Nanofibrous Polycaprolactone Membrane with Bioactive Glass and Atorvastatin for Wound Healing: Preparation and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
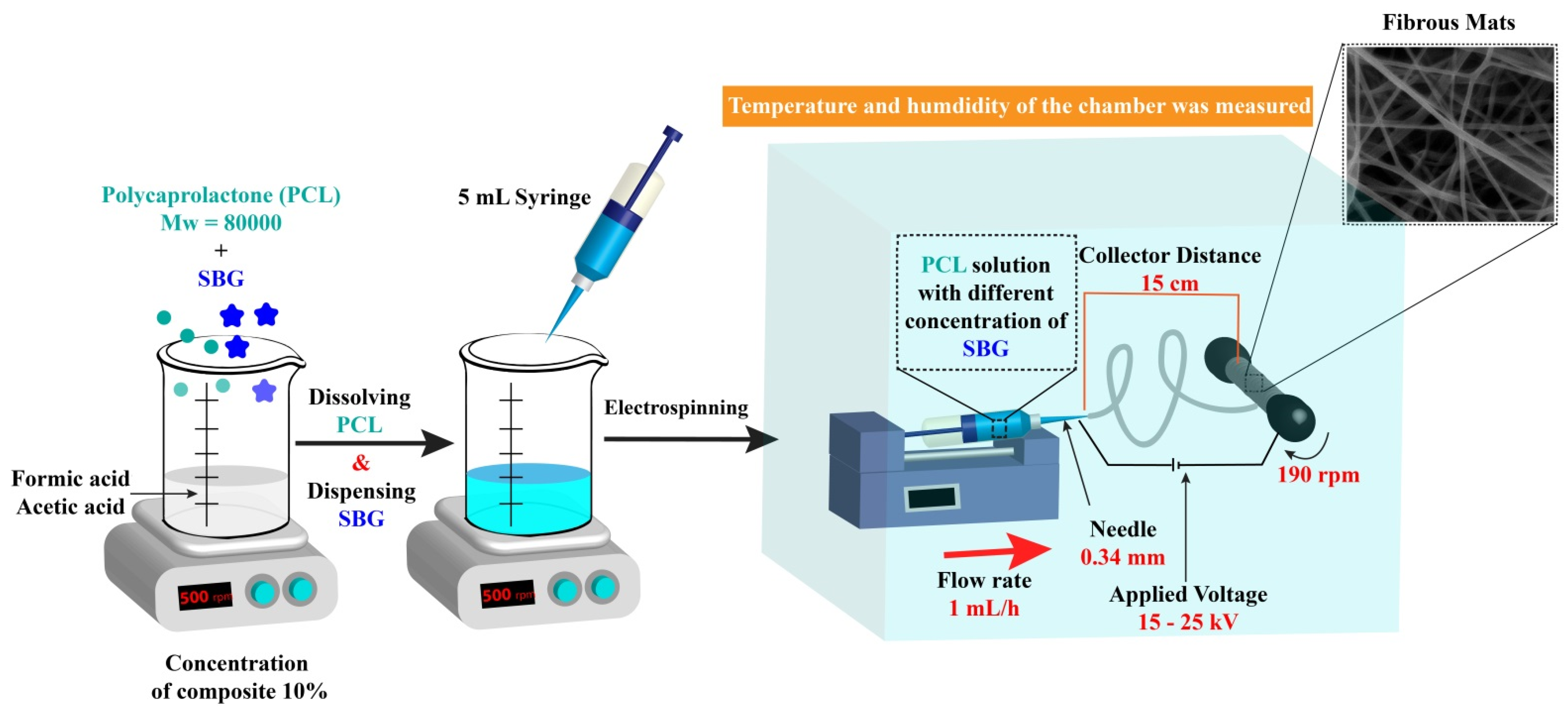
2.2.1. Preparation of the Electrospinning Solutions
2.2.2. Electrospinning Process
2.2.3. Characterization of the Prepared Nanofibers
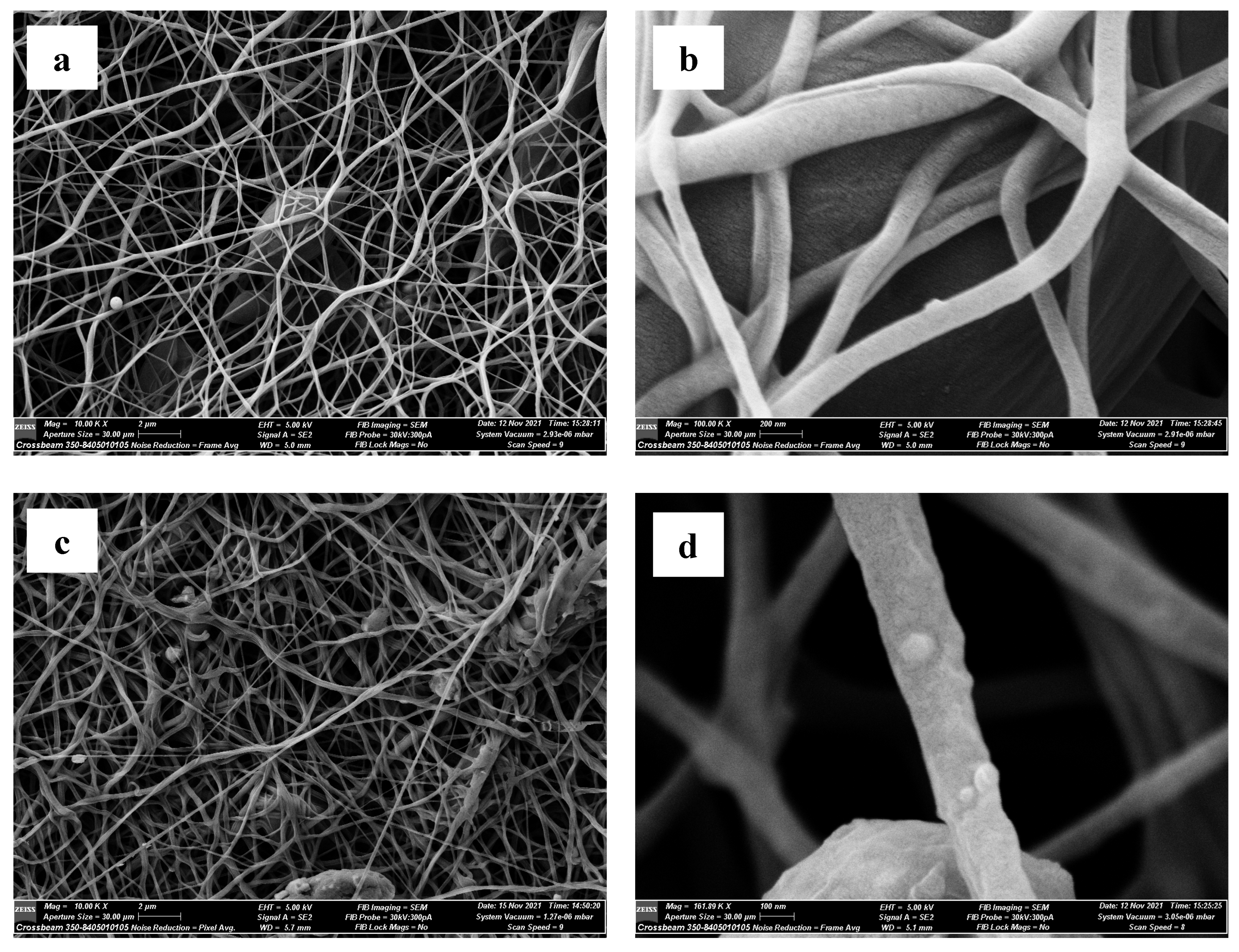
Scanning Electron Microscopy (SEM)
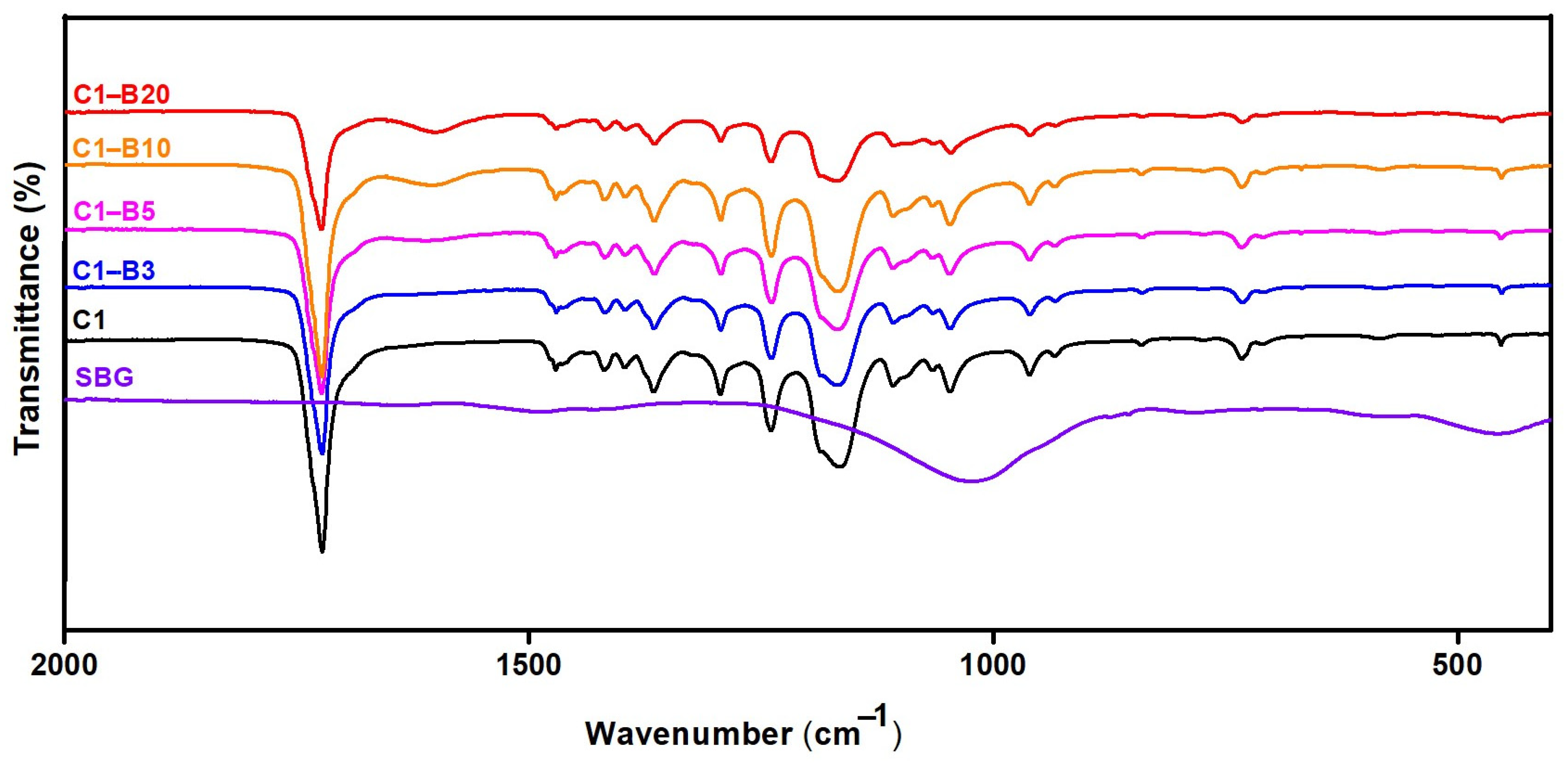
Fourier Transform Infrared (FT-IR) Spectra and X-ray Diffraction (XRD) Analysis
Mechanical Tests
2.2.4. Loading Atorvastatin on the Nanofibers
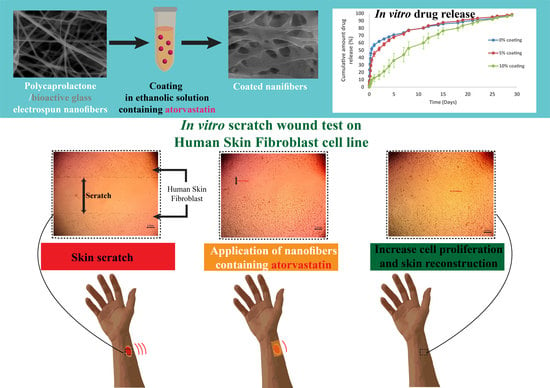
2.2.5. In Vitro Drug Release
2.2.6. Ex Vivo Studies on the Human Skin Fibroblast Cell Line
Cytotoxicity Assay (MTT Assay)
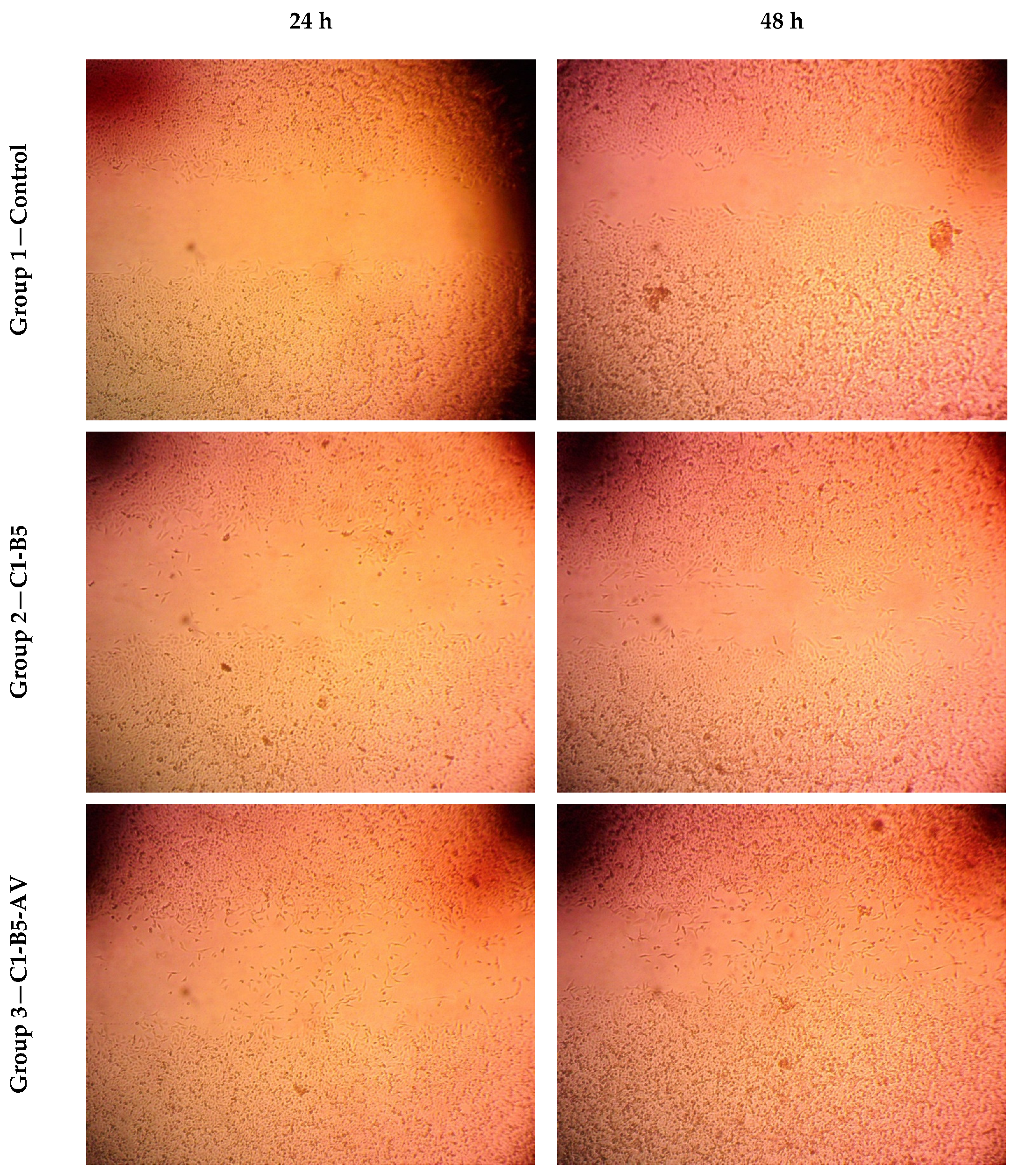
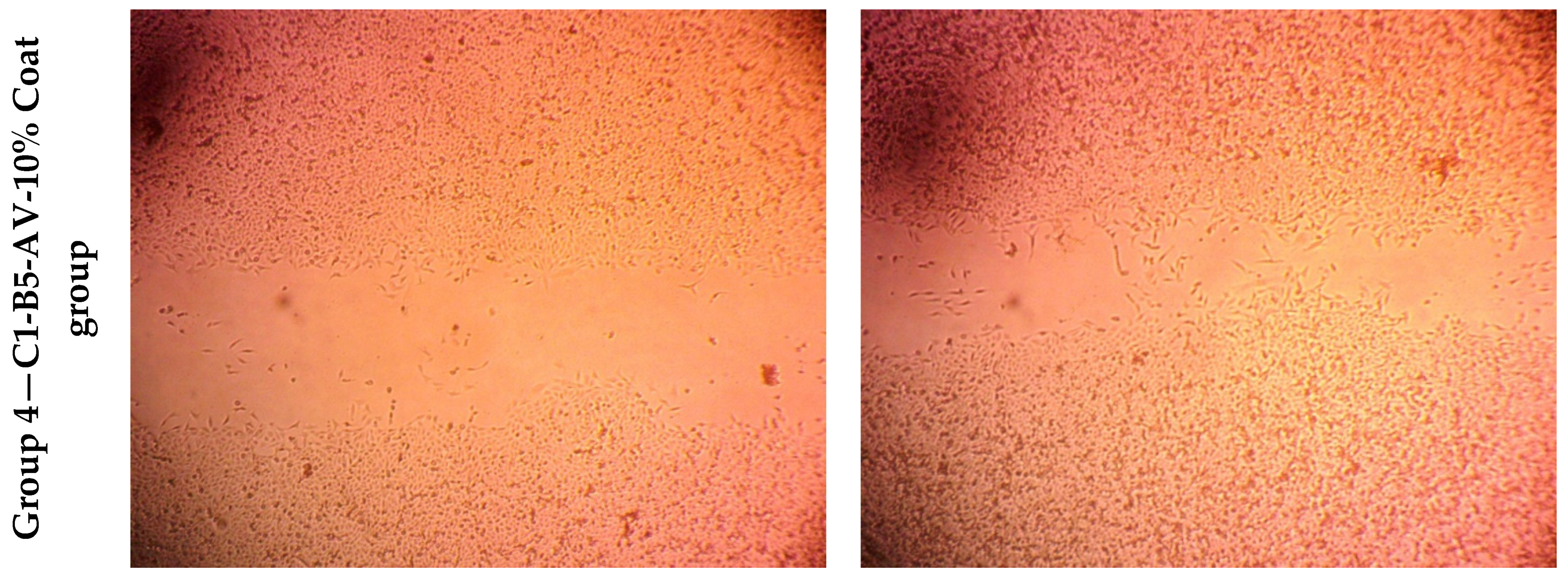
Wound Healing Assay
3. Results and Discussion
3.1. Preparation of the Electrospun Nanofibers
3.2. Characterization of the Prepared Nanofibers
3.2.1. Viscosity of the Spinning Solutions
3.2.2. Characterization of Nanofibers
Scanning Electron Microscopy (SEM)
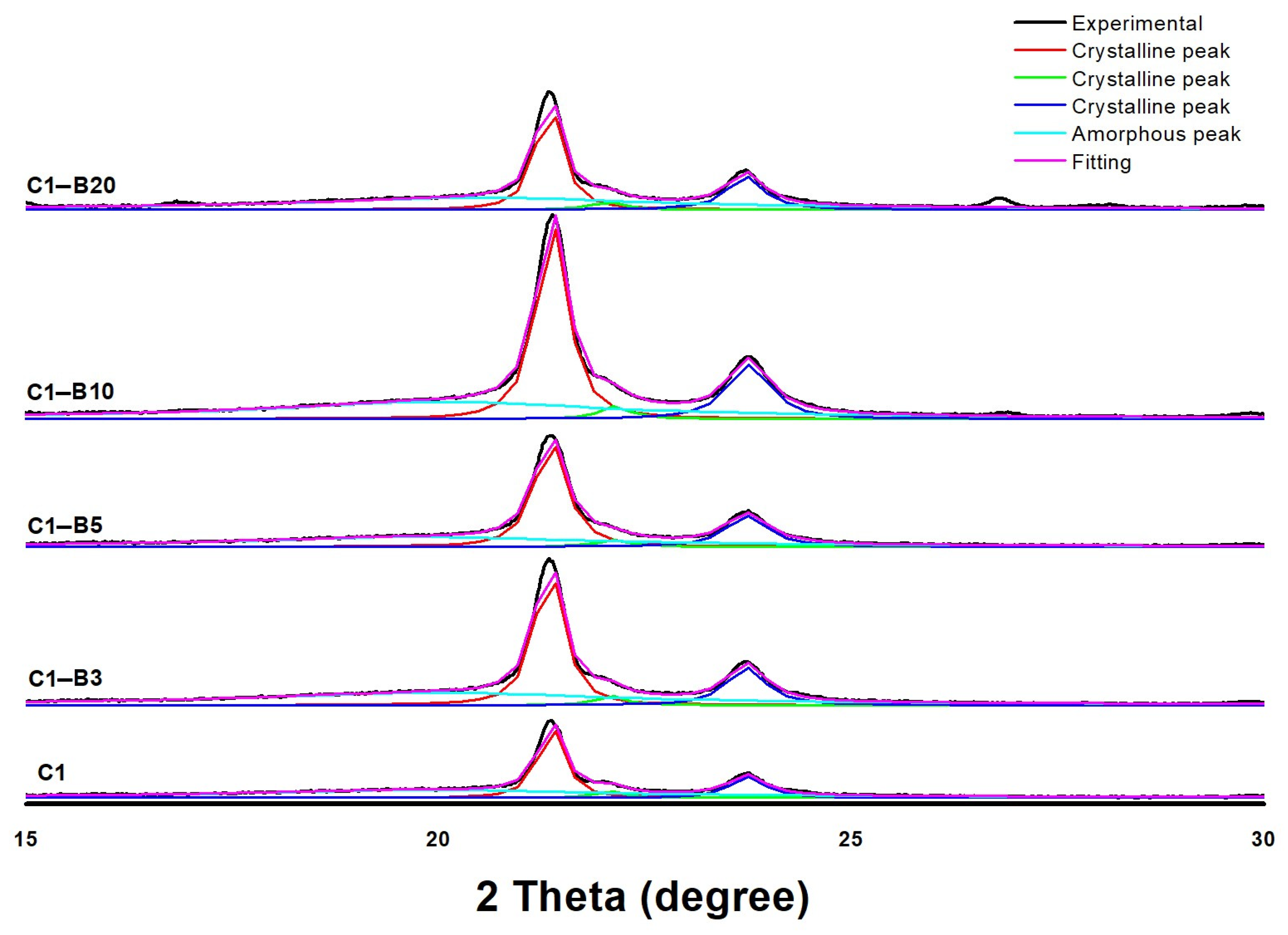
Fourier Transform Infrared (FT-IR) Spectra and X-ray Diffraction (XRD) Analysis
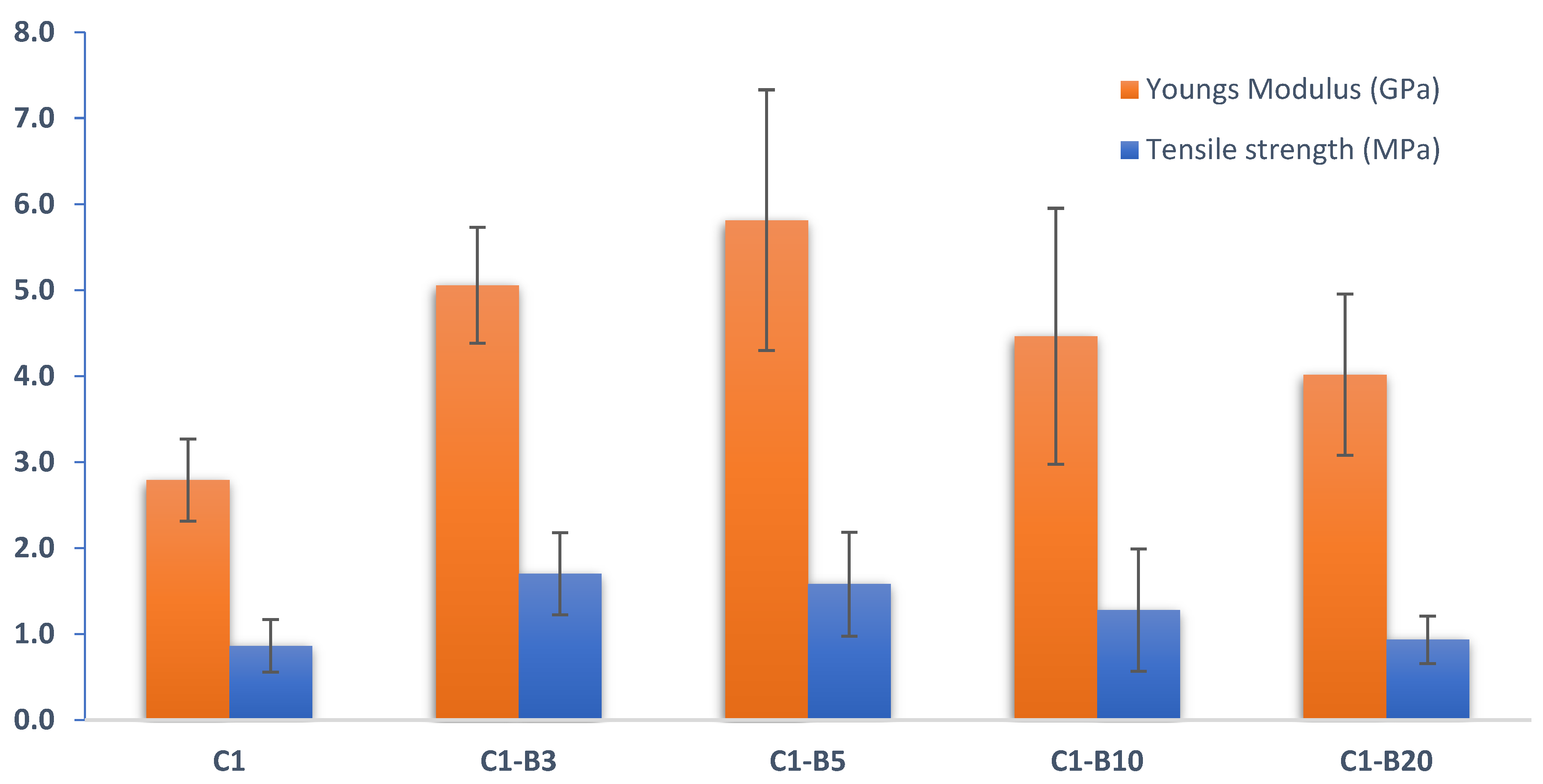
Mechanical Tests
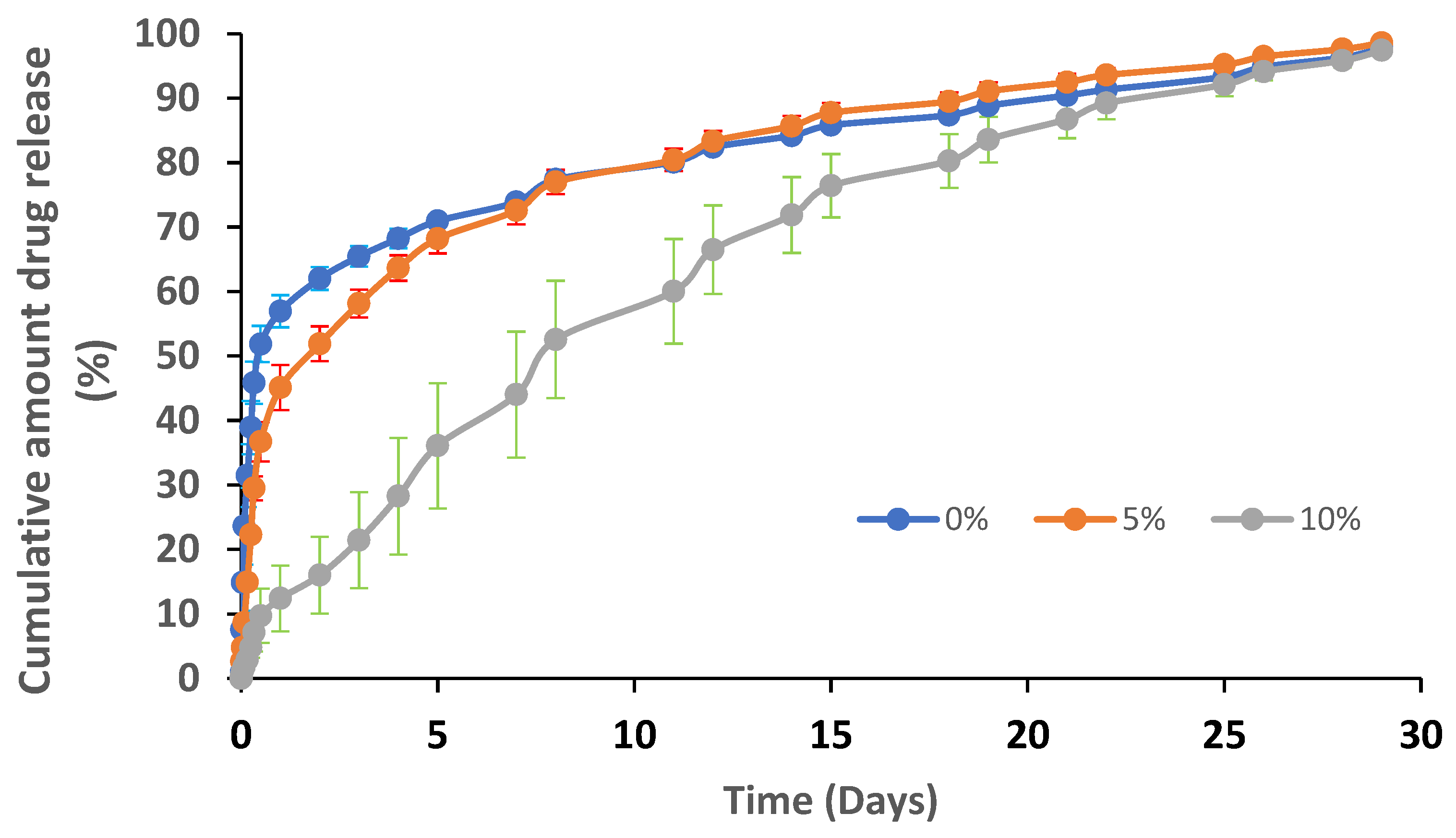
3.2.3. Loading Atorvastatin on the Nanofibers and In Vitro Drug Release
3.2.4. Ex Vivo Studies on the Human Skin Fibroblast Cell Line
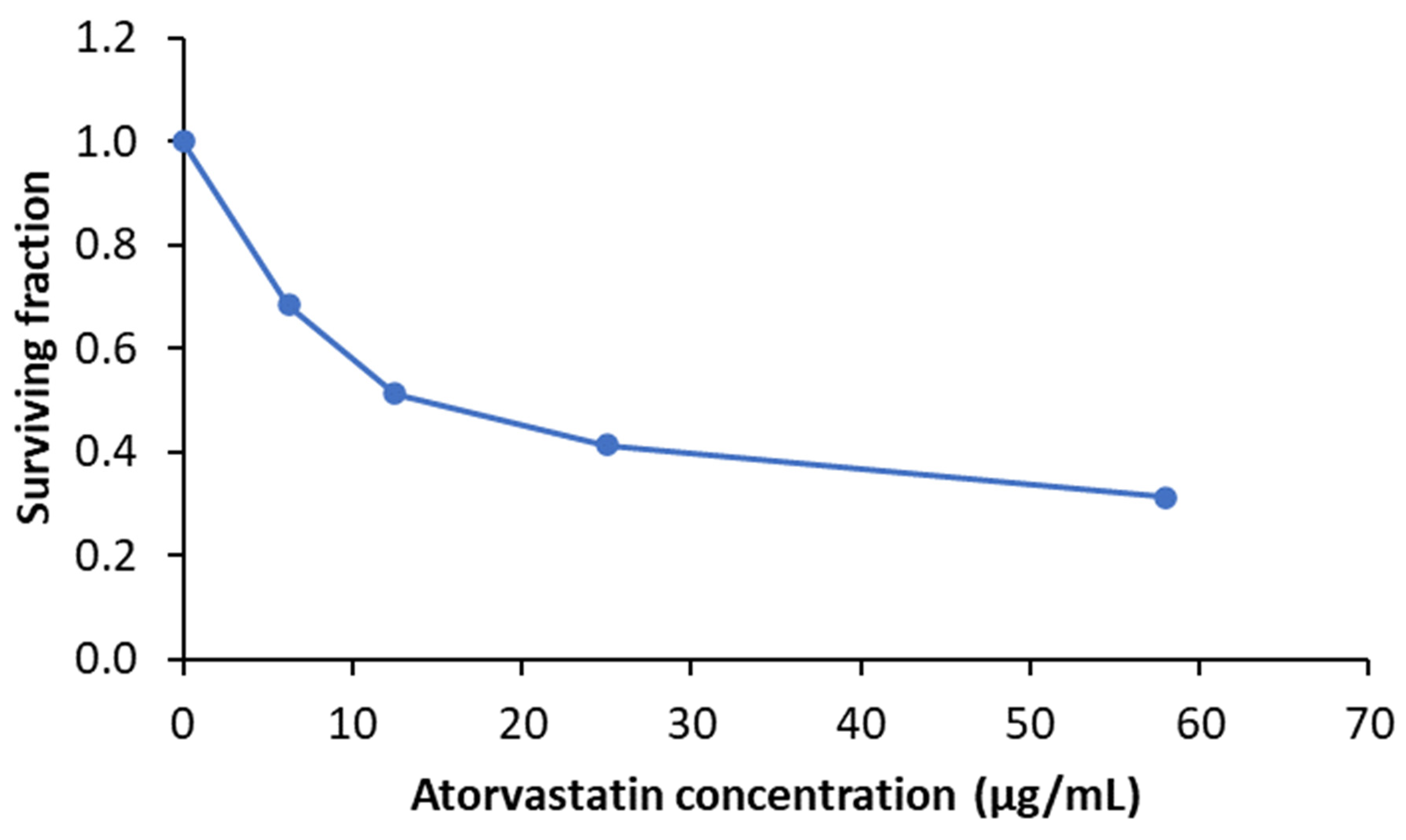
Cytotoxicity Assay (MTT Assay)
Wound Healing Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ather, S.; Harding, K.; Tate, S. Wound management and dressings. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Woodhead: Cambridge, UK, 2019; pp. 1–22. [Google Scholar]
- Armstrong, D.G.; Wrobel, J.; Robbins, J.M. Guest Editorial: Are diabetes-related wounds and amputations worse than cancer? Int. Wound J. 2007, 4, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Richmond, N.A.; Lamel, S.A.; Davidson, J.M.; Martins-Green, M.; Sen, C.K.; Tomic-Canic, M.; Vivas, A.C.; Braun, L.R.; Kirsner, R.S. US-National Institutes of Health-funded research for cutaneous wounds in 2012. Wound Repair Regen. 2013, 21, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Van Koppen, C.J.; Hartmann, R.W. Advances in the treatment of chronic wounds: A patent review. Expert Opin. Ther. Pat. 2015, 25, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Li, J.; Tu, J.; Yang, H.; Chen, Q.; Liu, H. Fabrication of porous chitosan membranes composed of nanofibers by low temperature thermally induced phase separation, and their adsorption behavior for Cu2+. Carbohydr. Polym. 2017, 178, 338–346. [Google Scholar] [CrossRef]
- Kamin, Z.; Abdulrahim, N.; Misson, M.; Chiam, C.; Sarbatly, R.; Krishnaiah, D.; Bono, A. Use of melt blown polypropylene nanofiber templates to obtain homogenous pore channels in glycidyl methacrylate/ethyl dimethacrylate-based monoliths. Chem. Eng. Commun. 2021, 208, 661–672. [Google Scholar] [CrossRef]
- MacNeil, S. Biomaterials for tissue engineering of skin. Mater. Today 2008, 11, 26–35. [Google Scholar] [CrossRef]
- Kanungo, I.; Fathima, N.N.; Rao, J.R.; Nair, B.U. Influence of PCL on the material properties of collagen based biocomposites and in vitro evaluation of drug release. Mater. Sci. Eng. C 2013, 33, 4651–4659. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.G. Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- Rafiq, M.; Rather, S.-u.; Wani, T.U.; Rather, A.H.; Khan, R.S.; Khan, A.E.; Hamid, I.; Khan, H.A.; Alhomida, A.S.; Sheikh, F.A. Recent progress in MXenes incorporated into electrospun nanofibers for biomedical application: Study focusing from 2017 to 2022. Chin. Chem. Lett. 2023, 34, 108463. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Toriello, M.; Afsari, M.; Shon, H.K.; Tijing, L.D. Progress on the Fabrication and Application of Electrospun Nanofiber Composites. Membranes 2020, 10, 204. [Google Scholar] [CrossRef]
- Akhmetova, A.; Heinz, A. Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges. Pharmaceutics 2020, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Amajuoyi, J.N.; Ilomuanya, M.O.; Asantewaa-Osei, Y.; Azubuike, C.P.; Adeosun, S.O.; Igwilo, C.I. Development of electrospun keratin/coenzyme Q10/poly vinyl alcohol nanofibrous scaffold containing mupirocin as potential dressing for infected wounds. Future J. Pharm. Sci. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Ceonzo, K.; Gaynor, A.; Shaffer, L.; Kojima, K.; Vacanti, C.A.; Stahl, G.L. Polyglycolic acid-induced inflammation: Role of hydrolysis and resulting complement activation. Tissue Eng. 2006, 12, 301–308. [Google Scholar] [CrossRef]
- Bhullar, S.K.; Rana, D.; Lekesiz, H.; Bedeloglu, A.C.; Ko, J.; Cho, Y.; Aytac, Z.; Uyar, T.; Jun, M.; Ramalingam, M. Design and fabrication of auxetic PCL nanofiber membranes for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 334–340. [Google Scholar] [CrossRef]
- Ma, K.; Liao, C.; Huang, L.; Liang, R.; Zhao, J.; Zheng, L.; Su, W. Electrospun PCL/MoS(2) Nanofiber Membranes Combined with NIR-Triggered Photothermal Therapy to Accelerate Bone Regeneration. Small 2021, 17, e2104747. [Google Scholar] [CrossRef]
- Xue, J.; He, M.; Liang, Y.; Crawford, A.; Coates, P.; Chen, D.; Shi, R.; Zhang, L. Fabrication and evaluation of electrospun PCL-gelatin micro-/nanofiber membranes for anti-infective GTR implants. J. Mater. Chem. B 2014, 2, 6867–6877. [Google Scholar] [CrossRef]
- Ravichandran, S.; Radhakrishnan, J. Anticancer efficacy of lupeol incorporated electrospun Polycaprolactone/gelatin nanocomposite nanofibrous mats. Nanotechnology 2022, 33, 295104. [Google Scholar] [CrossRef] [PubMed]
- Haroosh, H.J.; Dong, Y.; Jasim, S.; Ramakrishna, S. Morphological Structures and Drug Release Effect of Multiple Electrospun Nanofibre Membrane Systems Based on PLA, PCL, and PCL/Magnetic Nanoparticle Composites. J. Nanomater. 2022, 2022, 5190163. [Google Scholar] [CrossRef]
- McIver, L.A.; Siddique, M.S. Atorvastatin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lipitor. Atorvastatin Calcium Tablets: Description; Pfizer Ireland Pharmaceuticals Dublin: Dublin, Ireland, 2009. [Google Scholar]
- Suzuki-Banhesse, V.F.; Azevedo, F.F.; Araujo, E.P.; do Amaral, M.E.; Caricilli, A.M.; Saad, M.J.; Lima, M.H. Effect of atorvastatin on wound healing in rats. Biol. Res. Nurs. 2015, 17, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liao, J.K. Pleiotropic effects of statins—Basic research and clinical perspectives. Circ. J. 2010, 74, 818–826. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent update on nanoemulgel as topical drug delivery system. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef]
- Farsaei, S.; Khalili, H.; Farboud, E.S. Potential role of statins on wound healing: Review of the literature. Int. Wound J. 2012, 9, 238–247. [Google Scholar] [CrossRef]
- Tejaswini, T.; Keerthana, M.; Vidyavathi, M.; Kumar, R. Design and evaluation of atorvastatin-loaded chitosan-hydroxyapatite composite bioscaffolds for wound-healing activity. Future J. Pharm. Sci. 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Zaazou, M.H.; Chow, L.C.; Mahmoud, A.A.; Zaki, D.Y.; Basha, M.; Hamid, M.A.A.; Khallaf, M.E.; Sharaf, N.F.; Hamdy, T.M. Injectable nanoamorphous calcium phosphate based in situ gel systems for the treatment of periapical lesions. Biomed. Mater. 2015, 10, 065006. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Latif, R.G.; Nair, A.B.; Venugopala, K.N.; Ahmed, A.F.; Elsewedy, H.S.; Shehata, T.M. Preparation and Evaluation of Atorvastatin-Loaded Nanoemulgel on Wound-Healing Efficacy. Pharmaceutics 2019, 11, 609. [Google Scholar] [CrossRef]
- Abootorabi, S.; Akbari, J.; Saeedi, M.; Seyedabadi, M.; Ranaee, M.; Asare-Addo, K.; Nokhodchi, A. Atorvastatin Entrapped Noisome (Atrosome): Green Preparation Approach for Wound Healing. AAPS PharmSciTech 2022, 23, 81. [Google Scholar] [CrossRef]
- Zeng, W.; Cheng, N.M.; Liang, X.; Hu, H.; Luo, F.; Jin, J.; Li, Y.W. Electrospun polycaprolactone nanofibrous membranes loaded with baicalin for antibacterial wound dressing. Sci. Rep. 2022, 12, 10900. [Google Scholar] [CrossRef] [PubMed]
- Naseri, S.; Lepry, W.C.; Nazhat, S.N. Bioactive glasses in wound healing: Hope or hype? J. Mater. Chem. B 2017, 5, 6167–6174. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, C. Bioactive inorganic particles-based biomaterials for skin tissue engineering. In Exploration; Wiley Online Library: Hoboken, NJ, USA, 2022. [Google Scholar]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005, 11, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M.; Boccaccini, A.R. Effect of particulate bioactive glasses on human macrophages and monocytes in vitro. J. Biomed. Mater. Res. Part A 2005, 73, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, J.; Yu, H.; Green, C.R.; Chang, J. Bioglass promotes wound healing by affecting gap junction connexin 43 mediated endothelial cell behavior. Biomaterials 2016, 84, 64–75. [Google Scholar] [CrossRef]
- Stoor, P.; Soderling, E.; Salonen, J.I. Antibacterial effects of a bioactive glass paste on oral microorganisms. Acta Odontol. Scand. 1998, 56, 161–165. [Google Scholar] [CrossRef]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.; Evans, B.A.; Thompson, R.P.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Finnson, K.W.; McLean, S.; Di Guglielmo, G.M.; Philip, A. Dynamics of Transforming Growth Factor Beta Signaling in Wound Healing and Scarring. Adv. Wound Care New Rochelle 2013, 2, 195–214. [Google Scholar] [CrossRef]
- Xie, W.; Chen, X.; Miao, G.; Tang, J.; Fu, X. Regulation of cellular behaviors of fibroblasts related to wound healing by sol-gel derived bioactive glass particles. J. Biomed. Mater. Res. A 2016, 104, 2420–2429. [Google Scholar] [CrossRef]
- Elshazly, N.; Saad, M.M.; El Backly, R.M.; Hamdy, A.; Patruno, M.; Nouh, S.; Saha, S.; Chakraborty, J.; Marei, M.K. Nanoscale borosilicate bioactive glass for regenerative therapy of full-thickness skin defects in rabbit animal model. Front. Bioeng. Biotechnol. 2023, 11, 1036125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, T.; Xie, C.; Li, S.; Lei, B. Multi-layer-structured bioactive glass nanopowder for multistage-stimulated hemostasis and wound repair. Bioact. Mater. 2023, 25, 319–332. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.M.; Mostafa, A.A.; Gaafar, A.M.; El Hotaby, W.; Hamzawy, E.M.; El-Okaily, M.S.; Gamal-Eldeen, A.M. In vitro kinetic investigations on the bioactivity and cytocompatibility of bioactive glasses prepared via melting and sol-gel techniques for bone regeneration applications. Biomed. Mater. 2017, 12, 015029. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Mostafa, A.; Oudadesse, H.; Wers, E.; Lucas-Girot, A.; El-Gohary, M.I. Comparative study of nanobioactive glass quaternary system 46S6. Bioceram. Dev. Appl. 2014, 4, 1000072. [Google Scholar]
- Mendyk, A.; Jachowicz, R.; Fijorek, K.; Dorożyński, P.; Kulinowski, P.; Polak, S. KinetDS: An open source software for dissolution test data analysis. Dissolution Technol. 2012, 19, 6–11. [Google Scholar] [CrossRef]
- Podczeck, F. Comparison of in vitro dissolution profiles by calculating mean dissolution time (MDT) or mean residence time (MRT). Int. J. Pharm. 1993, 97, 93–100. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Wu, Y.K.; Wang, L.; Fan, J.; Shou, W.; Zhou, B.M.; Liu, Y. Multi-Jet Electrospinning with Auxiliary Electrode: The Influence of Solution Properties. Polymers 2018, 10, 572. [Google Scholar] [CrossRef]
- Denis, P.; Dulnik, J.; Sajkiewicz, P. Electrospinning and structure of bicomponent polycaprolactone/gelatin nanofibers obtained using alternative solvent system. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 354–364. [Google Scholar] [CrossRef]
- Luo, C.; Stride, E.; Edirisinghe, M. Mapping the influence of solubility and dielectric constant on electrospinning polycaprolactone solutions. Macromolecules 2012, 45, 4669–4680. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng. Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Steckl, A.J. Coaxial electrospinning formation of complex polymer fibers and their applications. ChemPlusChem 2019, 84, 1453–1497. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013, 2013, 789289. [Google Scholar] [CrossRef]
- Medeiros, G.B.; Lima, F.d.A.; de Almeida, D.S.; Guerra, V.G.; Aguiar, M.L. Modification and Functionalization of Fibers Formed by Electrospinning: A Review. Membranes 2022, 12, 861. [Google Scholar] [CrossRef] [PubMed]
- El-Okaily, M.S.; El-Rafei, A.M.; Basha, M.; Abdel Ghani, N.T.; El-Sayed, M.M.H.; Bhaumik, A.; Mostafa, A.A. Efficient drug delivery vehicles of environmentally benign nano-fibers comprising bioactive glass/chitosan/polyvinyl alcohol composites. Int. J. Biol. Macromol. 2021, 182, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Hussain, M.; Mao, J.J. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials 2007, 28, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef]
- Ramirez-Cedillo, E.; Ortega-Lara, W.; Rocha-Pizana, M.R.; Gutierrez-Uribe, J.A.; Elias-Zuniga, A.; Rodriguez, C.A. Electrospun Polycaprolactone Fibrous Membranes Containing Ag, TiO(2) and Na(2)Ti(6)O(13) Particles for Potential Use in Bone Regeneration. Membranes 2019, 9, 12. [Google Scholar] [CrossRef]
- Alahmmar, M.; Prabhakaran, P.; Jaganathan, S.; Nik, N.A.N. Fabrication and Characterization of Polycaprolactone with Retinoic Acid and Cerium Oxide for Anticancer Applications. Biointerface Res. Appl. Chem. 2023, 13, 1–15. [Google Scholar]
- Kołbuk, D.; Sajkiewicz, P.; Maniura-Weber, K.; Fortunato, G. Structure and morphology of electrospun polycaprolactone/gelatine nanofibres. Eur. Polym. J. 2013, 49, 2052–2061. [Google Scholar] [CrossRef]
- Otadi, M.; Mohebbi-Kalhori, D. Evaluate of different bioactive glass on mechanical properties of nanocomposites prepared using electrospinning method. Procedia Mater. Sci. 2015, 11, 196–201. [Google Scholar] [CrossRef]
- Balaji, S.; Kumar, R.; Sripriya, R.; Kakkar, P.; Ramesh, D.V.; Reddy, P.N.K.; Sehgal, P. Preparation and comparative characterization of keratin–chitosan and keratin–gelatin composite scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2012, 32, 975–982. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Hosseini, S.M.; Mofid, V.; Ramezani, S.; Ghorbani, M.; Ehsani, A.; Mortazavian, A.M. Electrospun ethyl cellulose/poly caprolactone/gelatin nanofibers: The investigation of mechanical, antioxidant, and antifungal properties for food packaging. Int. J. Biol. Macromol. 2021, 191, 457–464. [Google Scholar] [CrossRef]
- Lopez-Jornet, P.; Camacho-Alonso, F.; Gomez-Garcia, F.; Molina Minano, F.; Canas, X.; Serafin, A.; Castillo, J.; Vicente-Ortega, V. Effects of potassium apigenin and verbena extract on the wound healing process of SKH-1 mouse skin. Int. Wound J. 2014, 11, 489–495. [Google Scholar] [CrossRef]
- Suntar, I.; Kupeli Akkol, E.; Keles, H.; Yesilada, E.; Sarker, S.D. Exploration of the wound healing potential of Helichrysum graveolens (Bieb.) Sweet: Isolation of apigenin as an active component. J. Ethnopharmacol. 2013, 149, 103–110. [Google Scholar] [CrossRef]
- Hulkower, K.I.; Herber, R.L. Cell migration and invasion assays as tools for drug discovery. Pharmaceutics 2011, 3, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.; Abdelkhalek, A.A.; Mahmoud, A.A.; Salah, S.; Ammar, M.M.; Ghorab, M.M. Mesenchymal stem cells associated with chitosan scaffolds loaded with rosuvastatin to improve wound healing. Eur. J. Pharm. Sci. 2019, 127, 185–198. [Google Scholar] [CrossRef]
- Tomic-Canic, M. The Role of Statins in Cutaneous Wound Healing; NIH 2012, R21 AR; University of Miami School of Medicine: Coral Gables, FL, USA, 2012. [Google Scholar]


| Spinning Polymeric Solution | Applied Voltage (kV) | |||
|---|---|---|---|---|
| Sample Code | Solid Content Composition (% w/w) * | Viscosity (mPas) ± SD | ||
| PCL | SBG | |||
| C1 | 10.0 | 0.0 | 507.86 ± 6.79 | 15 |
| C1-B3 | 9.7 | 0.3 | 491.43 ± 5.92 | 17 |
| C1-B5 | 9.5 | 0.5 | 489.13 ± 7.85 | 19 |
| C1-B10 | 9.0 | 1.0 | 478.30 ± 10.80 | 21 |
| C1-B20 | 8.0 | 2.0 | 337.30 ± 7.41 | 25 |
| Sample Code | PCL Crystallinity |
|---|---|
| C1 | 0.37 |
| C1-B3 | 0.44 |
| C1-B5 | 0.47 |
| C1-B10 | 0.48 |
| C1-B20 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Okaily, M.S.; Mostafa, A.A.; Dulnik, J.; Denis, P.; Sajkiewicz, P.; Mahmoud, A.A.; Dawood, R.; Maged, A. Nanofibrous Polycaprolactone Membrane with Bioactive Glass and Atorvastatin for Wound Healing: Preparation and Characterization. Pharmaceutics 2023, 15, 1990. https://doi.org/10.3390/pharmaceutics15071990
El-Okaily MS, Mostafa AA, Dulnik J, Denis P, Sajkiewicz P, Mahmoud AA, Dawood R, Maged A. Nanofibrous Polycaprolactone Membrane with Bioactive Glass and Atorvastatin for Wound Healing: Preparation and Characterization. Pharmaceutics. 2023; 15(7):1990. https://doi.org/10.3390/pharmaceutics15071990
Chicago/Turabian StyleEl-Okaily, Mohamed S., Amany A. Mostafa, Judyta Dulnik, Piotr Denis, Paweł Sajkiewicz, Azza A. Mahmoud, Reham Dawood, and Amr Maged. 2023. "Nanofibrous Polycaprolactone Membrane with Bioactive Glass and Atorvastatin for Wound Healing: Preparation and Characterization" Pharmaceutics 15, no. 7: 1990. https://doi.org/10.3390/pharmaceutics15071990
APA StyleEl-Okaily, M. S., Mostafa, A. A., Dulnik, J., Denis, P., Sajkiewicz, P., Mahmoud, A. A., Dawood, R., & Maged, A. (2023). Nanofibrous Polycaprolactone Membrane with Bioactive Glass and Atorvastatin for Wound Healing: Preparation and Characterization. Pharmaceutics, 15(7), 1990. https://doi.org/10.3390/pharmaceutics15071990