Experimental and Computational Observations of Immunogenic Cobalt Porphyrin Lipid Bilayers: Nanodomain-Enhanced Antigen Association
Abstract
1. Introduction
2. Materials and Methods
2.1. Liposome Preparation and Characterization
2.2. Absorption Spectra of Cobalt Tetrapyrroles
2.3. Characterization of Histidine Binding to Cobalt Tetrapyrroles
2.4. Cryo-Electron Microscopy (Cryo-EM)
2.5. Fluorescence Quenching of Proteins and Peptides
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Antigen Uptake Study
2.8. Murine Immunization
2.9. Antibody Analysis
2.10. Molecular Dynamics Simulations
3. Results and Discussion
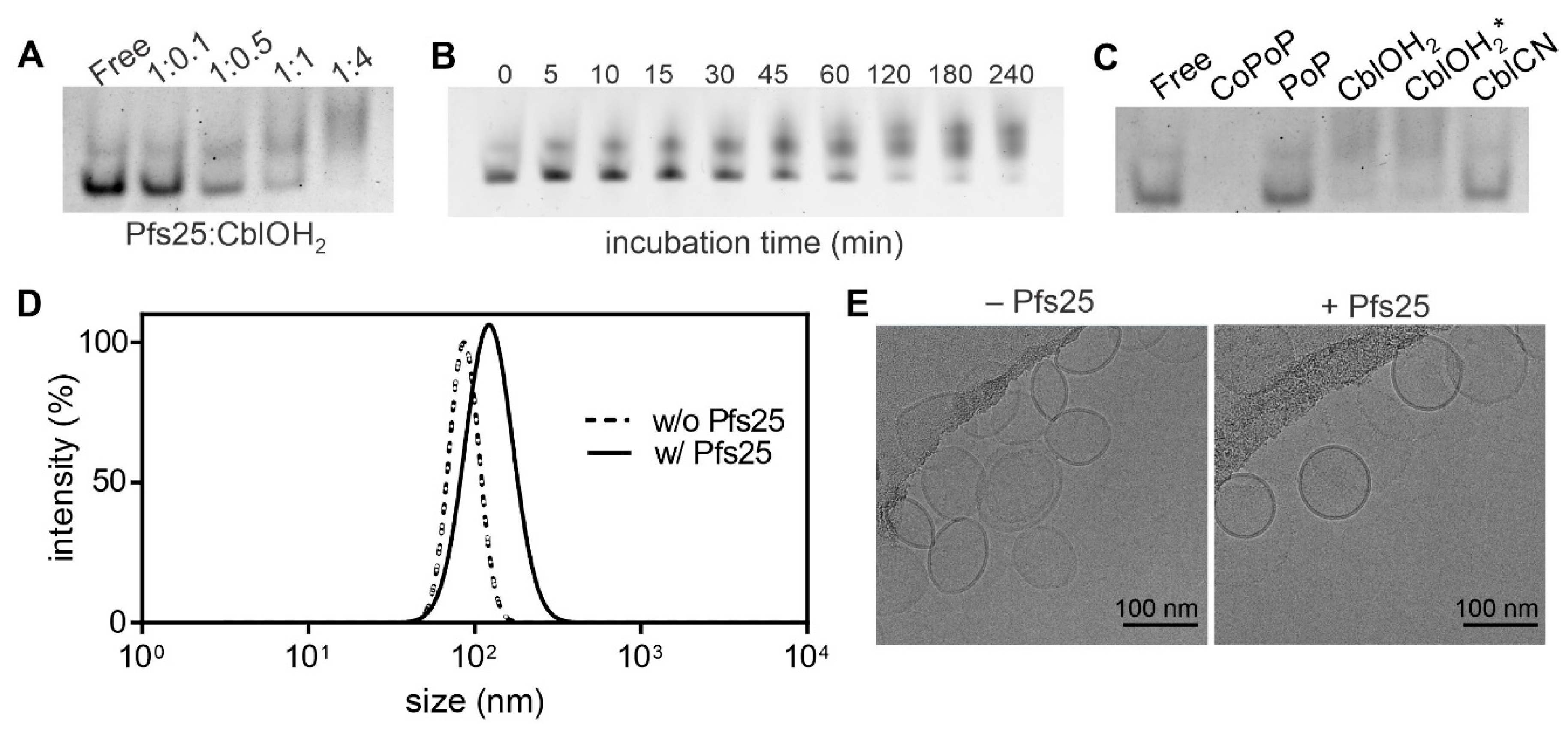
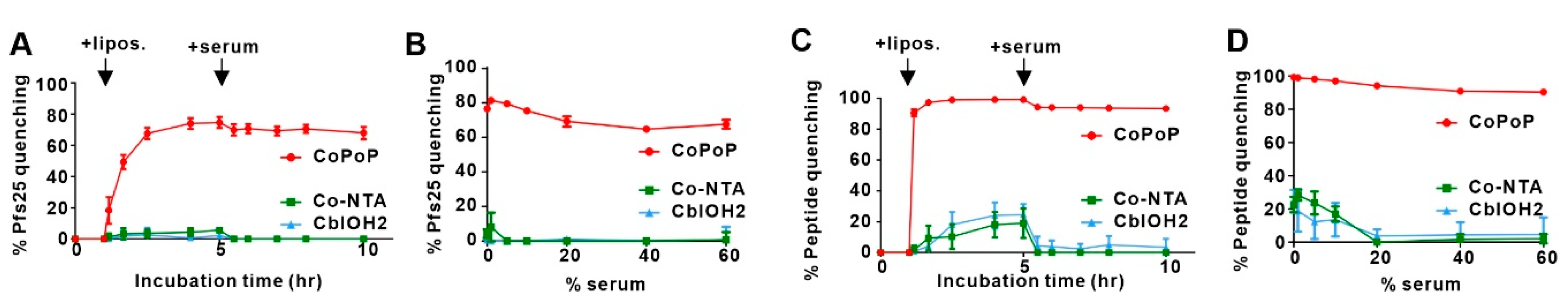
3.1. Experimental Results
3.2. Simulation Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Draper, S.J.; Angov, E.; Horii, T.; Miller, L.H.; Srinivasan, P.; Theisen, M.; Biswas, S. Recent advances in recombinant protein-based malaria vaccines. Vaccine 2015, 33, 7433–7443. [Google Scholar] [CrossRef] [PubMed]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- Oakes, R.S.; Froimchuk, E.; Jewell, C.M. Engineering Biomaterials to Direct Innate Immunity. Adv. Ther. 2019, 2, 1800157. [Google Scholar] [CrossRef] [PubMed]
- Bookstaver, M.L.; Tsai, S.J.; Bromberg, J.S.; Jewell, C.M. Improving Vaccine and Immunotherapy Design Using Biomaterials. Trends Immunol. 2018, 39, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, B.; Goodman, J.T.; Ramirez, J.E.V. Rational Design of Targeted Next-Generation Carriers for Drug and Vaccine Delivery. Annu. Rev. Biomed. Eng. 2016, 18, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Welder, J.H.; Torres, M.P.; Kipper, M.J.; Mallapragada, S.K.; Wannemuehler, M.J.; Narasimhan, B. Vaccine adjuvants: Current challenges and future approaches. J. Pharm. Sci. 2009, 98, 1278–1316. [Google Scholar] [CrossRef] [PubMed]
- Alving, C.R.; Rao, M.; Steers, N.J.; Matyas, G.R.; Mayorov, A.V. Liposomes containing lipid A: An effective, safe, generic adjuvant system for synthetic vaccines. Expert Rev. Vaccines 2012, 11, 733–744. [Google Scholar] [CrossRef]
- Schmidt, S.T.; Foged, C.; Korsholm, K.S.; Rades, T.; Christensen, D. Liposome-Based Adjuvants for Subunit Vaccines: Formulation Strategies for Subunit Antigens and Immunostimulators. Pharmaceutics 2016, 8, 7. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Watson, D.S.; Endsley, A.N.; Huang, L. Design considerations for liposomal vaccines: Influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine 2012, 30, 2256–2272. [Google Scholar] [CrossRef]
- Serre, K.; Machy, P.; Grivel, J.C.; Jolly, G.; Brun, N.; Barbet, J.; Leserman, L. Efficient presentation of multivalent antigens targeted to various cell surface molecules of dendritic cells and surface Ig of antigen-specific B cells. J. Immunol. 1998, 161, 6059–6067. [Google Scholar] [PubMed]
- Křupka, M.; Mašek, J.; Bartheldyová, E.; Knötigová, P.T.; Plocková, J.; Korvasová, Z.; Škrabalová, M.; Koudelka, Š.; Kulich, P.; Zachová, K.; et al. Enhancement of immune response towards non-lipidized Borrelia burgdorferi recombinant OspC antigen by binding onto the surface of metallochelating nanoliposomes with entrapped lipophilic derivatives of norAbuMDP. J. Control. Release 2012, 160, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Rajendiran, V.; Lovell, J.F. Metalloporphyrin nanoparticles: Coordinating diverse theranostic functions. Co-Ord. Chem. Rev. 2019, 379, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Geng, J.; Yi, H.A.; Gogia, S.; Neelamegham, S.; Jacobs, A.; Lovell, J.F. Functionalization of cobalt porphyrin–phospholipid bilayers with his-tagged ligands and antigens. Nat. Chem. 2015, 7, 438–446. [Google Scholar] [CrossRef]
- Bunker, A.; Magarkar, A.; Viitala, T. Rational design of liposomal drug delivery systems, a review: Combined experimental and computational studies of lipid membranes, liposomes and their PEGylation. Biochim. Biophys. Acta 2016, 1858, 2334–2352. [Google Scholar] [CrossRef]
- Huang, W.-C.; Deng, B.; Lin, C.; Carter, K.A.; Geng, J.; Razi, A.; He, X.; Chitgupi, U.; Federizon, J.; Sun, B.; et al. A malaria vaccine adjuvant based on recombinant antigen binding to liposomes. Nat. Nanotechnol. 2018, 13, 1174–1181. [Google Scholar] [CrossRef]
- Kumar, R.; Ray, P.C.; Datta, D.; Bansal, G.P.; Angov, E.; Kumar, N. Nanovaccines for malaria using Plasmodium falciparum antigen Pfs25 attached gold nanoparticles. Vaccine 2015, 33, 5064–5071. [Google Scholar] [CrossRef]
- Kumar, R.; Ledet, G.; Graves, R.A.; Datta, D.; Robinson, S.; Bansal, G.P.; Mandal, T.K.; Kumar, N. Potent Functional Immunogenicity of Plasmodium falciparum Transmission-Blocking Antigen (Pfs25) Delivered with Nanoemulsion and Porous Polymeric Nanoparticles. Pharm. Res. 2015, 32, 3827–3836. [Google Scholar] [CrossRef]
- Jones, R.M.; Chichester, J.A.; Mett, V.; Jaje, J.; Tottey, S.; Manceva, S.; Casta, L.J.; Gibbs, S.K.; Musiychuk, K.; Shamloul, M.; et al. A Plant-Produced Pfs25 VLP Malaria Vaccine Candidate Induces Persistent Transmission Blocking Antibodies against Plasmodium falciparum in Immunized Mice. PLoS ONE 2013, 8, e79538. [Google Scholar] [CrossRef]
- Li, Y.; Leneghan, D.B.; Miura, K.; Nikolaeva, D.; Brian, I.J.; Dicks, M.D.J.; Fyfe, A.J.; Zakutansky, S.E.; De Cassan, S.; Long, C.A.; et al. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci. Rep. 2016, 6, 18848. [Google Scholar] [CrossRef]
- Brune, K.D.; Leneghan, D.B.; Brian, I.J.; Ishizuka, A.S.; Bachmann, M.F.; Draper, S.J.; Biswas, S.; Howarth, M. Plug-and-Display: Decoration of Virus-Like Particles via isopeptide bonds for modular immunization. Sci. Rep. 2016, 6, 19234. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Deng, B.; Seffouh, A.; Ortega, J.; Long, C.A.; Suresh, R.V.; He, X.; Miura, K.; Lee, S.-M.; Wu, Y.; et al. Antibody response of a particle-inducing, liposome vaccine adjuvant admixed with a Pfs230 fragment. NPJ Vaccines 2020, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Deng, B.; Mabrouk, M.T.; Seffouh, A.; Ortega, J.; Long, C.; Miura, K.; Wu, Y.; Lovell, J.F. Particle-based, Pfs230 and Pfs25 immunization is effective, but not improved by duplexing at fixed total antigen dose. Malar. J. 2020, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Zhou, S.; He, X.; Chiem, K.; Mabrouk, M.T.; Nissly, R.H.; Bird, I.M.; Strauss, M.; Sambhara, S.; Ortega, J.; et al. SARS-CoV-2 RBD Neutralizing Antibody Induction is Enhanced by Particulate Vaccination. Adv. Mater. 2020, 32, e2005637. [Google Scholar] [CrossRef]
- Mabrouk, M.T.; Huang, W.-C.; Deng, B.; Li-Purcell, N.; Seffouh, A.; Ortega, J.; Atilla-Gokcumen, G.E.; Long, C.A.; Miura, K.; Lovell, J.F. Lyophilized, antigen-bound liposomes with reduced MPLA and enhanced thermostability. Int. J. Pharm. 2020, 589, 119843. [Google Scholar] [CrossRef]
- Federizon, J.; Frye, A.; Huang, W.-C.; Hart, T.M.; He, X.; Beltran, C.; Marcinkiewicz, A.L.; Mainprize, I.L.; Wills, M.K.; Lin, Y.-P.; et al. Immunogenicity of the Lyme disease antigen OspA, particleized by cobalt porphyrin-phospholipid liposomes. Vaccine 2020, 38, 942–950. [Google Scholar] [CrossRef]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef]
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-microparticles as immune adjuvants: Correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef]
- Shah, R.R.; O’Hagan, D.T.; Amiji, M.; Brito, L.A. The impact of size on particulate vaccine adjuvants. Nanomedicine 2014, 9, 2671–2681. [Google Scholar] [CrossRef]
- Perrie, Y.; Crofts, F.; Devitt, A.; Griffiths, H.R.; Kastner, E.; Nadella, V. Designing liposomal adjuvants for the next generation of vaccines. Adv. Drug Deliv. Rev. 2016, 99, 85–96. [Google Scholar] [CrossRef]
- Lee, S.-M.; Wu, C.-K.; Plieskatt, J.; McAdams, D.H.; Miura, K.; Ockenhouse, C.F.; King, C.R. Assessment of Pfs25 expressed from multiple soluble expression platforms for use as transmission-blocking vaccine candidates. Malar. J. 2016, 15, 405. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Q.; Palovcak, E.; Armache, J.-P.; Verba, K.A.; Cheng, Y.; Agard, D. MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 2017, 14, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Marques, H.M.; Ngoma, B.; Egan, T.; Brown, K. Parameters for the amber force field for the molecular mechanics modeling of the cobalt corrinoids. J. Mol. Struct. 2001, 561, 71–91. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Lee, S.; Tran, A.; Allsopp, M.; Lim, J.B.; Hénin, J.; Klauda, J. CHARMM36 United Atom Chain Model for Lipids and Surfactants. J. Phys. Chem. B 2014, 118, 547–556. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Dykhuizen, D.E.; Polin, D.S.; Dunn, J.J.; Wilske, B.; Preac-Mursic, V.; Dattwyler, R.J.; Luft, B.J. Borrelia burgdorferi is clonal: Implications for taxonomy and vaccine development. Proc. Natl. Acad. Sci. USA 1993, 90, 10163–10167. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Darden, T.A.; York, D.M.; Pedersen, L. Particle mesh Ewald: AnN log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Hess, B. P-LINCS: A Parallel Linear Constraint Solver for Molecular Simulation. J. Chem. Theory Comput. 2007, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Friedrich, W. Vitamins; De Gruyter, Inc.: Berlin, Germany, 2013. [Google Scholar]
- Pratt, J.M. Inorganic Chemistry of Vitamin B12; Academic Press: London, UK, 1972; p. ix+347. [Google Scholar]
- Fedosov, S.N.; Berglund, L.; Fedosova, N.U.; Nexø, E.; Petersen, T.E. Comparative Analysis of Cobalamin Binding Kinetics and Ligand Protection for Intrinsic Factor, Transcobalamin, and Haptocorrin. J. Biol. Chem. 2002, 277, 9989–9996. [Google Scholar] [CrossRef]
- Butler, C.C.; Vidal-Alaball, J.; Cannings-John, R.; McCaddon, A.; Hood, K.; Papaioannou, A.; McDowell, I.; Goringe, A. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: A systematic review of randomized controlled trials. Fam. Pr. 2006, 23, 279–285. [Google Scholar] [CrossRef]
- Frey, P.A.; Hegeman, A.D. Enzymatic Reaction Mechanisms; Oxford University Press (OUP): New York, NY, USA, 2007. [Google Scholar]
- Neale, G. B12 binding proteins. Gut 1990, 31, 59–63. [Google Scholar] [CrossRef]
- Vermeulen, A.N.; Ponnudurai, T.; Beckers, P.J.; Verhave, J.P.; Smits, M.A.; Meuwissen, J.H. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J. Exp. Med. 1985, 162, 1460–1476. [Google Scholar] [CrossRef]
- Pradel, G. Proteins of the malaria parasite sexual stages: Expression, function and potential for transmission blocking strategies. Parasitology 2007, 134, 1911–1929. [Google Scholar] [CrossRef]
- Javanainen, M.; Martinez-Seara, H. Efficient preparation and analysis of membrane and membrane protein systems. Biochim. Biophys. Acta 2016, 1858, 2468–2482. [Google Scholar] [CrossRef]
- Pavlova, A.; Parks, J.; Gumbart, J.C. Development of CHARMM-Compatible Force-Field Parameters for Cobalamin and Related Cofactors from Quantum Mechanical Calculations. J. Chem. Theory Comput. 2018, 14, 784–798. [Google Scholar] [CrossRef]
- Kratky, C.; Faerber, G.; Gruber, K.; Wilson, K.; Dauter, Z.; Nolting, H.-F.; Konrat, R.; Kraeutler, B. Accurate Structural Data Demystify B12: High-Resolution Solid-State Structure of Aquocobalamin Perchlorate and Structure Analysis of the Aquocobalamin Ion in Solution. J. Am. Chem. Soc. 1995, 117, 4654–4670. [Google Scholar] [CrossRef]
- Buchoux, S. FATSLiM: A fast and robust software to analyze MD simulations of membranes. Bioinformatics 2016, 33, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Ghysels, A.; Krämer, A.; Venable, R.M.; Teague, W.E.; Lyman, E.; Gawrisch, K.; Pastor, R.W. Permeability of membranes in the liquid ordered and liquid disordered phases. Nat. Commun. 2019, 10, 5616. [Google Scholar] [CrossRef] [PubMed]
- Ermilova, I.; Lyubartsev, A.P. Cholesterol in phospholipid bilayers: Positions and orientations inside membranes with different unsaturation degrees. Soft Matter 2019, 15, 78–93. [Google Scholar] [CrossRef] [PubMed]
- MacDermaid, C.M.; Kashyap, H.K.; DeVane, R.H.; Shinoda, W.; Klauda, J.B.; Klein, M.L.; Fiorin, G. Molecular dynamics simulations of cholesterol-rich membranes using a coarse-grained force field for cyclic alkanes. J. Chem. Phys. 2015, 143, 243144. [Google Scholar] [CrossRef]
- Porasso, R.D.; Ale, N.M.; Aloia, F.C.; Masone, D.; Del Pópolo, M.G.; Ben Altabef, A.; Gomez-Zavaglia, A.; Diaz, S.B.; Vila, J.A. Interaction of glycine, lysine, proline and histidine with dipalmitoylphosphatidylcholine lipid bilayers: A theoretical and experimental study. RSC Adv. 2015, 5, 43537–43546. [Google Scholar] [CrossRef]
- Sapay, N.; Bennett, W.F.D.; Tieleman, D.P. Thermodynamics of flip-flop and desorption for a systematic series of phosphatidylcholine lipids. Soft Matter 2009, 5, 3295–3302. [Google Scholar] [CrossRef]
- Shao, S.; Do, T.N.; Razi, A.; Chitgupi, U.; Geng, J.; Alsop, R.J.; Dzikovski, B.G.; Rheinstädter, M.C.; Ortega, J.; Karttunen, M.M.; et al. Design of Hydrated Porphyrin-Phospholipid Bilayers with Enhanced Magnetic Resonance Contrast. Small 2017, 13, 1602505. [Google Scholar] [CrossRef]
- Carter, K.A.; Shao, S.; Hoopes, M.I.; Luo, D.; Ahsan, B.; Grigoryants, V.M.; Song, W.; Huang, H.; Zhang, G.G.; Pandey, R.K.; et al. Porphyrin–phospholipid liposomes permeabilized by near-infrared light. Nat. Commun. 2014, 5, 3546. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Federizon, J.; Feugmo, C.G.T.; Huang, W.-C.; He, X.; Miura, K.; Razi, A.; Ortega, J.; Karttunen, M.; Lovell, J.F. Experimental and Computational Observations of Immunogenic Cobalt Porphyrin Lipid Bilayers: Nanodomain-Enhanced Antigen Association. Pharmaceutics 2021, 13, 98. https://doi.org/10.3390/pharmaceutics13010098
Federizon J, Feugmo CGT, Huang W-C, He X, Miura K, Razi A, Ortega J, Karttunen M, Lovell JF. Experimental and Computational Observations of Immunogenic Cobalt Porphyrin Lipid Bilayers: Nanodomain-Enhanced Antigen Association. Pharmaceutics. 2021; 13(1):98. https://doi.org/10.3390/pharmaceutics13010098
Chicago/Turabian StyleFederizon, Jasmin, Conrard Giresse Tetsassi Feugmo, Wei-Chiao Huang, Xuedan He, Kazutoyo Miura, Aida Razi, Joaquin Ortega, Mikko Karttunen, and Jonathan F. Lovell. 2021. "Experimental and Computational Observations of Immunogenic Cobalt Porphyrin Lipid Bilayers: Nanodomain-Enhanced Antigen Association" Pharmaceutics 13, no. 1: 98. https://doi.org/10.3390/pharmaceutics13010098
APA StyleFederizon, J., Feugmo, C. G. T., Huang, W.-C., He, X., Miura, K., Razi, A., Ortega, J., Karttunen, M., & Lovell, J. F. (2021). Experimental and Computational Observations of Immunogenic Cobalt Porphyrin Lipid Bilayers: Nanodomain-Enhanced Antigen Association. Pharmaceutics, 13(1), 98. https://doi.org/10.3390/pharmaceutics13010098





