Abstract
The Bunyaviridae is a family of arboviruses including both plant- and vertebrate-infecting representatives. The Tospovirus genus accommodates plant-infecting bunyaviruses, which not only replicate in their plant host, but also in their insect thrips vector during persistent propagative transmission. For this reason, they are generally assumed to encounter antiviral RNA silencing in plants and insects. Here we present an overview on how tospovirus nonstructural NSs protein counteracts antiviral RNA silencing in plants and what is known so far in insects. Like tospoviruses, members of the related vertebrate-infecting bunyaviruses classified in the genera Orthobunyavirus, Hantavirus and Phlebovirus also code for a NSs protein. However, for none of them RNA silencing suppressor activity has been unambiguously demonstrated in neither vertebrate host nor arthropod vector. The second part of this review will briefly describe the role of these NSs proteins in modulation of innate immune responses in mammals and elaborate on a hypothetical scenario to explain if and how NSs proteins from vertebrate-infecting bunyaviruses affect RNA silencing. If so, why this discovery has been hampered so far.
Keywords:
RNAi; RNA silencing; innate immunity; bunyavirus; NSs; tospovirus; orthobunyavirus; RNA silencing suppression 1. Introduction—The Family of Bunyaviridae
The Bunyaviridae, with more than 350 identified species, is divided in five genera and contains several important viruses that cause major problems in human/animal health and agriculture production systems. All five genera of this family contain viruses pathogenic to either animals/humans (Orthobunyavirus, Phlebovirus, Nairovirus and Hantavirus) or plants (Tospovirus). Most bunyaviruses are arthropod-borne viruses (arboviruses), as they replicate in the arthropods by which they are transmitted (Figure 1). Hantaviruses present an exception, as they are rodent-borne and no arthropod vector has been identified so far.
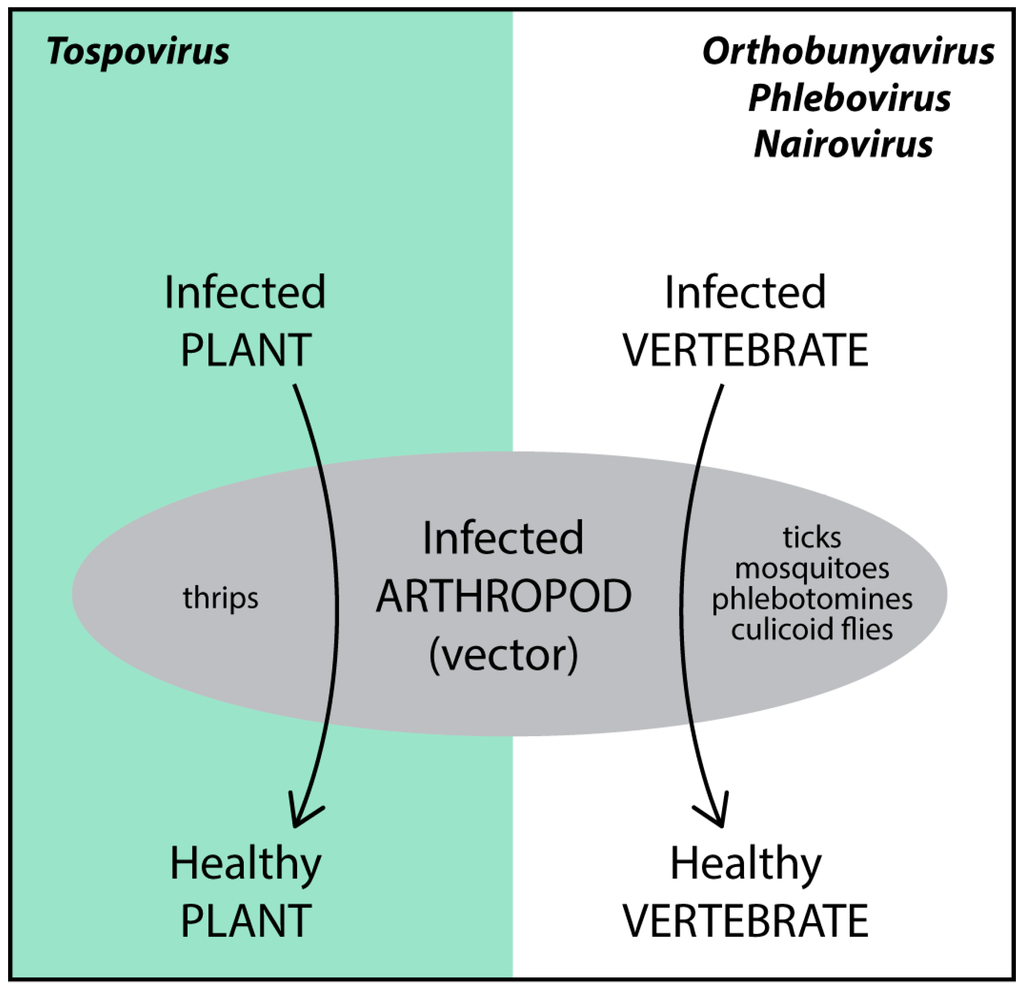
Figure 1.
Bunyaviruses and their arthropod vectors.
Members of all five genera in the Bunyaviridae share several features. Bunyavirus particles are enveloped and generally spherical. Viral glycoproteins are embedded in the envelope membrane and presented as spikes on the outside. The core of virus particles contains the single-stranded (ss)RNA genome that is encapsidated by a nucleocapsid (N) protein and small amounts of the viral RNA-dependent RNA polymerase (RdRp, also denoted L protein). The bunyavirus RNA genome is tripartite and segments have either a negative or ambisense polarity (Figure 2). Genome organization strategies vary among members of different genera and may diversify even among members within a genus, as observed with orthobunyaviruses and phleboviruses. In general, though, the bunyavirus genome codes for four structural and up to two non-structural proteins. The L RNA is of complete negative polarity and contains a single open reading frame (ORF) on the viral complementary (vc)RNA that encodes the RdRp. With the exception of tospoviruses, the M RNA of all other bunyaviruses is of negative polarity and contains one single ORF on the vc-strand coding for the precursor to the two glycoproteins (Gn and Gc), and in a few cases an additional non-structural protein NSm. The M RNA of tospoviruses contains an ambisense gene arrangement, and encodes a NSm on the viral (v)RNA strand and the glycoprotein precursor on the vcRNA. Tospovirus NSm protein facilitates the movement of viral ribonucleoproteins (RNPs) from cell-to-cell and presents an adaptation of this group of viruses to plants as a host. The S RNA segment is of negative polarity for members of the genera Orthobunyavirus, Hantavirus and Nairovirus, or ambisense for members of the genera Phlebovirus and Tospovirus [1,2,3,4,5,6]. The negative polarity S RNA encodes the major structural N protein on the vcRNA strand and, in certain members of orthobunyaviruses and hantaviruses, an additional non-structural protein (NSs) in an overlapping reading frame. For members of genera with ambisense S RNA, the NSs protein is encoded, separate from the N gene, by a second non-overlapping ORF on the vRNA strand (Figure 2).
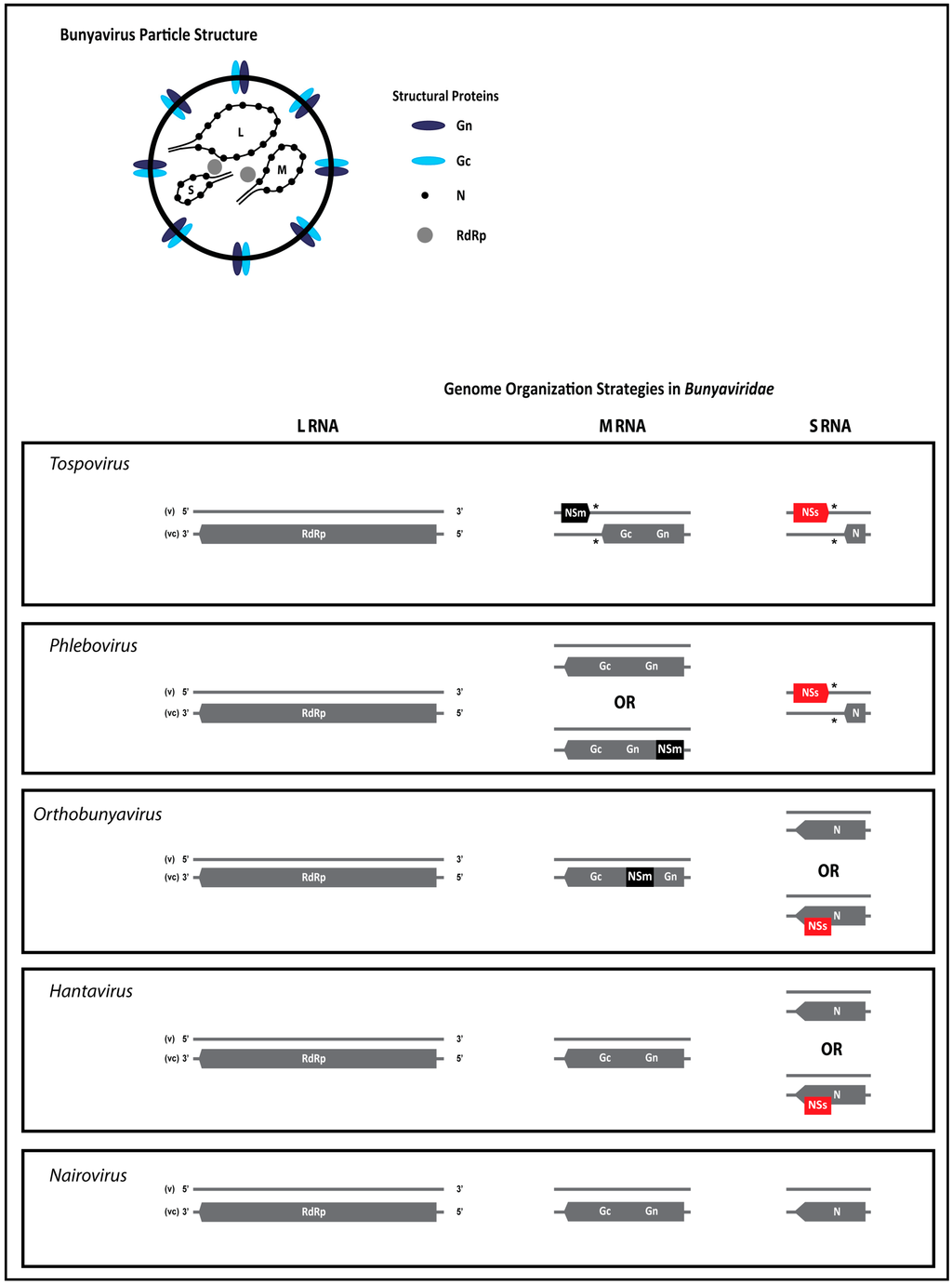
Figure 2.
Particle structure and genome organization of bunyaviruses. L, M, and S RNA (large, medium, and small RNA segments, respectively), RdRP (viral RNA-dependent RNA polymerase), Gn and Gc (glycoproteins derived from the N-terminus and C-terminus of the precursor protein, respectively), NSm (non-structural protein of the M RNA), N (nucleocapsid protein), NSs (non-structural protein of the S RNA). (*) intergenic region (IGR).
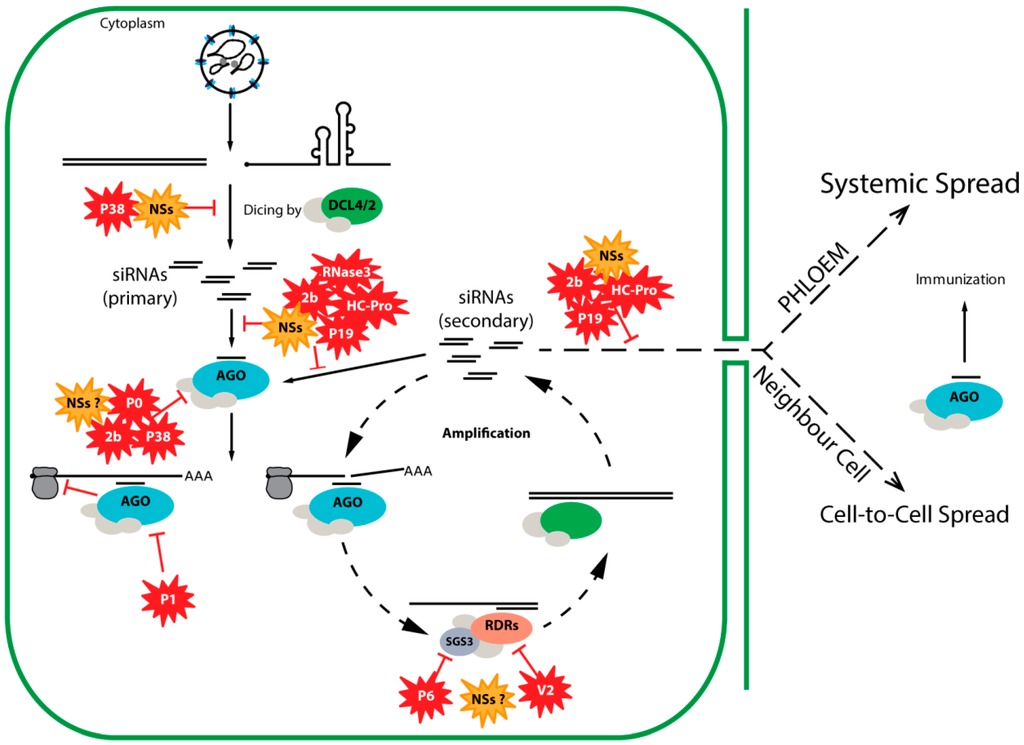
During their infection cycle, all viruses encounter the host innate immune system as one of the first lines of host defense. In response to that, viruses have evolved various strategies to counteract the host innate immune system. In the case of bunyaviruses, the NSs protein is a knowingly important modulator of host innate immune responses, and a virulence factor [1,2,3,4,5,6,7]. In vertebrates, the interferon (IFN) pathway plays a major role in antiviral defense, and accordingly IFN-antagonist activity is found in the NSs of vertebrate-infecting bunyaviruses (reviewed by [7]). In plants and arthropods, where IFN pathway is not present, RNA silencing is an important part of antiviral innate immunity, and during infection of plants (by tospoviruses) and arthropods (by arthropod-borne bunyaviruses), members of the Bunyaviridae are targeted by antiviral RNA silencing. However, so far only the NSs protein of tospoviruses has been irrefutably demonstrated to have RNA silencing suppression (RSS also known as viral suppressors of silencing, VSRs) activity [8,9,10], which is relevant for the establishment of a successful infection of plant hosts. Concerning the arthropod vector, information related to the possible effect of NSs on antiviral RNA silencing during infection in arthropods remains scarce for both plant- and vertebrate-infecting bunyaviruses likewise. In this review we will present the state of the art on the modulation of host defense responses by bunyavirus NSs proteins, with emphasis on its effect on antiviral RNA silencing, to finally discuss the enigma surrounding NSs from bunyaviruses and its (possible) effect on arthropod and mammalian antiviral RNA silencing.
4. Arthropod RNA Silencing and Arboviruses
After the discovery of RNA silencing in Caenorhabditis elegans and plants, one of the first evidence of natural antiviral silencing in insect species came in 2002 during studies on flock house virus (FHV) infection of Drosophila melanogaster cells, in which FHV B2 protein was identified as a RSS [82]. The presence of this (gene regulation) mechanism has also been shown in other arthropods, including flies (e.g., fruit fly), mosquitoes, spiders and ticks [83,84]. Drosophila is one of the insect species where RNA silencing is probably best characterized, and although the mechanism is similar to the one of plants, it also shows important differences. Drosophila contains two Dicer proteins, three Argonaute proteins and to date no RDR has been identified in insects (reviewed in [85]). In the case of antiviral RNA silencing Dicer-2 recognizes and cleaves dsRNA structures (generated during virus replication) into siRNAs, from which one strand activates a RISC complex containing AGO2 to surveil the cell for ssRNA with sequence complementarity to the siRNA [14,86,87,88]. The pathway also includes other proteins such as Loquacious (Loqs, which increases affinity of Dicer-2 to dsRNA), R2D2 (aids siRNA loading in AGO2 and RISC assembly), component 3 promoter of RISC (C3PO, facilitates AGO2 endonucleolytic cleavage of the siRNA passenger strand) and HEN1 (stabilizes the guide strand by methylating its 3′ terminal nucleotide) (a deeper review of arthropod RNA silencing can be found in [89,90,91]). Similar to plant-infecting viruses, several arthropod-infecting viruses have been shown to counteract arthropod antiviral RNA silencing by expressing RSS proteins that can target different steps of the RNA silencing machinery. B2 from drosophila-infecting nodavirus FHV, one of the first RSS identified in arthropods, binds dsRNA size-independently and inhibits Dicer cleavage [92], but also directly interacts with the PAZ domain of Drosophila Dicer proteins [93]. B2 from Wuhan nodavirus (WhNV) has also been shown to bind dsRNA (small and long) (reviewed in [94]). Another example is protein 1A from cricket paralysis virus (CrPV), which suppresses RNA silencing by directly interacting with AGO2 [95], while 1A from Drosophila C virus (DCV) binds dsRNA and blocks Dicer-2 processing of dsRNA into siRNAs but does not bind siRNAs [96].
Arthropods are vectors for viruses (arboviruses) from several families (including Flaviviridae, Togaviridae, Reoviridae and Bunyaviridae). During persistent-propagative transmission, both plant- and animal-infecting arboviruses replicate in the arthropod vector [97,98], following a similar route of infection. The primary infection site is the midgut epithelium and in order to further disseminate in the arthropod organism and reach the salivary glands the virus must pass the midgut barrier, a determinant factor for vector competence (reviewed in [97,98,99]). Several studies indicate that RNA silencing is a relevant part of the midgut barrier and plays an important role in arbovirus infection and vector competence [100,101]. However, the antiviral role of midgut RNA silencing remains under debate and may also vary according to the arthropod species, as a recent investigation of alphavirus infection in Anopheles gambiae mosquitoes presents evidence that RNA silencing is active however it does not seem to have antiviral impact on the initial midgut infection, but only at later stages of infection [102]. Supporting the importance of antiviral RNA silencing in modulation of arbovirus infection, in arthropods where RNA silencing has been compromised or suppressed (e.g., by the action of a strong RNA silencing suppressor), arboviruses replicate to higher titers and the infection becomes pathogenic [103,104], which indicates that arthropod RNA silencing also modulates the equilibrium of an arbovirus persistent infection of the vector. Some strategies used by arboviruses to modulate antiviral RNA silencing include decoy strategies, e.g., the flavivirus small structured non-coding RNA from the viral 3′UTR referred to as subgenomic flavivirus RNA (sfRNA) [105] and the nucleic acid mediated decoy mechanisms of alphaviruses [106].
Members of the Bunyaviridae are arboviruses, and as such replicate in both the host (plant or vertebrate) as well as in the arthropod vector. While infecting arthropods, bunyaviruses are targeted by RNA silencing, as illustrated by production of virus-specific siRNAs (Table 2) [107,108,109]. It has not yet been demonstrated whether arthropod infection by bunyavirus requires viral expression of a suppressor of arthropod RNA silencing. For plant-infecting tospoviruses, the NSs protein suppresses RNA silencing in plants [8,9] and this protein is expected to also modulate antiviral RNA silencing in thrips (although clear evidence for this is still needed). On the other hand, information on the effect of vertebrate-infecting bunyaviruses in either vertebrate or arthropod RNA silencing is scarce and limited to a few reports. Below, we review the current knowledge on the activity of NSs from bunyaviruses in the different environments where replication takes place.

Table 2.
Reports on RNA silencing responses and production of virus-derived siRNAs during infection with members of Bunyaviridae.
7. TSWV NSs as Effector of Plant NLR-Mediated Intracellular Innate Immunity Response
Besides RNA silencing, another layer of the innate immunity system is represented by resistance genes and their R protein products. In plants the major class of dominant resistance (R) genes codes for the NB-LRR type, which are proteins consisting of three main domains. The N-terminal end is presented by a coiled-coil (CC) or Toll and interleukin-1 receptor (TIR) domain, followed by an internal nucleotide binding site (NBS) domain and a leucine-rich repeat (LRR) at the C-terminal end [119,120]. R proteins act as intracellular sensors of innate immunity and are highly pathogen specific. They are able to directly or indirectly perceive the presence of a pathogen by recognizing one of its effector proteins that often play a role in virulence and are referred to as avirulence factors (Avr). R proteins act like molecular switches that upon effector/Avr recognition trigger a resistance mechanism concomitant with a programmed cell death, leading to the appearance of small necrotic lesions (hypersensitive response, HR) at the site of pathogen entry. The establishment of HR prevents further dissemination of the pathogen throughout the plant host [121,122,123,124]. Some R proteins recognize viral RSS proteins, thereby acting as a plant counter-counter-defense against the viral counter-defense (RNA silencing suppression) against plant antiviral RNA silencing [125].
Recently, TSWV NSs protein was identified as the Avr for the single dominant Tsw resistance gene product, a protein that is also thought to belong to the class of NB-LRR genes [126]. In the constant battle between viruses and plants, viruses continuously keep on evolving and mutations on key amino acid residues of NSs lead to the appearance of so-called TSWV resistance-breaking (RB) isolates that do not trigger the Tsw-mediated HR response. Considering the role of NSs in counter defense against RNA silencing, mutations within this protein are likely fine-tuned to preserve (some) viral fitness and virulence, preventing virus clearance from the plant by antiviral RNA silencing. A study on engineered NSs mutants indicated that NSs RSS and Avr functions can be uncoupled. Furthermore, some of the resistance-breaking NSs mutants (both natural and engineered), mutated in predicted RNA-binding or putative Argonaute-binding domains, still suppress systemic silencing spread while having lost the ability to suppress local silencing [10,127]. Altogether, this supports the idea that the virus and its NSs protein can evolve to fine tune Avr and (local/systemic) RSS functions in order to lose (some of) its avirulence features while preserving a certain level of fitness [10,127].
9. NSs from Vertebrate-Infecting Bunyaviruses—Antagonizing Mammalian Innate Immunity
Vertebrate-infecting viruses must deal with the innate immunity in their mammalian hosts, including the well-known IFN-based defense [139,140]. NSs from several vertebrate-infecting bunyaviruses are well reported for their IFN-antagonist activity. For orthobunyavirus Bunyamwera virus (BUNV), La Crosse virus (LACV) and phlebovirus Rift Valley fever virus (RVFV), the NSs protein inhibits the type I IFN system by blocking RNA polymerase II transcription, consequently leading to a shutoff of the antiviral response genes [110,141,142,143,144]. RVFV NSs has additionally been shown to induce specific degradation of dsRNA-dependent protein kinase (PKR) [145], a process that occurs independently from the NSs-mediated blocking of host gene transcription [146]. IFN-antagonistic activity has also been identified in the NSs of tick-borne phleboviruses, e.g., the NSs of Uukuniemi virus (UUKV) was shown to have weak IFN-antagonistic activity [147]. In the case of the tick-borne severe fever with thrombocytopenia syndrome virus (SFTSV), its NSs has been shown to form cytoplasmic inclusions involved in sequestration of host factors involved in RIG-I signaling as well as IFN signaling [148,149,150,151]. A more complete description of the diverse strategies employed by different phlebovirus NSs proteins to evade host IFN defense can be found in [152]. Several hantaviruses have been reported to contain, like orthobunyaviruses, an ORF overlapping the N gene and encoding a NSs protein with weak IFN-antagonistic properties [1]. For an extensive description on this subject readers are referred to excellent literature reviews [7,153]. In nairoviruses, a NSs protein has been identified only in Crimean-Congo hemorrhagic fever virus (CCHFV) and shown to induce apoptosis [154], however its effect on IFN pathway remains to be investigated.
Besides the IFN-induced innate immune responses, mammals contain additional layers of innate immunity that act against viruses, including antiviral RNA silencing [20,21]. Proteins from mammalian-infecting viruses have earlier been observed to possess RSS activity too, amongst which human immunodeficiency virus type 1 (HIV-1) trans-activator of transcription (Tat, Dicer interaction and inhibition), hepatitis C virus (HCV) core (Dicer interaction) and envelope E2 (AGO2 interaction), human influenza A NS1, Ebola virus VP35 and vaccinia virus E3L (all binding dsRNA), adenovirus VA (a non-coding RNA that folds into a stem loop structure and acts as a decoy for Dicer). Interestingly, all these very same viral proteins are known to act as IFN antagonists as well [155].
Thus far, only two papers have appeared on the identification of RSS activity in NSs proteins from vertebrate-infecting bunyaviruses in mammals, both with LACV NSs, but with contradictory outcomes. In one paper, RNA silencing was triggered by transfecting cells (human cell line 293T) with siRNAs, showing that in the additional presence of (transiently) over-expressed LACV NSs an apparent decrease of siRNA-triggered silencing was observed, which tempted the authors to suggest that LACV NSs exhibits RSS activity in mammals [156]. In another paper, researchers used LACV and recombinant LACVdelNSs viruses, and observed the outcome during infection in IFN-competent and IFN-deficient mammalian cell cultures (mouse embryo fibroblasts, MEFs) and mammalian animals (mice) (in vivo). In this case, however, LACV NSs did not provide an advantage to the virus [110].
10. NSs from Vertebrate-Infecting Bunyaviruses in Their Arthropod Vectors
Vertebrate-infecting bunyaviruses, with the exception of hantaviruses, are transmitted by arthropod vectors, including arachnids (ticks) and insects (mosquitoes, phlebotomines and culicoid flies) where, similarly to tospoviruses, they replicate and establish a persistent infection [157]. During infection of the arthropod vector, vertebrate-infecting bunyaviruses are targeted by antiviral RNA silencing, as indicated by the production of bunyavirus-specific small RNAs during bunyavirus infection (Table 2) [107,109,111]. The importance of RNA silencing in modulating bunyavirus replication and the establishment of a persistent infection in arthropods is supported by the fact that, at least for phlebovirus RVFV, persistency is only achieved in cells with active Dicer-2-based RNA silencing [109]. Similar investigations are needed to verify whether this is general to other Bunyaviridae genera.
Still, little is known regarding the role of NSs during propagative transmission of vertebrate-infecting bunyaviruses in the arthropod vector. Analysis of BUNV and a recombinant NSs-deletion BUNV indicates that NSs is required for efficient replication in cell lines (including mosquito Aedes albopictus cell line U4.4), and infection with NSs-deletion virus in mosquito Aedes aegypti revealed lower titers and delayed dissemination to salivary glands when compared to wild-type virus, suggesting that in the absence of NSs the virus has difficulties in overcoming cellular defenses in the midgut [158]. Considering the role of RNA silencing in the midgut barrier [101,159], it can be speculated that BUNV NSs counteracts the RNA silencing component of the midgut barrier. BUNV NSs was also observed to be non-essential in mosquito cell lines with impaired RNA silencing, further supporting that this proteins may have RSS activity [158]. NSs from orthobunyavirus LACV and phlebovirus RVFV have also been analyzed to some extent regarding their effect on RNA silencing in arthropods (respectively, mosquito and tick cell lines), however in both cases suppression of silencing was not observed [110,135].
Altogether, available data indicate NSs is relevant during bunyavirus infection of arthropod vectors, however clear proof for presence (or absence) of RSS activity with NSs from vertebrate-infecting bunyaviruses in arthropods is still lacking.
11. The Enigma of NSs: Questions and Perspectives
While bunyaviruses are targeted by antiviral RNA silencing in plants, vertebrates and arthropods, currently only the NSs from plant-infecting tospoviruses has been clearly shown to contain RNA silencing suppressor activity. Considering the close ancestral relation of bunyaviruses, as well as many structural and functional similarities, the lack of clear proof on suppression of RNA silencing by the NSs from vertebrate-infecting bunyaviruses in both arthropods and vertebrates remains a matter for debate.
In light of this it is important to highlight that the two reported (contradictory) studies on the effect of orthobunyavirus LACV NSs in mammalian RNA silencing made use of different experimental set ups to induce silencing and to express NSs. The first study by Soldan et al. [156], in which synthetic siRNAs were transfected into mammalian cells (human cell line 293T) already expressing LACV NSs (from plasmid constructs transfected 24 h prior transfection of siRNAs), likely has used the best conditions to test LACV NSs for the ability to suppress RNA silencing due to a couple of reasons. Firstly, NSs was expressed a priori, being readily available at the time when siRNAs were transfected and allowing it to directly interfere with RNA silencing. This strategy has earlier been successfully used to demonstrate RSS activity with viral proteins [160,161]. Secondly, transfection of RNA duplex molecules smaller than 30 bp activates only the RNA silencing pathway, and not the interferon pathway [162,163]. As such, a transfection with siRNAs (21 nt) will effectively trigger RNA silencing only, and therefore results obtained with LACV NSs solely reflect interference on RNA silencing, not on IFN-induced defense responses. Altogether, this supports the observations made by Soldan et al. on the presence of RSS activity with LACV NSs. The downside of the study by Soldan et al. is that it involves a transient system and this does not reflect an authentic viral infection. In the second, contradictory study by Blakqori et al. [110], infections were performed with LACV recombinant viruses that either contained or lacked NSs. However, during this study it was only assumed that LACV NSs does not interfere with mammalian silencing because viruses had similar growth patterns and titers regardless of NSs, but the effect of NSs on the siRNA profile was not investigated. This second study used MEFs, instead of the human 293T cells used in the first study. Therefore, based on this result it would be too premature to discard the possibility of RSS activity in LACV NSs, nor with other bunyaviral NSs proteins. Future studies should also take into consideration the effect of NSs on small RNA profiles. Considering the recent success in verifying antiviral RNA silencing in mammals [20], future investigations on NSs from vertebrate-infecting bunyaviruses would also benefit from using a similar approach with undifferentiated cells, in which the production of virus-derived siRNAs is stronger and the IFN response is lacking or reduced [20,21].
Another reason why studies on this point for the vertebrate-infecting bunyaviruses so far have remained unresolved is that in mammalian cells the IFN-pathway is a major antiviral mechanism, while antiviral silencing is still being debated by some [164] and might be more secondary or limited to undifferentiated cells or certain cell types [20]. In addition, NSs from vertebrate-infecting bunyaviruses might not present a strong suppressor of RNA silencing [164]. Within the insect vector, evidence points to a role of RNA silencing in the midgut barrier where it influences vector competence [100,101] and modulates a persistent viral infection as supported by the observation that persistent viruses become pathogenic if an RSS active against insect RNA silencing is co-expressed [95,104]. Although speculative, in case bunyavirus NSs would present a strong suppressor of arthropod RNA silencing, it could thus disrupt the equilibrium of a persistent infection and turn it into a pathogenic one that might be fatal to the arthropod. A low level of RSS activity might thus be preferred and, although maybe more difficult to proof experimentally, be sufficient to suppress RNA silencing in the midgut and support dissemination in the vector. Thus, whether vertebrate-infecting bunyaviruses truly need to counteract antiviral RNA silencing remains an issue that still requires further investigation and should not be ruled out. Based on the hypothesis that negative-sense RNA viruses may have their ancestry in arthropods [165], it is possible to speculate on the origin of NSs, which could have evolved as an adaptation to allow the ancestry insect-specific bunyavirus to cross and adapt to the plant or vertebrate secondary host. However, it is not possible to dismiss that NSs evolved initially in the ancestral arthropod host playing a role in modulation of arthropod antiviral RNA silencing. Further investigation of the several recently identified insect-specific bunyaviruses [166], some without a NSs ORF [167,168,169] while others seem to harbor a NSs (unknown function) [170], might shed light on the evolutionary history of bunyaviruses as well as on the role played by NSs.
In conclusion, NSs from bunyaviruses remains an enigmatic protein, being a virulence factor in different cellular environments. However, as reviewed above, its interference on antiviral RNA silencing is only clearly described for tospovirus infections in plants, while still being debated in arthropods and vertebrates. As new data are gathered on the relevance of RNA silencing during infection in mammals as well as during persistent infections in arthropods, this may eventually also contribute to a deeper understanding of how bunyaviruses possibly affect this antiviral defense mechanism. The advancement in deep-sequencing technologies and the use of undifferentiated cell lines may provide further tools to functionally analyze NSs during bunyavirus infection. Considering the importance of NSs for plant- and vertebrate-infecting bunyaviruses, understanding its role during infection and its modus operandi will remain a continuing challenge for many years to come.
Acknowledgments
The present research was supported by the Brazilian National Council for Scientific and Technological Development (CNPq; MH).
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Jaaskelainen, K.M.; Kaukinen, P.; Minskaya, E.S.; Plyusnina, A.; Vapalahti, O.; Elliott, R.M.; Weber, F.; Vaheri, A.; Plyusnin, A. Tula and Puumala hantavirus NSs ORFs are functional and the products inhibit activation of the interferon-beta promoter. J. Med. Virol. 2007, 79, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Kormelink, R.; Garcia, M.L.; Goodin, M.; Sasaya, T.; Haenni, A.L. Negative-strand RNA viruses: The plant-infecting counterparts. Virus Res. 2011, 162, 184–202. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier/Academic Press: London, UK, 2012; p. 1338. [Google Scholar]
- Bente, D.A.; Forrester, N.L.; Watts, D.M.; McAuley, A.J.; Whitehouse, C.A.; Bray, M. Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013, 100, 159–189. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M. Orthobunyaviruses: recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M.; Brennan, B. Emerging phleboviruses. 2014, 5, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Eifan, S.; Schnettler, E.; Dietrich, I.; Kohl, A.; Blomstrom, A.L. Non-structural proteins of arthropod-borne bunyaviruses: Roles and functions. Viruses 2013, 5, 2447–2468. [Google Scholar] [CrossRef] [PubMed]
- Bucher, E.; Sijen, T.; De Haan, P.; Goldbach, R.; Prins, M. Negative-strand tospoviruses and tenuiviruses carry a gene for a suppressor of gene silencing at analogous genomic positions. J. Virol. 2003, 77, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Sugiyama, K.; Nagano, H.; Mori, M.; Kaido, M.; Mise, K.; Tsuda, S.; Okuno, T. Identification of a novel RNA silencing suppressor, NSs protein of Tomato spotted wilt virus. FEBS Lett. 2002, 532, 75–79. [Google Scholar] [CrossRef]
- Hedil, M.; Sterken, M.G.; de Ronde, D.; Lohuis, D.; Kormelink, R. Analysis of Tospovirus NSs Proteins in Suppression of Systemic Silencing. PLoS ONE 2015, 10, e0134517. [Google Scholar] [CrossRef] [PubMed]
- Molnar, A.; Csorba, T.; Lakatos, L.; Varallyay, E.; Lacomme, C.; Burgyan, J. Plant virus-derived small interfering RNAs originate predominantly from highly structured single-stranded viral RNAs. J. Virol. 2005, 79, 7812–7818. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.J.; Baulcombe, D.C. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 1999, 286, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Nakahara, K.; Pham, J.W.; Kim, K.; He, Z.; Sontheimer, E.J.; Carthew, R.W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 2004, 117, 69–81. [Google Scholar] [CrossRef]
- Kim, Y.J.; Maizel, A.; Chen, X. Traffic into silence: Endomembranes and post-transcriptional RNA silencing. EMBO J. 2014, 33, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Wassenegger, M.; Krczal, G. Nomenclature and functions of RNA-directed RNA polymerases. Trends Plant Sci. 2006, 11, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Barnard, A.C.; Nijhof, A.M.; Fick, W.; Stutzer, C.; Maritz-Olivier, C. RNAi in Arthropods: Insight into the Machinery and Applications for Understanding the Pathogen-Vector Interface. Genes 2012, 3, 702–741. [Google Scholar] [CrossRef] [PubMed]
- Pumplin, N.; Voinnet, O. RNA silencing suppression by plant pathogens: Defence, counter-defence and counter-counter-defence. Nat. Rev. Microbiol. 2013, 11, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Bologna, N.G.; Voinnet, O. The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu Rev. Plant Biol. 2014, 65, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Maillard, P.V.; Ciaudo, C.; Marchais, A.; Li, Y.; Jay, F.; Ding, S.W.; Voinnet, O. Antiviral RNA interference in mammalian cells. Science 2013, 342, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, J.; Han, Y.; Fan, X.; Ding, S.W. RNA interference functions as an antiviral immunity mechanism in mammals. Science 2013, 342, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Chen, J.; Janda, M.; Sullivan, M.; den Boon, J.; Ahlquist, P. A positive-strand RNA virus replication complex parallels form and function of retrovirus capsids. Mol. Cell 2002, 9, 505–514. [Google Scholar] [CrossRef]
- Blevins, T.; Rajeswaran, R.; Aregger, M.; Borah, B.K.; Schepetilnikov, M.; Baerlocher, L.; Farinelli, L.; Meins, F., Jr.; Hohn, T.; Pooggin, M.M. Massive production of small RNAs from a non-coding region of Cauliflower mosaic virus in plant defense and viral counter-defense. Nucleic Acids Res. 2011, 39, 5003–5014. [Google Scholar] [CrossRef] [PubMed]
- Csorba, T.; Kontra, L.; Burgyan, J. viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 2015, 479–480, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Glick, E.; Zrachya, A.; Levy, Y.; Mett, A.; Gidoni, D.; Belausov, E.; Citovsky, V.; Gafni, Y. Interaction with host SGS3 is required for suppression of RNA silencing by tomato yellow leaf curl virus V2 protein. Proc. Natl. Acad. Sci. USA 2008, 105, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, R.; Doudna, J.A. dsRNA with 5′ overhangs contributes to endogenous and antiviral RNA silencing pathways in plants. EMBO J. 2009, 28, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Zrachya, A.; Glick, E.; Levy, Y.; Arazi, T.; Citovsky, V.; Gafni, Y. Suppressor of RNA silencing encoded by Tomato yellow leaf curl virus-Israel. Virology 2007, 358, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, C.; Li, Z.; Zhou, X. Suppression of RNA silencing by a plant DNA virus satellite requires a host calmodulin-like protein to repress RDR6 expression. PLoS Pathog. 2014, 10, e1003921. [Google Scholar] [CrossRef] [PubMed]
- Hemmes, H.; Lakatos, L.; Goldbach, R.; Burgyan, J.; Prins, M. The NS3 protein of Rice hoja blanca tenuivirus suppresses RNA silencing in plant and insect hosts by efficiently binding both siRNAs and miRNAs. RNA 2007, 13, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Xu, Y.; Jia, R.; Zhou, X.; Ye, K. Size-independent and noncooperative recognition of dsRNA by the Rice stripe virus RNA silencing suppressor NS3. J. Mol. Biol. 2010, 404, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Wu, J.; Zhou, Y.; Zhou, X. Characterization and subcellular localization of an RNA silencing suppressor encoded by Rice stripe tenuivirus. Virology 2009, 387, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Schnettler, E.; Hemmes, H.; Huismann, R.; Goldbach, R.; Prins, M.; Kormelink, R. Diverging affinity of tospovirus RNA silencing suppressor proteins, NSs, for various RNA duplex molecules. J. Virol. 2010, 84, 11542–11554. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Sahana, N.; Pandey, V.; Doblas, P.; Jain, R.K.; Palukaitis, P.; Canto, T.; Praveen, S. Interference in plant defense and development by non-structural protein NSs of Groundnut bud necrosis virus. Virus Res. 2012, 163, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.S.; Johnson, K.N.; Dietzgen, R.G. Cytorhabdovirus phosphoprotein shows RNA silencing suppressor activity in plants, but not in insect cells. Virology 2015, 476, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.S.; Johnson, K.N.; Carroll, B.J.; Dietzgen, R.G. Cytorhabdovirus P protein suppresses RISC-mediated cleavage and RNA silencing amplification in planta. Virology 2016, 490, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Song, X.; Xie, C.; Huo, Y.; Zhang, F.; Chen, X.; Geng, Y.; Fang, R. Rice yellow stunt rhabdovirus protein 6 suppresses systemic RNA silencing by blocking RDR6-mediated secondary siRNA synthesis. Mol. Plant-Microbe Interact. 2013, 26, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kobori, T.; Kosaka, Y.; Natsuaki, T.; Masuta, C. Characterization of silencing suppressor 2b of cucumber mosaic virus based on examination of its small RNA-binding abilities. Plant Cell Physiol. 2007, 48, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, Y.R.; Pei, Y.; Lin, S.S.; Tuschl, T.; Patel, D.J.; Chua, N.H. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 2006, 20, 3255–3268. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, I.; Martinez, L.; Rakitina, D.V.; Lewsey, M.G.; Atencio, F.A.; Llave, C.; Kalinina, N.O.; Carr, J.P.; Palukaitis, P.; Canto, T. Cucumber mosaic virus 2b protein subcellular targets and interactions: Their significance to RNA silencing suppressor activity. Mol. Plant-Microbe Interact. 2010, 23, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.G.; Fang, Y.Y.; Zhou, B.J.; Zhao, J.H.; Hou, W.N.; Zhu, H.; Ding, S.W.; Guo, H.S. Suppression of Arabidopsis ARGONAUTE1-mediated slicing, transgene-induced RNA silencing, and DNA methylation by distinct domains of the Cucumber mosaic virus 2b protein. Plant Cell 2012, 24, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Hamera, S.; Song, X.; Su, L.; Chen, X.; Fang, R. Cucumber mosaic virus suppressor 2b binds to AGO4-related small RNAs and impairs AGO4 activities. Plant J. 2012, 69, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Varallyay, E.; Havelda, Z. Unrelated viral suppressors of RNA silencing mediate the control of ARGONAUTE1 level. Mol. Plant Pathol. 2013, 14, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Pendon, J.A.; Li, F.; Li, W.X.; Ding, S.W. Suppression of antiviral silencing by cucumber mosaic virus 2b protein in Arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs. Plant Cell 2007, 19, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Yang, J.; Lin, C.; Yuan, Y.A. Structural basis for RNA-silencing suppression by Tomato aspermy virus protein 2b. EMBO Rep. 2008, 9, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, W.J.; Kreuze, J.F.; Rajamaki, M.L.; Cruzado, K.R.; Untiveros, M.; Valkonen, J.P. Elimination of antiviral defense by viral RNase III. Proc. Natl. Acad. Sci. USA 2009, 106, 10354–10358. [Google Scholar] [CrossRef] [PubMed]
- Weinheimer, I.; Jiu, Y.; Rajamaki, M.L.; Matilainen, O.; Kallijarvi, J.; Cuellar, W.J.; Lu, R.; Saarma, M.; Holmberg, C.I.; Jantti, J.; et al. Suppression of RNAi by dsRNA-degrading RNaseIII enzymes of viruses in animals and plants. PLoS Pathog. 2015, 11, e1004711. [Google Scholar] [CrossRef] [PubMed]
- Merai, Z.; Kerenyi, Z.; Kertesz, S.; Magna, M.; Lakatos, L.; Silhavy, D. Double-stranded RNA binding may be a general plant RNA viral strategy to suppress RNA silencing. J. Virol. 2006, 80, 5747–5756. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, L.; Csorba, T.; Pantaleo, V.; Chapman, E.J.; Carrington, J.C.; Liu, Y.P.; Dolja, V.V.; Calvino, L.F.; Lopez-Moya, J.J.; Burgyan, J. Small RNA binding is a common strategy to suppress RNA silencing by several viral suppressors. EMBO J. 2006, 25, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.J.; Prokhnevsky, A.I.; Gopinath, K.; Dolja, V.V.; Carrington, J.C. Viral RNA silencing suppressors inhibit the microRNA pathway at an intermediate step. Genes Dev. 2004, 18, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Chapman, E.J.; Yang, Z.; Carrington, J.C.; Chen, X. Transgenically expressed viral RNA silencing suppressors interfere with microRNA methylation in Arabidopsis. FEBS Lett. 2006, 580, 3117–3120. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Folimonov, A.; Shintaku, M.; Li, W.X.; Falk, B.W.; Dawson, W.O.; Ding, S.W. Three distinct suppressors of RNA silencing encoded by a 20-kb viral RNA genome. Proc. Natl. Acad. Sci. USA 2004, 101, 15742–15747. [Google Scholar] [CrossRef] [PubMed]
- Fagoaga, C.; Pensabene-Bellavia, G.; Moreno, P.; Navarro, L.; Flores, R.; Pena, L. Ectopic expression of the p23 silencing suppressor of Citrus tristeza virus differentially modifies viral accumulation and tropism in two transgenic woody hosts. Mol. Plant Pathol. 2011, 12, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, A.F.; Correa, R.L.; Nakasugi, K.; Jackson, C.; Kawchuk, L.; Vaslin, M.F.; Waterhouse, P.M. The Enamovirus P0 protein is a silencing suppressor which inhibits local and systemic RNA silencing through AGO1 degradation. Virology 2012, 426, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.; Dunoyer, P.; Heim, F.; Richards, K.E.; Jonard, G.; Ziegler-Graff, V. P0 of beet Western yellows virus is a suppressor of posttranscriptional gene silencing. J. Virol. 2002, 76, 6815–6824. [Google Scholar] [CrossRef] [PubMed]
- Csorba, T.; Lozsa, R.; Hutvagner, G.; Burgyan, J. Polerovirus protein P0 prevents the assembly of small RNA-containing RISC complexes and leads to degradation of ARGONAUTE1. Plant J. 2010, 62, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Bortolamiol, D.; Pazhouhandeh, M.; Marrocco, K.; Genschik, P.; Ziegler-Graff, V. The Polerovirus F box protein P0 targets ARGONAUTE1 to suppress RNA silencing. Curr. Biol. 2007, 17, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, N.; Tsai, C.H.; Lie, M.; Havecker, E.; Baulcombe, D.C. The Polerovirus silencing suppressor P0 targets ARGONAUTE proteins for degradation. Curr. Biol. 2007, 17, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Derrien, B.; Baumberger, N.; Schepetilnikov, M.; Viotti, C.; De Cillia, J.; Ziegler-Graff, V.; Isono, E.; Schumacher, K.; Genschik, P. Degradation of the antiviral component ARGONAUTE1 by the autophagy pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 15942–15946. [Google Scholar] [CrossRef] [PubMed]
- Delfosse, V.C.; Agrofoglio, Y.C.; Casse, M.F.; Kresic, I.B.; Hopp, H.E.; Ziegler-Graff, V.; Distefano, A.J. The P0 protein encoded by cotton leafroll dwarf virus (CLRDV) inhibits local but not systemic RNA silencing. Virus Res. 2014, 180, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Pazhouhandeh, M.; Dieterle, M.; Marrocco, K.; Lechner, E.; Berry, B.; Brault, V.; Hemmer, O.; Kretsch, T.; Richards, K.E.; Genschik, P.; et al. F-box-like domain in the polerovirus protein P0 is required for silencing suppressor function. Proc. Natl. Acad. Sci. USA 2006, 103, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Mangwende, T.; Wang, M.L.; Borth, W.; Hu, J.; Moore, P.H.; Mirkov, T.E.; Albert, H.H. The P0 gene of Sugarcane yellow leaf virus encodes an RNA silencing suppressor with unique activities. Virology 2009, 384, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Giner, A.; Lakatos, L.; Garcia-Chapa, M.; Lopez-Moya, J.J.; Burgyan, J. Viral protein inhibits RISC activity by argonaute binding through conserved WG/GW motifs. PLoS Pathog. 2010, 6, e1000996. [Google Scholar] [CrossRef] [PubMed]
- Lozsa, R.; Csorba, T.; Lakatos, L.; Burgyan, J. Inhibition of 3' modification of small RNAs in virus-infected plants require spatial and temporal co-expression of small RNAs and viral silencing-suppressor proteins. Nucleic Acids Res. 2008, 36, 4099–4107. [Google Scholar] [CrossRef] [PubMed]
- Ebhardt, H.A.; Thi, E.P.; Wang, M.B.; Unrau, P.J. Extensive 3′ modification of plant small RNAs is modulated by helper component-proteinase expression. Proc. Natl. Acad. Sci. USA 2005, 102, 13398–13403. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.W.; Gregory, B.D.; Gao, Z.; Foreman, A.W.; Mlotshwa, S.; Ge, X.; Pruss, G.J.; Ecker, J.R.; Bowman, L.H.; Vance, V. Two plant viral suppressors of silencing require the ethylene-inducible host transcription factor RAV2 to block RNA silencing. PLoS Pathog. 2010, 6, e1000729. [Google Scholar] [CrossRef] [PubMed]
- Kasschau, K.D.; Xie, Z.; Allen, E.; Llave, C.; Chapman, E.J.; Krizan, K.A.; Carrington, J.C. P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA unction. Dev. Cell 2003, 4, 205–217. [Google Scholar] [CrossRef]
- Jamous, R.M.; Boonrod, K.; Fuellgrabe, M.W.; Ali-Shtayeh, M.S.; Krczal, G.; Wassenegger, M. The helper component-proteinase of the Zucchini yellow mosaic virus inhibits the Hua Enhancer 1 methyltransferase activity in vitro. J. Gen. Virol. 2011, 92, 2222–2226. [Google Scholar] [CrossRef] [PubMed]
- Shiboleth, Y.M.; Haronsky, E.; Leibman, D.; Arazi, T.; Wassenegger, M.; Whitham, S.A.; Gaba, V.; Gal-On, A. The conserved FRNK box in HC-Pro, a plant viral suppressor of gene silencing, is required for small RNA binding and mediates symptom development. J. Virol. 2007, 81, 13135–13148. [Google Scholar] [CrossRef] [PubMed]
- Fuellgrabe, M.W.; Boonrod, K.; Jamous, R.; Moser, M.; Shiboleth, Y.; Krczal, G.; Wassenegger, M. Expression, purification and functional characterization of recombinant Zucchini yellow mosaic virus HC-Pro. Protein Expr. Purif. 2011, 75, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Merai, Z.; Kerenyi, Z.; Molnar, A.; Barta, E.; Valoczi, A.; Bisztray, G.; Havelda, Z.; Burgyan, J.; Silhavy, D. Aureusvirus P14 is an efficient RNA silencing suppressor that binds double-stranded RNAs without size specificity. J. Virol. 2005, 79, 7217–7226. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.; Garcia, D.; Pontier, D.; Ohnesorge, S.; Yu, A.; Garcia, S.; Braun, L.; Bergdoll, M.; Hakimi, M.A.; Lagrange, T.; et al. Argonaute quenching and global changes in Dicer homeostasis caused by a pathogen-encoded GW repeat protein. Genes Dev. 2010, 24, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Vargason, J.M.; Szittya, G.; Burgyan, J.; Hall, T.M. Size selective recognition of siRNA by an RNA silencing suppressor. Cell 2003, 115, 799–811. [Google Scholar] [CrossRef]
- Rawlings, R.A.; Krishnan, V.; Walter, N.G. Viral RNAi suppressor reversibly binds siRNA to outcompete Dicer and RISC via multiple turnover. J. Mol. Biol. 2011, 408, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Varallyay, E.; Olah, E.; Havelda, Z. Independent parallel functions of p19 plant viral suppressor of RNA silencing required for effective suppressor activity. Nucleic Acids Res. 2014, 42, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, D.; Molnar, A.; Lucioli, A.; Szittya, G.; Hornyik, C.; Tavazza, M.; Burgyan, J. A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J. 2002, 21, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Varallyay, E.; Valoczi, A.; Agyi, A.; Burgyan, J.; Havelda, Z. Plant virus-mediated induction of miR168 is associated with repression of ARGONAUTE1 accumulation. EMBO J. 2010, 29, 3507–3519. [Google Scholar] [CrossRef] [PubMed]
- Csorba, T.; Bovi, A.; Dalmay, T.; Burgyan, J. The p122 subunit of Tobacco Mosaic Virus replicase is a potent silencing suppressor and compromises both small interfering RNA- and microRNA-mediated pathways. J. Virol. 2007, 81, 11768–11780. [Google Scholar] [CrossRef] [PubMed]
- Vogler, H.; Akbergenov, R.; Shivaprasad, P.V.; Dang, V.; Fasler, M.; Kwon, M.O.; Zhanybekova, S.; Hohn, T.; Heinlein, M. Modification of small RNAs associated with suppression of RNA silencing by tobamovirus replicase protein. J. Virol. 2007, 81, 10379–10388. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Lin, S.S.; Hung, T.H.; Li, T.K.; Lin, N.C.; Shen, T.L. Multiple Domains of the Tobacco mosaic virus p126 Protein Can Independently Suppress Local and Systemic RNA Silencing. Mol. Plant-Microbe Interact. 2012, 25, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Haas, G.; Azevedo, J.; Moissiard, G.; Geldreich, A.; Himber, C.; Bureau, M.; Fukuhara, T.; Keller, M.; Voinnet, O. Nuclear import of CaMV P6 is required for infection and suppression of the RNA silencing factor DRB4. EMBO J. 2008, 27, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.; McInally, C.; Carr, C.; Doddiah, S.; Yates, G.; Chrysanthou, E.; Khattab, A.; Love, A.J.; Geri, C.; Sadanandom, A.; et al. Identification of the domains of cauliflower mosaic virus protein P6 responsible for suppression of RNA silencing and salicylic acid signalling. J. Gen. Virol. 2013, 94, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, W.X.; Ding, S.W. Induction and suppression of RNA silencing by an animal virus. Science 2002, 296, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Ding, S.W. Antiviral silencing in animals. FEBS Lett 2005, 579, 5965–5973. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Billecocq, A.; Crance, J.M.; Munderloh, U.; Garin, D.; Bouloy, M. Nairovirus RNA sequences expressed by a Semliki Forest virus replicon induce RNA interference in tick cells. J. Virol. 2005, 79, 8942–8947. [Google Scholar] [CrossRef] [PubMed]
- Karlikow, M.; Goic, B.; Saleh, M.C. RNAi and antiviral defense in Drosophila: Setting up a systemic immune response. Dev. Comp. Immunol. 2014, 42, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Ishizuka, A.; Siomi, H.; Siomi, M.C. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004, 18, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Rand, T.A.; Ginalski, K.; Grishin, N.V.; Wang, X. Biochemical identification of Argonaute 2 as the sole protein required for RNA-induced silencing complex activity. Proc. Natl. Acad. Sci. USA 2004, 101, 14385–14389. [Google Scholar] [CrossRef] [PubMed]
- Rand, T.A.; Petersen, S.; Du, F.H.; Wang, X.D. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell 2005, 123, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, A.W.; van Rij, R.P. The long and short of antiviral defense: Small RNA-based immunity in insects. Curr. Opin. Virol. 2014, 7, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Czech, B.; Hannon, G.J. Small RNA sorting: Matchmaking for Argonautes. Nat. Rev. Genet. 2011, 12, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Weng, K.F.; Shih, S.R.; Brewer, G. The evolving world of small RNAs from RNA viruses. Wiley Interdiscip. Rev. RNA 2016. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.A.; Lee, J.H.; Chapados, B.R.; Debler, E.W.; Schneemann, A.; Williamson, J.R. Dual modes of RNA-silencing suppression by Flock House virus protein B2. Nat. Struct Mol. Biol. 2005, 12, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Popli, S.; Hari, Y.; Malhotra, P.; Mukherjee, S.; Bhatnagar, R.K. Suppression of RNA silencing by Flock house virus B2 protein is mediated through its interaction with the PAZ domain of Dicer. FASEB J. 2009, 23, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Gammon, D.B.; Mello, C.C. RNA interference-mediated antiviral defense in insects. Curr. Opin. Insect Sci. 2015, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Berry, B.; Tassetto, M.; Kunitomi, M.; Acevedo, A.; Deng, C.; Krutchinsky, A.; Gross, J.; Antoniewski, C.; Andino, R. Cricket paralysis virus antagonizes Argonaute 2 to modulate antiviral defense in Drosophila. Nat. Struct. Mol. Biol. 2010, 17, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Van Rij, R.P.; Saleh, M.C.; Berry, B.; Foo, C.; Houk, A.; Antoniewski, C.; Andino, R. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006, 20, 2985–2995. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.M.; Banerjee, N. Mechanisms of arthropod transmission of plant and animal viruses. Microbiol Mol. Biol. Rev. 1999, 63, 128–148. [Google Scholar] [PubMed]
- Hogenhout, S.A.; Ammar el, D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.W.; Kantor, A.M.; Passarelli, A.L.; Clem, R.J. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Chen, H.; Liu, Y.; Jiang, C.; Mao, Q.; Jia, D.; Chen, Q.; Wei, T. Small Interfering RNA Pathway Modulates Initial Viral Infection in Midgut Epithelium of Insect after Ingestion of Virus. J. Virol. 2015, 90, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.C.; Piper, J.; Sanchez-Vargas, I.; Olson, K.E.; Franz, A.W. The RNA interference pathway affects midgut infection- and escape barriers for Sindbis virus in Aedes aegypti. BMC Microbiol. 2010, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Carissimo, G.; Pondeville, E.; McFarlane, M.; Dietrich, I.; Mitri, C.; Bischoff, E.; Antoniewski, C.; Bourgouin, C.; Failloux, A.B.; Kohl, A.; et al. Antiviral immunity of Anopheles gambiae is highly compartmentalized, with distinct roles for RNA interference and gut microbiota. Proc. Natl. Acad. Sci. USA 2015, 112, E176–E185. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.D. Mosquito RNAi is the major innate immune pathway controlling arbovirus infection and transmission. Future Microbiol. 2011, 6, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Cirimotich, C.M.; Scott, J.C.; Phillips, A.T.; Geiss, B.J.; Olson, K.E. Suppression of RNA interference increases alphavirus replication and virus-associated mortality in Aedes aegypti mosquitoes. BMC Microbiol. 2009, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.D.; Roby, J.A.; Slonchak, A.; Khromykh, A.A. Functional non-coding RNAs derived from the flavivirus 3′ untranslated region. Virus Res. 2015, 206, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Siu, R.W.; Fragkoudis, R.; Simmonds, P.; Donald, C.L.; Chase-Topping, M.E.; Barry, G.; Attarzadeh-Yazdi, G.; Rodriguez-Andres, J.; Nash, A.A.; Merits, A.; et al. Antiviral RNA interference responses induced by Semliki Forest virus infection of mosquito cells: Characterization, origin, and frequency-dependent functions of virus-derived small interfering RNAs. J. Virol. 2011, 85, 2907–2917. [Google Scholar] [CrossRef] [PubMed]
- Schnettler, E.; Ratinier, M.; Watson, M.; Shaw, A.E.; McFarlane, M.; Varela, M.; Elliott, R.M.; Palmarini, M.; Kohl, A. RNA interference targets arbovirus replication in Culicoides cells. J. Virol. 2013, 87, 2441–2454. [Google Scholar] [CrossRef] [PubMed]
- Sabin, L.R.; Zheng, Q.; Thekkat, P.; Yang, J.; Hannon, G.J.; Gregory, B.D.; Tudor, M.; Cherry, S. Dicer-2 Processes Diverse Viral RNA Species. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Leger, P.; Lara, E.; Jagla, B.; Sismeiro, O.; Mansuroglu, Z.; Coppee, J.Y.; Bonnefoy, E.; Bouloy, M. Dicer-2- and Piwi-mediated RNA interference in Rift Valley fever virus-infected mosquito cells. J. Virol. 2013, 87, 1631–1648. [Google Scholar] [CrossRef] [PubMed]
- Blakqori, G.; Delhaye, S.; Habjan, M.; Blair, C.D.; Sanchez-Vargas, I.; Olson, K.E.; Attarzadeh-Yazdi, G.; Fragkoudis, R.; Kohl, A.; Kalinke, U.; et al. La Crosse bunyavirus nonstructural protein NSs serves to suppress the type I interferon system of mammalian hosts. J. Virol. 2007, 81, 4991–4999. [Google Scholar] [CrossRef] [PubMed]
- Brackney, D.E.; Scott, J.C.; Sagawa, F.; Woodward, J.E.; Miller, N.A.; Schilkey, F.D.; Mudge, J.; Wilusz, J.; Olson, K.E.; Blair, C.D.; et al. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl. Trop. Dis. 2010, 4, e856. [Google Scholar] [CrossRef] [PubMed]
- Hedil, M.; Hassani-Mehraban, A.; Lohuis, D.; Kormelink, R. Analysis of the A-U rich hairpin from the intergenic region of tospovirus S RNA as target and inducer of RNA silencing. PLoS ONE 2014, 9, e106027. [Google Scholar] [CrossRef] [PubMed]
- Margaria, P.; Miozzi, L.; Rosa, C.; Axtell, M.J.; Pappu, H.R.; Turina, M. Small RNA profiles of wild-type and silencing suppressor-deficient tomato spotted wilt virus infected Nicotiana benthamiana. Virus Res. 2015, 208, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Koundal, V.; Williams, S.; Pappu, H. Differential expression of tomato spotted wilt virus-derived viral small RNAs in infected commercial and experimental host plants. PLoS ONE 2013, 8, e76276. [Google Scholar] [CrossRef] [PubMed]
- Margaria, P.; Miozzi, L.; Ciuffo, M.; Rosa, C.; Axtell, M.J.; Pappu, H.R.; Turina, M. Comparison of small RNA profiles in Nicotiana benthamiana and Solanum lycopersicum infected by polygonum ringspot tospovirus reveals host-specific responses to viral infection. Virus Res. 2015, 211, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kormelink, R.; Kitajima, E.W.; De Haan, P.; Zuidema, D.; Peters, D.; Goldbach, R. The nonstructural protein (NSs) encoded by the ambisense S RNA segment of tomato spotted wilt virus is associated with fibrous structures in infected plant cells. Virology 1991, 181, 459–468. [Google Scholar] [CrossRef]
- Hedil, M.; de Ronde, D.; Kormelink, R. Biochemical analysis of NSs from different tospoviruses. Manuscript in preparation. NSs of TSWV, GRSV and TYRV biochemically analysed using electrophoretic mobility shift assays (EMSA) to investigate their affinity to small and long dsRNA.
- Vaucheret, H.; Vazquez, F.; Crete, P.; Bartel, D.P. The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev. 2004, 18, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Moffett, P. Mechanisms of recognition in dominant R gene mediated resistance. Adv. Virus Res. 2009, 75, 1–33. [Google Scholar] [PubMed]
- De Ronde, D.; Butterbach, P.; Kormelink, R. Dominant resistance against plant viruses. Front. Plant Sci. 2014, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Qu, F.; Ren, T.; Ye, X.; Morris, T.J. RNA silencing-suppressor function of Turnip crinkle virus coat protein cannot be attributed to its interaction with the Arabidopsis protein TIP. J. Gen. Virol. 2004, 85, 3415–3420. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.H.; Khatabi, B.; Ashfield, T.; Saghai Maroof, M.A.; Hajimorad, M.R. The HC-Pro and P3 cistrons of an avirulent Soybean mosaic virus are recognized by different resistance genes at the complex Rsv1 locus. Mol. Plant-Microbe Interact. 2013, 26, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Angel, C.A.; Hsieh, Y.C.; Schoelz, J.E. Comparative analysis of the capacity of tombusvirus P22 and P19 proteins to function as avirulence determinants in Nicotiana species. Mol. Plant-Microbe Interact. 2011, 24, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.P.; Valkonen, J.P. Genetic determinants of Potato virus Y required to overcome or trigger hypersensitive resistance to PVY strain group O controlled by the gene Ny in potato. Mol. Plant-Microbe Interact. 2013, 26, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, A.S.; Pooggin, M.M. Silencing and Innate Immunity in Plant Defense Against Viral and Non-Viral Pathogens. Viruses 2012, 4, 2578–2597. [Google Scholar] [CrossRef] [PubMed]
- De Ronde, D.; Butterbach, P.; Lohuis, D.; Hedil, M.; van Lent, J.W.; Kormelink, R. Tsw gene-based resistance is triggered by a functional RNA silencing suppressor protein of the Tomato spotted wilt virus. Mol. Plant Pathol. 2013, 14, 405–415. [Google Scholar] [CrossRef] [PubMed]
- De Ronde, D.; Pasquier, A.; Ying, S.; Butterbach, P.; Lohuis, D.; Kormelink, R. Analysis of Tomato spotted wilt virus NSs protein indicates the importance of the N-terminal domain for avirulence and RNA silencing suppression. Mol. Plant Pathol. 2014, 15, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.W. RNA-based antiviral immunity. Nat. Rev. Immunol. 2010, 10, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Vargas, I.E.; Rotenberg, D.; Schneweis, B.A.; Whitfield, A.E. RNA interference tools for the western flower thrips, Frankliniella occidentalis. J. Insect Physiol. 2015, 76, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Whitten, M.M.; Facey, P.D.; Del Sol, R.; Fernandez-Martinez, L.T.; Evans, M.C.; Mitchell, J.J.; Bodger, O.G.; Dyson, P.J. Symbiont-mediated RNA interference in insects. Proc. Biol. Sci. 2016, 283, 20160042. [Google Scholar] [CrossRef] [PubMed]
- Ullman, D.E.; German, T.L.; Sherwood, J.L.; Westcot, D.M.; Cantone, F.A. Tospovirus replication in insect vector cells: Immunocytochemical evidence that the nonstructural protein encoded by the S RNA of tomato spotted wilt tospovirus is present in thrips vector cells. Phytopathology 1993, 83, 456–463. [Google Scholar] [CrossRef]
- Ullman, D.E.; Westcot, D.M.; Chenault, K.D.; Sherwood, J.L.; German, T.L.; Bandla, M.D.; Cantone, F.A.; Duer, H.L. Compartmentalization, Intracellular Transport, and Autophagy of Tomato Spotted Wilt Tospovirus Proteins in Infected Thrips Cells. Phytopathology 1995, 85, 644–654. [Google Scholar] [CrossRef]
- Wijkamp, I.; van Lent, J.; Kormelink, R.; Goldbach, R.; Peters, D. Multiplication of tomato spotted wilt virus in its insect vector, Frankliniella occidentalis. J. Gen. Virol. 1993, 74 (Pt. 3), 341–349. [Google Scholar] [CrossRef] [PubMed]
- Margaria, P.; Bosco, L.; Vallino, M.; Ciuffo, M.; Mautino, G.C.; Tavella, L.; Turina, M. The NSs protein of tomato spotted wilt virus is required for persistent infection and transmission by Frankliniella occidentalis. J. Virol. 2014, 88, 5788–5802. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Billecocq, A.; Crance, J.M.; Prins, M.; Garin, D.; Bouloy, M. Viral suppressors of RNA interference impair RNA silencing induced by a Semliki Forest virus replicon in tick cells. J. Gen. Virol. 2006, 87, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.C.; Bartasson, L.; de Castro, M.E.; Correa, J.R.; Ribeiro, B.M.; Resende, R.O. A silencing suppressor protein (NSs) of a tospovirus enhances baculovirus replication in permissive and semipermissive insect cell lines. Virus Res. 2011, 155, 259–267. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, V.C.; da Silva Morgado, F.; Ardisson-Araujo, D.M.; Resende, R.O.; Ribeiro, B.M. The silencing suppressor (NSs) protein of the plant virus Tomato spotted wilt virus enhances heterologous protein expression and baculovirus pathogenicity in cells and lepidopteran insects. Arch. Virol. 2015, 160, 2873–2879. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.B.; Resende Rde, O.; de Avila, A.C. The plant virus Tomato Spotted Wilt Tospovirus activates the immune system of its main insect vector, Frankliniella occidentalis. J. Virol. 2004, 78, 4976–4982. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.H.; Schneider, W.M.; Rice, C.M. Interferons and viruses: An evolutionary arms race of molecular interactions. Trends Immunol. 2015, 36, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Billecocq, A.; Spiegel, M.; Vialat, P.; Kohl, A.; Weber, F.; Bouloy, M.; Haller, O. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J. Virol. 2004, 78, 9798–9806. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.; Bridgen, A.; Fazakerley, J.K.; Streitenfeld, H.; Kessler, N.; Randall, R.E.; Elliott, R.M. Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J. Virol. 2002, 76, 7949–7955. [Google Scholar] [CrossRef] [PubMed]
- Hollidge, B.S.; Weiss, S.R.; Soldan, S.S. The role of interferon antagonist, non-structural proteins in the pathogenesis and emergence of arboviruses. Viruses 2011, 3, 629–658. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Blakqori, G.; Wagner, V.; Banholzer, M.; Kessler, N.; Elliott, R.M.; Haller, O.; Weber, F. Inhibition of RNA polymerase II phosphorylation by a viral interferon antagonist. J. Biol. Chem. 2004, 279, 31471–31477. [Google Scholar] [CrossRef] [PubMed]
- Habjan, M.; Pichlmair, A.; Elliott, R.M.; Overby, A.K.; Glatter, T.; Gstaiger, M.; Superti-Furga, G.; Unger, H.; Weber, F. NSs protein of rift valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J. Virol. 2009, 83, 4365–4375. [Google Scholar] [CrossRef] [PubMed]
- Kalveram, B.; Lihoradova, O.; Ikegami, T. NSs protein of rift valley fever virus promotes posttranslational downregulation of the TFIIH subunit p62. J. Virol. 2011, 85, 6234–6243. [Google Scholar] [CrossRef] [PubMed]
- Rezelj, V.V.; Overby, A.K.; Elliott, R.M. Generation of mutant Uukuniemi viruses lacking the nonstructural protein NSs by reverse genetics indicates that NSs is a weak interferon antagonist. J. Virol. 2015, 89, 4849–4856. [Google Scholar] [CrossRef] [PubMed]
- Santiago, F.W.; Covaleda, L.M.; Sanchez-Aparicio, M.T.; Silvas, J.A.; Diaz-Vizarreta, A.C.; Patel, J.R.; Popov, V.; Yu, X.J.; Garcia-Sastre, A.; Aguilar, P.V. Hijacking of RIG-I signaling proteins into virus-induced cytoplasmic structures correlates with the inhibition of type I interferon responses. J. Virol. 2014, 88, 4572–4585. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.J.; Wang, M.; Deng, M.; Shen, S.; Liu, W.; Cao, W.C.; Deng, F.; Wang, Y.Y.; Hu, Z.; Wang, H. Viral suppression of innate immunity via spatial isolation of TBK1/IKKepsilon from mitochondrial antiviral platform. J. Mol. Cell Biol. 2014, 6, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Zhang, S.; Yuen, K.S.; Li, C.; Lui, P.Y.; Fung, S.Y.; Wang, P.H.; Chan, C.P.; Li, D.; Kok, K.H.; et al. Suppression of type I and type III interferon signalling by NSs protein of severe fever-with-thrombocytopenia syndrome virus through inhibition of STAT1 phosphorylation and activation. J. Gen. Virol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.J.; Feng, K.; Min, Y.Q.; Cao, W.C.; Wang, M.; Deng, F.; Hu, Z.; Wang, H. Disruption of type I interferon signaling by the nonstructural protein of severe fever with thrombocytopenia syndrome virus via the hijacking of STAT2 and STAT1 into inclusion bodies. J. Virol. 2015, 89, 4227–4236. [Google Scholar] [CrossRef] [PubMed]
- Wuerth, J.D.; Weber, F. Phleboviruses and the Type I Interferon Response. Viruses 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.T.; Barr, J.N. Recent advances in the molecular and cellular biology of bunyaviruses. J. Gen. Virol. 2011, 92, 2467–2484. [Google Scholar] [CrossRef] [PubMed]
- Barnwal, B.; Karlberg, H.; Mirazimi, A.; Tan, Y.J. The Non-structural Protein of Crimean-Congo Hemorrhagic Fever Virus Disrupts the Mitochondrial Membrane Potential and Induces Apoptosis. J. Biol. Chem. 2016, 291, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Bivalkar-Mehla, S.; Vakharia, J.; Mehla, R.; Abreha, M.; Kanwar, J.R.; Tikoo, A.; Chauhan, A. Viral RNA silencing suppressors (RSS): Novel strategy of viruses to ablate the host RNA interference (RNAi) defense system. Virus Res. 2011, 155, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Soldan, S.S.; Plassmeyer, M.L.; Matukonis, M.K.; Gonzalez-Scarano, F. La Crosse virus nonstructural protein NSs counteracts the effects of short interfering RNA. J. Virol. 2005, 79, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Horne, K.M.; Vanlandingham, D.L. Bunyavirus-vector interactions. Viruses 2014, 6, 4373–4397. [Google Scholar] [CrossRef] [PubMed]
- Szemiel, A.M.; Failloux, A.B.; Elliott, R.M. Role of Bunyamwera Orthobunyavirus NSs protein in infection of mosquito cells. PLoS Negl. Trop. Dis. 2012, 6, e1823. [Google Scholar] [CrossRef] [PubMed]
- Brackney, D.E.; Beane, J.E.; Ebel, G.D. RNAi targeting of West Nile virus in mosquito midguts promotes virus diversification. PLoS Pathog. 2009, 5, e1000502. [Google Scholar] [CrossRef] [PubMed]
- Schnettler, E.; Hemmes, H.; Goldbach, R.; Prins, M. The NS3 protein of rice hoja blanca virus suppresses RNA silencing in mammalian cells. J. Gen. Virol. 2008, 89, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Li, H.; Lu, R.; Li, F.; Dus, M.; Atkinson, P.; Brydon, E.W.; Johnson, K.L.; Garcia-Sastre, A.; Ball, L.A.; et al. Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing. Proc. Natl. Acad. Sci. USA 2004, 101, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Behlke, M.A.; Rose, S.D.; Chang, M.S.; Choi, S.; Rossi, J.J. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat. Biotechnol. 2005, 23, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Siolas, D.; Lerner, C.; Burchard, J.; Ge, W.; Linsley, P.S.; Paddison, P.J.; Hannon, G.J.; Cleary, M.A. Synthetic shRNAs as potent RNAi triggers. Nat. Biotechnol. 2005, 23, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.R.; Cherry, S.; tenOever, B.R. Is RNA interference a physiologically relevant innate antiviral immune response in mammals? Cell Host Microbe 2013, 14, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Shi, M.; Tian, J.H.; Lin, X.D.; Kang, Y.J.; Chen, L.J.; Qin, X.C.; Xu, J.; Holmes, E.C.; Zhang, Y.Z. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.G.; Weaver, S.C.; Tesh, R.B.; Vasilakis, N. Insect-specific virus discovery: Significance for the arbovirus community. Viruses 2015, 7, 4911–4928. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.J.; Carrington, C.V.; Forrester, N.L.; Popov, V.L.; Guzman, H.; Widen, S.G.; Wood, T.G.; Weaver, S.C.; Tesh, R.B. Characterization of a novel Negevirus and a novel Bunyavirus isolated from Culex (Culex) declarator mosquitoes in Trinidad. J. Gen. Virol. 2014, 95, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Marklewitz, M.; Handrick, S.; Grasse, W.; Kurth, A.; Lukashev, A.; Drosten, C.; Ellerbrok, H.; Leendertz, F.H.; Pauli, G.; Junglen, S. Gouleako virus isolated from West African mosquitoes constitutes a proposed novel genus in the family Bunyaviridae. J. Virol. 2011, 85, 9227–9234. [Google Scholar] [CrossRef] [PubMed]
- Marklewitz, M.; Zirkel, F.; Rwego, I.B.; Heidemann, H.; Trippner, P.; Kurth, A.; Kallies, R.; Briese, T.; Lipkin, W.I.; Drosten, C.; et al. Discovery of a unique novel clade of mosquito-associated bunyaviruses. J. Virol. 2013, 87, 12850–12865. [Google Scholar] [CrossRef] [PubMed]
- Marklewitz, M.; Zirkel, F.; Kurth, A.; Drosten, C.; Junglen, S. Evolutionary and phenotypic analysis of live virus isolates suggests arthropod origin of a pathogenic RNA virus family. Proc. Natl. Acad. Sci. USA 2015, 112, 7536–7541. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).