Biological Obstacles for Identifying In Vitro-In Vivo Correlations of Orally Inhaled Formulations
Abstract
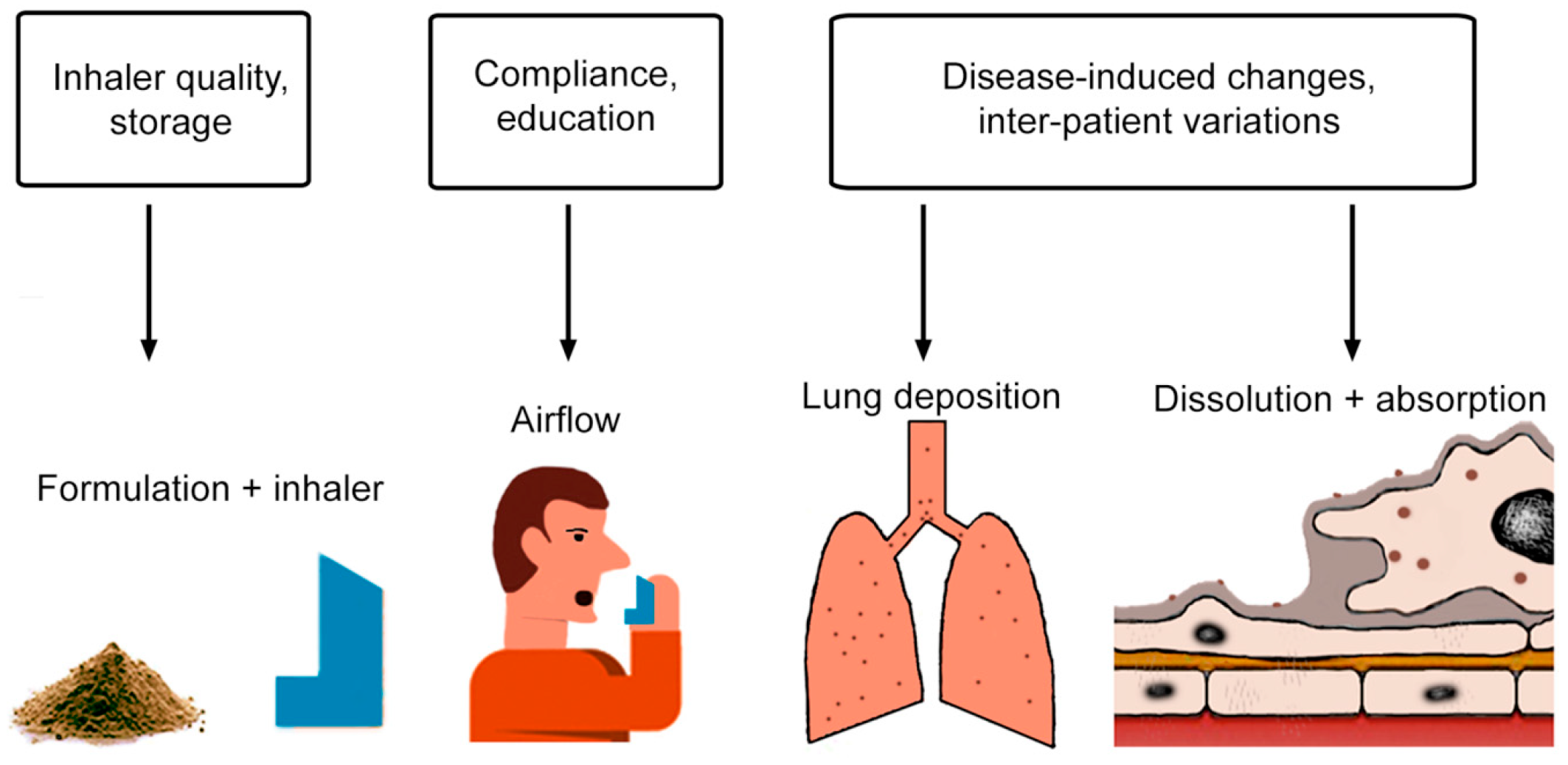
1. Introduction
2. Patient Inhaler Interaction
2.1. Role of Patient
2.2. Role of Inhalers
3. Particle Action in the Deep Lung
3.1. Deposition
3.2. Clearance
3.3. Drug Absorption
4. In Vitro Models to Assess Biological Processes
4.1. Deposition
4.2. Clearance
4.3. Particle Dissolution
4.4. Drug Absorption
5. In Vivo Parameters for Drug Action
5.1. Plasma Levels
5.2. Therapeutic Efficacy
6. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Tavares, M.; Aguiar-Ricardo, A. Sustainable strategies for nano-in-micro particle engineering for pulmonary delivery. J. Nanopart. Res. 2014, 16, 2602. [Google Scholar] [CrossRef]
- Pulmonary Drug Delivery Market. Available online: https://www.gilberttechnologies.eu/our-business/ (accessed on 2 April 2019).
- D’Urzo, A.; Chapman, K.; Donohue, J.; Kardos, P.; Maleki-Yazdi, R.; Price, D. Inhaler Devices for Delivery of LABA/LAMA Fixed-Dose Combinations in Patients with COPD. Pulm. Ther. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Cardot, J.M.; Davit, B.M. In Vitro-In Vivo correlations: Tricks and traps. Aaps J. 2012, 14, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Emami, J. In Vitro-In Vivo correlation: From theory to applications. J. Pharm. Pharm. Sci. 2006, 9, 169–189. [Google Scholar] [PubMed]
- Hochhaus, G.; Horhota, S.; Hendeles, L.; Suarez, S.; Rebello, J. Pharmacokinetics of Orally Inhaled Drug Products. Aaps J. 2015, 17, 769–775. [Google Scholar] [CrossRef]
- Forbes, B.; Backman, P.; Christopher, D.; Dolovich, M.; Li, B.V.; Morgan, B. In Vitro Testing for Orally Inhaled Products: Developments in Science-Based Regulatory Approaches. Aaps J. 2015, 17, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Verma, R.; Garcia-Contreras, L. Inhalation drug delivery devices: Technology update. Med. Devices 2015, 8, 131–139. [Google Scholar]
- Biddiscombe, M.F.; Usmani, O.S. Is there room for further innovation in inhaled therapy for airways disease? Breathe 2018, 14, 216–224. [Google Scholar] [CrossRef]
- Sanchis, J.; Corrigan, C.; Levy, M.L.; Viejo, J.L. Inhaler devices-from theory to practice. Respir. Med. 2013, 107, 495–502. [Google Scholar] [CrossRef]
- Lavorini, F.; Fontana, G.A.; Usmani, O.S. New inhaler devices-the good, the bad and the ugly. Respiration 2014, 88, 3–15. [Google Scholar] [CrossRef]
- De Boer, A.; Eber, E. Pulmonary. In Practical Pharmaceutics; Bouwan-Boer, Y., Fenton-May, V., Le Brun, P., Eds.; Springer: Cham, Switzerland, 2008; pp. 99–129. [Google Scholar]
- Lindsay, J.T.; Heaney, L.G. Nonadherence in difficult asthma-facts, myths, and a time to act. Patient Prefer. Adherence 2013, 7, 329–336. [Google Scholar]
- Sanchis, J.; Gich, I.; Pedersen, S. Systematic Review of Errors in Inhaler Use: Has Patient Technique Improved Over Time? Chest 2016, 150, 394–406. [Google Scholar] [CrossRef]
- Mahon, J.; Fitzgerald, A.; Glanville, J.; Dekhuijzen, R.; Glatte, J.; Glanemann, S.; Torvinen, S. Misuse and/or treatment delivery failure of inhalers among patients with asthma or COPD: A review and recommendations for the conduct of future research. Respir. Med. 2017, 129, 98–116. [Google Scholar] [CrossRef]
- Chrystyn, H.; van der Palen, J.; Sharma, R.; Barnes, N.; Delafont, B.; Mahajan, A.; Thomas, M. Device errors in asthma and COPD: Systematic literature review and meta-analysis. Npj Prim. Care Respir. Med. 2017, 27, 22. [Google Scholar] [CrossRef]
- Usmani, O.S.; Lavorini, F.; Marshall, J.; Dunlop, W.C.N.; Heron, L.; Farrington, E.; Dekhuijzen, R. Critical inhaler errors in asthma and COPD: A systematic review of impact on health outcomes. Respir. Res. 2018, 19, 10. [Google Scholar] [CrossRef]
- Derom, E.; Thorsson, L. Factors Affecting the Clinical Outcome of Aerosol Therapy. In Drug Delivery to the Lung; Bisgaard, H., Callaghan, C., Smaldone, G., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2001; pp. 143–172. [Google Scholar]
- Everard, M.L.; Devadason, S.G.; Summers, Q.A.; Le Souef, P.N. Factors affecting total and “respirable” dose delivered by a salbutamol metered dose inhaler. Thorax 1995, 50, 746–749. [Google Scholar] [CrossRef][Green Version]
- Dal Negro, R.W. Dry powder inhalers and the right things to remember: A concept review. Multidiscip. Respir. Med. 2015, 10, 13. [Google Scholar] [CrossRef]
- Mireles-Cabodevila, E.; Chatburn, R. Section II Applied Anatomy and Physiology. Ventilation. In Egan’s Fundamentals of Respiratory Care; Kacmarek, R., Stoller, J., Heuer, A., Eds.; Elsevier: St. Louis, MO, USA, 2017; pp. 226–246. [Google Scholar]
- Mutuku, J.; Chen, W. Flow Characterization in Healthy Airways and Airways with Chronic Obstructive Pulmonary Disease (COPD) during Different Inhalation Conditions. Aerosol Air Qual. Res. 2018, 18, 2680–2694. [Google Scholar] [CrossRef]
- Stein, S.; Myrdal, P. The relative influence of atomization and evaporation on metered dose inhaler drug delivery efficiency. Aerosol Sci. Technol. 2006, 40, 335–347. [Google Scholar] [CrossRef]
- Shemirani, F.M.; Hoe, S.; Lewis, D.; Church, T.; Vehring, R.; Finlay, W.H. In vitro investigation of the effect of ambient humidity on regional delivered dose with solution and suspension MDIs. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 215–222. [Google Scholar] [CrossRef]
- Demoly, P.; Hagedoorn, P.; de Boer, A.H.; Frijlink, H.W. The clinical relevance of dry powder inhaler performance for drug delivery. Respir. Med. 2014, 108, 1195–1203. [Google Scholar] [CrossRef]
- Preparation for Nebulization: Characterization. In European Pharmacopeia; Council of Europe: Strasbourg, France, 2006; Volume 18, pp. 280–283.
- Copley, M. Regulatory challenges of inhaler testing. USP Pharmacopeial Forum 2006, 32, 1348–1352. [Google Scholar]
- Physical Tests and Determinations—Aerosols, Nasal Sprays, Metered-Dose Inhalers and Dry Powder Inhalers. In United States Pharmacopeia; United States Pharmacopeial Convention: North Bethesda, MD, USA, 2003.
- United States Pharmacopeial Convention. Chapter 601: Aerosols, Nasal Sprays, Metered-Dose Inhalers and Dry Powder Inhalers; United States Pharmacopeia: Rockville, MD, USA, 2012. [Google Scholar]
- Nocella, M.; Kilber, E.; Witmer, B.; Karlage, K.; Myrdal, P.B. A Comparison of Pharmaceutical Product Performance of Albuterol Inhalers Available in the United States and Those Obtained in a Mexican Border Town. J. Pharm. Tech. 2015, 31, 289–295. [Google Scholar] [CrossRef]
- Burmeister Getz, E.; Carroll, K.J.; Jones, B.; Benet, L.Z. Batch-to-batch pharmacokinetic variability confounds current bioequivalence regulations: A dry powder inhaler randomized clinical trial. Clin. Pharmacol. Ther. 2016, 100, 223–231. [Google Scholar] [CrossRef]
- Lipworth, B.J. Airway and systemic effects of inhaled corticosteroids in asthma: Dose response relationship. Pulm. Pharmacol. 1996, 9, 19–27. [Google Scholar] [CrossRef]
- Van Oort, M. In Vitro Testing of Dry Powder Inhalers. Aerosol Sci. Technol. 1995, 22, 364–373. [Google Scholar] [CrossRef]
- Canonica, G.W.; Arp, J.; Keegstra, J.R.; Chrystyn, H. Spiromax, a New Dry Powder Inhaler: Dose Consistency under Simulated Real-World Conditions. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 309–319. [Google Scholar] [CrossRef]
- De Vries, T.W.; Rottier, B.L.; Gjaltema, D.; Hagedoorn, P.; Frijlink, H.W.; de Boer, A.H. Comparative In Vitro evaluation of four corticosteroid metered dose inhalers: Consistency of delivered dose and particle size distribution. Respir. Med. 2009, 103, 1167–1173. [Google Scholar] [CrossRef]
- Committee for Proprietary Medicinal Products. Guideline on Stability Testing: Stability Testing of Existing Active Substances and Related Finished Products; CPMP/QWP/122/02; European Medical Agency: London, UK, 2003. [Google Scholar]
- Pleasants, R.A. Dry Powder Inhalers and Humidity: Another Factor to Consider to Ensure Adequate Lung Delivery. Ann. Am. Thorac Soc. 2017, 14, 1602. [Google Scholar] [CrossRef]
- Meakin, B.J.; Cainey, J.; Woodcock, P.M. Effect of exposure to humidity on terbutaline delivery from turbuhaler dry power inhalation devices. Eur. Respir. J. 1993, 6, 760–761. [Google Scholar]
- Le, V.N.; Hoang Thi, T.H.; Robins, E.; Flament, M.P. Dry powder inhalers: Study of the parameters influencing adhesion and dispersion of fluticasone propionate. Aaps Pharmscitech 2012, 13, 477–484. [Google Scholar] [CrossRef]
- ICRP. Human Respiratory Tract Model for Radiological Protection. Ann. Icrp 1994, 24, 1–3. [Google Scholar] [CrossRef]
- Brown, J. Deposition of particles. In Comparative Biology of the Normal Lung; Parent, R., Ed.; Elsevier: London, UK, 2015; pp. 513–536. [Google Scholar]
- Hofmann, W. Modelling inhaled particle deposition in the human lung-A review. J. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Rostami, A.A. Computational modeling of aerosol deposition in respiratory tract: A review. Inhal. Toxicol. 2009, 21, 262–290. [Google Scholar] [CrossRef]
- Stuart, B.O. Deposition and clearance of inhaled particles. Environ. Health Perspect. 1984, 55, 369–390. [Google Scholar] [CrossRef]
- Haddrell, A.E.; Lewis, D.; Church, T.; Vehring, R.; Murnane, D.; Reid, J.P. Pulmonary aerosol delivery and the importance of growth dynamics. Ther. Deliv. 2017, 8, 1051–1061. [Google Scholar] [CrossRef]
- Rohatagi, S.; Wang, Y.; Agenti, D. Mass Balance Studies. In Pharmacokinetics in Drug Development Vol 1: Clinical Study Design and Analysis; Bonate, P., Howard, D., Eds.; AAPS Press: Arlington, VA, USA, 2004; pp. 121–148. [Google Scholar]
- Leach, C.L.; Kuehl, P.J.; Chand, R.; McDonald, J.D. Respiratory Tract Deposition of HFA-Beclomethasone and HFA-Fluticasone in Asthmatic Patients. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 127–133. [Google Scholar] [CrossRef]
- Borgstrom, L.; Asking, L.; Lipniunas, P. An in vivo and in vitro comparison of two powder inhalers following storage at hot/humid conditions. J. Aerosol Med. 2005, 18, 304–310. [Google Scholar] [CrossRef]
- Darquenne, C.; Fleming, J.S.; Katz, I.; Martin, A.R.; Schroeter, J.; Usmani, O.S.; Venegas, J.; Schmid, O. Bridging the Gap Between Science and Clinical Efficacy: Physiology, Imaging, and Modeling of Aerosols in the Lung. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 107–126. [Google Scholar] [CrossRef]
- Verbanck, S.; Ghorbaniasl, G.; Biddiscombe, M.F.; Dragojlovic, D.; Ricks, N.; Lacor, C.; Ilsen, B.; de Mey, J.; Schuermans, D.; Underwood, S.R.; et al. Inhaled Aerosol Distribution in Human Airways: A Scintigraphy-Guided Study in a 3D Printed Model. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 525–533. [Google Scholar] [CrossRef]
- Weibel, E.R.; Sapoval, B.; Filoche, M. Design of peripheral airways for efficient gas exchange. Respir. Physiol. Neurobiol. 2005, 148, 3–21. [Google Scholar] [CrossRef]
- Pu, J.; Leader, J.K.; Meng, X.; Whiting, B.; Wilson, D.; Sciurba, F.C.; Reilly, J.J.; Bigbee, W.L.; Siegfried, J.; Gur, D. Three-dimensional airway tree architecture and pulmonary function. Acad. Radiol. 2012, 19, 1395–1401. [Google Scholar] [CrossRef][Green Version]
- Smith, B.M.; Traboulsi, H.; Austin, J.H.M.; Manichaikul, A.; Hoffman, E.A.; Bleecker, E.R.; Cardoso, W.V.; Cooper, C.; Couper, D.J.; Dashnaw, S.M.; et al. Human airway branch variation and chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 2018, 115, E974–E981. [Google Scholar] [CrossRef]
- Balashazy, I.; Hofmann, W.; Heistracher, T. Local particle deposition patterns may play a key role in the development of lung cancer. J. Appl. Physiol. 2003, 94, 1719–1725. [Google Scholar] [CrossRef]
- Bennett, W.D.; Xie, M.; Zeman, K.; Hurd, H.; Donaldson, S. Heterogeneity of Particle Deposition by Pixel Analysis of 2D Gamma Scintigraphy Images. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 211–218. [Google Scholar] [CrossRef]
- Lippmann, M.; Yeates, D.B.; Albert, R.E. Deposition, retention, and clearance of inhaled particles. Br. J. Ind. Med. 1980, 37, 337–362. [Google Scholar] [CrossRef]
- Smith, D.J.; Gaffney, E.A.; Blake, J.R. Modelling mucociliary clearance. Respir. Physiol. Neurobiol. 2008, 163, 178–188. [Google Scholar] [CrossRef]
- Munkholm, M.; Mortensen, J. Mucociliary clearance: Pathophysiological aspects. Clin. Physiol. Funct. Imaging 2014, 34, 171–177. [Google Scholar] [CrossRef]
- Chatelain, R.; Anne-Archard, D.; Murris, E.; Sanchez, D.; Thinet, M.; Didier, A.; Poncet, P. Modeling Cystic Fibrosis and Mucociliary Clearance. In Modeling of Microscale Transport in Biological Processes; Baker, S., Ed.; Elsevier: London, UK, 2016. [Google Scholar]
- King, M. Experimental models for studying mucociliary clearance. Eur. Respir. J. 1998, 11, 222–228. [Google Scholar] [CrossRef]
- Ito, J.T.; Ramos, D.; Lima, F.F.; Rodrigues, F.M.; Gomes, P.R.; Moreira, G.L.; Macchione, M.; Toledo, A.C.; Ramos, E.M. Nasal Mucociliary Clearance in Subjects With COPD After Smoking Cessation. Respir. Care 2015, 60, 399–405. [Google Scholar] [CrossRef]
- Calverley, P. Cough in chronic obstructive pulmonary disease: Is it important and what are the effects of treatment? Cough 2013, 24, 17. [Google Scholar] [CrossRef]
- Fröhlich, E. Toxicity of orally inhaled drug formulations at the alveolar barrier: Parameters for initial biological screening. Drug Deliv. 2017, 24, 891–905. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bassler, K.; Aschenbrenner, A.C.; Fujii, W.; Schultze, J.L. Dysregulated Functions of Lung Macrophage Populations in COPD. J. Immunol. Res. 2018, 2018, 2349045. [Google Scholar] [CrossRef]
- Fricker, M.; Gibson, P.G. Macrophage dysfunction in the pathogenesis and treatment of asthma. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- Berenson, C.S.; Kruzel, R.L.; Eberhardt, E.; Sethi, S. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J. Infect. Dis. 2013, 208, 2036–2045. [Google Scholar] [CrossRef]
- Haussermann, S.; Acerbi, D.; Brand, P.; Herpich, C.; Poli, G.; Sommerer, K.; Meyer, T. Lung deposition of formoterol HFA (Atimos/Forair) in healthy volunteers, asthmatic and COPD patients. J. Aerosol Med. 2007, 20, 331–341. [Google Scholar] [CrossRef]
- Mariotti, F.; Sergio, F.; Acerbi, D.; Meyer, T.; Herpich, C. Lung deposition of the extra fine dry powder fixed combination beclomethasone dipropionate plus formoterol fumarate via the NEXT DPI® in healthy subjects, asthmatic and COPD patients. Eur. Respir. J. 2011, 38, 830. [Google Scholar]
- Zanen, P.; Laube, B. Targeting the lungs with therapeutic aerosols. Drug delivery to the lung. In Lung Biology in Human and Disease; Bisgaard, H., O’Callaghan, C., Smaldone, G., Eds.; CRC Press: Boca Raton, FL, USA, 2002; Volume 162, pp. 211–268. [Google Scholar]
- Farkas, A.; Lewis, D.; Church, T.; Tweedie, A.; Mason, F.; Haddrell, A.E.; Reid, J.P.; Horvath, A.; Balashazy, I. Experimental and computational study of the effect of breath-actuated mechanism built in the NEXThaler((R)) dry powder inhaler. Int. J. Pharm. 2017, 533, 225–235. [Google Scholar] [CrossRef]
- Jabbal, S.; Poli, G.; Lipworth, B. Does size really matter?: Relationship of particle size to lung deposition and exhaled fraction. J. Allergy Clin. Immunol. 2017, 139, 2013–2014. [Google Scholar] [CrossRef]
- Roth, C.; Kreyling, W.; Scheuch, G.; Busch, B.; Stahlhofen, W. Deposition and clearance of fine particles in the human respiratory tract. Ann. Occup. Hyg. 1997, 41, 503–508. [Google Scholar]
- Heyder, J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc. Am. Thorac Soc. 2004, 1, 315–320. [Google Scholar] [CrossRef]
- Longest, P.W.; McLeskey, J.T., Jr.; Hindle, M. Characterization of Nanoaerosol Size Change During Enhanced Condensational Growth. Aerosol Sci. Technol. 2010, 44, 473–483. [Google Scholar] [CrossRef]
- Shaheen, M.A.; Mahmoud, M.A.; Abdel Aziz, M.M.; El Morsy, H.I.; Abdel Khalik, K.A. Sputum dipalmitoylphosphatidylcholine level as a novel airway inflammatory marker in asthmatic children. Clin. Respir. J. 2009, 3, 95–101. [Google Scholar] [CrossRef]
- Haslam, P.; Postle, A.; Raymondos, K.; Baker, C. Measurement of pulmonary surfactant components and function in bronchoalveolar lavage fluid. Report of the ERS Task Force: Guidelines for measurement of acellular components and recommendations for standardization of bronchoalveolar lavage (BAL). Eur. Respir. Rev. 1999, 9, 43–69. [Google Scholar]
- Pison, U.; Obertacke, U.; Brand, M.; Seeger, W.; Joka, T.; Bruch, J.; Schmit-Neuerburg, K.P. Altered pulmonary surfactant in uncomplicated and septicemia-complicated courses of acute respiratory failure. J. Trauma 1990, 30, 19–26. [Google Scholar] [CrossRef]
- Meyer, K.C.; Sharma, A.; Brown, R.; Weatherly, M.; Moya, F.R.; Lewandoski, J.; Zimmerman, J.J. Function and composition of pulmonary surfactant and surfactant-derived fatty acid profiles are altered in young adults with cystic fibrosis. Chest 2000, 118, 164–174. [Google Scholar] [CrossRef]
- Ohashi, T.; Pinkerton, K.; Ikegami, M.; Jobe, A.H. Changes in alveolar surface area, surfactant protein A, and saturated phosphatidylcholine with postnatal rat lung growth. Pediatr. Res. 1994, 35, 685–689. [Google Scholar] [CrossRef][Green Version]
- Putz, G.; Goerke, J.; Clements, J.A. Surface activity of rabbit pulmonary surfactant subfractions at different concentrations in a captive bubble. J. Appl. Physiol. 1994, 77, 597–605. [Google Scholar] [CrossRef]
- Pacifici, G.M. Clinical pharmacology of fentanyl in preterm infants. A review. Pediatr. Neonatol. 2015, 56, 143–148. [Google Scholar] [CrossRef]
- Hamm, H.; Kroegel, C.; Hohlfeld, J. Surfactant: A review of its functions and relevance in adult respiratory disorders. Respir. Med. 1996, 90, 251–270. [Google Scholar] [CrossRef]
- Sutinen, S.; Riska, H.; Backman, R.; Sutinen, S.H.; Froseth, B. Alveolar lavage fluid (ALF) of normal volunteer subjects: Cytologic, immunocytochemical, and biochemical reference values. Respir. Med. 1995, 89, 85–92. [Google Scholar] [CrossRef][Green Version]
- Roberts, C.M.; Cairns, D.; Bryant, D.H.; Burke, W.M.; Yeates, M.; Blake, H.; Penny, R.; Shelley, L.; Zaunders, J.J.; Breit, S.N. Changes in epithelial lining fluid albumin associated with smoking and interstitial lung disease. Eur. Respir. J. 1993, 6, 110–115. [Google Scholar]
- Muller-Serieys, C.; Bancal, C.; Dombret, M.C.; Soler, P.; Murciano, G.; Aubier, M.; Bergogne-Berezin, E. Penetration of cefpodoxime proxetil in lung parenchyma and epithelial lining fluid of noninfected patients. Antimicrob. Agents Chemother. 1992, 36, 2099–2103. [Google Scholar] [CrossRef][Green Version]
- Lamer, C.; de Beco, V.; Soler, P.; Calvat, S.; Fagon, J.Y.; Dombret, M.C.; Farinotti, R.; Chastre, J.; Gibert, C. Analysis of vancomycin entry into pulmonary lining fluid by bronchoalveolar lavage in critically ill patients. Antimicrob. Agents Chemother. 1993, 37, 281–286. [Google Scholar] [CrossRef]
- Furuie, H.; Tanioka, S.; Shimizu, K.; Manita, S.; Nishimura, M.; Yoshida, H. Intrapulmonary Pharmacokinetics of Lascufloxacin in Healthy Adult Volunteers. Antimicrob. Agents Chemother. 2018, 62, e02169–e02217. [Google Scholar] [CrossRef]
- Carcas, A.J.; Garcia-Satue, J.L.; Zapater, P.; Frias-Iniesta, J. Tobramycin penetration into epithelial lining fluid of patients with pneumonia. Clin. Pharmacol. Ther. 1999, 65, 245–250. [Google Scholar] [CrossRef]
- Rodvold, K.A.; Gotfried, M.H.; Still, J.G.; Clark, K.; Fernandes, P. Comparison of plasma, epithelial lining fluid, and alveolar macrophage concentrations of solithromycin (CEM-101) in healthy adult subjects. Antimicrob. Agents Chemother. 2012, 56, 5076–5081. [Google Scholar] [CrossRef]
- Ortega, V.E.; Hawkins, G.A.; Peters, S.P.; Bleecker, E.R. Pharmacogenetics of the beta 2-adrenergic receptor gene. Immunol. Allergy Clin. N. Am. 2007, 27, 665–684. [Google Scholar] [CrossRef]
- Bai, T.R.; Zhou, D.; Aubert, J.D.; Lizee, G.; Hayashi, S.; Bondy, G.P. Expression of beta 2-adrenergic receptor mRNA in peripheral lung in asthma and chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 1993, 8, 325–333. [Google Scholar] [CrossRef]
- Mitchell, J. Section VII: Drug Product Testing, Aerodynamic Particle Size Testing. In Pharmaceutical Inhalation Technology; Hickey, A., da Rocha, S., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 541–590. [Google Scholar]
- Wei, X.; Bormann, K.; Byron, P. Predicting Variations in Aerodynamic Particle Size Distribution of the Lung Dose from Budelin Novolizer. RDD Europe 2015 2015, 2, 533–536. [Google Scholar]
- de Boer, A.H.; Gjaltema, D.; Hagedoorn, P.; Frijlink, H.W. Characterization of inhalation aerosols: A critical evaluation of cascade impactor analysis and laser diffraction technique. Int. J. Pharm. 2002, 249, 219–231. [Google Scholar] [CrossRef]
- Lizal, F.; Elcner, J.; Hopke, P.K.; Jedelsky, J.; Jicha, M. Development of a realistic human airway model. Proc. Inst. Mech. Eng. H 2012, 226, 197–207. [Google Scholar] [CrossRef]
- Choi, S.; Miyawaki, S.; Lin, C.L. A Feasible Computational Fluid Dynamics Study for Relationships of Structural and Functional Alterations with Particle Depositions in Severe Asthmatic Lungs. Comput. Math. Methods Med. 2018, 2018, 6564854. [Google Scholar] [CrossRef]
- Tian, G.; Hindle, M.; Lee, S.; Longest, P.W. Validating CFD Predictions of Pharmaceutical Aerosol Deposition with In Vivo Data. Pharm. Res. 2015, 32, 3170–3187. [Google Scholar] [CrossRef]
- Dolovich, M.; Kuttler, A.; Dimke, T.; Usmani, O. Biophysical Model to Predict Lung Delivery from a Dual Bronchodilator Dry-powder Inhaler. Int. J. Pharm. X 2019, 1, 100018. [Google Scholar] [CrossRef]
- Benam, K.H.; Vladar, E.K.; Janssen, W.J.; Evans, C.M. Mucociliary Defense: Emerging Cellular, Molecular, and Animal Models. Ann. Am. Thorac Soc. 2018, 15, S210–S215. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Taylor, L.; Lee, H.; Cowley, S.A.; James, W.S.; Iqbal, A.J.; Greaves, D.R. A novel real time imaging platform to quantify macrophage phagocytosis. Biochem. Pharmacol. 2016, 116, 107–119. [Google Scholar] [CrossRef]
- Radivojev, S.; Zellnitz, S.; Paudel, A.; Fröhlich, E. Searching for physiologically relevant in vitro dissolution techniques for orally inhaled drugs. Int. J. Pharm. 2019, 556, 45–56. [Google Scholar] [CrossRef]
- Gunther, A.; Schmidt, R.; Nix, F.; Yabut-Perez, M.; Guth, C.; Rosseau, S.; Siebert, C.; Grimminger, F.; Morr, H.; Velcovsky, H.G.; et al. Surfactant abnormalities in idiopathic pulmonary fibrosis, hypersensitivity pneumonitis and sarcoidosis. Eur. Respir. J. 1999, 14, 565–573. [Google Scholar] [CrossRef]
- Ochs, M.; Schuttler, M.; Stichtenoth, G.; Herting, E. Morphological alterations of exogenous surfactant inhibited by meconium can be prevented by dextran. Respir. Res. 2006, 7, 86. [Google Scholar] [CrossRef][Green Version]
- Morales, J.O.; Peters, J.I.; Williams, R.O., 3rd. Surfactants: Their critical role in enhancing drug delivery to the lungs. Ther. Deliv. 2011, 2, 623–641. [Google Scholar] [CrossRef]
- Hidalgo, A.; Cruz, A.; Perez-Gil, J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur. J. Pharm. Biopharm. 2015, 95, 117–127. [Google Scholar] [CrossRef]
- Floroiu, A.; Klein, M.; Krämer, J.; Lehr, C. Towards Standardized Dissolution Techniques for In Vitro Performance Testing of Dry Powder Inhalers. Dissolut. Technol. 2018, 25, 6–18. [Google Scholar] [CrossRef]
- Meindl, C.; Stranzinger, S.; Dzidic, N.; Salar-Behzadi, S.; Mohr, S.; Zimmer, A.; Fröhlich, E. Permeation of Therapeutic Drugs in Different Formulations across the Airway Epithelium In Vitro. PLoS ONE 2015, 10, e0135690. [Google Scholar] [CrossRef]
- Fröhlich, E. Comparison of conventional and advanced in vitro models in the toxicity testing of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1091–1107. [Google Scholar] [CrossRef]
- Konar, D.; Devarasetty, M.; Yildiz, D.V.; Atala, A.; Murphy, S.V. Lung-On-A-Chip Technologies for Disease Modeling and Drug Development. Biomed. Eng. Comput. Biol. 2016, 7, 17–27. [Google Scholar] [CrossRef]
- Young, B.; Ritchie, A.; Golshahi, L.; Heise, R. Section IX Preclinical testing. 3D in Vitro/Ex Vivo Studies. In Pharmaceutical Aerosol Technology; Hickley, A., da Rocha, E., Eds.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Lipworth, B.J. New perspectives on inhaled drug delivery and systemic bioactivity. Thorax 1995, 50, 105–110. [Google Scholar] [CrossRef]
- Bondesson, E.; Bengtsson, T.; Borgstrom, L.; Nilsson, L.E.; Norrgren, K.; Trofast, E.; Wollmer, P. Planar gamma scintigraphy--points to consider when quantifying pulmonary dry powder aerosol deposition. Int. J. Pharm. 2003, 258, 227–240. [Google Scholar] [CrossRef]
- Thorsson, L.; Edsbacker, S.; Conradson, T.B. Lung deposition of budesonide from Turbuhaler is twice that from a pressurized metered-dose inhaler P-MDI. Eur. Respir. J. 1994, 7, 1839–1844. [Google Scholar] [CrossRef]
- Borghardt, J.M.; Kloft, C.; Sharma, A. Inhaled Therapy in Respiratory Disease: The Complex Interplay of Pulmonary Kinetic Processes. Can. Respir. J. 2018, 2018, 2732017. [Google Scholar] [CrossRef]
- Vauquelin, G. Cell membranes... and how long drugs may exert beneficial pharmacological activity in vivo. Br. J. Clin. Pharmacol. 2016, 82, 673–682. [Google Scholar] [CrossRef]
- Honeybourne, D. Antibiotic penetration into lung tissues. Thorax 1994, 49, 104–106. [Google Scholar] [CrossRef][Green Version]
- Kiem, S.; Schentag, J.J. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 2008, 52, 24–36. [Google Scholar] [CrossRef]
- Marchand, S.; Gregoire, N.; Brillault, J.; Lamarche, I.; Gobin, P.; Couet, W. Biopharmaceutical Characterization of Nebulized Antimicrobial Agents in Rats: 3. Tobramycin. Antimicrob. Agents Chemother. 2015, 59, 6646–6647. [Google Scholar] [CrossRef]
- Esmailpour, N.; Hogger, P.; Rabe, K.F.; Heitmann, U.; Nakashima, M.; Rohdewald, P. Distribution of inhaled fluticasone propionate between human lung tissue and serum in vivo. Eur. Respir. J. 1997, 10, 1496–1499. [Google Scholar] [CrossRef]
- Fischer, H. Function of Proton Channels in Lung Epithelia. Wiley Interdiscip. Rev. Membr. Transp. Signal 2012, 1, 247–258. [Google Scholar] [CrossRef]
- Jayaraman, S.; Song, Y.; Verkman, A.S. Airway surface liquid pH in well-differentiated airway epithelial cell cultures and mouse trachea. Am. J. Physiol. Cell. Physiol. 2001, 281, C1504–C1511. [Google Scholar] [CrossRef]
- Barnes, P.J. Distribution of receptor targets in the lung. Proc. Am. Thorac Soc. 2004, 1, 345–351. [Google Scholar] [CrossRef]
- Alagha, K.; Palot, A.; Sofalvi, T.; Pahus, L.; Gouitaa, M.; Tummino, C.; Martinez, S.; Charpin, D.; Bourdin, A.; Chanez, P. Long-acting muscarinic receptor antagonists for the treatment of chronic airway diseases. Ther. Adv. Chronic Dis. 2014, 5, 85–98. [Google Scholar] [CrossRef]
- Pujolsa, L.; Mullol, J.; Picado, C. Glucocorticoid receptor in human respiratory epithelial cells. Neuroimmunomodulation 2009, 16, 290–299. [Google Scholar] [CrossRef]
- Yanai, M.; Sekizawa, K.; Ohrui, T.; Sasaki, H.; Takishima, T. Site of airway obstruction in pulmonary disease: Direct measurement of intrabronchial pressure. J. Appl. Physiol. 1992, 72, 1016–1023. [Google Scholar] [CrossRef]
- Bodzenta-Lukaszyk, A.; Kokot, M. Pharmacological consequences of inhaled drug delivery to small airways in the treatment of asthma. Adv. Ther. 2014, 31, 803–816. [Google Scholar] [CrossRef]
- Meynell, H.; Capstick, T. Role of dual and triple fixed-dose combination inhalers in the treatment of chronic obstructive pulmonary disease. Clinical Pharmacist, 5 November 2018. [Google Scholar]
- Chawes, B.L.; Piccinno, A.; Kreiner-Moller, E.; Vissing, N.H.; Poorisrisak, P.; Mortensen, L.; Nilson, E.; Bisgaard, A.; Dossing, A.; Deleuran, M.; et al. Pharmacokinetic comparison of inhaled fixed combination vs. the free combination of beclomethasone and formoterol pMDIs in asthmatic children. Br. J. Clin. Pharmacol. 2013, 75, 1081–1088. [Google Scholar] [CrossRef]
- Usmani, O.S.; Biddiscombe, M.F.; Barnes, P.J. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am. J. Respir. Crit. Care Med. 2005, 172, 1497–1504. [Google Scholar] [CrossRef]
- Bai, T. Beta2 Adrenergic Receptors in Asthma: A Current Perspective. Lung 1992, 170, 125–141. [Google Scholar] [CrossRef]
- Gumbleton, M. Process of Drug Handling by the Body. In Smith and Williams Introduction to the Principles of Drug Design and Action; Smith, H., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–32. [Google Scholar]
- Williams, L.K.; Peterson, E.L.; Wells, K.; Ahmedani, B.K.; Kumar, R.; Burchard, E.G.; Chowdhry, V.K.; Favro, D.; Lanfear, D.E.; Pladevall, M. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J. Allergy Clin. Immunol. 2011, 128, 1185–1191. [Google Scholar] [CrossRef]
- Levy, M.L.; Dekhuijzen, P.N.; Barnes, P.J.; Broeders, M.; Corrigan, C.J.; Chawes, B.L.; Corbetta, L.; Dubus, J.C.; Hausen, T.; Lavorini, F.; et al. Inhaler technique: Facts and fantasies. A view from the Aerosol Drug Management Improvement Team (ADMIT). Npj Prim. Care Respir. Med. 2016, 26, 16017. [Google Scholar] [CrossRef]
- Lindsay, D.A.; Russell, N.L.; Thompson, J.E.; Warnock, T.H.; Shellshear, I.D.; Buchanan, P.R. A multicentre comparison of the efficacy of terbutaline Turbuhaler and salbutamol pressurized metered dose inhaler in hot, humid regions. Eur. Respir. J. 1994, 7, 342–345. [Google Scholar] [CrossRef]
- Proskocil, B.J.; Fryer, A.D. Beta2-agonist and anticholinergic drugs in the treatment of lung disease. Proc. Am. Thorac Soc. 2005, 2, 305–310. [Google Scholar] [CrossRef]
- Delea, T.E.; Stanford, R.H.; Hagiwara, M.; Stempel, D.A. Association between adherence with fixed dose combination fluticasone propionate/salmeterol on asthma outcomes and costs. Curr. Med. Res. Opin. 2008, 24, 3435–3442. [Google Scholar] [CrossRef]
- Baldwin, D.R.; Wise, R.; Andrews, J.M.; Gill, M.; Honeybourne, D. Comparative bronchoalveolar concentrations of ciprofloxacin and lomefloxacin following oral administration. Respir. Med. 1993, 87, 595–601. [Google Scholar] [CrossRef]
- Choudhury, D.; Tanner, M.G.; McAughtrie, S.; Yu, F.; Mills, B.; Choudhary, T.R.; Seth, S.; Craven, T.H.; Stone, J.M.; Mati, I.K.; et al. Endoscopic sensing of alveolar pH. Biomed. Opt. Express 2017, 8, 243–259. [Google Scholar] [CrossRef]
| Parameter | Changes | Comments |
|---|---|---|
| Lack of patients’ adherence to therapy, incorrect use of inhalers | Improved information to patients about the need for treatment adherence and repeated training of patients | The problem is known for years and no improvement has been noted |
| Inhaler quality | Improved product quality, more detailed testing, fixed dose combinations | Improvement appears realistic |
| In vitro testing for lung deposition | Physiologically more relevant testing using impactors with breath simulation and mouth-throat model Testing under relevant moisture conditions Computation fluid dynamics simulations based on images | Both methods appear suitable to improve the prediction of deposition and to personalize the profile |
| Particle clearance and permeation in vitro | Cells and engineered tissues | Physiologically more relevant cellular models are being developed and improvement is expected |
| Dissolution | Identification of physiologically more relevant fluids | Studies are underway to solve these issues |
| In vivo parameters | Better imaging techniques for deposition New technologies for drug levels in ELF (sensors) | Improvement appears realistic |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröhlich, E. Biological Obstacles for Identifying In Vitro-In Vivo Correlations of Orally Inhaled Formulations. Pharmaceutics 2019, 11, 316. https://doi.org/10.3390/pharmaceutics11070316
Fröhlich E. Biological Obstacles for Identifying In Vitro-In Vivo Correlations of Orally Inhaled Formulations. Pharmaceutics. 2019; 11(7):316. https://doi.org/10.3390/pharmaceutics11070316
Chicago/Turabian StyleFröhlich, Eleonore. 2019. "Biological Obstacles for Identifying In Vitro-In Vivo Correlations of Orally Inhaled Formulations" Pharmaceutics 11, no. 7: 316. https://doi.org/10.3390/pharmaceutics11070316
APA StyleFröhlich, E. (2019). Biological Obstacles for Identifying In Vitro-In Vivo Correlations of Orally Inhaled Formulations. Pharmaceutics, 11(7), 316. https://doi.org/10.3390/pharmaceutics11070316





