Abstract
This integrative review addresses the potential of the Endocannabinoid System (ES) and cannabinoids in the pathogenesis and treatment of periodontal disease (PD). Cannabinoid receptors are expressed in healthy and inflamed periodontal tissues, indicating a potential regulatory role for SEC in oral homeostasis. Healthy periodontal cells express more CB1 receptors, while inflamed sites show increased CB2 receptors. This suggests a dynamic involvement of the SEC in the inflammatory response associated with PD. Cannabinoids such as cannabidiol (CBD) and cannabinoid receptor agonists such as HU-308, anandamide (AEA), and methanamide (Meta-AEA) have demonstrated promising therapeutic potential in studies. CBD has been associated with the control of bone resorption, antibacterial activity, and increased production of gingival fibroblasts, indicating effects in mitigating the progression of PD. HU-308 demonstrated preventive effects against alveolar bone loss, and anti-inflammatory, osteoprotective, and pro-homeostatic properties in animal models of periodontitis. AEA and Meta-AEA have anti-inflammatory effects by reducing pro-inflammatory mediators such as IL-1, IL-6, and TNF-α. The activation of cannabinoid receptors attenuates inflammatory processes, inhibits alveolar bone loss, exerts antibacterial effects, and promotes tissue repair. However, clinical trials are especially needed to validate these results and explore the therapeutic potential of cannabinoids in the treatment of PD in humans.
1. Introduction
Periodontal disease (PD) is a chronic inflammatory condition that compromises the teeth’s supporting tissues. This occurs primarily through the immunoinflammatory responses that are triggered by interactions between pathogenic bacteria and host-derived mediators, and contribute to the exacerbation of inflammation and subsequent tissue destruction [1,2]. Some biological systems, i.e., the Endocannabinoid System (ECS), are potential candidates for controlling PD through the modulation of conditions such as pain, inflammation, antimicrobial properties, and tissue repair. This system is a complex signaling network that plays a crucial role in regulating various physiological processes, including immune response, pain modulation, and bone metabolism [3,4]. It is composed of endogenous cannabinoids (endocannabinoids), cannabinoid receptors, and enzymes responsible for the synthesis and degradation of endocannabinoids [5]. They have roles in the cellular activities of osteoblasts, osteocytes, osteoclasts, and gingival fibroblasts which are important for oral homeostasis, as they assist in tissue remodeling [6]. In addition, this system, in isolation or when associated with different types of cannabinoids, has also been purported to hold therapeutic potential for periodontal diseases [6,7].
In general, conventional treatment for PD includes oral hygiene guidance, combined with scaling and root planning that may or may not be associated with adjunctive therapies, such as antimicrobial photodynamic therapy [8,9,10]. It may also require surgical intervention to reduce the depth of periodontal pockets [11]. Furthermore, the local or systemic administration of cannabinoids (substances that bind to Endocannabinoid System (ECS) receptors) has been reported to influence disease control by reducing the expression of inflammatory mediators such as IL-6 and TNF-α [12,13]. CB1 and CB2 receptors are proteins found on types of cells such as B and T lymphocytes, natural killer (NK) cells, monocytes, neutrophils, CD8+ white blood cells, and CD4+ white blood cells. This means that both natural and external cannabinoids have the ability to directly influence how the immune system works by affecting processes like cytokine release, cell growth, and enzyme activation, in effector cells [4].
In this context, cannabinoids comprise a diverse group of molecules that can be endogenous (anandamide (AEA), 2-arachidonylglycerol (2-AG), and Npalmitoylethanolamide (PEA)), synthetic (methanandamide—Meta-AEA- and HU-308) or derived from plants (phytocannabinoids; Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), cannabichromene (CBC), cannabinol (CBN), among others) [14]. These substances may contribute to the pathogenesis and healing of periodontal tissues, as an increase in the expression of cannabinoid receptors has been shown at diseased periodontal sites, as well as a decrease in inflammatory mediators and greater cell proliferation, following exposure to CBD and AEA, for example [15,16]. Due to their potential immunomodulatory, antibacterial, and regenerative effects, cannabinoids may offer advantages in the treatment of periodontal disease and have been suggested as an adjuvant therapeutic alternative to complement other established therapies [4]. Their effects could potentially augment the benefits already obtained through conventional treatments.
Some studies have investigated the relationship between the ECS, the cannabinoids themselves, and periodontal disease [7,17,18]. Most of these are in vitro studies and preclinical trials that (samples of human tissues/cells or other animals) identify the possible contributions of this system to the progression and/or management of inflamed periodontal tissues, although its role is not clear [3,15,19]. Therefore, the aim of the present study was to evaluate, through an integrative review of in vivo and in vitro studies, the role of ECS in the pathogenesis of PD and to determine the current evidence justifying or recommending the use of cannabinoids in periodontal therapy.
2. Materials and Methods
This integrative literature review aimed to identify the role of ECS in the pathogenesis and therapy of PD in in vitro and in vivo studies. For this purpose, electronic searches were performed in PubMed, Embase, Scopus, Web of Science, and Lilacs databases up to December 2023, using descriptors and terms controlled by MeSH (Medical Subject Headings) and DeCS (Descriptors in Health Sciences), as shown in Table 1.

Table 1.
Search strategy.
2.1. Eligibility Criteria
Due to the diversity among the studies, an adaptation of the PICOS strategy was employed to consider the eligibility of studies using P, I, and O:
- P: Population (bacteria, cells, and other structures inherent in diseased and/or healthy periodontal tissue—from humans, mice, or rabbits)
- I: Intervention (natural, endogenous, and/or synthetic cannabinoids)
- O: Outcome (anti-inflammatory, antibacterial, or repair-related effects and prevention of soft and hard tissue damage).
2.2. Inclusion Criteria
Only in vitro and in vivo studies that evaluated the role of the ECS in tissues, cells, or microorganisms inherent to the periodontium were considered. Restrictions on language, publication time, and geographical scope were not employed.
2.3. Exclusion Criteria
Descriptive studies, such as literature reviews, case reports, clinical trials, projects/protocols, opinion articles, letters, posters, and conference abstracts were excluded.
2.4. Data Extraction and Analysis
For the selection of studies, the Rayyan Qatar Computing Research Institute (QCRI) application was used in two phases. In phase one, duplicate articles were identified, and titles and abstracts were independently screened to exclude studies irrelevant to this review. In phase two, the texts were fully read, applying the previously established inclusion and exclusion criteria. The data collected were as follows: (1) Author and year of publication; (2) Type of study; (3) Intervention; (4) Objective; (5) Cannabinoid dose/administration; (6) Results (main outcomes of the articles related to anti-inflammatory, antibacterial, and tissue repair response); (7) Conclusion.
3. Results
3.1. Selection of Studies
After an electronic search in five databases (PubMed, Web of Science, Scopus, Embase, and Lilacs), 270 articles were retrieved, and after the removal of duplicates, 196 remained for us to read the title and abstract. Of these, 21 were included based on the eligibility criteria (Figure 1).
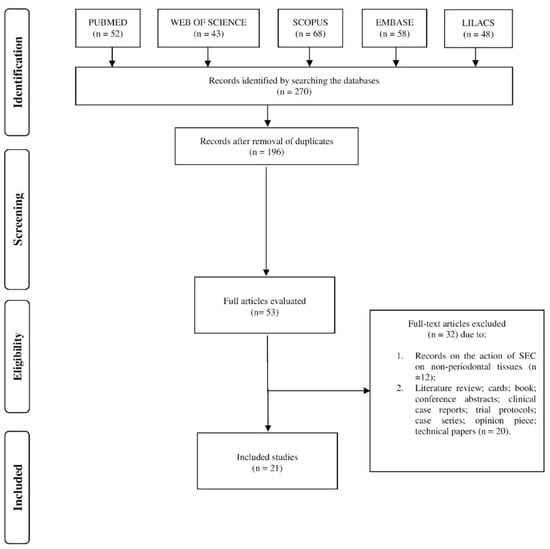
Figure 1.
Flow diagram of the selected studies.
3.2. Characteristics of the Studies
The included studies were published between 2005 and 2022. The majority, eleven (52%), had been published in the last 5 years; three (15%) in the last 10 years, and seven (33%) more than 10 years ago. Regarding the study design, fifteen (71%) articles presented in vitro methodologies; four (19%) in vivo, and two (10%) had both methodologies (Table 1).
With regard to the therapeutic potential of ECS and its ligands in periodontal disease, 10 studies investigated the anti-inflammatory performance of different types and representatives of cannabinoids in structures inherent to the periodontal tissue of humans and mice [3,6,12,15,19,20,21,22,23,24]. Another three studies evaluated their antibacterial activity [21,25,26]; six evaluated the tissue capacity of repair/cell proliferation [15,19,23,27,28,29]; and another six studies investigated the role of these substances in preventing alveolar bone resorption [3,6,13,22,24,30].
Regarding the participation of this system in the pathogenesis of periodontal disease, five studies evaluated the expression of CB1 and CB2 receptors in healthy or diseased periodontal tissue samples [16,17,27,31,32]. Considering the types of cannabinoids used in the studies, at least 13 different types were observed. The most studied were the endocannabinoid, anandamide [12,15,20,23,24,27,32,33], the phytocannabinoid CBD [12,15,20,23,24,27,32], and the synthetic cannabinoid, HU-308 [3,12,13]. The chemical structure and effects of these molecules on the periodontium are illustrated in Figure 2. Figure 3 illustrates the possible mechanisms on the effects of cannabinoids and their derivatives on periodontal disease.
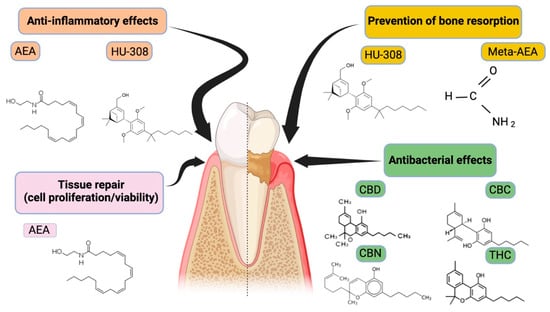
Figure 2.
Representation of the main cannabinoid molecules and their respective effects on periodontal disease. Cannabinoids comprise a diverse group of molecules that can be endogenous (anandamide (AEA), synthetic (methanandamide (Meta-AEA), and CB2-specific agonist (HU-308)) or derived from plants (phytocannabinoids; Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), cannabichromene (CBC), cannabinol (CBN)).
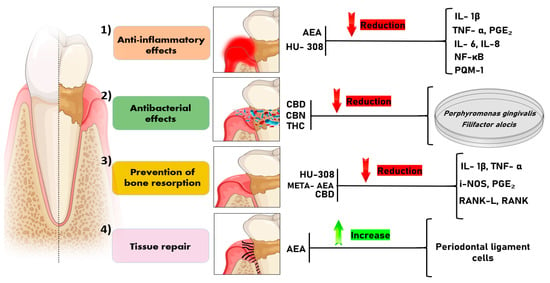
Figure 3.
Illustrates the possible mechanisms described in the literature on the effects of cannabinoids and their derivatives on periodontal disease. (1) Anti-inflammatory effect: AEA and HU-308 act to reduce the levels of cytokines involved in the inflammatory cascade (IL-1β, TNF-α, PGE₂, IL-6, IL-8, NF-κB, MCP-1). (2) Antibacterial effect: CBD, CBN, and THC have an inhibitory effect on the growth of periodontopathogenic bacteria, such as Porphyromonas gingivalis and Filifactor alocis. (3) Prevention of bone resorption: HU-308, META-AEA, and CBD demonstrate the ability to reduce alveolar bone loss by reducing levels of mediators (IL-1β, TNF-α, iNOS, PGE₂, RANKL, RANK) that induce bone resorption. (4) Tissue repair: AEA demonstrates an increase in tissue repair capacity due to its action on the proliferation of periodontal ligament cells. Legend: AEA: anandamide; HU-308: CB2-specific agonist; CBD: Cannabidiol; CBN: Cannabinol; THC: Δ9-Tetrahydrocannabinol; META-AEA: methanandamide; IL-1β: interleukin-1β; TNF-α: tumor necrosis factor alpha; PGE₂: Prostaglandin E2; IL-6: interleukin 6; IL-8: interleukin 8; NF-κB: Nuclear Factor Kappa-light-chain-enhancer of Activated B Cells; MCP-1: Monocyte Chemoattractant Protein-1; iNOS: Inducible Nitric Oxide Synthase; RANK: Receptor Activator of Nuclear Factor Kappa B; RANKL: Receptor Activator of Nuclear Factor Kappa B Ligand.
3.3. Prevention of Bone Resorption by Cannabinoids
Among the six studies that evaluated the prevention of bone resorption, four used synthetic cannabinoids, including HU-308 [3,13,30] and Meta-AEA [6]. HU-308, employed in in vivo studies, significantly reduced alveolar bone loss in animals subjected in parallel to bacterial LPS (4.52 ± 0.19 mm) stimulation, compared with animals receiving LPS alone (5.22 ± 0.14 mm) [13], and similar results were found for Meta-AEA [6]. The only study that investigated the role of a phytocannabinoid concluded that rats subjected to experimental periodontitis and systemic CBD injection showed less bone loss in the furcation region (0.5 mm2), compared with those treated with saline (0.9 mm2) [22].
3.4. Anti-/Pro-Inflammatory Effects of Cannabinoids
Of the studies that investigated the anti-inflammatory efficacy of cannabinoids, seven investigated the performance of synthetic cannabinoids, especially HU-308 [3,12,13,20,30] and Meta-AEA [6,19]. Five studies evaluated the effects of the endocannabinoid AEA [12,15,20,23,24] while two investigated the phytocannabinoids, especially CBD [21,22]. CBD administered in animals subjected to experimental periodontitis [22] and in cells stimulated by LPS from P. gingivalis [21] reduced the levels of cytokines such as RANK, RANKL, TNF- α, IL-1β, IL-6, IL-12 p40, and IL-8 [21,22]. The synthetic cannabinoid Meta-AEA, when applied to gingival tissue samples from mouse [6] and human periodontal ligament cells [19] exposed to bacterial LPS, significantly reduced TNF-α, PGE2 [6], IL-6, IL-8, and MCP-1 levels [19]. Similar results were found for HU-308, which demonstrated efficacy mainly in reducing IL-6, TNF-α, and IL-1β in inflamed periodontal tissues [3,12,20]. In contrast, the injection of anandamide in rats with experimental periodontitis significantly reduced TNF-α and IL-1β levels compared with animals treated with saline and the antagonists, AM251 and AM630 [24].
3.5. Tissue Repair (Cell Proliferation/Viability) by Cannabinoids
Those studies that analyzed tissue repair capacity (as assessed by cell proliferation or molecule expression) mainly investigated the effects of the endocannabinoid AEA [15,19,27]. In human periodontal ligament cells not exposed to bacterial LPS, anandamide had no significant effect on the proliferation/viability of these cells, while in those submitted to LPS, AEA (10–20 µM) induced a significant increase in proliferation/viability [23]. In addition, the phytocannabinoid, CBD, at low concentrations (0.01–0.05 µM), increased (by 40%) the production of transforming growth factor β (TGF-β), an important cytokine that controls cell proliferation and differentiation [28]. This relationship between receptors and periodontal tissue is illustrated in Figure 4.
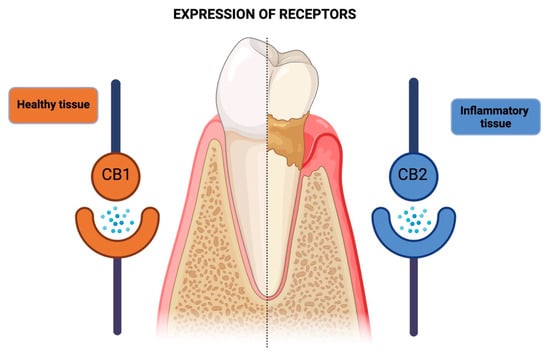
Figure 4.
Schematic representation of the differential expression of cannabinoid receptors (CB1 and CB2) in healthy and diseased periodontal tissues. In healthy periodontal tissues, CB1 receptor expression predominates, while inflamed tissues exhibit a higher tendency for CB2 receptor expression. This shift in receptor expression suggests a potential regulatory role of the Endocannabinoid System in the pathogenesis and progression of periodontal disease.
3.6. Antibacterial Effects of Cannabinoids
With regard to the antibacterial activities of cannabinoids, only the phytocannabinoids have been studied, especially CBD, CBC, CBN, and THC [21,25]. In addition, one study investigated the effect of CBD and CBG when infused in mouthwashes [25]. Exposure of the oral biofilm to the phytocannabinoid, CBD (12.5%), resulted in lower bacterial colony counts in samples taken from patients with gingivitis (mean colony count (MCC) = 5), calculus-associated gingivitis (MCC = 4.9), and from patients with severe PD (MCC = 1.5); these values differed from those of samples from patients with severe periodontitis submitted to Oral B (MCC = 29.8). Meanwhile, CBD (12.5%) administration, especially in patients with periodontitis (CMC = 3.1), also lowered bacterial growth averages, compared with those presented by groups treated with Oral B (CMC = 27.3) or Colgate (CMC = 27.7) [25]. The direct effects of CBD, CBN, and THC on P. gingivalis were also investigated. CBD, at concentrations of 5.0 and 10 µg/mL, significantly inhibited bacterial logarithmic growth up to 38 h after exposure, and similar results were observed for CBN and THC [21]. On the other hand, CBD and CBG, when infused in mouthwashes, showed a similar inhibition of bacterial growth to that of 0.2% chlorhexidine, and greater inhibition compared with the inhibition resulting from alcohol-based rinses with essential oils such as thymol, eucalyptol, and menthol (Product A) or fluoride- and potassium nitrate-based products (Product B) [26].
3.7. Expression of Receptors (CB1 and CB2)
The expressions of CB1 and CB2 receptors were analyzed to assess the role of the Endocannabinoid System in the pathogenesis of periodontal disease in five studies [21,25]. Compared with CB1, there was a greater tendency towards CB2 expression in healthy [17], inflammatory [16,27,32,33], and healing conditions [31]. Additionally, human gingival fibroblasts exposed to P. gingivalis LPS showed a marked expression of CB1 and CB2 messenger RNAs [32]. However, in healthy cells and tissues, CB1 levels were higher compared with CB2 [31,32] although the expression of this receptor was lower or absent in some studies [17,27].
The materials and data for this study are openly available on the Open Science Framework (OSF): https://doi.org/10.17605/OSF.IO/GFDZ2.
The studies summaries are included in Table 2.

Table 2.
Summary of included studies.
4. Discussion
The pathogenesis and therapeutics of PD are still being studied, with the aim of making its prevention and management more efficient. Currently, there are a variety of cannabinoid-based oral products on the market that promise analgesic, anti-inflammatory, and antibacterial efficacy; such products include chewing gums, dentifrices, oils, capsules, sprays, and mouthwashes [34]. However, no evidence or clinical studies are available that directly involve the use of these products for the prevention or treatment of this condition. Thus, this integrative review of preclinical studies investigated the role of the ECS and different types of cannabinoids in the pathogenesis and therapy of periodontal disease.
Since 2006, in vitro and in vivo studies have been investigating the performance of natural, synthetic, and endogenous cannabinoids, with regard to their anti-inflammatory, antibacterial properties, participation in tissue repair, and prevention of alveolar bone loss, in relation to periodontal disease [13,15,16,21,32]. Through this review, the role of cannabinoids in the prevention of alveolar bone resorption and the modulation of inflammatory responses, tissue repair, and antimicrobial effects was synthesized. The authors identified the potential of endogenous, synthetic, and natural cannabinoids in reducing inflammatory processes, exhibiting antibacterial activity, reducing alveolar bone loss, and promoting tissue repair in both in vitro and in vivo studies. However, none of the included studies directly investigated the analgesic potential of different types of cannabinoids on periodontal tissues. This may be justified by the profile of the studies (mostly in vitro), but is also due to the characteristics of periodontal disease, as the patient usually does not manifest pain, except in advanced and/or acute stages [35].
Proportionally, most studies have investigated the anti-inflammatory capacity of synthetic and endogenous cannabinoids and, to a lesser extent, phytocannabinoids. The findings reveal that the exposure of rat or human periodontal tissue/cells to these substances significantly reduces the expressions of cytokines relevant to periodontal destruction, such as TNF-α and IL-1β [3,12,20,24]. The importance of these findings is supported by evidence, for example, that TNF-α participates in alveolar bone resorption through different pathways, such as osteoclast differentiation and maturation [36]. IL-1β increases the expression of collagenolytic enzymes and matrix metalloproteinases (MMPs), contributing to the degradation of the extracellular matrix and, in turn, leading to bone resorption and tissue destruction [37]. As such, these anti-inflammatory findings align with the observed decrease in alveolar bone loss observed in animals that underwent cannabinoid treatment for experimental periodontitis [6,13,22].
With regard to the healing, repair, and regeneration of periodontal tissues, the effects of endogenous and synthetic cannabinoids on the proliferation and cell viability of human gingival fibroblasts and periodontal ligament cells [15,23,27] were mainly investigated. This may represent an important indicator, as gingival fibroblasts participate in tissue repair and remodeling, after procedures such as scaling and root planning and periodontal surgery. Furthermore, in general, periodontal ligament cells constitute the supporting tissue of the teeth in the alveolus, participate in the sensory function due to their abundant innervation, contribute to the dissemination of occlusal forces to the supporting bone, and favor cell formation and nutrition of bone, cementum, and gingiva [27].
The role of CBD in the therapy of several diseases (rheumatoid arthritis, multiple sclerosis, Alzheimer’s disease, anxiety disorders) is consolidated in the medical literature due to its analgesic, antitumor, anti-inflammatory, and central nervous system effects, among others [38]. However, only 5 of the 21 studies included in this review studied this phytocannabinoid with regard to its effects on the control of bone resorption and repair [16,22], antibacterial activity [21,25,26], and cell proliferation [28]. Traditionally, the main objective when studying the management of PD is to reduce the microbial load, especially periodontopathogens. Phytocannabinoids (CBD and THC) have already shown efficacy against gram-positive microorganisms such as Staphylococcus aureus [39] and, similarly, CBD reduced the growth of some gram-positive and negative microorganisms (P. gingivalis and Filifactor alocis) [19]. In addition, phytocannabinoids (isolated or infused in mouthwashes) also demonstrated antimicrobial activity that was superior or equal to that of traditional oral products such as 0.2% chlorhexidine and dentifrices, suggesting that these substances hold antibacterial potential [25,26].
With regard to the expressions of CB1 and CB2, there was no consensus among the studies, and differences were observed in the expressions of these receptors according to the characteristics of periodontal tissues [17,31]. This may be related to the types of stimuli examined, such as experimental periodontitis [27] and mechanical stress [31] or due to differences in cells and tissues, as some studies evaluated gingival tissue from rats [17,27] and humans [31], as well as gingival fibroblasts [16,32] and periodontal ligament cells from humans [31].
Despite the potential role of cannabinoids in preventing alveolar bone resorption, modulating inflammatory responses, promoting tissue repair, and exerting antimicrobial effects, some disadvantages should be considered. The long-term effects of cannabinoids on periodontal tissues remain unknown, and predicting their effectiveness and appropriate dosage is challenging due to the close relationship between the actions of these substances and the individual’s ECS and immune system. Moreover, the lack of standardization in dosing inherent to cannabinoid-based products may lead to inconsistent therapeutic outcomes [4].
The clinical relevance of these findings suggests potential improvements in periodontal clinical parameters, which may prevent, reduce, or limit the destruction of periodontal tissues inherent in the immune-inflammatory response of periodontal disease. However, although in vitro and in vivo studies provide contributions to the development of clinical trials, the limitations of these trials include the irreproducibility of the interaction between the products and substances tested with the human host, since the tests are restricted to inoculation in groups of cells isolated from humans or other animals and to cultivation in tubes or glass plates, for example. Finally, other limitations exist, such as the lack of standardization of studies, techniques, and analyses, which could provide greater data replicability, as well as the fact that laboratory conditions do not always occur simultaneously with real conditions. Further randomized and controlled clinical studies are needed to confirm the safety, tolerability, toxicity, efficacy, and optimal dosages of these compounds.
5. Conclusions
Preclinical studies evidence the positive role of the Endocannabinoid System (ECS) and different types of cannabinoids—such as endogenous (anandamide (AEA)), synthetic (methanandamide (Meta-AEA), and HU-308), and natural (Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD))—in the pathogenesis and therapeutics of conditions affecting oral tissues and cells. Notable contributions of this system and its ligands include the potential for preventing bone resorption, anti-inflammatory effects, tissue repair capabilities, and antimicrobial effects. However, future applications must consider limitations, such as the safety of natural, synthetic, and/or endogenous cannabinoids and their products, optimal dosages, and interactions between products, which need to be verified for the treatment of periodontal disease.
Author Contributions
Conceptualization, J.C.M.V. and B.C.d.V.G.; validation, G.E.d.S.G., F.J.D.O., L.N.M.d.A. and G.T.; resources, G.T. and C.F.M.; data curation, G.E.d.S.G., F.J.D.O., L.N.M.d.A., G.T. and B.C.d.V.G.; preparation of original draft, J.C.M.V., G.E.d.S.G., F.J.D.O., L.N.M.d.A., G.T., C.F.M. and B.C.d.V.G.; review and editing of draft, C.F.M. and B.C.d.V.G.; supervision, C.F.M. and B.C.d.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Balta, M.G.; Papathanasiou, E.; Blix, I.J.; Van Dyke, T.E. Host Modulation and Treatment of Periodontal Disease. J. Dent. Res. 2021, 100, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, X.; Wang, D.; Zheng, J.; Chen, L.; Xie, Q.; Liu, X.; Niu, S.; Qu, G.; Lan, J.; et al. Periodontal Inflammation-Triggered by Periodontal Ligament Stem Cell Pyroptosis Exacerbates Periodontitis. Front. Cell Dev. Biol. 2021, 9, 663037. [Google Scholar] [CrossRef] [PubMed]
- Ossola, C.A.; Surkin, P.N.; Mohn, C.E.; Elverdin, J.C.; Fernández-Solari, J. Anti-Inflammatory and Osteoprotective Effects of Cannabinoid-2 Receptor Agonist HU-308 in a Rat Model of Lipopolysaccharide-Induced Periodontitis. J. Periodontol. 2016, 87, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Carmona Rendón, Y.; Garzón, H.S.; Bueno-Silva, B.; Arce, R.M.; Suárez, L.J. Cannabinoids in Periodontology: Where Are We Now? Antibiotics 2023, 12, 1687. [Google Scholar] [CrossRef] [PubMed]
- David, C.; Elizalde-Hernández, A.; Barboza, A.S.; Cardoso, G.C.; Santos, M.B.F.; Moraes, R.R. Cannabidiol in Dentistry: A Scoping Review. Dent. J. 2022, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Ossola, C.A.; Surkin, P.N.; Pugnaloni, A.; Mohn, C.E.; Elverdin, J.C.; Fernandez-Solari, J. Long-term treatment with methanandamide attenuates LPS-induced periodontitis in rats. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2012, 61, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Abidi, A.H.; Abhyankar, V.; Alghamdi, S.S.; Tipton, D.A.; Dabbous, M. Phytocannabinoids regulate inflammation in IL-1β-stimulated human gingival fibroblasts. J. Periodontal Res. 2022, 57, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- de Melo Soares, M.S.; D’Almeida Borges, C.; de Mendonça Invernici, M.; Frantz, F.G.; de Figueiredo, L.C.; de Souza, S.L.S.; Taba, M.; Messora, M.R.; Novaes, A.B. Antimicrobial photodynamic therapy as adjunct to non-surgical periodontal treatment in smokers: A randomized clinical trial. Clin. Oral Investig. 2019, 23, 3173–3182. [Google Scholar] [CrossRef]
- Deutscher, H.; Derman, S.; Barbe, A.G.; Seemann, R.; Noack, M.J. The effect of professional tooth cleaning or non-surgical periodontal therapy on oral halitosis in patients with periodontal diseases. A systematic review. Int. J. Dent. Hyg. 2018, 16, 36–47. [Google Scholar] [CrossRef]
- Yan, Y.; Zhan, Y.; Wang, X.; Hou, J. Clinical evaluation of ultrasonic subgingival debridement versus ultrasonic subgingival scaling combined with manual root planing in the treatment of periodontitis: Study protocol for a randomized controlled trial. Trials 2020, 21, 113. [Google Scholar] [CrossRef]
- Graziani, F.; Karapetsa, D.; Mardas, N.; Leow, N.; Donos, N. Surgical treatment of the residual periodontal pocket. Periodontol. 2000 2018, 76, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Abidi, A.H.; Alghamdi, S.S.; Dabbous, M.K.; Tipton, D.A.; Mustafa, S.M.; Moore, B.M. Cannabinoid type-2 receptor agonist, inverse agonist, and anandamide regulation of inflammatory responses in IL-1β stimulated primary human periodontal ligament fibroblasts. J. Periodontal Res. 2020, 55, 762–783. [Google Scholar] [CrossRef] [PubMed]
- Ossola, C.A.; Rodas, J.A.; Balcarcel, N.B.; Astrauskas, J.I.; Elverdin, J.C.; Fernández-Solari, J. Signs of alveolar bone damage in early stages of periodontitis and its prevention by stimulation of cannabinoid receptor 2. Model in rats. Acta Odontol. Latinoam. 2020, 33, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W.; Nedamat, K. The Current and Potential Application of Medicinal Cannabis Products in Dentistry. Dent. J. 2021, 9, 106. [Google Scholar] [CrossRef]
- Jäger, A.; Setiawan, M.; Beins, E.; Schmidt-Wolf, I.; Konermann, A. Analogous modulation of inflammatory responses by the endocannabinoid system in periodontal ligament cells and microglia. Head Face Med. 2020, 16, 26. [Google Scholar] [CrossRef]
- Navarro-Saiz, L.M.; Bernal-Cepeda, L.J.; Castellanos, J.E. Immune challenges upregulate the expression of cannabinoid receptors in cultured human odontoblasts and gingival fibroblasts. Acta Odontol. Latinoam. 2022, 35, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qi, X.; Alhabeil, J.; Lu, H.; Zhou, Z. Activation of cannabinoid receptors promote periodontal cell adhesion and migration. J. Clin. Periodontol. 2019, 46, 1264–1272. [Google Scholar] [CrossRef]
- Pellegrini, G.; Carmagnola, D.; Toma, M.; Rasperini, G.; Orioli, M.; Dellavia, C. Involvement of the endocannabinoid system in current and recurrent periodontitis: A human study. J. Periodontal Res. 2023, 58, 422–432. [Google Scholar] [CrossRef]
- Zhang, F.; Özdemir, B.; Nguyen, P.Q.; Andrukhov, O.; Rausch-Fan, X. Methanandamide diminish the Porphyromonas gingivalis lipopolysaccharide induced response in human periodontal ligament cells. BMC Oral Health 2020, 20, 107. [Google Scholar] [CrossRef]
- Abidi, A.H.; Presley, C.S.; Dabbous, M.; Tipton, D.A.; Mustafa, S.M.; Moore, B.M., 2nd. Anti-inflammatory activity of cannabinoid receptor 2 ligands in primary hPDL fibroblasts. Arch. Oral Biol. 2018, 87, 79–85. [Google Scholar] [CrossRef]
- Gu, Z.; Singh, S.; Niyogi, R.G.; Lamont, G.J.; Wang, H.; Lamont, R.J.; Scott, D.A. Marijuana-Derived Cannabinoids Trigger a CB2/PI3K Axis of Suppression of the Innate Response to Oral Pathogens. Front. Immunol. 2019, 10, 2288. [Google Scholar] [CrossRef]
- Napimoga, M.H.; Benatti, B.B.; Lima, F.O.; Alves, P.M.; Campos, A.C.; Pena-dos-Santos, D.R.; Severino, F.P.; Cunha, F.Q.; Guimarães, F.S. Cannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and pro-inflammatory cytokines during experimental periodontitis in rats. Int. Immunopharmacol. 2009, 9, 216–222. [Google Scholar] [CrossRef]
- Özdemir, B.; Shi, B.; Bantleon, H.P.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Endocannabinoids and inflammatory response in periodontal ligament cells. PLoS ONE 2014, 9, e107407. [Google Scholar] [CrossRef]
- Rettori, E.; De Laurentiis, A.; Zorrilla Zubilete, M.; Rettori, V.; Elverdin, J.C. Anti-inflammatory effect of the endocannabinoid anandamide in experimental periodontitis and stress in the rat. Neuroimmunomodulation 2012, 19, 293–303. [Google Scholar] [CrossRef]
- Stahl, V.; Vasudevan, K. Comparison of Efficacy of Cannabinoids versus Commercial Oral Care Products in Reducing Bacterial Content from Dental Plaque: A Preliminary Observation. Cureus 2020, 12, e6809. [Google Scholar] [CrossRef]
- Vasudevan, K.; Stahl, V. Cannabinoids infused mouthwash products are as effective as chlorhexidine on inhibition of total-culturable bacterial content in dental plaque samples. J. Cannabis Res. 2020, 2, 20. [Google Scholar] [CrossRef]
- Kozono, S.; Matsuyama, T.; Biwasa, K.K.; Kawahara, K.; Nakajima, Y.; Yoshimoto, T.; Yonamine, Y.; Kadomatsu, H.; Tancharoen, S.; Hashiguchi, T.; et al. Involvement of the endocannabinoid system in periodontal healing. Biochem. Biophys. Res. Commun. 2010, 394, 928–933. [Google Scholar] [CrossRef]
- Rawal, S.Y.; Dabbous, M.K.; Tipton, D.A. Effect of cannabidiol on human gingival fibroblast extracellular matrix metabolism: MMP production and activity, and production of fibronectin and transforming growth factor β. J. Periodontal Res. 2012, 47, 320–329. [Google Scholar] [CrossRef]
- Yan, W.; Li, L.; Ge, L.; Zhang, F.; Fan, Z.; Hu, L. The cannabinoid receptor I (CB1) enhanced the osteogenic differentiation of BMSCs by rescue impaired mitochondrial metabolism function under inflammatory condition. Stem Cell Res. Ther. 2022, 13, 22. [Google Scholar] [CrossRef]
- Qian, H.; Zhao, Y.; Peng, Y.; Han, C.; Li, S.; Huo, N.; Ding, Y.; Duan, Y.; Xiong, L.; Sang, H. Activation of cannabinoid receptor CB2 regulates osteogenic and osteoclastogenic gene expression in human periodontal ligament cells. J. Periodontal Res. 2010, 45, 504–511. [Google Scholar] [CrossRef]
- Konermann, A.; Jäger, A.; Held, S.A.E.; Brossart, P.; Schmöle, A. In Vivo and In Vitro Identification of Endocannabinoid Signaling in Periodontal Tissues and Their Potential Role in Local Pathophysiology. Cell. Mol. Neurobiol. 2017, 37, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Furuichi, Y.; Biswas, K.K.; Hashiguchi, T.; Kawahara, K.; Yamaji, K.; Uchimura, T.; Izumi, Y.; Maruyama, I. Endocannabinoid, anandamide in gingival tissue regulates the periodontal inflammation through NF-kappaB pathway inhibition. FEBS Lett. 2006, 580, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Cao, Y.; Yang, H.; Han, N.; Zhu, X.; Fan, Z.; Du, J.; Zhang, F. CB1 enhanced the osteo/dentinogenic differentiation ability of periodontal ligament stem cells via p38 MAPK and JNK in an inflammatory environment. Cell Prolif. 2019, 52, e12691. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, B.C.d.V.; Borges, S.B.; Borges, R.E.A.; Calderon, P.D.S. COVID-19: Perspectives for the management of dental care and education. J. Appl. Oral Sci. 2020, 28, e20200358. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Dias-Pereira, A.C.; Branco-de-Almeida, L.S.; Martins, C.C.; Paiva, S.M. Impact of periodontal disease on quality of life: A systematic review. J. Periodontal Res. 2017, 52, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-related cytokines regulate osteoclast formation and bone resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Wu, Z.; Li, M.; Shao, M.; Hu, T. Interleukin-1β is a potential therapeutic target for periodontitis: A narrative review. Int. J. Oral Sci. 2020, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA J. Am. Med. Assoc. 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- van Klingeren, B.; ten Ham, M. Antibacterial activity of Δ9-tetrahydrocannabinol and cannabidiol. Antonie Van Leeuwenhoek 1976, 42, 9–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).