Formulation and Optimization of Metronidazole and Lactobacillus spp. Layered Suppositories via a Three-Variable, Five-Level Central Composite Design for the Management of Bacterial Vaginosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of Experiment
2.2. Artificial Neural Network Modelling
2.3. Goodness of Fit Assessment of the ANN Model
2.4. Optimisation of Responses
2.5. Preparation of Layered Suppositories
2.6. Physicochemical Characterization of the Layered Suppositories
2.7. Stability Testing of Optimized Suppositories
2.8. In Vitro Drug Release Analysis of Layered Suppositories
2.9. Lactobacilli Cell Viability of the Formulated Suppositories
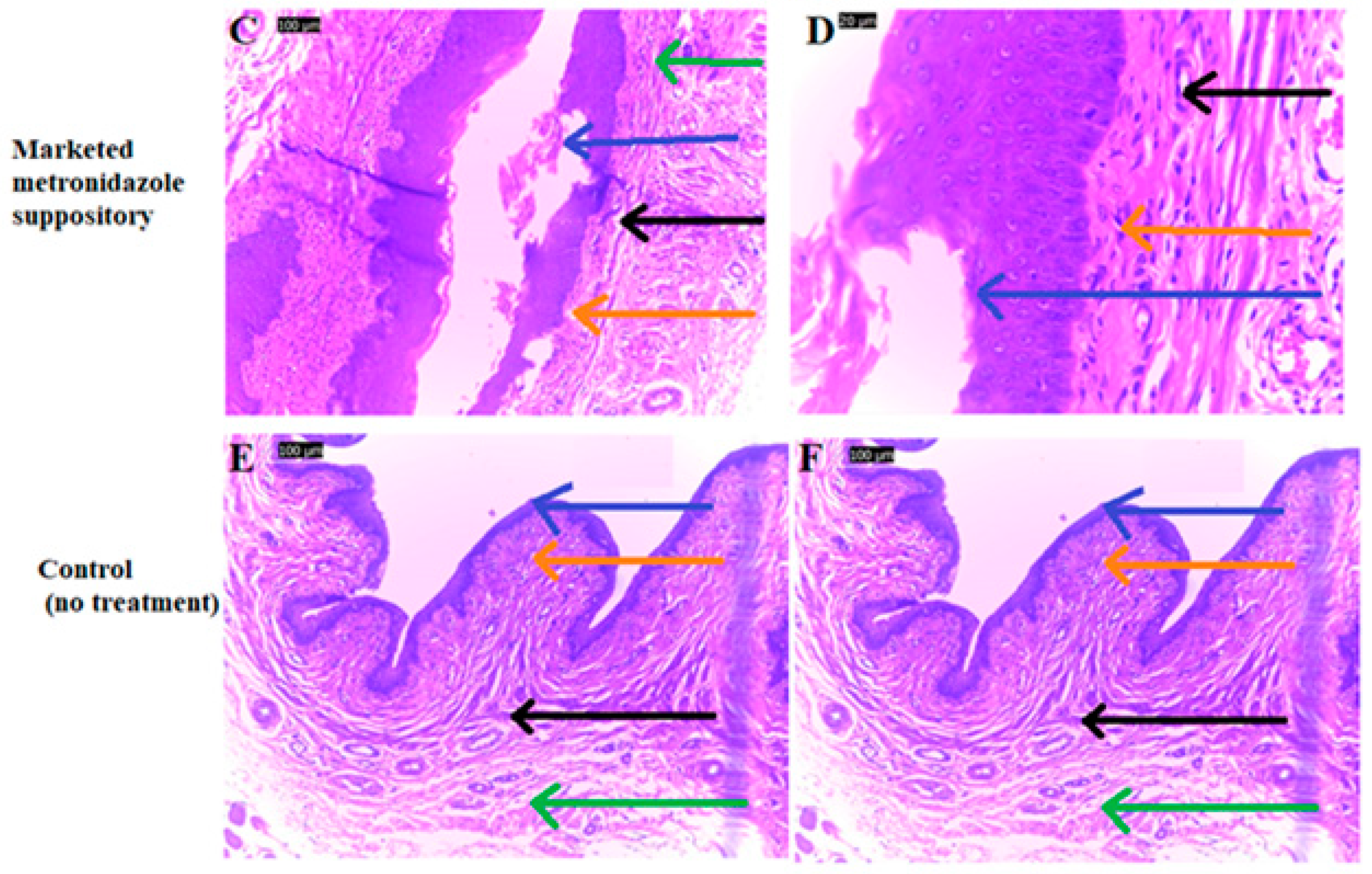
2.10. Assessment of Vagina Epithelium Exposed to Metronidazole Containing Lactobacillus spp. Formulation
2.11. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of the Layered Suppositories
3.2. ANN Architecture and Training
3.3. Validation of ANN Model Predictions
3.4. Effect of Input Factors on the Responses
3.5. Optimization of Input Factors and Responses
3.6. Assessment of Vagina Epithelium Exposed to Metronidazole Containing Lactobacillus spp. Formulation
3.7. Stability Testing
4. Discussion
4.1. Physicochemical Characterization of the Layered Suppositories
4.2. Effect of Input Factors on the Responses after Validation Using ANN Model Predictions
4.3. Optimization of Input Factors and Responses
4.4. Assessment of Vagina Epithelium Exposed to Metronidazole Containing Lactobacillus spp. Formulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BV | Bacterial vaginosis (BV) |
| RSM | response surface methodology |
| CCD | central composite design |
| ANN | artificial neural networks |
| MNFF | multilayer normal feed forward |
| MFFF | multilayer full feed forward |
| IBP | incremental back propagation algorithm |
| BBP | batch back propagation algorithm |
| QP | quick propagation algorithm |
| LM | Levenberg–Marquadt algorithm |
| MSE | mean square error |
| RMSE | root mean square error |
| SEP | standard error of prediction |
| MAE | mean absolute error |
| AAD | average absolute deviation |
| ICH | International Council for Harmonisation |
| HREC | Health Research Ethical Committee |
| ANOVA | Analysis of variance |
References
- Centre for Disease Control and Prevention (CDC 2020). Bacterial Vaginosis [BV] Statistics. Available online: http://www.cdc.gov/std/bv/stats.html (accessed on 12 April 2020).
- Taylor, B.D.; Darville, T.; Haggerty, C.L. Does bacterial vaginosis cause pelvic inflammatory disease. Sex Transm. Dis. 2013, 40, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.M.; Bukar, M.; Galadima, G.B.; Audu, B.M.; Ibrahim, H.A. Prevalence of bacterial vaginosis in pregnant women in Maiduguri, North-Eastern Nigeria. Niger. J. Clin. Pract. 2014, 17, 154–158. Available online: http://www.njcponline.com/text.asp?2014/17/2/154/127424 (accessed on 12 February 2022). [CrossRef] [PubMed]
- Ilomuanya, M.O.; Ifudu, N.D.; Odulaja, J.; Igwilo, C. Assessment of the effect of base type and surfactant on the release properties and kinetics of Paracetamol suppositories. J. Chem. Pharm. Res. 2012, 4, 3280–3286. [Google Scholar]
- Hanson, J.M.; McGregor, J.A.; Hillier, S.L.; Eschenbach, D.A.; Kreutner, A.K.; Galask, R.P.; Martens, M. Metronidazole for bacterial vaginosis. A comparison of vaginal gel vs. oral therapy. J. Reprod. Med. 2000, 45, 889–896. Available online: http://www.medwelljournals.com/abstract/?doi=rjmsci.2007.195.198 (accessed on 15 March 2022). [CrossRef]
- Löfmark, S.; Edlund, C.; Nord, C.E. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin. Infect. Dis. 2010, 50 (Suppl. 1), S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.G.; Brandsborg, E.; Forsum, U.; Pendharkar, S.; Andersen, K.K.; Nasic, S.; Hammarström, L.; Marcotte, H. Extended antimicrobial treatment of bacterial vaginosis combined with human lactobacilli to find the best treatment and minimize the risk of relapses. BMC Infect. Dis. 2011, 11, 223. [Google Scholar] [CrossRef]
- Falagas, M.; Betsi, G.I.; Athanasiou, S. Probiotics for the treatment of women with bacterial vaginosis. Clin. Microbiol. Infect. 2007, 13, 657–664. [Google Scholar] [CrossRef]
- FAO; WHO. Evaluation of health and nutritional properties of probiotics in food, including powder milk with live lactic acid bacteria. FAO/WHO Expert Consult. Rep. 2001, 2001, 1–34. [Google Scholar]
- Rodrigues, F.; Maia, M.J.; das Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B. Vaginal suppositories containing Lactobacillus acidophilus: Development and characterization. Drug Dev. Ind. Pharmacy 2015, 41, 1518–1525. [Google Scholar] [CrossRef]
- Ilomuanya, M.; Onwubuya, C.P.; Amenaghawon, N.A. Development and optimization of antioxidant polyherbal cream using artificial neural network aided response surface methodology. J. Pharm. Technol. 2020, 1, 46–53. [Google Scholar] [CrossRef]
- Amenaghawon, N.A.; Kazeem, J.O. Evaluation of the Comparative Performance of Response Surface Methodology, Artificial Neural Network and Adaptive Neuro Fuzzy Inference System for Modelling and Optimising Oxalic Acid Production from Pineapple Waste. FUW Trends Sci. Technol. J. 2020, 5, 255–263. [Google Scholar]
- Betiku, E.; Odude, V.O.; Ishola, N.B.; Bamimore, A.; Osunleke, A.S.; Okeleye, A.A. Predictive capability evaluation of RSM, ANFIS and ANN: A case of reduction of high free fatty acid of palm kernel oil via esterification process. Energy Convers. Manag. 2016, 124, 219–230. [Google Scholar] [CrossRef]
- Amenaghawon, N.A.; Agbedor, M. A Multilayer Full Feed Forward Neural Network for Modelling and Optimizing Nutrient Medium Composition for Oxalic Acid Production from Sweet Potato Peels. ATBU J. Sci. Technol. Edu. 2021, 9, 44–53. [Google Scholar]
- Ilomuanya, M.O.; Elesho, R.; Amenaghawon, A.; Adetuyi, A.; Velusamy, V.; Akanmu, S. Development of trigger sensitive hyaluronic acid/palm oil-based organogel for in vitro release of HIV/AIDS microbicides using artificial neural networks. Future J. Pharm. Sci. 2020, 6, 1. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, S.; Diao, C.; Liu, M.; Li, G.; Zhang, H. Simplifying Neural Network Based Model for Ship Motion Prediction: A Comparative Study of Sensitivity Analysis. In Proceedings of the ASME 2017 36th International Conference on Ocean, Offshore and Arctic Engineering, Trondheim, Norway, 25–30 June 2017; p. V001T01A016. [Google Scholar]
- Sankar, V.R.; Reddy, Y.D.; Reddy, G.H.; Reddy, G.S.K. Formulation and In-vitro characterisation of sustained release metronidazole cocoa butter suppositories. Inventi. Impact Pharm. Tech. 2012, 4, 275–280. [Google Scholar]
- Persaud, S.; Eid, S.; Swiderski, N.; Serris, I.; Cho, H. Preparations of Rectal Suppositories Containing Artesunate. Pharmaceutics 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. NC3Rs Reporting Guidelines Working Group Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Adesiji, Y.O.; Taiwo, S.S.; Adekanle, D.A.; Oboro, V.O.; Fayemiwo, S.A.; Opaleye, O.O. Bacterial vaginosis and pregnancy outcome in Osogbo, Nigeria. Res. J. Med. Sci. 2007, 1, 195–198. [Google Scholar]
- Koumans, E.H.; Sternberg, M.; Bruce, C.; McQuillan, G.; Kendrick, J.; Sutton, M.; Markowitz, L.E. The Prevalence of Bacterial Vaginosis in the United States, 2001–2004; Associations With Symptoms, Sexual Behaviors, and Reproductive Health. Sex. Transm. Dis. 2007, 34, 864–869. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, P.R.; Parolin, C.; Abruzzo, A.; Luppi, B.; Protti, M.; Mercolini, L.; Silva, J.A.; Giordani, B.; Marangoni, A.; Nader-Macías, M.E.; et al. Biosurfactant from vaginal Lactobacillus crispatus BC1 as a promising agent to interfere with Candida adhesion. Microb. Cell Fact. 2020, 19, 133. [Google Scholar] [CrossRef]
- Cardoso-Daodu, M.; Ilomuanya, M.O.; Amenaghawon, A.N.; Azubuike, C.P. Artificial neural network for optimizing the formulation of curcumin-loaded liposomes from statistically designed experiments. Prog. Biomater. 2022, 11, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ilomuanya, M.O.; Hameedat, A.T.; Akang, E.N.; Ekama, S.O.; Silva, B.O.; Akanmu, A.S. Development and evaluation of mucoadhesive bigel containing tenofovir and maraviroc for HIV prophylaxis. Futur. J. Pharm. Sci. 2020, 6, 81. [Google Scholar] [CrossRef]




| Physical Characteristics | A | B | C | D | E | F | G | H | I | J | K | L | M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Color and Opacity | Yellow and White Opaque | ||||||||||||
| Shape | Conical | ||||||||||||
| Homogeneity | Homogenous | ||||||||||||
| Weight Variation * (gram) | 1.17 ± 0.05 | 1.14 ± 0.05 | 1.2 ± 0 | 1.19 ± 0.03 | 1.13 ± 0.05 | 1.13 ± 0.05 | 1.12 ± 0.04 | 1.12 ± 0.04 | 1.16 ± 0.05 | 1.19 ± 0.03 | 1.19 ± 0.03 | 1.13 ± 0.09 | 1.14 ± 0.05 |
| Hardness * (kilogram) | 0.8 ± 0.28 | 0.9 ± 0.42 | 1.3 ± 0.14 | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.8 ± 0.28 | 0.7 ± 0.14 | 0.6 ± 0 | 0.9 ± 0.14 | 0.8 ± 0 | 0.74 ± 0.14 | 0.8 ± 0.28 | 0.6 ± 0.0 |
| Melting Point range (°C) | 32–36.5 | 33.5–38 | 35–38 | 31.5–37 | 32.5–377 | 32–36.5 | 32–36.5 | 30.45–37 | 32–36.5 | 32–37 | 35–37.5 | 34–36.5 | 32–36.5 |
| Solidification Point * (°C) | 38.5 ± 0.71 | 34.5 ± 0.71 | 38.9 ± 1.56 | 39.5 ± 4.95 | 37.4 ± 0.91 | 36.0 ± 1.41 | 36.5 ± 2.12 | 35.0 ± 1.41 | 36.5 ± 0.71 | 37.5 ± 2.12 | 39.0 ± 1.41 | 39.0 ± 1.41 | 36.5 ± 0.71 |
| Disintegration time * (minutes) | 10.83 ± 0.56 | 10.84 ± 0.45 | 12.76 ± 0.37 | 10.78 ± 0.69 | 10.55 ± 0.04 | 11.02 ± 0.01 | 10.07 ± 0.09 | 7.55 ± 0.02 | 9.95 ± 0.35 | 10.65 ± 0.70 | 11.29 ± 0.23 | 10.2 ± 0.13 | 9.87 ± 0.99 |
| Transfer Function | Drug Metronidazole Release (%) | Lactobacilli Viability (%) | ||
|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | |
| Sigmoid | 0.9767 | 1.0436 | 0.9998 | 0.3485 |
| Tanh | 0.9989 | 0.2297 | 0.9999 | 0.0225 |
| Gaussian | 0.9990 | 0.2161 | 0.9992 | 0.2548 |
| Linear | 0.8304 | 2.8136 | 0.1631 | 25.1260 |
| Threshold linear | 0.0014 | 6.8313 | 0.6115 | 17.1200 |
| Bipolar linear | 0.0014 | 6.8313 | 0.6642 | 15.9150 |
| Network Architecture | Training Algorithm | Response | |||
|---|---|---|---|---|---|
| Drug Metronidazole Release (%) | Lactobacilli Viability (%) | ||||
| R2 | RMSE | R2 | RMSE | ||
| MNFF | IBP | 0.9990 | 0.2161 | 0.9999 | 0.0225 |
| BBP | 0.8644 | 2.5152 | 0.9999 | 0.0250 | |
| QP | 0.9889 | 0.7208 | 0.9999 | 0.1919 | |
| GA | 0.9861 | 0.8067 | 0.9999 | 0.0337 | |
| LM | 0.3402 | 5.5489 | 0.0434 | 26.8630 | |
| MFFF | IBP | 0.9995 | 0.2161 | 1.0000 | 0.0225 |
| BBP | 0.9972 | 0.3590 | 1.0000 | 0.0232 | |
| QP | 0.9661 | 1.2581 | 1.0000 | 0.0233 | |
| GA | 0.9990 | 0.2162 | 0.9999 | 0.0337 | |
| LM | 0.9990 | 0.2162 | 0.0434 | 26.8630 | |
| Run | Blends | Factors | Responses | ||||
|---|---|---|---|---|---|---|---|
| Drug Metronidazole Release (%) | Lactobacilli Viability (%) | ||||||
| Ovucire/PEG | Surfactant Concentration (%w/w) | Experiment | Predicted | Experiment | Predicted | ||
| 1 | A | 2.25 | 0.25 | 86.64 | 86.59 | 30.96 | 30.95 |
| 2 | B | 3.49 | 0.07 | 81.85 | 81.85 | 37.21 | 37.21 |
| 3 | C | 1.01 | 0.07 | 96.91 | 96.91 | 96.64 | 96.64 |
| 4 | D | 2.25 | 0.25 | 85.95 | 86.59 | 30.96 | 30.95 |
| 5 | E | 1.01 | 0.43 | 92.54 | 92.54 | 80.45 | 80.45 |
| 6 | F | 2.25 | 0.25 | 86.64 | 86.59 | 30.96 | 30.95 |
| 7 | G | 4.00 | 0.25 | 75.17 | 75.17 | 88.34 | 88.34 |
| 8 | H | 3.49 | 0.43 | 70.28 | 70.28 | 67.92 | 67.92 |
| 9 | I | 2.25 | 0.50 | 84.90 | 84.90 | 80.44 | 80.44 |
| 10 | J | 0.50 | 0.25 | 89.37 | 89.37 | 85.71 | 85.71 |
| 11 | K | 2.25 | 0.25 | 86.86 | 86.59 | 30.98 | 30.95 |
| 12 | L | 2.25 | 0.00 | 88.10 | 88.10 | 30.57 | 30.57 |
| 13 | M | 2.25 | 0.25 | 86.86 | 86.59 | 30.88 | 30.88 |
| Parameter | Response | |
|---|---|---|
| Drug Metronidazole Release | Lactobacilli Viability | |
| R | 0.9997 | 0.9999 |
| R2 | 0.9995 | 0.9999 |
| Adjusted R2 | 0.9995 | 0.9999 |
| MSE | 0.0467 | 0.0005 |
| RMSE | 0.2161 | 0.0225 |
| SEP | 0.2427 | 0.0191 |
| MAE | 0.0985 | 0.0052 |
| AAD | 0.1140 | 0.0169 |
| Variables | Optimization Algorithms | |||||
|---|---|---|---|---|---|---|
| GA | RIO | PSO | ||||
| Drug Metronidazole Release (%) | Lactobacilli Viability (%) | Drug Metronidazole Release (%) | Lactobacilli Viability (%) | Drug Metronidazole Release (%) | Lactobacilli Viability (%) | |
| Ovucire/PEG | 1.086 | 1.042 | 1.087 | 1.038 | 1.087 | 1.041 |
| Surfactant concentration (%w/w) | 0.046 | 0.000342 | 0.046 | 0.000113 | 0.046 | 0.000483 |
| Predicted maximum response value (%) | 97.029 | 97.410 | 97.029 | 97.410 | 97.029 | 97.410 |
| Actual maximum response value (%) | 97.63 ± 0.22 | 97.40 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilomuanya, M.O.; Salako, B.B.; Ologunagba, M.O.; Shonekan, O.O.; Owodeha-Ashaka, K.; Osahon, E.S.; Amenaghawon, A.N. Formulation and Optimization of Metronidazole and Lactobacillus spp. Layered Suppositories via a Three-Variable, Five-Level Central Composite Design for the Management of Bacterial Vaginosis. Pharmaceutics 2022, 14, 2337. https://doi.org/10.3390/pharmaceutics14112337
Ilomuanya MO, Salako BB, Ologunagba MO, Shonekan OO, Owodeha-Ashaka K, Osahon ES, Amenaghawon AN. Formulation and Optimization of Metronidazole and Lactobacillus spp. Layered Suppositories via a Three-Variable, Five-Level Central Composite Design for the Management of Bacterial Vaginosis. Pharmaceutics. 2022; 14(11):2337. https://doi.org/10.3390/pharmaceutics14112337
Chicago/Turabian StyleIlomuanya, Margaret O., Busayo B. Salako, Modupe O. Ologunagba, Omonike O. Shonekan, Kruga Owodeha-Ashaka, Eseosa S. Osahon, and Andrew N. Amenaghawon. 2022. "Formulation and Optimization of Metronidazole and Lactobacillus spp. Layered Suppositories via a Three-Variable, Five-Level Central Composite Design for the Management of Bacterial Vaginosis" Pharmaceutics 14, no. 11: 2337. https://doi.org/10.3390/pharmaceutics14112337
APA StyleIlomuanya, M. O., Salako, B. B., Ologunagba, M. O., Shonekan, O. O., Owodeha-Ashaka, K., Osahon, E. S., & Amenaghawon, A. N. (2022). Formulation and Optimization of Metronidazole and Lactobacillus spp. Layered Suppositories via a Three-Variable, Five-Level Central Composite Design for the Management of Bacterial Vaginosis. Pharmaceutics, 14(11), 2337. https://doi.org/10.3390/pharmaceutics14112337








