Abstract
Since its emergence in late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has continuously evolved, giving rise to multiple variants that have significantly altered the trajectory of the COVID-19 pandemic. These variants have resulted in multiple waves of the pandemic, exhibiting characteristic mutations in the spike (S) protein that may have affected receptor interaction, tissue tropism, and cell entry mechanisms. While the virus was shown to primarily utilize the angiotensin-converting enzyme 2 (ACE2) receptor and host proteases such as transmembrane serine protease 2 (TMPRSS2) for entry into host cells, alterations in the S protein have resulted in changes to receptor binding affinity and use of alternative receptors, potentially expanding the virus’s ability to infect different cell types or tissues, contributing to shifts in clinical presentation. These changes have been linked to variations in disease severity, the emergence of new clinical manifestations, and altered transmission dynamics. In this paper, we overview the evolving receptor utilization strategies of SARS-CoV-2, focusing on how mutations in the S protein may have influenced viral entry mechanisms and clinical outcomes across the ongoing pandemic waves.
1. Introduction
Coronaviruses (CoVs) represent a family of RNA viruses that affect humans and a broad range of animal species, inducing a spectrum of diseases ranging from mild respiratory infections to severe life-threatening conditions [1]. Over the years, CoVs have undergone significant evolutionary adaptations, enhancing their infectivity and transmissibility. They have been responsible for multiple significant outbreaks in the last two decades, notably, severe acute respiratory syndrome (SARS) in 2002–2003, Middle East respiratory syndrome (MERS) in 2012, porcine epidemic diarrhea coronavirus (PEDV) outbreak in 2013, and recently, the coronavirus disease of 2019 (COVID-19) pandemic [2]. Given their high reproduction rates and accelerated evolutionary trends, these viruses are capable of crossing species barriers and pose a significant public health burden due to their frequent zoonotic transmission.
Among the four identified coronavirus genera, human coronaviruses (HCoVs) belong to the Alpha and Beta coronavirus genera [3]. Seven coronavirus strains are known to cause diseases in humans, with four strains—229E, NL63, OC43, and HKU1—causing seasonal mild infections [4,5,6,7], while SARS-CoV, MERS-CoV, and SARS-CoV-2 may lead to the development of severe and potentially lethal infections, often causing recurring pandemics and constituting great social and economic burdens [8,9,10]. Bats and other intermediate hosts are considered to be the source of the three virulent viruses due to congruence in the genetic constitution [11]. For instance, SARS-CoV depicts a 99% sequence identity with a virus infecting civet cats [12], while MERS-CoV was transmitted to humans by dromedary camels [13]. SARS-CoV-2 shows 98% sequence identity to the bat coronavirus strain RaTG13 and to human SARS-CoV with 79% genetic identity [14].
Compared to DNA viruses, RNA viruses, like coronaviruses, have a high rate of mutation, with vital consequences for fast adaptation and cross-species transmissions [15,16,17]. Frequent human contact with wildlife and domesticated animals has played a significant role in their evolution and in the selection of mutations that favor receptor adaptability, moreover, the continuous exposure to animal reservoirs allows these pathogens to test various receptor interactions until they evolve mutations that enable the cross-species jump [18,19].
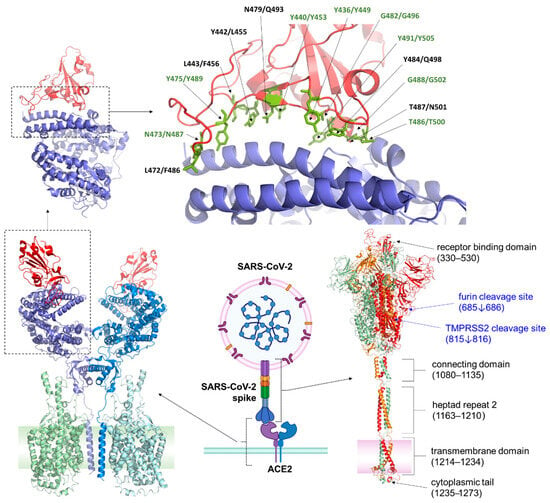
Key aspects of coronavirus permissivity, pathogenicity, and host specificity are their mechanism of entry, which is mediated by interactions between the viral spike (S) protein and specific receptors on host cell surfaces. This entry process is critical for viral infection, initiating the fusion between viral and host membranes, allowing the viral genome to enter host cells [20]. The entry receptor-binding mechanism involves complex interactions of the receptor-binding domains (RBDs) of the S proteins, which undergo conformational shifts to achieve optimal binding affinity with host target receptors [20]. The S protein is a trimeric transmembrane protein comprising two subunits, S1 and S2. S1 contains the RBD that recognizes and binds the target receptor on host cells, while the S2 subunit enables viral fusion with the host cell membrane upon priming by host proteases; such as transmembrane protease serine 2 (TMPRSS2) [21,22]. The role of cellular proteases in priming viral glycoproteins for entry is not unique to SARS-CoVs. Like the SARS-CoV-2 S protein, the influenza virus utilizes its hemagglutinin (HA) protein to recognize and bind host cell receptors, typically sialic acid residues. Following receptor binding, both viruses require host proteases for priming; SARS-CoV-2 depends on TMPRSS2 or cathepsins at the entry process, while influenza HA undergoes cleavage by host proteases such as trypsin or furin before assembly. This cleavage triggers conformational changes that facilitate membrane fusion, allowing viral RNA to enter the cytoplasm for replication [23]. Thus, while the specific receptors and fusion mechanisms differ, both viruses exploit host proteases and glycoprotein-mediated entry to infect cells.
RBD of the S protein is known to undergo mutations that can significantly influence host specificity and infectivity. Research shows that small mutations within the RBD can allow SARS-like coronaviruses to switch hosts, and to bind to human angiotensin-converting enzyme 2 (ACE2), despite their animal origin [17]. For example, several variants of SARS-CoV-2 have emerged with mutations in the RBD that increase the binding efficiency to ACE2, enhancing their ability to spread between humans and potentially cross to new hosts [24].
Coronaviruses exhibit a notable adaptability in receptor usage, exploiting both proteins and carbohydrate molecules on the cell surface. Commonly used and well-characterized receptors include ACE2, dipeptidyl peptidase 4 (DPP4), and aminopeptidase N (APN), utilized, respectively, by SARS-CoV-1 and -2, MERS-CoV, and HCoV-229E. Some coronaviruses use alternative receptors, such as sugars and cell adhesion molecules [25]. Coronaviruses HKU1 and OC43 employ 9-O-acetylated sialic acids for attachment [26], while heparin sulfate is used by NL63 for attachment in addition to ACE2 for cell entry [27]. HKU1 uses TMPRSS2 as a protein receptor, while there is no known protein receptor for OC43 [28]. A summary of human pathogenic coronaviruses, including their associated diseases, so far identified primary receptors, and major outbreak years, is presented in Table 1.

Table 1.
Human pathogenic coronaviruses and their primary receptors.
Compared to non-enveloped viruses, enveloped viruses exhibit a greater potential for transmission across different host species. Zoonotic viruses, which belong to diverse viral families, include SARS-CoV-2 (Coronaviridae), influenza (Orthomyxoviridae), and HIV (Retroviridae) [33]. This increased potential for evolving towards the use of novel receptors is attributed to the relatively low rigidity of the envelope proteins. The ability to utilize alternative receptors expands the range of host cells and species, thereby increasing the risk of cross-species transmission and zoonosis. A correlation between the number of alternative receptors and the host range has been established, with various proteins serving as receptors for entry across diverse viral families [34].
3. Role of Other Receptors
Certain cell surface proteins, besides primary receptors, could play a crucial role in aiding coronavirus entry by acting as attachment factors or co-receptors.
3.1. Dipeptidyl Peptidase 4 (DPP4)
Also known as CD26, DPP4 serves as the entry receptor for MERS-CoV [13]. It is a type II transmembrane serine exopeptidase expressed in the respiratory tract, liver, kidney, and intestinal cells, and on the surface of immune cells. This protease catalyzes the digestion of multiple chemokines, neuropeptides, and regulatory peptides, and plays crucial metabolic roles, such as regulating glucose levels by inactivating incretins and modulating adenosine levels through its interaction with adenosine deaminase [103,104]. DPP4 is structurally more diverse across species compared to ACE2. Structural studies on DPP4 variants across species demonstrated that MERS-CoV can engage DPP4 from camels, horses, and bats, but not from mice, due to minor structural variations of the DPP4 protein across species [8]. Related bat coronaviruses, such as BatCoV-HKU4, have also shown an affinity for DPP4, suggesting evolutionary adaptations. Although distantly related coronaviruses, like SARS-CoV and SARS-CoV-2, were initially thought not to utilize DPP4 due to differences in the CTD loop structures that affect the receptor-binding interface [105], recent findings, along with the fast pace at which mutations appear in the variants, may suggest otherwise [106].
In silico studies have shown that amino acid residues in the DPP4 can interact with, and are capable of, binding residues in the S RBD, with an affinity of −34.8 kcal/mol, comparable to the binding affinity for ACE2 (−39.2 kcal/mol). The amino acid residues in S RBD that were shown to be involved in the binding with DPP4 were Q498, D405, E484, Y489/N487, N501, and Y505. Mutations in the E484 or adjacent residues were found to be critical for the DPP4-binding ability of SARS-CoV-2 [107]. In regard to variants of SARS-CoV-2, computational analysis revealed that L452R and T478K mutations in the Delta variant directly participate in the interaction with DPP4. Also, the E484K mutation found in Beta and Gamma variants was also found to interact with the receptor [106]. Given that most of those mutations are found in the Omicron variant or its descendants, it is conceivable that further in silico analysis might also reveal similar interactions of the Omicron variants with the DPP4. While in vitro/in vivo evidence of utilization of DPP4 by SARS-CoV-2 remains lacking, clinical studies using DPP4 inhibitors have shown a reduction in respiratory complications, a decrease in mortality, and complications in COVID-19 patients [108,109]. A recent study demonstrated that knockdown of DPP4 in astrocytes and pericytes did not significantly impact infection rates by SARS-CoV-2 (wild-type virus); however, it led to a reduction in the mRNA levels of the N and S proteins compared to the DPP4-expressing control cells, suggesting a decreased replication potential [110].
3.2. Neuropilin-1 (NRP1)
Neuropilin-1 (NRP1) is a transmembrane glycoprotein that is involved in many physiological processes, such as axon guidance in the central nervous system, angiogenesis, and immune regulation [111]. While its role in infection by other coronaviruses remains inconclusive, early studies into the pandemic showed that it may act as a direct receptor for SARS-CoV-2 [112] or as an auxiliary factor that enhances viral entry into host cells, particularly in tissues with low ACE2 expression, especially in the CNS [113,114]. An interesting molecular dynamics simulation study [115] showed that the Delta variant exhibited the strongest binding to NRP1, whereas the early Omicron variant BA.1 showed reduced affinity for NRP1 compared to the wild-type. The authors further speculated that viral evolution is unlikely to enhance NRP1 binding affinity, suggesting a potential shift away from the utilization of this receptor.
3.3. Aminopeptidase N (APN)
Aminopeptidase N, also termed CD13, is a type II transmembrane metallopeptidase that cleaves amino acids from the N-terminus of peptides. It plays an important role in protein digestion, immune response regulation, angiogenesis, and cellular adhesion. This protein is expressed in epithelial cells of the intestines, the nervous system, and immune cells such as monocytes and dendritic cells. APN is also found in a soluble form in the plasma [116]. It is the primary target receptor for many Alpha coronaviruses, facilitating viral entry through binding to the coronavirus S subunit S1 CTD [117]. Human coronavirus HCoV-229E, transmissible gastroenteritis virus (TGEV), and related feline and canine coronaviruses (FCoV and CCoV) all utilize APN to infect host cells, each adapting distinct molecular interactions to engage the receptors [118]. Interestingly, while APN was initially thought to be unique to Alpha coronaviruses, studies have shown that porcine Delta coronaviruses (PDCoV) can also utilize APN for entry, where the interaction with APN is species-specific, influenced by variations in glycosylation patterns that affect receptor recognition and binding affinity [119]. In regard to Beta coronaviruses and SARS-CoV-2 in particular, there is little evidence to suggest the utilization of this metalloprotease by the virus. Gene expression analysis in human tissue revealed that expression of peptidases such as alanyl and glutamyl aminopeptidases shows a similar pattern to that of ACE2 [120]. Given the similarity in the sequence of the S protein between Alpha and Beta coronaviruses, while it may not be a primary target receptor, for lack of evidence, it is conceivable that it may function as a secondary or auxiliary receptor during infection. Known for its role in the regulation of immune response, others have even hypothesized that APN might influence SARS-CoV-2 pathogenesis through alternative pathways, such as amplifying the immune response, thereby limiting infectivity [121,122].
3.4. Glucose-Regulated Protein 78 (GRP78)
As a chaperone protein, besides its main function in protein homeostasis in the endoplasmic reticulum, GRP78 is also involved in many biological processes, such as signaling, inflammation, and cell survival [123]. During the response to ER stress or inflammation, this chaperone protein is also present on the cell surface, where it mediates binding to various ligands [124,125]. At the cell surface, GRP78 was shown to facilitate infection by SARS-CoV-2 through the formation of a complex with the S protein and the host receptor ACE2 [125,126]. In immortalized human monocyte-like THP-1 cells, GRP78 was found to facilitate entry of pseudovirions pseudotyped with the wild-type S protein in an ACE2-independent manner [127]. Utilizing the surface plasmon resonance technique, a research group found direct interaction between GRP78 and the S protein, and the affinity between GRP78 and the S protein of the Omicron variant was approximately two times higher than that of the wild-type. Given that GRP78 is a chaperone protein that is expressed in a wide variety of tissues and cells, this may greatly aid viral entry into non-target/off-target sites.
While there is a lack of studies on this receptor in association with SARS-CoV-2, there is a reason to believe that other variants, such as Delta or the newer emerging Omicron variants, also utilize this molecule for entry, especially given the diversity of COVID-19 symptoms presented by patients infected with those variants.
3.5. Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 (CEACAM1)
CEACAM1, a member of the immunoglobulin superfamily, is expressed mainly in the liver and central nervous system, and is involved in many physiological functions, such as cellular adhesion, development, and immune modulation [128]. The murine coronavirus mouse hepatitis virus (MHV) strain A59, a prototype species for the Betacoronavirus genus, utilizes murine carcinoembryonic antigen-related cell adhesion molecule 1 (mCEACAM1) as its primary receptor [129]. Unlike most coronaviruses that utilize the S protein’s C-terminal domain for receptor binding, MHV-A59 uses the S protein’s N-terminal domain instead (NTD). Structural studies suggest that this NTD usage phenomenon might have evolved from a host-derived galectin domain, retaining a carbohydrate-binding function [130]. To date, there is no evidence to suggest utilization of this protein by SARS-CoV-2 as an entry receptor, although transcriptomic analysis of a limited number of autopsy samples obtained from COVID-19 diseased patients revealed upregulation of CEACAM1, which correlated with viral levels [131]. This certainly does not imply an association between SARS-CoV-2 and CEACAM, and may be a result of immune dysregulation, given that the patients succumbed to COVID-19.
4. Other Potential Receptors
Heparan sulfate proteoglycans (HSPGs) are negatively charged macromolecules that are found on the cell surface and extracellular space, facilitating cellular adhesion, division, and migration [132]. In the context of infection with SARS-CoV-2, they were found to act as auxiliary attachment factors that complement ACE2, enhancing the efficiency of SARS-CoV-2’s binding and entry into host cells. This mechanism is particularly important to facilitate viral infection in tissues where expression of ACE2 is low [133,134]. This interaction appears to occur with the S1/S2 interface of each monomer in the trimeric S protein, and at residues 453–459 [135]. Similar interactions have been observed in feline and canine coronaviruses (FCoVs and CCoVs), as well as in human coronaviruses like OC43 and NL63 [7,32]. Other viruses also utilize HSPGs for cell entry, such as HIV, wherein interaction with the HIV envelope glycoprotein gp120 augments viral accumulation at the cell surface, enhancing the binding affinity to CD4 and the co-receptors CCR5 and CXCR4 [136].
Basigin (CD147) is a transmembrane glycoprotein belonging to the immunoglobulin family that is involved in various physiological processes, such as immune response and cell signaling, and has been implicated in assisting entry of pathogens [137]. While direct involvement with SARS-CoV-2 S protein was not proven, it was postulated that it may act as an auxiliary receptor or co-factor for SARS-CoV-2’s S protein, providing an alternative mechanism for viral attachment to the host cell surface, perhaps even independent of ACE2 via activation of the endocytotic pathway [138,139].
C-type lectins, such as DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3) and L-SIGN (liver/lymph node-specific intracellular adhesion molecule), are known to facilitate the entry of HIV-1 by allowing dendritic cells to capture the virus and transfer it to T cells in lymphoid tissues, thereby enhancing infection [140]. In regard to coronaviruses, studies have shown that DC-SIGN and L-SIGN can assist in the entry of various strains, including SARS-CoV, HCoV-229E, and infectious bronchitis virus (IBV), by promoting viral attachment to host cells [141].
Coronaviruses can also exploit toll-like receptors (TLRs) on host cells to enhance infection and trigger immune responses. TLRs, a family of receptors involved in recognizing pathogens, detect viral components like RNA and S proteins, leading to immune activation. Some studies suggest that SARS-CoV-2, along with other coronaviruses, may engage TLRs to support viral entry or amplify inflammatory responses, especially in lung tissues. For example, TLR2 and TLR4 are thought to interact with coronavirus structural proteins, potentially intensifying inflammation, which is common in severe COVID-19 cases. This TLR activation not only aids viral entry, but may also lead to excessive immune responses, contributing to disease severity [142].
More recently, histamine receptor H1 (HRH1) was found to act as an auxiliary receptor for SARS-CoV-2, directly binding to the viral S protein and enhancing ACE2-mediated viral entry [143]. In that study, antihistamines were able to block infection of HEK cells expressing ACE2 by almost all SARS-CoV-2 variants, albeit at higher micromolar concentrations that are not typically achieved with the usual dosage of the drugs [144].
5. Receptor-Mediated Endocytotic Pathways
Receptor-mediated endocytosis is a highly specific cellular uptake mechanism, enabling cells to internalize extracellular molecules through the interaction of ligands with their corresponding cell surface receptors. Following binding of the ligands to their receptors, recruitment of intracellular adaptor proteins ensues, triggering a cascade of events that leads to internalization of the particles into cells [145]. The acidic environment of the endosome often facilitates the dissociation of ligands from their receptors, leading to either receptor recycling to the plasma membrane, or trafficking to lysosomes for degradation. While clathrin-mediated endocytosis is the most well-characterized and prevalent pathway, alternative mechanisms also exist, including the caveolae-mediated pathway and clathrin- and caveolin-independent routes [146]. Importantly, several viruses exploit these pathways to gain entry into host cells. Once internalized, these viruses manipulate endosomal maturation and pH-dependent conformational changes to trigger membrane fusion or escape into the cytoplasm, enabling subsequent replication.
Research has shown that caveolae and lipid raft microdomains in the cell membrane play a crucial role in viral endocytosis, facilitating viral entry. Being rich in cholesterol, sphingolipids, and glycosylphosphatidylinositol-anchored proteins, they provide a cholesterol-rich, receptor-dense platform that many viruses utilize for efficient entry and infection. In fact, some viruses, such as certain types of human papillomaviruses [147] and Simian virus 40 (SV40), are known to exploit caveolin-1-dependent pathways for endocytosis [148], while HIV and influenza require the cholesterol-rich domains for membrane fusion and entry [149,150].
Clathrin-mediated endocytosis, on the other hand, is a key cellular process that is also exploited by certain viruses. Under the membrane, clathrin forms a lattice that drives membrane invagination, leading to the formation of clathrin-coated vesicles, thereafter, dynamin mediates vesicle scission, releasing the contents of the vesicles into the cytoplasm followed by fusion with early endosomes [151]. Given that the traffic here is typically directed towards lysosomes, this pathway is favorable for viruses that require low pH for uncoating, such as dengue and adenoviruses [152,153].
In regard to SARS-CoV-2, studies have shown that SARS-CoV-2 utilizes the clathrin-mediated endocytotic pathway after interacting with ACE2 [154,155], although SARS-CoV-2 may also enter cells via clathrin-mediated endocytosis in a manner that is independent of ACE2, potentially involving other receptors.
The transferrin receptor (TFRC or CD71), a membrane protein, plays a key role in binding and internalizing iron-bound transferrin into cells, making it essential for both immune function and cell growth [156]. Recent studies indicate that certain viruses, including the influenza virus, exploit this receptor for cell entry [157,158], although, mechanistic interaction between the virus and the receptor has not yet been explored. More recently, TFRC was identified as a receptor for SARS-CoV-2, facilitating viral entry by directly binding to the S protein in an ACE2 knockdown Calu-3 cell model [159]. To support their findings, the authors utilized soluble anti-TFRC antibodies and specially designed peptides to disrupt the interaction between TFRC and the virus, which was found to abrogate viral infection.
6. Mutations in Omicron Subvariants: Implications for Receptor Binding and Viral Entry
It is noteworthy that the Omicron variant contains over 30 mutations, significantly more than any of the previous variants, which has led some to speculate that evolution in a non-human host might be responsible [160]. Mutations such as Q493R, N501Y, and Y505H were found to be particularly well-suited to the ACE2 receptor of mouse origin, rather than that of humans, suggesting a potential mouse origin for the variant.
Novel mutations in the subvariants reflect key changes in the S protein. Mutations such as the “flip mutations”, wherein the amino acid at a given position is “flipped” or changed to a different one, often switching between two possible amino acids, are thought to affect transmissibility, immune escape, or potentially impact vaccine efficacy. Examples of such mutations include L452R, F486V, and Q493R, which are common across several subvariants. These mutations have been shown to significantly impact the variant’s susceptibility to both polyclonal and monoclonal antibodies, enhancing their ability to evade immune responses [161].
Other mutations, such as S371L, and S373P, are also commonly observed in Omicron-related subvariants. Although these mutations were computationally predicted to destabilize the RBD of the S protein in the closed conformation, the cross-RBD interactions that stabilize one RBD in the open conformation within the S trimer, along with compensatory mutations in the ACE2-binding site, may collectively enhance the variant’s affinity to the receptor, likely contributing to increased infectivity [162,163].
The increased transmissibility of newer variants is a complex phenomenon. The enhanced binding affinity of the Omicron RBD to ACE2 may improve the variant’s ability to infect the respiratory tract, where ACE2 expression is relatively low. Mutations such as S477N, N501Y, and T478K have been shown to strengthen the binding to ACE2 [164], which may, in turn, shift the viral tropism, promoting infection and replication in the upper respiratory tract and accelerating the spread of the infection [165]. In addition, mutations that facilitate immune evasion contribute significantly to the rapid spread and dominance of Omicron and subsequent variants over earlier strains. Studies have demonstrated that Omicron exhibits substantial resistance to neutralizing antibodies, with both post-convalescent and post-vaccination sera showing significantly reduced neutralization efficacy compared to previous variants [166].
Further evidence suggests that Omicron has altered its preferred cell entry mechanism, shifting from TMPRSS2-mediated surface fusion of the spike protein to cathepsin-mediated endosomal fusion, independent of TMPRSS2 [167]. This adaptation may indicate a shift in pathogenicity, potentially mitigating severe lung infection. In a study assessing the infection of mice and hamsters with several Omicron subvariants, the authors found reduced infection in immunocompetent and human ACE2-expressing mice and hamsters compared to previous SARS-CoV-2 variants [168]. Despite evidence that the Omicron S protein binds more strongly to mouse ACE2, the infected transgenic mice and hamsters showed limited pathogenesis and lower viral loads in both upper and lower respiratory tracts, hinting at the course of mild infections. This observation is consistent with current clinical data, which indicates that the majority of current COVID-19 cases present with mild symptoms and reduced severity compared to earlier variants. Overall, the immune evasion mechanisms and the shift in Omicron’s cell entry pathways likely contribute to its reduced pathogenicity, as it induces less severe disease while maintaining high transmissibility.
A summary of the most recent SARS-CoV-2 variants, along with their significant mutations in the S protein, is provided in Table 3. These mutations highlight the ongoing evolution of the virus and underscore their potential impact on transmissibility, and immune escape.

Table 3.
Key Omicron subvariants and their characteristic mutations in the S protein. Data was collected from [66,169,170,171,172]. * Cumulative prevalence is the ratio of the sequences collected since the identification of the variant based on data from GISAID Initiative.
7. Evidence of Shift in Receptor Utilization from the Changing Clinical Spectrum of Infection
To date, it is evident that the pattern of symptoms associated with COVID-19 varied over time as the pandemic progressed, with shifts in the symptom clusters linked to different viral variants (Table 4). During the initial phase, infection with the prototypic Wuhan strain typically involved the lower respiratory tract, resulting in severe pneumonia, dyspnea, and acute respiratory distress syndrome (ARDS) [170]. The hallmark of early COVID-19 during the pandemic was the reduced or lost sense of smell and taste. While nearly two-thirds of patients infected with the Alpha and Delta variants reported these symptoms, their prevalence significantly decreased with Omicron. Evidence suggests that the lack of anosmia or ageusia is associated with the Omicron variant’s decreased affinity for the olfactory epithelium [170]. As the pandemic advanced, common upper respiratory cold-like symptoms such as nasal congestion, sneezing and sore throat became more prevalent with the Delta, Omicron, and its subsequent subvariants [170,173], in fact, studies demonstrated higher viral loads in the upper respiratory tract, particularly in the nasal cavity, trachea, and throat, compared to the lower respiratory tract [174]. Moreover, long-term sequelae of COVID-19 were more frequently reported after infection with the prototypical and earlier Alpha and Delta variants, more so compared to the more recent Omicron and its subvariants [175]. This difference is likely attributable to the heightened inflammatory response and greater severity of infection associated with the earlier variants, rather than differences in tissue tropism.

Table 4.
Common and prominent symptoms of SARS-CoV-2 variants [176,177,178].
8. Therapeutic Implications
As previously discussed, mutations in the S protein have greatly impacted viral infectivity and immune evasion, most importantly, altering the effectiveness of therapeutic interventions, particularly susceptibility to monoclonal antibodies (mAbs). This necessitated the development of updated vaccine formulations to target both ancestral and emerging variants, resulting in a constant “catch-up” scenario in vaccine development, with the virus maintaining an advantage. The updated 2024 to 2025 formula mRNA COVID-19 vaccines are still based on the S protein of KP.2, which has already become outdated [179].
The emerging Omicron subvariants, harboring variant-specific critical mutations in the RBD such as S371L, D405N, K417N, N440K, G446S, F486V, E484K/A, and Q493R, were shown to reduce the neutralization potency of several mAbs by altering key antigenic sites in vitro, which may translate into therapeutic escape [180]. Tixagevimab/Cilgavimab (Evusheld) is a monoclonal antibody cocktail that gained emergency authorization in December of 2021 for the treatment and pre-exposure prophylaxis of COVID-19 in individuals who are immunocompromised or otherwise unable to mount an adequate immune response to vaccination. In the face of emerging Omicron variants, clinical and in vitro data showed a significant loss of efficacy, especially against BA.4/BA.5, BA.2, BQ.1, XBB.1.5, XBB.1.16, and EG.5 variants [181,182], which led to the loss of FDA authorization for use in the United States [183].
Pemivibart (Pemgarda) is a more recent monoclonal antibody that was granted Emergency Use Authorization (EUA) by the FDA in August of 2024, and so far had demonstrated notable efficacy in neutralizing BA.1, BA.2, BA.5, BQ.1.1, XBB.1.5, and JN.1-lineage variants (KP.2/KP.3/KP.3.1.1/XEC/LP8.1), with EC50 values ranging from 0.198 to 14.3 nM [184].
Research on hybrid immunity (a combination of infection and vaccination) has provided compelling insights. The SC27 monoclonal antibody, presumed to be generated through mRNA vaccination in an infection-naïve individual, showed a marked enhancement in potency following a hybrid immune response triggered by a subsequent infection. This antibody was found to effectively neutralize both the prototypical and current variants of concern of SARS-CoV-2, as well as a range of antigenically distinct zoonotic sarbecoviruses that may pose a future risk to humans. Its neutralization potency, as measured by IC50 values, ranged from 13 to 264 ng/mL [185]. It remains in the research and development phase, and as of the time of writing this review, it has not yet received EUA or full approval for widespread clinical application.
Given the pivotal role of the ACE2 receptor in mediating infectivity by SARS-CoV-2, the idea of using decoy ACE2 (soluble serum form) to target the virus’s S protein, preventing it from attaching to human cells gained traction earlier on at the start of the pandemic [186,187]. Utilizing minimal receptor fragments that mimic the host ACE2 receptor without triggering an immune response, however, is key. The challenge lies in identifying the most efficient minimal sequences of ACE2 that retain the ability to bind the virus while avoiding immune recognition. Mutations in the RBD of current variants, or perhaps the shift toward a completely different primary receptor, have cast doubt on that strategy. However, through computational design, an affinity-enhanced ACE2 decoy demonstrated strong binding to both the SARS-CoV-2 Delta and Omicron variants, suggesting its potential as an effective therapeutic against a wide range of SARS-CoV-2 variants and other sarbecoviruses [188], including BQ.1 and XBB.1 [189]. Beyond ACE2 affinity modulation, the evidence pointing to the utilization of alternative receptors, such as neuropilin-1 and heparan sulfate, to facilitate infection by SARS-CoV-2 highlights the urgent need for surveillance of S protein evolution and the development of broadly neutralizing antibodies targeting conserved viral epitopes to mitigate the risk of immune escape and receptor switching.
9. Conclusions
The wide variability in receptor utilization by coronaviruses underscores their adaptability and capacity to cross species barriers. In this review, we explored the receptors utilized by the SARS-CoV-2 spike protein and their impact on infectivity and tissue tropism across variants, which collectively shaped the trajectory of the COVID-19 pandemic. This shift in receptor utilization could explain the wide clinical spectrum of infection by different variants throughout the pandemic. Studies on the effects of mutations on the interaction between the S protein and potential receptors are of great importance to understanding the entry pathways of coronaviruses. A deeper understanding of how emerging variants adapt to different receptors is crucial for predicting tissue tropism, clinical outcomes, and transmission patterns. Furthermore, such knowledge will undoubtedly lay a critical foundation for developing novel therapeutic strategies and preventive interventions targeting viral entry mechanisms. There are indeed broader implications from a public health perspective, vaccine development, and pandemic preparedness. Understanding viral evolution and receptor interactions would undoubtedly aid in the robust and adaptable vaccine designs, offering broader protection against emerging strains. Moreover, this knowledge could improve surveillance efforts, allowing for more rapid identification of emerging variants. The shifting of receptor utilization dynamics highlights the need for adaptive strategies. Ongoing and future research could benefit greatly from using structural and molecular modeling tools to predict the interaction between the S protein and current/potential receptors across coronaviruses and other viral families. Such tools would enable identification and analysis of viral variants, receptor mapping and interaction modeling. In this era of artificial intelligence, AlphaFold and machine learning algorithms, would further enhance predictive modeling, and help pinpoint critical sites that would enhance the development of vaccines targeting viral entry.
Author Contributions
M.M. and J.T.: conceptualization; M.M., I.W.K., A.S.A.-M., J.A.M., J.T. and A.T.: writing of manuscript; J.A.M. and G.H.: preparation of figures and in silico simulations. All authors have read and agreed to the published version of the manuscript.
Funding
Project no. TKP2021-EGA-20 has been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA funding scheme. NKFIH K132623 and POST-COVID2021-33 were also implemented. This project was supported in part by the NKFIH Advanced Grant 150532 (to J.T.). János A. Mótyán is supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00110/23/5). Supported by the University of Debrecen (UD) Program for Scientific Publication. Gyula Hoffka was supported by the EKÖP-24-4 University Research Fellowship program of the Ministry for Culture and Innovation [EKÖP-24-4-I-DE-435]. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
List of Abbreviations
SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; COVID-19: Coronavirus disease of 2019; S:Spike protein; ACE2: Angiotensin-converting enzyme 2; TMPRSS2: Transmembrane serine protease 2; CoVs: Coronaviruses; SARS: Severe acute respiratory syndrome; MERS: Middle East respiratory syndrome; PEDV: Porcine epidemic diarrhea coronavirus; HCoVs: Human coronaviruses; RBD: Receptor-binding domains; HA: Hemagglutinin; DPP4: Dipeptidyl peptidase 4; APN: Aminopeptidase N; HIV: Human immunodeficiency virus; CLD: Collectrin-like domain; sACE2: Soluble agiotensin-converting enzyme 2; ADAM17: A disintegrin and metalloprotease 17; NRP1: Neuropilin-1; CNS: Central nervous system; GRP78: Glucose-regulated protein 78; CEACAM1: Carcinoembryonic antigen-related cell adhesion molecule 1; MHV: Mose hepatitis virus; mCEACAM1: Mrine carcinoembryonic antigen-related cell adhesion molecule 1; NTD:N-terminal domain; HSPGs: Heparan sulfate proteoglycans; FCoVs: Feline coronaviruses; CCoVs: Canine coronaviruses; gp120: Glycoprotein 120; CCR5: C-C chemokine receptor type 5; CXCR4: C-X-C chemokine receptor type 4; DC-SIGN: Dendritic cell-specific intercellular adhesion molecule-3; IBV: Infectious bronchitis virus; TLRs: Toll-like receptors; HRH1: Histamine receptor H1; SV40: Simian virus 40; ARDS: Acute respiratory distress syndrome; EUA: Emergency Use Authorization; FDA: Food and Drug Administration.
References
- Peiris, J.S.M. Coronaviruses. In Medical Microbiology, 18th ed.; Churchill Livingstone: London, UK, 2012. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, F.; Wang, R.; Yange, M.; Guan, K.; Jiang, T.; Xu, G.; Sun, J.; Chang, C. The deadly coronaviruses: The 2003 sars pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020, 109, 102434. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Enjuanes, L.; Ziebuhr, J.; Snijder, E.J. Nidovirales: Evolving the largest rna virus genome. Virus Res. 2006, 117, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, Y.; Michelow, I.C.; Choe, Y.J. Global seasonality of human coronaviruses: A systematic review. Open Forum Infect. Dis. 2020, 7, ofaa443. [Google Scholar] [CrossRef] [PubMed]
- Pfefferle, S.; Oppong, S.; Drexler, J.F.; Gloza-Rausch, F.; Ipsen, A.; Seebens, A.; Muller, M.A.; Annan, A.; Vallo, P.; Adu-Sarkodie, Y.; et al. Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229e in bats, ghana. Emerg. Infect. Dis. 2009, 15, 1377–1384. [Google Scholar] [CrossRef]
- Vijgen, L.; Keyaerts, E.; Moes, E.; Thoelen, I.; Wollants, E.; Lemey, P.; Vandamme, A.M.; Van Ranst, M. Complete genomic sequence of human coronavirus OC43: Molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 2005, 79, 1595–1604. [Google Scholar] [CrossRef]
- Milewska, A.; Zarebski, M.; Nowak, P.; Stozek, K.; Potempa, J.; Pyrc, K. Human coronavirus nl63 utilizes heparan sulfate proteoglycans for attachment to target cells. J. Virol. 2014, 88, 13221–13230. [Google Scholar] [CrossRef]
- Barlan, A.; Zhao, J.; Sarkar, M.K.; Li, K.; McCray, P.B., Jr.; Perlman, S.; Gallagher, T. Receptor variation and susceptibility to middle east respiratory syndrome coronavirus infection. J. Virol. 2014, 88, 4953–4961. [Google Scholar] [CrossRef]
- Faramarzi, A.; Norouzi, S.; Dehdarirad, H.; Aghlmand, S.; Yusefzadeh, H.; Javan-Noughabi, J. The global economic burden of COVID-19 disease: A comprehensive systematic review and meta-analysis. Syst. Rev. 2024, 13, 68. [Google Scholar] [CrossRef]
- Kirtipal, N.; Bharadwaj, S.; Kang, S.G. From sars to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2020, 85, 104502. [Google Scholar]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats are natural reservoirs of SARS-like coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef]
- Wang, L.F.; Eaton, B.T. Bats, civets and the emergence of sars. Curr. Top. Microbiol. Immunol. 2007, 315, 325–344. [Google Scholar] [PubMed]
- de Groot, R.J.; Baker, S.C.; Baric, R.S.; Brown, C.S.; Drosten, C.; Enjuanes, L.; Fouchier, R.A.; Galiano, M.; Gorbalenya, A.E.; Memish, Z.A.; et al. Middle east respiratory syndrome coronavirus (MERS-CoV): Announcement of the coronavirus study group. J. Virol. 2013, 87, 7790–7792. [Google Scholar] [CrossRef]
- Wrobel, A.G.; Benton, D.J.; Xu, P.; Roustan, C.; Martin, S.R.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effects. Nat. Struct. Mol. Biol. 2020, 27, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.W.; Holland, J.J. Mutation rates among RNA viruses. Proc. Natl. Acad. Sci. USA 1999, 96, 13910–13913. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Garcia-Crespo, C.; Lobo-Vega, R.; Perales, C. Mutation rates, mutation frequencies, and proofreading-repair activities in RNA virus genetics. Viruses 2021, 13, 1882. [Google Scholar] [CrossRef]
- Graham, R.L.; Baric, R.S. Recombination, reservoirs, and the modular spike: Mechanisms of coronavirus cross-species transmission. J. Virol. 2010, 84, 3134–3146. [Google Scholar] [CrossRef]
- Everest, H.; Stevenson-Leggett, P.; Bailey, D.; Bickerton, E.; Keep, S. Known cellular and receptor interactions of animal and human coronaviruses: A review. Viruses 2022, 14, 351. [Google Scholar] [CrossRef]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef]
- Brian, D.A.; Baric, R.S. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 2005, 287, 1–30. [Google Scholar]
- EA, J.A.; Jones, I.M. Membrane binding proteins of coronaviruses. Future Virol. 2019, 14, 275–286. [Google Scholar]
- Edinger, T.O.; Pohl, M.O.; Stertz, S. Entry of influenza a virus: Host factors and antiviral targets. J. Gen. Virol. 2014, 95, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lv, F.; Li, Z.; Zhao, C.; Wang, X.; Zhu, P.; Zhou, X. Cross-species susceptibility of emerging variants of SARS-CoV-2 spike. Genes 2024, 15, 1321. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.; Ibrahim, I.M.; Amin, F.G.; Magdy, M.; Elgharib, A.M.; Azzam, E.B.; Nasser, F.; Yousry, K.; Shamkh, I.M.; Mahdy, S.M.; et al. A review of human coronaviruses’ receptors: The host-cell targets for the crown bearing viruses. Molecules 2021, 26, 6455. [Google Scholar] [CrossRef]
- Hulswit, R.J.G.; Lang, Y.; Bakkers, M.J.G.; Li, W.; Li, Z.; Schouten, A.; Ophorst, B.; van Kuppeveld, F.J.M.; Boons, G.J.; Bosch, B.J.; et al. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc. Natl. Acad. Sci. USA 2019, 116, 2681–2690. [Google Scholar] [CrossRef]
- Hofmann, H.; Pyrc, K.; van der Hoek, L.; Geier, M.; Berkhout, B.; Pohlmann, S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. USA 2005, 102, 7988–7993. [Google Scholar] [CrossRef]
- Saunders, N.; Fernandez, I.; Planchais, C.; Michel, V.; Rajah, M.M.; Baquero Salazar, E.; Postal, J.; Porrot, F.; Guivel-Benhassine, F.; Blanc, C.; et al. TMPRSS2 is a functional receptor for human coronavirus HKU1. Nature 2023, 624, 207–214. [Google Scholar] [CrossRef]
- Wentworth, D.E.; Holmes, K.V. Molecular determinants of species specificity in the coronavirus receptor aminopeptidase N (CD13): Influence of N-linked glycosylation. J. Virol. 2001, 75, 9741–9752. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Sui, J.; Kuhn, J.H.; Moore, M.J.; Luo, S.; Wong, S.K.; Huang, I.C.; Xu, K.; Vasilieva, N.; et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005, 24, 1634–1643. [Google Scholar] [CrossRef]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Muller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell 2020, 183, 1043–1057.e15. [Google Scholar] [CrossRef] [PubMed]
- Valero-Rello, A.; Sanjuan, R. Enveloped viruses show increased propensity to cross-species transmission and zoonosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2215600119. [Google Scholar] [CrossRef]
- Valero-Rello, A.; Baeza-Delgado, C.; Andreu-Moreno, I.; Sanjuan, R. Cellular receptors for mammalian viruses. PLoS Pathog. 2024, 20, e1012021. [Google Scholar] [CrossRef]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000, 87, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Menach, E.; Hashida, Y.; Yasukawa, K.; Inouye, K. Effects of conversion of the zinc-binding motif sequence of thermolysin, HEXXH, to that of dipeptidyl peptidase III, HEXXXH, on the activity and stability of thermolysin. Biosci. Biotechnol. Biochem. 2013, 77, 1901–1906. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Patel, S.K.; Velkoska, E.; Burrell, L.M. Emerging markers in cardiovascular disease: Where does angiotensin-converting enzyme 2 fit in? Clin. Exp. Pharmacol. Physiol. 2013, 40, 551–559. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Yang, L.; You, R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol. Res. 2020, 157, 104833. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–30119. [Google Scholar] [CrossRef]
- Zoufaly, A.; Poglitsch, M.; Aberle, J.H.; Hoepler, W.; Seitz, T.; Traugott, M.; Grieb, A.; Pawelka, E.; Laferl, H.; Wenisch, C.; et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020, 8, 1154–1158. [Google Scholar] [CrossRef]
- Hikmet, F.; Mear, L.; Edvinsson, A.; Micke, P.; Uhlen, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for sars coronavirus. A first step in understanding sars pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Crackower, M.A.; Sarao, R.; Oudit, G.Y.; Yagil, C.; Kozieradzki, I.; Scanga, S.E.; Oliveira-dos-Santos, A.J.; da Costa, J.; Zhang, L.; Pei, Y.; et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002, 417, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Chua, R.L.; Lukassen, S.; Trump, S.; Hennig, B.P.; Wendisch, D.; Pott, F.; Debnath, O.; Thurmann, L.; Kurth, F.; Volker, M.T.; et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020, 38, 970–979. [Google Scholar] [CrossRef]
- Blume, C.; Jackson, C.L.; Spalluto, C.M.; Legebeke, J.; Nazlamova, L.; Conforti, F.; Perotin, J.M.; Frank, M.; Butler, J.; Crispin, M.; et al. A novel ACE2 isoform is expressed in human respiratory epithelia and is upregulated in response to interferons and RNA respiratory virus infection. Nat. Genet. 2021, 53, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef]
- Ma, X.; Lu, M.; Gorman, J.; Terry, D.S.; Hong, X.; Zhou, Z.; Zhao, H.; Altman, R.B.; Arthos, J.; Blanchard, S.C.; et al. HIV-1 env trimer opens through an asymmetric intermediate in which individual protomers adopt distinct conformations. eLife 2018, 7, e34271. [Google Scholar] [CrossRef]
- Cervantes, M.; Hess, T.; Morbioli, G.G.; Sengar, A.; Kasson, P.M. The ACE2 receptor accelerates but is not biochemically required for SARS-CoV-2 membrane fusion. Chem. Sci. 2023, 14, 6997–7004. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Yu, S.; Zheng, X.; Zhou, B.; Li, J.; Chen, M.; Deng, R.; Wong, G.; Lavillette, D.; Meng, G. SARS-CoV-2 spike engagement of ACE2 primes s2′ site cleavage and fusion initiation. Proc. Natl. Acad. Sci. USA 2022, 119, e2111199119. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Lan, Q.; Su, S.; Wang, X.; Xu, W.; Liu, Z.; Zhu, Y.; Wang, Q.; Lu, L.; Jiang, S. The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin. Signal Transduct. Target. Ther. 2020, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Almehdi, A.M.; Khoder, G.; Alchakee, A.S.; Alsayyid, A.T.; Sarg, N.H.; Soliman, S.S.M. SARS-CoV-2 spike protein: Pathogenesis, vaccines, and potential therapies. Infection 2021, 49, 855–876. [Google Scholar] [CrossRef]
- Ismail, A.M.; Elfiky, A.A. SARS-CoV-2 spike behavior in situ: A Cryo-EM images for a better understanding of the COVID-19 pandemic. Signal Transduct. Target. Ther. 2020, 5, 252. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 183, 1735. [Google Scholar] [CrossRef]
- Casalino, L.; Gaieb, Z.; Goldsmith, J.A.; Hjorth, C.K.; Dommer, A.C.; Harbison, A.M.; Fogarty, C.A.; Barros, E.P.; Taylor, B.C.; McLellan, J.S.; et al. Beyond shielding: The roles of glycans in the SARS-CoV-2 spike protein. ACS Cent. Sci. 2020, 6, 1722–1734. [Google Scholar] [CrossRef]
- Liu, H.; Wei, P.; Kappler, J.W.; Marrack, P.; Zhang, G. SARS-CoV-2 variants of concern and variants of interest receptor binding domain mutations and virus infectivity. Front. Immunol. 2022, 13, 825256. [Google Scholar]
- Cherian, S.; Potdar, V.; Jadhav, S.; Yadav, P.; Gupta, N.; Das, M.; Rakshit, P.; Singh, S.; Abraham, P.; Panda, S.; et al. SARS-CoV-2 spike mutations, L452R, T478K, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. Microorganisms 2021, 9, 1542. [Google Scholar] [CrossRef]
- Le, K.; Kannappan, S.; Kim, T.; Lee, J.H.; Lee, H.R.; Kim, K.K. Structural understanding of SARS-CoV-2 virus entry to host cells. Front. Mol. Biosci. 2023, 10, 1288686. [Google Scholar] [CrossRef] [PubMed]
- Cele, S.; Karim, F.; Lustig, G.; San, J.E.; Hermanus, T.; Tegally, H.; Snyman, J.; Moyo-Gwete, T.; Wilkinson, E.; Bernstein, M.; et al. SARS-CoV-2 prolonged infection during advanced HIV disease evolves extensive immune escape. Cell Host Microbe 2022, 30, 154–162.e5. [Google Scholar] [CrossRef] [PubMed]
- Hattab, D.; Amer, M.F.A.; Al-Alami, Z.M.; Bakhtiar, A. SARS-CoV-2 journey: From alpha variant to omicron and its sub-variants. Infection 2024, 52, 767–786. [Google Scholar] [CrossRef]
- Tzou, P.L.; Tao, K.; Pond, S.L.K.; Shafer, R.W. Coronavirus resistance database (CoV-RDB): SARS-CoV-2 susceptibility to monoclonal antibodies, convalescent plasma, and plasma from vaccinated persons. PLoS ONE 2022, 17, e0261045. [Google Scholar] [CrossRef]
- Niu, S.; Wang, J.; Bai, B.; Wu, L.; Zheng, A.; Chen, Q.; Du, P.; Han, P.; Zhang, Y.; Jia, Y.; et al. Molecular basis of cross-species ACE2 interactions with SARS-CoV-2-like viruses of pangolin origin. EMBO J. 2021, 40, e107786. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Monteiro da Silva, G.; Cui, J.Y.; Dalgarno, D.C.; Lisi, G.P.; Rubenstein, B.M. High-throughput prediction of protein conformational distributions with subsampled AlphaFold2. Nat. Commun. 2024, 15, 2464. [Google Scholar] [CrossRef]
- Singh, A.; Copeland, M.M.; Kundrotas, P.J.; Vakser, I.A. Gramm web server for protein docking. Methods Mol. Biol. 2024, 2714, 101–112. [Google Scholar]
- Fraser, B.J.; Beldar, S.; Seitova, A.; Hutchinson, A.; Mannar, D.; Li, Y.; Kwon, D.; Tan, R.; Wilson, R.P.; Leopold, K.; et al. Structure and activity of human TMPRSS2 protease implicated in SARS-CoV-2 activation. Nat. Chem. Biol. 2022, 18, 963–971. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARA-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Piva, F.; Sabanovic, B.; Cecati, M.; Giulietti, M. Expression and co-expression analyses of TMPRSS2, a key element in COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2021, 40, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Limburg, H.; Harbig, A.; Bestle, D.; Stein, D.A.; Moulton, H.M.; Jaeger, J.; Janga, H.; Hardes, K.; Koepke, J.; Schulte, L.; et al. TMPRSS2 is the major activating protease of influenza a virus in primary human airway cells and influenza B virus in human type II pneumocytes. J. Virol. 2019, 93, e00649-19. [Google Scholar] [CrossRef]
- Shulla, A.; Heald-Sargent, T.; Subramanya, G.; Zhao, J.; Perlman, S.; Gallagher, T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011, 85, 873–882. [Google Scholar] [CrossRef]
- Shirato, K.; Kawase, M.; Matsuyama, S. Wild-type human coronaviruses prefer cell-surface tmprss2 to endosomal cathepsins for cell entry. Virology 2018, 517, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, X.; Zhang, X.; Zhao, Z.; Lu, Y.; Pu, D.; Zhang, Z.; Chen, J.; Wang, Y.; Li, M.; et al. Tmprss2 and glycan receptors synergistically facilitate coronavirus entry. Cell 2024, 187, 4261–4271.e17. [Google Scholar] [CrossRef]
- Lu, G.; Wang, Q.; Gao, G.F. Bat-to-human: Spike features determining ‘host jump’ of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015, 23, 468–478. [Google Scholar] [CrossRef]
- Ou, T.; Mou, H.; Zhang, L.; Ojha, A.; Choe, H.; Farzan, M. Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2. PLoS Pathog. 2021, 17, e1009212. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Johnson, B.A.; Xia, H.; Ku, Z.; Schindewolf, C.; Widen, S.G.; An, Z.; Weaver, S.C.; Menachery, V.D.; et al. Delta spike P681R mutation enhances SARS-CoV-2 fitness over alpha variant. Cell Rep. 2022, 39, 110829. [Google Scholar] [CrossRef]
- Furusawa, Y.; Kiso, M.; Iida, S.; Uraki, R.; Hirata, Y.; Imai, M.; Suzuki, T.; Yamayoshi, S.; Kawaoka, Y. In SARS-CoV-2 delta variants, spike-P681R and D950N promote membrane fusion, spike-P681R enhances spike cleavage, but neither substitution affects pathogenicity in hamsters. EBioMedicine 2023, 91, 104561. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Khan, M.; Chiliveri, S.C.; Hu, X.; Irvin, P.; Leek, M.; Grieshaber, A.; Hu, Z.; Jang, E.S.; Bax, A.; et al. SARS-CoV-2 omicron variants harbor spike protein mutations responsible for their attenuated fusogenic phenotype. Commun. Biol. 2023, 6, 556. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.Z.; Sathi, F.A.; Nooruzzaman, M.; Parvin, R. Molecular insights into the SARS-CoV-2 omicron variant from bangladesh suggest diverse and continuous evolution. Virology 2023, 587, 109882. [Google Scholar] [CrossRef] [PubMed]
- Escalera, A.; Gonzalez-Reiche, A.S.; Aslam, S.; Mena, I.; Laporte, M.; Pearl, R.L.; Fossati, A.; Rathnasinghe, R.; Alshammary, H.; van de Guchte, A.; et al. Mutations in SARS-CoV-2 variants of concern link to increased spike cleavage and virus transmission. Cell Host Microbe 2022, 30, 373–387.e7. [Google Scholar] [CrossRef]
- Takeda, M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol. Immunol. 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Chakraborty, C.; Saha, A.; Bhattacharya, M.; Dhama, K.; Agoramoorthy, G. Natural selection of the D614G mutation in SARS-CoV-2 Omicron (B.1.1.529) variant and its subvariants. Mol. Therapy Nucleic Acids 2023, 31, 437–439. [Google Scholar] [CrossRef]
- Gellenoncourt, S.; Saunders, N.; Robinot, R.; Auguste, L.; Rajah, M.M.; Kervevan, J.; Jeger-Madiot, R.; Staropoli, I.; Planchais, C.; Mouquet, H.; et al. The spike-stabilizing D614G mutation interacts with S1/S2 cleavage site mutations to promote the infectious potential of SARS-CoV-2 variants. J. Virol. 2022, 96, e0130122. [Google Scholar] [CrossRef]
- Laporte, M.; Raeymaekers, V.; Van Berwaer, R.; Vandeput, J.; Marchand-Casas, I.; Thibaut, H.J.; Van Looveren, D.; Martens, K.; Hoffmann, M.; Maes, P.; et al. The SARS-CoV-2 and other human coronavirus spike proteins are fine-tuned towards temperature and proteases of the human airways. PLoS Pathog. 2021, 17, e1009500. [Google Scholar] [CrossRef]
- Scheepers, C.; Everatt, J.; Amoako, D.G.; Tegally, H.; Wibmer, C.K.; Mnguni, A.; Ismail, A.; Mahlangu, B.; Lambson, B.E.; Martin, D.P.; et al. Emergence and phenotypic characterization of the global SARS-CoV-2 C.1.2 lineage. Nat. Commun. 2022, 13, 1976. [Google Scholar] [CrossRef]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin l prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef]
- Maciewicz, R.A.; Etherington, D.J.; Kos, J.; Turk, V. Collagenolytic cathepsins of rabbit spleen—A kinetic-analysis of collagen degradation and inhibition by chicken cystatin. Collagen Relat. Res. 1987, 7, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Mason, R.W.; Chen, P.; Joseph, L.J.; Sukhatme, V.P.; Yee, R.; Chapman, H.A. Synthesis and processing of cathepsin-L, an elastase, by human alveolar macrophages. Biochem. J. 1989, 257, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Zhu, Y.; Zhang, L.; Zhong, G.; Tai, L.; Liu, S.; Yin, G.; Lu, J.; He, Q.; Li, M.J.; et al. Novel cleavage sites identified in SARS-CoV-2 spike protein reveal mechanism for cathepsin L-facilitated viral infection and treatment strategies. Cell Discov. 2022, 8, 53. [Google Scholar] [CrossRef]
- Park, J.E.; Li, K.; Barlan, A.; Fehr, A.R.; Perlman, S.; McCray, P.B., Jr.; Gallagher, T. Proteolytic processing of middle east respiratory syndrome coronavirus spikes expands virus tropism. Proc. Natl. Acad. Sci. USA 2016, 113, 12262–12267. [Google Scholar] [CrossRef] [PubMed]
- Bosch, B.J.; Bartelink, W.; Rottier, P.J. Cathepsin l functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J. Virol. 2008, 82, 8887–8890. [Google Scholar] [CrossRef]
- Bestle, D.; Heindl, M.R.; Limburg, H.; Van Lam van, T.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance 2020, 3, e202000786. [Google Scholar] [CrossRef]
- Sasaki, M.; Uemura, K.; Sato, A.; Toba, S.; Sanaki, T.; Maenaka, K.; Hall, W.W.; Orba, Y.; Sawa, H. SARS-CoV-2 variants with mutations at the S1/S2 cleavage site are generated in vitro during propagation in TMPRSS2-deficient cells. PLoS Pathog. 2021, 17, e1009233. [Google Scholar] [CrossRef]
- Sarker, J.; Das, P.; Sarker, S.; Roy, A.K.; Momen, A. A review on expression, pathological roles, and inhibition of TMPRSS2, the serine protease responsible for SARS-CoV-2 spike protein activation. Scientifica 2021, 2021, 2706789. [Google Scholar] [CrossRef]
- Suzuki, R.; Yamasoba, D.; Kimura, I.; Wang, L.; Kishimoto, M.; Ito, J.; Morioka, Y.; Nao, N.; Nasser, H.; Uriu, K.; et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature 2022, 603, 700–705. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, L.; Peng, Z.; Chen, L.L.; Meng, X.; Zhang, C.; Ip, J.D.; Chan, W.M.; Chu, A.W.; Chan, K.H.; et al. SARS-CoV-2 omicron variant shows less efficient replication and fusion activity when compared with delta variant in TMPRSS2-expressed cells. Emerg. Microbes Infect. 2022, 11, 277–283. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Kakizaki, M.; Shiwa-Sudo, N.; Okura, T.; Tahara, M.; Fukushi, S.; Maeda, K.; Kawase, M.; Asanuma, H.; Tomita, Y.; et al. Essential role of TMPRSS2 in SARS-CoV-2 infection in murine airways. Nat. Commun. 2022, 13, 6100. [Google Scholar] [CrossRef] [PubMed]
- Meyerholz, D.K.; Lambertz, A.M.; McCray, P.B., Jr. Dipeptidyl peptidase 4 distribution in the human respiratory tract: Implications for the middle east respiratory syndrome. Am. J. Pathol. 2016, 186, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Sebastian-Martin, A.; Sanchez, B.G.; Mora-Rodriguez, J.M.; Bort, A.; Diaz-Laviada, I. Role of dipeptidyl peptidase-4 (DPP4) on COVID-19 physiopathology. Biomedicines 2022, 10, 2026. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L.; Liu, C.; Wang, L.; Ma, C.; Tang, J.; Baric, R.S.; Jiang, S.; Li, F. Receptor usage and cell entry of bat coronavirus hku4 provide insight into bat-to-human transmission of mers coronavirus. Proc. Natl. Acad. Sci. USA 2014, 111, 12516–12521. [Google Scholar] [CrossRef]
- Roy, A.N.; Gupta, A.M.; Banerjee, D.; Chakrabarti, J.; Raghavendra, P.B. Unraveling dpp4 receptor interactions with SARS-CoV-2 variants and mers-cov: Insights into pulmonary disorders via immunoinformatics and molecular dynamics. Viruses 2023, 15, 2056. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Yang, L.; Lian, X.; Xie, Y.; Li, S.; Xin, S.; Cao, P.; Lu, J. The Mers-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience 2020, 23, 101160. [Google Scholar] [CrossRef] [PubMed]
- Nyland, J.E.; Raja-Khan, N.T.; Bettermann, K.; Haouzi, P.A.; Leslie, D.L.; Kraschnewski, J.L.; Parent, L.J.; Grigson, P.S. Diabetes, drug treatment, and mortality in COVID-19: A multinational retrospective cohort study. Diabetes 2021, 70, 2903–2916. [Google Scholar] [CrossRef]
- Abbasi, F.; Adatorwovor, R.; Davarpanah, M.A.; Mansoori, Y.; Hajiani, M.; Azodi, F.; Sefidbakht, S.; Davoudi, S.; Rezaei, F.; Mohammadmoradi, S.; et al. A randomized trial of sitagliptin and spironolactone with combination therapy in hospitalized adults with COVID-19. J. Endocr. Soc. 2022, 6, bvac017. [Google Scholar] [CrossRef]
- Martinez, T.E.; Mayilsamy, K.; Mohapatra, S.S.; Mohapatra, S. Modulation of paracellular permeability in SARS-CoV-2 blood-to-brain transcytosis. Viruses 2024, 16, 785. [Google Scholar] [CrossRef]
- Pellet-Many, C.; Frankel, P.; Jia, H.; Zachary, I. Neuropilins: Structure, function and role in disease. Biochem. J. 2008, 411, 211–226. [Google Scholar] [CrossRef]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Anton-Plagaro, C.; Shoemark, D.K.; Simon-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Cao, W.; Kim, S.; Cui, X.; Ziarnik, M.; Im, W.; Zhang, X.F. Biophysical investigation of interactions between SARS-CoV-2 spike protein and neuropilin-1. Protein Sci. A Publ. Protein Soc. 2023, 32, e4773. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Montano, M.; Corley, M.J.; Helmy, E.; Kobayashi, H.; Kinisu, M.; Suryawanshi, R.; Luo, X.; Royer, L.A.; Roan, N.R.; et al. Neuropilin-1 mediates SARS-CoV-2 infection of astrocytes in brain organoids, inducing inflammation leading to dysfunction and death of neurons. mBio 2022, 13, e0230822. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Hieu, H.K.; Nguyen, T.Q.; Nhung, N.T.A.; Li, M.S. Neuropilin-1 protein may serve as a receptor for SARS-CoV-2 infection: Evidence from molecular dynamics simulations. J. Phys. Chem. B 2024, 128, 7141–7147. [Google Scholar] [CrossRef]
- Morgan, R.L.; Behbahani-Nejad, N.; Endres, J.; Amin, M.A.; Lepore, N.J.; Du, Y.; Urquhart, A.; Chung, K.C.; Fox, D.A. Localization, shedding, regulation and function of aminopeptidase N/CD13 on fibroblast like synoviocytes. PLoS ONE 2016, 11, e0162008. [Google Scholar] [CrossRef]
- Delmas, B.; Gelfi, J.; Sjostrom, H.; Noren, O.; Laude, H. Further characterization of aminopeptidase-N as a receptor for coronaviruses. Adv. Exp. Med. Biol. 1993, 342, 293–298. [Google Scholar]
- Tresnan, D.B.; Holmes, K.V. Feline aminopeptidase N is a receptor for all group I coronaviruses. Coronaviruses Arter. 1998, 440, 69–75. [Google Scholar]
- Chen, L.; Lin, Y.L.; Peng, G.; Li, F. Structural basis for multifunctional roles of mammalian aminopeptidase n. Proc. Natl. Acad. Sci. USA 2012, 109, 17966–17971. [Google Scholar] [CrossRef]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef]
- Devarakonda, C.K.V.; Meredith, E.; Ghosh, M.; Shapiro, L.H. Coronavirus receptors as immune modulators. J. Immunol. 2021, 206, 923–929. [Google Scholar] [CrossRef]
- Alves, M.; Mahnke, L.C.; Macedo, T.C.; Silva, T.; Carvalho Junior, L.B. The enzymes in COVID-19: A review. Biochimie 2022, 197, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Gopal, U.; Pizzo, S.V. Cell surface GRP78 signaling: An emerging role as a transcriptional modulator in cancer. J. Cell. Physiol. 2021, 236, 2352–2363. [Google Scholar] [CrossRef]
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Toyoda, S.; Fukuhara, A.; Shimomura, I. GRP78, a novel host factor for SARS-CoV-2: The emerging roles in COVID-19 related to metabolic risk factors. Biomedicines 2022, 10, 1995. [Google Scholar] [CrossRef] [PubMed]
- Carlos, A.J.; Ha, D.P.; Yeh, D.W.; Van Krieken, R.; Tseng, C.C.; Zhang, P.; Gill, P.; Machida, K.; Lee, A.S. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. J. Biol. Chem. 2021, 296, 100759. [Google Scholar] [CrossRef]
- Han, B.; Lv, Y.; Moser, D.; Zhou, X.; Woehrle, T.; Han, L.; Osterman, A.; Rudelius, M.; Chouker, A.; Lei, P. ACE2-independent SARS-CoV-2 virus entry through cell surface GRP78 on monocytes—Evidence from a translational clinical and experimental approach. EBioMedicine 2023, 98, 104869. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Qiao, S.W.; Nagaishi, T.; Blumberg, R.S. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J. Immunol. 2008, 180, 6085–6093. [Google Scholar] [CrossRef]
- Dveksler, G.S.; Pensiero, M.N.; Cardellichio, C.B.; Williams, R.K.; Jiang, G.S.; Holmes, K.V.; Dieffenbach, C.W. Cloning of the mouse hepatitis virus (MHV) receptor: Expression in human and hamster cell lines confers susceptibility to MHV. J. Virol. 1991, 65, 6881–6891. [Google Scholar] [CrossRef]
- Peng, G.; Sun, D.; Rajashankar, K.R.; Qian, Z.; Holmes, K.V.; Li, F. Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 10696–10701. [Google Scholar] [CrossRef]
- Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Mdkhana, B.; Al Heialy, S.; Alsafar, H.S.; Hamoudi, R.; Hamid, Q.; Halwani, R. Enhanced expression of immune checkpoint receptors during SARS-CoV-2 viral infection. Mol. Ther. Methods Clin. Dev. 2021, 20, 109–121. [Google Scholar] [CrossRef]
- Elgundi, Z.; Papanicolaou, M.; Major, G.; Cox, T.R.; Melrose, J.; Whitelock, J.M.; Farrugia, B.L. Cancer metastasis: The role of the extracellular matrix and the heparan sulfate proteoglycan perlecan. Front. Oncol. 2019, 9, 1482. [Google Scholar] [CrossRef]
- Zhang, Q.; Pavlinov, I.; Ye, Y.; Zheng, W. Therapeutic development targeting host heparan sulfate proteoglycan in SARS-CoV-2 infection. Front. Med. 2024, 11, 1364657. [Google Scholar]
- Yue, J.; Jin, W.; Yang, H.; Faulkner, J.; Song, X.; Qiu, H.; Teng, M.; Azadi, P.; Zhang, F.; Linhardt, R.J.; et al. Heparan sulfate facilitates spike protein-mediated SARS-CoV-2 host cell invasion and contributes to increased infection of SARS-CoV-2 G614 mutant and in lung cancer. Front. Mol. Biosci. 2021, 8, 649575. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jin, W.; Sood, A.; Montgomery, D.W.; Grant, O.C.; Fuster, M.M.; Fu, L.; Dordick, J.S.; Woods, R.J.; Zhang, F.; et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antivir. Res. 2020, 181, 104873. [Google Scholar] [CrossRef] [PubMed]
- Connell, B.J.; Lortat-Jacob, H. Human immunodeficiency virus and heparan sulfate: From attachment to entry inhibition. Front. Immunol. 2013, 4, 385. [Google Scholar] [CrossRef]
- Fenizia, C.; Galbiati, S.; Vanetti, C.; Vago, R.; Clerici, M.; Tacchetti, C.; Daniele, T. SARS-CoV-2 entry: At the crossroads of CD147 and ACE2. Cells 2021, 10, 1434. [Google Scholar] [CrossRef]
- Shilts, J.; Crozier, T.W.M.; Greenwood, E.J.D.; Lehner, P.J.; Wright, G.J. No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor. Sci. Rep. 2021, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Wang, K.; Wang, X.Y.; Cui, H.Y.; Zhao, Y.; Zhu, P.; Chen, Z.N. SARS-CoV-2 pseudovirus enters the host cells through spike protein-CD147 in an Arf6-dependent manner. Emerg. Microbes Infect. 2022, 11, 1135–1144. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.H.; van Kooyk, Y. Pathogens target DC-SIGN to influence their fate—DC-SIGN functions as a pathogen receptor with broad specificity. APMIS 2003, 111, 698–714. [Google Scholar] [CrossRef]
- Jeffers, S.A.; Tusell, S.M.; Gillim-Ross, L.; Hemmila, E.M.; Achenbach, J.E.; Babcock, G.J.; Thomas, W.D.; Thackray, L.B.; Young, M.D.; Mason, R.J.; et al. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. USA 2004, 101, 15748–15753. [Google Scholar] [CrossRef]
- Choudhury, A.; Mukherjee, S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ace-2 receptor homologs and human tlrs. J. Med. Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, X.; Ou, H.; Li, X.; Liu, R.; Lv, X.; Xiao, S.; Hu, M.; Liang, T.; Chen, T.; et al. The histamine receptor H1 acts as an alternative receptor for SARS-CoV-2. mBio 2024, 15, e0108824. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Drug Approvals and Databases. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases (accessed on 20 February 2025).
- Mak, T.W.; Saunders, M.E. The immune response basic and clinical principles preface. In Immune Response: Basic and Clinical Principles; Academic Press: New York, NY, USA, 2006; Volume VII. [Google Scholar]
- Chaudhary, N.; Gomez, G.A.; Howes, M.T.; Lo, H.P.; McMahon, K.A.; Rae, J.A.; Schieber, N.L.; Hill, M.M.; Gaus, K.; Yap, A.S.; et al. Endocytic crosstalk: Cavins, caveolins, and caveolae regulate clathrin-independent endocytosis. PLoS Biol. 2014, 12, e1001832. [Google Scholar] [CrossRef]
- Smith, J.L.; Campos, S.K.; Wandinger-Ness, A.; Ozbun, M.A. Caveolin-1-dependent infectious entry of human papillomavirus Type 31 in human keratinocytes proceeds to the endosomal pathway for pH-dependent uncoating. J. Virol. 2008, 82, 9505–9512. [Google Scholar] [CrossRef]
- Norkin, L.C.; Anderson, H.A.; Wolfrom, S.A.; Oppenheim, A. Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J. Virol. 2002, 76, 5156–5166. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Kiessling, V.; Simmons, J.A.; White, J.M.; Tamm, L.K. HIV gp41-mediated membrane fusion occurs at edges of cholesterol-rich lipid domains. Nat. Chem. Biol. 2015, 11, 424–431. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, C.Y.; Yang, J.H.; Chiu, Y.F. Modulating cholesterol-rich lipid rafts to disrupt influenza a virus infection. Front. Immunol. 2022, 13, 982264. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. reviews. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- van der Schaar, H.M.; Rust, M.J.; Chen, C.; van der Ende-Metselaar, H.; Wilschut, J.; Zhuang, X.; Smit, J.M. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 2008, 4, e1000244. [Google Scholar] [CrossRef]
- Meier, O.; Greber, U.F. Adenovirus endocytosis. J. Gene Med. 2004, 6 (Suppl. S1), S152–S163. [Google Scholar] [CrossRef]
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021, 296, 100306. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, K.; Susumu, K.; Chen, J.; Xu, M.; Pradhan, M.; Zhu, W.; Hu, X.; Breger, J.C.; Wolak, M.; Oh, E. Quantum dot-conjugated SARS-CoV-2 spike pseudo-virions enable tracking of angiotensin converting enzyme 2 binding and endocytosis. ACS Nano 2020, 14, 12234–12247. [Google Scholar] [CrossRef]
- Ponka, P.; Lok, C.N. The transferrin receptor: Role in health and disease. Int. J. Biochem. Cell Biol. 1999, 31, 1111–1137. [Google Scholar] [CrossRef] [PubMed]
- Mazel-Sanchez, B.; Niu, C.; Williams, N.; Bachmann, M.; Choltus, H.; Silva, F.; Serre-Beinier, V.; Karenovics, W.; Iwaszkiewicz, J.; Zoete, V.; et al. Influenza a virus exploits transferrin receptor recycling to enter host cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2214936120. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.L.; Murphy, W.J.; Wang, D.; O’Brien, S.J.; Parrish, C.R. Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells. J. Virol. 2001, 75, 3896–3902. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, C.; Tang, X.; Yang, M.; Duan, Z.; Liu, L.; Lu, S.; Ma, L.; Cheng, R.; Wang, G.; et al. Human transferrin receptor can mediate SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2024, 121, e2317026121. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, K.; Geng, Q.; Ye, G.; Aihara, H.; Li, F. Structural basis for mouse receptor recognition by SARS-CoV-2 omicron variant. Proc. Natl. Acad. Sci. USA 2022, 119, e2206509119. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, H.; Hu, Y.; Zheng, X.; Chang, F.; Liu, Y.; Pan, Z.; Wang, Q.; Tang, F.; Qian, J.; et al. Immune evasion of omicron variants JN.1, KP.2, and KP.3 to the polyclonal and monoclonal antibodies from COVID-19 convalescents and vaccine recipients. Antivir. Res. 2025, 235, 106092. [Google Scholar] [CrossRef]
- Zheng, B.; Xiao, Y.; Tong, B.; Mao, Y.; Ge, R.; Tian, F.; Dong, X.; Zheng, P. S373P mutation stabilizes the receptor-binding domain of the spike protein in omicron and promotes binding. JACS Au 2023, 3, 1902–1910. [Google Scholar] [CrossRef]
- Yin, W.; Xu, Y.; Xu, P.; Cao, X.; Wu, C.; Gu, C.; He, X.; Wang, X.; Huang, S.; Yuan, Q.; et al. Structures of the omicron spike trimer with ACE2 and an anti-omicron antibody. Science 2022, 375, 1048–1053. [Google Scholar] [CrossRef]
- Geng, Q.; Shi, K.; Ye, G.; Zhang, W.; Aihara, H.; Li, F. Structural basis for human receptor recognition by SARS-CoV-2 omicron variant ba.1. J. Virol. 2022, 96, e0024922. [Google Scholar] [CrossRef] [PubMed]
- Wickenhagen, A.; Flagg, M.; Port, J.R.; Yinda, C.K.; Goldin, K.; Gallogly, S.; Schulz, J.E.; Lutterman, T.; Williamson, B.N.; Kaiser, F.; et al. Evolution of omicron lineage towards increased fitness in the upper respiratory tract in the absence of severe lung pathology. Nat. Commun. 2025, 16, 594. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hofmann-Winkler, H.; Kruger, N.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Arora, P.; Sidarovich, A.; Moldenhauer, A.S.; Winkler, M.S.; et al. SARS-CoV-2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination. Cell Rep. 2021, 36, 109415. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 omicron is an immune escape variant with an altered cell entry pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar] [CrossRef]
- Halfmann, P.J.; Iida, S.; Iwatsuki-Horimoto, K.; Maemura, T.; Kiso, M.; Scheaffer, S.M.; Darling, T.L.; Joshi, A.; Loeber, S.; Singh, G.; et al. SARS-CoV-2 omicron virus causes attenuated disease in mice and hamsters. Nature 2022, 603, 687–692. [Google Scholar] [CrossRef]
- Kaku, Y.; Uriu, K.; Kosugi, Y.; Okumura, K.; Yamasoba, D.; Uwamino, Y.; Kuramochi, J.; Sadamasu, K.; Yoshimura, K.; Asakura, H.; et al. Virological characteristics of the SARS-CoV-2 KP.2 variant. Lancet. Infect. Dis. 2024, 24, e416. [Google Scholar] [CrossRef]
- Gangavarapu, K.; Latif, A.A.; Mullen, J.L.; Alkuzweny, M.; Hufbauer, E.; Tsueng, G.; Haag, E.; Zeller, M.; Aceves, C.M.; Zaiets, K.; et al. Outbreak.Info genomic reports: Scalable and dynamic surveillance of SARS-CoV-2 variants and mutations. Nat. Methods 2023, 20, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Tsueng, G.; Mullen, J.L.; Alkuzweny, M.; Cano, M.; Rush, B.; Haag, E.; Lin, J.; Welzel, D.J.; Zhou, X.; Qian, Z.; et al. Outbreak.Info research library: A standardized, searchable platform to discover and explore COVID-19 resources. Nat. Methods 2023, 20, 536–540. [Google Scholar] [CrossRef]
- Shu, Y.; McCauley, J. Gisaid: Global initiative on sharing all influenza data—From vision to reality. Euro Surveill. 2017, 22, 30494. [Google Scholar] [CrossRef]
- Schulze, H.; Bayer, W. Changes in symptoms experienced by SARS-CoV-2-infected individuals—From the first wave to the Omicron variant. Front. Virol. 2022, 2, 880707. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, X.; Zhang, J.; Fu, S.; Ding, D.; Tao, Z. Differences in clinical characteristics between Delta variant and wild-type SARS-CoV-2 infected patients. Front. Med. 2021, 8, 792135. [Google Scholar]
- Thi Khanh, H.N.; Cornelissen, L.; Castanares-Zapatero, D.; De Pauw, R.; Van Cauteren, D.; Demarest, S.; Drieskens, S.; Devleesschauwer, B.; De Ridder, K.; Charafeddine, R.; et al. Association between SARS-CoV-2 variants and post COVID-19 condition: Findings from a longitudinal cohort study in the belgian adult population. BMC Infect. Dis. 2023, 23, 774. [Google Scholar] [CrossRef] [PubMed]
- von Bartheld, C.S.; Wang, L. Prevalence of olfactory dysfunction with the omicron variant of SARS-CoV-2: A systematic review and meta-analysis. Cells 2023, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Torabi, S.H.; Riahi, S.M.; Ebrahimzadeh, A.; Salmani, F. Changes in symptoms and characteristics of COVID-19 patients across different variants: Two years study using neural network analysis. BMC Infect. Dis. 2023, 23, 838. [Google Scholar] [CrossRef]
- Fyles, M.; Vihta, K.D.; Sudre, C.H.; Long, H.; Das, R.; Jay, C.; Wingfield, T.; Cumming, F.; Green, W.; Hadjipantelis, P.; et al. Diversity of symptom phenotypes in SARS-CoV-2 community infections observed in multiple large datasets. Sci. Rep. 2023, 13, 21705. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves and Authorizes Updated mRNA COVID-19 Vaccines to Better Protect Against Currently Circulating Variants. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-and-authorizes-updated-mrna-COVID-19-vaccines-better-protect-against-currently (accessed on 22 August 2024).
- Cox, M.; Peacock, T.P.; Harvey, W.T.; Hughes, J.; Wright, D.W.; Consortium, C.-G.U.; Willett, B.J.; Thomson, E.; Gupta, R.K.; Peacock, S.J.; et al. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nat. Rev. Microbiol. 2023, 21, 112–124. [Google Scholar] [CrossRef]
- Gidari, A.; Sabbatini, S.; Bastianelli, S.; Pierucci, S.; Busti, C.; Svizzeretto, E.; Tommasi, A.; Pallotto, C.; Schiaroli, E.; Francisci, D. Tixagevimab/cilgavimab: Still a valid prophylaxis against COVID-19 new variants? Viruses 2024, 16, 354. [Google Scholar] [CrossRef]
- Focosi, D.; Casadevall, A. A critical analysis of the use of cilgavimab plus tixagevimab monoclonal antibody cocktail (evusheld) for COVID-19 prophylaxis and treatment. Viruses 2022, 14, 1999. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Announces Evusheld Is Not Currently Authorized for Emergency Use in the U.S. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us (accessed on 16 March 2025).
- U.S. Food and Drug Administration. Fact Sheet for Healthcare Providers: Emergency Use Authorization of Pemgarda (Pemivibart). Invivyd, Inc. Available online: https://www.fda.gov/media/177067/download (accessed on 16 March 2025).
- Voss, W.N.; Mallory, M.A.; Byrne, P.O.; Marchioni, J.M.; Knudson, S.A.; Powers, J.M.; Leist, S.R.; Dadonaite, B.; Townsend, D.R.; Kain, J.; et al. Hybrid immunity to SARS-CoV-2 arises from serological recall of IgG antibodies distinctly imprinted by infection or vaccination. Cell Rep. Med. 2024, 5, 101668. [Google Scholar] [CrossRef]
- Nagy, B., Jr.; Fejes, Z.; Szentkereszty, Z.; Suto, R.; Varkonyi, I.; Ajzner, E.; Kappelmayer, J.; Papp, Z.; Toth, A.; Fagyas, M. A dramatic rise in serum ACE2 activity in a critically ill COVID-19 patient. Int. J. Infect. Dis. 2021, 103, 412–414. [Google Scholar] [CrossRef]
- Urano, E.; Itoh, Y.; Suzuki, T.; Sasaki, T.; Kishikawa, J.I.; Akamatsu, K.; Higuchi, Y.; Sakai, Y.; Okamura, T.; Mitoma, S.; et al. An inhaled ACE2 decoy confers protection against SARS-CoV-2 infection in preclinical models. Sci. Transl. Med. 2023, 15, eadi2623. [Google Scholar] [CrossRef] [PubMed]
- Havranek, B.; Lindsey, G.W.; Higuchi, Y.; Itoh, Y.; Suzuki, T.; Okamoto, T.; Hoshino, A.; Procko, E.; Islam, S.M. A computationally designed ACE2 decoy has broad efficacy against SARS-CoV-2 omicron variants and related viruses in vitro and in vivo. Commun. Biol. 2023, 6, 513. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yao, W.; Li, Y.; Ma, D.; Zhang, Z.; Wang, H.; Tang, X.; Wang, Y.; Li, C.; Cheng, D.; et al. Broadly effective ACE2 decoy proteins protect mice from lethal SARS-CoV-2 infection. Microbiol. Spectr. 2023, 11, e0110023. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).