Effect of a Cationic Surfactant on Microemulsion Globules and Drug Release from Hydrogel Contact Lenses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Microemulsions
2.3. Characterization of Microemulsions
2.3.1. Dynamic Light Scattering
2.3.2. Transmission Electron Microscopy (TEM)
2.4. Fabrication of Microemulsion-Laden Contact Lenses
2.5. Fabrication of Cationic Surfactant-Loaded Contact Lenses (No Microemulsion)
2.6. Drug Loading in Contact Lenses
2.7. In Vitro Drug Release Experiments
2.8. Characterization of Microemulsion-laden Contact Lenses
2.8.1. Optical Transparency
2.8.2. Water Content
3. Results and Discussion
3.1. Characterization of Microemulsions
3.1.1. Globule Size by Dynamic Light Scattering
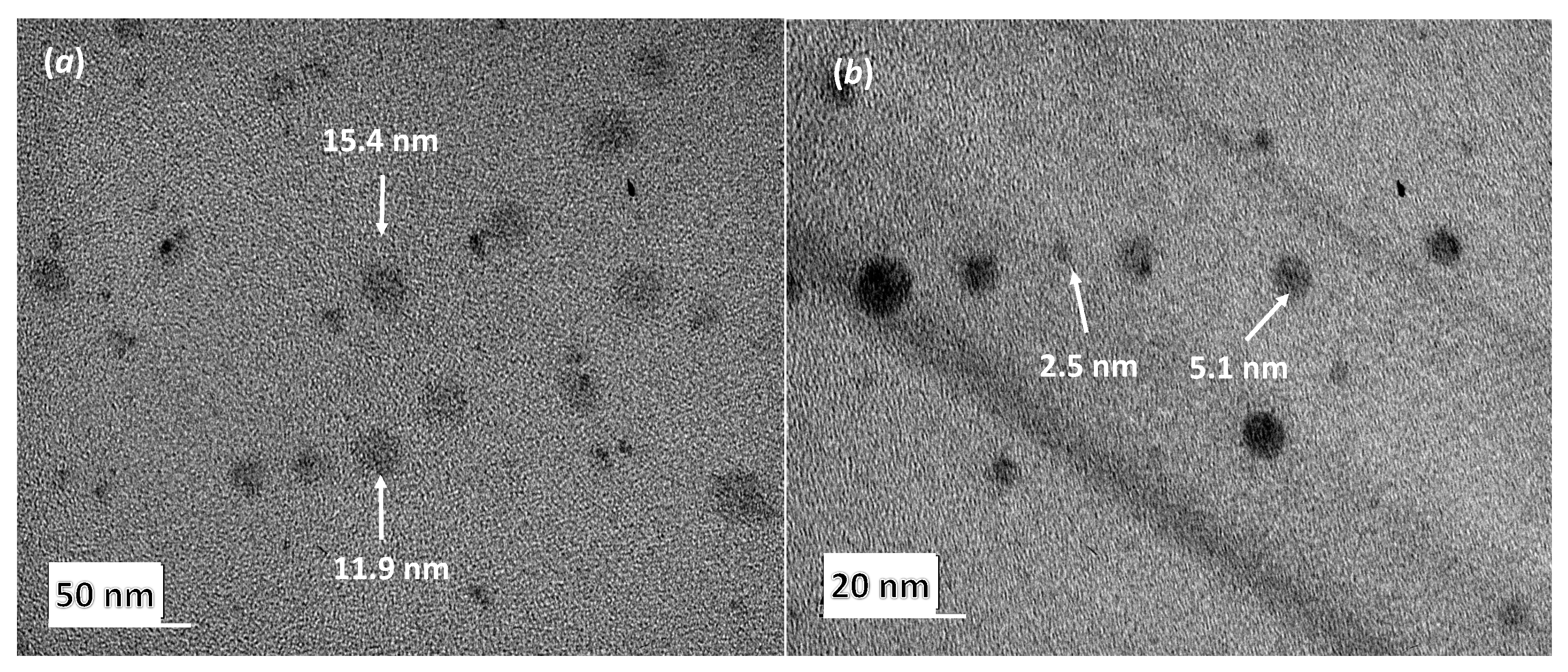
3.1.2. Globule Morphology by Transmission Electron Microscopy
3.2. In Vitro Drug Release
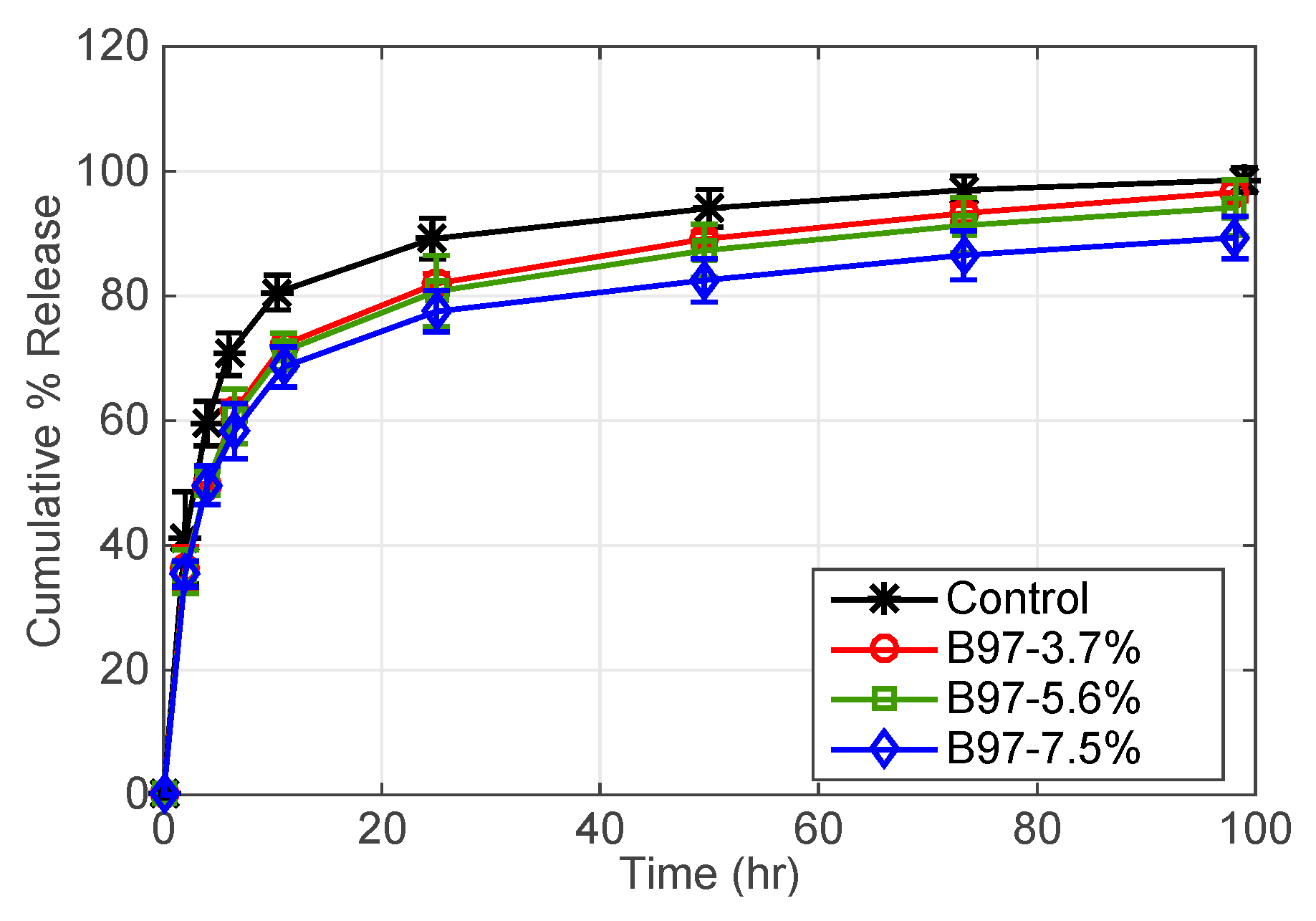
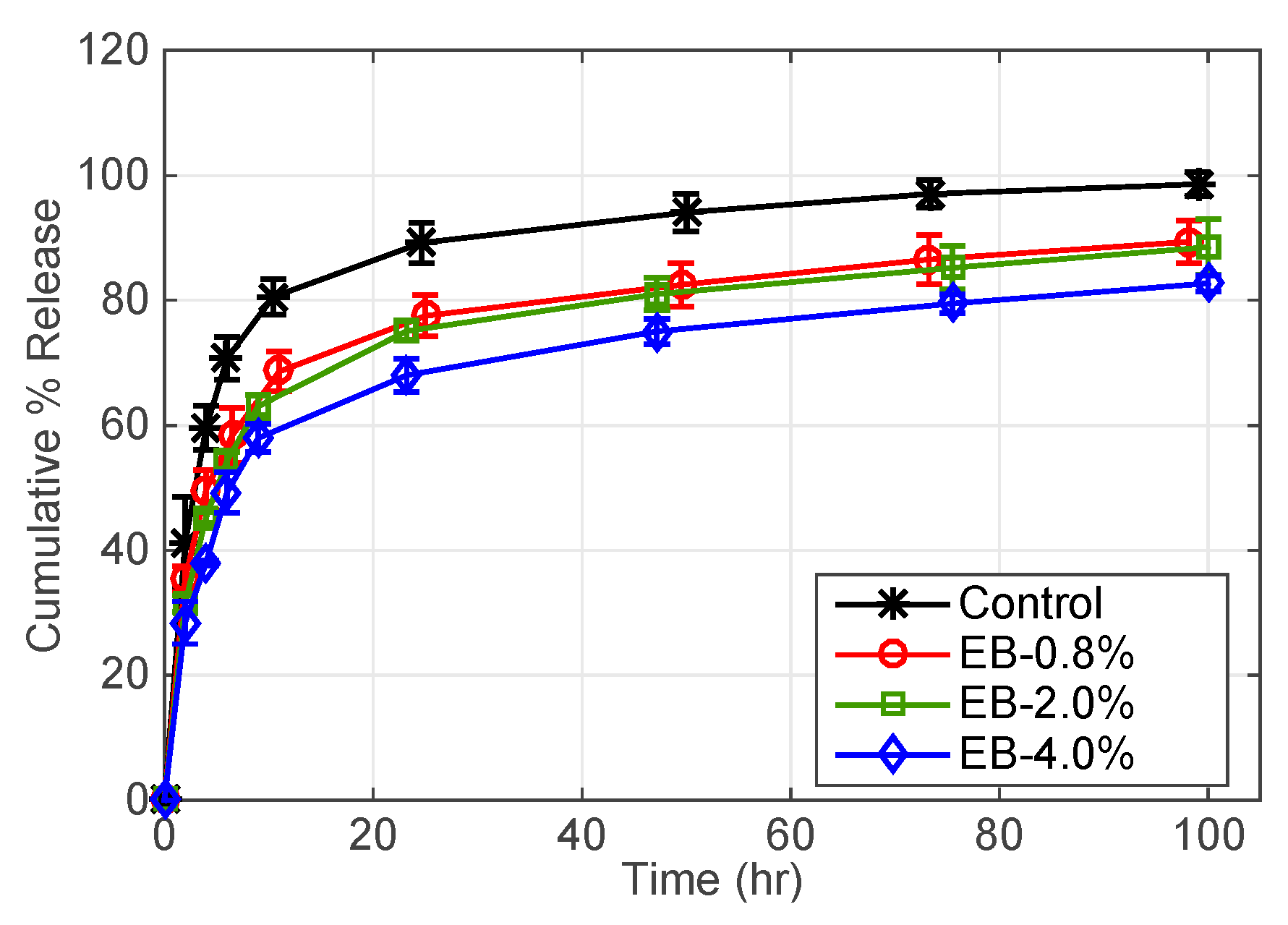
3.2.1. Effect of Surfactant Weight % (B97) and Oil Weight % (EB) in Microemulsion
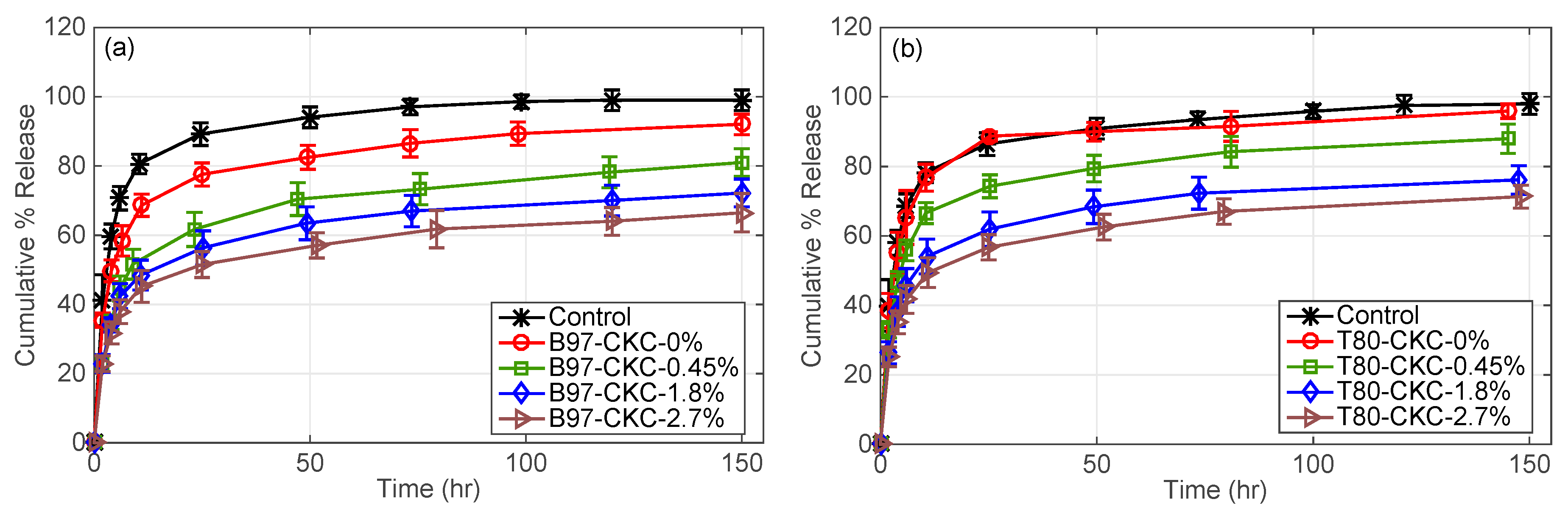
3.2.2. Effect of Surfactant Type and Cationic Surfactant Weight % in Microemulsion
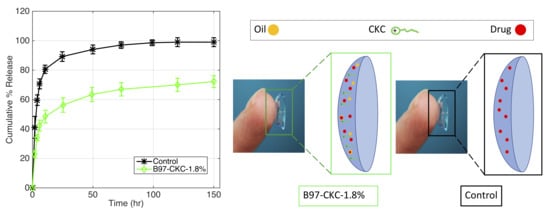
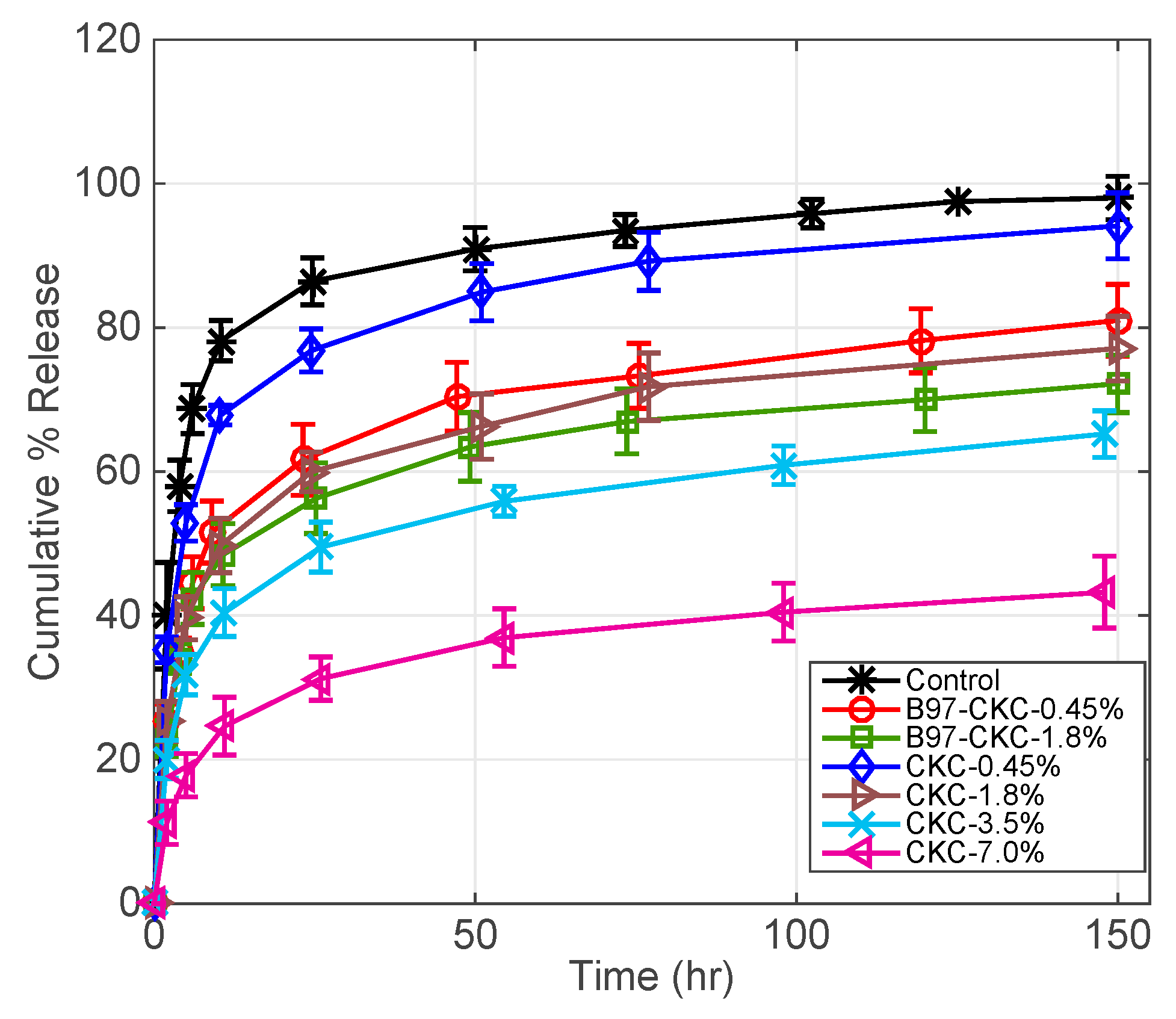
3.2.3. CKC-Microemulsion-laden and CKC-only Contact Lenses Comparison
3.3. Characterization of Contact Lenses
3.3.1. Optical Transmission
3.3.2. Water Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lang, J.C. Ocular drug delivery conventional ocular formulations. Adv. Drug Deliv. Rev. 1995, 16, 39–43. [Google Scholar] [CrossRef]
- Le Bourlais, C.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic drug delivery systems—Recent advances. Prog. Retin. Eye Res. 1998, 17, 33–58. [Google Scholar] [CrossRef]
- Peng, C.C.; Kim, J.; Chauhan, A. Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing Vitamin E diffusion barriers. Biomaterials 2010, 31, 4032–4047. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Peng, C.C.; Chauhan, A. Extended release of dexamethasone from silicone-hydrogel contact lenses containing vitamin E. J. Control. Release 2010, 148, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Reimondez-Troitiño, S.; Csaba, N.; Alonso, M.J.; de la Fuente, M. Nanotherapies for the treatment of ocular diseases. Eur. J. Pharm. Biopharm. 2015, 95, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Hiratani, H.; Gómez-Amoza, J.L.; Martínez-Pacheco, R.; Souto, C.; Concheiro, A. Soft Contact Lenses Capable of Sustained Delivery of Timolol. J. Pharm. Sci. 2002, 91, 2182–2192. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, J.B.; Stefanescu, C.F.; Ross, A.E.; Salvador-Culla, B.; Cortez, P.; Ford, E.M.; Wymbs, K.A.; Sprague, S.L.; Mascoop, D.R.; Rudina, S.S.; et al. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 2014, 35, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, P.; Serro, A.P.; Saramago, B.; Colaço, R.; Chauhan, A. Controlled Release of Antibiotics from Vitamin E–Loaded Silicone-Hydrogel Contact Lenses. J. Pharm. Sci. 2016, 105, 1164–1172. [Google Scholar] [CrossRef]
- Peng, C.C.; Burke, M.T.; Carbia, B.E.; Plummer, C.; Chauhan, A. Extended drug delivery by contact lenses for glaucoma therapy. J. Control. Release 2012, 162, 152–158. [Google Scholar] [CrossRef]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J. Control. Release 2013, 165, 82–89. [Google Scholar] [CrossRef]
- Hiratani, H.; Alvarez-Lorenzo, C. Timolol uptake and release by imprinted soft contact lenses made of N,N-diethylacrylamide and methacrylic acid. J. Control. Release 2002, 83, 223–230. [Google Scholar] [CrossRef]
- Ciolino, J.B.; Hoare, T.R.; Iwata, N.G.; Behlau, I.; Dohlman, C.H.; Langer, R.; Kohane, D.S. A Drug-Eluting Contact Lens. Investig. Opthalmol. Vis. Sci. 2009, 50, 3346. [Google Scholar] [CrossRef] [PubMed]
- Sekar, P.; Chauhan, A. Effect of vitamin-E integration on delivery of prostaglandin analogs from therapeutic lenses. J. Colloid Interface Sci. 2019, 539, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Hu, N.; Tammareddy, T.; Domszy, R.; Yang, J.; Wang, N.S.; Yang, A. Extended delivery of non-steroidal anti-inflammatory drugs through contact lenses loaded with vitamin E and cationic surfactants. Contact Lens Anterior Eye 2019. [Google Scholar] [CrossRef]
- Pall, B.; Gomes, P.; Yi, F.; Torkildsen, G. Management of Ocular Allergy Itch with an Antihistamine-Releasing Contact Lens. Cornea 2019, 38, 713–717. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). January 18, 2018–August 2019. Identifier NCT02852057, Effectiveness and Safety of Timolol and Dorzolamide Loaded Contact Lenses. Available online: https://clinicaltrials.gov/ct2/show/NCT02852057 (accessed on 29 March 2019).
- Peng, C.C.; Bengani, L.C.; Jung, H.J.; Leclerc, J.; Gupta, C.; Chauhan, A. Emulsions and microemulsions for ocular drug delivery. J. Drug Deliv. Sci. Technol. 2011, 21, 111–121. [Google Scholar] [CrossRef]
- Hegde, R.R.; Verma, A.; Ghosh, A. Microemulsion: New Insights into the Ocular Drug Delivery. ISRN Pharm. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Vandamme, T. Microemulsions as ocular drug delivery systems: Recent developments and future challenges. Prog. Retin. Eye Res. 2002, 21, 15–34. [Google Scholar] [CrossRef]
- Singh, P.K.; Iqubal, M.K.; Shukla, V.K.; Shuaib, M. Microemulsions: Current trends in novel drug delivery systems. J. Pharm. Chem. Biol. Sci. 2014, 1, 39–51. [Google Scholar]
- Klang, S.; Abdulrazik, M.; Benita, S. Influence of Emulsion Droplet Surface Charge on Indomethacin Ocular Tissue Distribution. Pharm. Dev. Technol. 2000, 5, 521–532. [Google Scholar] [CrossRef]
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.S. Successfully Improving Ocular Drug Delivery Using the Cationic Nanoemulsion, Novasorb. J. Drug Deliv. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Daull, P.; Lallemand, F.; Philips, B.; Lambert, G.; Buggage, R.; Garrigue, J.S. Distribution of cyclosporine A in ocular tissues after topical administration of cyclosporine A cationic emulsions to pigmented rabbits. Cornea 2013, 32, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Baudouin, C.; Daull, P.; Garrigue, J.S.; Buggage, R.; Brignole-Baudouin, F. In vitro and in vivo evaluation of a preservative-free cationic emulsion of latanoprost in corneal wound healing models. Cornea 2012, 31, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Amrane, M.; Creuzot-Garcher, C.; Robert, P.Y.; Ismail, D.; Garrigue, J.S.; Pisella, P.J.; Baudouin, C. Ocular tolerability and efficacy of a cationic emulsion in patients with mild to moderate dry eye disease—A randomised comparative study. J. Fr. Ophtalmol. 2014, 37, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Gulsen, D.; Chauhan, A. Dispersion of microemulsion drops in HEMA hydrogel: A potential ophthalmic drug delivery vehicle. Int. J. Pharm. 2005, 292, 95–117. [Google Scholar] [CrossRef]
- Li, C.C.; Abrahamson, M.; Kapoor, Y.; Chauhan, A. Timolol transport from microemulsions trapped in HEMA gels. J. Colloid Interface Sci. 2007, 315, 297–306. [Google Scholar] [CrossRef]
- Kapoor, Y.; Chauhan, A. Ophthalmic delivery of Cyclosporine A from Brij-97 microemulsion and surfactant-laden p-HEMA hydrogels. Int. J. Pharm. 2008, 361, 222–229. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Mangukiya, M.A.; Patel, P.A.; Vaidya, R.J.; Koli, A.R.; Ranch, K.M.; Shah, D.O. Extended release of ketotifen from silica shell nanoparticle-laden hydrogel contact lenses: In vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2016, 27, 113. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Desai, A.R.; Choksi, H.H.; Patil, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. Effect of surfactant chain length on drug release kinetics from microemulsion-laden contact lenses. Int. J. Pharm. 2017, 524, 193–204. [Google Scholar] [CrossRef]
- Hajjar, B.; Zier, K.I.; Khalid, N.; Azarmi, S.; Löbenberg, R. Evaluation of a microemulsion-based gel formulation for topical drug delivery of diclofenac sodium. J. Pharm. Investig. 2018, 48, 351–362. [Google Scholar] [CrossRef]
- Habib, F.; El-Mahdy, M.; Maher, S. Microemulsions for ocular delivery: Evaluation and characterization. J. Drug Deliv. Sci. Technol. 2011, 21, 485–489. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, C.K. Preparation and evaluation of flurbiprofen-loaded microemulsion for parenteral delivery. Int. J. Pharm. 1999, 181, 173–179. [Google Scholar] [CrossRef]
- Daull, P.; Lallemand, F.; Garrigue, J.S. Benefits of cetalkonium chloride cationic oil-in-water nanoemulsions for topical ophthalmic drug delivery: Cationic emulsion and ocular drug delivery. J. Pharm. Pharmacol. 2014, 66, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Bengani, L.C.; Chauhan, A. Extended delivery of an anionic drug by contact lens loaded with a cationic surfactant. Biomaterials 2013, 34, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Avdeef, A.; Box, K.J.; Comer, J.E.; Hibbert, C.; Tam, K.Y. Determination of liposomal membrane water partition coefficients of ionizable drugs. Pharm. Res. 1998, 15, 802–806. [Google Scholar] [CrossRef]
- Willis, S.L.; Court, J.L.; Redman, R.P.; Wang, J.-H.; Leppard, S.W.; O’Byrne, V.J.; Small, S.A.; Lewis, A.L.; Jones, S.A.; Stratford, P.W. A novel phosphorylcholine-coated contact lens for extended wear use. Biomaterials 2001, 22, 3261–3272. [Google Scholar] [CrossRef]
- Holden, D.A.; Merrz, G.W. Critical Oxygen Levels to Avoid Corneol Edema for Daily and Extended Wear Contact Lenses. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1161–1167. [Google Scholar] [CrossRef]
- Horst, C.R.; Brodland, B.; Jones, L.W.; Brodland, G.W. Measuring the Modulus of Silicone Hydrogel Contact Lenses. Optom. Vis. Sci. 2012, 89, 1468–1476. [Google Scholar] [CrossRef]
- El-Hadidy, G.N.; Ibrahim, H.K.; Mohamed, M.I.; El-Milligi, M.F. Microemulsions as vehicles for topical administration of voriconazole: Formulation and in vitro evaluation. Drug Dev. Ind. Pharm. 2012, 38, 64–72. [Google Scholar] [CrossRef]
- Thakur, S.S.; Solloway, J.; Stikkelman, A.; Seyfoddin, A.; Rupenthal, I.D. Phase transition of a microemulsion upon addition of cyclodextrin—Applications in drug delivery. Pharm. Dev. Technol. 2018, 23, 167–175. [Google Scholar] [CrossRef]
- Baudouin, C.; Labbé, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Y.; Liu, X.; Wang, N.; Song, Z.; Wu, K. A Comparison of the Effects of Benzalkonium Chloride on Ocular Surfaces between C57BL/6 and BALB/c Mice. Int. J. Mol. Sci. 2017, 18, 509. [Google Scholar] [CrossRef] [PubMed]
- Liang, H. Reduction of quaternary ammonium-induced ocular surface toxicity by emulsions: An in vivo study in rabbits. Mol. Vis. 2008, 14, 204–216. [Google Scholar] [PubMed]
- Guzman-Aranguez, A.; Colligris, B.; Pintor, J. Contact Lenses: Promising Devices for Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 189–199. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Oil | Surfactant | Cationic Surfactant | |
|---|---|---|---|---|
| EB (µL) | Brij 97 (g) | Tween 80 (g) | CKC (mg) | |
| B97-3.7% | 100 | 1 | - | - |
| B97-5.6% | 100 | 1.5 | - | - |
| B97-7.5% * | 100 | 2 | - | - |
| EB-2% ** | 250 | 2 | - | - |
| EB-4% ** | 500 | 2 | - | - |
| B97-CKC-0.45% | 100 | 2 | - | 125 |
| B97-CKC -1.8% | 100 | 2 | - | 500 |
| B97-CKC -2.7% | 100 | 2 | - | 750 |
| T80-CKC-0% | 100 | - | 2 | - |
| T80-CKC-0.45% | 100 | - | 2 | 125 |
| T80-CKC-1.8% | 100 | - | 2 | 500 |
| T80-CKC-2.7% | 100 | - | 2 | 750 |
| Formulation | Average Oil Globule Size (nm) |
|---|---|
| B97-CKC-0% | 12.1 ± 1.8 |
| B97-CKC-0.45% | 3.4 ± 0.3 |
| B97-CKC-1.8% | 2.4 ± 0.2 |
| T80-CKC-0% | 18.1 ± 3.2 |
| T80-CKC-0.45% | 5.0 ± 0.4 |
| T80-CKC-1.8% | 2.7 ± 0.6 |
| Formulation | Transmittance (%) | Water Content (%) |
|---|---|---|
| Control | 99.5 ± 0.4 | 36.7 ± 4.8 |
| EB-4% | 94.7 ± 4.9 | 27.6 ± 4.1 |
| T80-CKC-0% | 30.8 ± 5.3 | 33.1 ± 5.8 |
| T80-CKC-0.45% | 43.8 ± 5.8 | 39.7 ± 1.4 |
| T80-CKC-1.8% | 85.7 ± 7.1 | 34.8 ± 1.6 |
| B97-CKC-0% | 95.5 ± 2.2 | 39.2 ± 0.7 |
| B97-CKC-0.45% | 94.7 ± 4.6 | 38.8 ± 0.5 |
| B97-CKC-1.8% | 95.2 ± 4.0 | 36.9 ± 2.3 |
| CKC-3.5% | 99.1 ± 0.4 | 33.9 ± 5.0 |
| CKC-7.0% | 99.5 ± 0.2 | 33.5 ± 1.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Luna, C.; Hu, N.; Koolivand, A.; Fan, X.; Zhu, Y.; Domszy, R.; Yang, J.; Yang, A.; Wang, N.S. Effect of a Cationic Surfactant on Microemulsion Globules and Drug Release from Hydrogel Contact Lenses. Pharmaceutics 2019, 11, 262. https://doi.org/10.3390/pharmaceutics11060262
Torres-Luna C, Hu N, Koolivand A, Fan X, Zhu Y, Domszy R, Yang J, Yang A, Wang NS. Effect of a Cationic Surfactant on Microemulsion Globules and Drug Release from Hydrogel Contact Lenses. Pharmaceutics. 2019; 11(6):262. https://doi.org/10.3390/pharmaceutics11060262
Chicago/Turabian StyleTorres-Luna, Cesar, Naiping Hu, Abdollah Koolivand, Xin Fan, Yuli Zhu, Roman Domszy, Jeff Yang, Arthur Yang, and Nam Sun Wang. 2019. "Effect of a Cationic Surfactant on Microemulsion Globules and Drug Release from Hydrogel Contact Lenses" Pharmaceutics 11, no. 6: 262. https://doi.org/10.3390/pharmaceutics11060262
APA StyleTorres-Luna, C., Hu, N., Koolivand, A., Fan, X., Zhu, Y., Domszy, R., Yang, J., Yang, A., & Wang, N. S. (2019). Effect of a Cationic Surfactant on Microemulsion Globules and Drug Release from Hydrogel Contact Lenses. Pharmaceutics, 11(6), 262. https://doi.org/10.3390/pharmaceutics11060262