also dual- and multi-stimulus-responsive systems can operate precisely even in complex physiological environments, making them promising for the treatment of refractory tumors [41]. Combining these stimuli-responsive nanoparticles with immunotherapeutic agents can contribute to the induction of long-term immune memory and the prevention of tumor recurrence, while simultaneously improving the nonspecific distribution and rapid drug metabolism of existing treatments [42]. Therefore, introducing smart design into PCL, PLA, and PLGA-based nanoparticles is considered a key strategy to enhance the selectivity, therapeutic durability, and in vivo efficacy of anticancer drug delivery systems [43].
Collectively, precise control of polymer-level polymerization strategies, surface functionalization, and stimuli-responsive design, provides a crucial foundation for maximizing the therapeutic efficacy and biocompatibility of PCL-, PLA-, and PLGA-based drug delivery systems.
4. Nano- and Micro-Scale Drug Delivery Systems Based on PCL, PLA, and PLGA
4.1. Polymeric Micelles: Self-Assembly and Drug Encapsulation Efficiency
Functionalized polymers such as PCL, PLA, and PLGA, synthesized through polymer synthesis and surface modification, can self-assemble into nanoscale structures, forming unique nanoparticles called micelles [44]. Specifically, the physical interaction between the hydrophobic and hydrophilic moieties of block copolymers spontaneously forms stable micelles in aqueous solutions [45]. These micellar structures enhance drug solubility and prevent drug degradation in the body, significantly enhancing drug encapsulation efficiency [46].
PCL-based micelles offer high drug encapsulation efficiency based on their strong affinity for hydrophobic drugs. PCL-PEG copolymer-based micelles stably encapsulate hydrophobic drugs in their core, while the hydrophilic PEG layer on the outer surface increases the micelle’s in vivo stability and blood circulation time. This allows them to deliver long-term, sustained drug release profiles for various therapeutics, including cancer treatment [47]. PLA-based micelles are widely used, particularly for anticancer drug delivery, due to their rapid and predictable degradation characteristics. Micelles composed of PLA-PEG block copolymers facilitate the introduction of cell-targeting ligands through surface modification, and their combination of high biocompatibility and hydrolytic properties enables efficient and selective intracellular delivery. Furthermore, lactic acid generated from PLA degradation is readily eliminated from the body, minimizing toxicity [48].
PLGA-based micelles offer the advantage of precisely adjusting the monomer composition ratio to achieve a wide range of control over the rate and duration of drug release. Specifically, PLGA-PEG-based micelles exhibit rapid hydrolysis and offer a tunable release profile in vivo, suppressing burst release and maintaining consistent drug concentrations over time. These micelles can be tailored to achieve optimal drug release rates depending on the therapeutic objective and conditions, making them effective in the treatment of various diseases [49]. Thus, micellar structures formed by synthetic and functionalized polymers are establishing themselves as powerful platforms in the field of drug delivery to maximize drug solubility, stability, selective targeting, and in vivo delivery efficiency.
A representative study reported micelles prepared by free radical polymerization of star-shaped PCL/PEG (SSMPEG-PCL) copolymers. This structure maintained high stability based on a low critical micelle concentration (CMC), and exhibited excellent dosorubicin (Dox) anticancer efficacy both in vitro and in vivo, along with pH-dependent drug release [50]. Furthermore, PLA–PEG–Fol micelles modified with folic acid (Fol) increased the solubility and intracellular uptake of curcumin (CUR), thereby enhancing its targeting and cytotoxicity against HepG2 cells [51]. Meanwhile, PLGA–PEG–PLGA triblock copolymer micelles prepared by a solvent-dialysis method effectively improved drug biodistribution by extending drug loading efficiency and blood half-life and reducing nonspecific capture by the reticuloendothelial system (RES) [52] (Table 2).

Table 2.
Representative Studies on Polymeric Micelles for Drug Delivery.
4.2. Nanoparticles and Nanocapsules: Passive vs. Active Targeting Approaches
While polymeric micelles primarily entrap and deliver drugs through the self-assembly of block copolymers, nanoparticle and nanocapsule systems have evolved into platforms that enable more precise control of drug delivery and release through more sophisticated structural design and surface modification. Nanoparticles and nanocapsules typically measure less than a few hundred nanometers and can be used for passive and active targeting strategies, enabling concentrated drug delivery to specific lesion sites [53,54].
PCL-based nanoparticles, with their high hydrophobicity and exceptional structural stability, can effectively utilize the Enhanced Permeability and Retention (EPR) effect, which allows them to passively accumulate in tumor tissues while circulating in the blood for extended periods. Furthermore, surface modification, incorporating specific ligands such as peptides, allows for selective binding to specific receptors on the tumor cell surface, enabling active targeting. This enhances drug therapeutic efficacy and minimizes toxicity in normal tissues [55,56]. PLA-based nanoparticles are biodegradable and biocompatible, and their rapid hydrolysis allows for rapid drug release at the target site. In particular, PLA nanoparticles readily conjugate targeting ligands, such as antibodies or peptides, to their surface, enabling highly efficient selective drug delivery to cancer cells that overexpress specific receptors, such as HER2 or EGFR. This active targeting contributes to enhanced cancer cell specificity and minimizing systemic drug side effects [57,58].
PLGA-based nanocapsules possess a unique structure with separate internal and external structures, allowing for stable encapsulation of liquid drugs or active substances. PLGA nanocapsules can precisely control drug release rates by adjusting their degradation rate, and the introduction of various functional groups on their surface can induce specific interactions with specific cell receptors. This functionalization helps maximize therapeutic efficacy by simultaneously optimizing passive EPR effects and active targeting [59,60]. In conclusion, nanoparticle and nanocapsule systems are attracting attention as drug delivery technologies that can effectively deliver drugs to target sites through more precise and diverse targeting strategies compared to micellar systems, while minimizing side effects in normal tissues.
A representative study reported nanoparticles prepared using ultrasonic emulsification and solvent evaporation using a PCL–PEG–PCL triblock copolymer. This formulation exhibited a particle size of approximately 60 nm and a high encapsulation efficiency of approximately 95%, and exhibited sustained curcumin (CUR) release for up to 96 h, along with an AUC more than four times higher than free CUR and a prolonged blood circulation time [61]. In addition, PEG-PTX/PTX hybrid nanoparticles simultaneously demonstrated improved stability and tumor accumulation rate, potent antitumor efficacy, and low toxicity compared to PEG-PLA/PTX micelles and Taxol® [62]. In addition, PLGA-based paclitaxel (PTX) nanoparticles prepared by a nanoprecipitation method had a spherical particle shape of less than 200 nm and a drug loading efficiency of approximately 90%. They also exhibited a two-stage release pattern combining initial rapid release and sustained release, demonstrating superior cytotoxicity compared to Taxol®, confirming their applicability as an intravenous formulation [63] (Table 3).

Table 3.
Representative Studies on nanoparticles for Drug Delivery.
4.3. Hydrogels and Scaffolds: Applications in Localized Drug Delivery
While the previously discussed nanoparticles and nanocapsules are suitable for selective drug delivery to desired target sites via systemic circulation, hydrogels and scaffolds are larger-scale drug delivery systems that can deliver drugs to specific, stable, and long-term sites, or even serve as scaffolds for tissue regeneration and restoration [64]. Hydrogels are gels formed by three-dimensional cross-linking of hydrophilic polymer networks. Under physiological conditions, they contain a large amount of water and are soft and flexible [65]. Conversely, scaffolds, with their porous structures, provide structural stability that promotes cell penetration and tissue regeneration, while also providing the ability to stably encapsulate and release drugs over a long period of time [66].
PCL-based hydrogels and scaffolds, in particular, offer both prolonged drug release and excellent flexibility, making them widely used in tissue engineering and wound healing [67]. The slow biodegradability of PCL allows for localized, slow drug release, providing long-term, sustained therapeutic effects. Its excellent flexibility facilitates application in biological environments requiring flexibility, such as skin and soft tissues [68].
PLA-based hydrogels and scaffolds are ideal for relatively rapid drug release due to their high mechanical strength and rapid, predictable degradation rates, while simultaneously providing the initial structural support necessary for tissue regeneration and restoration [69]. Specifically, PLA hydrogels degrade along with drug release, supplying biocompatible lactic acid to the localized area, which promotes rapid regeneration of surrounding tissues [70].
PLGA-based scaffolds can precisely control drug release rates by adjusting their composition and porous structure, providing an optimal environment for cell growth and tissue regeneration [71]. Furthermore, PLGA releases acidic byproducts during degradation. Careful design of PLGA scaffolds, which carefully consider drug release and tissue regeneration rates, can maintain an appropriate pH environment in the surrounding tissue and maximize therapeutic efficacy [72]. Thus, hydrogels and scaffolds are larger than nanoscale particle systems and combine structural functions that promote tissue regeneration, making them essential platforms for a variety of medical applications requiring localized and long-term drug delivery and treatment.
A representative study reported a composite incorporating porphyrin into a four-branched PEG–PCL thermosensitive hydrogel (POR–PEG–PCL). This system enabled real-time imaging of drug release through its dual fluorescence properties, and demonstrated effective tumor suppression along with the sustained release of doxorubicin (Dox) [73]. Furthermore, a chitosan-based composite scaffold (alginate/chitosan/PLA-H scaffold) coated with PLA-H loaded with VEGF-containing microspheres achieved controlled release for approximately 5 weeks, and induced angiogenesis and bone regeneration while maintaining over 90% biological activity [74]. Meanwhile, a PLGA–PVA/collagen dual-network hydrogel (PTX–NPs–DN hydrogel) enabled the sustained local release of paclitaxel (PTX) for more than 10 days, reducing systemic toxicity and demonstrating potent antitumor efficacy after surgery [75] (Table 4).

Table 4.
Representative Studies on Polymeric Hydrogels and Scaffolds for Drug Delivery.
4.4. Microspheres and Implants: Sustained Drug Release for Long-Term Therapy
While hydrogels and scaffolds are soft, porous structures that promote tissue regeneration while delivering localized drugs, microspheres and implants are designed with more robust and stable structures and are primarily used to treat various chronic diseases requiring prolonged drug release. These systems are designed as particles or implantable structures sized from a few micrometers to several hundred micrometers in size and are optimized to release drugs slowly and at a constant rate over a very long period of time [76,77].
PCL-based microspheres and implants possess high hydrophobicity and crystallinity, leading to very slow hydrolysis. This allows them to release drugs consistently over very long periods of time, from months to years. Therefore, they are ideal for applications requiring sustained and stable drug release, such as diabetes treatments, contraceptives, and long-term hormone therapy. Furthermore, PCL’s biocompatibility and low inflammatory response provide significant advantages in ensuring safety during long-term implantation [78,79]. PLA-based microspheres and implants exhibit relatively rapid and predictable degradation rates, making them ideal for use as intermediate-duration drug delivery systems. In particular, the high mechanical strength of PLA enhances structural durability when manufactured as implants, enabling long-term drug release without physical damage. This is particularly effective for applications requiring the slow delivery of drugs, such as anticancer drugs or local anesthetics, over weeks or months. The lactic acid byproduct generated after degradation is safely metabolized in vivo [80,81].
PLGA-based microspheres and implants can be precisely tuned to achieve precise drug release over a desired timeframe. PLGA biodegradation occurs rapidly and consistently in vivo, and can be designed to suppress the initial rapid drug release. Therefore, PLGA-based systems can be optimized for drug release for a variety of durations and purposes, and are widely used in therapeutic areas requiring precise drug release, such as anticancer treatment and immunotherapy delivery [82,83]. Microspheres and implants are positioned as innovative solutions for chronic disease management and long-term drug therapy due to their ability to maintain consistent drug concentrations over long periods of time, and their precise design, leveraging the biodegradability and mechanical properties of polymers, has enabled successful application in a variety of therapeutic settings.
A representative study reported doxycycline-loaded PCL microspheres prepared by a single emulsion-solvent evaporation method. This formulation demonstrated sustained antibiotic release for approximately 3 months, and the release rate was primarily controlled by diffusion, which was tunable depending on the molecular weight of PCL (14 kDa vs. 65 kDa) [84]. Furthermore, chitosan-lipid composite implants containing PLA–PEG/PLA nanoparticles locally released paclitaxel (PTX) in ascites for up to 4 weeks, and showed a high correlation between in vitro and in vivo release, demonstrating significant tumor suppression in an ovarian cancer model [85]. Meanwhile, PLA–PEG–PLA microspheres prepared by the O/W solvent evaporation method achieved approximately 50% PTX release for approximately 1 month due to enhanced porosity caused by the introduction of hydrophilic PEG blocks, and exhibited better biocompatibility than PLGA microspheres [86]. Finally, porous PLGA microspheres prepared by W/O/W composite emulsification using ammonium bicarbonate induced synergistic cytotoxicity through dual drug loading of dosorubicin (DOX) and paclitaxel (PTX), effectively reducing systemic toxicity while suggesting an optimal drug ratio (DOX:PTX = 2:1) suitable for inhalational treatment of lung cancer [87] (Table 5).

Table 5.
Representative Studies on Polymeric Microspheres and Implants for Drug Delivery.
5. Mechanisms of Drug Release and Controlled Delivery
5.1. Diffusion, Degradation, and Erosion-Controlled Release
Drug release refers to the process by which a drug moves from its initial location within a drug delivery system to the external environment. It is generally categorized as immediate release and controlled release. Controlled-release systems can maximize therapeutic efficacy and minimize side effects by maintaining a stable release rate over a certain period of time or inducing selective release at specific biological sites [88].
In biodegradable polymer-based systems such as PCL, PLA, and PLGA, drug release is primarily controlled by diffusion, polymer degradation, and erosion mechanisms. Diffusion is based on the movement of a drug from a high concentration to a low concentration according to Fick’s law. Diffusion is dominant in the initial phase, with polymer degradation and erosion gradually taking over over time [89,90].
Due to its high crystallinity and hydrophobicity, PCL restricts water penetration, and its slow hydrolysis rate, rather than diffusion, is the primary limiting factor in the release rate. Gentle bulk erosion dominates over surface erosion, and limited pore formation results in minimal initial release and a relatively stable zero-order release pattern [91]. These characteristics enable PCL-based systems to provide prolonged release for months or even years, making them suitable for formulations requiring sustained, consistent concentrations, such as hormones, contraceptives, and long-term anticancer drugs. Strategies such as copolymerization, surface activation, and hydrophilic filler incorporation are utilized to control the degradation rate [92].
In PLA, diffusion through water-filled pores is the most common release mechanism. PLA exhibits prominent bulk erosion characteristics, resulting in a three-stage release profile: drug diffusion without change in appearance during internal degradation, followed by a rapid increase in release rate in the latter stages as structural collapse begins. The degradation rate and release pattern are controlled by copolymer composition, molecular weight, crystallinity, and hydrophilicity [93,94]. PLGA also exhibits bulk erosion properties, and its hydrophilicity and degradation rate can be precisely controlled, particularly by varying the LA:GA ratio [95]. In matrices such as PLGA hydrogels, swelling and network relaxation due to water absorption significantly affect the release rate [96]. Structural characteristics (e.g., porosity, degree of cross-linking) are key determinants of the release rate. In drug-polymer conjugates, the drug is linked to the polymer chain via ester or amide bonds, and is cleaved and released by external stimuli such as hydrolysis, redox, and enzymatic action [97].
Furthermore, Monte Carlo simulation models can quantitatively predict release time by distinguishing between diffusion-dominated and reaction-dominated regimes, making them particularly useful in systems with limited matrix erosion [98]. As a result, the diffusion, decomposition, and erosion-based release mechanisms show different aspects depending on the physical and chemical properties and structures of PCL, PLA, and PLGA, respectively, and by precisely designing and controlling these, long-term and precise anticancer drug release can be realized.
5.2. Stimuli-Responsive Systems (pH-Sensitive, Temperature-Sensitive, Enzyme-Triggered)
Stimuli-responsive polymeric nanocarriers are smart drug delivery systems designed to selectively release drugs in response to specific endogenous stimuli (e.g., pH, enzymes, redox) or exogenous stimuli (e.g., temperature, light, ultrasound, magnetic fields, and electric fields) [99,100]. Nanoparticles based on PCL, PLA, and PLGA can induce precise drug release in response to characteristics of the tumor microenvironment, such as low pH, high glutathione (GSH) concentration, and overexpression of specific enzymes [101].
Ph-sensitive systems utilize acid-cleavable bonds such as hydrazone, imine, and cis-aconityl to release drugs in acidic environments such as endosomes and lysosomes. Polyacrylic acid (PAA), polymethacrylic acid (PMAA), and poly(N,N-dimethylaminoethyl methacrylate, PDMAEMA) are used for this purpose [102]. PLA and PLGA-based systems can be designed to rapidly cleave in acidic environments, while PCL-based systems can be surface-modified and block copolymerized to impart pH-sensitive functional groups for accelerated release [103].
Temperature-responsive systems, when the hydrophobicity of the polymer increases above the lower critical solution temperature (LCST), result in nanostructure collapse and drug release. Representative examples include PNIPAAm, Pluronic (PEO–PPO–PEO block copolymer), gelatin, and chitosan. Copolymers incorporating PCL or PLA blocks can simultaneously exhibit temperature sensitivity and biodegradability [104].
Enzyme-responsive systems are designed to cleave the peptide linker or polymer backbone by enzymes overexpressed in tumor tissues, such as matrix metalloproteinases (MMPs), cathepsin B, and esterases. Examples include PLGLAG sequences, hyaluronidase (HAase)-sensitive hyaluronic acid coatings, and plasmin-responsive protein-based structures. Incorporating these linkers into PLA and PLGA matrices allows for selective release at the tumor site, and PCL-based systems can also be surface functionalized to impart enzyme sensitivity [105,106].
These stimuli-responsive carriers maintain stability in normal tissues while releasing drugs only at the target site, preventing premature leakage and enhancing therapeutic efficacy while minimizing side effects. Furthermore, multimodal platforms that respond to dual or triple stimuli, such as pH/redox, pH/temperature, and light/redox, are also being developed [107]. These advanced systems offer precise control over the timing and rate of drug release depending on the stimulus conditions, and are attracting attention as next-generation anticancer treatment strategies.
5.3. Dual and Multi-Modal Drug Delivery Strategies
Dual and multi-modal drug delivery strategies are important approaches that overcome the limitations of single anticancer agents and simultaneously target not only cancer cells but also the tumor microenvironment (TME), thereby enhancing anticancer efficacy and therapeutic precision [108]. Biodegradable polymeric carriers based on PCL, PLA, and PLGA can be designed as theranostic systems that can integrate different therapeutic mechanisms, such as chemotherapy, photothermal therapy (PTT), photodynamic therapy (PDT), gene therapy, and immunotherapy, into a single platform. Incorporating functional nanomaterials such as polydopamine (PDA), black phosphorus (BP), indocyanine green (ICG), gold nanorods (GNR), and porous silicon nanoparticles (PSiNPs) enables simultaneous drug release, imaging diagnosis, and multiple therapeutics within a single formulation [109]. For example, PDA-based ICG-PDA-TPZ nanoparticles generated ROS upon near-infrared (NIR) irradiation, activating the hypoxia-responsive prodrug tirapazamine (TPZ) to induce DNA damage, resulting in trimodal therapeutic effects in combination with PTT–PDT–chemotherapy [110].
PLA and PLGA-based cross-linked nanogels exhibit superior biostability and controlled drug release compared to conventional micelles or liposomes, enabling simultaneous implementation of optical imaging, enzyme-mediated therapy, and chemotherapy [111]. Controlling the composition of PLGA and surface functionalization allows for precise treatment sequences (e.g., chemotherapy followed by PDT or immunotherapy), maximizing therapeutic synergy [112]. PCL-based carriers inherently leverage their slow degradation rate and high crystallinity, making them advantageous for multimodal designs requiring prolonged release (e.g., long-term chemotherapy + intermittent PTT/PDT). Surface modification, incorporating pH-, enzyme-, and photoresponsive materials, allows for controlled release tailored to multiple therapeutic environments [113].
These integrated strategies for polymer-based complex carriers play a crucial role in overcoming tumor heterogeneity and therapeutic resistance, and hold great potential for development into next-generation precision anticancer platforms combining therapeutics, diagnostics, and targeting [114]. In particular, PCL, PLA, and PLGA each offer distinct advantages in terms of in vivo stability, degradation rate, and ease of functionalization. Therefore, it is crucial to select and combine materials tailored to the therapeutic intent and disease characteristics.
6. Active and Passive Targeting Strategies for Enhanced Efficacy
6.1. Enhanced Permeability and Retention (EPR) Effect for Passive Targeting
Passive targeting is a targeted drug delivery strategy that leverages tumor-specific vascular architecture, based on the enhanced permeability and retention (EPR) effect [115]. Unlike normal tissue, cancer tissue blood vessels are incompletely formed during rapid angiogenesis, resulting in wide endothelial gaps and impaired lymphatic drainage. These structural and functional characteristics allow nanoparticles with diameters ranging from approximately 10–200 nm to selectively penetrate tumor tissue and remain there for extended periods [116].
PCL-based nanoparticles maintain long-term stability in the bloodstream due to their high hydrophobicity and long circulating half-life, enabling prolonged drug release within the tumor microenvironment (TME). These properties are advantageous for the design of anticancer drugs or sustained-release therapeutics requiring prolonged release [117]. PLA-based nanoparticles exhibit relatively rapid hydrolysis rates, allowing them to release drugs within a relatively short period of time after reaching tumor tissue, making them ideal for strategies that rapidly increase local drug concentrations and achieve rapid therapeutic effects [118].
PLGA-based nanoparticles can be precisely engineered to achieve both a degradation rate and release duration by adjusting the lactic acid:glycolic acid (LA:GA) ratio. This allows for stable drug release within tumor tissues while minimizing initial burst release, and maintains consistent concentrations over long periods, enhancing intratumoral therapeutic efficacy [119].
Recent research has actively developed strategies to maximize the EPR effect and enhance tumor selectivity by fine-tuning particle size, surface hydrophilicity/hydrophobicity ratio, and surface charge (zeta potential) [120]. This design optimization directly impacts nanoparticle dynamics (drug circulation, tissue distribution, and tumor penetration) and serves as a key factor in further enhancing the efficiency of passive targeting.
6.2. Ligand-Functionalized Nanoparticles for Receptor-Mediated Targeting
Active targeting is a strategy that achieves selective and efficient drug delivery to target cells by attaching ligands that can selectively bind to specific cell receptors to the nanoparticle surface [121].
PCL-based nanoparticles are actively being studied for targeting the αvβ3 integrin receptor by introducing specific ligands, such as cRGD peptides, onto their surface [122]. αvβ3 integrin is a receptor overexpressed on tumor cells and neovascular endothelial cells. After ligand binding, nanoparticles enter cells via receptor-mediated endocytosis, releasing the drug [123].
PLA-based nanoparticles are used by conjugating antibodies (e.g., trastuzumab, cetuximab) that target cancer cell surface receptors such as HER2 and EGFR (epidermal growth factor receptor) to their surface [124]. These antibody-based ligands bind to target receptors and induce endocytosis to deliver drugs into cells, demonstrating particularly effective therapeutic effects in cancers with high receptor expression, such as breast and lung cancer [125].
PLGA-based nanoparticles are effectively utilized for active targeting in the treatment of central nervous system diseases. When polysorbate 80 is attached to the surface, it specifically binds to the low-density lipoprotein (LDL) receptor within the blood–brain barrier (BBB), allowing the nanoparticles to cross the BBB via transcytosis via vascular endothelial cells. This enables efficient delivery of therapeutics for central nervous system diseases such as brain tumors and Alzheimer’s disease [126,127].
Consequently, PCL, PLA, and PLGA-based nanoparticle systems can precisely deliver drugs to target cells by optimizing their individual material properties and ligand-receptor binding strategies. This approach provides an important foundation for the development of customized nano-drug delivery platforms that minimize systemic drug toxicity and maximize therapeutic efficacy.
6.3. Multi-Functionalized Polymeric Carriers for Precision Medicine
Multifunctional polymeric carriers are emerging as a key strategy in precision medicine, serving as theranostics platforms that simultaneously deliver therapy and diagnosis. These systems are designed to combine multiple functional modules into a single carrier, enabling concurrent drug release, imaging diagnostics, and environmental stimulus-responsive therapy [128].
PLA-based multifunctional nanoparticles can simultaneously perform real-time diagnostics via magnetic resonance imaging (MRI) and magnetically induced hyperthermia by incorporating magnetic nanoparticles onto their surface. When magnetic nanoparticles are subjected to an external magnetic field, they locally generate heat, damaging cancer cells and simultaneously altering the structure of the PCL matrix, promoting drug release. This heat-release coupling maximizes therapeutic efficacy and enables sustained treatment by leveraging long-term release characteristics [129,130].
PLA-based multifunctional carriers are frequently utilized in designs that combine photodynamic therapy (PDT) with fluorescence imaging. PLA nanoparticles, which incorporate photosensitizers on their surface, are activated by light of a specific wavelength, generating reactive oxygen species (ROS) within the cell and selectively killing cancer cells. Simultaneously, they emit a fluorescent signal, enabling real-time visualization of tumor sites, enabling a truly theranostic approach that combines treatment and diagnosis [131,132].
PLGA-based multifunctional systems can easily incorporate smart functions that respond to environmental stimuli such as pH, temperature, and enzymes. For example, exposure to the acidic environment (lysosomes/endosomes) within the cell accelerates hydrolysis, rapidly releasing drugs. Furthermore, by incorporating thermosensitive polymers or enzyme-sensitive materials onto their surfaces, the release pattern can be actively controlled based on tumor microenvironmental conditions [133,134].
Thus, multifunctional polymer platforms based on PCL, PLA, and PLGA can simultaneously enable precise spatiotemporal control of drug release, real-time imaging diagnosis, and the simultaneous implementation of multiple therapeutic modalities. These characteristics provide the basis for establishing customized treatment strategies based on the patient’s disease status and target characteristics, and are positioned as a key technology for realizing next-generation precision medicine.
7. Clinical and Preclinical Applications in Anticancer Therapy
7.1. Current Status of FDA-Approved Formulations Based on PCL, PLA, and PLGA
Biodegradable polymers such as polycaprolactone (PCL), polylactic acid (PLA), and poly(lactic-co-glycolic acid) (PLGA) have been utilized as the basis for several FDA-approved drug delivery systems in the field of anticancer therapy due to their advantages of biocompatibility and controllable degradation rates [76]. In particular, PLA and PLGA have been applied to the development of long-acting formulations since the late 1980s, opening a new era in anticancer therapy [70]. Representative examples include leuprolide, a GnRH agonist, developed as a PLGA/PLA microsphere injectable (Lupron Depot®, North Chicago, IL, USA) and approved by the FDA in 1989, and goserelin, also approved as a biodegradable PLA implant (Zoladex®, Cambridge, UK), used for long-term hormone suppression treatment in hormone-dependent prostate cancer and some breast cancers. These formulations provide stable drug release for 1 to 3 months, reducing the number of administrations and improving treatment compliance [135,136]. Additionally, octreotide, a somatostatin analog, has been approved as a PLGA-based once-monthly injectable formulation (Sandostatin® LAR, East Hanover, NJ, USA) for the treatment of neuroendocrine tumors and carcinoid syndrome [137]. Meanwhile, PCL has a relatively slow degradation rate, making it suitable for long-term release implantable formulations, but its application in current anticancer therapeutics is limited compared to PLA and PLGA (Table 6). Overall, these injectable microspheres and implantable formulations have contributed to enhancing therapeutic efficacy by delivering drugs continuously and locally to tumor-related tissues, while reducing rapid fluctuations in blood concentrations and systemic toxicity [76]. Therefore, the clinical success of PCL-, PLA-, and PLGA-based formulations has laid the foundation for next-generation delivery strategies that can be expanded to tumor targeting, combination therapy, and smart formulations, which are closely linked to the advancement of subsequent preclinical studies.

Table 6.
FDA-Approved PLA/PLGA-Based Depot Formulations for Cancer Therapy.
As shown in Table 6, the current status of FDA-approved formulations is summarized to provide an overview of clinically available polymer-based drug delivery systems.
7.2. Recent Advancements in Preclinical Research and Animal Studies
Drug delivery systems utilizing biodegradable polymers such as PCL, PLA, and PLGA are being actively studied in various animal tumor models, and have shown promising results in terms of sustained drug release and tumor targeting. For example, in a mouse model study in which arenobufagin was loaded into PEG-PLA (polyethylene glycol-polylactic acid) micelles for tumor-targeted delivery, the tumor inhibition rate was 72.9%, which was 1.28 times higher than that of the free drug, and toxicity was also reduced [138]. Furthermore, when artemisinin (a natural antimalarial agent) was loaded into PLA (polylactic acid) nanoparticles and administered to a rat model of colon cancer, the number and size of tumors were significantly reduced compared to the free drug, demonstrating enhanced anticancer efficacy [139]. Meanwhile, an innovative delivery system for combined chemotherapy and phototherapy was developed by simultaneously loading cisplatin and upconversion nanomaterials onto PLGA nanoparticles surface-modified with RGD peptides, and evaluated in an animal lung cancer model [34]. This RGD-targeted PLGA nanoplatform exhibited antitumor efficacy 4.6 times higher than that of conventional cisplatin injections, along with sustained drug release over approximately 72 h, and also improved treatment safety by minimizing lung tissue damage [140]. In another study, a tumor-targeting ligand (EphA2) was conjugated to microspheres blended with PCL and PLGA, demonstrating excellent therapeutic efficacy by successfully inhibiting local tumor regrowth in triple-negative breast cancer through prolonged drug release for more than 90 days [77]. In particular, such injectable or implantable microsphere formulations have been proposed as a strategy for local, long-term drug delivery to tumor sites while minimizing systemic side effects [77]. In general, PCL, PLA, and PLGA have different degradation rates and physicochemical properties, and these differences are being utilized to precisely control drug release profiles. For example, PCL is slow to degrade, making it suitable for long-term implantable sustained-release formulations, PLGA’s degradation rate can be controlled by varying the lactic acid to glycolic acid ratio, and PLA is widely used as an FDA-approved drug delivery vehicle due to its excellent biocompatibility and mechanical strength [139]. Advances in these preclinical studies suggest that PCL-, PLA-, and PLGA-based systems can significantly enhance therapeutic efficacy in animal tumor models by incorporating innovative formulation strategies such as sustained-release formulations, tumor-targeted delivery (e.g., ligand conjugation, surface modification), and photoactivation systems or implantable depots [141].
The recent advancements in preclinical research and animal studies are summarized in Table 7 to highlight the progress of polymer-based therapeutic systems toward clinical translation.

Table 7.
Preclinical Applications of PCL, PLA, and PLGA-Based DDS for Cancer Therapy.
7.3. Challenges in Clinical Translation and Regulatory Considerations
Although PCL, PLA, and PLGA-based drug delivery systems have demonstrated excellent efficacy and safety in preclinical trials, they face technical, biological, and regulatory hurdles during clinical translation [142]. Key technical challenges include the complexity of formulation design, process reproducibility during mass production, maintaining drug stability over long-term release, and minimizing local environmental changes due to degradation products. In particular, ensuring consistency in particle size distribution and release rate during mass production is challenging, and the removal of residual solvents and metal catalysts during the manufacturing process is essential [143]. Biological considerations include heterogeneity in biodistribution, reduced target tissue delivery, heterogeneity in the tumor microenvironment, interactions with the immune system, and potential toxicity due to long-term retention. For example, nanoparticles accumulate in RES organs such as the liver and spleen through protein opsonin binding, reducing target delivery efficiency. Furthermore, local pH changes due to PLA/PLGA degradation products can affect drug stability and tissue response [144]. Regulatory delays include the lack of safety verification of polymer matrices and degradation products, GMP and quality control, long-term stability testing, sterility assurance, and standardized release testing methods [145]. In actual clinical trials, although PLA-based paclitaxel micelles (Genexol-PM®) demonstrated anticancer efficacy and low toxicity in phase 2 and 3, standardization of the manufacturing process and securing long-term safety remain challenges [146]. Among PLGA-based formulations, leuprolide sustained-release injection (Lupron Depot®) has been approved for phase 3 clinical trials and is being used in clinical settings [147], but development of novel platforms such as OncoGel™ and BIND-014 has been halted due to insufficient release reproducibility and consistency in mass production [148]. A PLGA-based nanovaccine (Precious-01), which is currently under development, also shows potential for enhanced immunogenicity, but thorough regulatory verification of long-term toxicity, immunotoxicity, and degradation product accumulation is required [149]. On the other hand, PCL has a slow degradation rate, making it advantageous for long-term release, but few cases have been clinically implemented as an anticancer drug delivery system [150]. In summary, although biodegradable polymer DDSs have successfully demonstrated clinical efficacy, significant challenges remain in meeting regulatory requirements for manufacturing, stability, and safety.
8. Conclusions
8.1. Summary of Key Findings
This paper comprehensively reviews the design, mechanism of action, applications, and targeting strategies of biodegradable polymer drug delivery systems based on PCL, PLA, and PLGA. These three polymers can be precisely engineered to tailor their degradation rates and drug release profiles by precisely controlling their physicochemical properties, such as molecular weight, composition, and crystallinity. To achieve this, various synthetic and functionalization strategies, including ring-opening polymerization, block copolymerization, surface modification, and the introduction of functional groups, are utilized. This design flexibility allows the polymers to be engineered into diverse platforms, including micelles, nanoparticles, nanocapsules, hydrogels, and microspheres. Each platform offers unique advantages, including enhanced solubility of poorly soluble drugs, prolonged circulation, local and systemic targeted delivery, and prolonged sustained release. Drug release is controlled by diffusion, polymer degradation/erosion, and stimuli-responsive mechanisms. In particular, stimuli-responsive designs that leverage environmental characteristics of the tumor microenvironment, such as low pH, high reducing power, and overexpression of specific enzymes, are emerging as key to enhancing therapeutic selectivity and efficacy. In terms of targeting strategies, passive targeting utilizing the EPR effect enables selective accumulation in tumor tissue, while active targeting via ligand binding enhances therapeutic efficacy by inducing receptor-mediated intracellular uptake. Furthermore, a multifunctional targeting strategy integrating passive and active targeting with stimulus-responsive release can simultaneously achieve target-site accumulation and selective drug release, minimizing side effects and maximizing therapeutic efficacy. These scientific evidences, along with preclinical and clinical examples, demonstrate that PCL, PLA, and PLGA-based systems represent flexible and powerful platforms for a wide range of therapeutic applications.
8.2. Implications for Future Research and Clinical Applications
Drug delivery systems based on PCL, PLA, and PLGA have the potential to play a crucial role in future precision medicine and combination therapy platforms. A key future development direction is to precisely design polymer structures and compositions to precisely control drug release rates, release durations, and targeting characteristics. Applying advanced synthetic strategies, such as block copolymer arrangements, branched/star-shaped structures, and the introduction of functional groups during the molecular design stage, can improve physicochemical stability and in vivo behavior. These strategies can enhance the solubility of poorly soluble drugs, increase target tissue accumulation efficiency, and ensure homogeneity in therapeutic responses. In terms of therapeutic strategies, these systems can be expanded to multimodal delivery systems, such as drug–drug, drug–gene, and drug–photo/thermal therapy, to maximize therapeutic efficacy and mitigate clinical challenges such as resistance and relapse. In particular, responsive designs that leverage endogenous stimuli, such as the low pH and high reducing power of the tumor microenvironment, and the overexpression of specific enzymes, enable selective and precise drug release at the target site, minimizing side effects. These characteristics can also be effectively utilized in combination with immunotherapies or gene therapies. Furthermore, personalized design that reflects the patient’s genetic information, disease characteristics, and tumor microenvironment at each stage can optimize targeting ligands and drug combinations for each individual patient, enabling a precision medicine approach. To facilitate clinical translation, standardized manufacturing processes are essential to ensure consistent quality during mass production, a long-term stability and safety verification system is established, and an approval strategy is established through early consultation with regulatory agencies. Furthermore, internationally standardized release testing methods and quality assessment guidelines should be established to lay the foundation for the successful transition of various research platforms into commercial products. If these scientific and technological advances are combined with regulatory optimization, biodegradable polymer-based drug delivery systems will be able to translate current research findings into clinical practice and fully realize their potential as next-generation precision treatment platforms.
Funding
This study was supported by Bio&Medical Technology Development Program of the National Research Foundation of Korea (NRF, RS-2024-00441289). This study was also supported by NRF of Korea (RS-2025-00512586).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| DDS | Drug Delivery System |
| PCL | Polycaprolactone |
| PLA | Polylactic Acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| LA | Lactic Acid |
| GA | Glycolic Acid |
| T_g | Glass Transition Temperature |
| MPa | Megapascal |
| GPa | Gigapascal |
| ROP | Ring-Opening Polymerization |
| PEG | Polyethylene Glycol |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| NHS | N-hydroxysuccinimide |
| EPR | Enhanced Permeability and Retention (tumor targeting effect) |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| EGFR | Epidermal Growth Factor Receptor |
| GSH | Glutathione |
| PAA | Poly(acrylic acid) |
| PMAA | Poly(methacrylic acid) |
| PDMAEMA | Poly(N,N-dimethylaminoethyl methacrylate) |
| TME | Tumor Microenvironment |
| PTT | Photothermal Therapy |
| PDT | Photodynamic Therapy |
| PDA | Polydopamine |
| BP | Black Phosphorus |
| ICG | Indocyanine Green |
| GNR | Gold Nanorods |
| PSiNPs | Porous Silicon Nanoparticles |
| TPZ | Tirapazamine |
| DNA | Deoxyribonucleic Acid |
| cRGD | Cyclic Arginine–Glycine–Aspartic Acid peptide |
| FDA | Food and Drug Administration |
| GNO | Glial Neuro-Oncology |
References
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 569–578. [Google Scholar] [CrossRef]
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone (PCL) based polymer and composites. Phys. Sci. Rev. 2023, 8, 4391–4414. [Google Scholar] [CrossRef]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Mahar, R.; Chakraborty, A.; Nainwal, N.; Bahuguna, R.; Sajwan, M.; Jakhmola, V. Application of PLGA as a Biodegradable and Biocompatible Polymer for Pulmonary Delivery of Drugs. AAPS PharmSciTech 2023, 24, 39. [Google Scholar] [CrossRef]
- Jana, P.; Shyam, M.; Singh, S.; Jayaprakash, V.; Dev, A.; Misra, S. Biodegradable polymers in drug delivery and oral vaccination. Eur. Polym. J. 2021, 142, 110155. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef] [PubMed]
- Geszke-Moritz, M.; Moritz, M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers 2024, 16, 2536. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Endogan Tanir, T.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Batakliev, T.; Georgiev, V.; Kalupgian, C.; Muñoz, P.A.R.; Ribeiro, H.; Fechine, G.J.M.; Andrade, R.J.E.; Ivanov, E.; Kotsilkova, R. Physico-chemical Characterization of PLA-based Composites Holding Carbon Nanofillers. Appl. Compos. Mater. 2021, 28, 1175–1192. [Google Scholar] [CrossRef]
- De, R.; Mahata, M.K.; Kim, K.-T. Structure-Based Varieties of Polymeric Nanocarriers and Influences of Their Physicochemical Properties on Drug Delivery Profiles. Adv. Sci. 2022, 9, e2105373. [Google Scholar] [CrossRef]
- Fernández-Tena, A.; Pérez-Camargo, R.A.; Dinçkal, S.; Yildiz, U.H. Effect of Molecular Weight on the Crystallization and Melt Memory of Poly(ε-caprolactone) (PCL). Macromolecules 2023, 56, 4602–4620. [Google Scholar] [CrossRef]
- Ruz-Cruz, M.A.; Herrera-Franco, P.J.; Flores-Johnson, E.A.; Moreno-Chulim, M.V.; Galera-Manzano, L.M.; Valadez-González, A. Thermal and mechanical properties of PLA-based multiscale cellulosic biocomposites. J. Mater. Res. Technol. 2022, 18, 485–495. [Google Scholar] [CrossRef]
- Cespi, M.; Bonacucina, G.; Tiboni, M.; Casettari, L.; Cambriani, A.; Fini, F.; Perinelli, D.R.; Palmieri, G.F. Insights in the rheological properties of PLGA-PEG-PLGA aqueous dispersions: Structural properties and temperature-dependent behaviour. Polymer 2021, 213, 123216. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Abu Bakar, A.A.; Zainuddin, M.Z.; Adam, A.N.; Noor, I.S.; Tamchek, N.B.; Alauddin, M.S.; Ghazali, M.I. The study of mechanical properties of poly(lactic) acid PLA-based 3D printed filament under temperature and environmental conditions. Mater. Today Proc. 2022, 67, 652–658. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Zeng, Q.; Guan, J.; Gao, H.; Zhang, L.; Zhong, J.; Lai, W.-Y.; Wang, Q. Designing Polymer Electrolytes via Ring-Opening Polymerization for Advanced Lithium Batteries. Adv. Energy Mater. 2023, 14, 2302876. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, K.; Wang, J.; Chen, M.; Dai, W.; Liu, R. Recent Advances and Future Developments in the Preparation of Polypeptides via N-Carboxyanhydride (NCA) Ring-Opening Polymerization. J. Am. Chem. Soc. 2024, 146, 24189–24208. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Storti, G.; Morbidelli, M. Kinetics of Ring-Opening Polymerization of L,L-Lactide. Ind. Eng. Chem. Res. 2011, 50, 7927–7940. [Google Scholar] [CrossRef]
- Nieboer, V.; Fanjul-Mosteirín, N.; Olsén, P.; Odelius, K. Mastering Macromolecular Architecture by Controlling Backbiting Kinetics during Anionic Ring-Opening Polymerization. Macromolecules 2024, 57, 3397–3406. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Tavtorkin, A.N.; Kosarev, M.A.; Gavrilov, D.E.; Komarov, P.D.; Ilyin, S.O.; Karchevsky, S.G.; Ivchenko, P.V. Mechanistic study of transesterification in TBD-catalyzed ring-opening polymerization of methyl ethylene phosphate. Eur. Polym. J. 2019, 118, 393–403. [Google Scholar] [CrossRef]
- Nieboer, V.; Fanjul-Mosteirín, N.; Olsén, P.; Odelius, K. Lewis-Pair Derived Activated Lactone Initiator (ALI) Complex for Rapid, Controlled, Bench Stable and Selective Ring-Opening Polymerization of (Macro)lactones. Eur. Polym. J. 2023, 201, 112594. [Google Scholar] [CrossRef]
- Fowler, H.R.; O’Shea, R.; Sefton, J.; Howard, S.C.; Muir, B.W.; Stockman, R.A.; Taresco, V.; Irvine, D.J. Rapid, Highly Sustainable Ring-Opening Polymerization via Resonant Acoustic Mixing. ACS Sustain. Chem. Eng. 2025, 13, 1916–1926. [Google Scholar] [CrossRef]
- Conen, N.; Fuchs, M.; Hoffmann, A.; Herres-Pawlis, S.; Jupke, A. Taking the Next Step—Model-Based Analysis of Robust and Non-Toxic Zn Catalysts for the Ring Opening Polymerization of Lactide in the Polymer Melt. Adv. Sustain. Syst. 2022, 7, 2200359. [Google Scholar] [CrossRef]
- Sharma, S.; Dang, S. Nanocarrier-Based Drug Delivery to Brain: Interventions of Surface Modification. Curr. Neuropharmacol. 2023, 21, 517–535. [Google Scholar] [CrossRef]
- Mannu, R.; Karthikeyan, V.; Velu, N.; Arumugam, C.; Roy, V.A.L.; Gopalan, A.-I.; Saianand, G.; Sonar, P.; Lee, K.-P.; Kim, W.-J.; et al. Polyethylene Glycol Coated Magnetic Nanoparticles: Hybrid Nanofluid Formulation, Properties and Drug Delivery Prospects. Nanomaterials 2021, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; El-Shatoury, E.H.; El-Araby, M.M.I. Antibacterial and anticancer activities of three novel lectin-conjugated chitosan nanoparticles. Appl. Microbiol. Biotechnol. 2024, 108, 524. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Wang, Y.; Xun, Z.; Li, S. Application of PLGA in Tumor Immunotherapy. Polymers 2024, 16, 1253. [Google Scholar] [CrossRef] [PubMed]
- El-Hammadi, M.M.; Arias, J.L. Recent Advances in the Surface Functionalization of PLGA-Based Nanomedicines. Nanomaterials 2022, 12, 354. [Google Scholar] [CrossRef]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.-F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef]
- Liang, H.-Y.; Lee, W.-K.; Hsu, J.-T.; Shih, J.-Y.; Ma, T.-L.; Vo, T.T.T.; Lee, C.-W.; Cheng, M.-T.; Lee, I.-T. Polycaprolactone in Bone Tissue Engineering: A Comprehensive Review of Innovations in Scaffold Fabrication and Surface Modifications. J. Funct. Biomater. 2024, 15, 243. [Google Scholar] [CrossRef]
- Młotek, M.; Gadomska-Gajadhur, A.; Sobczak, A.; Kruk, A.; Perron, M.; Krawczyk, K. Modification of PLA Scaffold Surface for Medical Applications. Appl. Sci. 2021, 11, 1815. [Google Scholar] [CrossRef]
- Balcerak-Woźniak, A.; Dzwonkowska-Zarzycka, M.; Kabatc-Borcz, J. A Comprehensive Review of Stimuli-Responsive Smart Polymer Materials-Recent Advances and Future Perspectives. Materials 2024, 17, 4255. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Chaharmahali, R.; Alizad, S.; Kaseem, M.; Dikici, B. A review of smart polymeric materials: Recent developments and prospects for medicine applications. Hybrid Adv. 2024, 5, 100178. [Google Scholar] [CrossRef]
- Yu, Z.; Shen, X.; Yu, H.; Tu, H.; Chittasupho, C.; Zhao, Y. Smart Polymeric Nanoparticles in Cancer Immunotherapy. Pharmaceutics 2023, 15, 775. [Google Scholar] [CrossRef]
- Liang, J.; Yang, B.; Zhou, X.; Han, Q.; Zou, J.; Cheng, L. Stimuli-responsive drug delivery systems for head and neck cancer therapy. Drug Deliv. 2021, 28, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Ghasemi, S.; Akbarian, M.; Hoseini-Ghahfarokhi, M.; Moghoofei, M.; Doroudian, M. Physically stimulus-responsive nanoparticles for therapy and diagnosis. Front. Chem. 2022, 10, 952675. [Google Scholar] [CrossRef] [PubMed]
- Dallaev, R. Smart and Biodegradable Polymers in Tissue Engineering and Interventional Devices: A Brief Review. Polymers 2025, 17, 1976. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zeng, Z.; Zhu, R.; Liu, A.; Huo, M. Polymerization-Induced Self-Assembly Toward Micelle-Crosslinked Tough and Ultrastretchable Hydrogels. Chem. Mater. 2022, 34, 6408–6419. [Google Scholar] [CrossRef]
- Hasannia, M.; Aliabadi, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Synthesis of block copolymers used in polymersome fabrication: Application in drug delivery. J. Control. Release 2022, 341, 95–117. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z. Improved encapsulation capacity of casein micelles with modified structure. J. Food Eng. 2022, 333, 111138. [Google Scholar] [CrossRef]
- Kocabay, Ö.G.; İsmail, O. Preparation and optimization of biodegradable self-assembled PCL-PEG-PCL nano-sized micelles for drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 328–337. [Google Scholar] [CrossRef]
- Kuru, M.M.; Dalgakıran, E.A.; Kaçar, G. Investigation of morphology, micelle properties, drug encapsulation and release behavior of self-assembled PEG-PLA-PEG block copolymers: A coarse-grained molecular simulations study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127445. [Google Scholar] [CrossRef]
- Wang, M.; Lin, Y.; Gao, J.; Liu, D. DPD simulations on morphologies and structures of blank PLGA-b-PEG-b-PLGA polymeric micelles and docetaxel-loaded PLGA-b-PEG-b-PLGA polymeric micelles. RSC Adv. 2022, 12, 12078–12088. [Google Scholar] [CrossRef]
- Gao, X.; Wang, W.; Rao, A.; Zhao, F.; Men, K.; Yang, B.; Liu, X.; Huang, M.; Gou, M.; Qian, Z.; et al. Preparation, characterization and application of star-shaped PCL/PEG micelles for the delivery of doxorubicin in the treatment of colon cancer. Int. J. Nanomed. 2013, 8, 971–982. [Google Scholar] [CrossRef]
- Phan, Q.T.; Le, M.H.; Le, T.T.H.; Tran, T.H.H.; Xuan, P.N.; Ha, P.T. Characteristics and cytotoxicity of folate-modified curcumin-loaded PLA-PEG micellar nano-systems with various PLA:PEG ratios. Int. J. Pharm. 2016, 507, 32–40. [Google Scholar] [CrossRef]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef]
- Argenziano, M.; Arpicco, S.; Brusa, P.; Cavalli, R.; Chirio, D.; Dosio, F.; Gallarate, M.; Peira, E.; Stella, B.; Ugazio, E. Developing Actively Targeted Nanoparticles to Fight Cancer: Focus on Italian Research. Pharmaceutics 2021, 13, 1538. [Google Scholar] [CrossRef] [PubMed]
- Keerikkadu, M.; Bangera, P.D.; Tippavajhala, V.K.; Rathnanand, M. An Overview on Lipid Nanocapsules: Exploring the Role in Precision Cancer Treatment and Lymphatic Drug Distribution. Adv. Pharm. Bull. 2025, 15, 248. [Google Scholar] [CrossRef] [PubMed]
- Agwa, M.M.; Marzouk, R.E.; Sabra, S.A. Advances in active targeting of ligand-directed polymeric nanomicelles via exploiting overexpressed cellular receptors for precise nanomedicine. RSC Adv. 2024, 14, 23520–23542. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.; Napit, P.R.; Briski, K.P.; Mattheolabakis, G. Oral Delivery of Nucleic Acids with Passive and Active Targeting to the Intestinal Tissue Using Polymer-Based Nanocarriers. Pharmaceutics 2021, 13, 1075. [Google Scholar] [CrossRef]
- Taiariol, L.; Chaix, C.; Farre, C.; Moreau, E. Click and Bioorthogonal Chemistry: The Future of Active Targeting of Nanoparticles for Nanomedicines? Chem. Rev. 2022, 122, 340–384. [Google Scholar] [CrossRef]
- Naik, H.; Sonju, J.J.; Singh, S.; Chatzistamou, I.; Shrestha, L.; Gauthier, T.; Jois, S. Lipidated Peptidomimetic Ligand-Functionalized HER2 Targeted Liposome as Nano-Carrier Designed for Doxorubicin Delivery in Cancer Therapy. Pharmaceuticals 2021, 14, 221. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, L.; Wang, J.; Zhang, H.; Zhang, Z.; Xing, G.; Wang, X.; Liu, M. Drug-loaded PEG-PLGA nanoparticles for cancer treatment. Front. Pharmacol. 2022, 13, 990505. [Google Scholar] [CrossRef]
- Chiu, H.I.; Abdul Samad, N.; Fang, L.; Lim, V. Cytotoxicity of targeted PLGA nanoparticles: A systematic review. RSC Adv. 2021, 11, 9433–9449. [Google Scholar] [CrossRef]
- Feng, R.; Song, Z.; Zhai, G. Preparation and in vivo pharmacokinetics of curcumin-loaded PCL-PEG-PCL triblock copolymeric nanoparticles. Int. J. Nanomed. 2012, 7, 4089–4098. [Google Scholar] [CrossRef]
- Lu, J.; Chuan, X.; Zhang, H.; Dai, W.; Wang, X.; Wang, X.; Zhang, Q. Free paclitaxel loaded PEGylated-paclitaxel nanoparticles: Preparation and comparison with other paclitaxel systems in vitro and in vivo. Int. J. Pharm. 2014, 471, 525–535. [Google Scholar] [CrossRef]
- Fonseca, C.; Simões, S.; Gaspar, R. Paclitaxel-loaded PLGA nanoparticles: Preparation, physicochemical characterization and in vitro anti-tumoral activity. J. Control. Release 2002, 83, 273–286. [Google Scholar] [CrossRef]
- Baretta, R.; Raucci, A.; Cinti, S.; Frasconi, M. Porous hydrogel scaffolds integrating Prussian Blue nanoparticles: A versatile strategy for electrochemical (bio)sensing. Sens. Actuators B Chem. 2023, 376, 132985. [Google Scholar] [CrossRef]
- Hsu, X.-L.; Wu, L.-C.; Hsieh, J.-Y.; Huang, Y.-Y. Nanoparticle-Hydrogel Composite Drug Delivery System for Potential Ocular Applications. Polymers 2021, 13, 642. [Google Scholar] [CrossRef] [PubMed]
- Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering. Appl. Sci. 2021, 11, 11369. [Google Scholar] [CrossRef]
- Koch, F.; Thaden, O.; Conrad, S.; Tröndle, K.; Finkenzeller, G.; Zengerle, R.; Kartmann, S.; Zimmermann, S.; Koltay, P. Mechanical properties of polycaprolactone (PCL) scaffolds for hybrid 3D-bioprinting with alginate-gelatin hydrogel. J. Mech. Behav. Biomed. Mater. 2022, 130, 105219. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021, 131, 314–325. [Google Scholar] [CrossRef]
- Das, M.; Sharabani-Yosef, O.; Eliaz, N.; Mandler, D. Hydrogel-integrated 3D-printed poly(lactic acid) scaffolds for bone tissue engineering. J. Mater. Res. 2021, 36, 3833–3842. [Google Scholar] [CrossRef]
- Nguyen, H.T.-T.; Nguyen, L.T.-T.; Ha, A.C.; Huynh, P.D. Evaluation of Ibuprofen Prolonged Release of Biomedical PLA-PEG-PLA Hydrogel via Degradation Mechanism. Int. J. Biomater. 2023, 2023, 5005316. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Development and Applications of PLGA Hydrogels for Sustained Delivery of Therapeutic Agents. Gels 2024, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zhang, Y.; Wang, Q.; Ren, G.; Wang, Y.; Zhou, S.; Wang, Q.; Peng, C.; Cheng, X. Thermosensitive vancomycin@PLGA-PEG-PLGA/HA hydrogel as an all-in-one treatment for osteomyelitis. Int. J. Pharm. 2022, 627, 122225. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chen, H.; Qin, J.; Wei, C.; Liang, J.; Liu, T.; Kong, D.; Lv, F. Thermosensitive porphyrin-incorporated hydrogel with four-arm PEG-PCL copolymer (II): Doxorubicin loaded hydrogel as a dual fluorescent drug delivery system for simultaneous imaging tracking in vivo. Drug Deliv. 2017, 24, 641–650. [Google Scholar] [CrossRef]
- De la Riva, B.; Nowak, C.; Sánchez, E.; Hernández, A.; Schulz-Siegmund, M.; Pec, M.K.; Delgado, A.; Evora, C. VEGF-controlled release within a bone defect from alginate/chitosan/PLA-H scaffolds. Eur. J. Pharm. Biopharm. 2009, 73, 50–58. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Li, W.; Zang, F.; Liu, X.; Qin, S. Paclitaxel-nanoparticles-loaded double network hydrogel for local treatment of breast cancer after surgical resection. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111046. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, Y.; Lu, W.; Zhang, Q.; Gao, S.; Sun, L.; Chen, S.; Hu, R. Development of a Long-Term Drug Delivery System with Biodegradable Polymers. J. Drug Deliv. Sci. Technol. 2022, 68, 102955. [Google Scholar] [CrossRef]
- Cai, H.; Li, A.; Qi, F.; Liu, R.; Tang, X.; Li, D.; Gu, Y.; Liu, J. Application of biodegradable microsphere injections: An anticancer perspective. Mater. Adv. 2024, 5, 3094–3112. [Google Scholar] [CrossRef]
- Nwazojie, C.C.; Obayemi, J.D.; Salifu, A.A.; Borbor-Sawyer, S.M.; Uzonwanne, V.O.; Onyekanne, C.E.; Akpan, U.M.; Onwudiwe, K.C.; Oparah, J.C.; Odusanya, O.S.; et al. Targeted drug-loaded PLGA-PCL microspheres for specific and localized treatment of triple negative breast cancer. J. Mater. Sci. Mater. Med. 2023, 34, 41. [Google Scholar] [CrossRef]
- Soares, I.; Faria, J.; Marques, A.; Ribeiro, I.A.C.; Baleizão, C.; Bettencourt, A.; Ferreira, I.M.M.; Baptista, A.C. Drug Delivery from PCL/Chitosan Multilayer Coatings for Metallic Implants. ACS Omega 2022, 7, 23096–23106. [Google Scholar] [CrossRef]
- Butreddy, A.; Gaddam, R.P.; Kommineni, N.; Dudhipala, N.; Voshavar, C. PLGA/PLA-Based Long-Acting Injectable Depot Microspheres in Clinical Use: Production and Characterization Overview for Protein/Peptide Delivery. Int. J. Mol. Sci. 2021, 22, 8884. [Google Scholar] [CrossRef]
- Wang, P.; Wang, M.; Wei, X.; Xie, L.; Tian, L.; Yang, Z.; Zhou, Z.; Chen, H. In vitro and in vivo degradation profile, biocompatibility of poly-L-lactic acid porous microspheres. Int. J. Biol. Macromol. 2024, 272, 132876. [Google Scholar] [CrossRef]
- Istratov, V.; Gomzyak, V.; Baranov, O.; Markova, G.; Mezhuev, Y.; Vasnev, V. Preparation and Hydrolytic Degradation of Hydroxyapatite-Filled PLGA Composite Microspheres. J. Compos. Sci. 2023, 7, 346. [Google Scholar] [CrossRef]
- Bassand, C.; Freitag, J.; Benabed, L.; Verin, J.; Siepmann, F.; Siepmann, J. PLGA implants for controlled drug release: Impact of the diameter. Eur. J. Pharm. Biopharm. 2022, 177, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Aydın, Ö.; Aydın, B.; Tezcaner, A.; Keskin, D. Study on physiochemical structure and in vitro release behaviors of doxycycline-loaded PCL microspheres. J. Appl. Polym. Sci. 2015, 132, 41768. [Google Scholar] [CrossRef]
- Soo, P.L.; Cho, J.; Grant, J.; Ho, E.; Piquette-Miller, M.; Allen, C. Drug release mechanism of paclitaxel from a chitosan-lipid implant system: Effect of swelling, degradation and morphology. Eur. J. Pharm. Biopharm. 2008, 69, 149–157. [Google Scholar]
- Ruan, G.; Feng, S.-S. Preparation and characterization of poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) (PLA–PEG–PLA) microspheres for controlled release of paclitaxel. Biomaterials 2003, 24, 5037–5044. [Google Scholar] [CrossRef]
- Feng, T.; Tian, H.; Xu, C.; Lin, L.; Xie, Z.; Lam, M.H.-W.; Liang, H.; Chen, X. Synergistic co-delivery of doxorubicin and paclitaxel by porous PLGA microspheres for pulmonary inhalation treatment. Eur. J. Pharm. Biopharm. 2014, 88, 1086–1093. [Google Scholar] [CrossRef]
- Bayer, I.S. Controlled Drug Release from Nanoengineered Polysaccharides. Pharmaceutics 2023, 15, 1364. [Google Scholar] [CrossRef]
- Lakshani, N.; Wijerathne, H.S.; Sandaruwan, C.; Kottegoda, N.; Karunarathne, V. Release Kinetic Models and Release Mechanisms of Controlled-Release and Slow-Release Fertilizers. ACS Agric. Sci. Technol. 2023, 3, 939–956. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Liyanage, A.; Qiu, J.; Thushara, D.; Bao, B.; Zhao, S. Collective diffusion of charged nanoparticles in a microchannel under electric field. Chem. Eng. Sci. 2022, 248, 117264. [Google Scholar] [CrossRef]
- Engler, L.G.; Farias, N.C.; Crespo, J.S.; Gately, N.M.; Major, I.; Pezzoli, R.; Devine, D.M. Designing Sustainable Polymer Blends: Tailoring Mechanical Properties and Degradation Behaviour in PHB/PLA/PCL Blends in a Seawater Environment. Polymers 2023, 15, 2874. [Google Scholar] [CrossRef]
- Haroosh, H.J.; Dong, Y.; Jasim, S.; Ramakrishna, S. Morphological Structures and Drug Release Effect of Multiple Electrospun Nanofibre Membrane Systems Based on PLA, PCL and PCL/Magnetic Nanoparticle Composites. J. Nanomater. 2022, 2022, 5190163. [Google Scholar] [CrossRef]
- Li, T.; Sun, H.; Han, H.; Zhang, C.; Li, B.; Huang, J.; Sun, D. Ultrafast Bulk Degradation of Polylactic Acid by Artificially Cultured Diatom Frustules. Compos. Sci. Technol. 2022, 223, 109410. [Google Scholar] [CrossRef]
- Moya-López, C.; Juan, A.; Donizeti, M.; Valcárcel, J.; Vázquez, J.A.; Solano, E.; Chapron, D.; Bourson, P.; Bravo, I.; Alonso-Moreno, C.; et al. Multifunctional PLA/Gelatin Bionanocomposites for Tailored Drug Delivery Systems. Pharmaceutics 2022, 14, 1138. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Koithan, J.A.; Muliana, A.H.; Pharr, M. Effect of Mechanical Loading on PLGA Biodegradation. Polym. Degrad. Stab. 2025, 240, 111485. [Google Scholar] [CrossRef]
- Bassand, C.; Vérin, J.; Lamatsch, M.; Siepmann, F.; Siepmann, J. How agarose gels surrounding PLGA implants limit swelling and slow down drug release. J. Control. Release 2022, 343, 255–266. [Google Scholar] [CrossRef]
- Sun, R.; Chen, Y.; Pei, Y.; Wang, W.; Zhu, Z.; Zheng, Z.; Yang, L.; Sun, L. The drug release of PLGA-based nanoparticles and their application in treatment of gastrointestinal cancers. Heliyon 2024, 10, e38165. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, S.; Cordero-Sánchez, S.; Mejía-Hernández, J.-G.; Villalobos-García, R.; Villegas-Cortez, J. Monte Carlo simulation methods-based models for analyzing the kinetics of drug delivery from controlled-release systems. Braz. J. Pharm. Sci. 2025, 61, e24249. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, Y.; Dheer, D.; Shankar, R. Stimuli responsiveness of recent biomacromolecular systems (concept to market): A review. Int. J. Biol. Macromol. 2024, 261, 129901. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Li, M.; Zhao, G.; Su, W.-K.; Shuai, Q. Enzyme-Responsive Nanoparticles for Anti-tumor Drug Delivery. Front. Chem. 2020, 8, 647. [Google Scholar] [CrossRef] [PubMed]
- Alsehli, M. Polymeric nanocarriers as stimuli-responsive systems for targeted tumor (cancer) therapy: Recent advances in drug delivery. Saudi Pharm. J. 2020, 28, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Dachani, S.R.; Vashi, A.; Mundada, A.B.; Mundada, P.A.; Suman, S.R. Innovative polymers in pharmaceutical chemistry: Revolutionizing drug delivery systems. Polym.-Plast. Technol. Mater. 2024, 63, 911–933. [Google Scholar] [CrossRef]
- Sethuraman, V.; Janakiraman, K.; Krishnaswami, V.; Kandasamy, R. Recent Progress in Stimuli-Responsive Intelligent Nano Scale Drug Delivery Systems: A Special Focus Towards pH-Sensitive Systems. Curr. Drug Targets 2021, 22, 947–966. [Google Scholar] [CrossRef]
- Ma, B.; Shi, J.; Zhang, Y.; Li, Z.; Yong, H.; Zhou, Y.-N.; Liu, S.; A, S.; Zhou, D. Enzymatically Activatable Polymers for Disease Diagnosis and Treatment. Adv. Mater. 2023, 36, e2306358. [Google Scholar] [CrossRef]
- Wei, D.; Sun, Y.; Zhu, H.; Fu, Q. Stimuli-Responsive Polymer-Based Nanosystems for Cancer Theranostics. ACS Nano 2023, 17, 23223–23261. [Google Scholar] [CrossRef]
- Sun, Y.; Davis, E. Nanoplatforms for Targeted Stimuli-Responsive Drug Delivery: A Review of Platform Materials and Stimuli-Responsive Release and Targeting Mechanisms. Nanomaterials 2021, 11, 746. [Google Scholar] [CrossRef]
- Yang, G.; Ding, J.; Chen, X. Bioactive poly(amino acid)s for multi-modal cancer therapy. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1985. [Google Scholar] [CrossRef]
- Li, M.; Xuan, Y.; Zhang, W.; Zhang, S.; An, J. Polydopamine-containing nano-systems for cancer multi-mode diagnoses and therapies: A review. Int. J. Biol. Macromol. 2023, 247, 125826. [Google Scholar] [CrossRef]
- Wu, H.; Wei, M.; Xu, Y.; Li, Y.; Zhai, X.; Su, P.; Ma, Q.; Zhang, H. PDA-Based Drug Delivery Nanosystems: A Potential Approach for Glioma Treatment. Int. J. Nanomed. 2022, 17, 3751–3775. [Google Scholar] [CrossRef]
- Neerooa, B.N.H.M.; Ooi, L.-T.; Shameli, K.; Dahlan, N.A.; Islam, J.M.M.; Pushpamalar, J.; Teow, S.-Y. Development of polymer-assisted nanoparticles and nanogels for cancer therapy: An update. Gels 2021, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Dwivedi, D.; Tiwari, S.; Singh, V.K. Recent Applications of Natural Polymers in the Formulation of Nanotechnology-Based Drug Delivery Systems. Curr. Drug Targets 2022, 22, 947–966. [Google Scholar]
- Noori, F.; Osanloo, M.; Moradi, H.R.; Ghaderi Jafarbeigloo, H.; Jirehnezhadyan, M.; Kouhpayeh, S.A.; Tirgare, M.; Bozorgi, A.; Goodarzi, A. Fabrication, characterization, and in vivo implantation of eugenol-loaded nanogels and PCL/Cs electrospun nanofibers for wound healing applications. J. Bioact. Compat. Polym. 2023, 38, 480–492. [Google Scholar] [CrossRef]
- Fletcher, N.L.; Kempe, K.; Thurecht, K.J. Next-Generation Polymeric Nanomedicines for Oncology: Perspectives and Future Directions. Macromol. Rapid Commun. 2020, 41, e2000319. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Henderson, A.R.; Ilan, I.S.; Lee, E. A bioengineered lymphatic vessel model for studying lymphatic endothelial cell–cell junction and barrier function. Microcirculation 2021, 28, e12730. [Google Scholar] [CrossRef]
- Visan, A.I.; Popescu-Pelin, G.; Gherasim, O.; Mihailescu, A.; Socol, M.; Zgura, I.; Chiritoiu, M.; Sima, L.E.; Antohe, F.; Ivan, L.; et al. Long-Term Evaluation of Dip-Coated PCL-Blend-PEG Coatings in Simulated Conditions. Polymers 2020, 12, 717. [Google Scholar] [CrossRef]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced permeability and retention effect: A key facilitator for solid tumor targeting by nanoparticles. Photodiagn. Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Sogomonyan, A.S.; Zelepukin, I.V.; Nikitin, M.P.; Deyev, S.M. PLGA Nanoparticles Decorated with Anti-HER2 Affibody for Targeted Delivery and Photoinduced Cell Death. Molecules 2021, 26, 3955. [Google Scholar] [CrossRef]
- Ejigah, V.; Owoseni, O.; Bataille-Backer, P.; Ogundipe, O.D.; Fisusi, F.A.; Adesina, S.K. Approaches to Improve Macromolecule and Nanoparticle Accumulation in the Tumor Microenvironment by the Enhanced Permeability and Retention Effect. Polymers 2022, 14, 2601. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Li, Y.-P.; Dang, S.-S. Precision targeting in hepatocellular carcinoma: Exploring ligand-receptor mediated nanotherapy. World J. Hepatol. 2024, 16, 164–176. [Google Scholar] [CrossRef]
- Kumar, V.; Tiwari, O.; Finkelstein-Zuta, G.; Rencus-Lazar, S.; Gazit, E. Design of Functional RGD Peptide-Based Biomaterials for Tissue Engineering. Pharmaceutics 2023, 15, 345. [Google Scholar] [CrossRef] [PubMed]
- Mennati, A.; Rostamizadeh, K.; Fathi, M. Dual silencing of integrin αvβ3 receptor and insulin-like growth factor 1 receptor using mPEG-PCL/DDAB hybrid nanoparticle loaded siRNA in breast cancer therapy: An in vitro study on MCF-7 cells. Int. J. Biol. Macromol. 2025, 294, 139334. [Google Scholar] [CrossRef] [PubMed]
- Ellebæk, S.; Brix, S.; Grandal, M.; Lantto, J.; Horak, I.D.; Kragh, M.; Poulsen, T.T. Pan-HER—An antibody mixture targeting EGFR, HER2 and HER3 abrogates preformed and ligand-induced EGFR homo- and heterodimers. Int. J. Cancer 2016, 139, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.; Tchistiakova, L.; Scott, N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs 2013, 5, 13–21. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, M.; Alrobaian, M.M.; Alghamdi, S.A.; Warsi, M.H.; Sultana, S.; Khan, R.A. Brain-targeted polysorbate-80 coated PLGA thymoquinone nanoparticles for the treatment of Alzheimer’s disease, with biomechanistic insights. J. Drug Deliv. Sci. Technol. 2021, 61, 102214. [Google Scholar] [CrossRef]
- Sharma, S.; Dang, S. Polysorbate 80 surface modified PLGA nanoparticles: An in-vitro evaluation of cellular uptake and cytotoxicity on Neuro-2a cells. J. Microencapsul. 2023, 40, 534–548. [Google Scholar] [CrossRef]
- Matiyani, M.; Jali, H.; Ghodrat, S.; Rahimi, M.; Hosseini, S.M.; Yousefi, M. Development of multi-functionalized graphene oxide based novel drug nanocarrier for delivery of quercetin and curcumin. Int. J. Biol. Macromol. 2023, 254, 124389. [Google Scholar]
- Eom, J.; Kwak, Y.; Nam, C. Electrospinning fabrication of magnetic nanoparticles-embedded polycaprolactone sorbent with enhanced sorption capacity and recovery speed for spilled oil removal. Chemosphere 2022, 303, 135063. [Google Scholar] [CrossRef]
- Vieira, J.; Maurmann, N.; Venturini, J.; Pranke, P.; Fernandes, D.; Pérez Bergmann, C. PCL-coated magnetic Fe3O4 nanoparticles: Synthesis, characterization and application in stem cells. Mater. Today Commun. 2022, 31, 103416. [Google Scholar] [CrossRef]
- Machado, M.G.C.; de Oliveira, M.A.; Lanna, E.G.; Siqueira, R.P.; Pound-Lana, G.; Branquinho, R.T.; Mosqueira, V.C.F. Photodynamic therapy with the dual-mode association of IR780 to PEG-PLA nanocapsules and the effects on human breast cancer cells. Biomed. Pharmacother. 2022, 145, 112464. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, C.; Shan, M.; Jia, H.; Zhang, S.; Chen, X.; Liu, W.; Liu, X.; Chen, J.; Wang, X. Synergistic poly(lactic acid) antibacterial surface combining superhydrophobicity for anti-adhesion and chlorophyll for photodynamic therapy. Langmuir 2022, 38, 8987–8998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, X.; Wang, J.; Zhang, Y.; Guo, P.; Lv, Y.; Ma, G.; Wei, W.; Wang, S. Versatile PLGA-Based Drug Delivery Systems for Tumor Immunotherapy. Small Methods 2025, 9, 2401623. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Chwalisz, K. Clinical development of the GnRH agonist leuprolide acetate depot. Fertil. Steril. Rep. 2022, 4, 33–39. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, R.; Wei, Y.; Huang, B.; Chen, Y.; Zhang, X.; Yao, J.; Wang, G.; Mao, H.; Shi, H.; et al. Multicenter, prospective, single-arm clinical study to investigate the efficacy and safety of Zoladex (Goserelin acetate) 10.8 mg prior to surgery in Chinese premenopausal women with symptomatic uterine fibroids. Gynecol. Endocrinol. 2024, 40, 2427190. [Google Scholar] [CrossRef]
- Han, B.; Tang, H.; Liang, Q.; Zhu, M.; Xie, Y.; Chen, J.; Li, Q.; Jia, J.; Li, Y.; Ren, Z.; et al. Preparation of long-acting microspheres loaded with octreotide for the treatment of portal hypertensive. Drug Deliv. 2021, 28, 719–732. [Google Scholar] [CrossRef]
- Wang, S.-J.; Wang, P.-X.; Ding, H.-L.; Qi, Y.-Y.; Song, Y.-R.; Mu, X.-N. Cellular and molecular mechanisms of arenobufagin in cancer therapy: A systematic review. Discov. Oncol. 2025, 16, 1207. [Google Scholar] [CrossRef]
- Dwidar, Z.; Awadin, W.F.; El-Adl, M.; Abomosallam, M.; ELzeer, A.A.; Abdellatif, A.M. Artemisinin-loaded polylactic acid nanoparticles alleviate 1, 2 N,N-dimethylhydrazine-induced colorectal cancer in Albino rats. Cancer Nanotechnol. 2025, 16, 18. [Google Scholar] [CrossRef]
- Yadav, B.; Chauhan, M.; Shekhar, S.; Kumar, A.; Mehata, A.K.; Nayak, A.K.; Dutt, R.; Garg, V.; Kailashiya, V.; Muthu, M.S.; et al. RGD-decorated PLGA nanoparticles improved effectiveness and safety of cisplatin for lung cancer therapy. Int. J. Pharm. 2023, 633, 122587. [Google Scholar] [CrossRef]
- Remmers, R.C.P.A.; Neumann, K. Reaching new lights: A review on photo-controlled nanomedicines and their in vivo evaluation. Biomater. Sci. 2023, 11, 1607–1624. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.; Yaqoob, A.; Rehman, K.; Shoaib, S.M.; Akash, M.S.H. PLGA-based nanoparticles for the treatment of cancer: Current strategies and perspectives. AAPS Open 2022, 8, 12. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-co-Glycolic Acid) and Progress of Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef] [PubMed]
- Tracey, S.R.; Smyth, P.; Barelle, C.J.; Scott, C.J. Development of next generation nanomedicine-based approaches for the treatment of cancer: We’ve barely scratched the surface. Biochem. Soc. Trans. 2021, 49, 2253–2269. [Google Scholar] [CrossRef]
- Varenne, F.; Vauthier, C. Practical guidelines for the characterization and quality control of nanoparticles in the pharmaceutical industry. In Emerging Technologies for Nanoparticle Manufacturing; Springer: Cham, Switzerland, 2021; pp. 487–508. [Google Scholar] [CrossRef]
- Park, I.-H.; Sohn, J.-H.; Kim, S.-B.; Lee, K.-S.; Chung, J.-S.; Lee, S.-H.; Kim, T.-Y.; Jung, K.-H.; Cho, E.-K.; Kim, Y.-S.; et al. An open-label, randomized, parallel, phase III trial evaluating the efficacy and safety of polymeric micelle-formulated paclitaxel compared to conventional Cremophor EL-based paclitaxel for recurrent or metastatic HER2-negative breast cancer. Cancer Res. Treat. 2017, 49, 569–577. [Google Scholar] [CrossRef]
- Kim, G.-Y.; Kim, J.-H.; Lee, T.; Bae, B.-C.; Baik, H.; Kim, T.; Kim, J.; Kang, D.-W.; Kim, J.-H.; Kim, D.; et al. In vitro and in vivo evaluations of a 3-month sustained-release microsphere depot formulation of leuprolide acetate. J. Pharm. Investig. 2022, 52, 129–138. [Google Scholar] [CrossRef]
- Martins, C.; Pacheco, C.; Faria, P.; Sarmento, B. Nanomedicine approaches for treating glioblastoma. Nanomedicine 2023, 18, 1135–1138. [Google Scholar] [CrossRef]
- Creemers, J.H.A.; Pawlitzky, I.; Grosios, K.; Gileadi, U.; Middleton, M.R.; Gerritsen, W.R.; Mehra, N.; Rivoltini, L.; Walters, I.; Figdor, C.G.; et al. Assessing the safety, tolerability and efficacy of PLGA-based immunomodulatory nanoparticles in patients with advanced NY-ESO-1-positive cancers: A first-in-human phase I open-label dose-escalation study protocol. BMJ Open 2021, 11, e050725. [Google Scholar] [CrossRef]
- Siqueira, D.D.; Luna, C.B.B.; Araújo, E.M.; Barros, A.B.S.; Wellen, R.M.R. Approaches on PCL/macaíba biocomposites: Mechanical, thermal, morphological properties and crystallization kinetics. Polym. Adv. Technol. 2021, 32, 3572–3585. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).