Innovation in the Development of Synthetic and Natural Ocular Drug Delivery Systems for Eye Diseases Treatment: Focusing on Drug-Loaded Ocular Inserts, Contacts, and Intraocular Lenses
Abstract
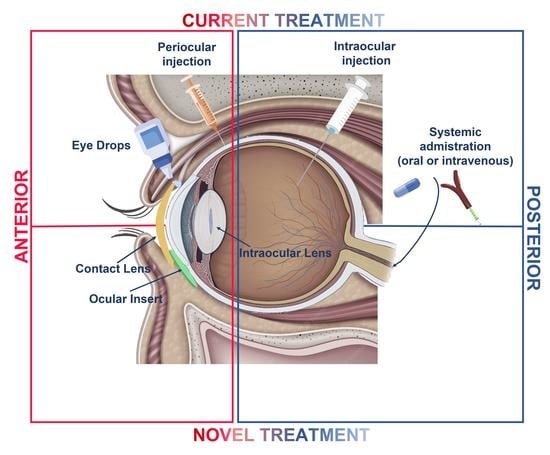
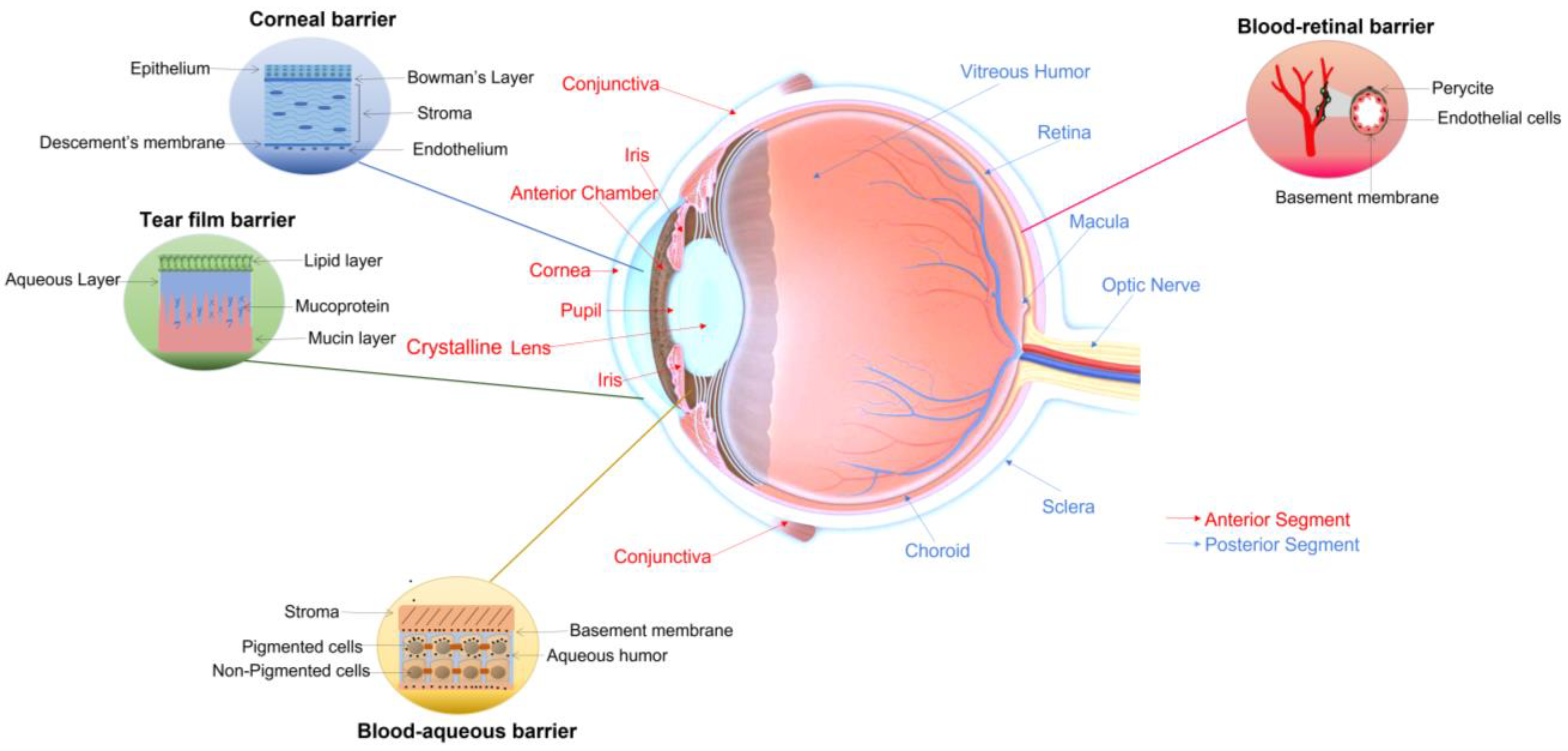
1. Introduction
2. Materials and Methods
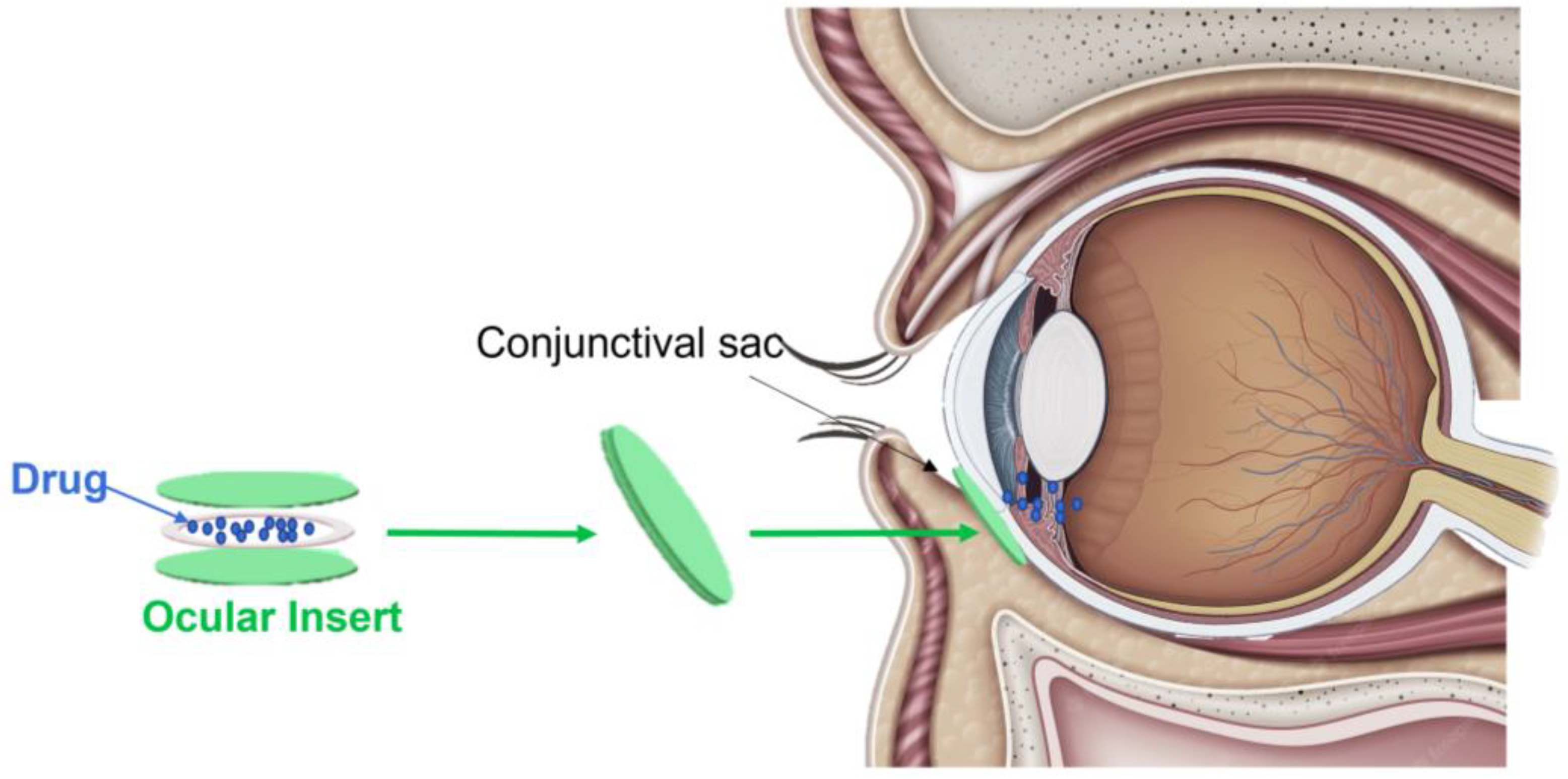
3. Ocular Inserts
3.1. Description and Mechanisms
3.2. Ocular Insert Drug Release Studies
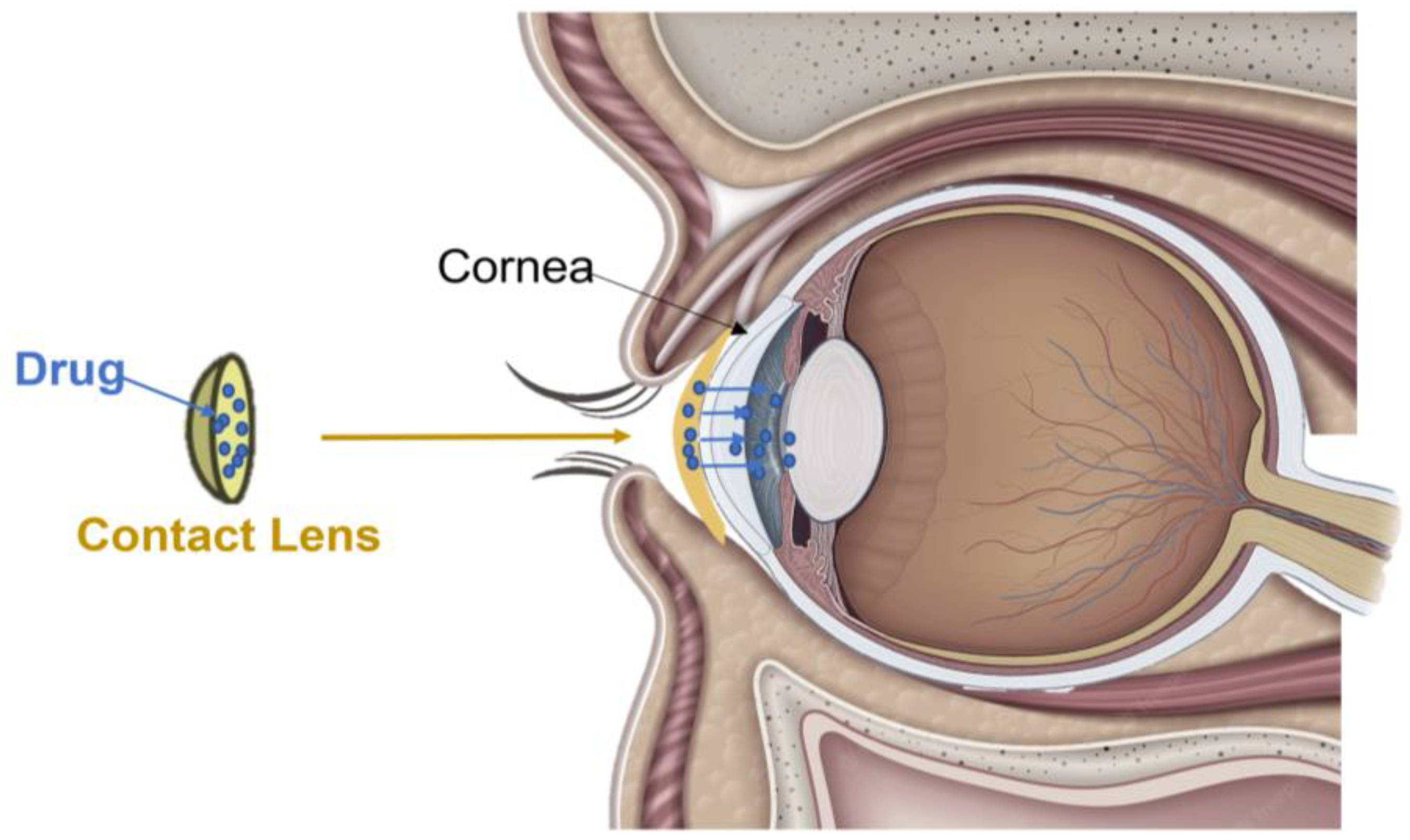
4. Contact Lenses
4.1. Description and Mechanisms
4.2. Contact Lens Drug Release Studies
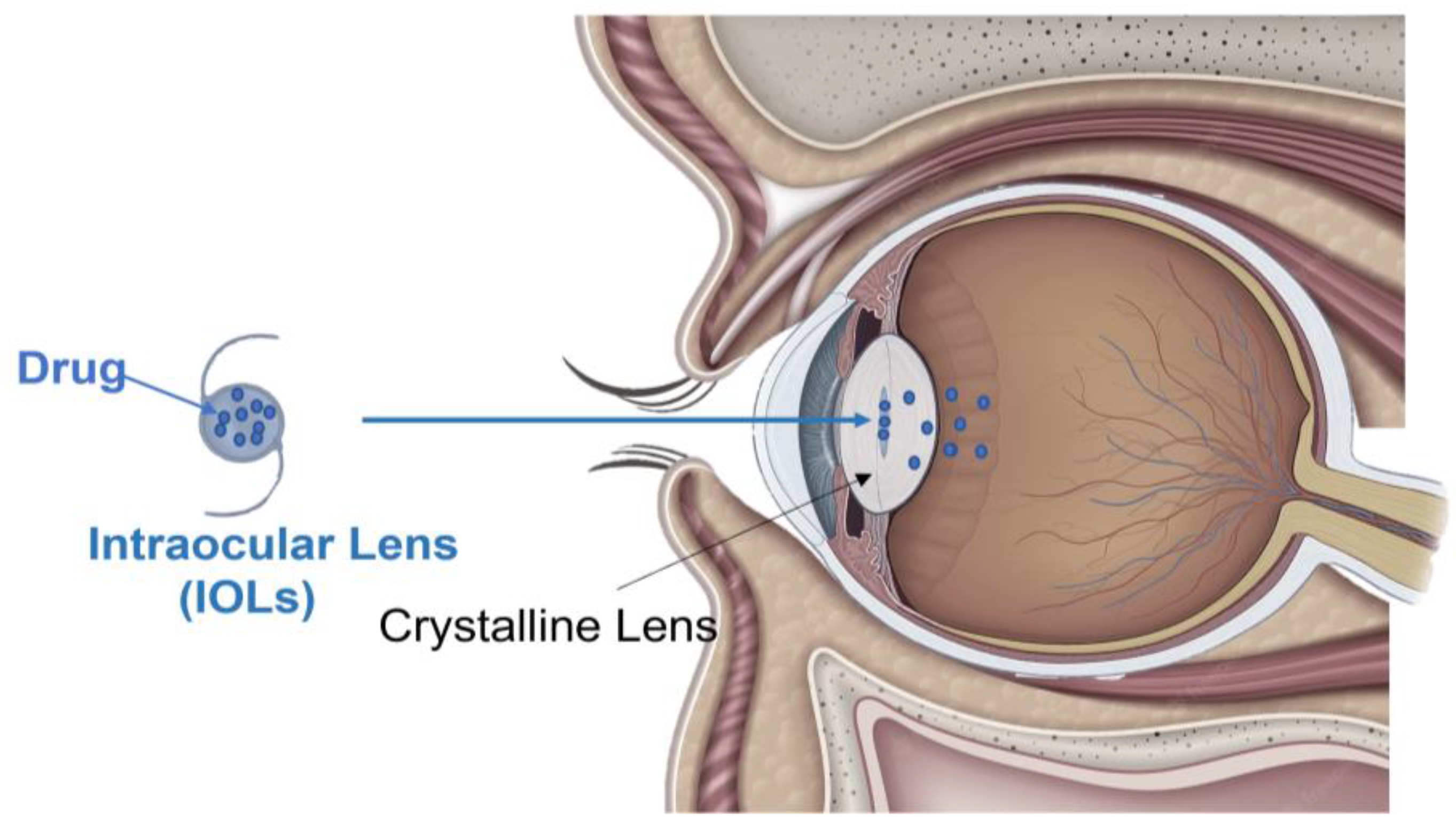
5. Intraocular Lenses (IOLs)
5.1. Description and Mechanisms
5.2. Intraocular Lens Drug Release Studies
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, C.-H.; Wang, P.-Y.; Lin, I.-C.; Huang, H.; Liu, G.-S.; Tseng, C.-L. Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv. Transl. Res. 2020, 11, 866–893. [Google Scholar] [CrossRef]
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Akhter, M.H.; Ahmad, I.; Alshahrani, M.Y.; Al-Harbi, A.I.; Khalilullah, H.; Afzal, O.; Altamimi, A.S.A.; Nasar Mir Najib Ullah, S.; Ojha, A.; Karim, S. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels 2022, 8, 82. [Google Scholar] [CrossRef]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.-L.; Dinu-Pîrvu, C.-E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Kaur, I.P.; Kakkar, S. Nanotherapy for posterior eye diseases. J. Control. Release 2014, 193, 100–112. [Google Scholar] [CrossRef]
- Peyman, G.A.; Lad, E.M.; Moshfeghi, D.M. Intravitreal Injection of Therapeutic Agents. Retina 2009, 29, 875–912. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Wróblewska, K.B.; Jadach, B.; Muszalska-Kolos, I. Progress in drug formulation design and delivery of medicinal substances used in ophthalmology. Int. J. Pharm. 2021, 607, 121012. [Google Scholar] [CrossRef]
- Grassiri, B.; Zambito, Y.; Bernkop-Schnürch, A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv. Colloid Interface Sci. 2020, 288, 102342. [Google Scholar] [CrossRef]
- Souto, E.B.; Dias-Ferreira, J.; López-Machado, A.; Ettcheto, M.; Cano, A.; Espuny, A.C.; Espina, M.; Garcia, M.L.; Sánchez-López, E. Advanced Formulation Approaches for Ocular Drug Delivery: State-Of-The-Art and Recent Patents. Pharmaceutics 2019, 11, 460. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in ocular drug delivery systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef]
- Lakhani, P.; Patil, A.; Majumdar, S. Recent advances in topical nano drug-delivery systems for the anterior ocular segment. Ther. Deliv. 2018, 9, 137–153. [Google Scholar] [CrossRef]
- Tundisi, L.; Mostaço, G.; Carricondo, P.; Petri, D. Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; Du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef]
- Omer, S.; Zelkó, R. A Systematic Review of Drug-Loaded Electrospun Nanofiber-Based Ophthalmic Inserts. Pharmaceutics 2021, 13, 1637. [Google Scholar] [CrossRef]
- Battaglia, L.; Serpe, L.; Foglietta, F.; Muntoni, E.; Gallarate, M.; Rodriguez, A.D.P.; Solinis, M.A. Application of lipid nanoparticles to ocular drug delivery. Expert Opin. Drug Deliv. 2016, 13, 1743–1757. [Google Scholar] [CrossRef]
- Asim, M.H.; Ijaz, M.; Mahmood, A.; Knoll, P.; Jalil, A.; Arshad, S.; Bernkop-Schnürch, A. Thiolated cyclodextrins: Mucoadhesive and permeation enhancing excipients for ocular drug delivery. Int. J. Pharm. 2021, 599, 120451. [Google Scholar] [CrossRef]
- Grassiri, B.; Knoll, P.; Fabiano, A.; Piras, A.M.; Zambito, Y.; Bernkop-Schnurch, A. Thiolated Hydroxypropyl-beta-cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2612. [Google Scholar] [CrossRef]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Rodrigues, L.B.; Bravo, R.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.A.; et al. Bimatoprost-Loaded Ocular Inserts as Sustained Release Drug Delivery Systems for Glaucoma Treatment: In Vitro and In Vivo Evaluation. PLoS ONE 2014, 9, e95461. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, P.K.; Garg, V.K.; Garg, G. Ocular inserts—Advancement in therapy of eye diseases. J. Adv. Pharm. Technol. Res. 2010, 1, 291–296. [Google Scholar] [CrossRef]
- Yashika, D.K.a.U. Ocular inserts: Novel approach for drug delivery into eyes. GSC Biol. Pharm. Sci. 2019, 7, 001–003. [Google Scholar]
- Morrison, P.W.; Khutoryanskiy, V.V. Advances in ophthalmic drug delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [CrossRef]
- Vyas, S.P.; Khar, R.H. Targeted and Controlled Drug Delivery Novel Carrier Systems; C.B.S. Publishers and Distributors, Inc.: Chennai, India, 2002. [Google Scholar]
- Dubald, M.; Bourgeois, S.; Andrieu, V.; Fessi, H. Ophthalmic Drug Delivery Systems for Antibiotherapy—A Review. Pharmaceutics 2018, 10, 10. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Nicoli, A.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C. Cyclosporine-loaded cross-linked inserts of sodium hyaluronan and hydroxypropyl-beta-cyclodextrin for ocular administration. Carbohydr. Polym. 2018, 201, 308–316. [Google Scholar] [CrossRef]
- Terreni, E.; Burgalassi, S.; Chetoni, P.; Tampucci, S.; Zucchetti, E.; Fais, R.; Ghelardi, E.; Lupetti, A.; Monti, D. Development and Characterization of a Novel Peptide-Loaded Antimicrobial Ocular Insert. Biomolecules 2020, 10, 664. [Google Scholar] [CrossRef]
- Sadeghi, A.M.; Farjadian, F.; Alipour, S. Sustained release of linezolid in ocular insert based on lipophilic modified structure of sodium alginate. Iran. J. Basic Med. Sci. 2021, 24, 331–340. [Google Scholar] [CrossRef]
- Polat, H.K.; Pehlivan, S.B.; Özkul, C.; Çalamak, S.; Öztürk, N.; Aytekin, E.; Fırat, A.; Ulubayram, K.; Kocabeyoğlu, S.; Irkeç, M.; et al. Development of besifloxacin HCl loaded nanofibrous ocular inserts for the treatment of bacterial keratitis: In vitro, ex vivo and in vivo evaluation. Int. J. Pharm. 2020, 585, 119552. [Google Scholar] [CrossRef]
- Khalil, I.A.; Ali, I.H.; El-Sherbiny, I.M. Noninvasive biodegradable nanoparticles-in-nanofibers single-dose ocular insert: In vitro, ex vivo and in vivo evaluation. Nanomedicine 2019, 14, 33–55. [Google Scholar] [CrossRef]
- Singla, J.; Bajaj, T.; Goyal, A.K.; Rath, G. Development of Nanofibrous Ocular Insert for Retinal Delivery of Fluocinolone Acetonide. Curr. Eye Res. 2019, 44, 541–550. [Google Scholar] [CrossRef]
- Alambiaga-Caravaca, A.M.; Domenech-Monsell, I.M.; Sebastián-Morelló, M.; Calatayud-Pascual, M.A.; Merino, V.; Rodilla, V.; López-Castellano, A. Development, characterization, and ex vivo evaluation of an insert for the ocular administration of progesterone. Int. J. Pharm. 2021, 606, 120921. [Google Scholar] [CrossRef]
- Mohammadi, G.; Mirzaeei, S.; Taghe, S.; Mohammadi, P. Preparation and Evaluation of Eudragit(R) L100 Nanoparticles Loaded Impregnated with KT Tromethamine Loaded PVA -HEC Insertions for Ophthalmic Drug Delivery. Adv. Pharm. Bull. 2019, 9, 593–600. [Google Scholar] [CrossRef]
- Girgis, G.N.S. Formulation and Evaluation of Atorvastatin Calcium-Poly-epsilon-Caprolactone Nanoparticles Loaded Ocular Inserts for Sustained Release and Antiinflammatory Efficacy. Curr. Pharm. Biotechnol. 2020, 21, 1688–1698. [Google Scholar] [CrossRef]
- Bertens, C.J.; Martino, C.; van Osch, M.C.; Lataster, A.; Dias, A.J.; Biggelaar, F.J.V.D.; Tuinier, R.; Nuijts, R.M.; Gijs, M. Design of the ocular coil, a new device for non-invasive drug delivery. Eur. J. Pharm. Biopharm. 2020, 150, 120–130. [Google Scholar] [CrossRef]
- Bertens, C.J.F.; Gijs, M.; Dias, A.A.J.; Biggelaar, F.J.H.M.V.D.; Ghosh, A.; Sethu, S.; Nuijts, R.M.M.A. Pharmacokinetics and efficacy of a ketorolac-loaded ocular coil in New Zealand white rabbits. Drug Deliv. 2021, 28, 400–407. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Concheiro, A.; Alvarez-Lorenzo, C. Crosslinked Hyaluronan Electrospun Nanofibers for Ferulic Acid Ocular Delivery. Pharmaceutics 2020, 12, 274. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Li, J.; Shi, Z. Grafting of methyl methacrylate onto sodium alginate initiated by potassium ditelluratoargentate(III). J. Appl. Polym. Sci. 2005, 97, 1688–1694. [Google Scholar] [CrossRef]
- Kurşun, N.I.F. Synthesis and characterization of graft copolymer of sodium alginate and poly(itaconic acid) by the redox system. Polym. Bull. 2013, 70, 1064–1084. [Google Scholar]
- Gagandeep; Garg, T.; Malik, B.; Rath, G.; Goyal, A.K. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur. J. Pharm. Sci. 2014, 53, 10–16. [Google Scholar] [CrossRef]
- Thakkar, S.; Misra, M. Electrospun polymeric nanofibers: New horizons in drug delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef]
- Suwantong, O. Biomedical applications of electrospun polycaprolactone fiber mats. Polym. Adv. Technol. 2016, 27, 1264–1273. [Google Scholar] [CrossRef]
- Nuzzi, R.; Scalabrin, S.; Becco, A.; Panzica, G. Sex Hormones and Optic Nerve Disorders: A Review. Front. Neurosci. 2019, 13, 57. [Google Scholar] [CrossRef]
- Lopez, A.M.R.; Roche, S.L.; Jackson, A.C.W.; Moloney, J.N.; Byrne, A.M.; Cotter, T.G. Pro-survival redox signalling in progesterone-mediated retinal neuroprotection. Eur. J. Neurosci. 2017, 46, 1663–1672. [Google Scholar] [CrossRef]
- Doonan, F.; O’Driscoll, C.; Kenna, P.; Cotter, T.G. Enhancing survival of photoreceptor cells in vivo using the synthetic progestin Norgestrel. J. Neurochem. 2011, 118, 915–927. [Google Scholar] [CrossRef]
- Rathod, L.V.; Kapadia, R.; Sawant, K.K. A novel nanoparticles impregnated ocular insert for enhanced bioavailability to posterior segment of eye: In vitro, in vivo and stability studies. Mater. Sci. Eng. C 2017, 71, 529–540. [Google Scholar] [CrossRef]
- Durgun, M.E.; Gungor, S.; Ozsoy, Y. Micelles: Promising Ocular Drug Carriers for Anterior and Posterior Segment Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 323–341. [Google Scholar] [CrossRef]
- Naik, J.B.; Lokhade, A.B.; Mishra, S.; Kulkarni, R.D. Development of Sustained Release Micro/Nano Particles Using Different Solvent Emulsification Techniques: A Review. Int. J. Pharm. Bio. Sci. 2012, 3, 573–590. [Google Scholar]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2012, 10, 131–149. [Google Scholar] [CrossRef]
- Deepalekshmi Ponnamma, N.N.; Thomas, S. Carbon Nanotube Tube Filled Polymer Nanocomposites and Their Applications in Tissue Engineering. Appl. Nanomater. 2018, 13, 391–414. [Google Scholar]
- Terreni, E.; Chetoni, P.; Burgalassi, S.; Tampucci, S.; Zucchetti, E.; Chipala, E.; Alany, R.G.; Al-Kinani, A.A.; Monti, D. A hybrid ocular delivery system of cyclosporine-A comprising nanomicelle-laden polymeric inserts with improved efficacy and tolerability. Biomater. Sci. 2021, 9, 8235–8248. [Google Scholar] [CrossRef]
- Roslan, S.A.H.; Zaki, M.; Rasid, Z.A.; Aidi Bani, N.; Sarip, S.; Md Daud, M.Y.; Muhammad-Sukki, F. Mode I fracture toughness of optimized alkali-treated Bambusa Vulgaris bamboo by Box-Behnken design. In Advances in Material Sciences and Engineering: Selected Papers from the Proceedings of the 4th International Conference on Mechanical, Manufacturing and Plant Engineering (ICMMPE 2018); Springer: Berlin, Germany, 2018; pp. 565–575. [Google Scholar]
- Shukr, M.H.; Ismail, S.; El-Hossary, G.G.; El-Shazly, A.H. Spanlastics nanovesicular ocular insert as a novel ocular delivery of travoprost: Optimization using Box–Behnken design and in vivo evaluation. J. Liposome Res. 2022, 32, 354–364. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Contact Lenses as Ophthalmic Drug Delivery Systems: A Review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef]
- Carvalho, I.; Marques, C.; Oliveira, R.; Coelho, P.; Costa, P.; Ferreira, D. Sustained drug release by contact lenses for glaucoma treatment—A review. J. Control. Release 2015, 202, 76–82. [Google Scholar] [CrossRef]
- Gulsen, D.; Chauhan, A. Ophthalmic Drug Delivery through Contact Lenses. Investig. Opthalmology Vis. Sci. 2004, 45, 2342–2347. [Google Scholar] [CrossRef]
- Nguyen, D.C.T.; Dowling, J.; Ryan, R.; McLoughlin, P.; Fitzhenry, L. Pharmaceutical-loaded contact lenses as an ocular drug delivery system: A review of critical lens characterization methodologies with reference to ISO standards. Contact Lens Anterior Eye 2021, 44. [Google Scholar] [CrossRef]
- Dixon, P.; Ghosh, T.; Mondal, K.; Konar, A.; Chauhan, A.; Hazra, S. Controlled delivery of pirfenidone through vitamin E-loaded contact lens ameliorates corneal inflammation. Drug Deliv. Transl. Res. 2018, 8, 1114–1126. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Hu, N.; Tammareddy, T.; Domszy, R.; Yang, J.; Wang, N.S.; Yang, A. Extended delivery of non-steroidal anti-inflammatory drugs through contact lenses loaded with Vitamin E and cationic surfactants. Contact Lens Anterior Eye 2019, 42, 546–552. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Hu, N.; Koolivand, A.; Fan, X.; Zhu, Y.; Domszy, R.; Yang, J.; Yang, A.; Wang, N.S. Effect of a Cationic Surfactant on Microemulsion Globules and Drug Release from Hydrogel Contact Lenses. Pharmaceutics 2019, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Zhang, X.; Sheng, R.; Lin, Q.; Song, W.; Hao, L. Novel Contact Lenses Embedded with Drug-Loaded Zwitterionic Nanogels for Extended Ophthalmic Drug Delivery. Nanomaterials 2021, 11, 2328. [Google Scholar] [CrossRef] [PubMed]
- Varela-Garcia, A.; Gomez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C. Imprinted Contact Lenses for Ocular Administration of Antiviral Drugs. Polymers 2020, 12, 2026. [Google Scholar] [CrossRef] [PubMed]
- Gade, S.K.; Nirmal, J.; Garg, P.; Venuganti, V.V.K. Corneal delivery of moxifloxacin and dexamethasone combination using drug-eluting mucoadhesive contact lens to treat ocular infections. Int. J. Pharm. 2020, 591, 120023. [Google Scholar] [CrossRef]
- Nguyen, D.; Dowling, J.; Ryan, R.; McLoughlin, P.; Fitzhenry, L. Controlled release of naringenin from soft hydrogel contact lens: An investigation into lens critical properties and in vitro release. Int. J. Pharm. 2022, 621. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Concheiro, A.; Alvarez-Lorenzo, C. Epalrestat-loaded silicone hydrogels as contact lenses to address diabetic-eye complications. Eur. J. Pharm. Biopharm. 2018, 122, 126–136. [Google Scholar] [CrossRef]
- Mohamdeen, Y.M.G.; Tabriz, A.G.; Tighsazzadeh, M.; Nandi, U.; Khalaj, R.; Andreadis, I.; Boateng, J.S.; Douroumis, D. Development of 3D printed drug-eluting contact lenses. J. Pharm. Pharmacol. 2021, 74, 1467–1476. [Google Scholar] [CrossRef]
- De Guzman, L.M.C.; De Guzman, G.Q.; Borromeo, E.C. Brinzolamide-loaded soft contact lens for ophthalmic delivery. Ther. Deliv. 2022, 13, 233–247. [Google Scholar] [CrossRef]
- Mun, J.; Mok, J.W.; Jeong, S.; Cho, S.; Joo, C.-K.; Hahn, S.K. Drug-eluting contact lens containing cyclosporine-loaded cholesterol-hyaluronate micelles for dry eye syndrome. RSC Adv. 2019, 9, 16578–16585. [Google Scholar] [CrossRef]
- Khanum, B.N.M.K.; Guha, R.; Sur, V.P.; Nandi, S.; Basak, S.K.; Konar, A.; Hazra, S. Pirfenidone inhibits post-traumatic proliferative vitreoretinopathy. Eye 2017, 31, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-C.; Kim, J.; Chauhan, A. Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing Vitamin E diffusion barriers. Biomaterials 2010, 31, 4032–4047. [Google Scholar] [CrossRef]
- Peng, C.-C.; Burke, M.T.; Chauhan, A. Transport of Topical Anesthetics in Vitamin E Loaded Silicone Hydrogel Contact Lenses. Langmuir 2011, 28, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Soni, T.G.; Shan, D.O. Effect of timolol maleate concentration on uptake and release from hydrogel contact lenses using soaking method. J. Pharm. Appl. Sci. 2014, 1, 1. [Google Scholar]
- Dixon, P.; Fentzke, R.C.; Bhattacharya, A.; Konar, A.; Hazra, S.; Chauhan, A. In vitro drug release and in vivo safety of vitamin E and cysteamine loaded contact lenses. Int. J. Pharm. 2018, 544, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Nien, C.J.; Flynn, K.J.; Jester, J.V. Evaluation of topical cysteamine therapy in the CTNS(-/-) knockout mouse using in vivo confocal microscopy. Mol. Vis. 2011, 17, 2649–2654. [Google Scholar] [PubMed]
- Koganti, R.; Yadavalli, T.; Shukla, D. Current and Emerging Therapies for Ocular Herpes Simplex Virus Type-1 Infections. Microorganisms 2019, 7, 429. [Google Scholar] [CrossRef] [PubMed]
- Kalezic, T.; Mazen, M.; Kuklinski, E.; Asbell, P. Herpetic eye disease study: Lessons learned. Curr. Opin. Ophthalmol. 2018, 29, 340–346. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Choksi, H.H.; Desai, A.R.; Patel, A.S.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. pH triggered controlled drug delivery from contact lenses: Addressing the challenges of drug leaching during sterilization and storage. Colloids Surf. B Biointerfaces 2017, 157, 72–82. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef]
- Zidan, G.; Greene, C.A.; Etxabide, A.; Rupenthal, I.D.; Seyfoddin, A. Gelatine-based drug-eluting bandage contact lenses: Effect of PEGDA concentration and manufacturing technique. Int. J. Pharm. 2021, 599, 120452. [Google Scholar] [CrossRef]
- McAvoy, K.; Jones, D.; Thakur, R.R.S. Synthesis and Characterisation of Photocrosslinked poly(ethylene glycol) diacrylate Implants for Sustained Ocular Drug Delivery. Pharm. Res. 2018, 35, 1–17. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhang, Y.; Fang, D.; Xu, B.; Zhang, L.; Chen, T.; Ren, K.; Nie, Y.; Yao, S.; et al. Liposomes as a Novel Ocular Delivery System for Brinzolamide: In Vitro and In Vivo Studies. AAPS PharmSciTech 2015, 17, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.A.; Borja, N.L. Epalrestat: An Aldose Reductase Inhibitor for the Treatment of Diabetic Neuropathy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2008, 28, 646–655. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J. Therapeutic Contact Lenses with Polymeric Vehicles for Ocular Drug Delivery: A Review. Materials 2018, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Bastiancich, C.; Danhier, P.; Préat, V. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J. Control. Release 2016, 243, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Obireddy, S.R.; Lai, W.-F. Preparation and use of nanogels as carriers of drugs. Drug Deliv. 2021, 28, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Swift, K.J.; Booker, J.D. Casting Processes. In Manufacturing Process Selection Handbook; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Dang, H.; Dong, C.; Zhang, L. Sustained latanoprost release from PEGylated solid lipid nanoparticle-laden soft contact lens to treat glaucoma. Pharm. Dev. Technol. 2022, 27, 127–133. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H. In vitro and in vivo evaluation of brimonidine loaded silica nanoparticles-laden silicone contact lenses to manage glaucoma. J. Biomater. Appl. 2022, 37, 333–343. [Google Scholar] [CrossRef]
- Werner, L. Intraocular Lenses: Overview of Designs, Materials, and Pathophysiologic Features. Ophthalmology 2021, 128, e74–e93. [Google Scholar] [CrossRef]
- Werner, L. Biocompatibility of intraocular lens materials. Curr. Opin. Ophthalmol. 2008, 19, 41–49. [Google Scholar] [CrossRef]
- Lambert, S.R.; Cotsonis, G.; DuBois, L.; Nizam Ms, A.; Kruger, S.J.; Hartmann, E.E.; Weakley, D.R., Jr.; Drews-Botsch, C.G. Infant Aphakia Treatment Study, Long-term Effect of Intraocular Lens vs. Contact Lens Correction on Visual Acuity After Cataract Surgery During Infancy: A Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 365–372. [Google Scholar] [CrossRef]
- Liu, H.; Wu, L.; Fu, S.; Hou, Y.; Liu, P.; Cui, H.; Liu, J.; Xing, L.; Zhang, X. Polylactide-glycoli acid and rapamycin coating intraocular lens prevent posterior capsular opacification in rabbit eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 247, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Wong, T.T.; Mehta, J.S. Intraocular lens as a drug delivery reservoir. Curr. Opin. Ophthalmol. 2013, 24, 53–59. [Google Scholar] [CrossRef]
- Topete, A.; Saramago, B.; Serro, A.P. Intraocular lenses as drug delivery devices. Int. J. Pharm. 2021, 602, 120613. [Google Scholar] [CrossRef] [PubMed]
- Filipe, H.P.; Bozukova, D.; Pimenta, A.; Vieira, A.P.; Oliveira, A.; Galante, R.; Topete, A.; Másson, M.; Alves, P.; Coimbra, P.; et al. Moxifloxacin-loaded acrylic intraocular lenses: In vitro and in vivo performance. J. Cataract. Refract. Surg. 2019, 45, 1808–1817. [Google Scholar] [CrossRef]
- Ongkasin, K.; Masmoudi, Y.; Wertheimer, C.M.; Hillenmayer, A.; Eibl-Lindner, K.H.; Badens, E. Supercritical fluid technology for the development of innovative ophthalmic medical devices: Drug loaded intraocular lenses to mitigate posterior capsule opacification. Eur. J. Pharm. Biopharm. 2020, 149, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhu, S.; Liu, D.; Wen, S.; Lin, Q. Antiproliferative drug-loaded multi-functionalized intraocular lens for reducing posterior capsular opacification. J. Biomater. Sci. Polym. Ed. 2021, 32, 735–748. [Google Scholar] [CrossRef]
- Topete, A.; Tang, J.; Ding, X.; Filipe, H.P.; Saraiva, J.A.; Serro, A.P.; Lin, Q.; Saramago, B. Dual drug delivery from hydrophobic and hydrophilic intraocular lenses: In-vitro and in-vivo studies. J. Control. Release 2020, 326, 245–255. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Cai, J.-P.; Ma, X.-Y.; Li, Y.; Cheng, J.-W.; Wei, R.-L. Sustained Release of 5-Fluorouracil from Chitosan Nanoparticles Surface Modified Intra Ocular Lens to Prevent Posterior Capsule Opacification: An In Vitro and In Vivo Study. J. Ocul. Pharmacol. Ther. 2013, 29, 208–215. [Google Scholar] [CrossRef]
- Xiang, Y.; Zou, M.; Zhang, Y.; Jin, R.; Nie, Y. Drug-loaded and Blue-ray Filtered Hydrogel as a Potential Intraocular Lens for Cataract Treatment. Pharm. Nanotechnol. 2020, 8, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Mohammadi, S.S.; Falavarjani, K.G. Intravitreal Methotrexate. J. Ophthalmic Vis. Res. 2021, 657–669. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.-M.; Tassaing, T.; Jérôme, C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: A review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Toffoletto, N.; Salema-Oom, M.; Igea, S.A.; Alvarez-Lorenzo, C.; Saramago, B.; Serro, A. Drug-Loaded Hydrogels for Intraocular Lenses with Prophylactic Action against Pseudophakic Cystoid Macular Edema. Pharmaceutics 2021, 13, 976. [Google Scholar] [CrossRef] [PubMed]
- Wielders, L.H.; Lambermont, V.A.; Schouten, J.S.; Biggelaar, F.J.V.D.; Worthy, G.; Simons, R.W.; Winkens, B.; Nuijts, R.M. Prevention of Cystoid Macular Edema After Cataract Surgery in Nondiabetic and Diabetic Patients: A Systematic Review and Meta-Analysis. Am. J. Ophthalmol. 2015, 160, 968–981.e33. [Google Scholar] [CrossRef]
- Topete, A.; Barahona, I.; Santos, L.F.; Pinto, C.A.; Saraiva, J.A.; Serro, A.P.; Saramago, B. The effects of addition of functional monomers and molecular imprinting on dual drug release from intraocular lens material. Int. J. Pharm. 2021, 600, 120513. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Fichman, G.; Schneider, J. Dopamine Self-Polymerization as a Simple and Powerful Tool to Modulate the Viscoelastic Mechanical Properties of Peptide-Based Gels. Molecules 2021, 26, 1363. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, X.; Tang, J.; Han, Y.; Lin, Q. Drug-Eluting Hydrophilic Coating Modification of Intraocular Lens via Facile Dopamine Self-Polymerization for Posterior Capsular Opacification Prevention. ACS Biomater. Sci. Eng. 2021, 7, 1065–1073. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Nubile, M.; Acerra, G.; Detta, N.; Pelusi, L.; Lanzini, M.; Mattioli, S.; Santalucia, M.; Pietrangelo, L.; Allegretti, M.; et al. Bioengineered Human Stromal Lenticule for Recombinant Human Nerve Growth Factor Release: A Potential Biocompatible Ocular Drug Delivery System. Front. Bioeng. Biotechnol. 2022, 10, 887414. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M. Small incision lenticule extraction (SMILE) history, fundamentals of a new refractive surgery technique and clinical outcomes. Eye Vis. 2014, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, L.; Lanzini, M.; Dua, H.S.; Uffizi, A.D.; Di Nicola, M.; Calienno, R.; Bondì, J.; Said, D.G.; Nubile, M. In Vivo Evaluation of Corneal Nerves and Epithelial Healing After Treatment with Recombinant Nerve Growth Factor for Neurotrophic Keratopathy. Am. J. Ophthalmol. 2020, 217, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, M.; Lambiase, A.; Schmidl, D.; Schmetterer, L.; Ferrari, M.; Mantelli, F.; Allegretti, M.; Garhoefer, G. Effect of recombinant human nerve growth factor eye drops in patients with dry eye: A phase IIa, open label, multiple-dose study. Br. J. Ophthalmol. 2020, 104, 127–135. [Google Scholar] [CrossRef] [PubMed]
| Clinical Application | OIs Materials | OIs Fabrication Methods | Drug-Loaded | Drug-Loading Technique | Reference |
|---|---|---|---|---|---|
| Ocular Infections | Sodium Hyaluronate (HA) combined with Hydroxypropyl-β-Cyclodextrin (Permeation Enhancer) | Cross-linking | Cyclosporine (CyS) | Soaking | [30] |
| Hydroxypropyl Methylcellulose (HPMC) combined with Hyaluronate (HA) | Freeze-Drying Technique | N-Terminus of Human Lactoferrin (hLF 1-11) | Directly addition to polymeric solution | [31] | |
| Alginate combined with Butyl Methacrylate (BMC) Or Lauryl Methacrylate (LMC) | Grafting Method | Linezolid (LNZ) | Directly addition to polymeric solution | [32] | |
| Polycaprolactone (PCL)/Polyethylene Glycol (PEG) combined with Sodium Alginate (SA) Or Thiolated Sodium Alginate (TSA) | Electrospinning | Besifloxacin Hcl (BH) | Permeation Enhancer Hydroxypropyl- β -Cyclodextrin | [33] | |
| Polyvinylpyrrolidone (PVP) nanofibers | Electrospinning | Azithromycin (AZM) | Poly-lactic-co-glycolic acid (PLGA) Nanoparticles | [34] | |
| Posterior Ocular Diseases | Polycaprolactone (PCL) nanofibers | Electrospinning | Fluocinolone Acetonide (FA) | Directly addition to polymeric solution | [35] |
| Polyvinyl Alcohol (PVA) combined with Polyvinylpyrrolidone K30 (PVP-K30) and Propylene Glycol (PGL) | Grafting Method | Progesterone (PG) | Permeation Enhancer β -Cyclodextrin | [36] | |
| Hydroxyethyl Cellulose (HEC) and Polyvinyl Alcohol (PVA) | Spontaneous emulsification | Ketorolac Tromethamine (KT) | Eudragit® L100 commercial Nanoparticles | [37] | |
| Ocular Inflammatory conditions | Methylcellulose (MC) and Polyvinyl Alcohol (PVA) | Solvent casting | Atorvastatin Calcium (ATC) | Polycaprolactone (PCL) Nanoparticles | [38] |
| Coated stainless steel | Commercial | Ketorolac Tromethamine (KT) | Polymethyl methacrylate (PMMA) Microspheres | [39,40] |
| Clinical Application | OIs Materials | OIs Fabrication Methods | Drug-Loaded | Drug-Loading Technique | Reference |
|---|---|---|---|---|---|
| Ocular Inflammatory conditions | Silicon Hydrogel | Commercial (ACUVUE® OASYS® and ACUVUE TruEyeTM) | Pirfenidone (PFD), Ketorolac Tromethamine (KT) and Flurbiprofen Sodium (FS) | Vitamin E coating | [62,63] |
| Poly-2-Hydroxyethyl Methacrylate(pHEMA) | Microemulsion | Diclofenac sodium (DFNa) | Soaking | [64] | |
| 2-HydroxyethylMethacrylate (HEMA) nd N-Vinyl-2-Pyrrolidone (NVP) | Solvent Casting | Levofloxacin (LEV) | Poly (sulfobetaine methacrylate) (PSBMA) based Nanogel | [65] | |
| Ocular Infections | Hydrogel: Methacrylic acid (MAA) | Polymerization of monomers mixture | Acyclovir (ACV) and Valacyclovir (VACV) | Soaking | [66] |
| Chitosan, Glycerol and Polyethylene Glycol (PEG) | Cast molding method | Moxifloxacin (MF) and dexamethasone (DM) | Drugs solution added to polymeric formulation | [67] | |
| Posterior Ocular Diseases | Hydrogel: N-vinyl pyrrolidone (NVP), 2-hydroxyethyl methacrylate (HEMA) ethylene glycol dimethacrylate (EGDMA), allyl methacrylate (AMA), e-Butyl-hydroxycyclohexyl (TBE) and Poloxamer | Polymerization monomers mixture | Naringenin (NAR) | Direct Drug Entrapment and Soaking | [68] |
| Hydrogel: 2-Hydroxyethyl Methacrylate (HEMA) and Monomethacryloxypropyl-Sym-Polydimethylsiloxane Hydroxypropyl terminated (MCS-MC12) | Polymerization monomers mixture | Epalrestat (EPS) | Soaking | [69] | |
| Glaucoma | Polylactic Acid (PLA) | 3D printing | Timolol Maleate (TM) | Drugs solution added to polymeric formulation | [70] |
| Silicone | Polymerizaztion of monomers mixture | Brinzolamide (BRZ) | Soaking | [71] | |
| Dry Eye Syndrome | Hydroxyethyl Methacrylate (HEMA) and Ethylene Glycol Dimethacrylate (EGDMA) | Photopolymerization | Cyclosporine (CyS) | Cholesterol-Hyaluronate (C-HA) Micelles | [72] |
| Hydrogel: Poly-2-Hydroxyethyl Methacrylate(pHEMA) | Microemulsion | Diclofenac sodium (DFNa) | Soaking | [64] |
| Clinical Application | OIs Materials | OIs Fabrication Methods | Drug-Loaded | Drug-Loading Technique | Reference |
|---|---|---|---|---|---|
| Ocular Infections | 2-Hydroxylethyl methacrylate (HEMA) and Methyl Methacrylate (MMA) | Cross-linking | Moxifloxacin (MXF) | Soaking | [99] |
| Acrylic | Commercial | Methotrexate (MTX) | Supercritical impregnation technology | [100] | |
| Posterior Capsule Opacification | Hyaluronic Acid (HA) and Chitosan (CHI) | Layer by Layer (LbL) | Paclitaxel (Pac) | Chemical Bonding | [101] |
| Acrylic G-free® material [ethylene glycol phenyl ether acrylate (EGPE), 2-hydroxyethyl methacrylate (HEMA) and poly (propylene glycol) dimethacrylate (PPGDMA)] | Commercial | Moxifloxacin (MXF) and Diclofenac (DFN) | Molecular Imprinting | [102] | |
| Poly-Methyl-Methacrylate (PMMA) | Commercial | 5-fluorouracil (5-Fu) | Chitosan Nanoparticles | [103] | |
| Ocular Inflammatory conditions | Hydrogel: 2-hydroxyethyl methacrylate (HEMA), Methyl Methacrylate (MMA), Methacrylic acid (MAA) | Free-radical polymerization | Indomethacin (IND) | Directly addition to polymeric solution | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelusi, L.; Mandatori, D.; Mastropasqua, L.; Agnifili, L.; Allegretti, M.; Nubile, M.; Pandolfi, A. Innovation in the Development of Synthetic and Natural Ocular Drug Delivery Systems for Eye Diseases Treatment: Focusing on Drug-Loaded Ocular Inserts, Contacts, and Intraocular Lenses. Pharmaceutics 2023, 15, 625. https://doi.org/10.3390/pharmaceutics15020625
Pelusi L, Mandatori D, Mastropasqua L, Agnifili L, Allegretti M, Nubile M, Pandolfi A. Innovation in the Development of Synthetic and Natural Ocular Drug Delivery Systems for Eye Diseases Treatment: Focusing on Drug-Loaded Ocular Inserts, Contacts, and Intraocular Lenses. Pharmaceutics. 2023; 15(2):625. https://doi.org/10.3390/pharmaceutics15020625
Chicago/Turabian StylePelusi, Letizia, Domitilla Mandatori, Leonardo Mastropasqua, Luca Agnifili, Marcello Allegretti, Mario Nubile, and Assunta Pandolfi. 2023. "Innovation in the Development of Synthetic and Natural Ocular Drug Delivery Systems for Eye Diseases Treatment: Focusing on Drug-Loaded Ocular Inserts, Contacts, and Intraocular Lenses" Pharmaceutics 15, no. 2: 625. https://doi.org/10.3390/pharmaceutics15020625
APA StylePelusi, L., Mandatori, D., Mastropasqua, L., Agnifili, L., Allegretti, M., Nubile, M., & Pandolfi, A. (2023). Innovation in the Development of Synthetic and Natural Ocular Drug Delivery Systems for Eye Diseases Treatment: Focusing on Drug-Loaded Ocular Inserts, Contacts, and Intraocular Lenses. Pharmaceutics, 15(2), 625. https://doi.org/10.3390/pharmaceutics15020625