Onchocerciasis Drug Discovery: In Vitro Evaluation of FDA-Approved Drugs against Onchocerca gutturosa in Gambia
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasites—Onchocerca gutturosa
2.2. Origin of Drugs Tested
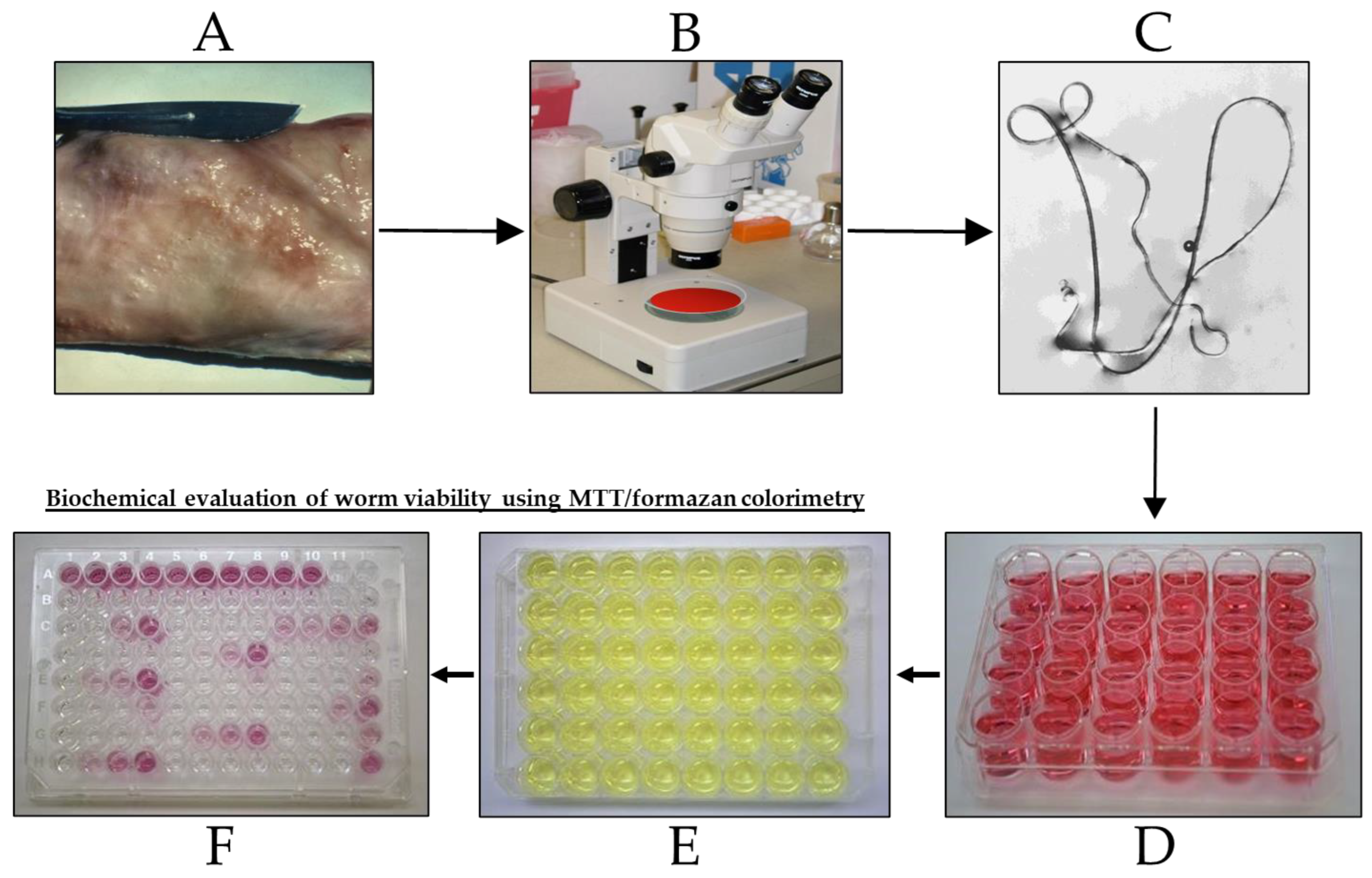
2.3. In Vitro Drug Activity against O. gutturosa Adult Worms as Described by Townson et al. [13]
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brattig, N.W.; Cheke, R.A.; Garms, R. Onchocerciasis (river blindness)—More than a century of research and control. Acta Trop. 2021, 218, 105677. [Google Scholar] [CrossRef]
- WHO. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1 (accessed on 15 October 2023).
- Duke, B.O.; Zea-Flores, G.; Muñoz, B. The embryogenesis of Onchocerca volvulus over the first year after a single dose of ivermectin. Trop. Med. Parasitol. 1991, 42, 175–180. [Google Scholar]
- Basáñez, M.G.; Pion, S.D.; Boakes, E.; Filipe, J.A.; Churcher, T.S.; Boussinesq, M. Effect of single-dose ivermectin on Onchocerca volvulus: A systematic review and meta-analysis. Lancet Infect. Dis. 2008, 8, 310–322. [Google Scholar] [CrossRef]
- Plaisier, A.P.; van Oortmarssen, G.J.; Remme, J.; Habbema, J.D. The reproductive lifespan of Onchocerca volvulus in West African savanna. Acta Trop. 1991, 48, 271–284. [Google Scholar] [CrossRef]
- Geerts, S.; Gryseels, B. Drug resistance in human helminths: Current situation and lessons from livestock. Clin. Microbiol. Rev. 2000, 13, 207–222. [Google Scholar] [CrossRef]
- von Samson-Himmelstjerna, G.; Harder, A.; Sangster, N.C.; Coles, G.C. Efficacy of two cyclooctadepsipeptides, PF1022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitology 2005, 130, 343–347. [Google Scholar] [CrossRef]
- Prichard, R.K.; Geary, T.G. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 69–83. [Google Scholar] [CrossRef]
- Osei-Atweneboana, M.Y.; Awadzi, K.; Attah, S.K.; Boakye, D.A.; Gyapong, J.O.; Prichard, R.K. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 2011, 5, e998. [Google Scholar] [CrossRef]
- Nana-Djeunga, H.C.; Bourguinat, C.; Pion, S.D.; Bopda, J.; Kengne-Ouafo, J.A.; Njiokou, F.; Prichard, R.K.; Wanji, S.; Kamgno, J.; Boussinesq, M. Reproductive status of Onchocerca volvulus after ivermectin treatment in an ivermectin-naïve and a frequently treated population from Cameroon. PLoS Negl. Trop. Dis. 2014, 8, e2824. [Google Scholar] [CrossRef]
- Milton, P.; Hamley, J.I.D.; Walker, M.; Basáñez, M.G. Moxidectin: An oral treatment for human onchocerciasis. Expert Rev. Anti Infect. Ther. 2020, 18, 1067–1081. [Google Scholar] [CrossRef]
- Pfarr, K.M.; Krome, A.K.; Al-Obaidi, I.; Batchelor, H.; Vaillant, M.; Hoerauf, A.; Opoku, N.O.; Kuesel, A.C. The pipeline for drugs for control and elimination of neglected tropical diseases: 2. Oral anti-infective drugs and drug combinations for off-label use. Parasit. Vectors 2023, 16, 394. [Google Scholar] [CrossRef]
- Townson, S.; Ramirez, B.; Fakorede, F.; Mouries, M.A.; Nwaka, S. Challenges in drug discovery for novel antifilarials. Expert Opin. Drug Discov. 2007, 2, S63–S73. [Google Scholar] [CrossRef]
- Tagboto, S.; Orish, V. Drug development for onchocerciasis-the past, the present and the future. Front. Trop. Dis. 2022, 3, 953061. [Google Scholar] [CrossRef]
- Ehrens, A.; Hoerauf, A.; Hübner, M.P. Current perspective of new anti-Wolbachial and direct-acting macrofilaricidal drugs as treatment strategies for human filariasis. GMS Infect. Dis. 2022, 10, Doc02. [Google Scholar] [CrossRef]
- Panic, G.; Vargas, M.; Scandale, I.; Keiser, J. Activity Profile of an FDA-Approved Compound Library against Schistosoma mansoni. PLoS Negl. Trop. Dis. 2015, 9, e0003962. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Halliday, A.; Gentil, K.; Hoerauf, A.; Pearlman, E.; Taylor, M.J. Onchocerciasis: The role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin. Microbiol. Rev. 2011, 24, 459–468. [Google Scholar] [CrossRef]
- Hoerauf, A. Filariasis: New drugs and new opportunities for lymphatic filariasis and onchocerciasis. Curr. Opin. Infect. Dis. 2008, 21, 673–681. [Google Scholar] [CrossRef]
- Johnston, K.L.; Cook, D.A.N.; Berry, N.G.; David Hong, W.; Clare, R.H.; Goddard, M.; Ford, L.; Nixon, G.L.; O’Neill, P.M.; Ward, S.A.; et al. Identification and prioritization of novel anti-Wolbachia chemotypes from screening a 10,000-compound diversity library. Sci. Adv. 2017, 3, eaao1551. [Google Scholar] [CrossRef]
- Townson, S.; Tagboto, S.; McGarry, H.F.; Egerton, G.L.; Taylor, M.J. Onchocerca parasites and Wolbachia endosymbionts: Evaluation of a spectrum of antibiotic types for activity against Onchocerca gutturosa in vitro. Filaria J. 2006, 5, 4. [Google Scholar] [CrossRef]
- Fenollar, F.; Maurin, M.; Raoult, D. Wolbachia pipientis growth kinetics and susceptibilities to 13 antibiotics determined by immunofluorescence staining and real-time PCR. Antimicrob. Agents Chemother. 2003, 47, 1665–1671. [Google Scholar] [CrossRef]
- Aljayyoussi, G.; Tyrer, H.E.; Ford, L.; Sjoberg, H.; Pionnier, N.; Waterhouse, D.; Davies, J.; Gamble, J.; Metuge, H.; Cook, D.A.N.; et al. Short-Course, High-Dose Rifampicin Achieves Wolbachia Depletion Predictive of Curative Outcomes in Preclinical Models of Lymphatic Filariasis and Onchocerciasis. Sci. Rep. 2017, 7, 210. [Google Scholar] [CrossRef]
- Wanji, S.; Hoerauf, A.; Klarmann-Schulz, U. ISRCTN38954299—The Efficacy of Rifampicin Plus Albendazole against River Blindness (Onchocerciasis) in Cameroon. Available online: https://www.isrctn.com/ISRCTN38954299 (accessed on 5 November 2023).
- Hübner, M.P.; Townson, S.; Gokool, S.; Tagboto, S.; Maclean, M.J.; Verocai, G.G.; Wolstenholme, A.J.; Frohberger, S.J.; Hoerauf, A.; Specht, S.; et al. Evaluation of the in vitro susceptibility of various filarial nematodes to emodepside. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 27–35. [Google Scholar] [CrossRef]
- Gillon, J.Y.; Dennison, J.; van den Berg, F.; Delhomme, S.; Dequatre Cheeseman, K.; Peña Rossi, C.; Strub Wourgaft, N.; Specht, S.; Pedrique, B.; Monnot, F.; et al. Safety, tolerability and pharmacokinetics of emodepside, a potential novel treatment for onchocerciasis (river blindness), in healthy male subjects. Br. J. Clin. Pharmacol. 2021, 87, 3949–3960. [Google Scholar] [CrossRef]
- DNDi. Available online: https://dndi.org/research-development/portfolio/emodepside/ (accessed on 15 October 2023).
- Hübner, M.P.; Martin, C.; Specht, S.; Koschel, M.; Dubben, B.; Frohberger, S.J.; Ehrens, A.; Fendler, M.; Struever, D.; Mitre, E.; et al. Oxfendazole mediates macrofilaricidal efficacy against the filarial nematode Litomosoides sigmodontis in vivo and inhibits Onchocerca spec. motility in vitro. PLoS Negl. Trop. Dis. 2020, 14, e0008427. [Google Scholar] [CrossRef]
- Risch, F.; Scheunemann, J.F.; Reichwald, J.J.; Lenz, B.; Ehrens, A.; Gal, J.; Fercoq, F.; Koschel, M.; Fendler, M.; Hoerauf, A.; et al. The efficacy of the benzimidazoles oxfendazole and flubendazole against Litomosoides sigmodontis is dependent on the adaptive and innate immune system. Front. Microbiol. 2023, 14, 1213143. [Google Scholar] [CrossRef]
- Bach, T.; Galbiati, S.; Kennedy, J.K.; Deye, G.; Nomicos, E.Y.H.; Codd, E.E.; Garcia, H.H.; Horton, J.; Gilman, R.H.; Gonzalez, A.E.; et al. Pharmacokinetics, Safety, and Tolerability of Oxfendazole in Healthy Adults in an Open-Label Phase 1 Multiple Ascending Dose and Food Effect Study. Antimicrob. Agents Chemother. 2020, 64, e01018. [Google Scholar] [CrossRef]
- eWHORM. Available online: https://ewhorm.org/ (accessed on 5 November 2023).
- Townson, S.; Connelly, C.; Dobinson, A.; Muller, R. Drug activity against Onchocerca gutturosa males in vitro: A model for chemotherapeutic research on onchocerciasis. J. Helminthol. 1987, 61, 271–281. [Google Scholar] [CrossRef]
- Strote, G.; Wieland, S.; Darge, K.; Comley, J.C. In vitro assessment of the activity of anthelmintic compounds on adults of Onchocerca volvulus. Acta Leiden. 1990, 59, 285–296. [Google Scholar] [PubMed]
- Dominguez-Vazquez, A.; Taylor, H.R.; Greene, B.M.; Ruvalcaba-Macias, A.M.; Rivas-Alcala, A.R.; Murphy, R.P.; Beltran-Hernandez, F. Comparison of flubendazole and diethylcarbamazine in treatment of onchocerciasis. Lancet 1983, 1, 139–143. [Google Scholar] [CrossRef]
- Mackenzie, C.D.; Geary, T.G. Flubendazole: A candidate macrofilaricide for lymphatic filariasis and onchocerciasis field programs. Expert Rev. Anti Infect. Ther. 2011, 9, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Hübner, M.P.; Ehrens, A.; Koschel, M.; Dubben, B.; Lenz, F.; Frohberger, S.J.; Specht, S.; Quirynen, L.; Lachau-Durand, S.; Tekle, F.; et al. Macrofilaricidal efficacy of single and repeated oral and subcutaneous doses of flubendazole in Litomosoides sigmodontis infected jirds. PLoS Negl. Trop. Dis. 2019, 13, e0006320. [Google Scholar] [CrossRef] [PubMed]
- Fissiha, W.; Kinde, M.Z. Anthelmintic Resistance and Its Mechanism: A Review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef] [PubMed]
- Kale, O. Small-scale trials of six drugs against Onchocerca volvulus. Tropenmed. Parasitol. 1978, 29, 163–167. [Google Scholar]
- Kura, K.; Milton, P.; Hamley, J.I.D.; Walker, M.; Bakajika, D.K.; Kanza, E.M.; Opoku, N.O.; Howard, H.; Nigo, M.M.; Asare, S.; et al. Can mass drug administration of moxidectin accelerate onchocerciasis elimination in Africa? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220277. [Google Scholar] [CrossRef]
- Baggish, A.L.; Hill, D.R. Antiparasitic agent atovaquone. Antimicrob. Agents Chemother. 2002, 46, 1163–1173. [Google Scholar] [CrossRef]
- Cheng, G.; Hardy, M.; Topchyan, P.; Zander, R.; Volberding, P.; Cui, W.; Kalyanaraman, B. Potent inhibition of tumour cell proliferation and immunoregulatory function by mitochondria-targeted atovaquone. Sci. Rep. 2020, 10, 17872. [Google Scholar] [CrossRef] [PubMed]
| Bioactivity | Drug |
|---|---|
| Antibacterial | Carbadox; Cefaclor; Cefamandole nafate; Cefoperazone; Cefoxitin sodium; Cefsulodin sodium; Ceftibuten; Cefuroxime sodium; Chlorhexidine dihydrochloride; Chloroxylenol; Demeclocycline hydrochloride; Doxycycline hydrochloride; Furazolidone; Gramicidin; Lasalocid sodium; Merbromin; Methacycline hydrochloride; Methenamine; Minocycline hydrochloride; Nitrofurantoin; Nitroxoline; Ofloxacin; Oxytetracycline; Rifampcin; Rifaximin; Sulfaquinoxaline sodium; Teicoplanin |
| Anticancer | Azaserine; Bleomycin; Daunorubicin; Doxorubicin; Epirubicin hydrochloride; Isotretinon; Lomustine; Mitoxantrone hydrochloride; Tretinoin |
| Antihypertensive/ vasodilator | Dipyridamole; Guanethidine; Losartan; Nicardipine hydrochloride; Nicotinyl alcohol tartrate; Nifedipine |
| Anti-infective | Benzethonium chloride; Broxyquinoline; Dequalinium chloride; Methylbenzethonium chloride; Nitrofurazone; Oxyquinoline hemisulfate; Phenylethyl alcohol; Resorcinol monoacetate |
| Anti-inflammatory/ antihistamine | Dexamethasone acetate; Doxylamine succinate; Meloxicam sodium; Prednisolone tebutate; Sulfasalazine |
| Antiparasitic | Amitraz; Atovaquone; Candicidin; Clorsulon; Diethylcarbamazine citrate; Flubendazole; Hexetidine; Homidium bromide; Iodoquinol; Levamisole hydrochloride; Moxidectin; Primaquine phosphate; Pyrantel pamoate; |
| Antiviral | Oseltamivir phosphate; Valganciclovir hydrochloride |
| Neurological | Acepromazine maleate; Almotriptan; Ampyzine sulfate; Apomorphine hydrochloride; Armodafinil; Bupropion; Chlorpromazine; Danazol; Desipramine hydrochloride; Dopamine hydrochloride; Isradipine (also antihypertensive/vasodilator); Methsuximide; Methylphenidate hydrochloride; Olanzapine; Oxidopamine hydrochloride; Penfluridol; Rivastigmine tartrate; Selegiline hydrochloride; Zaleplon |
| Various | Alendronate sodium; Anisindione; Ascorbyl palmitate; Bromhexine hydrochloride; Butacaine; β-Carotene; Clopidogrel sulfate; Dienestrol; Dioxybenzone; Docusate sodium; Fluorescein; Mangafodipir trisodium; Methylergonovine maleate; Propoxycaine hydrochloride; Riboflavin; Sennoside A; Tetrahydrozoline hydrochloride |
| Drug | Mot Red | MTT Red | ||
|---|---|---|---|---|
| % | p-Value | % | p-Value | |
| IMMITICIDE (positive control) | 100.00 | <0.0001 | 91.09 | <0.0001 |
| Acepromazine Maleate | 53.57 | <0.0001 | 57.67 | <0.001 |
| Amitraz | 89.29 | <0.0001 | 77.23 | <0.0001 |
| Ampyzine Sulfate | 78.57 | <0.0001 | 76.98 | <0.0001 |
| Apomorphine Hydrochloride | 78.57 | <0.0001 | 76.49 | <0.0001 |
| Armodafinil | 82.14 | <0.0001 | 84.65 | <0.0001 |
| Ascorbyl Palmitate | 57.14 | <0.0001 | 67.08 | <0.001 |
| Bleomycin | 53.57 | <0.0001 | 53.22 | <0.01 |
| Bromhexine Hydrochloride | 57.14 | <0.0001 | 56.93 | <0.01 |
| Broxyquinoline | 82.14 | <0.0001 | 82.18 | <0.0001 |
| Candicidin | 67.86 | <0.0001 | 72.03 | <0.001 |
| Carbadox | 60.71 | <0.0001 | 66.83 | <0.001 |
| Cefsulodin Sodium | 50.00 | <0.0001 | 21.04 | 0.18 |
| Ceftibuten | 75.00 | <0.0001 | 71.53 | <0.001 |
| Cefuroxime Sodium | 92.86 | <0.0001 | 79.70 | <0.0001 |
| Chlorpromazine | 82.14 | <0.0001 | 79.46 | <0.0001 |
| Clopidogrel Sulfate | 28.57 | <0.01 | 53.47 | <0.01 |
| Demeclocycline Hydrochloride | 82.14 | <0.0001 | 76.24 | <0.001 |
| Dexamethasone Acetate | 78.57 | <0.0001 | 61.14 | <0.001 |
| Dienestrol | 78.57 | <0.0001 | 75.74 | <0.0001 |
| Docusate Sodium | 75.00 | <0.0001 | 66.58 | <0.001 |
| Dopamine Hydrochloride | 82.14 | <0.0001 | 69.55 | <0.001 |
| Doxorubicin | 60.71 | <0.0001 | 64.36 | <0.001 |
| Doxycycline Hydrochloride | 53.57 | <0.0001 | 37.13 | <0.05 |
| Epirubicin Hydrochloride | 75.00 | <0.0001 | 77.48 | <0.0001 |
| Fluorescein | 50.00 | <0.0001 | 45.54 | <0.01 |
| Guanethidine | 78.57 | <0.0001 | 79.95 | <0.0001 |
| Mangafodipir Trisodium | 89.29 | <0.0001 | 76.49 | <0.0001 |
| Methenamine | 92.86 | <0.0001 | 78.47 | <0.0001 |
| Methsuximide | 71.43 | <0.0001 | 66.34 | <0.001 |
| Methylergonovine Maleate | 57.14 | <0.0001 | 55.45 | <0.001 |
| Methylphenidate Hydrochloride | 75.00 | <0.0001 | 88.12 | <0.0001 |
| Minocycline Hydrochloride | 78.57 | <0.0001 | 72.52 | <0.001 |
| Moxidectin | 78.57 | <0.0001 | 71.78 | <0.001 |
| Nicotinyl Alcohol Tartrate | 67.86 | <0.0001 | 70.30 | <0.001 |
| Nifedipine | 92.86 | <0.0001 | 82.92 | <0.0001 |
| Prednisolone Tebutate | 96.43 | <0.0001 | 82.18 | <0.0001 |
| Primaquine Phosphate | 92.86 | <0.0001 | 84.16 | <0.0001 |
| Propoxycaine Hydrochloride | 60.71 | <0.0001 | 60.15 | <0.001 |
| Riboflavin | 60.71 | <0.0001 | 56.19 | <0.01 |
| Rifampin | 57.14 | <0.0001 | 56.44 | <0.01 |
| Rivastigmine Tartrate | 92.86 | <0.0001 | 81.93 | <0.001 |
| Sennoside A | 53.57 | <0.0001 | 52.23 | <0.01 |
| Tetrahydrozoline Hydrochloride | 53.57 | <0.0001 | 53.22 | <0.01 |
| Valganciclovir Hydrochloride | 50.00 | <0.0001 | 53.71 | <0.01 |
| Drug (Molecular Weight/Bioactivity) | Molecular Structure | Trial 1 | Trial 2 | |||
|---|---|---|---|---|---|---|
| Mot Redn (%) | MTT Redn (%) | p-Value | Mot Redn (%) | MTT Redn (%) | ||
| IMMITICIDE, positive control (501.34/Anthelmintic) | 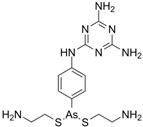 | 100.00 | 91.09 | <0.0001 | 100.00 | Range 77.19–98.88 |
| ATOVAQUONE (366.85/Antimalarial) |  | 100.00 | 90.10 | <0.0001 | 100.00 | 74.42 |
| BENZETHONIUM CHLORIDE (448.09/Antiinfective) | 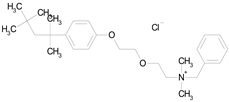 | 100.00 | 88.12 | <0.0001 | 100.00 | 71.91 |
| CHLORHEXIDINE DIHYDROCHLORIDE (578.38/Antibacterial) | 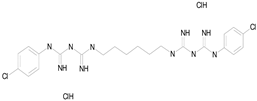 | 100.00 | 90.10 | <0.0001 | 100.00 | 100.00 |
| GRAMICIDIN, gramicidin A shown (1882.34/Antibacterial) | 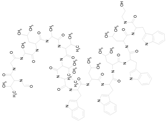 | 100.00 | 76.24 | <0.0001 | 100.00 | 84.27 |
| IODOQUINOL (396.96/Antiamoebic) |  | 100.00 | 80.20 | <0.0001 | 100.00 | 87.64 |
| ISRADIPINE (371.40/Calcium channel blocker) |  | 100.00 | 87.13 | <0.0001 | 100.00 | 78.95 |
| LASALOCID SODIUM (612.79/Antibacterial) | 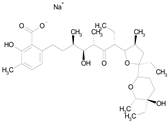 | 100.00 | 89.11 | <0.0001 | 73.33 | 90.70 |
| LEVAMISOLE HYDROCHLORIDE (240.76/Anthelmintic) | 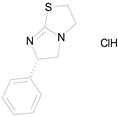 | 100.00 | 92.08 | <0.0001 | 100.00 | 55.81 |
| LOSARTAN (422.92/Antihypertensive) | 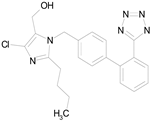 | 100.00 | 88.12 | <0.0001 | 100.00 | 82.56 |
| MELOXICAM SODIUM (373.39/Antiinflammatory) | 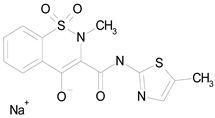 | 100.00 | 89.11 | <0.0001 | 100.00 | 47.67 |
| METHYLBENZETHONIUM CHLORIDE (462.12/Antiinfective) | 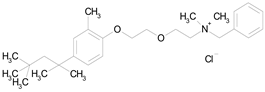 | 100.00 | 91.09 | <0.0001 | 100.00 | 67.44 |
| MITOXANTRONE HYDROCHLORIDE (517.41/Antineoplastic) | 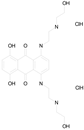 | 100.00 | 91.09 | <0.0001 | 100.00 | 70.93 |
| NITROFURANTOIN (238.16/Antibacterial) | 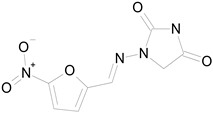 | 100.00 | 75.25 | <0.0001 | 100.00 | 92.70 |
| NITROFURAZONE (198.14/Antibacterial) | 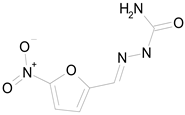 | 100.00 | 85.15 | <0.0001 | 100.00 | 95.51 |
| OXYQUINOLINE HEMISULFATE (243.24/Antiinfective) | 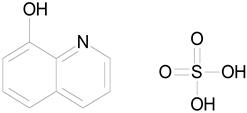 | 100.00 | 86.14 | <0.0001 | 100.00 | 91.57 |
| PYRANTEL PAMOATE (594.69/Anthelmintic) | 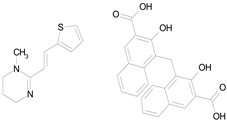 | 100.00 | 85.15 | <0.0001 | 100.00 | 90.45 |
| RIFAXIMIN (785.90/Antibacterial) | 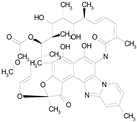 | 100.00 | 91.09 | <0.0001 | 100.00 | 68.60 |
| CEFACLOR (367.81/Antibacterial) | 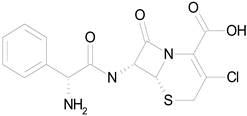 | nd | nd | nd | 100.00 | 96.51 |
| DEQUALINIUM CHLORIDE (527.59/Antiinfective) | 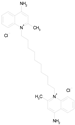 | nd | nd | nd | 100.00 | 61.40 |
| HEXETIDINE (339.61/Antifungal) | 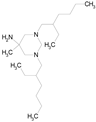 | nd | nd | nd | 100.00 | 88.60 |
| HOMIDIUM BROMIDE (394.32/Antiprotozoal) | 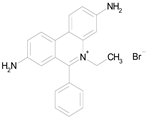 | nd | nd | nd | 100.00 | 81.58 |
| NITROXOLINE (190.16/Antibacterial) | 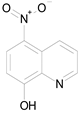 | nd | nd | nd | 100.00 | 82.46 |
| PENFLURIDOL (523.98/Antipsychotic) | 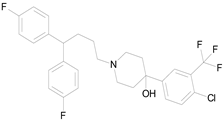 | nd | nd | nd | 100.00 | 64.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gokool, S.; Townson, S.; Freeman, A.; Siemienski-Kleyn, J.; Zubrzycki, J.; Tagboto, S.; Hübner, M.P.; Scandale, I. Onchocerciasis Drug Discovery: In Vitro Evaluation of FDA-Approved Drugs against Onchocerca gutturosa in Gambia. Pharmaceutics 2024, 16, 210. https://doi.org/10.3390/pharmaceutics16020210
Gokool S, Townson S, Freeman A, Siemienski-Kleyn J, Zubrzycki J, Tagboto S, Hübner MP, Scandale I. Onchocerciasis Drug Discovery: In Vitro Evaluation of FDA-Approved Drugs against Onchocerca gutturosa in Gambia. Pharmaceutics. 2024; 16(2):210. https://doi.org/10.3390/pharmaceutics16020210
Chicago/Turabian StyleGokool, Suzanne, Simon Townson, Andrew Freeman, Jadzia Siemienski-Kleyn, Jakub Zubrzycki, Senyo Tagboto, Marc P. Hübner, and Ivan Scandale. 2024. "Onchocerciasis Drug Discovery: In Vitro Evaluation of FDA-Approved Drugs against Onchocerca gutturosa in Gambia" Pharmaceutics 16, no. 2: 210. https://doi.org/10.3390/pharmaceutics16020210
APA StyleGokool, S., Townson, S., Freeman, A., Siemienski-Kleyn, J., Zubrzycki, J., Tagboto, S., Hübner, M. P., & Scandale, I. (2024). Onchocerciasis Drug Discovery: In Vitro Evaluation of FDA-Approved Drugs against Onchocerca gutturosa in Gambia. Pharmaceutics, 16(2), 210. https://doi.org/10.3390/pharmaceutics16020210








