Abstract
The clinical prevalence of antifungal drug resistance has been increasing over recent years, resulting in the failure of treatments. In an attempt to overcome this critical problem, we sought novel synergistic enhancers to restore the effectiveness of fluconazole against resistant Candida albicans. Based on the structural optimization of hit compound 8 from our in-house library, a series of novel 1,3,5-triazines derivatives was designed, synthesized, and biologically evaluated for synergistic activity in combination with fluconazole. Among them, compounds 10a–o, which contain thiosemicarbazides side chains, exhibited excellent in vitro synergistic antifungal potency (MIC80 = 0.125–2.0 μg/mL, FICI range from 0.127 to 0.25). Interestingly, compound 10l exhibited moderate C. albicans activity as monotherapy with an MIC80 value of 4.0 μg/mL, and also on several Cryptococcus strains (MIC80 ranging from ≤ 0.125–0.5 μg/mL) and C. glabrata (MIC80 ≤ 0.125 μg/mL). These effects were fungal-selective, with much lower levels of cytotoxicity towards human umbilical vein endothelial cells. Here, we report a series of thiosemicarbazides containing 1,3,5-triazines derivatives as potent synergists with fluconazole, and have preliminarily validated compound 10l as a promising antifungal lead for further investigation.
1. Introduction
Due to advances in modern medicine, antifungal drug resistance has become a widespread clinical problem that is frequently encountered. The associated morbidity and mortality rates of invasive fungal infections (IFIs) have increased dramatically in recent years [1,2]. Candida albicans is the most common opportunistic fungi, with mortality rates of 40–80% [3,4,5]. Moreover, IFIs generally occur in patients with critical comorbidities, such as viral pneumonitis (including SARS-CoV-2), and those living in an immunocompromised state, for example those living with AIDS or immunosuppressive therapy following organ transplant [6,7]. Currently, there are four major classes of antifungal drugs: polyenes, echinocandins, flucytosine, and azoles. Fluconazole is a widely available drug worldwide and has high bioavailability, good water solubility, low toxicity, and is relatively inexpensive. However, fluconazole has frequently been associated with the development of drug resistance. Current research to optimize the azoles, either through the development of a new generation of azoles (e.g., the tetrazoles) or use in combination therapy, has shown significant clinical results.
The drug combination has proven an effective strategy to overcome drug resistance in C. albicans [8]. Several small molecules have no or weak antifungal activity by themselves yet have been identified as successfully restoring the antifungal activity of fluconazole against resistant fungi when the two drugs are used in combination. These small molecules are regarded as enhancers or synergists. Our group had reported multiple studies on the use of drug combination, such as baicalein [9], curcumin [10], berberine [11], and a series of structural optimization of berberine [12,13,14]. Here, we have continued to persist with our search to discover new types of synergistic molecules.
Due to the prevalence of electronegative nitrogen atoms in polyazine systems, triazine, as an electron-deficient aromatic scaffold, has higher nucleophilic substitution/addition reactivity compared to benzene. Numerous routes have been proposed for the synthesis of triazine and its derivatives exhibiting various physical, chemical, and biological properties. These could be potential building blocks and start-ups in drug design and discovery research [15]. As an important class of the heterocyclic systems, 1,3,5-triazines (s-triazine) have been extensively studied in medicinal chemistry (Figure 1) on account of their pharmacological properties: being anticancer [16], antiviral [17], antibacterial [18], antimalarial [19] and anti-inflammatory [20]. 1,3,5-Triazines and their derivatives have also been reported to possess antifungal activity [21,22,23,24]; however, to the best of our knowledge, 1,3,5-triazines or their derivatives have not been used as antifungal synergists to restore the susceptibility of the antifungal triazoles to azole-resistant fungi.
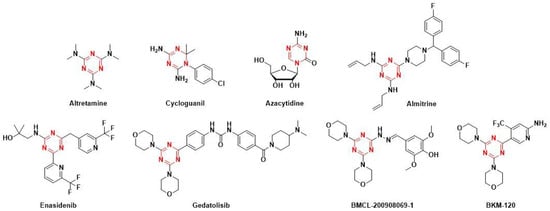
Figure 1.
1,3,5-Triazine containing drugs in market and derivatives in phase Ⅲ clinical trials.
In this study, we designed, synthesized, and evaluated the antifungal activity of four series of trisubstituted 1,3,5-triazines derivatives, which was based on the screening of our hit compound 8 from an in-house library (Figure 2). The PEG-containing side chains in hit compound 8 had no drug-like properties. Firstly, we replaced the PEG-containing side chains and retained the other two substituted groups, (4-fluorophenyl)methanamine and cyclohexanemethylamine. Then, by retrosynthetic analysis, we synthesized two key intermediates, acid 5 and hydrazide 6, to further react with various aromatic amines, isocyanate, and isothiocyanate, respectively. Through optimizing side chains, we obtained 42 target compounds in a facile way. Among them, the series compounds 10a–o containing a thiosemicarbazides moiety exhibited excellent synergists activity with fluconazole against fluconazole-resistant C. albicans. Compound 10l also possessed moderate activity against fluconazole-resistant C. albicans by itself, with selective anti-Cryptococcus and anti-C. glabrata activity. Overall, based on a hit-to-lead structural optimization, a class of novel thiosemicarbazides containing 1,3,5-triazines derivatives with excellent synergist activity were discovered in vitro.
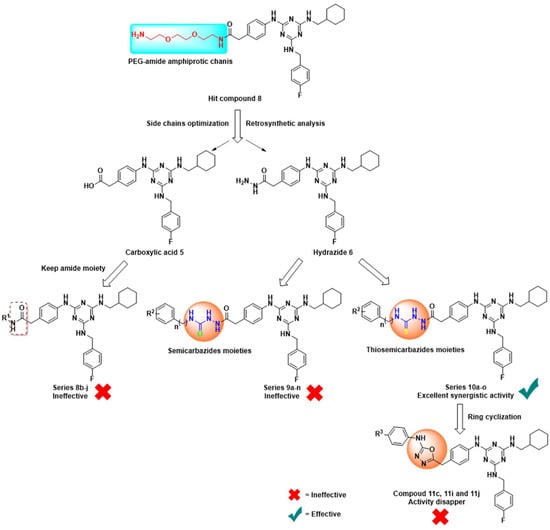
Figure 2.
Design strategies and structure modification of novel 1,3,5-triazines derivatives.
2. Experimental Section
2.1. Chemistry
1H and 13C NMR spectra were recorded in DMSO-d6 unless otherwise indicated with a Bruker AC-300P spectrometer, using tetramethylsilane (TMS) as an internal standard. Chemical shifts (δ values) and coupling constants (J values) are given in ppm and Hz, respectively. ESI mass spectra were performed on an Agilent Technologies 6120 Quadrupole LC-MS. TLC analysis was carried out on silica gel plates GF254 (Yantai Huanghai Chemical, Qingdao, China). The solvents and reagents were purchased from commercial vendors and were used as received, or dried prior to use as needed.
2.1.1. General Procedure for Synthesis of Methyl 2-(4-((4,6-Dichloro-1,3,5-triazin-2-yl)amino)phenyl)acetate (2)
Methyl 2-(4-aminophenyl)acetate (2.34 mL, 16.3 mmol) was added dropwise to a solution of cyanuric chloride (1) (3.00 g, 16.3 mmol) and NaHCO3 (1.36 g, 16.3 mmol) in THF (50 mL) in an ice-water bath. The solution was stirred at 0 °C for 3 h and then concentrated in vacuo. The mixture was recrystallized by MeOH/H2O, filtered, and washed with water to afford intermediate 2 as a white solid (4.21 g, 83%).
2.1.2. General Procedure for Synthesis of Methyl 2-(4-((4-Chloro-6-((4-fluorobenzyl)amino)-1,3,5-triazin-2-yl)amino)phenyl)acetate (3)
(4-fluorophenyl)methanamine (1.54 mL, 13.4 mmol) was added dropwise to a solution of intermediate 2 (4.20 g, 13.4 mmol) and NaHCO3 (1.13 g, 13.4 mmol) in THF (50 mL). The reaction was stirred at room temperature for 2 h. After the reaction was completed (monitored by TLC with PE/EtOAc = 4/1, v/v), the mixture was concentrated in vacuo and then extracted with ethyl acetate (3 × 30 mL). The organic phases were combined, washed with brine (2 × 50 mL), and dried over anhydrous Na2SO4. The crude product was purified by chromatography on silica gel (PE/EtOAc = 10:1~1:1) to obtain intermediate 3 as a white solid (3.67 g, 68%).
2.1.3. General Procedure for Synthesis of Methyl 2-(4-((4-((Cyclohexylmethyl)amino)-6-((4-fluorobenzyl)amino)-1,3,5-triazin-2-yl)amino)phenyl)acetate (4)
1,8-diazabicyclo [5.4.0]undec-7-ene (DBU, 2.23 mL, 14.96 mmol) was added to a mixture of disubstituted triazine 3 (3.00 g, 7.48 mmol) and cyclohexylmethanamine (1.26 mL, 9.72 mmol) in 1,4-dioxane (50 mL). The reaction was stirred at reflux conditions overnight. After the reaction was completed (monitored by TLC with DCM/MeOH = 15/1, v/v), the mixture was concentrated in vacuo and then extracted with ethyl acetate (3 × 30 mL). The organic phases were combined, washed with brine (2 × 50 mL), and dried over anhydrous Na2SO4. The crude product was purified by chromatography on silica gel (DCM/MeOH = 150:1~80:1) to obtain ester 4 as a semisolid (2.50 g, 70%).
Compound 4: 1H NMR (300 MHz, DMSO-d6) δ 9.05–8.73 (m, 1H), 7.68 (d, J = 8.5 Hz, 2H), 7.46–7.02 (m, 7H), 6.84 (t, J = 32.8 Hz, 1H), 4.44 (d, J = 5.7 Hz, 2H), 3.60 (s, 3H), 3.57 (s, 2H), 3.10–3.02 (m, 2H), 1.73–1. 40 (m, 6H), 1.23–0.99 (m, 3H), 0.99–0.68 (m, 2H). 13C NMR (75 MHz, DMSO-d6) δ 172.35, 166.43, 166.30, 166.18, 164.58, 163.08, 159.88, 139.98, 137.61, 137.33, 129.50, 129.42, 129.31, 127.25, 120.09, 119.97, 119.82, 115.38, 115.10, 99.99, 52.02, 46.98, 46.66, 43.27, 43.15, 42.98, 38.08, 37.92, 31.07, 26.65, 25.93. MS (ESI) m/z: 479.0 [M + H]+, C26H31FN6O2. Purity: 97.6% (LC-MS).
2.1.4. General Procedure for Synthesis of 2-(4-((4-((Cyclohexylmethyl)amino)-6-((4-fluorobenzyl)amino)-1,3,5-triazin-2-yl)amino)phenyl)acetic acid (5)
NaOH (2 mol/L, 20 mL) was added to a solution of ester 4 (2.00 g, 4.18 mmol) in MeOH (30 mL). The reaction was stirred at reflux for 5 h (monitored by TLC, DCM/MeOH = 20/1, v/v). The organic solvent was removed in vacuo and the residues were dissolved in water. The mixture was acidified with 2 mol/L HCl until the product precipitated. The precipitate was filtered and dried to afford acid 5 as a white solid (1.04 g, 54%).
Compound 5: 1H NMR (300 MHz, DMSO-d6) δ 12.20 (s, 1H), 9.03–8.63 (m, 1H), 7.75–7.53 (m, 2H), 7.46–7.01 (m, 7H), 6.83 (t, J = 30.7 Hz, 1H), 4.43 (d, J = 5.0 Hz, 2H), 3.45 (s, 2H), 3.10–3.02 (m, 2H), 1.74–1.36 (m, 6H), 1.23–0.97 (m, 3H), 0.95–0.61 (m, 2H). 13C NMR (75 MHz, DMSO-d6) δ 173.48, 166.39, 166.33, 166.28, 166.21, 166.17, 164.57, 163.09, 159.86, 139.74, 137.35, 129.52, 128.02, 120.11, 119.75, 115.39, 115.11, 46.99, 46.66, 43.23, 43.12, 43.04, 42.96, 37.95, 31.08, 26.65, 25.94. MS (ESI) m/z: 465.0 [M + H]+, C25H29FN6O2. Purity: 96.9% (LC-MS).
2.1.5. General Procedure for Synthesis of 2-(4-((4-((Cyclohexylmethyl)amino)-6-((4-fluorobenzyl)amino)-1,3,5-triazin-2-yl)amino)phenyl)acetohydrazide (6)
NH2-NH2.H2O (4 mL, 83.6 mmol) was added to a solution of ester 4 (2.00 g, 4.18 mmol) in MeOH (30 mL). The reaction was stirred at reflux overnight (monitored by TLC, DCM/MeOH = 15/1, v/v). The solution was restored to room temperature and poured onto ice water (20 mL). The precipitate was formed, filtered, and recrystallized by MeOH/H2O twice to obtain hydrazide 6 as a white solid (1.24 g, 62%).
Compound 6: 1H NMR (300 MHz, DMSO-d6) δ 9.15 (s, 1H), 8.95–8.63 (m, 1H), 7.74–7.52 (m, 2H), 7.42–7.07 (m, 7H), 6.84 (t, J = 34.0 Hz, 1H), 4.44 (d, J = 5.4 Hz, 2H), 4.23 (s, 2H), 3.25 (s, 2H), 3.15–2.95 (m, 2H), 1.73–1.46 (m, 6H), 1.19–1.03 (m, 3H), 0.99–0.68 (m, 2H). 13C NMR (75 MHz, DMSO-d6) δ 170.37, 166.42, 166.29, 166.19, 164.56, 163.06, 159.93, 159.86, 139.52, 137.39, 137.34, 129.41, 129.27, 129.12, 120.12, 119.69, 115.39, 115.11, 46.93, 46.61, 43.26, 37.95, 31.08, 26.65, 25.93. MS (ESI) m/z: 479.0 [M + H]+, C25H31FN8O. Purity: 98.2% (LC-MS).
2.1.6. General Procedure for Synthesis of Target Compounds (8 and 8a–j)
Benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP, 0.17 g, 0.33 mmol) and N, N-diisopropylethylamine (DIEA, 156 μL, 0.90 mmol) in DMF (5 mL) was added to the corresponding amine (0.30 mmol), to a solution of acid 5 (0.14 g, 0.30 mmol) and the mixture was stirred at room temperature for 3 h (monitored by TLC, DCM/MeOH = 20/1, v/v). The reaction solution was diluted with water (10 mL) and filtered. The precipitate was recrystallized by MeOH/H2O to obtain the target compound (8a–j).
To remove the Boc-protecting group, HCl (5.0 mL, 4.0 M in EtOAc) was added dropwise into a solution of tert-butyl (2-(2-(2-(2-(4-((4-((cyclohexylmethyl)amino)-6-((4-fluorobenzyl)amino)-1,3,5-triazin-2-yl)amino)phenyl)acetamido)ethoxy)ethoxy)ethyl)carbamate (8a) (0.15 g, 0.22 mmol) in EtOAc (5.0 mL), cooled by an ice-water bath. The ice bath was removed, and the mixture was stirred at room temperature over a 1 h period. After completion of the reaction (monitored by TLC, DCM/MeOH = 10/1, v/v), the precipitate was filtered quickly and washed with dry ethyl ether to afford the hit compound 8 (86.0 mg, 67%) as a white salt for store. In this paper, we used it as a free amido form.
Compound 8: 1H NMR (300 MHz, DMSO-d6) δ 10.43 (d, J = 105.8 Hz, 1H), 9.31–8.63 (m, 2H), 8.28–8.08 (m, 3H), 7.71–6.93 (m, 8H), 4.65–4.39 (m, 2H), 3.64–3.57 (m, 2H), 3.56–3.42 (m, 8H), 3.23–3.12 (m, 4H), 2.94 (dd, J = 10.5, 5.2 Hz, 2H), 1.81–1.44 (m, 6H), 1.30–1.02 (m, 3H), 1.03–0.71 (m, 2H). 13C NMR (75 MHz, DMSO-d6) δ 171.06, 166.41, 166.30, 164.55, 163.06, 159.85, 139.50, 137.35, 137.30, 129.43, 129.30, 129.12, 120.11, 119.86, 119.68, 115.39, 115.11, 70.41, 70.06, 69.97, 69.55, 46.93, 46.63, 43.26, 42.19, 37.96, 31.06, 26.65, 25.92. MS (ESI) m/z: 595.0 [M + H]+, C31H43FN8O3. Purity: 98.6% (LC-MS).
2.1.7. General Procedure for Synthesis of Target Compounds (9a–n)
The corresponding isocyanates (0.32 mmol) was added to a solution of hydrazide 6 (0.14 g, 0.30 mmol) in anhydrous THF (5 mL). The mixture was then stirred at room temperature for 2 h (monitored by TLC, DCM/MeOH = 10/1, v/v). The organic solvent was removed in vacuo and the residues were recrystallized by MeOH/H2O to give the target compounds (9a–n).
2.1.8. General Procedure for Synthesis of Target Compounds (10a–o)
The corresponding isothiocyanates (0.32 mmol) was added to a solution of hydrazide 6 (0.14 g, 0.30 mmol) in anhydrous EtOH (5 mL). The mixture was then stirred at reflux for 2 h (monitored by TLC, DCM/MeOH = 10/1, v/v). The organic solvent was removed in vacuo and the residues were recrystallized by MeOH/H2O to give target compounds (10a–o).
2.1.9. General Procedure for Synthesis of Target Compounds (11c, 11i and 11j)
A volume of 0.30 mmol of compound 10c, 10i and 10j and 0.90 mmol of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl) with catalytic amounts of hydroxybenzotriazole (HOBt) was added to 5 mL of anhydrous DMF. The reaction mixture was stirred at room temperature for 3–5 h under nitrogen atmospheric conditions. After the reaction was completed (monitored by TLC, DCM/MeOH = 15/1, v/v), the mixture was poured onto ice. The precipitate was filtered, washed with water, dried under high vacuum, and recrystallized by MeOH/H2O to give target compounds (11c, 11i and 11j).
2.2. Biological Activity
2.2.1. Strains and Culture Conditions
The fungi strains used in this study were obtained from the American-Type Culture Collection (ATCC) and Changhai Hospital of Shanghai, China. All the strains were stored in glycerol and grown in yeast extract–peptone–dextrose (YPD, 1% yeast extract, 2% peptone, and 2% dextrose) medium at 30◦C with shaking (200 rpm) for 16 h. All the target compounds were prepared as 6.4 mg/mL solutions with DMSO.
2.2.2. Antifungal Susceptibility Testing
The in vitro MIC80 values of the compounds against the fungi strains were determined using the microbroth dilution method, according to the protocols from CLSI document M27-A3. Briefly, fungal cells were resuspended in a RPMI 1640 medium and adjusted to 1 × 103 cells/mL. Antifungal compounds were serially 2-fold diluted, with concentrations ranging from 64.0 to 0.125 µg/mL. The final concentration of fluconazole was 8.0 μg/mL for the preliminary screening assay. For the checkerboard microdilution, the final concentration of fluconazole was set in the range of 16.0–0.5 μg/mL. The plates were incubated at 30 °C for 48 h for C. albicans, C. parasilosis, C. glabrata, C. tropicalis, and C. auris, and 72 h for C. neoformans and Aspergillus fumigates. The optical density at 630 nM (OD630 nm) of each well was measured by spectrophotometer.
2.2.3. Biofilm Formation Assay
Biofilm formation was performed according to the reported protocol [25]. C. albicans SC5314 strain was incubated to the exponential growth stage in YPD medium, then harvested and diluted in the RPMI 1640 medium at a concentration of 1 × 106 CFU/mL. The fungal cells were transferred to the 96-well culture plates and static cultured at 37 °C. After 1.5 h of adhesion, the cells were washed three times with phosphate-buffered saline (PBS) and the non-adherent cells were removed. Different concentrations of FCZ and compounds were added, and the cells were further incubated at 37 °C for 24 h. Finally, the XTT/menadione reduction assay was used to examine the formation of biofilms. The optical density at 492 nM (OD492 nm) in each well was measured by a spectrophotometer. The assay was performed in triplicate. The comparison between the two group was determined by Student’s t test.
2.2.4. Cytotoxicity Assays
The cytotoxicity of target compounds on human umbilical vein endothelial cell line (HUVECs) was determined according to previous work [26]. HUVECs were cultured in DMEM medium to 2 × 105 cells/mL. A volume 100 μL of cell suspension was plated in 96-well plates. After adhesion for 12 h, the cell supernatant was replaced with 100 μL fresh DMEM medium containing different concentrations of the tested compounds dissolved in DMSO. Subsequently, the HUVECs were cultured for an additional 24 h at 37 °C with 5% CO2. Then, 10 μL of CCK-8 regents was added to the 96-well plates and the HUVECs were incubated for another 2 h. The cell survival percentage was assessed by detecting absorbance at 450 nm with a microplate reader.
3. Results and Discussion
3.1. Chemistry
The complete synthesis routes of target compounds are described in Scheme 1, Scheme 2, Scheme 3 and Scheme 4.
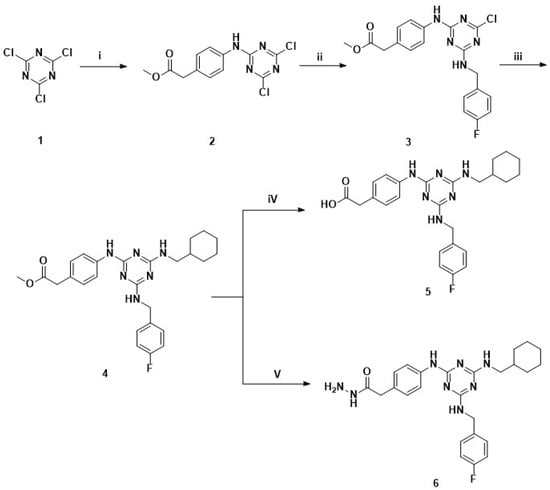
Scheme 1.
Synthetic pathway for key intermediate acid 5 and hydrazide 6. Reagents and conditions: (i) methyl 2-(4-aminophenyl)acetate, NaHCO3, THF, 0 °C, 3 h; (ii) (4-fluorophenyl)methanamine, NaHCO3, THF, 0 °C-r.t., 2 h; (iii) cyclohexylmethanamine, DBU, 1,4-dioxane, reflux, overnight; (iv) 2M NaOH (aq), MeOH, reflux, 5 h; (v) NH2-NH2 H2O, MeOH, reflux, overnight.
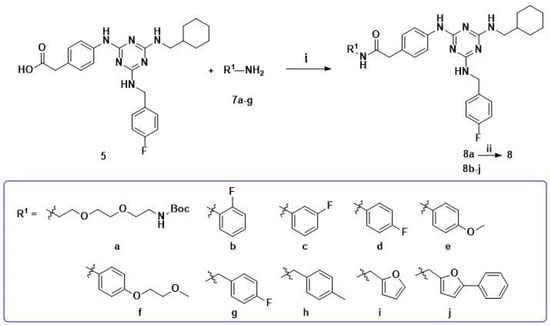
Scheme 2.
Synthetic pathway for hit compound 8 and 1,3,5-triazines derivatives 8b–j. Reagents and conditions: (i) PyBOP, DIEA, DMF, r.t., 3 h; (ii) 4 M HCl in ethyl acetate (EtOAc), EtOAc, 0 °C-r.t., 1 h.
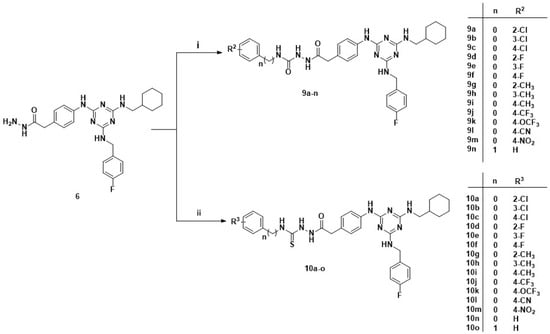
Scheme 3.
Synthetic pathway for 1,3,5-triazines derivatives 9a–n and 10a–o. Reagents and condition: (i) various isocyanate, THF, r.t., 2 h; (ii) various isothiocyanate, EtOH, reflux, 2 h.
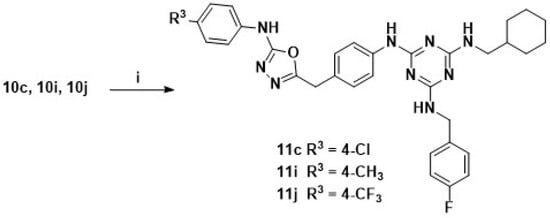
Scheme 4.
Synthetic pathway for the 1,3,5-triazines derivatives 11c, 11i and 11j. Reagents and condition: EDC·HCl, cat. HOBt, DMF, r.t., 3–5 h.
2,4,6-Trichloro-1,3,5-triazine (TCT, 1) is the starting material. The first nucleophile performs at 0 °C, while the second requires room temperature, and the third performs under reflux conditions. By carefully controlling the temperature stepwise in this way, we facilitated the consecutive nucleophilic substitutions with corresponding original aniline, benzylamine, primary amine in reaction activity order from low to high. Finally, we obtained the pure intermediate ester 4 with a relatively high yield, 70%. After regular hydrolysis and hydrazinolysis, the key intermediate acid 5 and hydrazide 6 were obtained, respectively (Scheme 1).
Subsequently, acid 5 and various aromatic amines underwent a condensation reaction using PyBOP as a coupling reagent to give series 8b–j. In this method, we also synthesized compound 8a, then cleaved the Boc protecting group with hydrochloric acid to create hit compound 8 as a control (Scheme 2).
The series 9a–n and 10a–o were prepared according to the procedures shown in Scheme 3. Hydrazide 6 reacted with various commercially available isocyanates and isothiocyanates by a nucleophilic addition to produce the corresponding semicarbazides and thiosemicarbazides moiety-containing target compounds.
EDC-catalyzed cyclization of part thiosemicarbazides of compounds 10c, 10i and 10j yielded the target compounds 11c, 11i and 11j, as illustrated in Scheme 4.
3.2. In Vitro Antifungal Evaluation and Structure–Activity Relationships Study
The in vitro synergistic antifungal activities of newly synthesized 1,3,5-triazine derivatives were tested with the broth microdilution susceptibility assay, guided by Clinical Laboratory Standards Institute (CLSI, USA) in CLSI document M38eA2 [27]. The individual MIC80 values were obtained by determining the minimum concentration of the target compound, or of fluconazole used alone, required to inhibit ≥80% growth of fungal cells compared to the drug-free control. The interaction MIC80 values were determined for wells containing a combination of target compounds and fluconazole. Two fluconazole-resistant C. albicans strains, 901 and 904, were chosen for testing the activity of drug combinations. For determining the interaction modes of 1,3,5-triazine derivatives with fluconazole as synergistic or indifferent, the fractional inhibitory concentration index (FICI) was defined according to FICI values of ≤0.5 or >0.5, respectively, and calculated by summing up the ratios of the MIC80 (combination)/MIC80 (alone) of each compound and fluconazole14. It should be noted that, when the MIC80 value was >64.0 μg/mL, 64.0 μg/mL was used in the calculation.
As shown in Table 1, hit compound 8 in combination with fluconazole had moderate activity against the fluconazole-resistant C. albicans 901 and 904, with an MIC80 = 32.0 μg/mL, and FICI values of 0.625 and 0.375, respectively. These results indicated it was necessary for the structure to be optimized, and that SAR studies would be a useful way to obtain new potential synergists based on compound 8. Thus, we displaced the PEG-containing amphiprotic side chains and kept the amide by coupling acid 5 with a series of amines, such as -F, -CH3, -OCH3, -OCH2CH2OCH3, substituents on the phenyl, and benzyl, furan and phenylfuran in series 8b–j. However, none of the amide compounds were synergistic in combination with fluconazole (FICI > 1). Replacing the long PEG-containing side chains with various aromatic amines had no effects on optimizing hit compound 8.

Table 1.
In vitro antifungal activity of 1,3,5-triazines derivatives used alone or in combination with FCZ (8.0 μg/mL) against FCZ-resistant C. albicans strains (MIC80, μg/mL, 48 h).
Next, we adjusted the side chains, and made use of NH2-NH2.H2O by hydrazinolysis of ester compound 4 to obtain hydrazide intermediate 6. Intermediate 6 was then reacted with various aromatic isocyanate or isothiocyanate to generate compounds 9a–n and compounds 10a–o. Surprisingly for two structurally similar series of compounds, 9a–n and 10a–o exhibited completely opposite results. All of the semicarbazide compounds 9a–n were ineffective when used alone or in combination with fluconazole against azole-resistant C. albicans, irrespective of the substituent on the terminal phenyl ring. In contrast, all thiosemicarbazides compounds 10a–o showed excellent synergistic activity. For C. albicans 901 and 904, the interaction MIC80 values of 10a–o were in the range of 0.125–2.0 μg/mL and the FICI values were 0.127–0.25, indicating that these compounds had a higher level of synergistic activity than hit compound 8. Moreover, it seemed that compounds 10a–m (R3 = Cl, F, CH3, CF3, OCF3, CN, NO2) which had a substituted group on the terminal phenyl, were more active compared to compound 10n (R3 = H) against C. albicans 901, apart from compound 10g (R3 = 2-CH3). The MIC80 of compound 10o was 2.0 μg/mL against isolates C. albicans 901 and 904, which was due to the introduction of a benzyl group causing decreased synergistic activity compared with phenyl isothiocyanate. Compound 10m (R3 = 4-NO2), which bears a strong electron withdrawing group, was the most active compound in our study, with the interaction of MIC80 0.125 μg/mL against C. albicans 901 and 904. In addition, compound 10l (R3 = 4-CN) unexpectedly displayed moderate activity against resistant C. albicans, with an MIC80 of 4.0 μg/mL.
Interestingly, as we introduced 1,3,4-oxadiazole moieties by cyclization of thiosemicarbazides (10c, 10i, and 10j) to afford the target compounds 11c, 11i, and 11j, the synergistic activities were totally lost. It was clear that the thiosemicarbazides moiety was the key pharmacophore and played an important role in exerting synergistic antifungal activity in these trisubstituted 1,3,5-triazines derivatives.
3.3. Checkerboard Microdilution Assay
During the preliminary screening with 8.0 μg/mL FCZ, compounds 10a–o displayed good in vitro synergistic antifungal activity. Thus, four representative active compounds 10a, 10j, 10l, and 10m were selected for a checkerboard microdilution assay. A series of FICI and interaction MIC80 values were determined for these compounds, and the lowest FICI value is shown in Table 2. At concentrations of 32.0–0.125 μg/mL, the MIC80 of tested compounds decreased when combined with fluconazole at concentrations 16.0–0.5 μg/mL, as can be seen in the Supporting Information Figure S1. The lowest FICI values of compounds 10a, 10j, 10l, and 10m were 0.023, 0.039, 0.141, and 0.016, respectively, and showed superior synergistic activity to compound 8 (0.508). The lowest MIC80 values, with fluconazole in the parentheses, were 0.5(1.0), 0.5(2.0), 0.5(1.0) and 0.5(0.5) μg/mL. Overall, compounds 10a, 10j, 10l and 10m at 0.5 μg/mL decreased the MIC80 of fluconazole from >64.0 μg/mL to 0.5–2.0 μg/mL.

Table 2.
Checkerboard microdilution assay of the 1,3,5-triazines derivatives and FCZ (MIC80, μg/mL, 48 h).
In summary, the above results further confirmed that the thiosemicarbazides containing 1,3,5-triazines derivatives possess synergistic activity with fluconazole. Furthermore, it is worthwhile noting that the higher FICI values of 10l (0.141) compared to 10a, 10j, and 10m (0.023, 0.039, 0.016) were due to the moderate antifungal activity of compound 10l alone against the fluconazole-resistant C. albicans. Interestingly, Verma AK et al. recently reported a molecular docking and molecular dynamics modeling to investigate the interaction between the derivatives of 1,2,4-triazine with the enzyme lanosterol 14-demethylase (CYP51) of C. albicans, a key enzyme in sterol biosynthesis and the target of azoles antifungal drugs. They concluded that 1,2,4-triazine and its derivatives can target CYP51, which may be beneficial for bringing into antifungal activity [28]. These findings inspire us to further investigate the mechanism of compound 10l.
3.4. Antifungal Spectrum Investigation of Compound 10l
Inspired by the antifungal activity of compound 101 against the FCZ-resistant C. albicans, we further investigated the in vitro susceptibility of 10l as a single agent against other pathogenic fungi including drug-resistant and -sensitive C. albicans, Cryptococcus neoformans, Candida parapsilosis, Candida glabrata, Candida tropicalis, Candida auris and Aspergillus fumigates (Table 3). Hit compound 8 showed broad-spectrum moderate antifungal activity (MIC80 16.0–32.0 μg/mL) against these strains. Although compound 10l had poor antifungal activity against many of the tested strains, it exhibited excellent and selective anti-Cryptococcus and anti-C. glabrata activity with an MIC80 below 0.125 μg/mL, lower than FCZ. To confirm the specific inhibitory effects of compound 10l against Cryptococcus, a further 8 clinical C. neoformans fungal pathogens were assayed. The results listed in Table S1 indicate that 10l did have potent antifungal activity against multiple strains of Cryptococcus (MIC80 range: ≤0.125–0.5 μg/mL), except for C. neoformans HN-15. Therefore, 10l demonstrated potential as a novel antifungal agent for the treatment of cryptococcal meningitis.

Table 3.
Antifungal spectrum of compound 10l (MIC80, μg/mL, 48 h).
3.5. Inhibitory Effects of Compounds 10l and 10m against the Formation of C. albicans Biofilms
Biofilms, an important virulence factor in C. albicans, are highly resistant to several clinical antifungal agents [29]. There is an intimate relationship between fungal biofilm formation and drug resistance. It was reported that the MIC values of fluconazole against different C. albicans strains under biofilm growth conditions were 60–4000 times higher than those against planktonic cells [30]. To further investigate the synergism of potent compounds 10l or 10m with FCZ on biofilm formation, we conducted a biofilm inhibitory assay using the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide salt (XTT) reduction method. Compounds 10l and 10m, used alone, did not inhibit biofilm formation in the range of 0.0625–1 μg/mL. When the concentration was above 2 μg/mL, both compounds exhibited weaker inhibitory effects on fungal biofilm formation. As a control, FCZ at concentrations of 2, 4, or 8 μg/mL blocked nearly 92% of biofilm formation, and when the concentration decreased to 0.0625 μg/mL, it showed a 37% inhibition rate (Figure 3A). In particular, the inhibitory activity of FCZ at 0.0625 μg/mL on biofilm formation was improved by over 65%, and 62% when it was used in combination with compound 10l or 10m at a lower concentration of 0.0625 μg/mL, respectively (Figure 3B). However, when the FCZ was used at 0.5 or 8 μg/mL, our compounds showed a limited synergism with FCZ, mainly due to the potent inhibitory effects of FCZ itself.
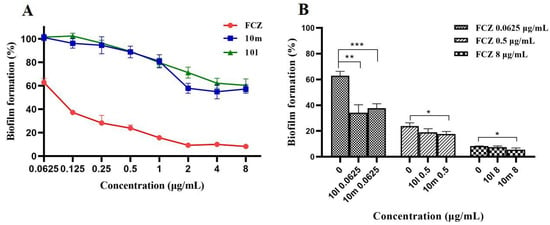
Figure 3.
(A) Inhibition of biofilm formation in C. albicans with the treatment of FCZ, 10l or 10m alone at the indicated concentrations. (B) Inhibition of biofilm formation in C. albicans treated with FCZ in combination with 10l or 10m. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to the corresponding FCZ-treated alone group.
3.6. In Vitro Cytotoxicity Assay
Due to the new types of triazine synergists, we examined whether the antifungal activity was caused by non-specific cytotoxicity. The cytotoxicity of active compounds 10a, 10j, and 10l were tested in a human umbilical vein endothelial cell line (HUVECs) by the CCK8 method. As shown in Figure 4, when compounds 10a and 10l were used alone at 32 μg/mL and 64 μg/mL, they displayed a slight inhibitory effect on the HUVEC cells (survival rate means: 83.19% and 81.88%, respectively), and at 4 μg/mL there was greater than 90% survival. In contrast, compound 10j showed moderate inhibitory effects on the HUVEC cells at 32 μg/mL (survival rate means: 65.28%), but greater than 90% survival at 4 μg/mL. Overall, the above results demonstrated that these compounds have a good safety profile at much lower MIC levels.
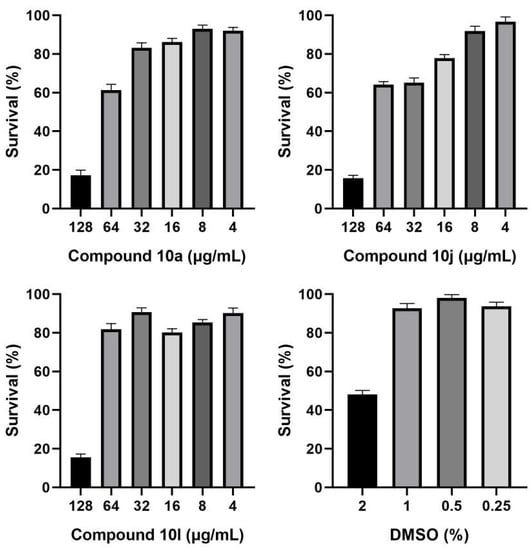
Figure 4.
Cytotoxicity of compound 10a, 10j and 10l against HUVEC cells. The cell survival (%) was measured by CCK8 assay.
3.7. In Silico Predictive Studies
Drug-likeness is a significant drug research and development parameter that promotes molecules to be potential drug candidates. Therefore, it is necessary to evaluate these newly synthesized compounds’ ability as drugs by in silico predictions. As shown in the bioavailability radar formed by the SwissADME [31] (Figure 5), the physicochemical characteristics, lipophilicity, water solubility, and drug-likeness properties of four active compounds 10a, 10j, 10l, and 10m were all outside in the pink area (optimal range), indicating that these compounds had poor physicochemical properties and drug-likeness, such as size bulkiness (molecular weight > 500 Da), high polarity (TPSA > 130 Å2), poor water solubility (Log S (ESOL) < −6.00) and being too flexible (more than 9 rotatable bonds). Although these triazine derivatives exhibited excellent synergistic antifungal effects, they still had a lot of room for us to ameliorate their drug ability through further structural modifications.
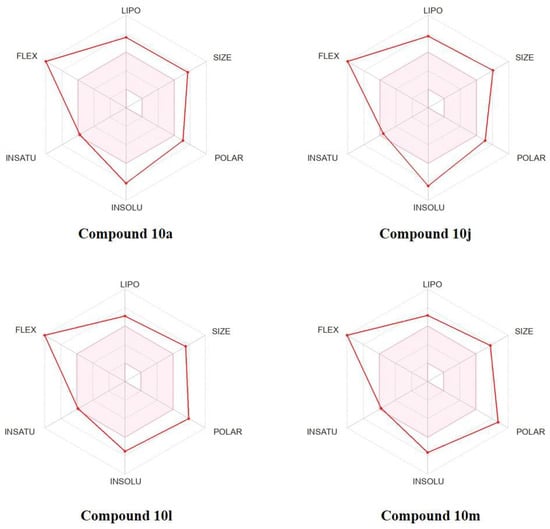
Figure 5.
Bioavailability radar of four active compounds. The pink area represents the optimal range for each property. Abbreviations: LIPO (Lipophilicity), SIZE (Molecular Weight), POLAR (Polarity), INSOLU (Insolubility), INSATU (Instauration), and FLIX (Rotatable bond flexibility).
4. Conclusions
In summary, we described the process of structural optimization of hit compound 8 with a focus on its side chains. A series of novel 1,3,5-triazines derivatives were designed, synthesized, and evaluated their potential as novel synergistic enhancers. Preliminary structure–activity relationship studies revealed that, when the PEG-containing side chains in compound 8 were replaced with the thiosemicarbazides moiety, the synergistic activity of compounds 10a–o was maintained and further improved (MIC80 = 0.125–2.0 μg/mL, FICI range from 0.127 to 0.25) in combination with fluconazole. 10a, 10j, 10l, and 10m were the four potent compounds in combination with fluconazole out of the 10a–o series, and so were tested by checkerboard microdilution assays. Results indicated that low concentrations (0.5 μg/mL) of these compounds could restore fluconazole from MIC80 > 64.0 μg/mL to 0.5–2.0 μg/mL, which in other words significantly sensitized fungi towards fluconazole. To our delight, compound 10l also exhibited moderate activity against resistant Candida (MIC80 = 4.0 μg/mL) and selective anti-Cryptococcus and anti-C. glabrata activity (MIC80 ≤ 0.125 μg/mL). 10l is a promising lead for the development of novel antifungal agents based on the new structure scaffold of the triazine nucleus. Overall, thiosemicarbazides containing 1,3,5-triazines derivatives (compounds 10a–o) possess excellent synergistic antifungal activity, and low levels of cytotoxicity; however, these derivatives had poor physicochemical properties and drug-likeness. Therefore, further structure optimizations, focusing on improving drug-likeness and preliminary mechanism studies to confirm therapeutic targets, are currently underway.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics14112334/s1, Figure S1: Checkerboard microdilution assay of FCZ with the target compounds 10a, 10j, 10l and 10m; Table S1: Antifungal activity of compound 10l against 8 Cryptococcus strains.
Author Contributions
Data curation, J.B.; Project administration, J.L. and Y.L.; Supervision, T.N., X.C., T.W., S.Y. and Y.J.; Writing—original draft, F.X. and Y.H.; Writing—review and editing, D.Z., L.L. and L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81830106; 82173786; 82173867), and Shanghai Pujiang Program (No. 21PJD081).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
These authors declare no conflict of interest.
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The Changing Epidemiology of Invasive Fungal Infections. Methods Mol. Biol. 2017, 1508, 17–65. [Google Scholar] [PubMed]
- Rodrigues, M.E.; Silva, S.; Azeredo, J.; Henriques, M. Novel strategies to fight Candida species infection. Crit. Rev. Microbiol. 2016, 42, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Calderone, R.; Sun, N.; Gay-Andrieu, F.; Groutas, W.; Weerawarna, P.; Prasad, S.; Alex, D.; Li, D. Antifungal drug discovery: The process and outcomes. Future Microbiol. 2014, 9, 791–805. [Google Scholar] [CrossRef]
- Denning, D.W.; Bromley, M.J. Infectious Disease. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef]
- Stewart, A.G.; Paterson, D.L. How urgent is the need for new antifungals? Expert Opin. Pharm. 2021, 22, 1857–1870. [Google Scholar] [CrossRef]
- Maddy, A.J.; Sanchez, N.; Shukla, B.S.; Maderal, A.D. Dermatological manifestations of fungal infection in patients with febrile neutropaenia: A review of the literature. Mycoses 2019, 62, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tu, J.; Ji, C.; Li, Z.; Han, G.; Liu, N.; Li, J.; Sheng, C. Discovery of piperidol derivatives for combinational treatment of azole-resistant candidiasis. ACS Infect. Dis. 2021, 7, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cao, Y.Y.; Dai, B.D.; Sun, X.R.; Zhu, Z.Y.; Cao, Y.B.; Wang, Y.; Gao, P.H.; Jiang, Y.Y. In vitro synergism of fluconazole and baicalein against clinical isolates of Candida albicans resistant to fluconazole. Biol. Pharm. Bull. 2008, 31, 2234–2236. [Google Scholar] [CrossRef]
- Zhao, F.; Dong, H.H.; Wang, Y.H.; Wang, T.Y.; Yan, Z.H.; Yan, F.; Zhang, D.Z.; Cao, Y.Y.; Jin, Y.S. Synthesis and synergistic antifungal effects of monoketone derivatives of curcumin against fluconazole-resistant Candida spp. Medchemcomm 2017, 8, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Cao, Y.Y.; Xu, Z.; Zhao, J.X.; Gao, P.H.; Qin, X.F.; Jiang, Y.Y. Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob. Agents Chemother. 2006, 50, 1096–1099. [Google Scholar] [CrossRef]
- Dai, L.; Zang, C.; Tian, S.; Liu, W.; Tan, S.; Cai, Z.; Ni, T.; An, M.; Li, R.; Gao, Y.; et al. Design, synthesis, and evaluation of caffeic acid amides as synergists to sensitize fluconazole-resistant Candida albicans to fluconazole. Bioorganic Med. Chem. Lett. 2015, 25, 34–37. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Li, Y.; Liu, J.; An, M.; Zhu, S.; Cao, Y.; Jiang, Z.; Zhao, M.; Cai, Z.; et al. Structural optimization of berberine as a synergist to restore antifungal activity of fluconazole against drug-resistant Candida albicans. ChemMedChem 2014, 9, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Ding, Z.; Hao, Y.; Ni, T.; Xie, F.; Zhao, J.; Li, R.; Yu, S.; Wang, T.; Chai, X.; et al. Design, synthesis, and SAR study of 3-(benzo[d][1,3]dioxol-5-yl)-N-benzylpropanamide as novel potent synergists against fluconazole-resistant Candida albicans. Bioorganic Med. Chem. Lett. 2017, 27, 4571–4575. [Google Scholar] [CrossRef] [PubMed]
- Mondal, J.; Sivaramakrishna, A. Functionalized triazines and tetrazines: Synthesis and applications. Top. Curr. Chem. (Cham) 2022, 380, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Long, S.; Rakesh, K.P.; Zha, G.F. Structure-activity relationships (SAR) of triazine derivatives: Promising antimicrobial agents. Eur. J. Med. Chem. 2020, 185, 111804. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mandal, M.K.; Masih, A.; Saha, A.; Ghosh, S.K.; Bhat, H.R.; Singh, U.P. 1,3,5-Triazine: A versatile pharmacophore with diverse biological activities. Arch. Pharm. (Weinh.) 2021, 354, e2000363. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorganic Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Srivastava, K.; Puri, S.K.; Chauhan, P.M. Syntheses of 2,4,6-trisubstituted triazines as antimalarial agents. Bioorganic Med. Chem. Lett. 2005, 15, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Lazewska, D.; Wiecek, M.; Ner, J.; Kaminska, K.; Kottke, T.; Schwed, J.S.; Zygmunt, M.; Karcz, T.; Olejarz, A.; Kuder, K.; et al. Aryl-1,3,5-triazine derivatives as histamine H4 receptor ligands. Eur. J. Med. Chem. 2014, 83, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Bhat, H.R.; Gahtori, P. Antifungal activity, SAR and physicochemical correlation of some thiazole-1,3,5-triazine derivatives. J. Mycol. Med. 2012, 22, 134–141. [Google Scholar] [CrossRef]
- Alhameed, R.A.; Almarhoon, Z.; Sholkamy, E.N.; Khan, S.A.; Ul-Haq, Z.; Sharma, A.; de la Torre, B.G.; Albericio, F.; El-Faham, A. Novel 4,6-disubstituted s-triazin-2-yl amino acid derivatives as promising antifungal agents. J. Fungi 2020, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- Haiba, N.S.; Khalil, H.H.; Moniem, M.A.; El-Wakil, M.H.; Bekhit, A.A.; Khattab, S.N. Design, synthesis and molecular modeling studies of new series of s-triazine derivatives as antimicrobial agents against multi-drug resistant clinical isolates. Bioorg. Chem. 2019, 89, 103013. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaydi, K.M.; Khalil, H.H.; El-Faham, A.; Khattab, S.N. Synthesis, characterization and evaluation of 1,3,5-triazine aminobenzoic acid derivatives for their antimicrobial activity. Chem. Cent. J. 2017, 11, 39. [Google Scholar] [CrossRef]
- Dong, H.H.; Wang, Y.H.; Peng, X.M.; Zhou, H.Y.; Zhao, F.; Jiang, Y.Y.; Zhang, D.Z.; Jin, Y.S. Synergistic antifungal effects of curcumin derivatives as fungal biofilm inhibitors with fluconazole. Chem. Biol. Drug Des. 2021, 97, 1079. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Xu, J.; Wu, J.; Wang, Y.; Qiu, X.; Zhang, Y.; Hou, W.; Yan, L.; An, M.; et al. The synergism of the small molecule ENOblock and fluconazole against fluconazole-resistant Candida albicans. Front. Microbiol. 2019, 10, 2071. [Google Scholar] [CrossRef]
- M38eA2; Clinical and Laboratory Standards Institute/National Committee for Clinical Laboratory Standards, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast. 2nd ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- Verma, A.K.; Majid, A.; Hossain, M.S.; Ahmed, S.F.; Ashid, M.; Bhojiya, A.A.; Upadhyay, S.K.; Vishvakarma, N.K.; Alam, M. Identification of 1,2,4-triazine and its derivatives against lanosterol 14-demethylase (CYP51) property of Candida albicans: Influence on the development of new antifungal therapeutic strategies. Front. Med. Technol. 2022, 4, 845322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, Z.; Yin, H.; Hu, J.; Xue, Y.; Zhang, G.; Zheng, X.; Chen, W.; Hu, X. Synergistic antibiofilm effects of pseudolaric acid A combined with fluconazole against Candida albicans via inhibition of adhesion and yeast-to-hypha transition. Microbiol. Spectr. 2022, 10, e01478-21. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Walle, K.V.; Wickes, B.L.; Lopez-Ribot, J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).