Carbon Dots with Tunable Charge as Mucus-Penetrating Gene Carriers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticle Synthesis
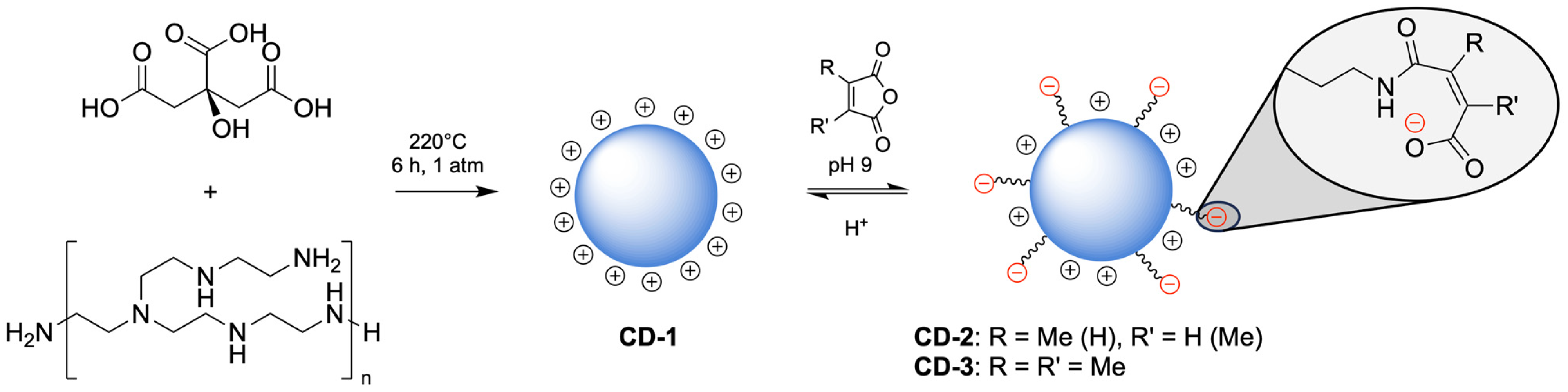
2.2.1. Synthesis of CD1
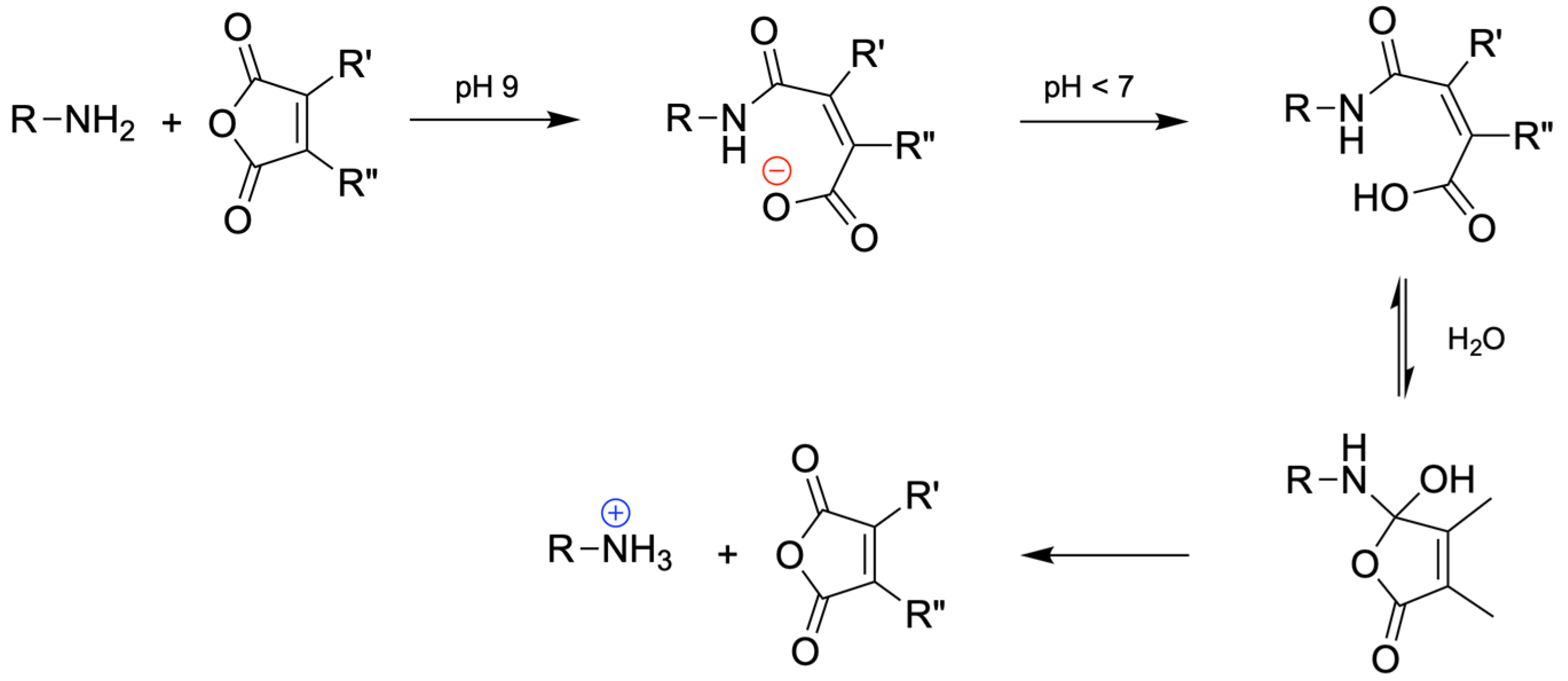
2.2.2. Synthesis of pH-Sensitive CDs
2.3. Nanoparticle Characterization
2.4. Preparation of CD/pDNA Complexes
2.5. pDNA Complexation Assay
2.6. Turbidimetric Measurements
2.7. Mucopenetration Measurements
2.8. Rheological Measurements
2.9. Cell Culture
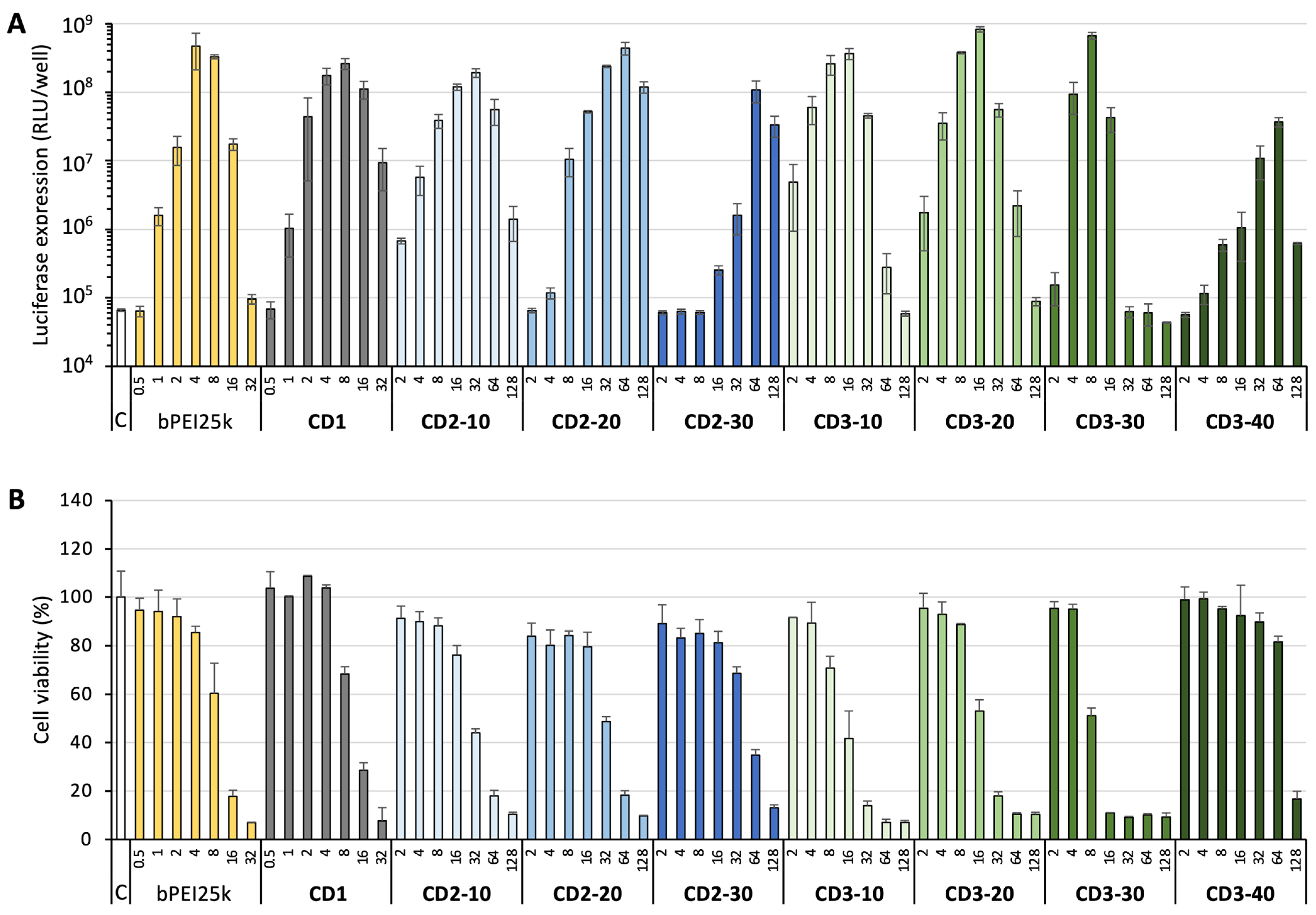
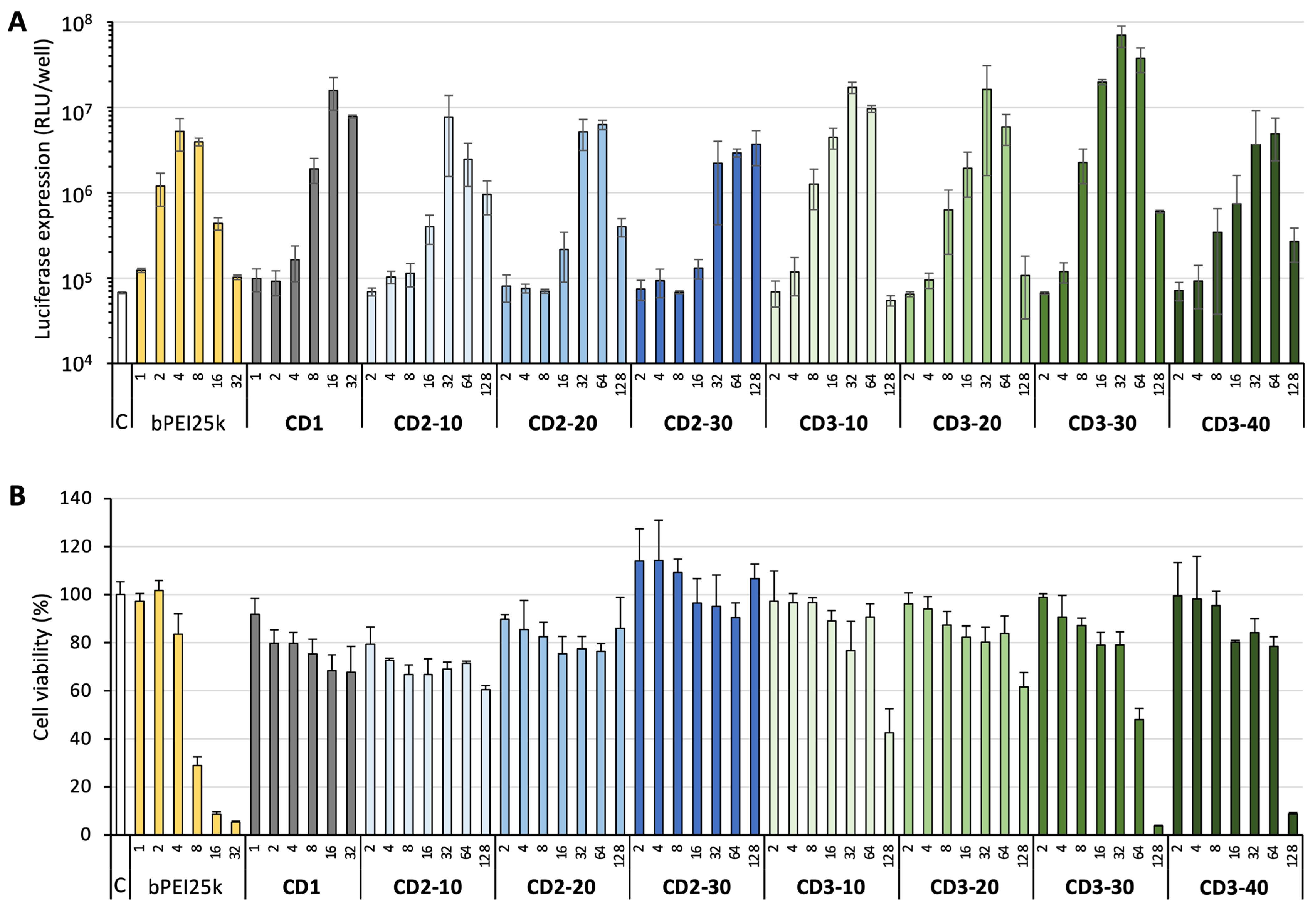
2.10. Transfection Assay
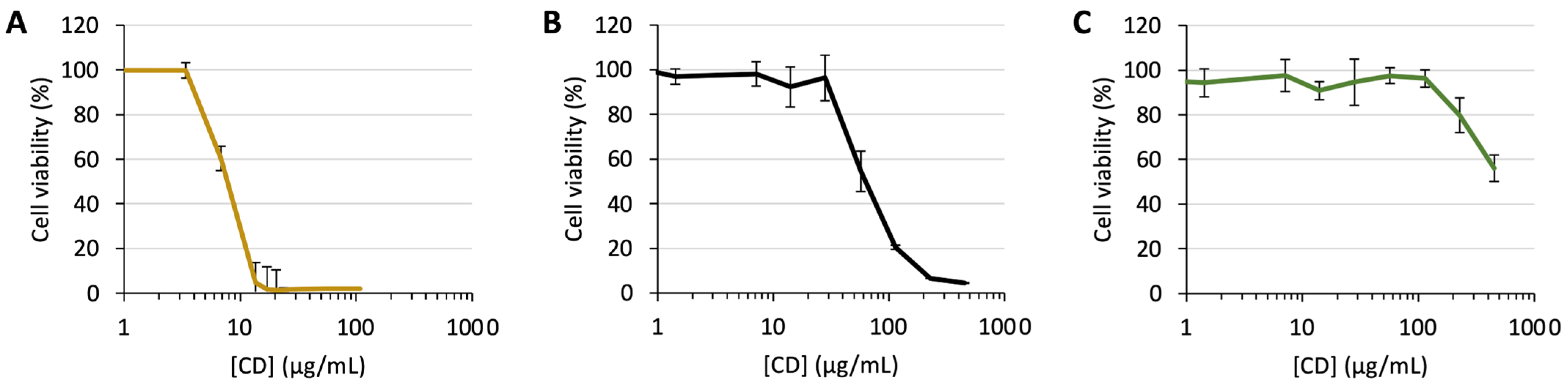
2.11. Cytotoxicity Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Design, Synthesis, and Characterization of CDs
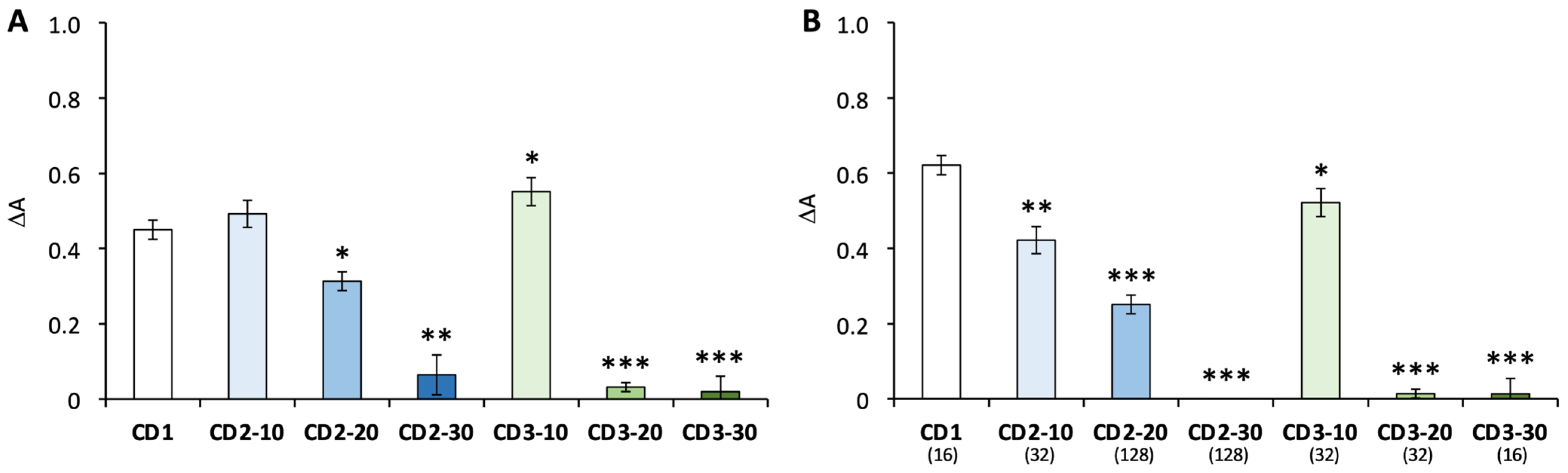
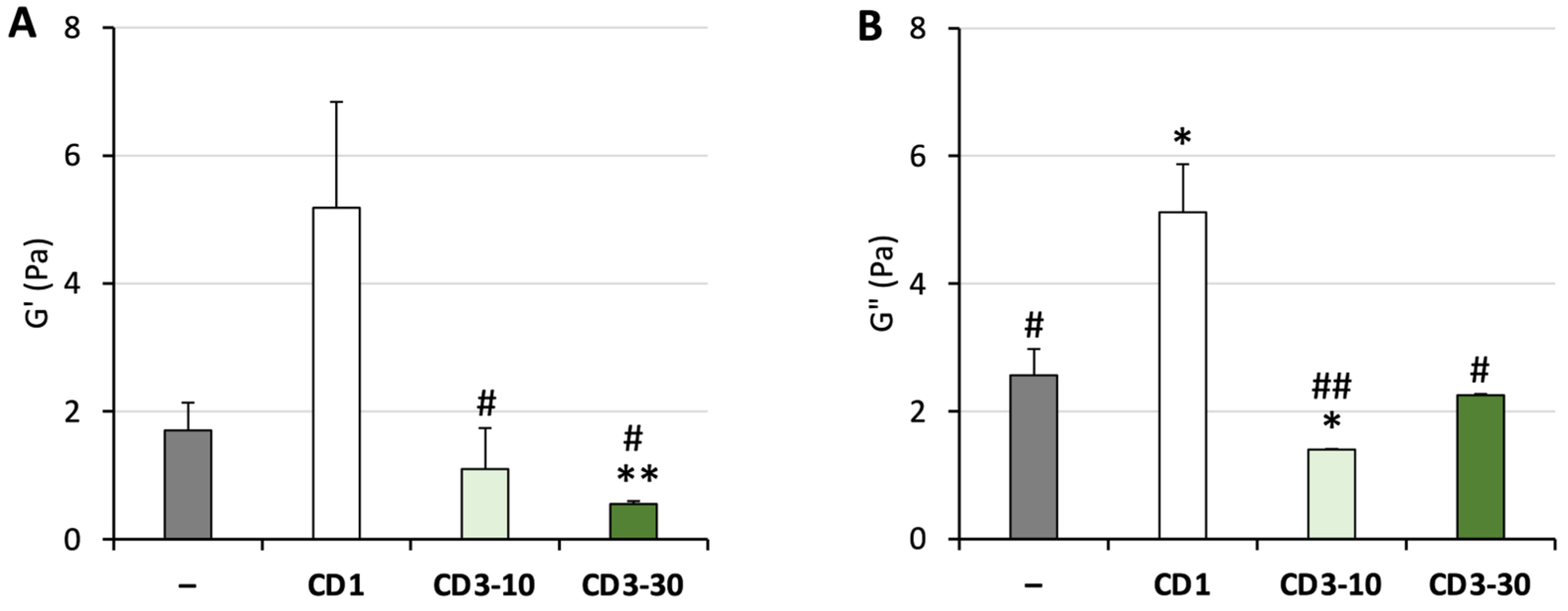
3.2. Nanoparticle–Mucus Interactions
3.3. DNA Delivery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALI | air–liquid interface |
| bPEI | branched polyethyleneimine |
| CDs | carbon dots |
| ctDNA | calf thymus DNA |
| DI | deionized water |
| DLS | dynamic light scattering |
| DMA | dimethyl maleic anhydride |
| DMEM | Dulbecco’s modified Eagle medium |
| DMSO | dimethyl sulfoxide |
| ELS | electrophoretic light scattering |
| FBS | Fetal bovine serum |
| FR | functionalization ratio |
| lPEI | linear polyethyleneimine |
| MMA | methyl maleic anhydride |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| MWCO | molecular weight cut-off |
| NMR | nuclear magnetic resonance |
| NPs | nanoparticles |
| PAA | poly(acrylic acid) |
| PBS | phosphate-buffer saline |
| pDNA | plasmid DNA |
| PEG | polyethylene glycol |
| PES | polyether sulfone |
| PGM | type III mucin from porcine stomach |
| ppm | parts per million |
| rhDNase | recombinant human deoxyribonuclease |
| RI | refractive index |
| SD | standard deviation |
References
- High, K.A.; Roncarolo, M.G. Gene therapy. N. Engl. J. Med. 2019, 381, 455–464. [Google Scholar] [CrossRef]
- Griffiths, G.; Gruenberg, J.; Marsh, M.; Wohlmann, J.; Jones, A.T.; Parton, R.G. Nanoparticle entry into cells: The cell biology weak link. Adv. Drug Deliv. Rev. 2022, 188, 114403. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Le, Y.; Zhang, Z.G.; Nian, X.X.; Liu, B.; Yang, X.M. Viral vector-based gene therapy. Int. J. Mol. Sci. 2023, 24, 7736. [Google Scholar] [CrossRef] [PubMed]
- Zu, H.; Gao, D.C. Non-viral vectors in gene therapy: Recent development, challenges, and prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.; Mozaffarian, M.; Pazuki, G.; Hadidi, N.; Villate-Beitia, I.; Zárate, J.; Puras, G.; Pedraz, J.L. Carbon-Based Nanostructures as emerging materials for gene delivery applications. Pharmaceutics 2024, 16, 288. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy—An overview. J. Clin. Diagn. Res. 2015, 9, GE1–GE6. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef]
- Shogren, R.; Gerken, T.A.; Jentoft, N. Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: Light-scattering-studies of ovine submaxillary mucin. Biochemistry 1989, 28, 5525–5536. [Google Scholar] [CrossRef]
- Kim, N.; Duncan, G.A.; Hanes, J.; Suk, J.S. Barriers to inhaled gene therapy of obstructive lung diseases: A review. J. Control. Release 2016, 240, 465–488. [Google Scholar] [CrossRef]
- Kolb, M.; Martin, G.; Medina, M.; Ask, K.; Gauldie, J. Gene therapy for pulmonary diseases. Chest 2006, 130, 879–884. [Google Scholar] [CrossRef]
- Pickles, R.J. Physical and biological barriers to viral vector-mediated delivery of genes to the airway epithelium. Proc. Am. Thorac. Soc. 2004, 1, 302–308. [Google Scholar] [CrossRef]
- Balsamo, R.; Lanata, L.; Egan, C.G. Mucoactive drugs. Eur. Respir. Rev. 2010, 19, 127–133. [Google Scholar] [CrossRef]
- Ghadiri, M.; Young, P.M.; Traini, D. Strategies to enhance drug absorption via nasal and pulmonary routes. Pharmaceutics 2019, 11, 113. [Google Scholar] [CrossRef]
- Laffleur, F.; Bernkop-Schnurch, A. Strategies for improving mucosal drug delivery. Nanomedicine 2013, 8, 2061–2075. [Google Scholar] [CrossRef]
- Watchorn, J.; Clasky, A.J.; Prakash, G.; Johnston, I.A.E.; Chen, P.Z.; Gu, F.X. Untangling mucosal drug delivery: Engineering, designing, and testing nanoparticles to overcome the mucus barrier. ACS Biomater. Sci. Eng. 2022, 8, 1396–1426. [Google Scholar] [CrossRef]
- Wu, L.; Shan, W.; Zhang, Z.; Huang, Y. Engineering nanomaterials to overcome the mucosal barrier by modulating surface properties. Adv. Drug Deliv. Rev. 2018, 124, 150–163. [Google Scholar] [CrossRef]
- Ensign, L.M.; Schneider, C.; Suk, J.S.; Cone, R.; Hanes, J. Mucus penetrating nanoparticles: Biophysical tool and method of drug and gene delivery. Adv. Mater. 2012, 24, 3887–3894. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.-Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef]
- Schuster, B.S.; Suk, J.S.; Woodworth, G.F.; Hanes, J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials 2013, 34, 3439–3446. [Google Scholar] [CrossRef]
- Suk, J.S.; Lai, S.K.; Wang, Y.-Y.; Ensign, L.M.; Zeitlin, P.L.; Boyle, M.P.; Hanes, J. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials 2009, 30, 2591–2597. [Google Scholar] [CrossRef]
- Kim, A.J.; Boylan, N.J.; Suk, J.S.; Hwangbo, M.; Yu, T.; Schuster, B.S.; Cebotaru, L.; Lesniak, W.G.; Oh, J.S.; Adstamongkonkul, P.; et al. Use of single-site-functionalized PEG dendrons to prepare gene vectors that penetrate human mucus barriers. Angew. Chem. Int. Ed. 2013, 52, 3985–3988. [Google Scholar] [CrossRef]
- Huckaby, J.T.; Lai, S.K. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev. 2018, 124, 125–139. [Google Scholar] [CrossRef]
- Henry, C.E.; Wang, Y.-Y.; Yang, Q.; Hoang, T.; Chattopadhyay, S.; Hoen, T.; Ensign, L.M.; Nunn, K.L.; Schroeder, H.; McCallen, J.; et al. Anti-PEG antibodies alter the mobility and biodistribution of densely PEGylated nanoparticles in mucus. Acta Biomater. 2016, 43, 61–70. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, J.; Liang, J.; Ge, C.; Zhou, Y.; Yin, L.; Ji, Y. Pulmonary RNA interference against acute lung injury me diate d by mucus- and cell-penetrating nanocomplexes. Acta Biomater. 2024, 177, 332–346. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, M.; Cui, Y.; Meng, Y.; Ding, J.; Zeng, W.; Zhou, W. Surface coating of pulmonary siRNA delivery vectors enabling mucus penetration, cell targeting, and intracellular radical ccavenging for enhanced acute lung injury therapy. ACS Appl. Mater. Interfaces 2022, 14, 5090–5100. [Google Scholar] [CrossRef]
- Li, Y.; Liu, R.Y.; Shi, Y.J.; Zhang, Z.Z.; Zhang, X. Zwitterionic poly(carboxybetaine)-based cationic liposomes for effective delivery of small interfering RNA therapeutics without accelerated blood clearance phenomenon. Theranostics 2015, 5, 583–596. [Google Scholar] [CrossRef]
- Peng, H.; Ji, W.H.; Zhao, R.C.; Lu, Z.G.; Yang, J.; Li, Y.; Zhang, X. pH-sensitive zwitterionic polycarboxybetaine as a potential non-viral vector for small interfering RNA delivery. RSC Adv. 2020, 10, 45059–45066. [Google Scholar] [CrossRef]
- Schattling, P.; Taipaleenmaki, E.; Zhang, Y.; Stadler, B. A polymer chemistry point of view on mucoadhesion and mucopenetration. Macromol. Biosci. 2017, 17, 1700060. [Google Scholar] [CrossRef]
- Sun, J.; Zeng, F.; Jian, H.L.; Wu, S.Z. Conjugation with betaine: A facile and effective approach to significant improvement of gene delivery properties of PEI. Biomacromolecules 2013, 14, 728–736. [Google Scholar] [CrossRef]
- Ferrari, S.; Kitson, C.; Farley, R.; Steel, R.; Marriott, C.; Parkins, D.A.; Scarpa, M.; Wainwright, B.; Evans, M.J.; Colledge, W.H.; et al. Mucus altering agents as adjuncts for nonviral gene transfer to airway epithelium. Gene Ther. 2001, 8, 1380–1386. [Google Scholar] [CrossRef]
- Kushwah, R.; Oliver, J.R.; Cao, H.; Hu, J. Nacystelyn enhances adenoviral vector-mediated gene delivery to mouse airways. Gene Ther. 2007, 14, 1243–1248. [Google Scholar] [CrossRef]
- Suk, J.S.; Boylan, N.J.; Trehan, K.; Tang, B.C.; Schneider, C.S.; Lin, J.M.G.; Boyle, M.P.; Zeitlin, P.L.; Lai, S.K.; Cooper, M.J.; et al. N-acetylcysteine enhances cystic fibrosis sputum penetration and airway gene transfer by highly compacted DNA nanoparticles. Mol. Ther. 2011, 19, 1981–1989. [Google Scholar] [CrossRef]
- Majima, Y.; Inagaki, M.; Hirata, K.; Takeuchi, K.; Morishita, A.; Sakakura, Y. The effect of an orally-administered proteolytic-enzyme on the elasticity and viscosity of nasal mucus. Arch. Oto-Rhino-Laryngol. 1988, 244, 355–359. [Google Scholar] [CrossRef]
- Dasgupta, B.; King, M. Reduction in viscoelasticity in cystic fibrosis sputum in vitro using combined treatment with nacystelyn and rhDNase. Pediatr. Pulmonol. 1996, 22, 161–166. [Google Scholar] [CrossRef]
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, L.; Tu, H.; Zhang, H.; Silvester, D.S.; Banks, C.E.; Zou, G.; Hou, H.; Ji, X. The development of carbon dots: From the perspective of materials chemistry. Mater. Today 2021, 51, 188–207. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.F.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, P.; Zhai, X.; Tian, F.; Li, W.; Yang, J.; Liu, Y.; Wang, H.; Wang, W.; Liu, W. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 2012, 33, 3604–3613. [Google Scholar] [CrossRef]
- Biswal, M.R.; Bhatia, S. Carbon Dot Nanoparticles: Exploring the potential use for gene delivery in ophthalmic diseases. Nanomaterials 2021, 11, 935. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Bhamore, J.R.; Koduru, J.R.; Park, T.J. Carbon dots as carriers for the development of controlled drug and gene delivery systems. In Biomedical Applications of Nanoparticles; Grumezescu, A.M., Ed.; William Andrew Publishing: New York, NY, USA, 2019; pp. 295–317. [Google Scholar]
- Zahed, Z.; Hadi, R.; Imanzadeh, G.; Ahmadian, Z.; Shafiei, S.; Zadeh, A.Z.; Karimi, H.; Akbarzadeh, A.; Abbaszadeh, M.; Ghadimi, L.S.; et al. Recent advances in fluorescence nanoparticles “quantum dots” as gene delivery system: A review. Int. J. Biol. Macromol. 2024, 254, 10. [Google Scholar] [CrossRef]
- Kirby, A.J.; Lancaster, P.W. Structure and efficiency in intramolecular and enzymic catalysis. Catalysis of amide hydrolysis by the carboxy-group of substituted maleamic acids. J. Chem. Soc. Perkin Trans. 1972, 2, 1206–1214. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrion, C.; Valcarcel, M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef]
- Mulkerns, N.M.C.; Hoffmann, W.H.; Ramos-Soriano, J.; de la Cruz, N.; Garcia-Millan, T.; Harniman, R.L.; Lindsay, I.D.; Seddon, A.M.; Galan, M.C.; Gersen, H. Measuring the refractive index and sub-nanometre surface functionalisation of nanoparticles in suspension. Nanoscale 2022, 14, 8145–8152. [Google Scholar] [CrossRef]
- Zhao, H. Refractive index dependent optical property of carbon dots integrated luminescent solar concentrators. J. Lumin. 2019, 211, 150–156. [Google Scholar] [CrossRef]
- Weiss, M.; Fan, J.H.; Claudel, M.; Sonntag, T.; Didier, P.; Ronzani, C.; Lebeau, L.; Pons, F. Density of surface charge is a more predictive factor of the toxicity of cationic carbon nanoparticles than zeta potential. J. Nanobiotechnol. 2021, 19, 5. [Google Scholar] [CrossRef]
- Porsio, B.; Craparo, E.F.; Mauro, N.; Giammona, G.; Cavallaro, G. Mucus and cell-penetrating nanoparticles embedded in nano-into-micro formulations for pulmonary delivery of Ivacaftor in patients with cystic fibrosis. ACS Appl. Mater. Interfaces 2018, 10, 165–181. [Google Scholar] [CrossRef]
- Claudel, M.; Fan, J.; Rapp, M.; Pons, F.; Lebeau, L. Influence of carbonization conditions on luminescence and gene delivery properties of nitrogen-doped carbon dots. Rsc Adv. 2019, 9, 3493–3502. [Google Scholar] [CrossRef]
- Pierrat, P.; Wang, R.R.; Kereselidze, D.; Lux, M.; Didier, P.; Kichler, A.; Pons, F.; Lebeau, L. Efficient in vitro and in vivo pulmonary delivery of nucleic acid by carbon dot-based nanocarriers. Biomaterials 2015, 51, 290–302. [Google Scholar] [CrossRef]
- Jiang, Q.; Nie, Y.; Chen, X.; He, Y.; Yue, D.; Gu, Z. pH-Triggered pinpointed cascading charge-conversion and redox-controlled gene release design: Modularized fabrication for nonviral gene transfection. Adv. Funct. Mater. 2017, 27, 1701571. [Google Scholar] [CrossRef]
- Su, S.; Du, F.-S.; Li, Z.-C. Synthesis and pH-dependent hydrolysis profiles of mono- and dialkyl substituted maleamic acids. Org. Biomol. Chem. 2017, 15, 8384–8392. [Google Scholar] [CrossRef]
- Vermeulen, L.M.P.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. The proton sponge hypothesis: Fable or fact? Eur. J. Pharm. Biopharm. 2018, 129, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Conese, M.; Hoekstra, D. Gene transfer by means of lipo- and polyplexes: Role of clathrin and caveolae-mediated endocytosis. J. Liposome Res. 2006, 16, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Arezki, Y.; Rapp, M.; Lebeau, L.; Ronzani, C.; Pons, F. Cationic carbon nanoparticles induce inflammasome-dependent pyroptosis in macrophages via lysosomal dysfunction. Front. Toxicol. 2022, 4, 11. [Google Scholar] [CrossRef]
- Sonntag, T.; Rapp, M.; Didier, P.; Lebeau, L.; Pons, F.; Casset, A. Mucus-producing epithelial models for investigating the activity of gene delivery systems in the lung. Int. J. Pharm. 2022, 614, 121423. [Google Scholar] [CrossRef]
- Arca, S.; Pons, F.; Lebeau, L. Engineered carbon dots for mucosalgene delivery. Eur. J. Pharm. Sci. 2025, 213, 107222. [Google Scholar] [CrossRef]



| NPs | FR * (mmol/g) | Size (nm) | ζ (mV) | Qek (mmol/g) | λmax (nm) | λex (nm) | λem (nm) |
|---|---|---|---|---|---|---|---|
| CD1 | na | 10.4 ± 0.2 | +22.4 ± 0.6 | 3.8 | 340 | 350 | 460 |
| CD2-10 | 0.80 | 5.0 ± 0.4 | +17.2 ± 4.1 | 2.7 | 345 | 360 | 460 |
| CD2-20 | 1.34 | 4.5 ± 0.4 | +10.2 ± 2.6 | 1.7 | 345 | 360 | 460 |
| CD2-30 | 2.01 | 4.6 ± 0.3 | +2.6 ± 0.5 | 0.1 | 345 | 360 | 460 |
| CD3-10 | 0.57 | 3.9 ± 0.7 | +18.4 ± 0.6 | 3.5 | 345 | 360 | 460 |
| CD3-20 | 1.13 | 4.5 ± 0.4 | +10.1 ± 0.6 | 3.3 | 345 | 360 | 460 |
| CD3-30 | 1.91 | 6.1 ± 0.5 | +7.5 ± 0.5 | 2.4 | 345 | 360 | 460 |
| CD3-40 | nd | 8.1 ± 0.7 | +7.0 ± 0.2 | 2.2 | 345 | 360 | 460 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arca, S.; Witjaksono, C.; Pons, F.; Lebeau, L. Carbon Dots with Tunable Charge as Mucus-Penetrating Gene Carriers. Pharmaceutics 2025, 17, 1330. https://doi.org/10.3390/pharmaceutics17101330
Arca S, Witjaksono C, Pons F, Lebeau L. Carbon Dots with Tunable Charge as Mucus-Penetrating Gene Carriers. Pharmaceutics. 2025; 17(10):1330. https://doi.org/10.3390/pharmaceutics17101330
Chicago/Turabian StyleArca, Samuel, Clea Witjaksono, Françoise Pons, and Luc Lebeau. 2025. "Carbon Dots with Tunable Charge as Mucus-Penetrating Gene Carriers" Pharmaceutics 17, no. 10: 1330. https://doi.org/10.3390/pharmaceutics17101330
APA StyleArca, S., Witjaksono, C., Pons, F., & Lebeau, L. (2025). Carbon Dots with Tunable Charge as Mucus-Penetrating Gene Carriers. Pharmaceutics, 17(10), 1330. https://doi.org/10.3390/pharmaceutics17101330







