Downstream Processing of Amorphous and Co-Amorphous Olanzapine Powder Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Amorphous and Co-Amorphous Olanzapine
2.2.2. Characterization of Pure Amorphous and Co-Amorphous Olanzapine
2.2.3. Formulation of Tablets
2.2.4. Blending
2.2.5. Tableting
2.2.6. Quantification of the Fraction of Amorphous and Co-Amorphous Olanzapine
2.2.7. Stability Studies
2.2.8. Characterization of Blends and Tablets
2.2.9. Statistical Analysis
3. Results and Discussion
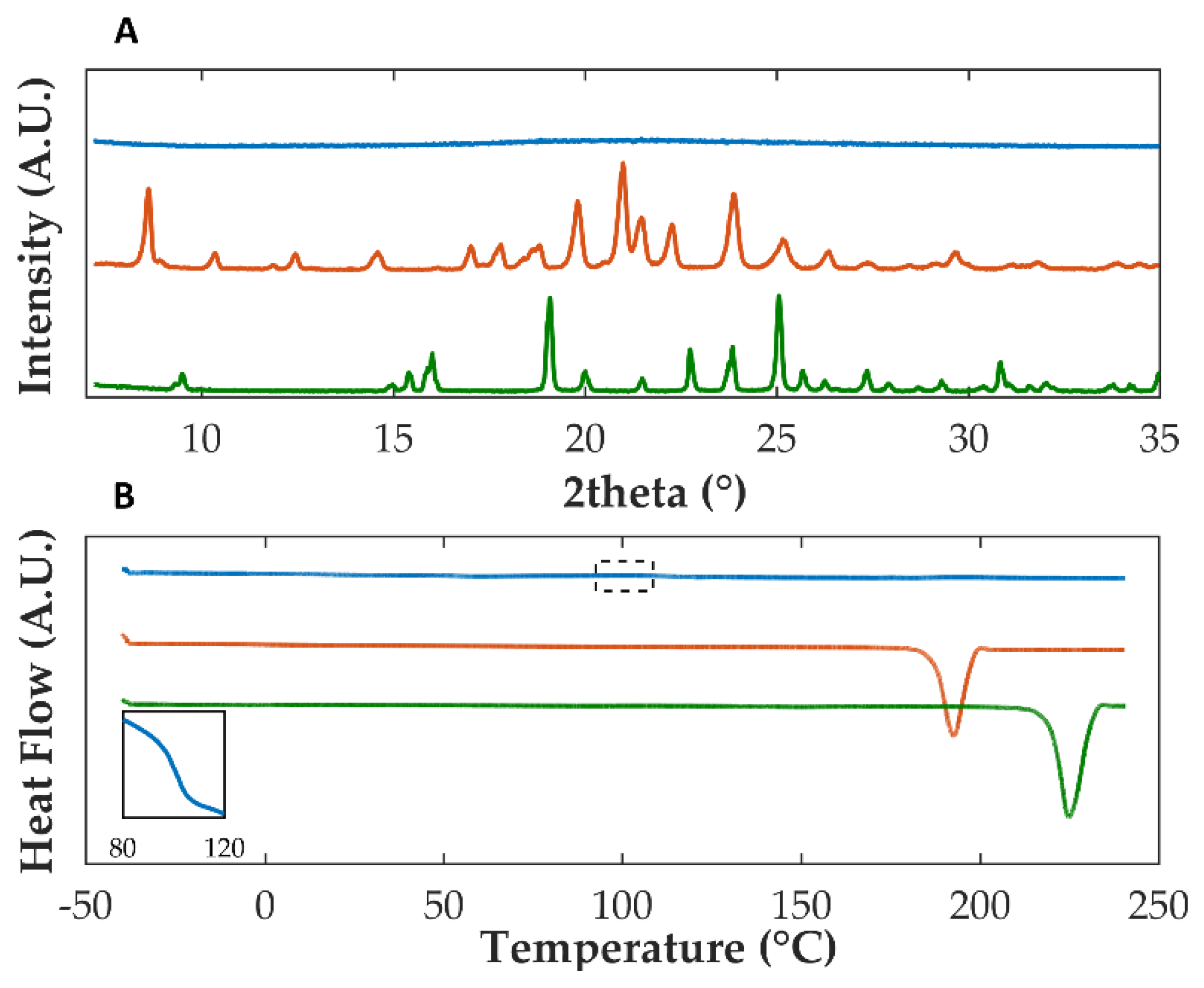
3.1. Assessment of the Amorphization and Co-Amorphization of Olanzapine
3.2. Rheological Characterization of Crystalline, Amorphous, and Co-Amorphous Olanzapine
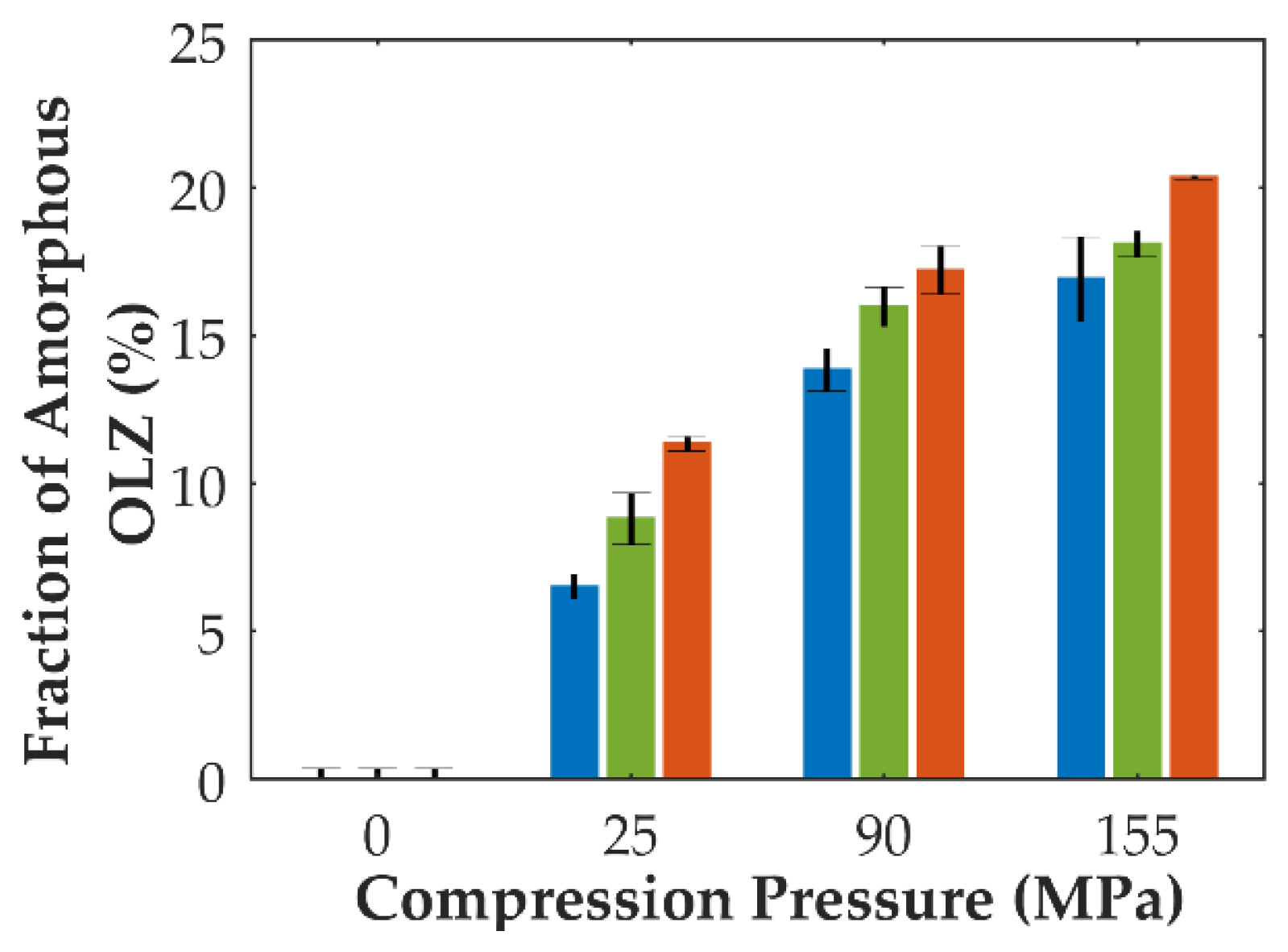
3.3. Pressure-Induced Amorphization
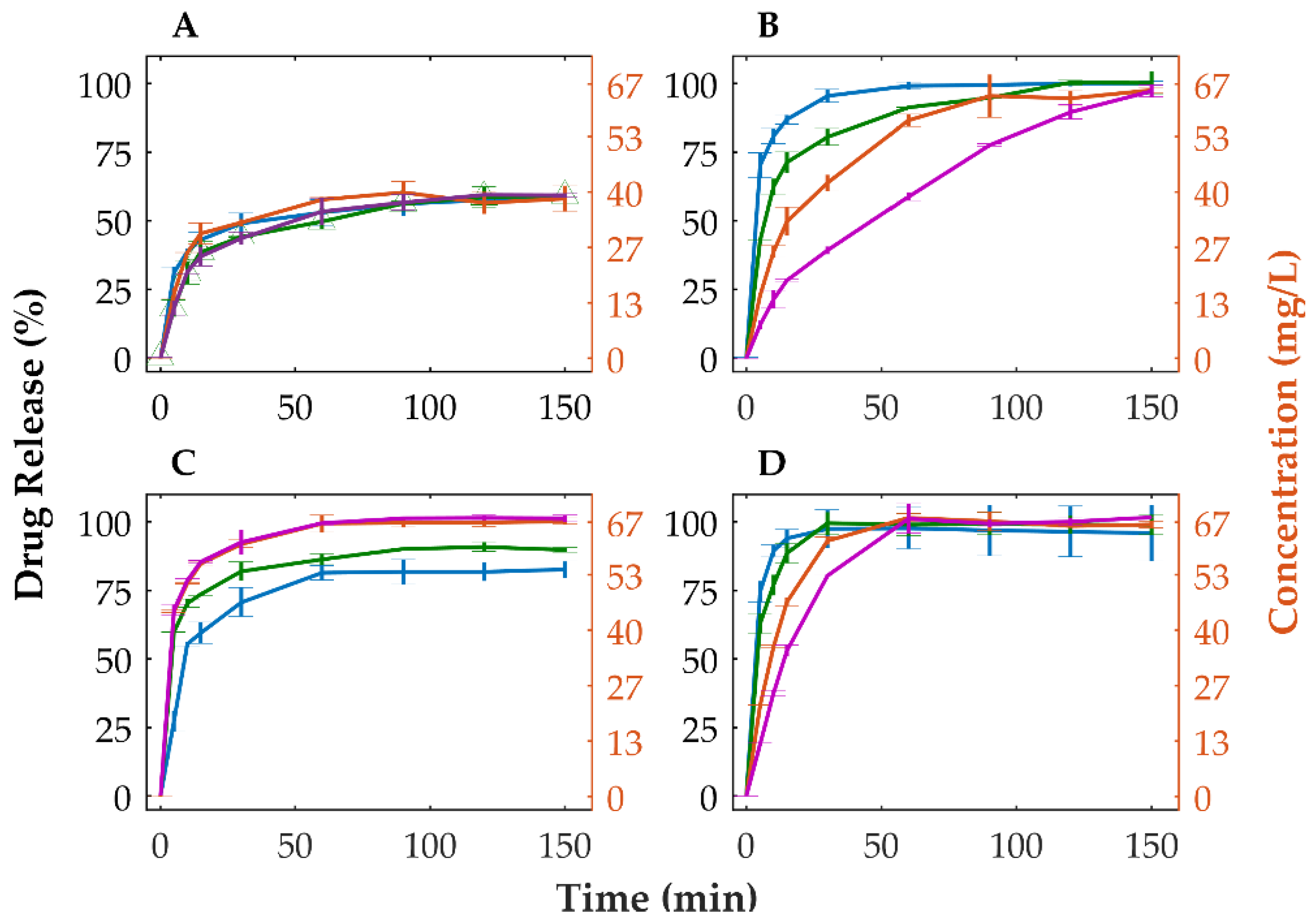
3.4. Tableting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Silva, F.L.O.; Marques, M.B.D.F.; Kato, K.C.; Carneiro, G. Nanonization techniques to overcome poor water-solubility with drugs. Expert Opin. Drug Discov. 2020, 15, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Thayer, A.M. Finding solutions. Chem. Eng. News 2010, 88, 13–18. [Google Scholar] [CrossRef]
- Mohammadzadeh, R.; Javadzadeh, Y. An overview on oral drug delivery via nano-based formulations. Pharm. Biomed. Res. 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Hetal, T.; Bindesh, P.; Sneha, T. A review on techniques for oral bioavailability enhancement of drugs. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 203–223. [Google Scholar]
- Pathak, K.; Raghuvanshi, S. Oral Bioavailability: Issues and Solutions via Nanoformulations. Clin. Pharmacokinet. 2015, 54, 325–357. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Vimalson, D.C.; Parimalakrishnan, S.; Jeganathan, N.S.; Anbazhagan, S. Techniques to enhance solubility of hydrophobic drugs: An overview. Asian J. Pharm. 2016, 10, S67–S75. [Google Scholar] [CrossRef]
- Liu, J.; Grohganz, H.; Löbmann, K.; Rades, T.; Hempel, N.-J.J. Co-Amorphous Drug Formulations in Numbers: Recent Advances in Co-Amorphous Drug Formulations with Focus on Co-Formability, Molar Ratio, Preparation Methods, Physical Stability, In Vitro and In Vivo Performance, and New Formulation Strategies. Pharmaceutics 2021, 13, 389. [Google Scholar] [CrossRef]
- Lim, L.M.; Park, J.-W.; Hadinoto, K. Benchmarking the Solubility Enhancement and Storage Stability of Amorphous Drug–Polyelectrolyte Nanoplex against Co-Amorphous Formulation of the Same Drug. Pharmaceutics 2022, 14, 979. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Ueda, H.; Takata, Y.; Minamihata, K.; Wakabayashi, R.; Kamiya, N.; Goto, M. Co-amorphous formation of piroxicam-citric acid to generate supersaturation and improve skin permeation. Eur. J. Pharm. Sci. 2021, 158, 105667. [Google Scholar] [CrossRef]
- Narala, S.; Nyavanandi, D.; Srinivasan, P.; Mandati, P.; Bandari, S.; Repka, M.A. Pharmaceutical Co-crystals, Salts, and Co-amorphous Systems: A novel opportunity of hot-melt extrusion. J. Drug Deliv. Sci. Technol. 2021, 61, 102209. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, R.; Löbmann, K.; Strachan, C.J.; Grohganz, H.; Rades, T. Emerging trends in the stabilization of amorphous drugs. Int. J. Pharm. 2013, 453, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ueda, H.; Löbmann, K.; Rades, T.; Grohganz, H. Organic acids as co-formers for co-amorphous systems—Influence of variation in molar ratio on the physicochemical properties of the co-amorphous systems. Eur. J. Pharm. Biopharm. 2018, 131, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rades, T.; Grohganz, H. The influence of moisture on the storage stability of co-amorphous systems. Int. J. Pharm. 2021, 605, 120802. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Feng, Y.; Yuan, D.; Xu, R.; Jiang, C.; Xiao, X.; Lu, S. Design and molecular insights of drug-active metabolite based co-amorphous formulation: A case study of toltrazuril-ponazuril co-amorphous. Int. J. Pharm. 2022, 615, 121475. [Google Scholar] [CrossRef]
- Löbmann, K.; Grohganz, H.; Laitinen, R.; Strachan, C.; Rades, T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs—Part 1: Preparation, stability and dissolution enhancement. Eur. J. Pharm. Biopharm. 2013, 85, 873–881. [Google Scholar] [CrossRef]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Löbmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.; Fan, H. Evaluation of Drug Dissolution Rate in Co-amorphous and Co-crystal Binary Drug Delivery Systems by Thermodynamic and Kinetic Methods. AAPS PharmSciTech 2021, 22, 21. [Google Scholar] [CrossRef]
- da Costa, N.F.; Fernandes, A.I.; Pinto, J.F. Measurement of the amorphous fraction of olanzapine incorporated in a co-amorphous formulation. Int. J. Pharm. 2020, 588, 119716. [Google Scholar] [CrossRef]
- Huang, C.; Klinzing, G.; Procopio, A.; Yang, F.; Ren, J.; Burlage, R.; Zhu, L.; Su, Y. Understanding Compression-Induced Amorphization of Crystalline Posaconazole. Mol. Pharm. 2019, 16, 825–833. [Google Scholar] [CrossRef]
- Bastos, A.C.; Santos, I.A.; Pinto, J.F.; Fernandes, A.I. Co-formability, solubility enhancement and stability of olanzapine co-amorphous systems produced with different co-formers. Ann. Med. 2021, 53, S112–S113. [Google Scholar] [CrossRef]
- Fael, H.; Demirel, A.L. Indomethacin co-amorphous drug-drug systems with improved solubility, supersaturation, dissolution rate and physical stability. Int. J. Pharm. 2021, 600, 120448. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, D.L.; Anand, V.S.K.; Nair, A.R.; Sree, K.N.; Dengale, S.J.; Bhat, K. Considerations for the selection of co-formers in the preparation of co-amorphous formulations. Int. J. Pharm. 2021, 602, 120649. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Q.; Wang, J.-R.R.; Lin, K.-L.L.; Mei, X. Amino acids as co-amorphous excipients for tackling the poor aqueous solubility of valsartan. Pharm. Dev. Technol. 2017, 22, 69–76. [Google Scholar] [CrossRef]
- Chavan, R.B.; Thipparaboina, R.; Kumar, D.; Shastri, N.R. Co amorphous systems: A product development perspective. Int. J. Pharm. 2016, 515, 403–415. [Google Scholar] [CrossRef]
- Ruponen, M.; Kettunen, K.; Pires, M.S.; Laitinen, R. Co-amorphous formulations of furosemide with arginine and p-glycoprotein inhibitor drugs. Pharmaceutics 2021, 13, 171. [Google Scholar] [CrossRef]
- Adahalli, S.B.; Talluri, M. Formulation and evaluation of tablet prepared by coamorphous system containing anti-hypertensive and anti-hyperlipidemic drug. Int. J. Pharm. Pharm. Sci. 2016, 8, 182–193. [Google Scholar] [CrossRef][Green Version]
- Lenz, E.; Jensen, K.T.; Blaabjerg, L.I.; Knop, K.; Grohganz, H.; Löbmann, K.; Rades, T.; Kleinebudde, P. Solid-state properties and dissolution behaviour of tablets containing co-amorphous indomethacin–arginine. Eur. J. Pharm. Biopharm. 2015, 96, 44–52. [Google Scholar] [CrossRef]
- Ojarinta, R.; Saarinen, J.; Strachan, C.J.; Korhonen, O.; Laitinen, R. Preparation and characterization of multi-component tablets containing co-amorphous salts: Combining multimodal non-linear optical imaging with established analytical methods. Eur. J. Pharm. Biopharm. 2018, 132, 112–126. [Google Scholar] [CrossRef]
- Balbão, M.S.; Hallak, J.E.C.; Nunes, E.A.; de Mello, M.H.; Triffoni-Melo, A.T.; Ferreira, F.I.S.; Chaves, C.; Durão, A.M.S.; Ramos, A.P.P.; Crippa, J.A.S.; et al. Olanzapine, weight change and metabolic effects: A naturalistic 12-month follow up. Ther. Adv. Psychopharmacol. 2014, 4, 30–36. [Google Scholar] [CrossRef]
- Mauri, M.C.; Volonteri, L.S.; Colasanti, A.; Fiorentini, A.; De Gaspari, I.F.; Bareggi, S.R. Clinical Pharmacokinetics of Atypical Antipsychotics. Clin. Pharmacokinet. 2007, 46, 359–388. [Google Scholar] [CrossRef]
- Sarmah, K.K.; Nath, N.; Rao, D.R.; Thakuria, R. Mechanochemical synthesis of drug–drug and drug–nutraceutical multicomponent solids of olanzapine. CrystEngComm 2020, 22, 1120–1130. [Google Scholar] [CrossRef]
- Sood, S.; Jawahar, N.; Jain, K.; Gowthamarajan, K.; Meyyanathan, S.N. Olanzapine Loaded Cationic Solid Lipid Nanoparticles for Improved Oral Bioavailability. Curr. Nanosci. 2013, 9, 26–34. [Google Scholar] [CrossRef]
- Cavallari, C.; Fini, A.; Ceschel, G. Design of olanzapine/lutrol solid dispersions of improved stability and performances. Pharmaceutics 2013, 5, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Aburub, A.; Sun, C.C. Direct Compression Tablet Containing 99% Active Ingredient—A Tale of Spherical Crystallization. J. Pharm. Sci. 2019, 108, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Fara, D.A.; Al-Hmoud, L.; Rashid, I.; Chowdhry, B.Z.; Badwan, A. Understanding the Performance of a Novel Direct Compression Excipient Comprising Roller Compacted Chitin. Mar. Drugs 2020, 18, 115. [Google Scholar] [CrossRef]
- Thalberg, K.; Lindholm, D.; Axelsson, A. Comparison of different flowability tests for powders for inhalation. Powder Technol. 2004, 146, 206–213. [Google Scholar] [CrossRef]
- Garg, V.; Mallick, S.S.; Garcia-Trinanes, P.; Berry, R.J. An investigation into the flowability of fine powders used in pharmaceutical industries. Powder Technol. 2018, 336, 375–382. [Google Scholar] [CrossRef]
- Sarraguça, M.C.; Cruz, A.V.; Soares, S.O.; Amaral, H.R.; Costa, P.C.; Lopes, J.A. Determination of flow properties of pharmaceutical powders by near infrared spectroscopy. J. Pharm. Biomed. Anal. 2010, 52, 484–492. [Google Scholar] [CrossRef]
- Jager, P.D.; Bramante, T.; Luner, P.E. Assessment of Pharmaceutical Powder Flowability using Shear Cell-Based Methods and Application of Jenike’s Methodology. J. Pharm. Sci. 2015, 104, 3804–3813. [Google Scholar] [CrossRef]
- De Campos, M.M.; Ferreira, M.D.C. A comparative analysis of the flow properties between two alumina-based dry powders. Adv. Mater. Sci. Eng. 2013, 2013, 519846. [Google Scholar] [CrossRef]
- Polla, G.I.; Vega, D.R.; Lanza, H.; Tombari, D.G.; Baggio, R.; Ayala, A.P.; Filho, J.M.; Fernández, D.; Leyva, G.; Dartayet, G. Thermal behaviour and stability in Olanzapine. Int. J. Pharm. 2005, 301, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, E.C.; Salam, A.M.; Aziz, A.R. Cohesiveness and Flowability Properties of Silica Gel Powder. Phys. Int. 2010, 1, 16–21. [Google Scholar] [CrossRef]
- Council of Europe. Pharmaceutical Technical Procedures; Council of Europe: Strasbourg, France, 2020. [Google Scholar]
- Fell, J.T.; Newton, J.M. Determination of Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Pina, M.F.; Zhao, M.; Pinto, J.F.; Sousa, J.J.; Craig, D.Q.M. The influence of drug physical state on the dissolution enhancement of solid dispersions prepared via hot-melt extrusion: A case study using olanzapine. J. Pharm. Sci. 2014, 103, 1214–1223. [Google Scholar] [CrossRef]
- Cruz-Angeles, J.; Videa, M.; Martínez, L.M. Highly Soluble Glimepiride and Irbesartan Co-amorphous Formulation with Potential Application in Combination Therapy. AAPS PharmSciTech 2019, 20, 144. [Google Scholar] [CrossRef]
- Santos, I.A.; Bastos, A.C.; Pinto, J.F.; Fernandes, A.I. Characterisation and stability of co-amorphous systems containing olanzapine and sulphonic acids. Ann. Med. 2021, 53 (Suppl. 1), S111. [Google Scholar] [CrossRef]
- Szabó, E.; Démuth, B.; Galata, D.L.; Vass, P.; Hirsch, E.; Csontos, I.; Marosi, G.; Nagy, Z.K. Continuous formulation approaches of amorphous solid dispersions: Significance of powder flow properties and feeding performance. Pharmaceutics 2019, 11, 654. [Google Scholar] [CrossRef]
- Fitzpatrick, J.J.; Hodnett, M.; Twomey, M.; Cerqueira, P.S.M.; O’Flynn, J.; Roos, Y.H. Glass transition and the flowability and caking of powders containing amorphous lactose. Powder Technol. 2007, 178, 119–128. [Google Scholar] [CrossRef]
- Brockbank, K.; Armstrong, B.; Chandorkar, Y.; Freeman, T. Understanding powder caking as a consequence of a range of mechanisms by means of powder rheometry. Part. Sci. Technol. 2015, 33, 102–108. [Google Scholar] [CrossRef]
- Freeman, T.; Brockbank, K.; Armstrong, B. Measurement and quantification of caking in powders. Procedia Eng. 2015, 102, 35–44. [Google Scholar] [CrossRef]
- Šimek, M.; Grünwaldová, V.; Kratochvíl, B. Comparison of compression and material properties of differently shaped and sized paracetamols. KONA Powder Part. J. 2017, 34, 197–206. [Google Scholar] [CrossRef]
- Kholodovych, V.; Welsh, W.J. Densities of Amorphous and Crystalline Polymers. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 611–617. ISBN 978-0-387-69002-5. [Google Scholar]
- Caccavo, D.; Cascone, S.; Apicella, P.; Lamberti, G.; Barba, A.A. HPMC-based granules for prolonged release of phytostrengtheners in agriculture. Chem. Eng. Commun. 2017, 204, 1333–1340. [Google Scholar] [CrossRef]
- Deb, S.K.; Wilding, M.; Somayazulu, M.; McMillan, P.F. Pressure-induced amorphization and an amorphous—Amorphous transition in densified porous silicon. Nature 2001, 414, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, B.; Wang, L.; Li, D.; Liu, R.; Zou, B.; Cui, T.; Zou, G.; Meng, Y.; Mao, H.-K.; et al. Pressure-induced amorphization and polyamorphism in one-dimensional single-crystal TiO2 nanomaterials. J. Phys. Chem. Lett. 2010, 1, 309–314. [Google Scholar] [CrossRef]
- Richet, P.; Gillet, P. Pressure-induced amorphization of minerals: A review. Eur. J. Mineral. 1997, 9, 907–933. [Google Scholar] [CrossRef]
- Yamanaka, T.; Nagai, T.; Tsuchiya, T. Mechanism of pressure-induced amorphization. Zeitschritt für Krist. 1997, 212, 401–410. [Google Scholar] [CrossRef]
- Ovid’ko, I.A. Amorphization and new mechanisms of plastic deformation in solids. J. Phys. D Appl. Phys. 1994, 27, 999–1007. [Google Scholar] [CrossRef]
- Petzoldt, C.; Bley, O.; Byard, S.J.; Andert, D.; Baumgartner, B.; Nagel, N.; Tappertzhofen, C.; Feth, M.P. An example of how to handle amorphous fractions in API during early pharmaceutical development: SAR114137—A successful approach. Eur. J. Pharm. Biopharm. 2014, 86, 337–350. [Google Scholar] [CrossRef]
- Wabuyele, B.W.; Sotthivirat, S.; Zhou, G.X.; Ash, J.; Dhareshwar, S.S. Dispersive Raman Spectroscopy for Quantifying Amorphous Drug Content in Intact Tablets. J. Pharm. Sci. 2017, 106, 579–588. [Google Scholar] [CrossRef]
- Ruegger, C.E.; Çelick, M. The effect of compression and decompression speed on the mechanical strength of compacts. Pharm. Dev. Technol. 2000, 5, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Schlack, H.; Bauer-Brandl, A.; Schubert, R.; Becker, D. Properties of fujicalin®, a new modified anhydrous dibasic calcium phosphate for direct compression: Comparison with dicalcium phosphate dihydrate. Drug Dev. Ind. Pharm. 2001, 27, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Thakral, N.K.; Thakral, S.; Stephenson, G.A.; Sedlock, R.; Suryanarayanan, R. Compression-Induced Polymorphic Transformation in Tablets: Role of Shear Stress and Development of Mitigation Strategies. J. Pharm. Sci. 2019, 108, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Pitt, K.G.; Heasley, M.G. Determination of the tensile strength of elongated tablets. Powder Technol. 2013, 238, 169–175. [Google Scholar] [CrossRef]
- Cespi, M.; Bonacucina, G.; Casettari, L.; Ronchi, S.; Palmieri, G.F. Effect of temperature increase during the tableting of pharmaceutical materials. Int. J. Pharm. 2013, 448, 320–326. [Google Scholar] [CrossRef]
- Mollan, M.J.; Çelik, M. The effects of lubrication on the compaction and post-compaction properties of directly compressible maltodextrins. Int. J. Pharm. 1996, 144, 1–9. [Google Scholar] [CrossRef]
- Yaakub, N.A.; Anuar, M.S.; Tahir, S.M. Compaction behaviour and mechanical strength of lactose-sodium starch glycolate and lactose-croscarmellose sodium binary tablets. IOP Conf. Ser. Mater. Sci. Eng. 2018, 342, 012026. [Google Scholar] [CrossRef]
- Uzondu, B.; Leung, L.Y.; Mao, C.; Yang, C.Y. A mechanistic study on tablet ejection force and its sensitivity to lubrication for pharmaceutical powders. Int. J. Pharm. 2018, 543, 234–244. [Google Scholar] [CrossRef]
- Kaur, G.; Grewal, J.; Jyoti, K.; Jain, U.K.; Chandra, R.; Madan, J. Oral controlled and sustained drug delivery systems: Concepts, advances, preclinical, and clinical status. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier Inc.: Oxford, UK, 2018; pp. 567–626. ISBN 9780128136898. [Google Scholar]
- FDA. Dissolution Testing and Acceptance Criteria for Immediate-Release Solid Oral Dosage Form Drug Products Containing High Solubility Drug Substances Guidance for Industry; FDA: Silver Spring, MD, USA, 2018.

| Substance | A | B |
|---|---|---|
| OLZ | 30 | 30 |
| SAC | 0 | 18 |
| Dibasic calcium phosphate anhydrous | 45 | 27 * |
| Microcrystalline cellulose | 20 | 20 |
| Polyvinylpyrrolidone | 5 | 5 |
| Cohesion Index (mm) | Cake Strength (g) | Angle of Repose (°) | |
|---|---|---|---|
| OLZ | |||
| Crystalline | 14.3 ± 0.6 | 174.7 ± 3.4 | 51.7 ± 0.1 |
| Amorphous | 15.8 ± 0.3 * | 181.4 ± 9.9 | 52.8 ± 0.3 * |
| OLZ:SAC | |||
| Crystalline | 12.7 ± 0.4 | 159.6 ± 6.9 | 51.0 ± 0.9 |
| Co-amorphous | 25.2 ± 1.2 ** | 180.7 ± 5.9 * | 54.6 ± 1.7 * |
| True Density (g/cm3) | Carr’s Index | |
|---|---|---|
| OLZ | ||
| Crystalline | 1.3053 ± 0.0022 | 39.7 ± 0.5 |
| Amorphous | 1.2764 ± 0.0014 ** | 41.3 ± 0.6 * |
| OLZ:SAC | ||
| Crystalline | 1.3897 ± 0.0017 | 34.0 ± 1.0 |
| Co-amorphous | 1.3501 ± 0.0058 ** | 36.7 ± 1.1 * |
| Tensile Strength (MPa) | Disintegration Time (s) | Work of Compaction (J) | Ejection Force (kN) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compaction Pressure (MPa) | 25 | 90 | 155 | 25 | 90 | 155 | 25 | 90 | 155 | 25 | 90 | 155 |
| Formulation A | ||||||||||||
| Crystalline | 0.12 ± 0.00 | 0.95 ± 0.01 | 1.64 ± 0.04 | 31.0 ± 2.7 | 26.2 ± 3.3 | 64.7 ± 3.1 | 0.184 ± 0.007 | 0.839 ± 0.018 | 1.357 ± 0.011 | 0.014 ± 0.001 | 0.093 ± 0.010 | 0.142 ± 0.014 |
| Amorphous | 0.29 ± 0.00 ** | 1.14 ± 0.02 ** | 2.11 ± 0.08 ** | 686.3 ± 63.2 ** | 2470.7 ± 45.0 ** | 7131.0 ± 201.06 ** | 0.212 ± 0.013 * | 0.890 ± 0.028 ** | 1.422 ± 0.018 ** | 0.018 ± 0.002 | 0.089 ± 0.007 | 0.133 ± 0.012 |
| Formulation B | ||||||||||||
| Crystalline | 0.16 ± 0.01 | 1.01 ± 0.05 | 1.84 ± 0.04 | 56.0 ± 3.6 | 48.7 ± 4.5 | 63.7 ± 5.9 | 0.192 ± 0.005 | 0.902 ± 0.028 | 1.382 ± 0.020 | 0.028 ± 0.004 | 0.163 ± 0.009 | 0.288 ± 0.013 |
| Co-amorphous | 0.59 ± 0.01 ** | 1.97 ± 0.01 ** | 2.74 ± 0.05 ** | 167.0 ± 4.2 ** | 420.0 ± 87.2 ** | 1364.5 ± 12.02 ** | 0.250 ± 0.017 * | 1.090 ± 0.034 ** | 1.754 ± 0.025 ** | 0.054 ± 0.014 | 0.234 ± 0.026 * | 0.433 ± 0.041 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa, N.F.; Daniels, R.; Fernandes, A.I.; Pinto, J.F. Downstream Processing of Amorphous and Co-Amorphous Olanzapine Powder Blends. Pharmaceutics 2022, 14, 1535. https://doi.org/10.3390/pharmaceutics14081535
da Costa NF, Daniels R, Fernandes AI, Pinto JF. Downstream Processing of Amorphous and Co-Amorphous Olanzapine Powder Blends. Pharmaceutics. 2022; 14(8):1535. https://doi.org/10.3390/pharmaceutics14081535
Chicago/Turabian Styleda Costa, Nuno F., Rolf Daniels, Ana I. Fernandes, and João F. Pinto. 2022. "Downstream Processing of Amorphous and Co-Amorphous Olanzapine Powder Blends" Pharmaceutics 14, no. 8: 1535. https://doi.org/10.3390/pharmaceutics14081535
APA Styleda Costa, N. F., Daniels, R., Fernandes, A. I., & Pinto, J. F. (2022). Downstream Processing of Amorphous and Co-Amorphous Olanzapine Powder Blends. Pharmaceutics, 14(8), 1535. https://doi.org/10.3390/pharmaceutics14081535