Preformulation Studies of Ezetimibe-Simvastatin Solid Dispersions in the Development of Fixed-Dose Combinations
Abstract
:1. Introduction
2. Materials and Methods
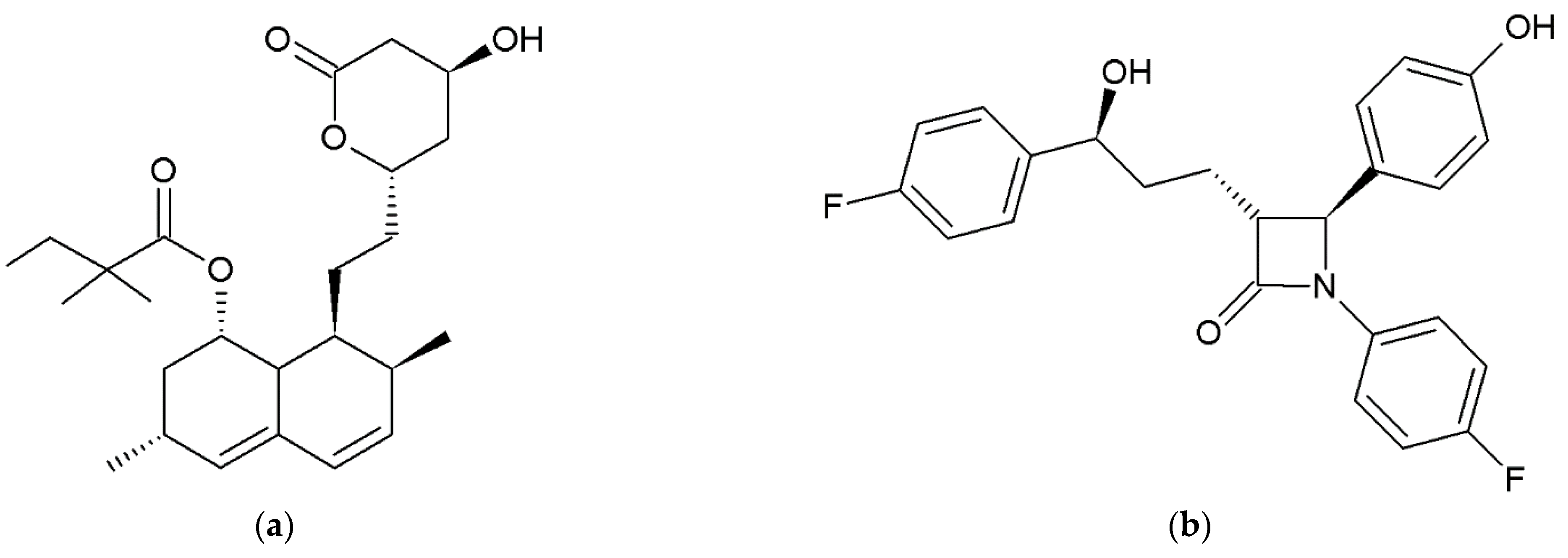
2.1. Materials
2.2. Preparation of SIM-EZT Dispersions
2.3. API Content Analysis
2.4. Differential Scanning Calorimetry (DSC) Measurements
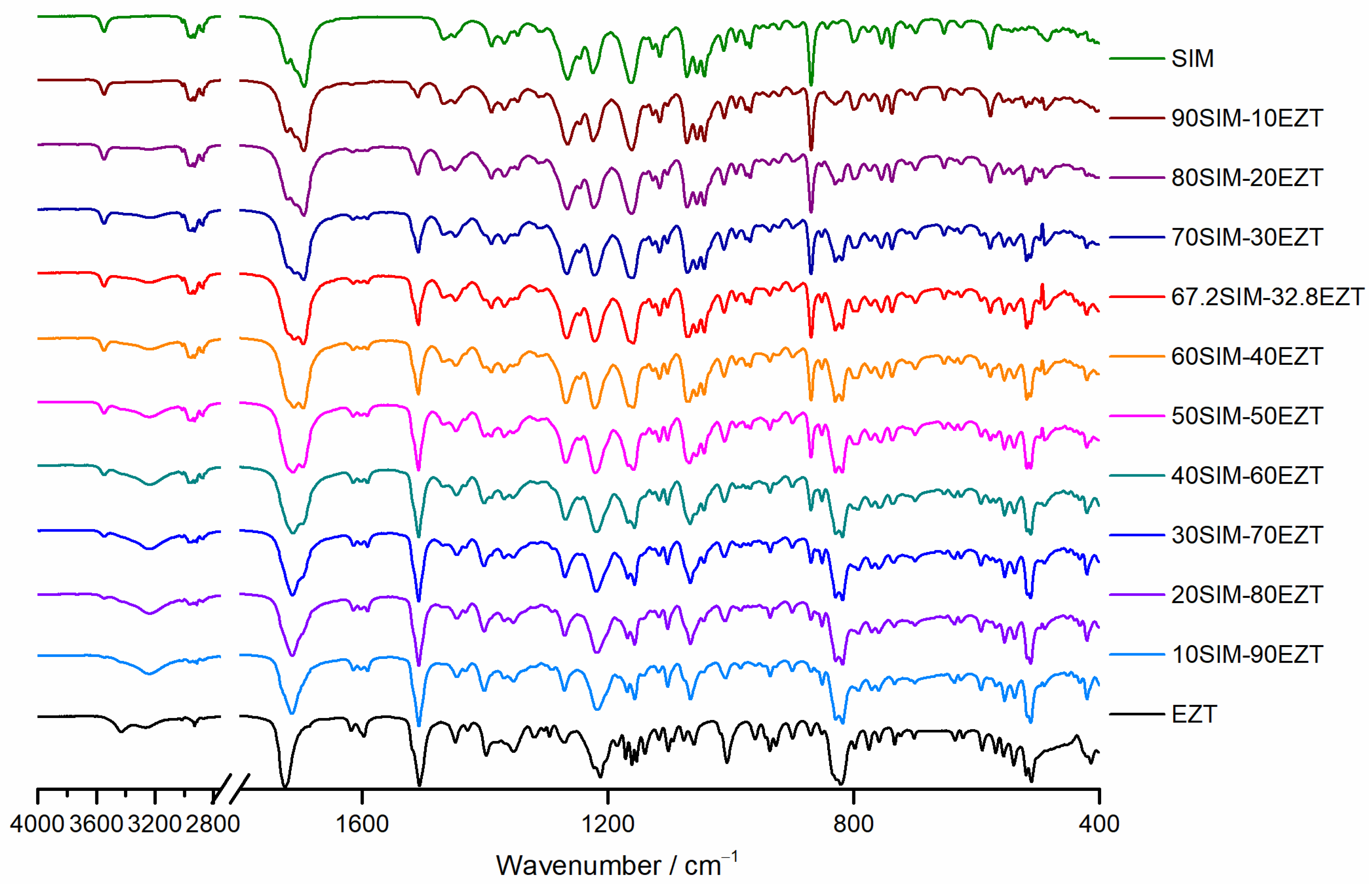
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
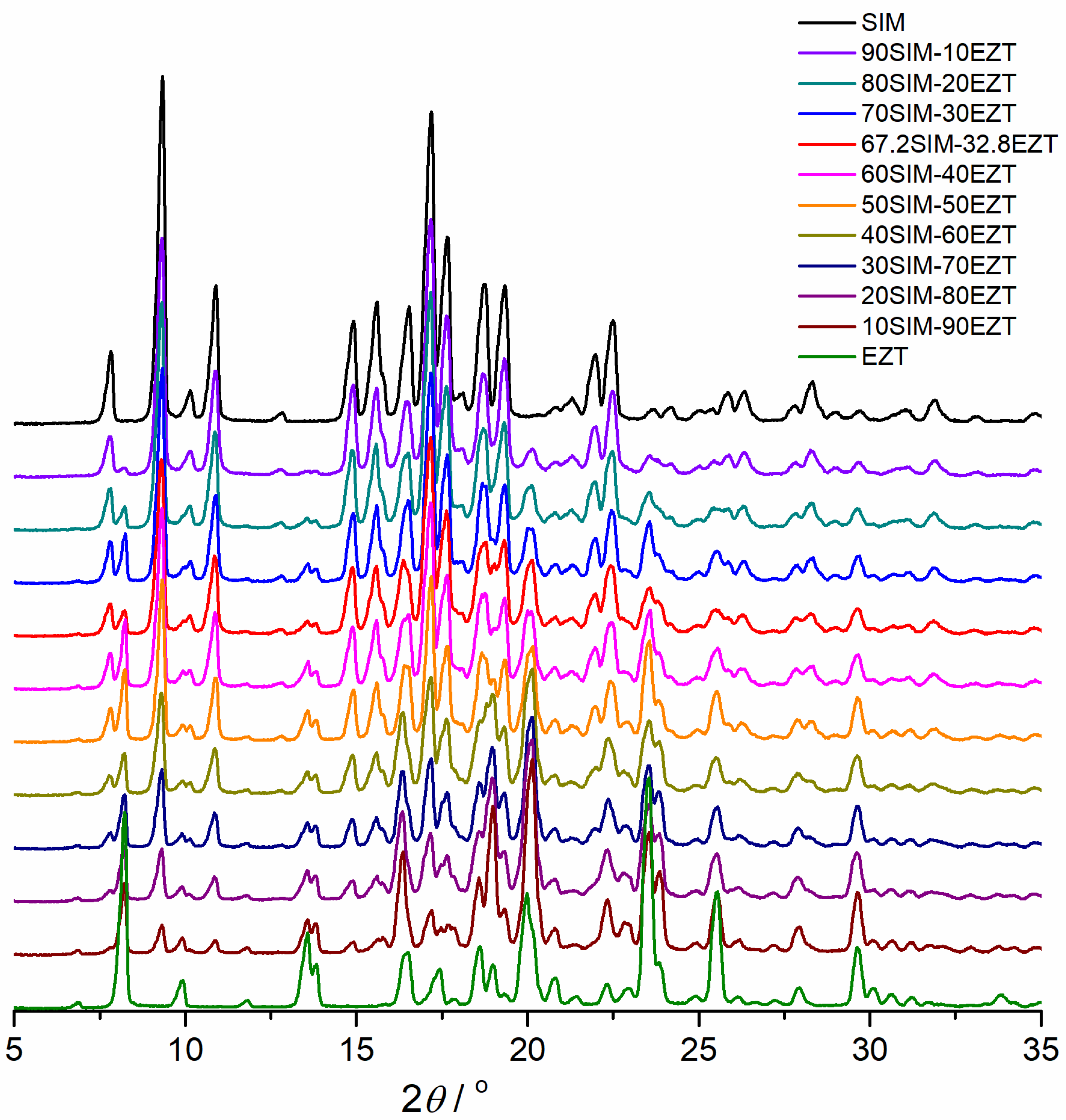
2.6. X-ray Powder Diffractometry (XRPD)
2.7. Scanning Electron Microscopy (SEM) Imaging
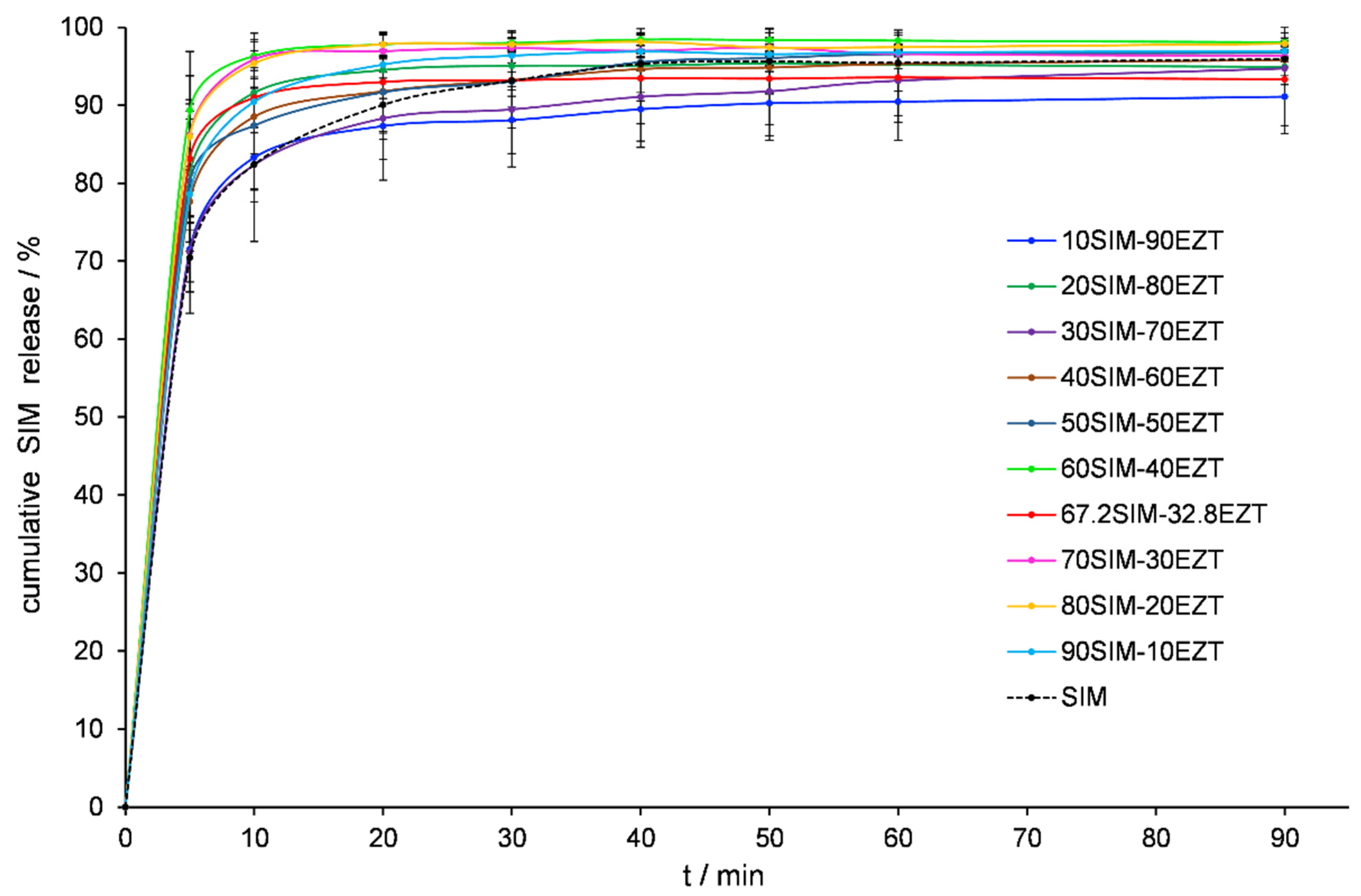
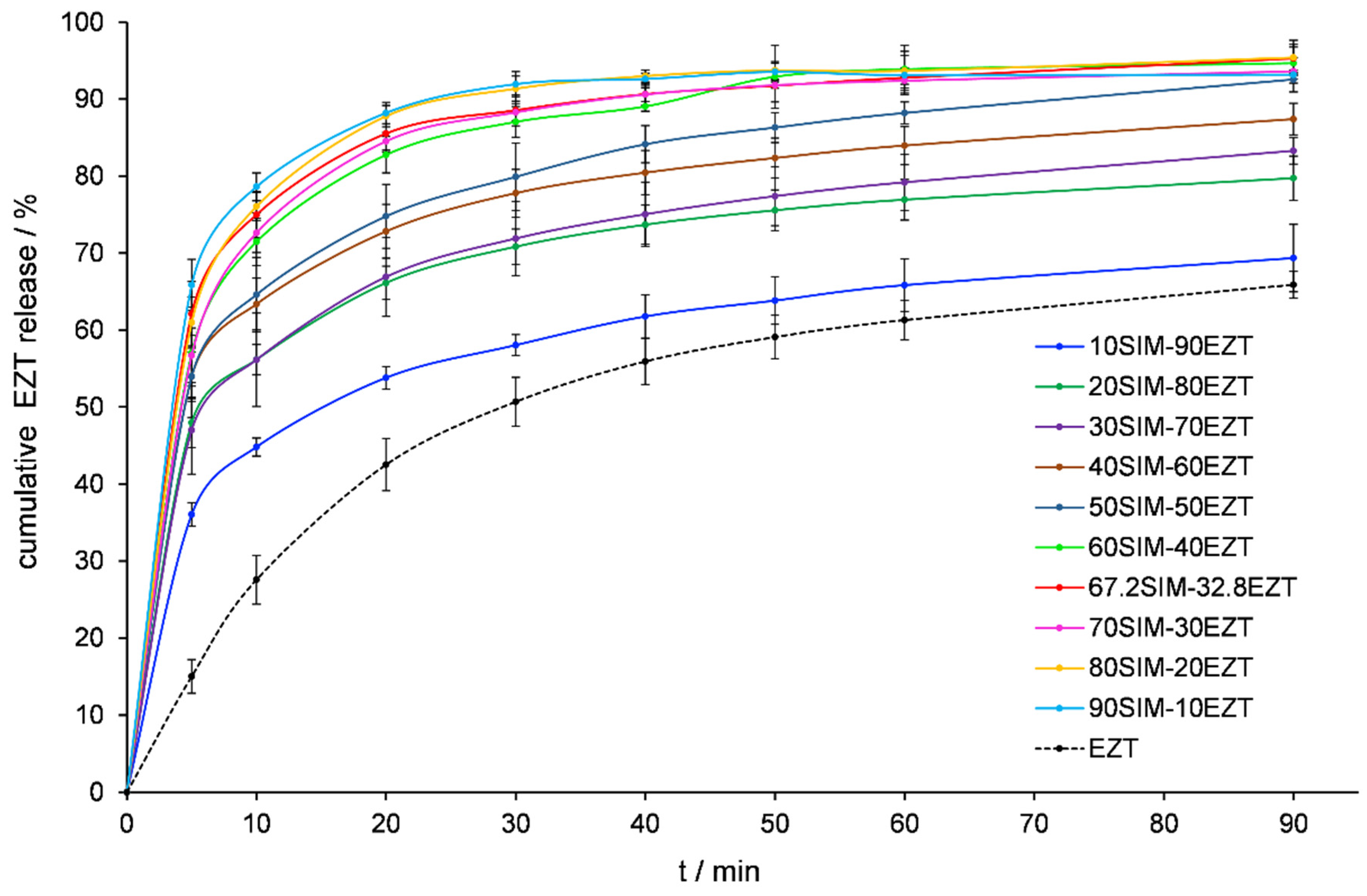
2.8. In Vitro Dissolution Testing
3. Results and Discussion
3.1. Drug Content
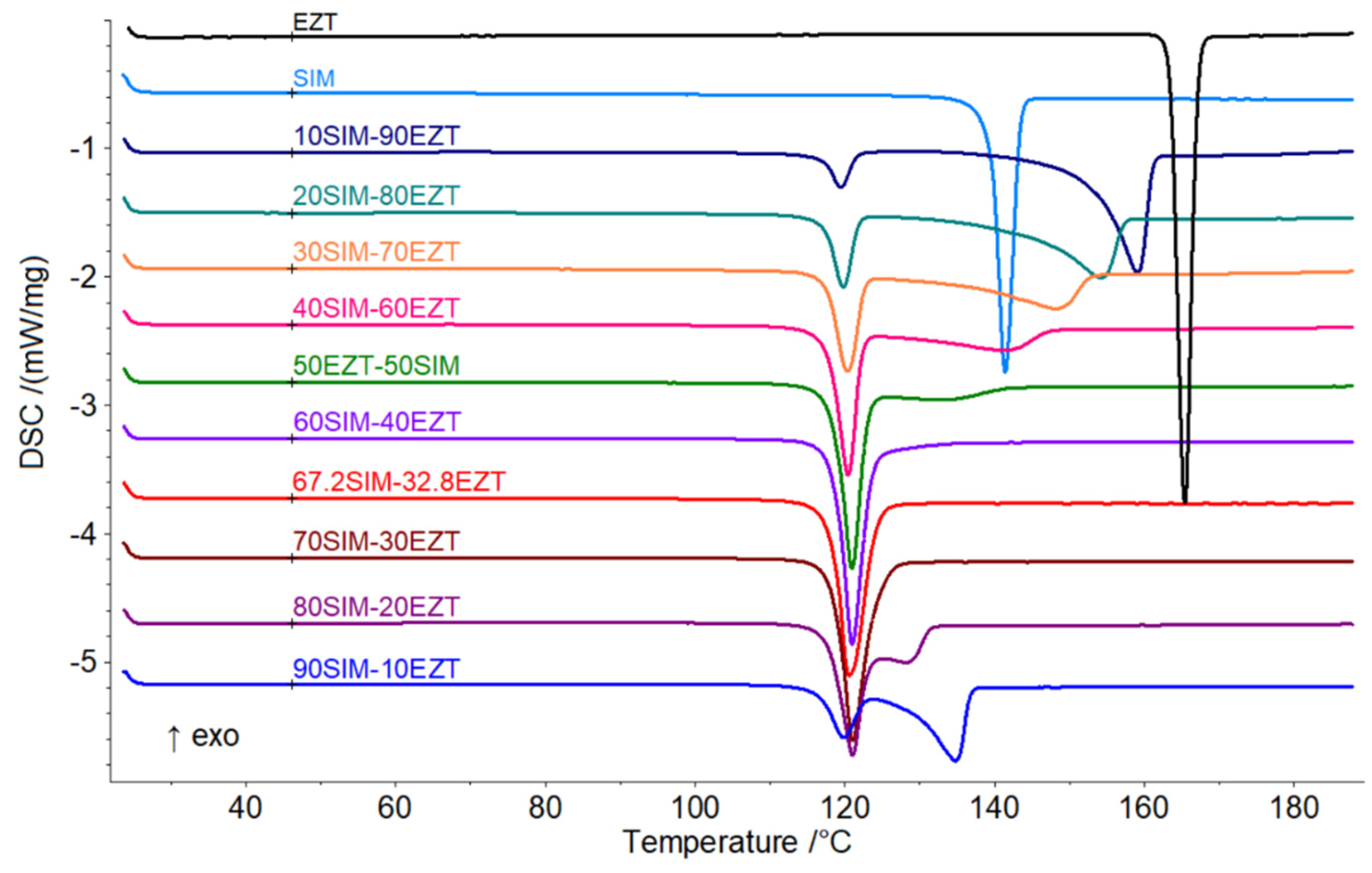
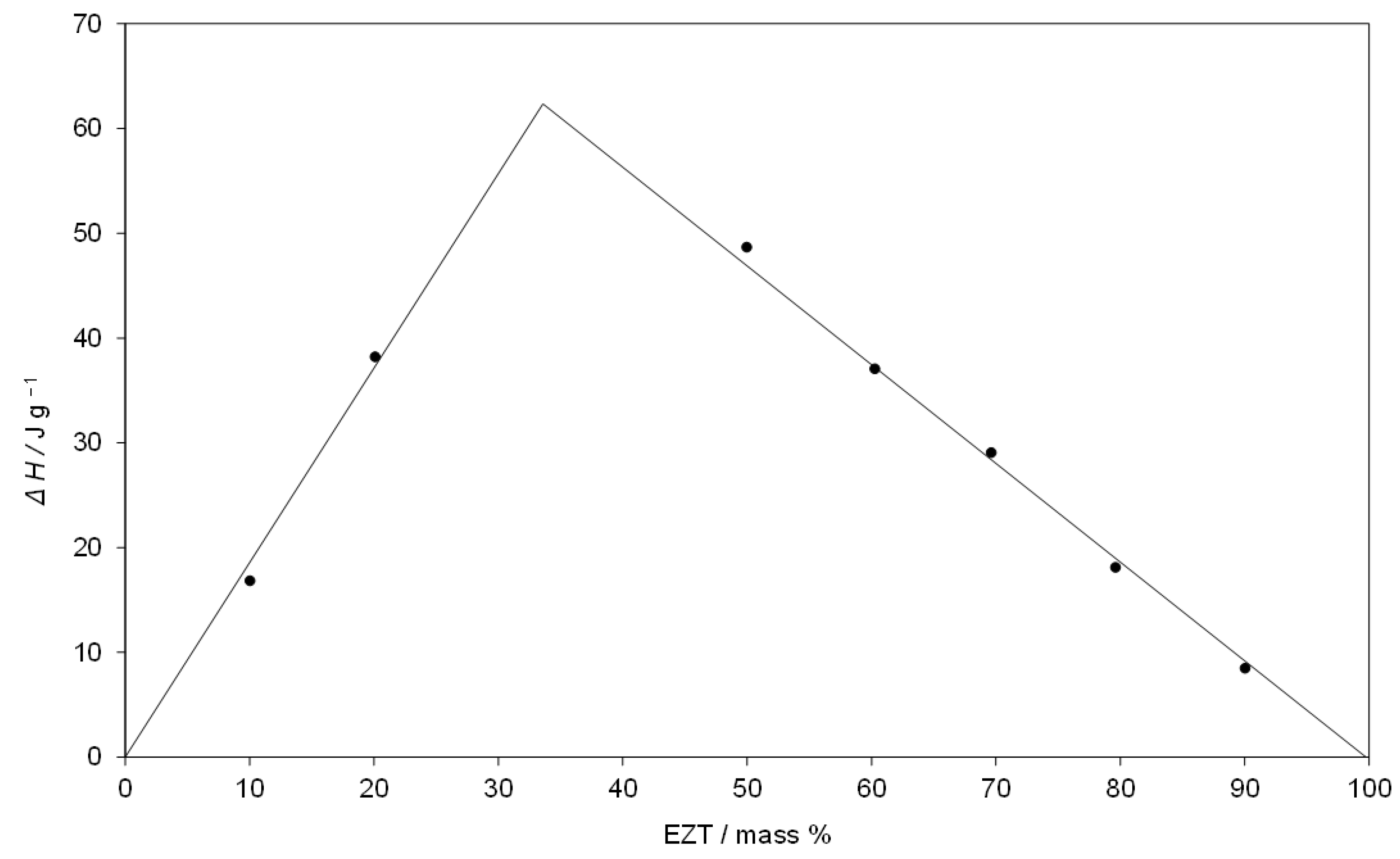
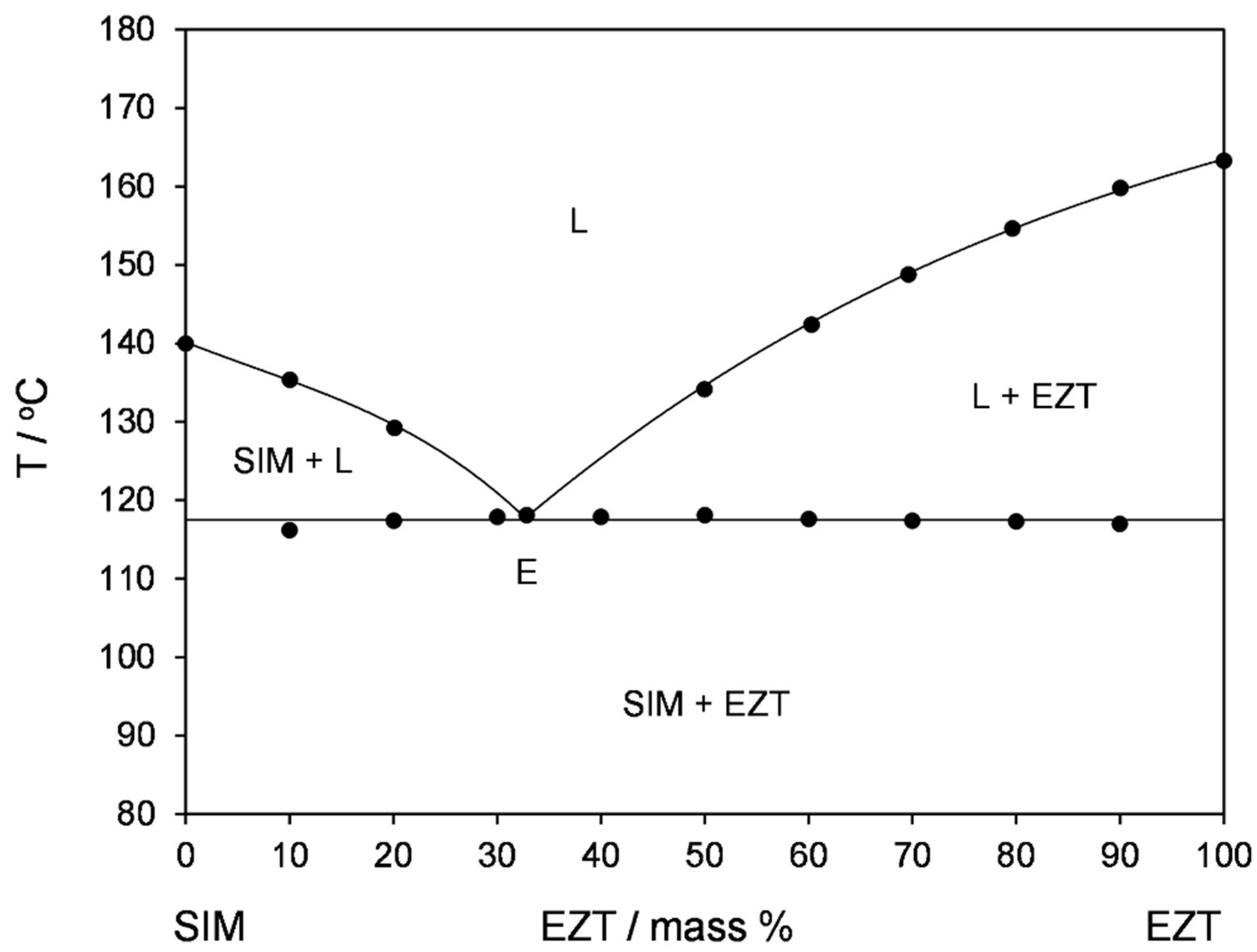
3.2. Thermal Analysis and Phase Transformation Behaviour
3.3. XRPD Studies
3.4. FTIR Spectroscopy Analysis
3.5. Shape and Surface Morphology
3.6. Dissolution Tests Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care. 2013, 40, 195–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M. Hypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndrome. Am. J. Cardiol. 1998, 81, 18B–25B. [Google Scholar] [CrossRef]
- Last, A.R.; Ference, J.D.; Falleroni, J. Pharmacologic treatment of hyperlipidemia. Am. Fam. Physician. 2011, 84, 551–558. [Google Scholar]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef] [Green Version]
- Zodda, D.; Giammona, R.; Schifilliti, S. Treatment Strategy for Dyslipidemia in Cardiovascular Disease Prevention: Focus on Old and New Drugs. Pharmacy 2018, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Schaiff, R.A.; Moe, R.M.; Krichbaum, D.W. An Overview of Cholesterol Management. Am. Health Drug Benefits 2008, 1, 39–48. [Google Scholar]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 4, 378–387. [Google Scholar] [CrossRef]
- Wang, D.Q.H. Regulation of intestinal cholesterol absorption. Ann. Rev. Physiol. 2007, 69, 221–248. [Google Scholar] [CrossRef]
- Garcia-Calvo, M.; Lisnock, J.; Bull, H.G.; Hawes, B.E.; Burnett, D.A.; Braun, M.P.; Crona, J.H.; Davis, H.R.; Dean, D.C.; Detmers, P.A.; et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 2005, 102, 8132–8137. [Google Scholar] [CrossRef] [Green Version]
- Ge, L.; Wang, J.; Qi, W.; Miao, H.H.; Cao, J.; Qu, Y.X.; Li, B.L.; Song, B.L. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008, 7, 508–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.J.; Song, B.L. Niemann-Pick C1-Like 1 and cholesterol uptake. Biochim. Biophys. Acta. 2012, 1821, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Phan, B.A.P.; Dayspring, T.D.; Toth, P.P. Ezetimibe therapy: Mechanism of action and clinical update. Vascular health and risk management. Vasc. Health Risk Manag. 2012, 8, 415–427. [Google Scholar] [PubMed] [Green Version]
- Hammersley, D.; Signy, M. Ezetimibe: An update on its clinical usefulness in specific patient groups. Ther. Adv. Chronic Dis. 2017, 8, 4–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banach, M.; Nikolic, D.; Rizzo, M.; Toth, P.P. IMPROVE-IT: What have we learned? Curr. Opin. Cardiol. 2016, 31, 426–433. [Google Scholar] [CrossRef]
- Vavlukis, M.; Vavlukis, A. Adding ezetimibe to statin therapy: Latest evidence and clinical implications. Drugs Context 2018, 7, 212534. [Google Scholar] [CrossRef] [Green Version]
- Saxon, D.R.; Eckel, R.H. Statin intolerance: A literature review and management strategies. Prog. Cardiovasc. Dis. 2016, 59, 153–164. [Google Scholar] [CrossRef]
- Montecucco, F.; Quercioli, A.; Mach, F. Ezetimibe/simvastatin. Expert Opin. Dug. Saf. 2009, 8, 715–724. [Google Scholar] [CrossRef]
- Kei, A.A.; Filippatos, T.D.; Elisaf, M.S. The safety of ezetimibe and simvastatin combination for the treatment of hypercholesterolemia. Expert Opin. Drug Saf. 2016, 15, 559–569. [Google Scholar] [CrossRef]
- Morrone, D.; Weintraub, W.S.; Toth, P.P.; Hanson, M.E.; Lowe, R.S.; Lin, J.; Shah, A.K.; Tershakovec, A.M. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: A pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis 2012, 223, 251–261. [Google Scholar] [CrossRef]
- Bays, H.E.; Ose, L.; Fraser, N.; Tribble, D.L.; Quinto, K.; Reyes, R.; Johnson-Levonas, A.O.; Sapre, A.; Donahue, S.R. A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin. Ther. 2004, 26, 1758–1773. [Google Scholar] [PubMed]
- Pradhan, A.; Bhandari, M.; Sethi, R. Ezetimibe and Improving Cardiovascular Outcomes: Current Evidence and Perspectives. Cardiol. Res. Prac. 2020, 2020, 9815016. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Weon, K.Y. Pharmaceutical application and development of fixed-dose combination: Dosage form review. J. Pharm. Investig. 2021, 51, 555–570. [Google Scholar] [CrossRef]
- Bangalore, S.; Kamalakkannan, G.; Parkar, S.; Messerli, F.H. Fixed-Dose Combinations Improve Medication Compliance: A Meta-Analysis. Am. J. Med. 2007, 120, 713–719. [Google Scholar] [CrossRef]
- Thipparaboina, R.; Thumuri, D.; Chavan, R.; Naidu, V.G.M.; Shastri, N.R. Fast dissolving drug-drug eutectics with improved compressibility and synergistic effects. Eur. J. Pharm. Sci. 2017, 104, 82–89. [Google Scholar] [CrossRef]
- Hennekens, C.H. Fixed-dose combination therapy with statins: Strengths, limitations, and clinical and regulatory considerations. Am. J. Cardiovasc. Drugs 2008, 8, 155–160. [Google Scholar] [CrossRef]
- Baumgartner, A.; Drame, K.; Geutjens, S.; Airaksinen, M. Does the Polypill Improve Patient Adherence Compared to Its Individual Formulations? A Systematic Review. Pharmaceutics 2020, 12, 190. [Google Scholar] [CrossRef] [Green Version]
- Webster, R.; Patel, A.; Selak, V.; Billot, L.; Bots, M.; Brown, A.; Bullen, C.; Cass, A.; Crengle, S.; Raina Elley, C.; et al. Effectiveness of fixed dose combination medication (‘polypills’) compared with usual care in patients with cardiovascular disease or at high risk: A prospective, individual patient data meta-analysis of 3140 patients in six countries. Int. J. Cardiol. 2016, 205, 147–156. [Google Scholar] [CrossRef]
- Pappa, E.; Rizos, C.V.; Filippatos, T.D.; Elisaf, M.S. Emerging Fixed-Dose Combination Treatments for Hyperlipidemia. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 315–322. [Google Scholar] [CrossRef]
- Bove, M.; Fogacci, F.; Cicero, A.F.G. Pharmacokinetic drug evaluation of ezetimibe + simvastatin for the treatment of hypercholesterolemia. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1099–1104. [Google Scholar] [CrossRef]
- Kastelein, J.J.; Sankatsing, R.R. Ezetimibe/simvastatin (INEGY) in the treatment of hyperlipidaemia. Int. J. Clin. Pract. 2005, 59, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J.; Radermecker, R.P. Drug of the month. Ezetimibe/simvastatin tablet (Inegy). Rev. Med. Liege. 2007, 62, 585–590. [Google Scholar]
- Lestari, M.L.; Ardiana, F.; Indrayanto, G. Ezetimibe. Profiles Drug Subst. Excip. Relat. Methodol. 2011, 36, 103–149. [Google Scholar] [PubMed]
- Jiang, T.; Han, N.; Zhao, B.; Xie, Y.; Wang, S. Enhanced dissolution rate and oral bioavailability of simvastatin nanocrystal prepared by sonoprecipitation. Drug. Dev. Ind. Pharm. 2012, 38, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G. Solubility enhancement of simvastatin: A review. Acta Pol. Pharm. 2012, 69, 581–590. [Google Scholar]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug. Discov. Today. 2007, 12, 1068–1075. [Google Scholar] [CrossRef]
- Vippagunta, S.R.; Wang, Z.; Hornung, S.; Krill, S.L. Factors affecting the formation of eutectic solid dispersions and their dissolution behavior. J. Pharm. Sci. 2007, 96, 294–304. [Google Scholar] [CrossRef]
- Petry, I.; Löbmann, K.; Grohganz, H.; Rades, T.; Leopold, C.S. Solid state properties and drug release behavior of co-amorphous indomethacin-arginine tablets coated with Kollicoat® Protect. Eur. J. Pharm. Biopharm. 2017, 119, 150–160. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Nangia, A. Eutectics as improved pharmaceutical materials: Design, properties and characterization. Chem. Commun. (Camb). 2014, 50, 906–923. [Google Scholar] [CrossRef]
- Bazzo, G.C.; Pezzini, B.R.; Stulzer, H.K. Eutectic mixtures as an approach to enhance solubility, dissolution rate and oral bioavailability of poorly water-soluble drugs. Int. J. Pharm. 2020, 588, 119741. [Google Scholar] [CrossRef]
- Haneef, J.; Ali, S.; Chadha, R. Emerging Multi-Drug Eutectics: Opportunities and Challenges. AAPS PharmSciTech 2021, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Al-Akayleh, F.; Mohammed Ali, H.H.; Ghareeb, M.M.; Al-Remawi, M. Therapeutic deep eutectic system of capric acid and menthol: Characterization and pharmaceutical application. J. Drug Deliv. Sci. Technol. 2019, 53, 101159. [Google Scholar] [CrossRef]
- Goud, N.R.; Suresh, K.; Sanphui, P.; Nangia, A. Fast dissolving eutectic compositions of curcumin. Int. J. Pharm. 2012, 439, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Riekes, M.K.; Engelen, A.; Appeltans, B.; Rombaut, P.; Stulzer, H.K.; Van den Mooter, G. New perspectives for fixed dose combinations of poorly water-soluble compounds: A case study with ezetimibe and lovastatin. Pharm. Res. 2016, 33, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Knapik-Kowalczuk, J.; Kramarczyk, D.; Jurkiewicz, K.; Chmiel, K.; Paluch, M. Ternary Eutectic Ezetimibe–Simvastatin–Fenofibrate System and the Physical Stability of Its Amorphous Form. Mol. Pharm. 2021, 18, 3588–3600. [Google Scholar] [CrossRef]
- Ledeţi, I.; Vlase, G.; Vlase, T.; Şuta, L.M.; Todea, A.; Fuliaş, A. Selection of solid-state excipients for simvastatin dosage forms through thermal and nonthermal techniques. J Therm. Anal Calorim 2015, 121, 1093–1102. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- Hendrani, A.D.; Adesiyun, T.; Quispe, R.; Jones, S.R.; Stone, N.J.; Blumenthal, R.S.; Martin, S.S. Dyslipidemia management in primary prevention of cardiovascular disease: Current guidelines and strategies. World J. Cardiol. 2016, 8, 201–210. [Google Scholar] [CrossRef]
- Brown, R.E.; Welsh, P.; Logue, J. Systematic review of clinical guidelines for lipid lowering in the secondary prevention of cardiovascular disease events. Open Heart 2020, 7, e001396. [Google Scholar] [CrossRef]
- Löbmann, K.; Strachan, C.; Grohganz, H.; Rades, T.; Korhonen, O.; Laitinen, R. Co-amorphous simvastatin and glipizide combinations show improved physical stability without evidence of intermolecular interactions. Eur. J. Pharm. Biopharm. 2012, 81, 159–169. [Google Scholar] [CrossRef]
- Dengale, S.J.; Ranjan, O.P.; Hussen, S.S.; Krishna, B.S.; Musmade, P.B.; Gautham Shenoy, G.; Bhat, K. Preparation and characterization of co-amorphous Ritonavir-Indomethacin systems by solvent evaporation technique: Improved dissolution behavior and physical stability without evidence of intermolecular interactions. Eur. J. Pharm. Sci. 2014, 62, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Xiao, D.; Ren, S.; Bi, S.; Wang, J.; Li, F. The Binary System of Ibuprofen-Nicotinamide under Nanoscale Confinement: From Cocrystal to Coamorphous State. J. Pharm. Sci. 2017, 106, 3150–3155. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Siahi-Shadbad, M.; Barzegar-Jalali, M.; Adibkia, K. Characterizing eutectic mixtures of gliclazide with succinic acid prepared by electrospray deposition and liquid assisted grinding methods. Drug Deliv. Sci. Technol. 2018, 45, 101–109. [Google Scholar] [CrossRef]
- Patel, R.D.; Raval, M.K.; Bagathariya, A.A.; Sheth, N.R. Functionality improvement of Nimesulide by eutectic formation with nicotinamide: Exploration using temperature-composition phase diagram. Adv. Powder. Technol. 2019, 30, 961–973. [Google Scholar] [CrossRef]
- Araya-Sibaja, A.M.; Vega-Baudrit, J.R.; Guillén-Girón, T.; Navarro-Hoyos, M.; Cuffini, S.L. Drug Solubility Enhancement through the Preparation of Multicomponent Organic Materials: Eutectics of Lovastatin with Carboxylic Acids. Pharmaceutics. 2019, 11, 112. [Google Scholar] [CrossRef] [Green Version]
- Castellano, J.M.; Sanz, G.; Penalvo, J.L.; Bansilal, S.; Fernández-Ortiz, A.; Alvarez, L.; Guzmán, L.; Linares, J.C.; García, F.; D’Aniello, F.; et al. A polypill strategy to improve adherence: Results from the FOCUS project. J. Am. Coll. Cardiol. 2014, 64, 2071–2082. [Google Scholar] [CrossRef] [Green Version]
- Bramlage, P.; Sims, H.; Minguet, J.; Ferrero, C. The polypill: An effective approach to increasing adherence and reducing cardiovascular event risk. Eur. J. Prev. Cardiol. 2017, 24, 297–310. [Google Scholar] [CrossRef]
- Roshandel, G.; Khoshnia, M.; Poustchi, H.; Hemming, K.; Kamangar, F.; Gharavi, A.; Ostovaneh, M.R.; Nateghi, A.; Majed, M.; Navabakhsh, B.; et al. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): A pragmatic, cluster-randomised trial. Lancet 2019, 394, 672–683. [Google Scholar] [CrossRef]

| Sample Code | Composition/Mass % | Average Content /% | ||
|---|---|---|---|---|
| Simvastatin | Ezetimibe | Simvastatin | Ezetimibe | |
| 90SIM-10EZT | 90.0 | 10.0 | 101.74 ± 0.03 | 101.71 ± 0.15 |
| 80SIM-20EZT | 80.0 | 20.0 | 97.44 ± 0.15 | 98.44 ± 0.15 |
| 70SIM-30EZT | 70.0 | 30.0 | 99.39 ± 0.04 | 100.53 ± 0.05 |
| 67.2SIM-32.8EZT | 67.2 | 32.8 | 99.39 ± 0.06 | 100.66 ± 0.05 |
| 60SIM-40EZT | 60.0 | 40.0 | 98.61 ± 0.03 | 100.02 ± 0.06 |
| 50EZT-50SIM | 50.0 | 50.0 | 98.17 ± 0.03 | 99.98 ± 0.06 |
| 40SIM-60EZT | 40.0 | 60.0 | 98.86 ± 0.14 | 100.41 ± 0.21 |
| 30SIM-70EZT | 30.0 | 70.0 | 98.78 ± 0.04 | 100.44 ± 0.19 |
| 20SIM-80EZT | 20.0 | 80.0 | 97.76 ± 0.05 | 99.27 ± 0.04 |
| 10SIM-90EZT | 10.0 | 90.0 | 97.56 ± 0.11 | 99.25 ± 0.06 |
| Sample Code | Eutectic Invariant | Liquidus | |
|---|---|---|---|
| T/°C | ΔH/J g−1 | /°C | |
| SIM | 140.0 ± 0.1 | ||
| 90SIM-10EZT | 116.2 ± 0.2 | 16.8 ± 0.6 | 135.3 ± 0.2 |
| 80SIM-20EZT | 117.4 ± 0.2 | 38.2 ± 1.3 | 129.2 ± 0.2 |
| 70SIM-30EZT | 117.9 ± 0.2 | - | |
| 67.2SIM-32.8EZT | 118.1 ± 0.3 | - | |
| 60-SIM-40EZT | 117.9 ± 0.1 | - | |
| 50SIM-50EZT | 118.1 ± 0.1 | 48.7 ± 1.0 | 134.2 ± 0.2 |
| 40SIM-60EZT | 117.6 ± 0.2 | 37.1 ± 0.5 | 142.4 ± 0.1 |
| 30SIM-70EZT | 116.9 ± 0.1 | 29.1 ± 0.2 | 148.8 ± 0.1 |
| 20SIM-80EZT | 117.3 ± 0.3 | 18.1 ± 0.9 | 154.7 ± 0.1 |
| 10SIM-90EZT | 117.0 ± 0.2 | 8.5 ± 0.1 | 159.8 ± 0.3 |
| EZT | 163.3 ± 0.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górniak, A.; Złocińska, A.; Trojan, M.; Pęcak, A.; Karolewicz, B. Preformulation Studies of Ezetimibe-Simvastatin Solid Dispersions in the Development of Fixed-Dose Combinations. Pharmaceutics 2022, 14, 912. https://doi.org/10.3390/pharmaceutics14050912
Górniak A, Złocińska A, Trojan M, Pęcak A, Karolewicz B. Preformulation Studies of Ezetimibe-Simvastatin Solid Dispersions in the Development of Fixed-Dose Combinations. Pharmaceutics. 2022; 14(5):912. https://doi.org/10.3390/pharmaceutics14050912
Chicago/Turabian StyleGórniak, Agata, Adrianna Złocińska, Mateusz Trojan, Adrianna Pęcak, and Bożena Karolewicz. 2022. "Preformulation Studies of Ezetimibe-Simvastatin Solid Dispersions in the Development of Fixed-Dose Combinations" Pharmaceutics 14, no. 5: 912. https://doi.org/10.3390/pharmaceutics14050912
APA StyleGórniak, A., Złocińska, A., Trojan, M., Pęcak, A., & Karolewicz, B. (2022). Preformulation Studies of Ezetimibe-Simvastatin Solid Dispersions in the Development of Fixed-Dose Combinations. Pharmaceutics, 14(5), 912. https://doi.org/10.3390/pharmaceutics14050912






