Abstract
Radiotherapy is an integral part of modern oncology, applied to more than half of all patients diagnosed with cancer. It can be used alone or in combination with surgery or chemotherapy. However, despite the high precision of radiation delivery, irradiation may affect surrounding healthy tissues leading to the development of toxicity. The most common and clinically significant toxicity of radiotherapy is acute and chronic radiation dermatitis, which could result in desquamation, wounds, nonhealing ulcers, and radionecrosis. Moreover, preoperative radiotherapy impairs wound healing after surgery and may lead to severe wound complications. In this review, we comprehensively discuss available types of dressings used in the management of acute and chronic radiation dermatitis and address their efficacy. The most effective ways of preventing acute radiation dermatitis are film dressings, whereas foam dressings were found effective in its treatment. Data regarding dressings in chronic radiation dermatitis are scarce. This manuscript also contains authors’ consensus.
1. Introduction
Radiation-induced dermatitis (RID) is the most frequent side effect of radiotherapy and affects approximately 90–95% of patients exposed to therapeutic radiation, with 87% of patients experiencing moderate-to-severe skin reactions [1]. Acute radiation dermatitis (ARD) usually occurs within 90 days of exposure to ionizing radiation, whereas chronic radiation dermatitis (CRD) may develop many years after the completion of treatment. Both ARD and CRD are associated with radiation exposure of 2–50 Gy [2,3]. ARD occurs mostly in particular sites, namely, the neck, face, extremities, chest, and abdomen [4]. The main symptoms of ARD include erythema, dry and wet desquamations, and ulceration [5]. The manifestation of CRD is more complex and covers events from mild fibrosis to secondary cancers [3].
Modern radiation techniques such as intensity-modulated radiotherapy (IMRT) and computed-tomography-based planning algorithms enabled a significant reduction in the rates of severe RID, mostly due to sparing of the skin and subcutaneous tissue [6]. Nevertheless, moderate and severe forms of RID can still result in serious impairments of patients’ quality of life and may also be a major cause of nonadherence or treatment interruptions [7]. Hence, appropriate and efficient management of RID plays a crucial role in radiation oncology supportive care.
Radiation-associated skin toxicity is complex and dependent on a variety of factors, such as total dose delivered, fractionation regimen, and volume of irradiated tissue, as well as concomitant systemic therapy and comorbidities. Therapeutic radiation exhibits biological effects within hours to weeks after exposure, causing extensive genetic damage irreversibly breaking double strands in nuclear and mitochondrial DNA, and inhibiting cells’ ability to divide and replicate. This damage, along with other structural tissue destruction, generation of reactive oxygen species, a decrease in the functional stem cell population, initiation of epidermal and dermal inflammatory responses, and skin cell necrosis, results in RID [8].
To date, no gold standards in the management of ARD and CRD have been established. Although numerous topical and systemic medications are available for treatment and prevention of radiation-associated skin reactions, conclusions of various clinical trials often contradict each other and lack universality, mostly due to the lack of high-quality and large-sample studies. Therefore, the management of RID is often empirical and commonly based on personal experience supported by weak scientific evidence [9,10].
Although the standard forms of RID management were previously based on topical agents, mostly aqueous or steroid creams, recently more attention has been turned towards various forms of dressings applied either as RID prophylaxis (from the beginning of radiation therapy) or as a treatment form (applied at the onset of skin damage signs). Their potential advantage over topical creams is their common feature of creating a stable moist environment that enables faster re-epithelialization of radiation-damaged skin. Some dressings also possess antimicrobial and anti-inflammatory features that additionally facilitate radiation damage prevention or healing [7,11].
The objective of this narrative review is to discuss various types of dressings previously studied for the treatment and prevention of RID and show options that have proved successful, resulting in satisfactory clinical outcomes. To clearly present this complex issue, we divided the text into the following five sections: description of the main types of dressings, dressings in ARD and CRD, novel technologies and summary with recommendations. The issues of wound healing complications after surgery with prior radiotherapy and vacuum-assisted closure therapy were out of the scope of this narrative review.
2. Types of Dressings and Wound Management
The development of surgery and various dressings subtypes enabled effective wound control. The simplest division covers two groups, non-absorbing (like films) and absorbing (such as foams). The introduction of additional materials or substances allow modification of wound microenvironment, namely exudate, microflora, epithelization, healing and scar formation [12]. The indication for a particular dressing may be based on the acronym T.I.M.E. that describes the general rules of wound preparation. That covers four domains, i.e., tissue management, inflammation and infection control, moisture balance, and epithelial edge advancement [13,14]. The proposed T.I.M.E. for RID was proposed in Supplementary Table S1.
However, the complexity and unpredictability of RID development and different pathophysiology compared to in the case of other wounds limit the easy application of T.I.M.E. into routine practice in radiation oncology. First, the traumatic factor, ionizing radiation, constantly affects tissues for several days. Second, all phenomena may occur at the same time, because various areas of the skin and subcutaneous tissue might receive different doses. Third, microflora of the skin may be also affected by radiation, immunosuppression, and concomitant systemic therapy. Moreover, natural reepithelization usually begins within ten days [15]. Finally, RID-related wound care should consider the possible effect of applied treatment (like thick foam dressing) to the skin that may lead to changes in dose distribution.
3. Acute Radiation Dermatitis
3.1. Definition and Classification
ARD may present in the form of erythema, dry and moist desquamation, skin necrosis, ulcers, as well as bleeding. The Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) have developed a standardized grading system to evaluate acute radiation-induced skin toxicity (Table 1) [16]. A measurement tool has also been created that helps evaluate ARD using both patient symptoms and healthcare professionals’ assessment scales. The tool is called the Radiation-Induced Skin Reaction Assessment Scale (RISRAS) [17]. Another important classification of ARD more frequently used in clinical trials than RTOG/EORTC scale is Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (Table 1). Figure 1 presents an example of grade 2 acute radiation dermatitis.

Table 1.
The classification of acute radiation dermatitis.
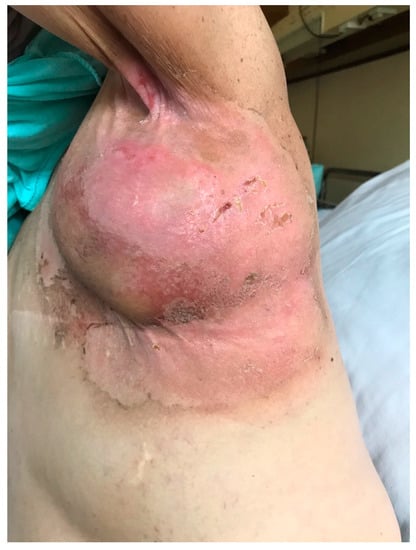
Figure 1.
Grade 2 acute radiation dermatitis.
3.2. Dressings in ARD Prevention and Treatment
Several dressings were investigated in both ARD prophylaxis and treatment. The main aim of their application is the reduction of ARD severity or severity of related symptoms (such as pain) and subsequent improvement of treatment tolerance. Our review focuses on available data of significant scientific evidence obtained from randomized controlled trials and cohort studies. The summary of discussed literature data regarding ARD prevention and treatment is presented in Supplementary Table S2.
3.2.1. Film and Membrane Dressings
Mepitel Film is a semi-permeable dressing/film based on Safetac technology. The film can be used prophylactically, starting from the first day of radiotherapy, and the transparency grants skin appearance assessment without the need for dressing removal [18]. Five randomized trials were conducted that assessed the potential reduction of ARD rates with Mepitel Film used as ARD prophylaxis. A Danish trial by Møller and colleagues involved 101 patients treated for breast cancer who were randomized to cover either the lateral or medial part of their chest with Mepitel film [19]. The primary endpoint was patient-reported symptoms and experience. A secondary endpoint was radiotherapy staff evaluation of dermatitis using the RTOG scale. Out of 79 patients included in the final statistical analysis, a statistically significant proportion reported a lower level of pain, itching, burning sensation as well as a reduced sensitivity within the area covered by Mepitel film. There was no statistical difference in staff-evaluated RID rates on the last day of radiotherapy among the whole study group. In selected groups of patients, i.e., post-mastectomy patients and patients treated with a total dose of 50 Gy, there was a significant difference in ARD rates. However, fourteen days after radiotherapy, the difference in ARD rates was overall non-significant in all groups of patients. The original randomized trial by Herst and colleagues provided evidence of Mepitel film effectiveness in reducing the severity of skin reactions in breast cancer patients [20]. A total of 78 participants contributed data for analysis. Lateral and medial halves of the skin areas to be irradiated were randomized to Mepitel Film or aqueous cream. Skin reaction severity was assessed using RISRAS and RTOG scales. Overall skin reaction severity (RISRAS) was reduced by 92% in favor of Mepitel Film. All patients developed some form of reaction in cream-treated skin, which progressed to moist desquamation in 26% of patients. Only 44% of patients had a skin reaction under the Mepitel Film, which did not progress to moist desquamation in any of the patients. Three other RCTs with relatively smaller sample groups have also been conducted that tested prophylactic use of Mepitel film in head and neck cancer patients. One of them did not reach its primary endpoint due to a limited tolerance of Mepitel film [21]. Two other studies demonstrated reduced risks of developing ARD and a decrease in ARD severity in the study groups [22,23]. The efficacy of Mepitel film in ARD prophylaxis was also demonstrated in a single-arm feasibility study by Yee and colleagues involving 30 patients irradiated for breast and chest wall neoplasms. Complete prevention of RTOG grade 3 ARD and a significant reduction in grade 2 cases were obtained. Moist desquamation, however, could not be completely prevented [24]. A retrospective review carried out by Oshin et al. showed significantly lower rates of moist desquamation after prophylactic use of Mepitel film in patients with breast cancer [25].
Two trials verifying the use of polymeric membrane dressings for ARD treatment in head and neck cancer patients were conducted. A randomized controlled trial by Scott presented mixed outcomes, demonstrating a significant reduction of self-reported pain and improved quality of life with no effect on ARD healing rates [26]. Another study, a single-arm controlled trial by Hegarty and Wong, confirmed polymeric membrane dressings’ superiority over standard care in ARD treatment [27]. Another type of polymeric dressing was also tested for ARD prevention. Two studies conducted by Schmeel et al. investigated the use of Hydrofilm (polyurethane film) dressing in ARD prophylaxis in breast cancer patients. A 2018 randomized controlled trial demonstrated a significant reduction in ARD rates in the study group [28]. Moreover, complete prevention of moist desquamation was also obtained. In a 2019 self-controlled trial, the authors confirmed these findings [29]. The study proved a reduction of staff-assessed dermatitis signs as well as complete prevention of moist desquamation. Furthermore, patient-reported symptoms such as itching, burning, and pain were also significantly diminished in the study group.
A large-sample Japanese study showed a beneficial effect of a thin-film dressing application for ARD prevention in patients undergoing proton beam therapy for prostate cancer [30].
Soft silicone film dressing was recently tested in a randomized controlled trial by Zou and colleagues [31]. The study involved 100 patients treated for various cancer types. The experimental group that received soft polysiloxane film for ARD prophylaxis presented a significantly lower incidence of dermatitis signs than the control group. Also, the healing of ARD lasted shorter in the experimental group.
3.2.2. Foam Dressings
Mepilex Lite (Molnlycke Health Care, Gothenburg, Sweden) is a thin dressing composed of an outer polyurethane film, an absorbent layer, and a soft silicone wound contact layer that enables adherence to wounds with low-to-medium exudate levels. It can be left in place for up to 14 days, whereas the secondary dressing can be changed as frequently as required to avoid irritation of the wound bed [32]. The efficacy of Mepilex Lite in RID management was studied in a clinical trial conducted by Zhong and colleagues who assessed a group of 88 head and neck cancer patients developing ARD during radiotherapy [33]. The study sample of 43 patients who received Mepilex Lite dressings showed significantly faster healing times than the control group (median healing time was 16 vs. 23 days, respectively; p = 0.009). Other parameters impacting patients’ quality of life were also assessed, among which patients’ sleep was significantly improved in the study group. Diggelmann and colleagues studied 24 patients irradiated for breast cancer who developed ARD [34]. Each of the erythematous areas (n = 34) was randomly divided into two groups; the first group was treated with Mepilex Lite dressing and the other with standard care, i.e., an aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas in which Mepilex Lite dressings were applied compared with the control areas. Paterson et al. also confirmed Mepilex Lite efficacy in ARD management [35]. Although the incidence rates of moist desquamation were equal for the study and control (aqueous cream) groups, their trial showed a significant reduction in overall skin reactions severity by 41% (p < 0.001), along with a reduction in the average moist desquamation score by 49% (p = 0.043) in favor of Mepilex-covered skin areas.
3.2.3. Gel Dressings
Hydrogel and hydrocolloid dressings have been used in treating moderate and severe forms of ARD that involve moist desquamation. They facilitate the maintenance of a wet environment over de-epithelialized skin, and thus are considered to accelerate healing.
Three randomized controlled trials investigated hydrogel dressings for treating moist desquamation, the first compared hydrogel to a gentian violet dressing in patients with breast or head and neck cancers [36], the second compared hydrogel to a simple dry dressing for people with ARD in the head and neck, breast and anorectal regions [37], and the third and largest study compared Hydrosorb® (hydrogel dressing without oil components) to a water-based spray in patients treated for breast cancer [38]. The only study that confirmed Hydrogel’s efficacy was the Gollins et al. trial, which showed hydrogel dressing superiority to a gentian violet dressing in terms of healing rates in patients with moist desquamation [36]. Two other trials, with significantly larger study groups, could not confirm these findings, with one study showing even prolonged healing times in the Hydrogel group compared to standard care, i.e., dry dressing.
StrataXRT is a silicone-based film-forming, self-drying, semi-occlusive, non-resorbable, topical gel preparation, consisting of polydimethylsiloxanes, siloxanes, and alkyl methyl silicones. The dressing is designed to promote a moist wound-healing environment. When applied topically, StrataXRT dries to form a thin, flexible, protective layer that is gas permeable and waterproof. This environment leads to rapid wound healing and faster skin recovery [39]. In a relatively large single-blind randomized controlled trial by Chan et al., a reduced risk of developing ARD was obtained with prophylactic application of StrataXRT dressings [40]. The study involved 197 head and neck cancer patients randomized to receive either standard care (Sorbolene cream) or StrataXRT dressing from the beginning of radiation therapy. There was a significantly lower incidence of RTOG grade 2 and 3 ARD in the study group. Also, delayed development of skin toxicity was demonstrated in the dressing group. There was no difference in patient-reported symptoms. Two other studies verifying the prophylactic use of StrataXRT confirmed these findings. An RCT involving 56 patients treated for breast cancer demonstrated a reduction in objectively measured ARD severity in the StrataXRT group [39]. The other study proved StrataXRT’s noninferiority to Mepitel film in ARD prevention [41]. The only trial studying the therapeutic use of StrataXRT for ARD was a prospective study by Quilis et al., which showed a significant improvement in RISRAS score for patients receiving StrataXRT dressings at the onset of dermatitis signs [42].
Application of 3M Cavilon Barrier Film was tested as a form of ARD prevention. Three randomized controlled studies were carried out that involved patients treated for breast cancer. Only one of them demonstrated the superiority of this dressing over sorbolene cream in reducing the rates of moist desquamation and pruritus [43]. Two newer randomized controlled trials did not confirm these findings [44,45].
3.2.4. Silver-Containing Dressings
Silver nylon dressings are nonadhesive nanocrystalline silver-coated material, used clinically as a burn dressing with satisfactory outcomes resulting from its antimicrobial activity against Gram-positive and Gram-negative bacteria as well as some fungal infections [46]. Aquino-Parsons et al. studied 196 patients treated with whole-breast radiation therapy [47]. They showed that there was no benefit of silver-leaf nylon dressings for the prevention of acute grade 3 ARD compared with patients who received standard skin care. However, the incidence of itching in the last week of radiation and one week post-treatment was lower among the patients who used the dressings. The Niazi et al. study compared prophylactic use of silver clear nylon dressing vs. no prophylaxis with sulfadiazine cream applied at the onset of RID [48]. The trial demonstrated a significant reduction in the severity of RID in the study group on the last day of treatment. However, the skin reaction assessed two weeks after treatment completion showed no statistically significant difference between the study and control groups. Prophylactic use of silver dressings also proved to be effective in a single-arm controlled trial by Vuong et al., which covered 30 patients treated for gynecological and anal cancers [49]. The mean dermatitis score was significantly lower in patients who used dressings for RID prevention in comparison with a historical control group. A self-controlled trial by Vavassis et al., which studied silver nylon dressings for the treatment of ARD in patients with head and neck cancers, showed no superiority of these dressings over the standard care in terms of RID severity measured using the RTOG scale [50]. There was, however, a significant improvement in pain control in favor of silver dressing.
3.2.5. Biodressings
Biodressings are highly advanced biomaterials that combine conventional fibers with bioactive molecules such as growth factors or stem cells [51]. The main aim of their use is to speed up the healing process. Such biomaterials were used in the management of severe ARD. In a small prospective observational study, Lee and colleagues demonstrated a significant acceleration in the healing of severe ARD in patients irradiated for head and neck cancers treated with a foam dressing containing epidermal growth factor [52]. A dressing in the form of a gauze impregnated with granulocyte-macrophage colony-stimulating factor tested by Kouvaris et al. showed to be efficacious in the prevention and treatment of ARD in women undergoing radiotherapy for vulvar cancer [53].
The use of lyophilized and irradiated human amniotic membrane as a biological dressing for grade 2 and 3 ARD treatment was evaluated in an Indian study by Lobo-Gajiwala and Sharma [54]. The authors used such a biodressing in fourteen patients who developed moist desquamation in groin folds and natal cleft after pelvic radiotherapy. They observed rapid healing of ARD in all treated patients, achieving a median of seven days after dressing application.
3.2.6. Other Dressings
A retrospective cohort analysis by Bonomo et al. showed an improvement in treatment tolerability in head and neck cancer patients undergoing radiotherapy with concurrent cetuximab who received calcium alginate dressings for the therapy of severe ARD involving moist desquamation [55]. In another analysis, the dry non-adherent absorbent dressing was shown to be ineffective in ARD treatment in patients with head and neck cancers [56].
4. Chronic Radiation Dermatitis
4.1. Definition and Classification
CRD is an irreversible and progressive complication of radiotherapy, which usually influences patients’ quality of life. It can occur suddenly even several years after irradiation. CRD covers chronic ulcers including necrosis, radiation-induced keratosis, telangiectasias, fibrosis, as well as secondary skin cancers [3]. The RTOG/EORTC classification of CRD was shown in Table 2. Figure 2 presents an example of grade 1 skin fibrosis with anthropic changes.

Table 2.
The classification of chronic radiation dermatitis according to the Radiation Therapy Oncology Group/the European Organization for Research and Treatment of Cancer (EORTC).
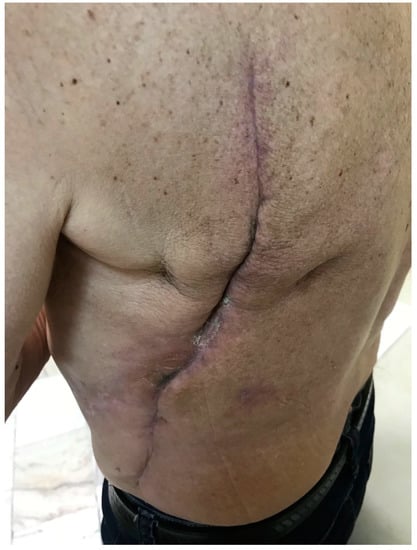
Figure 2.
Grade 1 chronic radiation dermatitis.
CTCAE classification does not directly mention the term CRD but describes its several related manifestations, namely, all events related to late fibrosis, ulceration, and necrosis.
4.2. Dressings in CRD Prevention and Treatment
Whereas radiation-induced fibrosis is mostly managed with other methods, the most frequent indications for dressings in CRD are chronic ulceration and necrosis. Unfortunately, the available data are scarce. There are no randomized nor single-arm prospective trials regarding the efficacy of modern dressings in the management of severe CRD. Thus, we did not prepare a similar summary of evidence as in the case of ARD. However, several authors reported such attempts in retrospective analyses and case reports. The most important and promising dressings for ulcers and necrosis related to CRD are biodressings. Below, we have described and discussed biodressings that were investigated in humans.
American researchers reported a case of a patient with chronic radiation necrosis treated with a lyopreserved placental membrane containing viable cells [57]. The 73-year-old woman received postoperative radiotherapy due to squamous cell carcinoma of the right medial ankle, and she developed a chronic necrotic wound refractory to conventional treatment (collagen dressings, honey-impregnated dressings, topical and oral antibiotics). It was decided to use vLPM (GrafixPL PRIME®; Osiris Therapeutics, Inc., Columbia, MD, USA). According to the description, this material contains a lyopreserved placental tissue allograft that retains the extracellular matrix, growth factors, and endogenous neonatal mesenchymal stem cells, fibroblasts, and epithelial cells of the native tissue. The dressing provided the desired effect (re-epithelialization and wound closure) after 98 days without any significant adverse events.
Another case report described a 59-year-old woman with radiation necrosis on the trunk which developed after total body electron irradiation with a relatively low dose of 36 Gy for cutaneous T-cell lymphoma [58]. CRD was confirmed by biopsy. The wound was treated with PDGF BB 0.01 gel (Regranex® 0.01 gelanssen-Cilag, Neuss, Germany), covered by a hydrophilic copolymer membrane (Omiderm®; Omikron Scientific Ltd., Rehovot, Israel, distributed by Idel Medical Service, Hamburg, Germany). The authors reported re-epithelialization and granulation as well as pain reduction.
A group of Italian researchers obtained excellent results in the treatment of 20 patients with severe CRD with autologous adipose-derived stem cells therapy, including patients with ulcers, necrosis, fibrosis, telangiectasia, atrophy, and retraction [59]. A significant clinical improvement was observed in all but one patient.
Recombinant human platelet-derived growth factor-BB (rhPDGF) gel was found effective in another 47-year-old patient who suffered from chronic radiation-induced ulceration of the neck area that persisted for 12 years [60]. He underwent radiochemotherapy for locally advanced nasopharyngeal carcinoma. Six months of RhPDGF gel application enabled satisfactory granulation to apply a split-thickness skin graft.
5. Novel Technologies
Recent developments in modern dressings used in cancer patients are focused on two main groups, namely, interactive dressings and biodressings. The first group includes various substances that affect wound microenvironment. Intensively studied and described examples are hyaluronic acid, alginate, and collagen-coated dressings [61]. However, none of them was proven effective in RID prevention or treatment.
Biodressings constitute the most promising group of modern dressings that may have a significant role in RID prevention and treatment in the future. Biodressings provide the gradual release of the integrated biomolecules that enable faster healing. An interesting and promising approach is the use of functional hydrogels that present complex properties ensuring proper wound healing [62,63]. Especially interesting are the multifunctional photoresponsive hydrogels that combine pro-healing effect of light-based therapy with properties of hydrogels. Other studies also describe the various hydrogels integrated with biopolymers that may accelerate healing of post-radiation wounds [64,65,66,67,68]. Several biodressing groups were studied and widely described in the literature [61,69,70,71,72]. However, this group is rarely investigated in patients who undergo radiotherapy. The reason could be the risk of decrease of lethal damage caused by ionizing radiation to cellular DNA. Photon radiotherapy acts mostly through free radicals. Thus, antioxidative properties of the biodressings may affect efficacy of radiotherapy [73]. As a result, this group of dressings should be used with caution in patients with ARD.
6. Summary and Recommendations
Available data suggest that the use of dressings as a preventive measure in RID management may be both efficacious and cost-effective, reducing skin-related complications and preventing treatment interruptions. Based on available evidence, film dressings could be a valuable prevention method against ARD. Foam dressings may be used for the treatment of severe ARD, whereas hydrogel dressings seem to be ineffective in that indication. Figure 3 presents authors’ consensus and recommendation regarding prevention and treatment of ARD.
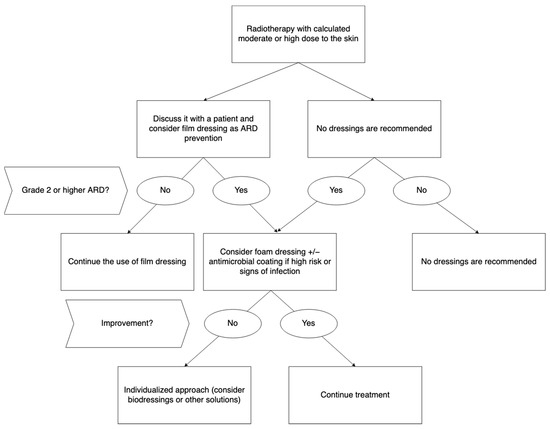
Figure 3.
Recommended dressings for prevention and treatment of acute radiation dermatitis. Abbreviations: ARD—acute radiation dermatitis.
Nevertheless, due to the high heterogeneity of RID manifestation, some patients require an individualized approach. Moreover, due to the lack of additional analyses (real clinical benefit measured in oncological outcomes, cost-effectiveness), any of the discussed dressings cannot be recommended as a part of routine clinical practice.
Evidence-based data on the use of any dressings in ulceration and necrosis as a manifestation of CRD are scarce. Biodressings are a promising group of modern dressing that showed preliminary efficacy in both ARD and CRD. However, further investigations of their potential are warranted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics14061204/s1, Table S1: Proposed T.I.M.E. wound bed preparation for acute and chronic radiation dermatitis, Table S2: Dressings for prevention and treatment of acute radiation dermatitis [19,20,21,22,23,24,25,26,27,28,29,30,31,33,34,35,36,37,38,39,40,42,43,44,45,47,48,49,50,52,53,54,55,56,74].
Author Contributions
Conceptualization, K.Z. and M.J.S.; methodology, K.Z. and M.J.S.; software, K.Z. and M.J.S.; validation, M.J.S.; formal analysis, K.Z. and M.J.S.; investigation, K.Z.; resources, K.Z. and M.J.S.; data curation, K.Z.; writing—original draft preparation, K.Z. and M.J.S.; writing—review and editing, K.Z., M.J.S. and P.R.; visualization, K.Z. and M.J.S.; supervision, M.J.S. and P.R.; project administration, K.Z. and M.J.S.; funding acquisition K.Z., M.J.S. and P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The processing charges cost was covered by the Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland.
Institutional Review Board Statement
The study does not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chan, R.J.; Webster, J.; Chung, B.; Marquart, L.; Ahmed, M.; Garantziotis, S. Prevention and Treatment of Acute Radiation-Induced Skin Reactions: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Cancer 2014, 14, 53. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.N.; Simmons, B.J.; Wolfson, A.H.; Nouri, K. Acute and Chronic Cutaneous Reactions to Ionizing Radiation Therapy. Dermatol. Ther. 2016, 6, 185–206. [Google Scholar] [CrossRef] [Green Version]
- Spałek, M. Chronic Radiation-Induced Dermatitis: Challenges and Solutions. Clin. Cosmet. Investig. Dermatol. 2016, 9, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.R.; Rzucidlo, E. Acute and Chronic Radiation Injury. J. Vasc. Surg. 2011, 53, 15S–21S. [Google Scholar] [CrossRef] [PubMed]
- Kiprian, D.; Szykut-Badaczewska, A.; Gradzińska, A.; Czuwara, J.; Rudnicka, L. How to Manage Radiation-Induced Dermatitis? Nowotw. J. Oncol. 2022, 72, 86–95. [Google Scholar] [CrossRef]
- Pignol, J.-P.; Olivotto, I.; Rakovitch, E.; Gardner, S.; Sixel, K.; Beckham, W.; Vu, T.T.T.; Truong, P.; Ackerman, I.; Paszat, L. A Multicenter Randomized Trial of Breast Intensity-Modulated Radiation Therapy to Reduce Acute Radiation Dermatitis. J. Clin. Oncol. 2008, 26, 2085–2092. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, A.; Israilevich, R.; Moy, R. Management of Acute Radiation Dermatitis: A Review of the Literature and Proposal for Treatment Algorithm. J. Am. Acad. Dermatol. 2019, 81, 558–567. [Google Scholar] [CrossRef]
- Fowble, B.; Yom, S.S.; Yuen, F.; Arron, S. Skin Care in Radiation Oncology: A Practical Guide; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-31460-0. [Google Scholar]
- Bolderston, A.; Lloyd, N.S.; Wong, R.K.S.; Holden, L.; Robb-Blenderman, L.; Supportive Care Guidelines Group of Cancer Care Ontario Program in Evidence-Based Care. The Prevention and Management of Acute Skin Reactions Related to Radiation Therapy: A Systematic Review and Practice Guideline. Support. Care Cancer 2006, 14, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, F.; Mathew, L.M.; Schwartz, R.A. Radiation Dermatitis: An Overview. Int. J. Dermatol. 2017, 56, 909–914. [Google Scholar] [CrossRef]
- Singh, M.; Alavi, A.; Wong, R.; Akita, S. Radiodermatitis: A Review of Our Current Understanding. Am. J. Clin. Dermatol. 2016, 17, 277–292. [Google Scholar] [CrossRef]
- A Practical Guide to the Most Commonly Used Dressings in Wound Care. Available online: https://www.thepmfajournal.com/error-page/a-practical-guide-to-the-most-commonly-used-dressings-in-wound-care (accessed on 31 May 2022).
- Halim, A.S.; Khoo, T.L.; Saad, A.Z.M. Wound Bed Preparation from a Clinical Perspective. Indian J. Plast. Surg. 2012, 45, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Wound Bed Preparation in Practice EWMA Position Document—Wounds International. Available online: https://www.woundsinternational.com/resources/details/wound-bed-preparation-practice-ewma-position-document (accessed on 1 June 2022).
- Mendelsohn, F.A.; Divino, C.M.; Reis, E.D.; Kerstein, M.D. Wound Care after Radiation Therapy. Adv. Skin Wound Care 2002, 15, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity Criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Noble-Adams, R. Radiation-Induced Skin Reactions. 2: Development of a Measurement Tool. Br. J. Nurs. 1999, 8, 1208–1211. [Google Scholar] [CrossRef]
- Fernández-Castro, M.; Martín-Gil, B.; Peña-García, I.; López-Vallecillo, M.; García-Puig, M.E. Effectiveness of Semi-Permeable Dressings to Treat Radiation-Induced Skin Reactions. A Systematic Review. Eur. J. Cancer Care 2017, 26, e12685. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.K.; Olling, K.; Berg, M.; Habæk, I.; Haislund, B.; Iversen, A.-M.; Ewertz, M.; Lorenzen, E.L.; Brink, C. Breast Cancer Patients Report Reduced Sensitivity and Pain Using a Barrier Film during Radiotherapy—A Danish Intra-Patient Randomized Multicentre Study. Tech. Innov. Patient Support Radiat. Oncol. 2018, 7, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Herst, P.M.; Bennett, N.C.; Sutherland, A.E.; Peszynski, R.I.; Paterson, D.B.; Jasperse, M.L. Prophylactic Use of Mepitel Film Prevents Radiation-Induced Moist Desquamation in an Intra-Patient Randomised Controlled Clinical Trial of 78 Breast Cancer Patients. Radiother. Oncol. 2014, 110, 137–143. [Google Scholar] [CrossRef]
- Rades, D.; Narvaez, C.A.; Splettstößer, L.; Dömer, C.; Setter, C.; Idel, C.; Ribbat-Idel, J.; Perner, S.; Bartscht, T.; Olbrich, D.; et al. A Randomized Trial (RAREST-01) Comparing Mepitel® Film and Standard Care for Prevention of Radiation Dermatitis in Patients Irradiated for Locally Advanced Squamous Cell Carcinoma of the Head-and-Neck (SCCHN). Radiother. Oncol. 2019, 139, 79–82. [Google Scholar] [CrossRef]
- Yan, J.; Yuan, L.; Wang, J.; Li, S.; Yao, M.; Wang, K.; Herst, P.M. Mepitel Film Is Superior to Biafine Cream in Managing Acute Radiation-Induced Skin Reactions in Head and Neck Cancer Patients: A Randomised Intra-Patient Controlled Clinical Trial. J. Med. Radiat. Sci. 2020, 67, 208–216. [Google Scholar] [CrossRef]
- Wooding, H.; Yan, J.; Yuan, L.; Chyou, T.-Y.; Gao, S.; Ward, I.; Herst, P.M. The Effect of Mepitel Film on Acute Radiation-Induced Skin Reactions in Head and Neck Cancer Patients: A Feasibility Study. Br. J. Radiol. 2018, 91, 20170298. [Google Scholar] [CrossRef]
- Yee, C.; Lam, E.; Gallant, F.; Karam, I.; Czarnota, G.; Soliman, H.; Wong, G.; Drost, L.; Vesprini, D.; Rakovitch, E.; et al. A Feasibility Study of Mepitel Film for the Prevention of Breast Radiation Dermatitis in a Canadian Center. Pract. Radiat. Oncol. 2021, 11, e36–e45. [Google Scholar] [CrossRef]
- Oshin, F.; McBrayne, L.; Bratt, M.; Lucier, M.; McKenzie, A.; Vasiliadis, S.; Agapito, C.; D’Alimonte, L. A Retrospective Chart Review on the Prophylactic Use of Mepitel Film for Breast Cancer Patients Undergoing Chest Wall Irradiation: A Single-Institution Experience. J. Med. Imaging Radiat. Sci. 2020, 51, S3–S4. [Google Scholar] [CrossRef]
- Scott, A. Polymeric Membrane Dressings for Radiotherapy-Induced Skin Damage. Br. J. Nurs. 2014, 23, S24, S26–S31. [Google Scholar] [CrossRef] [Green Version]
- Hegarty, F.; Wong, M. Polymeric Membrane Dressing for Radiotherapy-Induced Skin Reactions. Br. J. Nurs. 2014, 23 (Suppl. S20), S38–S46. [Google Scholar] [CrossRef]
- Schmeel, L.C.; Koch, D.; Stumpf, S.; Leitzen, C.; Simon, B.; Schüller, H.; Vornholt, S.; Schoroth, F.; Müdder, T.; Röhner, F.; et al. Prophylactically Applied Hydrofilm Polyurethane Film Dressings Reduce Radiation Dermatitis in Adjuvant Radiation Therapy of Breast Cancer Patients. Acta Oncol. 2018, 57, 908–915. [Google Scholar] [CrossRef] [Green Version]
- Schmeel, L.C.; Koch, D.; Schmeel, F.C.; Bücheler, B.; Leitzen, C.; Mahlmann, B.; Kunze, D.; Heimann, M.; Brüser, D.; Abramian, A.-V.; et al. Hydrofilm Polyurethane Films Reduce Radiation Dermatitis Severity in Hypofractionated Whole-Breast Irradiation: An Objective, Intra-Patient Randomized Dual-Center Assessment. Polymers 2019, 11, 2112. [Google Scholar] [CrossRef] [Green Version]
- Arimura, T.; Ogino, T.; Yoshiura, T.; Toi, Y.; Kawabata, M.; Chuman, I.; Wada, K.; Kondo, N.; Nagayama, S.; Hishikawa, Y. Effect of Film Dressing on Acute Radiation Dermatitis Secondary to Proton Beam Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 472–476. [Google Scholar] [CrossRef]
- Zou, M.Y.; Xu, D.J.; Zhang, R.; Wang, Y.X.; Li, L.; Huang, J.T.; Ji, X.Y. Study on Prevention of Acute Radiodermatitis with Soft Silicone Film Dressing. Indian J. Pharm. Sci. 2021, 83, 49–56. [Google Scholar] [CrossRef]
- White, R. Evidence for Atraumatic Soft Silicone Dressing Use. Wounds 2005, 1, 104–109. [Google Scholar]
- Zhong, W.-H.; Tang, Q.-F.; Hu, L.-Y.; Feng, H.-X. Mepilex Lite Dressings for Managing Acute Radiation Dermatitis in Nasopharyngeal Carcinoma Patients: A Systematic Controlled Clinical Trial. Med. Oncol. 2013, 30, 761. [Google Scholar] [CrossRef] [PubMed]
- Diggelmann, K.V.; Zytkovicz, A.E.; Tuaine, J.M.; Bennett, N.C.; Kelly, L.E.; Herst, P.M. Mepilex Lite Dressings for the Management of Radiation-Induced Erythema: A Systematic Inpatient Controlled Clinical Trial. Br. J. Radiol. 2010, 83, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Paterson, D.; Poonam, P.; Bennett, N.C.; Peszynski, R.; Beekhuizen, M.; Herst, P. Randomized Intra-Patient Controlled Trial of Mepilex Lite Dressings versus Aqueous Cream in Managing Radiation-Induced Skin Reactions Post-Mastectomy. J. Cancer Sci. Ther. 2012, 4, 347–356. [Google Scholar] [CrossRef]
- Gollins, S.; Gaffney, C.; Slade, S.; Swindell, R. RCT on Gentian Violet versus a Hydrogel Dressing for Radiotherapy-Induced Moist Skin Desquamation. J. Wound Care 2008, 17, 268–275. [Google Scholar] [CrossRef]
- Macmillan, M.S.; Wells, M.; MacBride, S.; Raab, G.M.; Munro, A.; MacDougall, H. Randomized Comparison of Dry Dressings versus Hydrogel in Management of Radiation-Induced Moist Desquamation. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 864–872. [Google Scholar] [CrossRef]
- Bazire, L.; Fromantin, I.; Diallo, A.; de la Lande, B.; Pernin, V.; Dendale, R.; Fourquet, A.; Savignoni, A.; Kirova, Y.M. Hydrosorb® versus Control (Water Based Spray) in the Management of Radio-Induced Skin Toxicity: Results of Multicentre Controlled Randomized Trial. Radiother. Oncol. 2015, 117, 229–233. [Google Scholar] [CrossRef]
- Ahn, S.; Sung, K.; Kim, H.J.; Choi, Y.E.; Lee, Y.K.; Kim, J.S.; Lee, S.K.; Roh, J.-Y. Reducing Radiation Dermatitis Using a Film-Forming Silicone Gel during Breast Radiotherapy: A Pilot Randomized-Controlled Trial. In Vivo 2020, 34, 413–422. [Google Scholar] [CrossRef]
- Chan, R.J.; Blades, R.; Jones, L.; Downer, T.-R.; Peet, S.C.; Button, E.; Wyld, D.; McPhail, S.; Doolan, M.; Yates, P. A Single-Blind, Randomised Controlled Trial of StrataXRT®—A Silicone-Based Film-Forming Gel Dressing for Prophylaxis and Management of Radiation Dermatitis in Patients with Head and Neck Cancer. Radiother. Oncol. 2019, 139, 72–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EP-1286 StrataXRT Is Non Inferior to Mepitel Film in Preventing Radiation Induced Moist Desquamation|Request PDF. Available online: https://www.researchgate.net/publication/333212705_EP-1286_StrataXRT_is_non_inferior_to_Mepitel_Film_in_preventing_radiation_induced_moist_desquamation (accessed on 31 March 2022).
- Quilis, A.; Martín, J.; Rodríguez, C.; Sánchez, P.; Ribes, J.L. Reducing Radiation Dermatitis during Ongoing Radiation Therapy: An Innovative Film-Forming Wound Dressing. J. Radiat. Oncol. 2018, 7, 255–264. [Google Scholar] [CrossRef]
- Graham, P.; Browne, L.; Capp, A.; Fox, C.; Graham, J.; Hollis, J.; Nasser, E. Randomized, Paired Comparison of No-Sting Barrier Film versus Sorbolene Cream (10% Glycerine) Skin Care during Postmastectomy Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 241–246. [Google Scholar] [CrossRef]
- Lam, A.C.; Yu, E.; Vanwynsberghe, D.; O’Neil, M.; D’Souza, D.; Cao, J.; Lock, M. Phase III Randomized Pair Comparison of a Barrier Film vs. Standard Skin Care in Preventing Radiation Dermatitis in Post-Lumpectomy Patients with Breast Cancer Receiving Adjuvant Radiation Therapy. Cureus 2019, 11, e4807. [Google Scholar] [CrossRef] [Green Version]
- Shaw, S.-Z.; Nien, H.-H.; Wu, C.-J.; Lui, L.T.; Su, J.-F.; Lang, C.-H. 3M Cavilon No-Sting Barrier Film or Topical Corticosteroid (Mometasone Furoate) for Protection against Radiation Dermatitis: A Clinical Trial. J. Formos. Med. Assoc. 2015, 114, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Ren, H.; Guo, X.; Hu, C.; Fu, J. Radiation-Induced Skin Injury: Pathogenesis, Treatment, and Management. Aging 2020, 12, 23379–23393. [Google Scholar] [CrossRef]
- Aquino-Parsons, C.; Lomas, S.; Smith, K.; Hayes, J.; Lew, S.; Bates, A.T.; Macdonald, A.G. Phase III Study of Silver Leaf Nylon Dressing vs Standard Care for Reduction of Inframammary Moist Desquamation in Patients Undergoing Adjuvant Whole Breast Radiation Therapy. J. Med. Imaging Radiat. Sci. 2010, 41, 215–221. [Google Scholar] [CrossRef]
- Niazi, T.M.; Vuong, T.; Azoulay, L.; Marijnen, C.; Bujko, K.; Nasr, E.; Lambert, C.; Duclos, M.; Faria, S.; David, M.; et al. Silver Clear Nylon Dressing Is Effective in Preventing Radiation-Induced Dermatitis in Patients with Lower Gastrointestinal Cancer: Results from a Phase III Study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e305–e310. [Google Scholar] [CrossRef]
- Vuong, T.; Franco, E.; Lehnert, S.; Lambert, C.; Portelance, L.; Nasr, E.; Faria, S.; Hay, J.; Larsson, S.; Shenouda, G.; et al. Silver Leaf Nylon Dressing to Prevent Radiation Dermatitis in Patients Undergoing Chemotherapy and External Beam Radiotherapy to the Perineum. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 809–814. [Google Scholar] [CrossRef]
- Vavassis, P.; Gelinas, M.; Chabot Tr, J.; Nguyen-Tân, P.F. Phase 2 Study of Silver Leaf Dressing for Treatment of Radiation-Induced Dermatitis in Patients Receiving Radiotherapy to the Head and Neck. J. Otolaryngol. Head Neck Surg. 2008, 37, 124–129. [Google Scholar]
- Beele, H. Artificial Skin: Past, Present and Future. Int. J. Artif. Organs 2002, 25, 163–173. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Hong, J.P.; Shon, M.W.; Ryu, S.; Ahn, S.D. Foam Dressing with Epidermal Growth Factor for Severe Radiation Dermatitis in Head and Neck Cancer Patients. Int. Wound J. 2014, 13, 390–393. [Google Scholar] [CrossRef]
- Kouvaris, J.R.; Kouloulias, V.E.; Plataniotis, G.A.; Balafouta, E.J.; Vlahos, L.J. Dermatitis during Radiation for Vulvar Carcinoma: Prevention and Treatment with Granulocyte-Macrophage Colony-Stimulating Factor Impregnated Gauze. Wound Repair Regen. 2001, 9, 187–193. [Google Scholar] [CrossRef]
- Lobo Gajiwala, A.; Sharma, V. Use of Irradiated Amnion as a Biological Dressing in the Treatment of Radiation Induced Ulcers. Cell Tissue Bank. 2003, 4, 147–150. [Google Scholar] [CrossRef]
- Bonomo, P.; Desideri, I.; Loi, M.; Ciccone, L.P.; Lo Russo, M.; Becherini, C.; Greto, D.; Simontacchi, G.; Pimpinelli, N.; Livi, L. Management of Severe Bio-Radiation Dermatitis Induced by Radiotherapy and Cetuximab in Patients with Head and Neck Cancer: Emphasizing the Role of Calcium Alginate Dressings. Support. Care Cancer 2019, 27, 2957–2967. [Google Scholar] [CrossRef]
- Mak, S.S.; Zee, C.Y.; Molassiotis, A.; Chan, S.J.; Leung, S.F.; Mo, K.F.; Johnson, P.J. A Comparison of Wound Treatments in Nasopharyngeal Cancer Patients Receiving Radiation Therapy. Cancer Nurs. 2005, 28, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Regulski, M.J.; Danilkovitch, A.; Saunders, M.C. Management of a Chronic Radiation Necrosis Wound with Lyopreserved Placental Membrane Containing Viable Cells. Clin. Case Rep. 2019, 7, 456–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollina, U.; Liebold, K.; Konrad, H. Treatment of Chronic Radiation Ulcers with Recombinant Platelet-Derived Growth Factor and a Hydrophilic Copolymer Membrane. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 455–457. [Google Scholar] [CrossRef]
- Rigotti, G.; Marchi, A.; Galiè, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical Treatment of Radiotherapy Tissue Damage by Lipoaspirate Transplant: A Healing Process Mediated by Adipose-Derived Adult Stem Cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef]
- Hom, D.B.; Manivel, J.C. Promoting Healing with Recombinant Human Platelet-Derived Growth Factor--BB in a Previously Irradiated Problem Wound. Laryngoscope 2003, 113, 1566–1571. [Google Scholar] [CrossRef]
- Pavel, T.I.; Chircov, C.; Rădulescu, M.; Grumezescu, A.M. Regenerative Wound Dressings for Skin Cancer. Cancers 2020, 12, 2954. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Maleki, A.; He, J.; Bochani, S.; Nosrati, V.; Shahbazi, M.-A.; Guo, B. Multifunctional Photoactive Hydrogels for Wound Healing Acceleration. ACS Nano 2021, 15, 18895–18930. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Fabrication of Apigenin Loaded Gellan Gum-Chitosan Hydrogels (GGCH-HGs) for Effective Diabetic Wound Healing. Int. J. Biol. Macromol. 2016, 91, 1110–1119. [Google Scholar] [CrossRef]
- Vedakumari, W.S.; Ayaz, N.; Karthick, A.S.; Senthil, R.; Sastry, T.P. Quercetin Impregnated Chitosan-Fibrin Composite Scaffolds as Potential Wound Dressing Materials—Fabrication, Characterization and in Vivo Analysis. Eur. J. Pharm. Sci. 2017, 97, 106–112. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.M.S. Synergic Formulation of Onion Peel Quercetin Loaded Chitosan-Cellulose Hydrogel with Green Zinc Oxide Nanoparticles towards Controlled Release, Biocompatibility, Antimicrobial and Anticancer Activity. Int. J. Biol. Macromol. 2019, 132, 784–794. [Google Scholar] [CrossRef]
- Jangde, R.; Srivastava, S.; Singh, M.R.; Singh, D. In Vitro and In Vivo Characterization of Quercetin Loaded Multiphase Hydrogel for Wound Healing Application. Int. J. Biol. Macromol. 2018, 115, 1211–1217. [Google Scholar] [CrossRef]
- Ajmal, G.; Bonde, G.V.; Thokala, S.; Mittal, P.; Khan, G.; Singh, J.; Pandey, V.K.; Mishra, B. Ciprofloxacin HCl and Quercetin Functionalized Electrospun Nanofiber Membrane: Fabrication and Its Evaluation in Full Thickness Wound Healing. Artif. Cells Nanomed. Biotechnol. 2019, 47, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound Dressings—A Review. Biomedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart Bandages: The Future of Wound Care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Konop, M.; Rybka, M.; Drapała, A. Keratin Biomaterials in Skin Wound Healing, an Old Player in Modern Medicine: A Mini Review. Pharmaceutics 2021, 13, 2029. [Google Scholar] [CrossRef]
- Konop, M.; Czuwara, J.; Kłodzińska, E.; Laskowska, A.K.; Sulejczak, D.; Damps, T.; Zielenkiewicz, U.; Brzozowska, I.; Sureda, A.; Kowalkowski, T.; et al. Evaluation of Keratin Biomaterial Containing Silver Nanoparticles as a Potential Wound Dressing in Full-Thickness Skin Wound Model in Diabetic Mice. J. Tissue Eng. Regen. Med. 2020, 14, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Borek, C. Antioxidants and Radiation Therapy. J. Nutr. 2004, 134, 3207S–3209S. [Google Scholar] [CrossRef] [Green Version]
- Chao, M.; Spencer, S.; Kai, C.; Baker, C.; Jassal, S.; Law, M.; Cheng, M.; Zantuck, N.; Yu, V.; Stoney, D.; et al. EP-1286 StrataXRT Is Non Inferior to Mepitel Film in Preventing Radiation Induced Moist Desquamation. Radiother. Oncol. 2019, 133, S704–S705. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).