Behavioral Dynamics, Genomic Insights, and Social Drivers of SARS-CoV-2 Waves and Variants in Cali, Colombia (2020–2023)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Library Construction, and Sequencing Methods
2.2. Viral Genome Assembly and Genome Sequence Dataset Retrieval
2.3. Number of Cases, Infection Fatality Rate (IFR), Vaccination, and Effective Reproduction Number (Re)
2.4. Phylogenetic Analysis and Mutation Site Identification
3. Results
3.1. Demographic Distribution
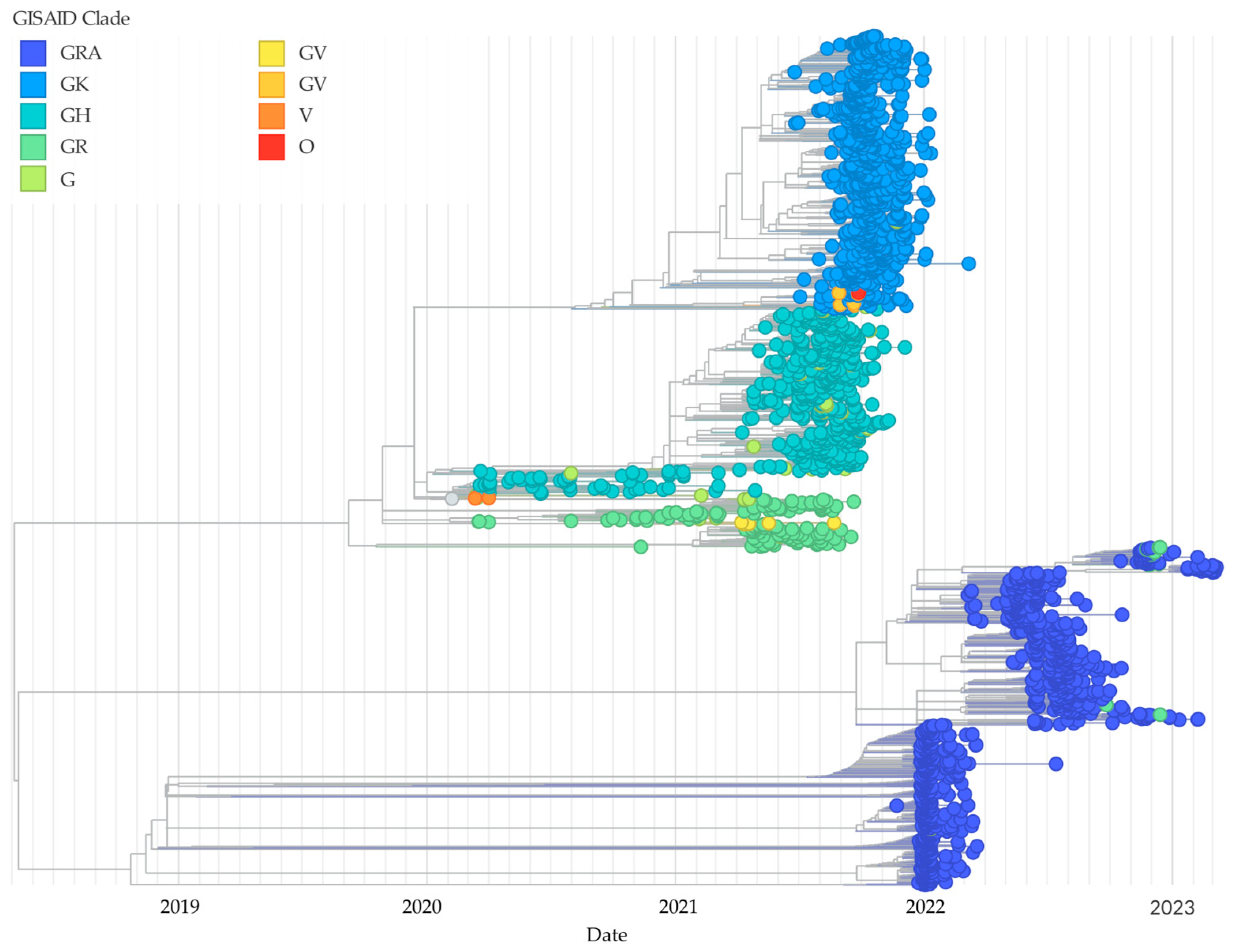
3.2. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS-CoV 2 | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | Coronavirus disease 2019 |
| VOCs | Variants of concern |
| VOIs | Variants of interest |
| VBMs | Variants being monitored |
| VTM | Viral transport medium |
| RP | RNase P gene |
| N | Nucleocapsid |
| Ct | Cycle threshold |
| WHO | World Health Organization |
| INS | Colombian National Institute of Health |
| IFR | Infection Fatality Rate |
| Re | Effective reproduction number |
| PAHO | Pan-American Health Organization |
| SNV | Single-nucleotide variant |
| RBD | Receptor-binding domain |
| NTD | N-terminal domain |
| NSP13 | Nonstructural protein 13 |
| NSP12b | Nonstructural protein 12 b |
| ORF3a | Open reading frame 3a |
| NSP3 | Nonstructural protein 3 |
| NSP2 | Nonstructural protein |
| S | Spike |
References
- Russell, T.W.; Wu, J.T.; Clifford, S.; Edmunds, W.J.; Kucharski, A.J.; Jit, M. Effect of internationally imported cases on internal spread of COVID-19: A mathematical modelling study. Lancet Public Health 2021, 6, e12–e20. [Google Scholar] [CrossRef] [PubMed]
- Shultz, J.M.; Berg, R.C.; Bernal Acevedo, O.A.; Ocampo Cañas, J.A.; Escobar, V.A.P.; Muñoz, O.; Espinel, Z.; Uribe-Restrepo, J.M. Complex correlates of Colombia’s COVID-19 surge. Lancet Reg. Health—Am. 2021, 3, 100072. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.; Patiño, L.H.; Muñoz, M.; Ballesteros, N.; Guerrero-Araya, E.; Paredes-Sabja, D.; Flórez, C.; Gomez, S.; Ramírez-Santana, C.; Salguero, G.; et al. Evolution and Epidemic Spread of SARS-CoV-2 in Colombia: A Year into the Pandemic. Vaccines 2021, 9, 837. [Google Scholar] [CrossRef]
- Tyson, J.R.; James, P.; Stoddart, D.; Sparks, N.; Wickenhagen, A.; Hall, G.; Choi, J.H.; Lapointe, H.; Kamelian, K.; Smith, A.D.; et al. Improvements to the ARTIC multiplex PCR method for SARS-CoV-2 genome sequencing using nanopore. BioRxiv Prepr. Serv. Biol. 2020. [Google Scholar] [CrossRef]
- Arévalo, M.T.; Karavis, M.A.; Katoski, S.E.; Harris, J.V.; Hill, J.M.; Deshpande, S.V.; Roth, P.A.; Liem, A.T.; Bernhards, R.C. A Rapid, Whole Genome Sequencing Assay for Detection and Characterization of Novel Coronavirus (SARS-CoV-2) Clinical Specimens Using Nanopore Sequencing. Front. Microbiol. 2022, 13, 910955. [Google Scholar] [CrossRef]
- Ramírez-Ortiz, J.; Castro-Quintero, D.; Lerma-Córdoba, C.; Yela-Ceballos, F.; Escobar-Córdoba, F. Consecuencias de la pandemia COVID 19 en la salud mental asociadas al aislamiento social. Colomb. J. Anesthesiol. 2020, 12, 1–8. Available online: http://www.scielo.org.co/pdf/rca/v48n4/es_2256-2087-rca-48-04-e301.pdf (accessed on 21 April 2025).
- Prada, S.I.; Garcia-Garcia, M.P.; Guzman, J. COVID-19 response in Colombia: Hits and misses. Health Policy Technol. 2022, 11, 100621. [Google Scholar] [CrossRef]
- Casos Positivos de COVID-19 en Colombia. | Datos Abiertos Colombia. Available online: https://www.datos.gov.co/Salud-y-Protecci-n-Social/Casos-positivos-de-COVID-19-en-Colombia-/gt2j-8ykr/about_data (accessed on 13 January 2025).
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- O’Toole, Á.; Pybus, O.G.; Abram, M.E.; Kelly, E.J.; Rambaut, A. Pango lineage designation and assignment using SARS-CoV-2 spike gene nucleotide sequences. BMC Genom. 2022, 23, 121. [Google Scholar] [CrossRef]
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. SARS-CoV-2 Variant Classifications and Definitions. 2021. Available online: https://stacks.cdc.gov/view/cdc/116721 (accessed on 26 April 2022).
- Galloway, S.E. Emergence of SARS-CoV-2 B.1.1.7 Lineage—United States, December 29, 2020–January 12, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 95–99. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Deka, K.; Nongrang, L.; Kalita, T. The Delta and Omicron Variants of SARS-CoV-2: What We Know So Far. Vaccines 2022, 10, 1926. [Google Scholar] [CrossRef] [PubMed]
- Laiton-Donato, K.; Franco-Muñoz, C.; Álvarez-Díaz, D.A.; Ruiz-Moreno, H.A.; Usme-Ciro, J.A.; Prada, D.A.; Reales-González, J.; Corchuelo, S.; Herrera-Sepúlveda, M.T.; Naizaque, J.; et al. Characterization of the emerging B.1.621 variant of interest of SARS-CoV-2. Infect. Genet. Evol. 2021, 95, 105038. [Google Scholar] [CrossRef]
- Boletín de prensa. INS: Confirmados casos colombianos con variante P1. Available online: https://www.ins.gov.co/BibliotecaDigital/boletin-prensa-ins-31-01-2021-casos-colombianos-variante-p1.pdf (accessed on 13 January 2025).
- Novel Sublineage Within B.1.1.1 Currently Expanding in Peru and Chile, with a Convergent Deletion in the ORF1a Gene (Δ3675-3677) and a Novel Deletion in the Spike Gene (Δ246-252, G75V, T76I, L452Q, F490S, T859N)—SARS-CoV-2 Coronavirus/nCoV-2019 Genomic Epidemiology. Available online: https://virological.org/t/novel-sublineage-within-b-1-1-1-currently-expanding-in-peru-and-chile-with-a-convergent-deletion-in-the-orf1a-gene-3675-3677-and-a-novel-deletion-in-the-spike-gene-246-252-g75v-t76i-l452q-f490s-t859n/685 (accessed on 13 January 2025).
- Colombia Confirma su Primer Caso de COVID-19. Available online: https://www.minsalud.gov.co/Paginas/Colombia-confirma-su-primer-caso-de-COVID-19.aspx (accessed on 13 January 2025).
- Colombia es el país de Suramérica que más rápido Reporta sus Secuencias de Genomas del SARS-CoV-2 a Bases de Datos Internacionales. Available online: https://www.ins.gov.co/Noticias/Paginas/Colombia-es-el-pais-de-Suramerica-que-mas-rapido-reporta-sus-secuencias-de-genomas-del-SARS-CoV-2.aspx (accessed on 4 February 2025).
- Colombia Confirma Presencia de la Variante Delta en el país. Available online: https://www.minsalud.gov.co/Paginas/Colombia-confirma-presencia-de-la-variante-Delta-en-el-pais-.aspx (accessed on 4 February 2025).
- INS Identificó Tres Casos de Variante Ómicron en Colombia. Available online: https://www.minsalud.gov.co/Paginas/INS-identifico-tres-casos-de-variante-Omicron-en-Colombia.aspx (accessed on 13 January 2025).
- INS confirma circulación viral de la variante ‘andina’ en Boyacá. Gob. Boyacá 2021. Available online: https://www.boyaca.gov.co/ins-confirma-circulacion-viral-de-la-variante-andina-en-boyaca/ (accessed on 2 February 2025).
- CDC C for DC and P. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. Available online: https://www.fda.gov/media/134922/download (accessed on 14 January 2025).
- Artic Network. Available online: https://artic.network/ncov-2019/ncov2019-bioinformatics-sop.html (accessed on 13 January 2025).
- Cori, A.; Ferguson, N.M.; Fraser, C.; Cauchemez, S. A New Framework and Software to Estimate Time-Varying Reproduction Numbers During Epidemics. Am. J. Epidemiol. 2013, 178, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Boletines Vacunación Covid. Available online: https://www.cali.gov.co/publicaciones/159073/boletines-vacunacion-covid/ (accessed on 4 February 2025).
- Registros de Vacunas Para COVID-19 Aplicadas en Santiago de Cali—Datos Abiertos Cali. Available online: http://datos.cali.gov.co/ca/dataset/registros-de-vacunas-para-covid-aplicadas (accessed on 4 February 2025).
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Sagulenko, P.; Bedford, T.; Neher, R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Sagulenko, P.; Puller, V.; Neher, R.A. TreeTime: Maximum-likelihood phylodynamic analysis. Virus Evol. 2018, 4, vex042. [Google Scholar] [CrossRef] [PubMed]
- Idrovo, A.J. More social discontent than pandemic-related risk perception in Colombia. Lancet 2021, 398, 211. [Google Scholar] [CrossRef]
- Funk, S.; Salathé, M.; Jansen, V.A.A. Modelling the influence of human behaviour on the spread of infectious diseases: A review. J. R. Soc. Interface 2010, 7, 1247–1256. [Google Scholar] [CrossRef]
- Quispe-Ricalde, M.A.; Castelán-Sánchez, H.G.; Meza-Rodríguez, P.M.; Dávila-Ramos, S.; Sierra, J.L.; Batista-Garcia, R.; Concha-Velasco, F.; Lucana, S.F.; De Santa Cruz, J.; Zea, V.; et al. Evidence of natural selection and dominance of SARS-CoV-2 variant Lambda (C.37) over variants of concern in Cusco, Peru. Arch. Virol. 2023, 168, 88. [Google Scholar] [CrossRef]
- First Identification of SARS-CoV-2 lambda (C.37) variant in Southern Brazil|Infection Control & Hospital Epidemiology | Cambridge Core. Available online: https://www.cambridge.org/core/journals/infection-control-and-hospital-epidemiology/article/first-identification-of-sarscov2-lambda-c37-variant-in-southern-brazil/42A50C4938E81BAAE1F4CAF1E03B9419 (accessed on 14 January 2025).
- Hossain, M.J.; Rabaan, A.A.; Mutair, A.A.; Alhumaid, S.; Emran, T.B.; Saikumar, G.; Mitra, S.; Dhama, K. Strategies to tackle SARS-CoV-2 Mu, a newly classified variant of interest likely to resist currently available COVID-19 vaccines. Hum. Vaccines Immunother. 2022, 18, 2027197. [Google Scholar] [CrossRef] [PubMed]
- Pascarella, S.; Bianchi, M.; Giovanetti, M.; Narzi, D.; Cauda, R.; Cassone, A.; Ciccozzi, M. The SARS-CoV-2 Mu variant should not be left aside: It warrants attention for its immuno-escaping ability. J. Med. Virol. 2022, 94, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P.; Zeng, C.; Qu, P.; Faraone, J.; Zheng, Y.-M.; Carlin, C.; Bednash, J.S.; Zhou, T.; Lozanski, G.; Mallampalli, R.; et al. Neutralization of SARS-CoV-2 Omicron sub-lineages BA.1, BA.1.1, and BA.2. Cell Host Microbe 2022, 30, 1093–1102.e3. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Montoya, J.; Rodríguez-Villamizar, L.A.; Idrovo, A.J. Massive social protests amid the pandemic in selected Colombian cities: Did they increase COVID-19 cases? Medrxiv 2021. [Google Scholar] [CrossRef]
- Patiño, L.H.; Castañeda, S.; Muñoz, M.; Ballesteros, N.; Ramirez, A.L.; Luna, N.; Guerrero-Araya, E.; Pérez, J.; Correa-Cárdenas, C.A.; Duque, M.C.; et al. Epidemiological Dynamics of SARS-CoV-2 Variants During Social Protests in Cali, Colombia. Front. Med. 2022, 9, 863911. [Google Scholar] [CrossRef]
- Fernández-Niño, J.A.; Peña, C. Potential Effects of Social Protests in Colombia on the Transmission of COVID-19. Soc. Sci. Res. Netw. 2021. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Alvarez, D.; Rivera-Franco, N.; Aristizabal, E.; Solarte, M.; Castillo, A.; Pardo, C.A.; Parra, B. Behavioral Dynamics, Genomic Insights, and Social Drivers of SARS-CoV-2 Waves and Variants in Cali, Colombia (2020–2023). Viruses 2025, 17, 800. https://doi.org/10.3390/v17060800
López-Alvarez D, Rivera-Franco N, Aristizabal E, Solarte M, Castillo A, Pardo CA, Parra B. Behavioral Dynamics, Genomic Insights, and Social Drivers of SARS-CoV-2 Waves and Variants in Cali, Colombia (2020–2023). Viruses. 2025; 17(6):800. https://doi.org/10.3390/v17060800
Chicago/Turabian StyleLópez-Alvarez, Diana, Nelson Rivera-Franco, Erica Aristizabal, Melissa Solarte, Andrés Castillo, Carlos A. Pardo, and Beatriz Parra. 2025. "Behavioral Dynamics, Genomic Insights, and Social Drivers of SARS-CoV-2 Waves and Variants in Cali, Colombia (2020–2023)" Viruses 17, no. 6: 800. https://doi.org/10.3390/v17060800
APA StyleLópez-Alvarez, D., Rivera-Franco, N., Aristizabal, E., Solarte, M., Castillo, A., Pardo, C. A., & Parra, B. (2025). Behavioral Dynamics, Genomic Insights, and Social Drivers of SARS-CoV-2 Waves and Variants in Cali, Colombia (2020–2023). Viruses, 17(6), 800. https://doi.org/10.3390/v17060800






