Abstract
The whitefly Bemisia tabaci is a major pest to agricultural crops. It transmits begomoviruses, such as Tomato yellow leaf curl virus (TYLCV), in a circular, persistent fashion. Transcriptome analyses revealed that B. tabaci knottin genes were responsive to various stresses. Upon ingestion of tomato begomoviruses, two of the four knottin genes were upregulated, knot-1 (with the highest expression) and knot-3. In this study, we examined the involvement of B. tabaci knottin genes in relation to TYLCV circulative transmission. Knottins were silenced by feeding whiteflies with knottin dsRNA via detached tomato leaves. Large amounts of knot-1 transcripts were present in the abdomen of whiteflies, an obligatory transit site of begomoviruses in their circulative transmission pathway; knot-1 silencing significantly depleted the abdomen from knot-1 transcripts. Knot-1 silencing led to an increase in the amounts of TYLCV ingested by the insects and transmitted to tomato test plants by several orders of magnitude. This effect was not observed following knot-3 silencing. Hence, knot-1 plays a role in restricting the quantity of virions an insect may acquire and transmit. We suggest that knot-1 protects B. tabaci against deleterious effects caused by TYLCV by limiting the amount of virus associated with the whitefly vector.
1. Introduction
The whitefly Bemisia tabaci is a major agricultural pest worldwide, damaging plants by sucking their phloem and by transmitting many viruses [1]. Among them, the begomoviruses (genus Begomovirus, family Geminiviridae) are the most harmful. Begomoviruses are characterized by a 22 × 38 nm geminate particle. Their genome consists of one (monopartite) or two (bipartite) circular ssDNA components of approximately 2800 nucleotides each. Tomato yellow leaf curl virus (TYLCV) is one of the most destructive monopartite begomoviruses [2]. The TYLCV complex includes several species and numerous isolates [3]. The virion-sense strand comprises two genes, while the complementary-sense strand comprises four genes. The role of the encoded proteins was summarized elsewhere [4]. The TYLCV disease is managed by frequent applications of insecticides to contain the whitefly populations in the field and greenhouses, and by breeding resistant cultivars [2].
B. tabaci transmits begomoviruses in a circular, persistent fashion. Once ingested during feeding on the phloem of infected plant, virions pass along the food canal in the stylet and reach the esophagus and the gut. Virions can cross to the haemolymph via the filter chamber and the midgut [5]. From there virions reach the salivary glands [6] and are transmitted to plants with the saliva. In many reported cases, but not all (e.g., [7]), begomoviruses are deleterious to their whitefly host, reducing their longevity and fertility [8,9,10]. Examination of the transcriptome of B. tabaci provided an insight into the genes responsive to various stresses [11,12,13]. Knottins were among the genes identified upon ingestion of TYLCV [11,14] and bacteria [15], and during parasitization by the wasp Eretmocerus mundus [12]. Knottins are 59 to 65 amino acids proteins named as such because the three disulphide bonds in their sequences form a three-dimensional knot-like structure [16]. Knottins have been described in arthropods, mollusks, plants and mammals, where they function as antimicrobial peptides, toxins, or insecticides [17].
During the last decade, post-transcriptional gene silencing (PTGS) or RNA interference (RNAi) has proven to be an exquisite tool for deciphering gene function in insects [18,19,20]. The factors that determine RNAi stability and efficacy have been examined [21,22,23]. In the first study, involving B. tabaci, we demonstrated that by injecting into the body cavity dsRNA directed against genes uniquely expressed in the midgut and salivary glands, the amounts of targeted mRNA in the different organs were depleted by up to 70% [24]. To overcome the complications inherent to microinjecting such as traumatism and high mortality [24], we have developed a high throughput procedure to silence whitefly genes using a leaf-mediated dsRNA feeding approach [25]. This method was applied to explore the roles of genes within the molting hormone ecdysone synthesis and signaling pathway. Gene silencing reduced survival and delayed development of the whitefly nymphal stages [25], demonstrating that disruption of whitefly gene expression may help control the deleterious effects of this insect.
In this work, we applied the leaf-mediated silencing approach to investigate the function of knottin genes in the interaction between TYLCV and its whitefly vector. Previous analyses of cDNA libraries of non-viruliferous whiteflies and of whiteflies that fed on tomato plants infected with the begomoviruses TYLCV and Tomato mottle virus revealed a family of four knottin-like genes, coined knot-1 to -4. Knot-1 and knot-3 were more abundant in the cDNA libraries from viruliferous than from non-viruliferous whiteflies [11,14]. Therefore, we investigated the role of these genes in B. tabaci ingestion and transmission of TYLCV. We found that silencing knot-1 increased by several orders of magnitude the quantity of virus acquired by the insects and transmitted to tomato test plants. Hence, it seems that knottin-1 is involved in restraining the amount of TYLCV particles acquired by B. tabaci during feeding and during the circulation of the virus in the insect body.
2. Materials and Methods
2.1. Plants and Whiteflies
Tomato plants (Solanum lycopersicum cv. Daniella) were grown in a greenhouse under controlled conditions. Whiteflies (Bemisia tabaci Middle East-Asia Minor 1, previously known as “B biotype” [26], were maintained on tomato plants (cv. Daniella) in insect-proof wood-framed cages in a climate-controlled room (14 h/8 h light/dark, 24–27 °C). Whitefly adults, seven days after emergence, were used to inoculate TYLCV tomato plants (cv. Daniella) at the 5–6 true leaf stage.
2.2. Isolation of RNA from B. tabaci, Preparation of cDNA and Quantitative RT-PCR (qPCR)
Total RNA was extracted from whiteflies using the TRI-reagent method (Merck, Grand Island, NU, USA) and quantified with a NanoDrop spectrophotometer (Thermo Fischer Scientific, Wilmington, DE, USA). RNA quality was appraised by subjecting the RNA samples to gel electrophoresis to verify rRNAs integrity. RNA was reverse transcribed using the EZ-First Strand cDNA synthesis Kit (Biological Industries, Bet-Haemek, Israel) with oligo-dT primers in a total reaction volume of 20 µL. The amount of transcripts was appraised by qPCR in three technical replicates for each of three biologically independent experiments. qRT-PCR was performed using the Rotor-Gene 6000 machine (Corbett Robotics Pty Ltd., Brisbane, Australia) with SYBR-Green detection (SYBR® Premix Ex TaqTMII, Takara, Kyoto, Japan). The accompanying software (Rotor Gene Q-Series Software V2.0) was used for qPCR data normalization and quantification. The whitefly β-actin gene was used as a calibrator gene (see primers used below). Similar levels of β-actin transcripts were obtained in both the dsRNA-treated and control whiteflies (data not shown), indicating that the expression of β-actin was not affected by the dsRNA treatment. The expression of each gene was tested in two technical replicates for each of three biologically independent experiments. The ∆∆Ct method for was applied for relative quantifications. All the primers generated a single peak in the real-time dissociation analysis and had the amplification efficiencies ranging from 0.95 to 1.0.
2.3. Isolation of DNA from B. tabaci and Tomato Plants; Measurement of TYLCV Amounts
Adult whiteflies were collected using an aspirator and stored at −80 °C until DNA extraction. DNA from groups of 25 insects was extracted using CTAB [27], including RNase treatment. Young leaves were collected from plants and DNA was extracted using the Wizard® genomic DNA purification Kit (Promega, Madison, WI, USA) following the manufacturer’s instructions. DNA was dissolved in 100 µL Rehydration buffer and its quality was appraised following agarose gel electrophoresis. The DNA concentrations were measured using a NanoDrop spectrophotometer. The amounts of TYLCV were measured by qPCR using TYLCV CP-specific primers in the presence of SYBR Green I (Takara, Kyoto, Japan) in a Corbett Research Rotor-Gene 6000 cycler. The reaction was as follows: 30 s at 94 °C followed by 40 cycles consisting of 10 s at 94 °C, 30 s at 59 °C, and 20 s at 72 °C. The primers used to amplify a 200 bp fragment of TYLCV were sense: 5′-GAAGCGACCAGGCGATATAA-3′ and anti-sense: 5′-GGAACATCAGG GCTTCGATA-3′. A 60 bp fragment of the tomato β-actin gene served as an internal reference; it was amplified using the primer pair: sense: 5′-TGGAGGA TCCATCCTTGCATCAC-3′ and complementary sense: 5′-TCGCCCTTTGAAAT CCACATCTGC-3′. A 200 bp fragment of the whitefly β-actin gene also served as an internal reference; it was amplified using the following primer pair: sense: 5′-TCTTCCAGCCATCCTTCTTG-3′ and complementary sense: 5′-CGGTGATTTC CTTCTGCATT-3′. For absolute quantification, standard curves were generated using ten-fold dilutions of a CP-carrying plasmid of known size and concentration.
2.4. dsRNA Synthesis
Primers for the synthesis of double stranded RNA were designed based on the sequences of the relevant genes (Figure 1). dsRNAs were synthesized using the AmpliScribe™ T7-Flash™ Transcription Kit (Epicentre Biotechnologies, Madison, WI, USA) as described previously [24]. The dsRNA obtained was diluted with nuclease-free water to a final concentration of 0.5 µg/µL. The dsRNA purity and quality was determined by agarose gel electrophoresis.

Figure 1.
Sequences of whitefly knottin genes. Sequences retrieved from GenBank. The whitefly tropomyosin gene served as control for gene silencing. The primers used to amplify the gene fragment used for dsRNA synthesis (green) and for measuring expressing by qPCR (yellow) are underlined.
To follow its path in leaf and insect, the dsRNA was Cy-labeled as follows. The candidate gene was subjected to PCR using specific primers conjugated to 27 bases of the T7 RNA polymerase promoter. PCR products were then purified (QIAquick PCR Purification Kit, Qiagen, Hilden, Germany) and used as template for dsRNA in vitro synthesis using the Ampliscribe T7 RNA transcription kit as above, and a fluorescent ribonucleotide (dCTP-cy3 at 2 mM with unlabeled 8 mM dCTP) for generating fluorescently labeled dsRNA molecules. The dsRNA was purified (RNeasy Mini Kit, Qiagen) and quantified using a spectrophotometer. Leaflets were soaked in tubes containing 0.5 mL of dsRNA at the concentration of 1 µg/µL labeled dsRNA in sterile water. Ingested dsRNA reached the leaf vascular system and was detected in the digestive system of insects feeding on these leaflets (Supplemental Figure S1, panels 3 and 5).
2.5. Gene Silencing by Leaf-Mediated dsRNA Feeding
A tomato leaflet was cut off from a tomato plant and placed in a 1.5 mL Eppendorf tube containing 0.5 mL of dsRNA at the concentration of 0.5 µg/µL for each targeted gene (distilled water was used as control). Then the tubes containing the dsRNA and leaves were transferred into a 50 mL plastic tube (Corning Inc., Corning, NY, USA) (Supplementary Figure S1, panel 2). Adult whiteflies were released into this silencing system and the tube was covered with a piece of paper towel tightly held with a rubber band [25]. The whiteflies were left to feed on the leaflet for two days. Three replicates were established in each of the treatments and the controls. After two days of feeding, samples (each containing 25 adult whiteflies) were collected for gene expression and silencing analysis by qRT-PCR as described above. The remaining whiteflies were transferred to a TYLCV infected leaf for two more days and then the whitefly samples were again taken for analysis to determine gene expression and silencing and amounts of TYLCV acquired. Three whiteflies were put in a cage and clipped to a leaf of a young tomato test plant (5–6 true leaf stage). Whiteflies were discarded after one day and the leaf was removed after one week.
2.6. Fluorescence in Situ Hybridization (FISH)
FISH was performed as previously described [28]. Briefly, specimens were fixed overnight in Carnoy’s fixative (chloroform-ethanol-glacial acetic acid, 6:3:1 (vol/vol/vol), decolorized in 6% H2O2 in ethanol for 2 h, washed in 100% ethanol, and hybridized overnight in hybridization buffer (20 mM Tris-HCl (pH 8.0), 0.9 M NaCl, 0.01% SDS, 30% formamide) containing 10 pmol of fluorescent probes per mL. The primer used for specific targeting of knottin-1 transcripts was Cy3-knot-1 (5′-Cy3-GTTCTTCTCAAGTTCACCAATAGAC-3′). The stained samples were mounted whole in hybridization buffer and viewed under an IX81 Olympus FluoView500 confocal microscope. The specificity of detection was appraised by performing the procedure without the probe.
2.7. Statistical Analysis of Data
For all statistical analyses, JMP software (Release 5.0, SAS Institute, Inc.). All data were subjected to one-way ANOVA. The significant differences among means were evaluated by application of the Tukey-Kramer honestly significant difference (HSD) test at p < 0.05. The data are presented as means ± SEM. In all of the figures, an asterisk was used to indicate significance between treatments.
3. Results
3.1. Out of the Four Whitefly Knottin Genes, knot-1 Shows the Highest Levels of Expression. It Is Upregulated upon Ingestion of TYLCV
The sequences of the B. tabaci knottin genes (knot-1 to -4) were retrieved from GenBank (accession numbers DQ308606.1, DQ308607.1, DQ308608.1, DQ308609.1, respectively) (Figure 1). Two different sets of primers were designed: one for the synthesis of silencing dsRNA, and the other for monitoring the amounts of knot transcripts by RT-qPCR (qPCR). For each gene, the region used for qPCR was separated (downstream) from the region that served as template for dsRNA synthesis.
The amounts of knottin transcripts (relative to β-actin) were followed by qPCR during the adult life of the insect (2, 6, 10, 14, 18, 21 and 24 days after emergence), using synchronized populations. Pools of 50 insects were used for each time point. Figure 2A shows that the expression of knot-1 and knot-3 increased upon aging, especially during the first 10–14 days after emergence. At all times, the levels of knot-1 transcripts were two to four times higher than those of knot-3. The levels of knot-4 were about one twentieth those of knot-1; they were too low to clearly point to a developmental pattern. Knot-2 transcripts were undetectable.
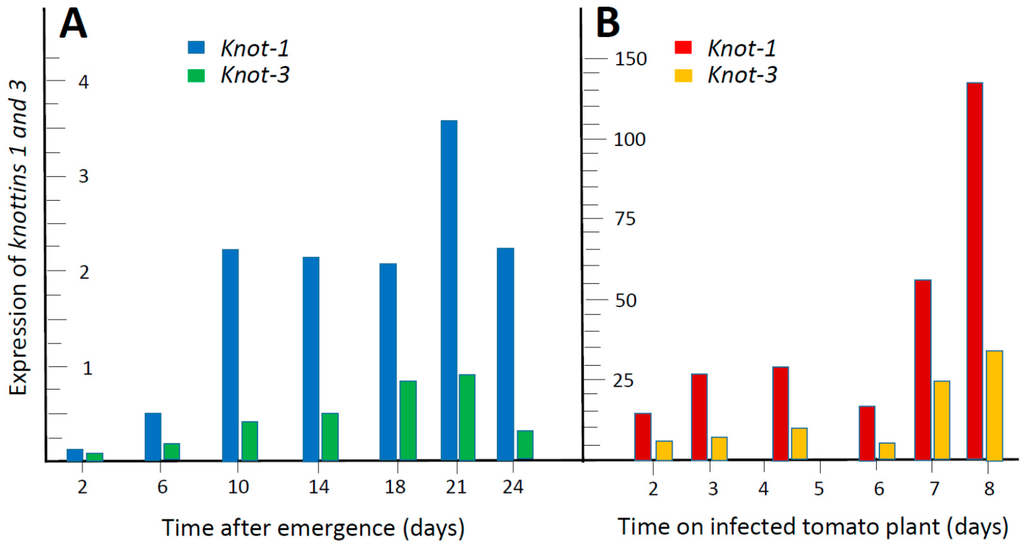
Figure 2.
Expression of knot-1 and knot-3 during adult whitefly development and upregulation by Tomato yellow leaf curl virus (TYLCV). (A) qPCR quantification of transcripts (relative to β-actin) in synchronized whitefly populations reared on tomato plants, collected at the time indicated after emergence; (B) Five- to seven-day-old whiteflies were caged with leaves of TYLCV-infected tomatoes and the amounts of transcripts were estimated by qPCR (relative to β-actin) at the time indicated thereafter. Pools of about 50 insects were used for each time point.
The effect of TYLCV on the expression of knot-1 and knot-3 was appraised (Figure 2B). Five to 7 days-old adult whiteflies were caged with leaves of TYLCV-infected tomatoes and the amounts of knot transcripts were estimated by qPCR 2, 3, 4–5, 6, 7 and 8 days thereafter. The presence of the virus was associated with a large increase (20-fold and above) in the expression of knot-1 and knot-3 (knot-1 remaining higher than knot-3).
3.2. Feeding Whiteflies on Tomato Leaflets Bathing in dsRNA Targeted against knot-1 and knot-3 Downregulated These Genes by about Two Thirds
The whitefly knottin genes were silenced by releasing whiteflies (5–7 days after emergence) into the silencing system consisting of detached tomato leaflets soaking in dsRNA directed against specific knottin transcripts (or in water as control). The flow chart of the experiments described in this article is shown in Supplemental Figure S1. We tested the effect of dsRNA at concentrations of 0.1, 0.5 and 1 μg/μL and found that 0.5 μg dsRNA/μL provided an optimal silencing effect in this system. We have shown previously that the dsRNA was readily detected in the tomato leaflets one day after the beginning of the treatment. Moreover, the dsRNA was stable in the aqueous solution and in the tomato leaflet for at least five days [25]. Feeding whiteflies on leaflets soaking in dsRNA (directed against knottins or any other gene, e.g., Tropomyosin, used here as an unrelated control) or water, did not significantly affected the survival of the insects.
After the whiteflies had fed on tomato leaflets bathing in knot-1 dsRNA, the amount of knot-1 transcript in their body was about one third that of whiteflies feeding on leaves soaking in water (Figure 3A). Similarly, feeding whiteflies on leaflets soaking in knot-3 and knot-4 depleted the amounts of knot-3 and knot-4 transcripts by approximately two-thirds. These results were confirmed by visualizing knot-1 transcripts in the abdomen of female whiteflies by FISH (Figure 3B). The knot-1 transcript signal conspicuous in the abdomen of female whiteflies was greatly reduced upon knot-1 silencing.
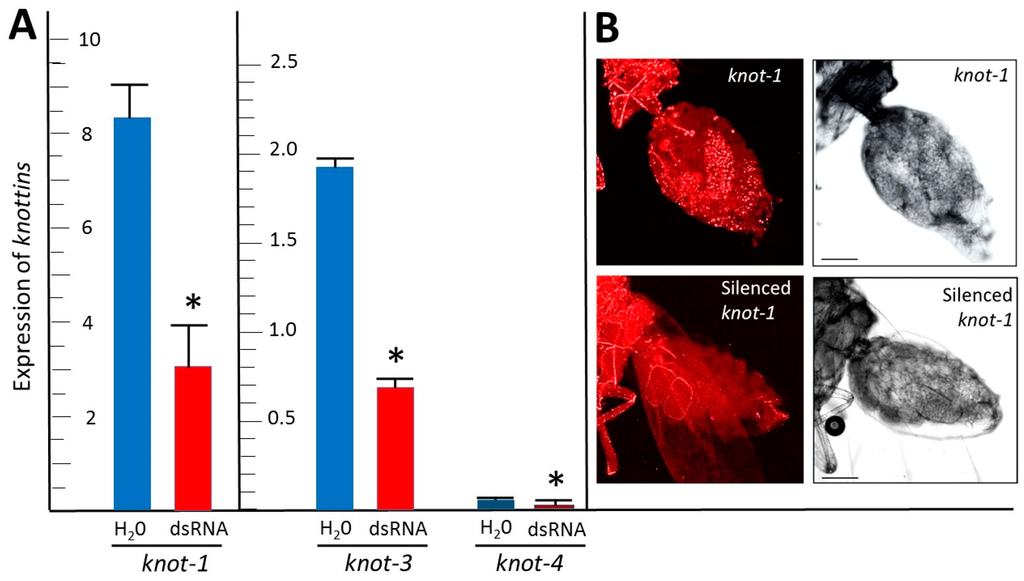
Figure 3.
Expression of members of the whitefly Knottin gene family in insects: effect of gene silencing. (A) qPCR quantification of knot-1; -3 and -4 transcripts (relative to β-actin) in adult whiteflies (collected 7 days after emergence) after caging the insects for 48 h with tomato leaflets bathing in water (H2O) or in knot-1; -3 and -4 dsRNA (dsRNA); (n = 3; mean ± SEM). An asterisk above the bars indicates significant differences between treatments at p < 0.05. (B) Confocal microscopy (FISH) of knot-1 transcripts in the abdomen of female whiteflies; before (upper panels) and after (lower panels) knot-1 silencing; using a Cy3-labeled knot-1 primer. Left picture: bright field; right picture: red signal associated with the presence of knot-1 transcripts. Bar: 100 μm.
3.3. Silencing knot-1 Has no Effect on the Expression of the Other Whitefly Knottin Genes
Since the knottin genes are members of the same family, we have measured the effect of knot-1 silencing on the expression of knot-1, knot-3 and knot-4 (Figure 4). While upon knot-1 silencing the expression of knot-1 was reduced by approximately 90%, knot-1 silencing did not significantly down-regulate the expression of knot-3 and knot-4. Hence, it is likely that the expression of the four knottin genes is not interconnected in a way where perturbations in one does not influence each other significantly.
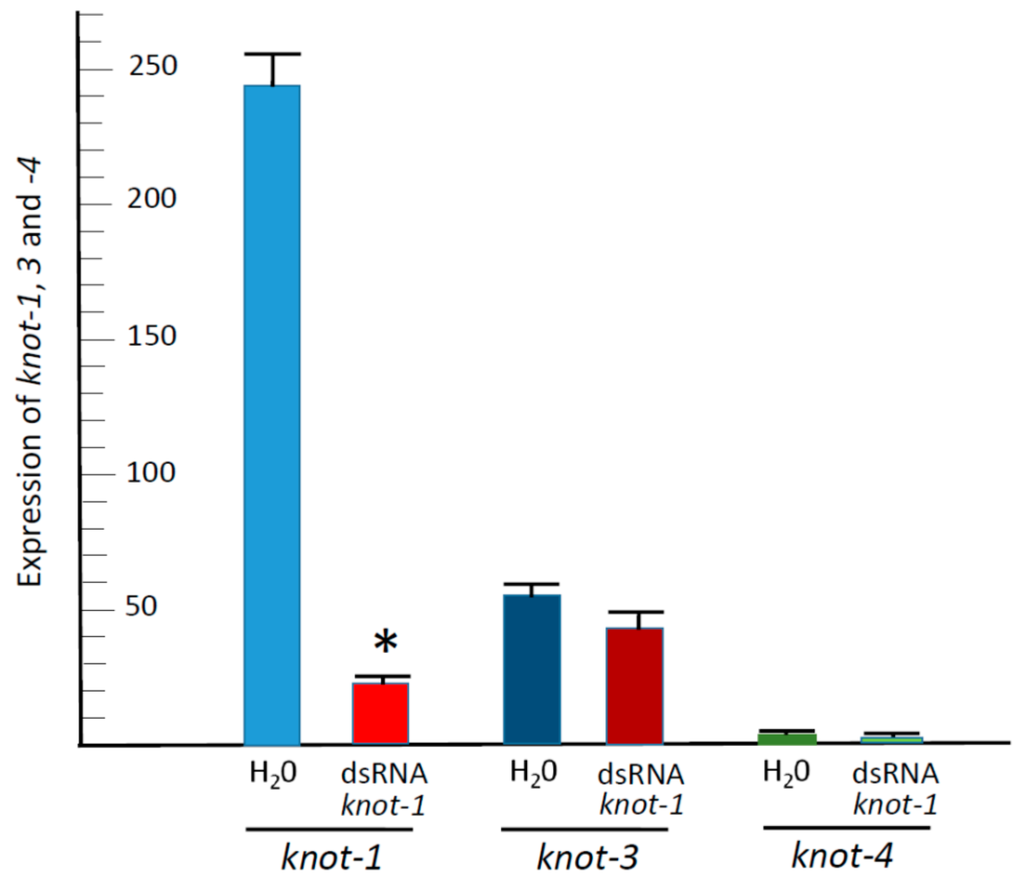
Figure 4.
Expression of knot-1; -3; and -4 upon knot-1 silencing. qPCR quantification of knot transcripts (relative to β-actin transcripts) from whiteflies caged for 48 h with tomato leaflets bathing in water (H2O) or in knot-1; -3 and -4 dsRNA (dsRNA); (n = 3; mean ± SEM). An asterisk above the bars indicates significant differences between treatments at p < 0.05.
3.4. Whiteflies with Silencing knot-1 Contain Increased Amounts of TYLCV upon Feeding on Leaves of Infected Tomato Plants
Following feeding on leaflets soaking in water and on knot-1 dsRNA as described above, the insects were caged with TYLCV-infected leaflets for a virus acquisition access period of 48 h, after which the expression of knot-1 was estimated. In the unsilenced whiteflies, the presence of TYLCV was accompanied by an increase of about one third in the level of knot-1 expression (Figure 5A). In comparison, the presence of TYLCV in knot-1-silenced whiteflies did not change the expression levels of knot-1. Altogether, in viruliferous insects, the expression levels of knot-1 in silenced whiteflies were about one third those measured in unsilenced whiteflies (Figure 5A).
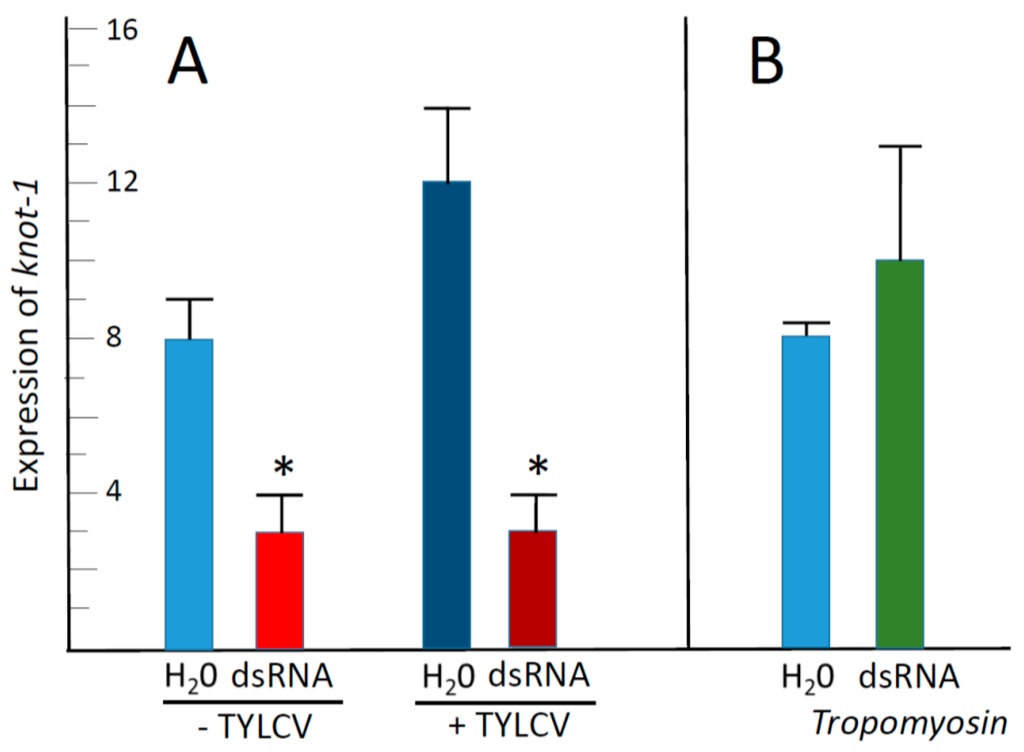
Figure 5.
Effect of TYLCV acquisition on the expression of knot-1; in whiteflies with and without silenced knot-1. (A) qPCR quantification of knot-1 transcript levels in adult whiteflies after caging the insects for 48 h with tomato bathing in water (H2O) or in knot-1 dsRNA (dsRNA); before (− TYLCV) and after caging with infected tomato leaflets (+ TYLCV) for a 48 h virus acquisition (n = 3; mean ± SEM). (B) knot-1 expression in whiteflies following caging the insects for 48 h with tomato bathing in water (H2O) or in tropomyosin dsRNA (dsRNA tropomyosin) (n = 3; mean ± SEM). An asterisk above the bars indicates significant differences between treatments at p < 0.05.
To eliminate the possibility that dsRNA by itself (directed against any gene) affected the expression of knot-1, whiteflies were caged for two days with tomato leaflets bathing in water and in dsRNA directed against the whitefly Tropomyosin gene. Then the levels of knot-1 expression were measured. Figure 5B shows that whitefly treatment with tropomyosin dsRNA did not significantly affect the expression of knot-1, indicating that the downregulation of knot-1 was specifically the result of targeting knot-1 transcripts with knot-1 dsRNA.
The effect of knot-1 silencing on the amount of TYLCV acquired by whiteflies during the 48 h feeding period was appraised (Figure 6). Whiteflies with silenced knot-1 acquired three orders of magnitudes more virus than untreated insects (Figure 6A). By comparison, whiteflies with silenced knot-3 acquired about the same amount of virus than untreated whiteflies. Insects with silenced Tropomyosin acquired 2–3 times less virus than untreated insects (Figure 6B). It is possible that muscle proteins such as tropomyosin, myosin and actin are also involved in TYLCV acquisition and movement in the insect body, an observation worth further investigation. These results indicated that the effect of knottin silencing on the acquisition of TYLCV were knot-1-specific.
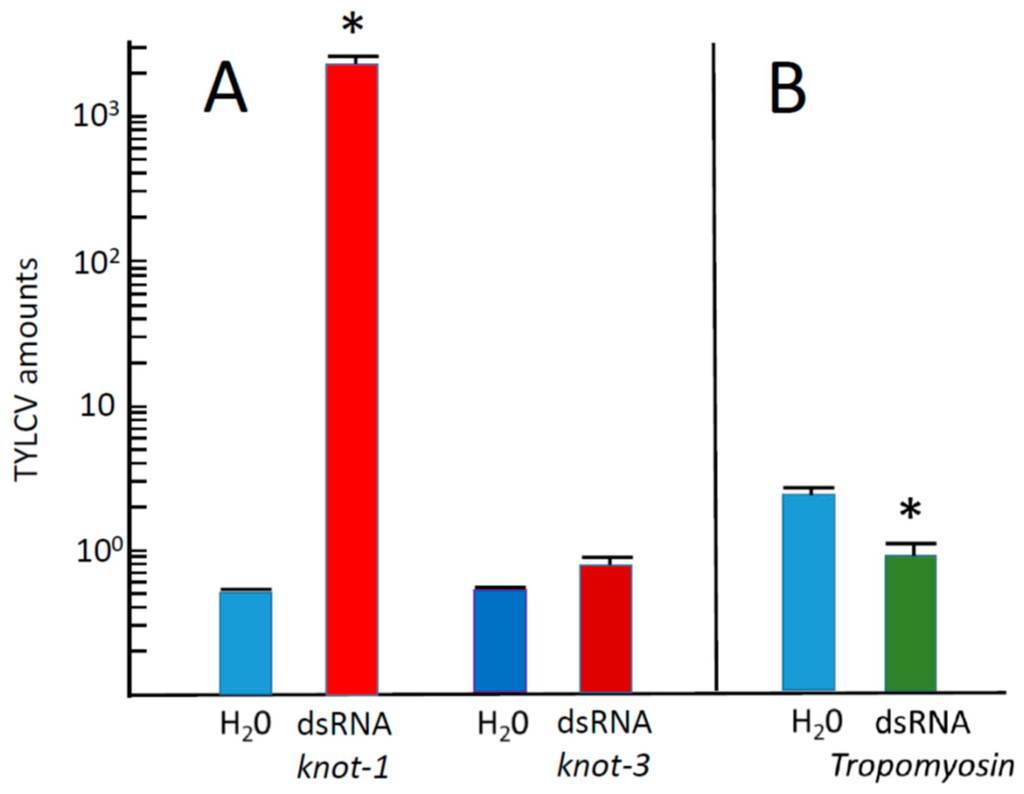
Figure 6.
TYLCV amounts acquired by whiteflies with down-regulated knot-1 and knot-3 during a 48 h acquisition period on infected tomato leaves. (A) Viral amounts (viral genomes per insect β-actin genes) in whiteflies caged with down-regulated knot-1 (dsRNA knot-1) or knot-3 (dsRNA knot-3) compared with control not-silenced insects (H2O); (B) viral amounts in whiteflies caged with down-regulated tropomyosin (dsRNA tropomyosin) compared with control insects (H2O) (n = 3; mean ± SEM). An asterisk above the bars indicates significant differences between treatments at p < 0.05.
3.5. Tomato Plants Inoculated by Viruliferous Whiteflies with Silenced knot-1 Presented Early Disease Symptoms and Contained Large Amounts of Virus
Following TYLCV acquisition, the two groups of insects (knot-1 silenced and not silenced) were caged with young leaves of tomato test plants (at the 5–6 true leaf stage) for a 24 h inoculation access period, 3 insects in a clip cage per plant (minimal conditions to ensure nearly 100% infection [29]. The amount of viral DNA associated with the test plants was measured 5, 9, 13, 16 and 19 days post-inoculation. Nineteen days after whitefly-mediated inoculation, the plants infected by the silenced knot-1 insects contained five orders of magnitude more virus than the control plants (Figure 7A). The typical disease symptoms of arrest of growth (61 vs. 90 cm high in average), yellowing and curling of upper leaves (Figure 7B) started to appear about 7–10 days earlier in plants infected by knot-1 silenced insects than by control whiteflies (12 vs. 21 days). These results indicated that the large amounts of TYLCV acquired by the knot-1 silenced whiteflies do not stay confined in the insect body, disconnected from virus the circulative pathway, but are transmitted to plants during feeding.
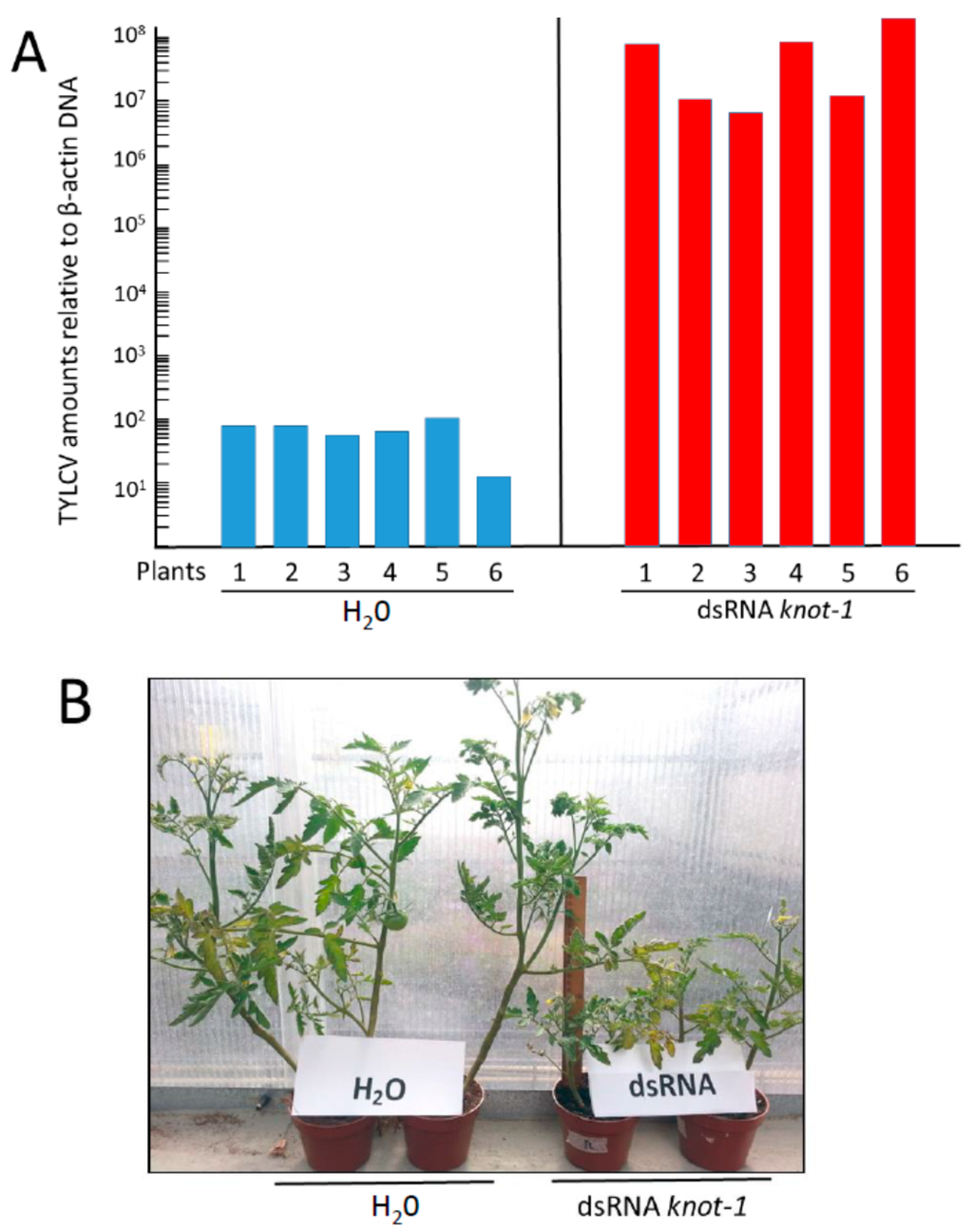
Figure 7.
TYLCV amounts and symptoms in tomato plants infected by viruliferous whiteflies without (H2O) and with knot-1 silenced gene. (A) Viral amounts (viral genomes per plant β-actin gene) in tomato plants 19 days after infection by viruliferous whiteflies without (H2O) and with knot-1 silenced (dsRNA knot-1); (B) symptom appearance of tomato plants 24 days after infection with not-silenced (H2O) and knot-1 silenced insects (dsRNA knot-1).
3.6. Inoculation of Tomato Plants by Whiteflies with Silenced knot-3 Significantly Modified Neither the Time of Symptom Appearance nor the Accumulation of TYLCV
The specificity of knot-1 in restraining virus acquisition and transmission was tested by comparing the effect of silencing knot-1 and knot-3. Upon feeding on tomato leaflets soaking in knot-3 dsRNA, knot-3 expression was reduced by about 2/3 (Figure 3A). The knot-3 silenced whiteflies contained approximately the same amount of virus than the non-treated insects (Figure 6A), in contrast to the three orders of magnitude observed between knot-1 silenced and control insects (Figure 7A). The knot-3 silenced whiteflies were able to transmit the TYLCV disease to tomato tests plants. However, contrary to the tomatoes infected with viruliferous knot-1 silenced whiteflies, which contained five to six orders of magnitude more virus than the plants infected by the control insects (Figure 7A), the plants infected with viruliferous knot-3 silenced whiteflies contained approximately the same amounts of viral DNA as the plants infected by viruliferous, untreated insects (Figure 8A). The plants presented similar symptoms of stunting and yellowing (Figure 8B). These results strongly supported the unique role of knot-1 in controlling the amount of TYLCV in the whitefly body.
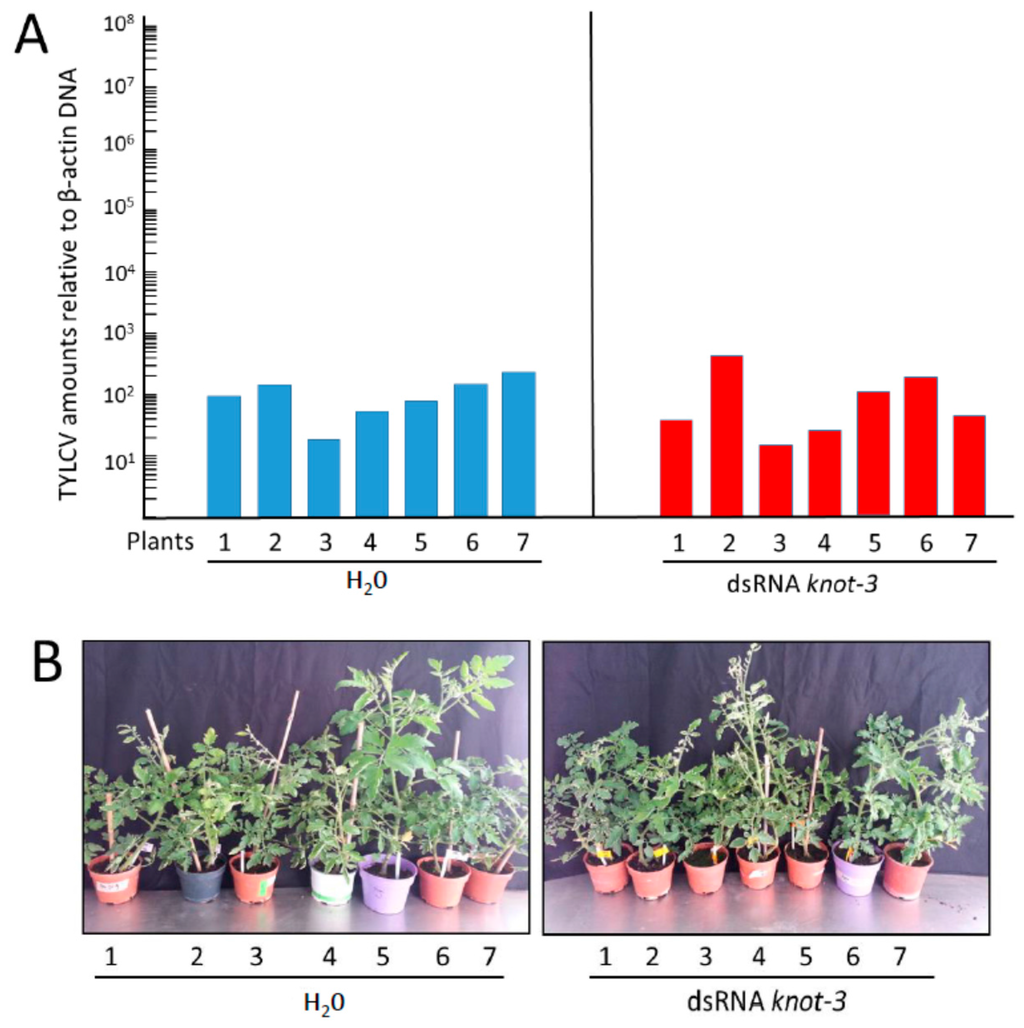
Figure 8.
TYLCV amounts and symptoms in tomato plants infected by viruliferous whiteflies without (H2O) and with silenced knot-3. (A) TYLCV amounts (viral genomes per plant β-actin genes) in tomato plants 19 days after infection by viruliferous whiteflies without (H2O) and with silenced knot-3 (dsRNA knot-3); (B) symptom appearance of tomato plants 24 days after infection with not-silenced (H2O) and knot-3 silenced insects (dsRNA knot-3).
4. Discussion
We examined the involvement of members of the whitefly knottin gene family in the interaction of TYLCV with its B. tabaci vector, applying RNAi-based gene silencing. The flow chart in Supplemental Figure S1 summarizes the experiments. The factors for a successful use of RNAi in insect science such as targeted species, choice of gene, mode of delivery, dsRNA dosage, have been reviewed recently [30]. To circumvent potential effects due to insect aging and different plant allelochemicals, we used synchronized whitefly populations raised on the very same tomato genotypes that were utilized for leaf-mediated silencing. The procedure we used to silence knottins (Supplemental Figure S1, panels 2 and 4) was developed to silence genes involved in whitefly nymphal development [25]. The uptake of dsRNA by the leaf vascular system and by the insects feeding on these leaves was visualized using Cy-labeled dsRNA (Supplemental Figure S1, panels 4 and 5). Several methods of dsRNA delivery have been applied to silence insect genes: by microinjection [24,31], with the diet [32,33], by topical application [34], by feeding on leaves of dsRNA-expressing transgenic plants [35,36], and by leaflet absorption [37]. In the latter case, similarly to our own experiments, leaflets were immersed in 200 µL water containing 10 µg dsRNA of various target genes.
Leaf-mediated feeding of knottin-specific dsRNA by whiteflies was associated with a downregulation of the knottin genes by more than 50%, whether knot-1, -3 or -4 (Figure 3A). The silencing of a given knottin gene was specific although the sequences of the knottin genes present a high level of similarity (Figure 1). Silencing of knot-1 did not affect the expression of knot-3 and knot-4 (Figure 4). Moreover, the silencing of knot-1 was specific and did not occur when an unrelated gene, such as tropomyosin, was silenced (Figure 5B).
Ingestion of virus led to the upregulation of knot-1 (and to a much lesser extent of knot-3) suggesting that this gene is responsive to the stress caused by the virus. In this perspective, silencing of knot-1 neutralized the stress-response to TYLCV (Figure 2 and Figure 5). As a result, whiteflies with depleted levels of knot-1 contained several orders of magnitude more virus than non-silenced insects (Figure 6). This phenomenon was specific for the knot-1 gene, and did not occur when knot-3 (or tropomyosin) was silenced. Tomato plants inoculated by knot-1 silenced insects contained several orders of magnitude more virus than plants inoculated with non-silenced insects. Moreover, the disease symptoms, stunting, leaf yellowing and curling, appeared 7 to 10 days earlier and were more pronounced (Figure 7). Silencing knot-3 was not accompanied by the severe effects associated with knot-1 silencing, such as a dramatic increase of virus acquisition and transmission to tomato plants (Figure 8). Although the amounts of virus inoculated by the different types of viruliferous whiteflies during the 24 h access feeding period are unknown, the results presented in Figure 7 and Figure 8 showed a direct correlation between the amounts of virus in the whiteflies and the amounts of virus in the plants inoculated by these whiteflies. These results suggest that the additional amounts of virus in knot-1 silenced whiteflies are not removed from the circulative pathway but are transmitted in a manner similar to the virus transmitted by unsilenced insects.
Since knot-1 transcripts are found mainly in the whitefly abdomen (Figure 3B), it is tempting to suggest that knot-1 regulates the number of virions in the hemolymph, an obligatory site of begomovirus transit from the digestive tract to the salivary glands [5]. How this control is exerted is not known. Knottins are present in diverse organisms and possess various biological functions. For instance, knottins from pea (Pisum sativum) have insecticide properties to certain species of cereal weevils, mosquitoes and aphids [38]; the venom of the spider Segesteria florentina contains the insecticidal toxin Sf1a, a knottin that blocks the pore of insect voltage-gated sodium channels [39]. Knottins from the beetle Psacothea hilaris present antimicrobial properties towards Gram-positive and Gram-negative bacterial strains [40]. Knot-1 may be involved in maintaining the amount of virions associated with the whitefly to a level where it is not overwhelmingly harmful to the insect. We are not aware of any other case of knottin or knottin-like genes directly or indirectly involved in the regulation of virus amounts in animal or plant cells.
We have previously suggested that some begomoviruses interact actively with its B. tabaci vector, to a point reminiscent of a host-pathogen relationship [41,42]. Indeed, in many instances (but not always) the long-term presence of begomoviruses (sometimes for the entire life) has deleterious effects on the longevity and fertility of the whitefly host [9]. In addition, begomoviruses affect the insect pattern of gene expression [43], and may replicate to some extent in their host [44]. It is possible that the geminivirus-vector partnership evolved towards a neutralization of any viral function that may negatively affect the insect. In this perspective, the role of the insect knottins, in particular knot-1, may be to regulate the amount of virus uptaken by the insect to levels the insect can handle without causing extensive deleterious effects. The long-term effect of knot-1 silencing on the biology of viruliferous whitefly, especially on longevity and fertility, warrants further research.
Beside knot-1, we expect that other genes are involved in the control of begomovirus circulative transmission. The comparison of the transcriptome of viruliferous vs. non-viruliferous whiteflies has identified a large number of genes upregulated and dowregulated upon TYLCV acquisition organized in 157 differentially regulated pathways [13,43]. In addition, TYLCV was associated with the activation of the whitefly immune responses, including autophagy (Atg3, 9 and 12), lysosome proteins (AP-1, cathepsin B and D, protein tyrosine phosphatase, saposin, phosphatidylcholine acyltransferase), melanization (dopa carboxylase) and antimicrobial peptides (including knottin-3). Hsp70 was strongly upregulated upon acquisition of TYLCV and of the bipartite Squash leaf curl begomovirus [28]. The wealth of publication on the use of RNAi to downregulate insect genes indicated that a strategy based on targeting whitefly genes involved in begomovirus transmission could be used to control the virus before it could be transmitted and infect target crops.
Supplementary Materials
The following figure is available online at http://www.mdpi.com/1999-4915/8/7/205/s1. Figure S1. Flow of described experiments.
Acknowledgments
Supported by the Israel Science Foundation (grant 1689/13 to H.C.) and the National Natural Science Foundation of China (grant 31361140356 to S.S.L.).
Author Contributions
Aliza Hariton Shalev, Shu-Sheng Liu and Henryk Czosnek designed research; Aliza Hariton Shalev, Iris Sobol and Murad Ghanim performed research; Aliza Hariton Shalev, Iris Sobol, Shu-Sheng Liu Murad Ghanim and Henryk Czosnek analyzed data; Aliza Hariton Shalev and Henryk Czosnek wrote the paper.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Stansley, P.A.; Naranjo, S.E. Bemisia: Bionomics and Management of a Global Pest; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Czosnek, H. Tomato Yellow Leaf Curl Virus Disease: Management, Molecular Biology, Breeding for Resistance; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Brown, J.K.; Zerbuni, F.M.; Navas-Castillo, J.; Moriones, E.; Ramos-Sobrinho, R.; Silva, J.C.F.; Fiallo-Olivé, E.; Briddon, R.W.; Hernández-Zepeda, C.; Idris, A. Revision of Begomovirus taxonomy based on pairwise sequence comparisons. Arch. Virol. 2014, 160, 1593–1619. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Pendon, J.A.; Caňizares, M.C.; Moriones, E.; Bejarano, E.R.; Czosnek, H.; Navas-Castillo, J. Tomato yellow leaf curl viruses: Ménage a trios between the virus complex, the plant and the whitefly vector. Mol. Plant Pathol. 2010, 11, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Czosnek, H.; Ghanim, M.; Ghanim, M. Circulative pathway of begomoviruses in the whitefly vector Bemisia tabaci—Insights from studies with Tomato yellow leaf curl virus. Ann. Appl. Biol. 2002, 140, 215–231. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, J.-J.; Zhang, T.; Li, F.-F.; Ghanim, M.; Zhou, X.-P.; Ye, G.-Y.; Liu, S.-S.; Wang, X.-W. Specific cells in the primary salivary glands of the whitefly Bemisia tabaci control retention and transmission of begomoviruses. J. Virol. 2014, 88, 13460–13468. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Hoshino, S. Effect of tomato yellow leaf curl disease on reproduction of Bemisia tabaci Q biotype (Hemiptera: Aleyrodidae) on tomato plants. Appl. Entomol. Zool. 2009, 44, 143–148. [Google Scholar] [CrossRef]
- Jiu, M.; Zhou, X.P.; Tong, L.; Xu, J.; Yang, X.; Wan, F.H.; Liu, S.-S. Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE 2007, 2, e182. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, G.; Czosnek, H. Long-term association of tomato yellow leaf curl virus (TYLCV) with its whitefly vector Bemisia tabaci: Effect on the insect transmission capacity, longevity and fecundity. J. Gen. Virol. 1997, 78, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Chu, D.; Liu, B.; Shi, X.; Guo, L.; Xie, W.; Carrière, Y.; Li, X.; Zhang, Y. Differential effects of an exotic plant virus on its two closely related vectors. Sci. Rep. 2013, 3, 2230. [Google Scholar] [CrossRef] [PubMed]
- Leshkowitz, D.; Gazit, S.; Reuveni, E.; Ghanim, M.; Czosnek, H.; McKenzie, C.; Shatters, R.G., Jr.; Brown, J.K. Whitefly (Bemisia tabaci) genome project: Analysis of sequenced clones from egg, instar, and adult (viruliferous and non-viruliferous) cDNA libraries. BMC Genom. 2006, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Mahadav, A.; Gerling, D.; Gottlieb, Y.; Czosnek, H.; Ghanim, M. Gene expression in the whitefly Bemisia tabaci pupae in response to parasitization by the wasp Eretmocerus mundus. BMC Genom. 2000, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.-B.; Li, J.-M.; Varela, N.; Wang, Y.-L.; Li, F.-F.; Bao, Y.-Y.; Zhang, C.-X.; Liu, S.-S.; Wang, X.-W. Global analysis of the transcriptional response of whitefly to Tomato Yellow leaf curl China virus reveals the relationship of coevolved adaptations. J. Virol. 2011, 85, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Shatters, R.G., Jr.; McKenzie, C.L.; Boykin, L.M.; Gazit, S.; Sinisterra, X.; Weathersbee, A.A.; Brown, J.K.; Czosnek, H. A knottin-like putative antimicrobial gene family in the whitefly Bemisia tabaci biotype B: Cloning and transcript regulation. J. Insect Sci. 2008, 8, 4. [Google Scholar]
- Zhang, C.-R.; Zhang, S.; Xia, J.; Li, F.-F.; Xia, W.-Q.; Liu, S.-S.; Wang, X.-W. The immune strategy and stress response of the Mediterranean species of the Bemisia tabaci complex to an orally delivered bacterial pathogen. PLoS ONE 2014, 9, e94477. [Google Scholar] [CrossRef] [PubMed]
- Gracy, J.; Chiche, L. Structure and modeling of knottins, a promising molecular scaffold for drug discovery. Curr. Pharm. Des. 2011, 17, 4337–4350. [Google Scholar] [CrossRef] [PubMed]
- Gelly, J.C.; Gracy, J.; Kaas, Q.; Le-Nguyen, D.; Heitz, A.; Chiche, L. The KNOTTIN website and database: A new information system dedicated to the knottin scaffold. Nucleic Acids Res. 2004, 32, D156–D159. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Mahaffey, J.P.; Lorenzen, M.D.; Denell, R.E.; Mahaffey, J.W. Using RNAi to investigate orthologous homeotic gene function during development of distantly related insects. Evol. Dev. 1999, 1, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Tomoyasu, Y.; Denell, R.E. Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev. Genes Evol. 2004, 214, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; Ihle, K.E.; Fondrk, M.K.; Page, R.E., Jr.; Amdam, G.V. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 2007, 5, e62. [Google Scholar] [CrossRef] [PubMed]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Coleman, A.D.; Wouters, R.H.M.; Mugford, S.T.; Hogenhout, S.A. Persistence and transgenerational effect of plant-mediated RNAi in aphids. J. Exp. Bot. 2015, 66, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, O.; Swevers, L.; Smagghe, G. dsRNA degradation in the pea aphid (Acyrthosiphon pisum) associated with lack of response in RNAi feeding and injection assay. Peptides 2014, 53, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, M.; Kontsedalov, S.; Czosnek, H. Tissue-specific gene silencing by RNA interference in the whitefly Bemisia tabaci (Gennadius). Insect Biochem. Mol. Biol. 2007, 37, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.-B.; Ghanim, M.; Liu, S.-S.; Czosnek, H. Silencing the ecdysone (synthesis and signaling) pathway genes disrupts nymphal development in the whitefly. Insect Biochem. Mol. Biol. 2013, 43, 740–746. [Google Scholar] [CrossRef] [PubMed]
- De Barro, P.J.; Liu, S.-S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A Statement of Species Status. Ann. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shahjahan, R.M.; Roger, K.J.H.; Leopold, R.A.; Devault, J.D. Lower incubation temperature increases yield of insect genomic DNA isolated by the CTAB method. Biotechniques 1995, 19, 332–339. [Google Scholar] [PubMed]
- Götz, M.; Popovski, S.; Kollenberg, M.; Gorovits, R.; Brown, J.K.; Cicero, J.M.; Czosnek, H.; Winter, S.; Ghanim, M. Implication of Bemisia tabaci heat shock protein 70 in begomovirus-whitefly interactions. J. Virol. 2012, 86, 13241–13252. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Nitzany, F.E. Transmission and host range of the tomato yellow leaf curl virus. Phytopathology 1966, 56, 1127–1131. [Google Scholar]
- Scott, J.G.; Michel, K.; Bartholomay, L.C.; Siegfried, B.D.; Hunter, W.B.; Smagghe, G.; Zhu, K.Y.; Douglas, A.E. Towards the elements of successful insect RNAi. J. Insect Physiol. 2013, 59, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Cai, Z.; Song, J.; Wu, Z.; Zhou, S. dsRNA uptake and persistence account for tissue-dependent susceptibility to RNA interference in the migratory locust, Locusta migratoria. Insect Mol. Biol. 2014, 23, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Asokan, R.; Rebijith, K.B.; Roopa, H.K.; Kumar, N.K. Non-invasive delivery of dsGST is lethal to the sweet potato whitefly, Bemisia tabaci (G.) (Hemiptera: Aleyrodidae). Appl. Biochem. Biotechnol. 2015, 175, 2288–2299. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Wan, P.-J.; Zhou, L.-T.; Mu, L.-L.; Li, G.-Q. RNA interference-mediated silencing of a Halloween gene spookier affects nymph performance in the small brown planthopper Laodelphax striatellus. Insect Sci. 2015, 22, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N.; Hajeri, S.; Tiwari, S.; Gowda, S.; Stelinski, L.L. Double-stranded RNA uptake through topical application, mediates silencing of five CYP4 genes and suppresses insecticide resistance in Diaphorina citri. PLoS ONE 2014, 9, e110536. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Upadhyay, S.K.; Verma, P.C.; Chandrashekar, K.; Tuli, R.; Singh, P.K. Enhanced whitefly resistance in transgenic tobacco plants expressing double stranded RNA of v-ATPase A gene. PLoS ONE 2014, 9, e87235. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Rajam, M.V. Targeting chitinase gene of Helicoverpa armigera by host-induced RNA interference confers insect resistance in tobacco and tomato. Plant Mol. Biol. 2016, 90, 281–292. [Google Scholar]
- Camargo, R.A.; Herai, R.H.; Santos, L.N.; Bento, F.M.M.; Lima, J.E.; Marques-Souza, H.; Figueira, A. De novo transcriptome assembly and analysis to identify potential gene targets for RNAi-mediated control of the tomato leafminer (Tuta absoluta). BMC Genom. 2015, 16, 635. [Google Scholar] [CrossRef] [PubMed]
- Chouabe, C.; Eyraud, V.; Da Silva, P.; Rahioui, I.; Royer, C.; Soulage, C.; Bonvallet, R.; Huss, M.; Gressent, F. New mode of action for a knottin protein bioinsecticide Pea albumin 1 subunit b (PA1b) is the first peptidic inhibitor of V-ATPase. J. Biol. Chem. 2011, 286, 36291–36296. [Google Scholar] [CrossRef] [PubMed]
- Bende, N.J.; Dziemborowicz, S.; Herzig, V.; Ramanujam, V.; Brown, G.W.; Bosmans, F.; Nicholson, G.M.; King, G.F.; Mobli, M. The insecticidal spider toxin SFI1 is a knottin peptide that blocks the pore of insect voltage-gated sodium channels via a large β-hairpin loop. FEBS J. 2015, 282, 904–920. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-S.; Lee, J.; Hwang, B.; Nam, S.-H.; Yun, E.-Y.; Kim, S.-R.; Lee, D.G. Isolation and characterization of Psacotheasin, a novel knottin-type antimicrobial peptide, from Psacothea hilaris. J. Microbiol. Biotech. 2010, 20, 708–711. [Google Scholar] [CrossRef]
- Czosnek, H.; Ghanim, M.; Rubinstein, G.; Morin, S.; Fridman, V.; Zeidan, M. Whiteflies: Vectors–or victims?–of geminiviruses. Adv. Virus Res. 2001, 57, 291–322. [Google Scholar] [PubMed]
- Czosnek, H.; Ghanim, M. Back to basics: Are begomoviruses whitefly pathogens? J. Integr. Agric. 2012, 11, 225–234. [Google Scholar] [CrossRef]
- Li, J.M.; Ruan, Y.M.; Li, F.F.; Liu, S.S.; Wang, X.W. Gene expression profiling of the whitefly (Bemisia tabaci) Middle East—Asia Minor 1 feeding on healthy and Tomato yellow leaf curl China virus-infected tobacco. Insect Sci. 2011, 18, 11–22. [Google Scholar] [CrossRef]
- Pakkianathan, B.C.; Kontsedalov, S.; Lebedev, G.; Mahadav, A.; Zeidan, M.; Czosnek, H.; Ghanim, M. Replication of Tomato yellow leaf curl in its whitefly vector Bemisia tabaci. J. Virol. 2015, 89, 9791–9803. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).