Abstract
A20 (TNFAIP3) is a ubiquitin-editing enzyme that plays a central role in the regulation of inflammation and cell death, primarily through modulation of NF-κB signaling. In the context of viral infection, A20 exhibits a dual nature: it can both suppress antiviral immune responses to facilitate viral replication and act as a host-protective factor to prevent immunopathology. This review synthesizes current findings on the context-dependent roles of A20, focusing on its capacity to switch between antiviral and proviral functions. We examine how specific determinants—including viral genetic makeup, the infected cell type, and the temporal stage of infection—dictate whether A20 protects the host or facilitates viral persistence. We propose a systematic framework for understanding A20 as a dynamic regulator that orchestrates the balance between pathogen clearance and tissue protection.
1. Introduction
Viral infections pose a major threat to human health, forcing host cells to rapidly sense invading pathogens and mount effective immune defenses. The innate immune system serves as the first line of protection, relying on pattern recognition receptors (PRRs) to detect viral nucleic acids such as double-stranded RNA and unmethylated CpG DNA [1,2]. Activation of these receptors initiates signaling cascades that induce type I interferons (IFNs), pro-inflammatory cytokines, and chemokines, establishing an antiviral state and recruiting immune cells [1,2].
Central to this response is the transcription factor nuclear factor-kappa B (NF-κB), a master regulator of genes involved in inflammation, immunity, and cell survival [3,4]. NF-κB activation ensures the production of cytokines, chemokines, and anti-apoptotic factors essential for controlling infection [3,4]. However, uncontrolled NF-κB signaling can result in chronic inflammation, autoimmune disease, and even cancer [5,6,7]. To prevent such immunopathology, its activity must be tightly regulated. A key negative feedback regulator is the ubiquitin-editing enzyme A20 (tumor necrosis factor alpha-induced protein 3, TNFAIP3), which balances effective antiviral responses with protection against tissue damage [1,2,3,4,8,9].
While A20 is broadly characterized as an anti-inflammatory and host-protective factor, assigning it a static label of “antiviral” or “proviral” fails to capture its dynamic nature. A growing body of evidence reveals that A20 functions as both a facilitator and inhibitor of viral infection, with outcomes that are profoundly dependent on biological context. Here, we define such context-dependence not merely as variability, but as a systematic functional switch wherein A20 aligns either with host defense or viral survival. This duality is governed by three principal biological determinants: the genetic makeup of the invading virus, the specific tissue or cell type infected, and the temporal stage of the immune response.
To provide a systematic framework for analyzing these varied roles, we classify A20’s interactions with viruses into four distinct categories. Proviral interactions occur when A20’s immunosuppressive functions are co-opted by the virus to promote its own replication and persistence. Antiviral interactions describe situations in which A20 acts to limit viral replication or prevent host pathogenesis, consistent with its canonical protective role. Dual interactions represent cases where the role of A20 shifts dynamically between antiviral and proviral states depending on the temporal or cellular context of infection. Finally, we address context-dependent effects, wherein A20’s influence extends beyond the direct virus–host conflict to affect systemic homeostasis, tissue integrity, and long-term immunological outcomes, as exemplified by chronic Human Immunodeficiency Virus (HIV) infection.
2. Molecular Mechanisms of A20 Regulation
2.1. Transcriptional Control of A20 Expression
The control of A20 expression is achieved through transcriptional mechanisms that respond to viral stimuli. Figure 1 illustrates the architecture and activation of the A20 promoter. At baseline, A20 expression is tightly restricted by the interplay of transcription factors Sp1 and upstream stimulatory factor 1 (USF-1), which maintain RNA polymerase II in a paused state. Upon immune stimulation, NF-κB binds to κB sites, displaces USF-1, and facilitates transcriptional elongation, enabling prompt A20 induction. This inducible promoter design allows A20 to function as a negative feedback regulator, becoming upregulated when NF-κB activity peaks and establishing a self-limiting loop in inflammatory signaling [3].
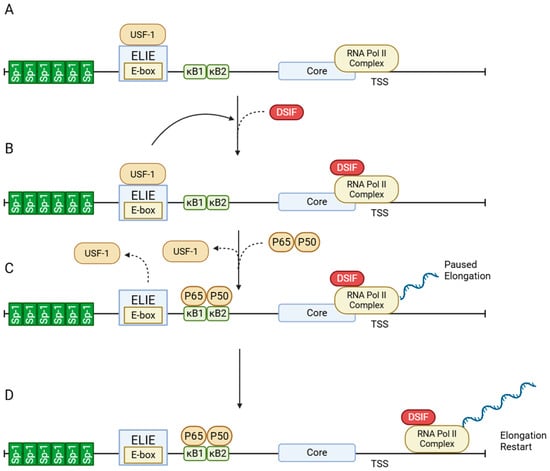
Figure 1.
Structure and activation of the A20 promoter. (A) Promoter architecture. Upstream of the core promoter, A20 harbors two NF-κB sites (κB1/κB2), six Sp1 sites, and an E-box overlapping the Elongation-Inhibitory Element (ELIE) element. (B) Basal state. RNA polymerase II and the general transcription factors are bound at the core promoter, while the ELIE–USF1 axis suppresses transcript elongation through DRB Sensitivity-Inducing Factor (DSIF). The Sp1 cluster supports promoter accessibility and initiation capacity, but productive elongation is limited by DSIF. (C) Upon stimulation. NF-κB binds κB1/κB2 and displaces USF1; control of DSIF shifts to the NF-κB/core-promoter module, which triggers pause release and enables multiple rounds of transcription. (D) Elongation restart. DSIF converts to a pro-elongation role, allowing RNA polymerase II to transition from promoter-proximal pausing to productive mRNA elongation. Solid curved arrows indicate regulatory interactions, while dashed curved arrows represent the displacement or binding of transcription factors. Figure created with BioRender.com (https://BioRender.com/96ii9dl, accessed on 12 December 2025).
2.2. Structural Organization and Catalytic Mechanisms
Structurally, A20 contains an N-terminal ovarian tumor (OTU) domain with deubiquitinase (DUB) activity and seven C-terminal zinc finger motifs, an arrangement that enables it to carry out two opposing enzymatic reactions simultaneously [3,4]. The structural organization of A20 and its regulatory complex provides the molecular foundation for its functional versatility.
The modular domain structure of A20 is depicted in Figure 2A. The N-terminal OTU domain mediates deubiquitination of Lys63-linked chains, while the C-terminal zinc finger motifs, particularly ZF4, catalyze Lys48-linked ubiquitination for proteasomal degradation. These domains cooperate to enable A20’s “ubiquitin-editing” function. Post-translational regulation further fine-tunes activity, as exemplified by IKKβ-mediated phosphorylation or MALT1 cleavage. Moreover, recruitment of adaptor proteins through the C-terminal zinc finger region facilitates assembly of the A20 regulatory complex, ensuring substrate specificity and spatial precision [4,10,11].
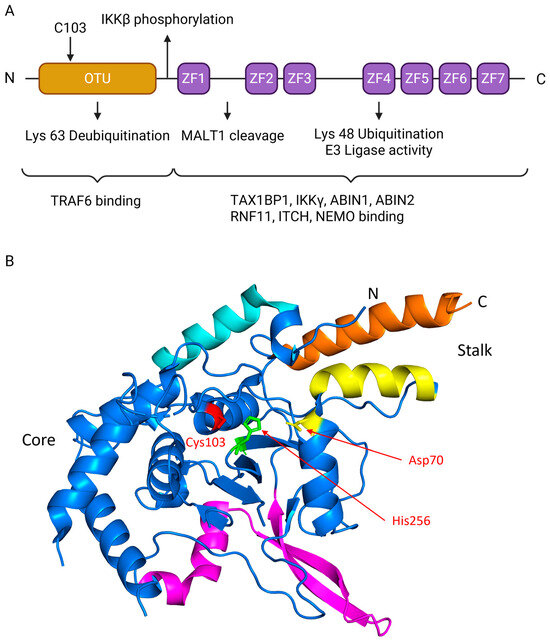
Figure 2.
Structure of A20 protein. (A) Schematic representation of A20 domains, where the N-terminal OTU domain is colored in gold and the C-terminal zinc fingers (ZF1-ZF7) are colored in purple. The N-terminal OTU DUB domain contains catalytic Cys103 that removes K63-linked ubiquitin from substrates (e.g., TRAF6), damping NF-κB signaling. A linker near ZF1 carries an IKKβ site (classically Ser381) that tunes activity. The C-terminus contains seven zinc fingers (ZF1–ZF7); ZF4 provides E3-ligase activity that builds K48-linked chains for proteasomal turnover. MALT1 cleaves A20 between ZF1 and ZF2 (≈Arg439), transiently reducing inhibition. Partner recruitment maps to regions: N-term binds TRAF6, while ZF4–ZF7 engage TAX1BP1, IKKγ/NEMO, ABIN1/2, RNF11, and ITCH to assemble the ubiquitin-editing complex. (B) The crystal structure of the human A20 OTU domain. The image was generated using PyMOL (version3.1.6.1), based on coordinate data from the Protein Data Bank (PDB ID: 2VFJ). The structure is colored to highlight key regions identified in the original study: the core domain (blue), the α1 helix (cyan), the helical stalk formed by helix α2 (yellow) and orange (α11)), and the insertion domain (magenta). The essential catalytic triad residues—Cysteine 103 (red), Histidine 256 (green), and Aspartate 70 (yellow)—are shown as stick representations and explicitly labeled. Panel A created with BioRender.com (https://BioRender.com/t4hi4jt, accessed on 12 December 2025).
To provide a structural foundation for understanding its function, we examined the crystal structure of the human A20 OTU domain, previously determined by Komander and Barford [12]. The structure, visualized from the Protein Data Bank (PDB ID: 2VFJ), reveals a complex fold characterized by a central core, a helical stalk, and a unique insertion region not commonly found in other OTU family members (Figure 2B). The active site is located within a conserved cleft and features a canonical catalytic triad essential for its deubiquitinase function. This triad is composed of Cysteine 103, which acts as the nucleophile, alongside Histidine 256 and Aspartate 70, which facilitate the catalytic reaction. This established structural framework of the OTU domain, complemented by the functions of the C-terminal zinc fingers, provides a basis for dissecting the multifaceted roles of A20 in ubiquitin editing and signal termination.
2.3. Functional Mechanisms in Cellular Signaling
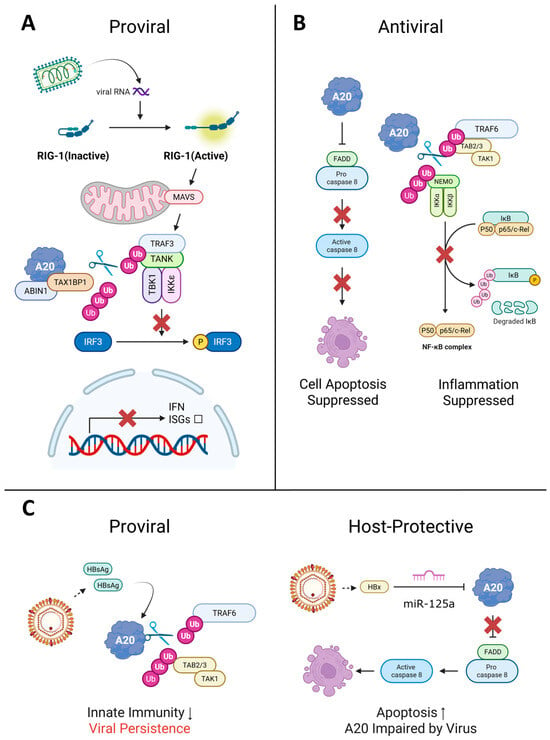
These structural insights inform understanding of A20’s functional mechanisms in cellular signaling pathways. A20’s best-characterized role is negative regulation of NF-κB signaling. Following activation of tumor necrosis factor receptor 1 (TNF-R1), A20 removes activating Lys63-linked ubiquitin chains from the adaptor kinase receptor-interacting protein 1 (RIP1) and then deposits degradative Lys48-linked chains on the same molecule, targeting it for proteasomal destruction [4]. In Toll-like receptor (TLR) pathways, A20 performs a similar ubiquitin-editing step on the signaling adaptor TNF receptor-associated factor 6 (TRAF6), stripping its Lys63-linked chains to dampen downstream NF-κB activation [9].
This ubiquitin-editing mechanism is essential for terminating NF-κB signaling. A20 operates within a regulatory complex comprising adaptor proteins such as A20-binding inhibitor of NF-κB activation 1 (ABIN1) and Tax1-binding protein 1 (TAX1BP1), together with RING-type E3 ligases including RING finger protein 11 (RNF11) and Itchy E3 ubiquitin protein ligase (ITCH). These partners confer substrate specificity and spatiotemporal control [1,2,13].
As illustrated in Figure 3, A20 regulates two major endosomal TLR pathways. In the TLR3–TRIF axis, the A20–TAX1BP1–ABIN1 complex removes K63-linked ubiquitin chains from TRIF and TRAF3, reducing TBK1 and IKKε activation and limiting IRF3/IRF7-mediated induction of IFNs and interferon-stimulated genes (ISGs). In the TLR7/8/9–MyD88 axis, A20 deubiquitinates TRAF6 and NEMO, promoting K48-linked ubiquitin-dependent degradation. This process suppresses IKK-driven IκB degradation and NF-κB transcriptional activity, while also dampening MyD88-dependent activation of IRF7.
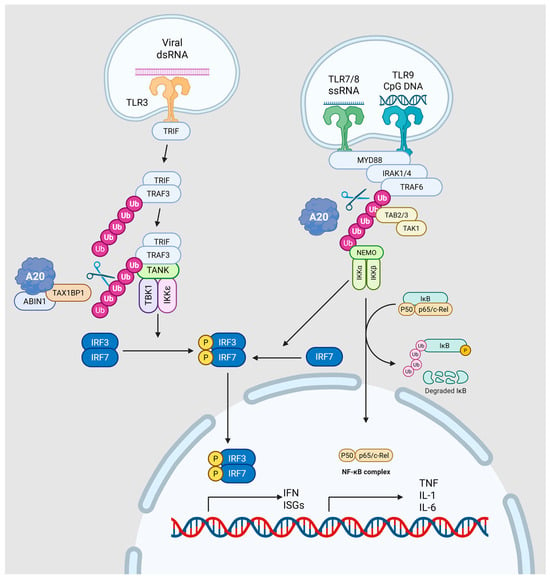
Figure 3.
Schematic of A20 control of endosomal TLR signaling. Left, TLR3–TRIF axis: viral dsRNA activates endosomal TLR3, recruiting TRIF and then TRAF3, which scaffolds the TANK–TBK1/IKKε module. K63-linked ubiquitin platforms enable TBK1/IKKε to phosphorylate IRF3/IRF7, inducing type I IFNs and ISGs. The A20 regulatory complex (A20–TAX1BP1–ABIN1) edits ubiquitin chains on TRIF/TRAF3, removing K63 linkages to dampen TBK1/IKKε activation and IRF3/7 signaling. Right, TLR7/8/9–MyD88 axis: MyD88 triggers IRAK1/4 → TRAF6 → TAB2/3–TAK1, activating the NEMO–IKKα/β complex, which phosphorylates and degrades IκB to unleash NF-κB and drive TNF, IL-1, and IL-6 expression; MyD88 also promotes IRF7 activation. A20 restrains this branch by deubiquitinating TRAF6/NEMO and redirecting ubiquitin toward K48-linked turnover, thereby limiting both NF-κB and IRF7 outputs. Solid arrows indicate the signal transduction pathway. Purple and pink spheres represent ubiquitin chains. Yellow circles labeled ‘P’ denote phosphorylation events. The scissors icon indicates the deubiquitinating activity of A20. Figure created with BioRender.com (https://BioRender.com/j6ekhsu, accessed on 12 December 2025).
2.4. Broader Biological Functions Beyond NF-κB
Recent studies show that A20’s influence extends far beyond NF-κB inhibition. Independently of NF-κB, A20 potently suppresses apoptotic and necroptotic cell death, preventing the release of damage-associated molecular patterns (DAMPs) that would otherwise amplify inflammation [5]. Through this cell-protective function, A20 also acts as a tumor suppressor. Loss-of-function mutations are frequent in various lymphomas and in colorectal carcinoma, underscoring its role in curbing aberrant survival and proliferation of malignant cells [6,7].
During viral infection, A20’s activities generate a complex interplay between host and pathogen. Many viruses exploit A20’s ability to dampen type I IFN production (e.g., TBK1/IKKε–IRF3 axis), creating a more permissive environment for replication and making A20 a proviral factor [1,2]. Conversely, by restraining excessive inflammatory cascades and lethal cytokine storms, A20 protects host tissues and improves survival, revealing a host-protective facet [8]. The outcome depends on the viral species, infected cell type, and stage of infection.
This review synthesizes the current knowledge regarding the dual and sometimes opposing roles of A20 during viral infections. We outline its proviral and antiviral functions across a range of viral pathogens and discuss how A20 modulates the balance of host–pathogen interactions that ultimately determines disease outcomes.
5. Dual Effects of A20
5.1. Zika Virus
Zika Virus (ZIKV) is a (+) single-stranded RNA virus in the family Flaviviridae, genus Orthoflavivirus. Primarily transmitted by Aedes aegypti mosquitoes, infamous for causing severe congenital malformations such as congenital Zika syndrome (CZS), particularly microcephaly [60]. During its replication, ZIKV remodels the endoplasmic reticulum (ER) membrane to form viral replication organelles, a process in which it actively utilizes host proteins [60].
A20 plays a context-dependent regulatory role during ZIKV infection, with distinct observations in different cell types. In HeLa cells, ZIKV infection downregulates A20 protein expression despite elevated TNFAIP3 mRNA levels. The reduction in A20 protein occurs through post-transcriptional mechanisms rather than post-translational degradation, as neither autophagy inhibitors (Bafilomycin A1, chloroquine) nor the proteasome inhibitor MG132 restored A20 levels, and no MALT1-mediated cleavage was detected. ZIKV NS5, which localizes to the nucleus, and the capsid protein, which shows partial nuclear localization, were both identified as viral components responsible for decreasing A20 expression without directly interacting with A20 itself [61].
In parallel, ZIKV infection in HeLa cells led to sustained NF-κB activation and increased expression of prosurvival genes including BIRC3, BCL-2, and MCL-1. When A20 was overexpressed in ZIKV-infected HeLa cells, markers of cell death (CASP3-p17 and GSDME-p34) increased, suggesting that A20 restoration can promote apoptotic pathways in this context [61]. However, whether A20 protein is downregulated in ZIKV-infected macrophages remains to be directly demonstrated, as the underlying mechanisms were established primarily in HeLa and HEK293T cells.
A20’s function in viral infections is known to be highly context-dependent. While it generally acts as an anti-inflammatory regulator that limits NF-κB signaling and can restrict cell death in many settings, tissue-specific and expression-level-dependent variations have been reported where A20 may promote apoptosis [5]. This suggests that A20’s role in ZIKV pathogenesis may vary across different cell types and infection conditions [47].
5.2. Influenza A Virus
Influenza A virus (IAV) belongs to the family Orthomyxoviridae genus Alphainfluenzavirus, and has a genome of eight (−) single-stranded RNA segments, each packed as a ribonucleoprotein (RNP) complex [62,63]. Subtypes are determined by the antigenicity of two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA) (e.g., H1N1, H3N2), with 18 HA and 11 NA subtypes reported to date [62]. Due to its segmented genome, genetic reassortment (antigenic shift) can occur when different viruses co-infect the same cell, potentially creating new subtypes and causing pandemics [62,63]. The precise packaging of the eight genome segments into new virions is essential for viral replication and is tightly regulated by complex RNA secondary structures and inter-segment interactions [63].
A A20 displays both proviral and antiviral activities during IAV infection. Several studies in A549 cells have demonstrated that IAV infection induces a time-dependent increase in both A20 mRNA and protein. This upregulation is partly mediated by enhanced expression of the miR-29 family, particularly miR-29c, which stabilizes A20 mRNA through interaction with its 3′-UTR [64,65]. Additionally, the viral nonstructural protein NS1 promotes A20 protein accumulation without affecting transcript levels, particularly in highly pathogenic H5N1 and H7N1 strains [66]. Elevated A20 levels inhibit IRF3 phosphorylation and ISRE promoter activity, suppressing IFN-β and downstream ISGs such as IFITM3, ISG20, OAS1, and OASL, which facilitates increased production of viral M and NP proteins and elevated extracellular titers [66].
Beyond supporting replication, A20 also enhances IAV pathogenesis. In mice lacking A20, early infection stages are marked by elevated cytokine production (CCL2, IL-6) following TNF/poly I:C stimulation. However, in later stages, these mice exhibit reduced lung tissue damage and vascular leakage, correlating with decreased inflammatory cytokines and improved tolerance to infection. Restoration of CCL2 reverses this tolerance, confirming A20’s contribution to IAV-induced pathogenicity [67]. Furthermore, macrophage-specific A20 deficiency results in enhanced NF-κB and IRF3 activation in response to IAV infection, leading to elevated secretion of IL-6, TNF, and IFN-β, again supporting A20’s role in dampening early innate responses and allowing viral persistence [68].
A20 also displays antiviral properties by limiting host immunopathology. Its expression in airway epithelial cells helps suppress NF-κB–mediated inflammation. Chronic low-dose LPS exposure further enhances A20 expression and elevates PPAR-α and PPAR-γ levels, which in turn inhibit NF-κB and NLRP3 inflammasome activation. This mechanism protects against lung inflammation during IAV infection, maintaining tissue homeostasis and host viability [69].
5.3. Hepatitis B Virus
Belonging to the family Hepadnaviridae, hepatitis B virus (HBV) has a partially double-stranded DNA genome [70,71]. After infecting a host cell, HBV transports its genome into the nucleus and forms a stable covalently closed circular DNA (cccDNA). This cccDNA serves as the template for viral replication and is highly stable, which is a key factor in causing chronic infection and making treatment difficult [70,71]. HBV is a major cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC), making it a significant public health burden affecting approximately 300 million people globally [70,71].
A20 has both proviral and antiviral activities during HBV infection. As a proviral factor, HBV exploits A20 for immune evasion by suppressing host innate immunity. During HBV infection in THP-1 monocytes, the viral surface antigen HBsAg drives A20 expression, which subsequently deubiquitinates TRAF6, prevents TRAF6–TAB2 complex assembly, and halts TLR4 signaling (Figure 4C). This viral manipulation of A20 blocks LPS-driven cytokine production, as demonstrated by restoration of cytokine responses when A20 is suppressed by siRNA [72].
A20 also functions as an antiviral factor through multiple mechanisms. Genetic studies reveal that individuals with a specific polymorphism (TT>A variant) that impairs A20 function exhibit higher susceptibility to chronic HBV infection, indicating that fully functional A20 contributes to preventing viral persistence and facilitating viral clearance during early infection stages [73]. Additionally, A20 controls viral infection through regulation of apoptosis pathways. The HBV pathogenic factor HBx protein targets A20 for downregulation by inducing upregulation of miR-125a, which directly targets A20 mRNA and suppresses its expression, leading to caspase-8 activation and increased apoptosis (Figure 4C) [74]. This viral targeting suggests that A20 normally protects hepatocytes and prevents pathological damage, requiring the virus to overcome this protective function to induce disease.
The context-dependent nature of A20 is evident in its dynamic regulation during different phases of infection and varying clinical conditions. During the natural course of chronic HBV infection, A20 expression levels increase during the immune clearance phase when immune responses are active, due to TNF-α/NF-κB signaling pathway activation and representing a physiological feedback mechanism to restrain excessive immune responses [75,76]. This dual nature is apparent in conditions such as acute-on-chronic hepatic failure (ACHBLF) caused by HBV. In ACHBLF patients, A20 mRNA levels show positive correlations with liver injury indicators (total bilirubin, INR, MELD scores) and negative correlations with albumin and prothrombin time activity. Simultaneously, A20 levels correlate positively with IL-10 and negatively with IL-6, indicating suppression of inflammatory responses and induction of an immunosuppressive state. Among patients who died from ACHBLF, A20 mRNA levels were higher compared to survivors, exhibiting immune paralysis characterized by low IL-6 and high IL-10 levels [77].
5.4. Epstein–Barr Virus
Epstein–Barr virus (EBV), also known as human herpesvirus 4 (HHV-4), is a gamma-herpesvirus infecting over 90% of adults worldwide [78]. It targets B cells and epithelial cells to establish lifelong latency, classified into stages I–III by viral antigen expression. In latency I, Burkitt lymphoma cells express only EBNA1 to evade immunity, while proteins such as LMPs activate NF-κB and block apoptosis, driving oncogenesis. Through these mechanisms, EBV contributes to cancers including Burkitt lymphoma, Hodgkin lymphoma, and nasopharyngeal carcinoma [78,79,80].
A20 exhibits complex and opposing roles during EBV infection, functioning as both a proviral factor that supports viral persistence and an antiviral tumor suppressor. These context-dependent regulatory mechanisms demonstrate the intricate balance between viral exploitation and host defense.
In its proviral capacity, A20 plays a crucial role in prolonging infected cell survival and inhibiting apoptosis. Expression of the EBV oncoprotein latent membrane protein-1 (LMP1) in EBV-negative B-cell lines drives a pronounced increase in A20 at both transcript and protein levels [81]. LMP1-driven A20 expression effectively blocks p53-mediated apoptosis induction, with cells expressing A20 alone showing suppressed apoptosis to the same level as LMP1-expressing cells, independent of LMP1 itself [82]. The DUB domain at the N terminus of A20 directly binds to IRF7 and removes K63-linked ubiquitination, thereby facilitating immune evasion and contributing to both immune escape and cell survival. Studies demonstrate that A20’s deubiquitinase activity is essential for IRF7 inhibition, as DUB-deficient mutants completely lose the ability to suppress IRF7, while A20’s C-terminal domains are dispensable for this function [83].
However, A20 simultaneously functions as an antiviral factor by inhibiting virus-induced oncogenesis. A20 acts as a classical tumor suppressor gene that prevents malignant transformation by various oncogenic viruses, requiring viruses to evade or disable A20’s suppressive function to achieve tumorigenesis. This antiviral role is evidenced by frequent deletions or loss-of-function mutations in the A20 gene observed in various EBV-associated lymphomas, particularly AIDS-related lymphomas (ARL) [84,85]. The context-dependent nature of A20 is further demonstrated through its participation in dynamic regulatory mechanisms that control viral activity via negative feedback loops. Following EBV infection, A20 is upregulated as part of the host response and subsequently acts as a key component of sophisticated negative feedback regulation that suppresses upstream signaling to modulate immune response intensity. During EBV infection, LMP1 upregulates A20 expression via the NF-κB pathway, but A20 then physically binds to the LMP1 signaling complex and displaces signaling molecules such as TRAF1 and TRADD, thereby inhibiting LMP1-mediated NF-κB activation [86]. Simultaneously, A20 targets the LMP1-IRF7 pathway through its deubiquitinase activity, removing activating K63-linked ubiquitin chains from IRF7 to prevent excessive IFN responses while maintaining viral latency [83]. These dual negative feedback mechanisms illustrate how EBV exploits cellular regulatory networks to achieve a delicate balance between viral persistence and host cell survival.
5.5. Human T-Cell Leukemia Virus Type 1
Human T-cell Leukemia Virus type 1 (HTLV-1), which carries a (+) single-stranded RNA genome, was the first discovered human retrovirus. It primarily targets CD4+ T-cells, causing severe diseases such as adult T-cell leukemia/lymphoma (ATLL) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [87,88]. Approximately 20 million people are infected worldwide, and while most remain asymptomatic carriers, about 5% develop disease [88]. ATLL is an aggressive malignancy with a very poor prognosis. Current treatment options include chemotherapy, targeted therapies, and allogeneic hematopoietic stem cell transplantation (allo-HSCT), while therapies using genetically modified stem cells are also under investigation [87].
A20 demonstrates paradoxical roles during HTLV-1 infection, serving simultaneously as a proviral factor essential for cell survival and as an antiviral tumor suppressor that must be overcome for oncogenic transformation. As a proviral factor, A20 is critical for prolonging cell survival and inhibiting apoptosis. Artificial depletion of A20 using shRNA in HTLV-1-positive cell lines results in significant growth inhibition, accompanied by activation and cleavage of caspase-8 and caspase-3/7. This protective function is mediated through A20’s formation of a stable physical complex with caspase-8 and FADD, an interaction facilitated by the zinc finger domain at the C-terminus of A20 [89,90].
Conversely, A20 also acts as a potent antiviral factor by inhibiting virus-induced oncogenesis. The key oncogenic protein Tax drives T-cell transformation through persistent NF-κB signaling, but for this oncogenic program to succeed, the virus must inactivate the A20 functional complex by exploiting the host molecule CADM1 [91]. This requirement for A20 inactivation demonstrates that A20 directly suppresses HTLV-1-mediated oncogenesis. The dual nature of A20 reveals the complex evolutionary pressure between HTLV-1’s need to maintain cell survival while simultaneously overcoming A20’s tumor suppressor activity to achieve malignant transformation.
6. Context-Dependent Effects of A20
Human Immunodeficiency Virus
Human Immunodeficiency Virus (HIV), a (+) single-stranded RNA retrovirus, enters cells via its Env glycoprotein complex of gp120 and gp41. gp120 binds CD4, while gp41 mediates membrane fusion through a six-helical bundle, a major antiviral target [92,93]. Although once a fatal and untreatable disease, the advent of antiretroviral therapies has transformed HIV into a manageable chronic condition. Vaccine efforts now focus on stabilizing Env trimers. Early designs used gp120–gp41 disulfide bonds, whereas newer interdomain lock trimers within gp120 block CD4 binding yet retain broadly neutralizing antibody recognition, offering improved vaccine potential [93].
In HIV infection, A20 serves as a context-dependent regulator of host homeostasis and tissue integrity rather than a simple pro- or antiviral factor. A20 expression is significantly downregulated in PBMCs from viremic, treatment-naïve individuals compared to healthy controls, and its levels are partially restored with highly active antiretroviral therapy (HAART) [94]. This dysregulation directly impacts intestinal barrier function, as untreated viremia-associated type I IFN (specifically IFNα) suppresses A20 expression in intestinal epithelial cells, leading to cell death and compromised barrier integrity. Following antiretroviral therapy initiation, A20 restoration positively correlates with epithelial barrier markers claudin-4 (CLDN4) and tight junction protein 1 (TJP1) [95].
However, A20 also acts as an endogenous regulator of dendritic cell activation. While this prevents excessive inflammatory responses during natural infection, it can limit vaccine efficacy. In a murine model, immunization with A20-silenced murine dendritic cells (DCs), which become hyperactivated, elicited superior anti-HIV immune responses and induced robust mucosal immunity compared to control DCs [96]. These observations demonstrate that A20 is a critical host-protective factor in HIV infection. Its downregulation during viremia contributes to immunopathology and tissue damage, while its regulatory role in antigen-presenting cells represents a double-edged mechanism that protects against excessive inflammation but may constrain vaccine-induced immunity.
7. Discussion
The evidence reviewed here establishes A20 as a central, yet paradoxical, regulator of host–virus interactions [3,4]. Far from being a simple negative regulator of NF-κB [9], A20 operates as a multifaceted molecular switch that can either promote viral persistence or safeguard host tissues, depending on the viral species, infected cell type, and stage of infection [1,2]. This duality explains why A20 has been alternatively described as both a proviral factor and an antiviral defense mechanism [5,13].
A clear pattern emerges from comparative analysis (Table 1). For many RNA viruses including HCV [16,17,18], HRSV [47], MeV [53], and VSV [32], A20 is consistently hijacked to suppress innate immune signaling and facilitate replication, highlighting its proviral role. By contrast, in poliovirus and ZIKV infections, A20 exerts direct antiviral activity by sustaining essential immune responses or promoting apoptosis of infected cells, forcing these viruses to evolve countermeasures that suppress its expression [56,61]. Still, other pathogens, such as IAV [65,66,67,68,69], HBV [72,74,75,76,77], EBV [81,82,83,84,85,86], and HTLV-1 [89], exploit A20 during certain infection phases while being restrained by it in others. HIV represents a distinct case, where dysregulated A20 expression contributes to gut pathology and vaccine inefficacy, illustrating how its regulatory role extends beyond viral clearance to broader immune homeostasis [94,95,96].

Table 1.
Effects of A20 on virus and host. A20 can act as a proviral, antiviral, or dual regulator depending on the virus and infection context. This table summarizes representative viruses in each category, their mechanisms of A20 modulation, and clinical/pathological implications. Symbols: → (leads to/results in); ↑ (increase/upregulation); ↓ (decrease/downregulation); *: (Highlight).
These findings also carry important clinical and translational implications. The ability of A20 to fine-tune inflammation places it at the crossroads of viral pathogenesis and host survival [3,5,17]. Excessive immune activation often drives pathology into acute infections, whereas insufficient responses enable viral persistence and oncogenesis. Thus, therapeutic strategies aimed at modulating A20 require careful consideration. Pharmacological inhibition could restore antiviral immunity in chronic infections such as HBV [72,75,76,77] or HCV [16,18], yet risks triggering uncontrolled inflammation. Conversely, enhancing A20 activity might protect against immunopathology in diseases like influenza [69] or COVID-19 [8], but could simultaneously impair viral clearance [68].
Future research should prioritize several directions. Mapping A20’s interactome across different viral infections will be necessary to identify when its inhibition or activation is beneficial [1,2]. Genetic and epigenetic variation in A20 among human populations should be studied as potential predictors of viral susceptibility and clinical outcomes [97,98]. The development of targeted modulators—such as cell type-specific agonists or transient inhibitors—could enable precision immunotherapy that leverages A20’s regulatory capacity without triggering systemic toxicity [5,8,68].
In conclusion, A20 is best understood not as inherently proviral or antiviral, but as a dynamic immunoregulatory hub. Its actions reflect an evolutionary balance between host defense and tissue protection, continuously exploited and countered by viral pathogens. By dissecting how A20 operates in different infectious contexts, future studies may uncover therapeutic opportunities to both enhance antiviral immunity and minimize immunopathology, advancing host-directed antiviral strategies.
Author Contributions
Conceptualization, H.J. and C.L.; Investigation, H.J. and C.L.; Resources, H.J. and C.L.; Data curation, H.J.; Writing—original draft preparation, H.J.; Writing—review and editing, H.J. and C.L.; Visualization, H.J.; Supervision, C.L.; Project administration, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to acknowledge the assistance of ChatGPT-4o/Gemini 2.0/Claude 3.5 Sonnet for its help in the translation process and in providing grammar and paraphrasing suggestions during the preparation of this review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Parvatiyar, K.; Barber, G.N.; Harhaj, E.W. TAX1BP1 and A20 Inhibit Antiviral Signaling by Targeting TBK1-IKKi Kinases*. J. Biol. Chem. 2010, 285, 14999–15009. [Google Scholar] [CrossRef]
- Gao, L.; Coope, H.; Grant, S.; Ma, A.; Ley, S.C.; Harhaj, E.W. ABIN1 Protein Cooperates with TAX1BP1 and A20 Proteins to Inhibit Antiviral Signaling*. J. Biol. Chem. 2011, 286, 36592–36602. [Google Scholar] [CrossRef]
- Verstrepen, L.; Verhelst, K.; van Loo, G.; Carpentier, I.; Ley, S.C.; Beyaert, R. Expression, biological activities and mechanisms of action of A20 (TNFAIP3). Biochem. Pharmacol. 2010, 80, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Heyninck, K.; Beyaert, R. A20 inhibits NF-κB activation by dual ubiquitin-editing functions. Trends Biochem. Sci. 2005, 30, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Priem, D.; van Loo, G.; Bertrand, M.J.M. A20 and Cell Death-driven Inflammation. Trends Immunol. 2020, 41, 421–435. [Google Scholar] [CrossRef]
- Ungerbäck, J.; Belenki, D.; Jawad ul-Hassan, A.; Fredrikson, M.; Fransén, K.; Elander, N.; Verma, D.; Söderkvist, P. Genetic variation and alterations of genes involved in NFκB/TNFAIP3- and NLRP3-inflammasome signaling affect susceptibility and outcome of colorectal cancer. Carcinogenesis 2012, 33, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Nocturne, G.; Boudaoud, S.; Miceli-Richard, C.; Viengchareun, S.; Lazure, T.; Nititham, J.; Taylor, K.E.; Ma, A.; Busato, F.; Melki, J.; et al. Germline and somatic genetic variations of TNFAIP3 in lymphoma complicating primary Sjögren’s syndrome. Blood 2013, 122, 4068–4076. [Google Scholar] [CrossRef]
- Wu, Y.; He, L.; Li, R.; Li, J.; Zhao, Q.; Shao, B. A20 as a Potential Therapeutic Target for COVID-19. Immun. Inflamm. Dis. 2025, 13, e70127. [Google Scholar] [CrossRef]
- Lin, S.-C.; Chung, J.Y.; Lamothe, B.; Rajashankar, K.; Lu, M.; Lo, Y.-C.; Lam, A.Y.; Darnay, B.G.; Wu, H. Molecular Basis for the Unique Deubiquitinating Activity of the NF-κB Inhibitor A20. J. Mol. Biol. 2008, 376, 526–540. [Google Scholar] [CrossRef]
- Wu, Y.; He, X.; Huang, N.; Yu, J.; Shao, B. A20: A master regulator of arthritis. Arthritis Res. Ther. 2020, 22, 220. [Google Scholar] [CrossRef]
- Ma, A.; Malynn, B.A. A20: Linking a complex regulator of ubiquitylation to immunity and human disease. Nat. Rev. Immunol. 2012, 12, 774–785. [Google Scholar] [CrossRef]
- Komander, D.; Barford, D. Structure of the A20 OTU domain and mechanistic insights into deubiquitination. Biochem. J. 2008, 409, 77–85. [Google Scholar] [CrossRef]
- Shembade, N.; Parvatiyar, K.; Harhaj, N.S.; Harhaj, E.W. The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-kappaB signalling. EMBO J. 2009, 28, 513–522. [Google Scholar] [CrossRef]
- Millman, A.J.; Nelson, N.P.; Vellozzi, C. Hepatitis C: Review of the Epidemiology, Clinical Care, and Continued Challenges in the Direct Acting Antiviral Era. Curr. Epidemiol. Rep. 2017, 4, 174–185. [Google Scholar] [CrossRef]
- Torrents de la Peña, A.; Sliepen, K.; Eshun-Wilson, L.; Newby, M.L.; Allen, J.D.; Zon, I.; Koekkoek, S.; Chumbe, A.; Crispin, M.; Schinkel, J.; et al. Structure of the hepatitis C virus E1E2 glycoprotein complex. Science 2022, 378, 263–269. [Google Scholar] [CrossRef]
- Lee, J.; Chan, S.T.; Kim, J.Y.; Ou, J.J. Hepatitis C Virus Induces the Ubiquitin-Editing Enzyme A20 via Depletion of the Transcription Factor Upstream Stimulatory Factor 1 To Support Its Replication. mBio 2019, 10, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zhang, Y.; Zhou, Y.; Li, B.; He, Y.; Guo, Y.; Jia, Z. Up-regulation of A20/ABIN1 contributes to inefficient M1 macrophage polarization during Hepatitis C virus infection. Virol. J. 2015, 12, 147. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, Y.; Zhang, Y.; Li, Y.; Guo, Y.; He, Y.; Wang, J.; Lian, J.; Hao, C.; Moorman, J.P.; et al. Role of A20 in interferon-α-mediated functional restoration of myeloid dendritic cells in patients with chronic hepatitis C. Immunology 2014, 143, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Li, J.; Chang, Y.-F.; Lin, W. Avian Leucosis Virus-Host Interaction: The Involvement of Host Factors in Viral Replication. Front. Immunol. 2022, 13, 907287. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Yang, Y.; Fang, C.; Liu, J.; Liang, X.; Yang, Y. A20 Enhances the Expression of the Proto-Oncogene C-Myc by Downregulating TRAF6 Ubiquitination after ALV-A Infection. Viruses 2022, 14, 2210. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Yang, Y.; Fang, C.; Liu, J.; Liang, X.; Yang, Y. Enhanced pathogenicity by up-regulation of A20 after avian leukemia subgroup a virus infection. Front. Vet. Sci. 2022, 9, 1031480. [Google Scholar] [CrossRef]
- Smith, D.B.; Meyers, G.; Bukh, J.; Gould, E.A.; Monath, T.; Scott Muerhoff, A.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.T.; Simmonds, P.; et al. Proposed revision to the taxonomy of the genus Pestivirus, family Flaviviridae. J. Gen. Virol. 2017, 98, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Romero, N.; Arias, C.F.; Verdugo-Rodríguez, A.; López, S.; Valenzuela-Moreno, L.F.; Cedillo-Peláez, C.; Basurto-Alcántara, F.J. Immune protection induced by E2 recombinant glycoprotein of bovine viral diarrhea virus in a murine model. Front. Vet. Sci. 2023, 10, 1168846. [Google Scholar] [CrossRef]
- Villalba, M.; Canales, N.; Maldonado, N.; Otth, C.; Fredericksen, F.; Garcés, P.; Stepke, C.; Arriagada, V.; Olavarría, V.H. Bovine A20 gene overexpression during bovine viral diarrhea virus-1 infection blocks NF-κB pathway in MDBK cells. Dev. Comp. Immunol. 2017, 77, 23–29. [Google Scholar] [CrossRef]
- Fredericksen, F.; Villalba, M.; Olavarría, V.H. Characterization of bovine A20 gene: Expression mediated by NF-κB pathway in MDBK cells infected with bovine viral diarrhea virus-1. Gene 2016, 581, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Loney, C.; Mottet-Osman, G.; Roux, L.; Bhella, D. Paramyxovirus Ultrastructure and Genome Packaging: Cryo-Electron Tomography of Sendai Virus. J. Virol. 2009, 83, 8191–8197. [Google Scholar] [CrossRef]
- Shimokata, K.; Nishiyama, Y.; Ito, Y.; Kimura, Y.; Nagata, I. Pathogenesis of Sendai virus infection in the central nervous system of mice. Infect. Immun. 1976, 13, 1497–1502. [Google Scholar] [CrossRef]
- Sakuma, R.; Morita, N.; Tanaka, Y.; Koide, N.; Komatsu, T. Sendai virus C protein affects macrophage function, which plays a critical role in modulating disease severity during Sendai virus infection in mice. Microbiol. Immunol. 2022, 66, 124–134. [Google Scholar] [CrossRef]
- Irie, T.; Nagata, N.; Yoshida, T.; Sakaguchi, T. Paramyxovirus Sendai virus C proteins are essential for maintenance of negative-sense RNA genome in virus particles. Virology 2008, 374, 495–505. [Google Scholar] [CrossRef]
- Kristensson, K.; Orvell, C.; Leestma, J.; Norrby, E. Sendai virus infection in the brains of mice: Distribution of viral antigens studied with monoclonal antibodies. J. Infect. Dis. 1983, 147, 297–301. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, L.; Han, K.J.; Zhai, Z.; Shu, H.B. A20 is a potent inhibitor of TLR3- and Sendai virus-induced activation of NF-kappaB and ISRE and IFN-beta promoter. FEBS Lett. 2004, 576, 86–90. [Google Scholar] [CrossRef]
- Lin, R.; Yang, L.; Nakhaei, P.; Sun, Q.; Sharif-Askari, E.; Julkunen, I.; Hiscott, J. Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J. Biol. Chem. 2006, 281, 2095–2103. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Okesanya, O.J.; Ukoaka, B.M.; Ibrahim, A.M.; Lucero-Prisno, D.E., 3rd. Vesicular Stomatitis Virus: Insights into Pathogenesis, Immune Evasion, and Technological Innovations in Oncolytic and Vaccine Development. Viruses 2024, 16, 1933. [Google Scholar] [CrossRef]
- Liu, G.; Cao, W.; Salawudeen, A.; Zhu, W.; Emeterio, K.; Safronetz, D.; Banadyga, L. Vesicular Stomatitis Virus: From Agricultural Pathogen to Vaccine Vector. Pathogens 2021, 10, 1092. [Google Scholar] [CrossRef]
- Mire, C.E.; White, J.M.; Whitt, M.A. A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating. PLOS Pathog. 2010, 6, e1000994. [Google Scholar] [CrossRef]
- Oshiumi, H.; Miyashita, M.; Inoue, N.; Okabe, M.; Matsumoto, M.; Seya, T. The Ubiquitin Ligase Riplet Is Essential for RIG-I-Dependent Innate Immune Responses to RNA Virus Infection. Cell Host Microbe 2010, 8, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Shi, Y.; Ding, W.; Niu, T.; Sun, L.; Tan, Y.; Chen, Y.; Shi, J.; Xiong, Q.; Huang, X.; et al. Cryo-EM analysis of the HCoV-229E spike glycoprotein reveals dynamic prefusion conformational changes. Nat. Commun. 2021, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Siragam, V.; Maltseva, M.; Castonguay, N.; Galipeau, Y.; Srinivasan Mrudhula, M.; Soto Justino, H.; Dankar, S.; Langlois, M.-A. Seasonal human coronaviruses OC43, 229E, and NL63 induce cell surface modulation of entry receptors and display host cell-specific viral replication kinetics. Microbiol. Spectr. 2024, 12, e04220–e04223. [Google Scholar] [CrossRef]
- Tsai, Y.-X.; Chien, Y.-C.; Hsu, M.-F.; Khoo, K.-H.; Hsu, S.-T.D. Molecular basis of host recognition of human coronavirus 229E. Nat. Commun. 2025, 16, 2045. [Google Scholar] [CrossRef]
- Poppe, M.; Wittig, S.; Jurida, L.; Bartkuhn, M.; Wilhelm, J.; Müller, H.; Beuerlein, K.; Karl, N.; Bhuju, S.; Ziebuhr, J.; et al. The NF-κB-dependent and -independent transcriptome and chromatin landscapes of human coronavirus 229E-infected cells. PLOS Pathog. 2017, 13, e1006286. [Google Scholar] [CrossRef]
- Sijmons, S.; Van Ranst, M.; Maes, P. Genomic and functional characteristics of human cytomegalovirus revealed by next-generation sequencing. Viruses 2014, 6, 1049–1072. [Google Scholar] [CrossRef]
- Shang, Z.; Li, X. Human cytomegalovirus: Pathogenesis, prevention, and treatment. Mol. Biomed. 2024, 5, 61. [Google Scholar] [CrossRef]
- Gu, S.Y.; Kim, Y.-E.; Kwon, K.M.; Han, T.-H.; Ahn, J.-H. Biphasic regulation of A20 gene expression during human cytomegalovirus infection. Virol. J. 2014, 11, 124. [Google Scholar] [CrossRef]
- Peng, R.; Chen, C.; Chen, Q.; Zhang, Y.; Huang, R.; Zhang, Y.; Li, J. Global progress in clinical research on human respiratory syncytial virus vaccines. Front. Microbiol. 2024, 15, 1457703. [Google Scholar] [CrossRef]
- Piñana, M.; González-Sánchez, A.; Andrés, C.; Vila, J.; Creus-Costa, A.; Prats-Méndez, I.; Arnedo-Muñoz, M.; Saubi, N.; Esperalba, J.; Rando, A.; et al. Genomic evolution of human respiratory syncytial virus during a decade (2013–2023): Bridging the path to monoclonal antibody surveillance. J. Infect. 2024, 88, 106153. [Google Scholar] [CrossRef]
- Martín-Vicente, M.; González-Sanz, R.; Cuesta, I.; Monzón, S.; Resino, S.; Martínez, I. Downregulation of A20 Expression Increases the Immune Response and Apoptosis and Reduces Virus Production in Cells Infected by the Human Respiratory Syncytial Virus. Vaccines 2020, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Descamps, D.; Peres de Oliveira, A.; Gonnin, L.; Madrières, S.; Fix, J.; Drajac, C.; Marquant, Q.; Bouguyon, E.; Pietralunga, V.; Iha, H.; et al. Depletion of TAX1BP1 Amplifies Innate Immune Responses during Respiratory Syncytial Virus Infection. J. Virol. 2021, 95, 10-1128. [Google Scholar] [CrossRef]
- Misin, A.; Antonello, R.M.; Di Bella, S.; Campisciano, G.; Zanotta, N.; Giacobbe, D.R.; Comar, M.; Luzzati, R. Measles: An Overview of a Re-Emerging Disease in Children and Immunocompromised Patients. Microorganisms 2020, 8, 276. [Google Scholar] [CrossRef]
- Fukuhara, H.; Mwaba, M.H.; Maenaka, K. Structural characteristics of measles virus entry. Curr. Opin. Virol. 2020, 41, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Moss, W.J.; Griffin, D.E. What’s going on with measles? J. Virol. 2024, 98, e00758-24. [Google Scholar] [CrossRef]
- Schmitz Katharina, S.; Handrejk, K.; Liepina, L.; Bauer, L.; Haas Griffin, D.; van Puijfelik, F.; Veldhuis Kroeze Edwin, J.B.; Riekstina, M.; Strautmanis, J.; Cao, H.; et al. Functional properties of measles virus proteins derived from a subacute sclerosing panencephalitis patient who received repeated remdesivir treatments. J. Virol. 2024, 98, e01874-23. [Google Scholar] [CrossRef]
- Yokota, S.; Okabayashi, T.; Yokosawa, N.; Fujii, N. Measles virus P protein suppresses Toll-like receptor signal through up-regulation of ubiquitin-modifying enzyme A20. FASEB J. 2008, 22, 74–83. [Google Scholar] [CrossRef]
- Bolaños-Martínez, O.C.; Strasser, R. Plant-made poliovirus vaccines—Safe alternatives for global vaccination. Front. Plant Sci. 2022, 13, 1046346. [Google Scholar] [CrossRef]
- Hogle, J.M. Poliovirus cell entry: Common structural themes in viral cell entry pathways. Annu. Rev. Microbiol. 2002, 56, 677–702. [Google Scholar] [CrossRef]
- Doukas, T.; Sarnow, P. Escape from transcriptional shutoff during poliovirus infection: NF-κB-responsive genes IκBa and A20. J. Virol. 2011, 85, 10101–10108. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Chen, S.; Sun, X.; Zhou, C. Immune mechanisms of group B coxsackievirus induced viral myocarditis. Virulence 2023, 14, 2180951. [Google Scholar] [CrossRef]
- Machado, R.S.; Tavares, F.N.; Sousa, I.P. Global landscape of coxsackieviruses in human health. Virus Res. 2024, 344, 199367. [Google Scholar] [CrossRef]
- Li, B.; Xie, X. A20 (TNFAIP3) alleviates viral myocarditis through ADAR1/miR-1a-3p-dependent regulation. BMC Cardiovasc. Disord. 2022, 22, 10. [Google Scholar] [CrossRef]
- Fishburn, A.T.; Florio, C.J.; Klaessens, T.N.; Prince, B.; Adia, N.A.B.; Lopez, N.J.; Beesabathuni, N.S.; Becker, S.S.; Cherkashchenko, L.; Haggard Arcé, S.T.; et al. Microcephaly protein ANKLE2 promotes Zika virus replication. mBio 2024, 16, e0268324. [Google Scholar] [CrossRef]
- Li, J.; Zhu, C.; Meng, Y.; Zhang, L.; Liu, C.; Qin, Y.; Chen, M. Zika virus inhibits cell death by inhibiting the expression of NLRP3 and A20. J. Virol. 2025, 99, e01980-24. [Google Scholar] [CrossRef]
- Poon, A.F.Y. Prospects for a sequence-based taxonomy of influenza A virus subtypes. Virus Evol. 2024, 10, veae064. [Google Scholar] [CrossRef]
- Yang, R.; Pan, M.; Guo, J.; Huang, Y.; Zhang, Q.C.; Deng, T.; Wang, J. Mapping of the influenza A virus genome RNA structure and interactions reveals essential elements of viral replication. Cell Rep. 2024, 43, 113833. [Google Scholar] [CrossRef]
- Guan, Z.; Shi, N.; Song, Y.; Zhang, X.; Zhang, M.; Duan, M. Induction of the cellular microRNA-29c by influenza virus contributes to virus-mediated apoptosis through repression of antiapoptotic factors BCL2L2. Biochem. Biophys. Res. Commun. 2012, 425, 662–667. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, C.; Sun, X.; Li, Z.; Zhang, M.; Guan, Z.; Duan, M. Induction of the cellular miR-29c by influenza virus inhibits the innate immune response through protection of A20 mRNA. Biochem. Biophys. Res. Commun. 2014, 450, 755–761. [Google Scholar] [CrossRef]
- Feng, W.; Sun, X.; Shi, N.; Zhang, M.; Guan, Z.; Duan, M. Influenza a virus NS1 protein induced A20 contributes to viral replication by suppressing interferon-induced antiviral response. Biochem. Biophys. Res. Commun. 2017, 482, 1107–1113. [Google Scholar] [CrossRef]
- Maelfait, J.; Roose, K.; Vereecke, L.; Mc Guire, C.; Sze, M.; Schuijs, M.J.; Willart, M.; Itati Ibañez, L.; Hammad, H.; Lambrecht, B.N.; et al. A20 Deficiency in Lung Epithelial Cells Protects against Influenza A Virus Infection. PLOS Pathog. 2016, 12, e1005410. [Google Scholar] [CrossRef]
- Maelfait, J.; Roose, K.; Bogaert, P.; Sze, M.; Saelens, X.; Pasparakis, M.; Carpentier, I.; van Loo, G.; Beyaert, R. A20 (Tnfaip3) Deficiency in Myeloid Cells Protects against Influenza A Virus Infection. PLOS Pathog. 2012, 8, e1002570. [Google Scholar] [CrossRef]
- Gu, Y.; Hsu, A.C.-Y.; Zuo, X.; Guo, X.; Zhou, Z.; Jiang, S.; Ouyang, Z.; Wang, F. Chronic exposure to low-level lipopolysaccharide dampens influenza-mediated inflammatory response via A20 and PPAR network. Front. Immunol. 2023, 14, 1119473. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Yin, Q.; Shen, T. A review of epidemiology and clinical relevance of Hepatitis B virus genotypes and subgenotypes. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102180. [Google Scholar] [CrossRef]
- Hu, J.L.; Huang, A.L. Classifying hepatitis B therapies with insights from covalently closed circular DNA dynamics. Virol. Sin. 2024, 39, 9–23. [Google Scholar] [CrossRef]
- Wang, C.; Huang, C.; Li, Y.; Bai, J.; Zhao, K.; Fang, Z.; Chen, J. Hepatitis B surface antigen impairs TLR4 signaling by upregulating A20 expression in monocytes. Microbiol. Spectr. 2024, 12, e00909-24. [Google Scholar] [CrossRef]
- Li, N.; Shi, Y.; Zhang, P.; Sang, J.; Li, F.; Deng, H.; Lv, Y.; Han, Q.; Liu, Z. Association of the tandem polymorphisms (rs148314165, rs200820567) in TNFAIP3 with chronic hepatitis B virus infection in Chinese Han population. Virol. J. 2017, 14, 148. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, C.; Wang, Y.; Lu, Z.; Zhuang, N.; Zhao, D.; He, J.; Shi, L. Hepatitis B Virus X Protein Sensitizes TRAIL-Induced Hepatocyte Apoptosis by Inhibiting the E3 Ubiquitin Ligase A20. PLoS ONE 2015, 10, e0127329. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Y.; Fan, Y.C.; Wang, N.; Xia, H.H.; Xiao, X.Y.; Wang, K. Increased A20 mRNA Level in Peripheral Blood Mononuclear Cells is Associated With Immune Phases of Patients With Chronic Hepatitis B. Medicine 2015, 94, e2428. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-C.; Zhang, Y.-Y.; Sun, Y.-Y.; Wang, N.; Xiao, X.-Y.; Wang, K. Altered expression of A20 gene in peripheral blood mononuclear cells is associated with the progression of chronic hepatitis B virus infection. Oncotarget 2016, 7, 68821–68832. [Google Scholar] [CrossRef]
- Fan, Y.-C.; Sun, Y.-Y.; Wang, N.; Xiao, X.-Y.; Wang, K. Up-regulation of A20 gene expression in peripheral blood mononuclear cells is associated with acute-on-chronic hepatitis B liver failure. J. Viral Hepat. 2016, 23, 180–190. [Google Scholar] [CrossRef]
- Kong, I.Y.; Giulino-Roth, L. Targeting latent viral infection in EBV-associated lymphomas. Front. Immunol. 2024, 15, 1342455. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.d.M.; Alves, C.E.d.C.; Pontes, G.S. Epstein-Barr virus: The mastermind of immune chaos. Front. Immunol. 2024, 15, 1297994. [Google Scholar] [CrossRef]
- Alsaadawe, M.; Radman, B.A.; Long, J.; Alsaadawi, M.; Fang, W.; Lyu, X. Epstein Barr virus: A cellular hijacker in cancer. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189218. [Google Scholar] [CrossRef]
- Laherty, C.D.; Hu, H.M.; Opipari, A.W.; Wang, F.; Dixit, V.M. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J. Biol. Chem. 1992, 267, 24157–24160. [Google Scholar] [CrossRef]
- Fries, K.L.; Miller, W.E.; Raab-Traub, N. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J. Virol. 1996, 70, 8653–8659. [Google Scholar] [CrossRef]
- Ning, S.; Pagano, J.S. The A20 deubiquitinase activity negatively regulates LMP1 activation of IRF7. J. Virol. 2010, 84, 6130–6138. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Sato, Y.; Takata, K.; Nomoto, J.; Nakamura, S.; Ohshima, K.; Takeuchi, T.; Orita, Y.; Kobayashi, Y.; Yoshino, T. A20 (TNFAIP3) deletion in Epstein-Barr virus-associated lymphoproliferative disorders/lymphomas. PLoS ONE 2013, 8, e56741. [Google Scholar] [CrossRef]
- Giulino, L.; Mathew, S.; Ballon, G.; Chadburn, A.; Barouk, S.; Antonicelli, G.; Leoncini, L.; Liu, Y.F.; Gogineni, S.; Tam, W.; et al. A20 (TNFAIP3) genetic alterations in EBV-associated AIDS-related lymphoma. Blood 2011, 117, 4852–4854. [Google Scholar] [CrossRef] [PubMed]
- Fries, K.L.; Miller, W.E.; Raab-Traub, N. The A20 protein interacts with the Epstein-Barr virus latent membrane protein 1 (LMP1) and alters the LMP1/TRAF1/TRADD complex. Virology 1999, 264, 159–166. [Google Scholar] [CrossRef]
- Gholamzad, A.; Khakpour, N.; Gholamzad, M.; Roudaki Sarvandani, M.R.; Khosroshahi, E.M.; Asadi, S.; Rashidi, M.; Hashemi, M. Stem cell therapy for HTLV-1 induced adult T-cell leukemia/lymphoma (ATLL): A comprehensive review. Pathol.—Res. Pract. 2024, 255, 155172. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Kang, X.; Niu, Y.; Zhao, T.; Wang, H. PCBP1 interacts with the HTLV-1 Tax oncoprotein to potentiate NF-κB activation. Front. Immunol. 2024, 15, 1375168. [Google Scholar] [CrossRef]
- Saitoh, Y.; Hamano, A.; Mochida, K.; Kakeya, A.; Uno, M.; Tsuruyama, E.; Ichikawa, H.; Tokunaga, F.; Utsunomiya, A.; Watanabe, T.; et al. A20 targets caspase-8 and FADD to protect HTLV-I-infected cells. Leukemia 2016, 30, 716–727. [Google Scholar] [CrossRef]
- Laherty, C.D.; Perkins, N.D.; Dixit, V.M. Human T cell leukemia virus type I Tax and phorbol 12-myristate 13-acetate induce expression of the A20 zinc finger protein by distinct mechanisms involving nuclear factor kappa B. J. Biol. Chem. 1993, 268, 5032–5039. [Google Scholar] [CrossRef]
- Pujari, R.; Hunte, R.; Thomas, R.; van der Weyden, L.; Rauch, D.; Ratner, L.; Nyborg, J.K.; Ramos, J.C.; Takai, Y.; Shembade, N. Human T-cell leukemia virus type 1 (HTLV-1) tax requires CADM1/TSLC1 for inactivation of the NF-κB inhibitor A20 and constitutive NF-κB signaling. PLoS Pathog. 2015, 11, e1004721. [Google Scholar] [CrossRef]
- Chan, D.C.; Fass, D.; Berger, J.M.; Kim, P.S. Core Structure of gp41 from the HIV Envelope Glycoprotein. Cell 1997, 89, 263–273. [Google Scholar] [CrossRef]
- Zhang, P.; Gorman, J.; Tsybovsky, Y.; Lu, M.; Liu, Q.; Gopan, V.; Singh, M.; Lin, Y.; Miao, H.; Seo, Y.; et al. Design of soluble HIV-1 envelope trimers free of covalent gp120-gp41 bonds with prevalent native-like conformation. Cell Rep. 2024, 43, 114518. [Google Scholar] [CrossRef]
- Peng, X.; Sun, T.; Yao, P.; Chen, B.; Lu, X.; Han, D.; Wu, N. Differential expression of innate immunity regulation genes in chronic HIV-1 infected adults. Cytokine 2020, 126, 154871. [Google Scholar] [CrossRef]
- Chitre, A.S.; Kattah, M.G.; Rosli, Y.Y.; Pao, M.; Deswal, M.; Deeks, S.G.; Hunt, P.W.; Abdel-Mohsen, M.; Montaner, L.J.; Kim, C.C.; et al. A20 upregulation during treated HIV disease is associated with intestinal epithelial cell recovery and function. PLoS Pathog. 2018, 14, e1006806. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Song, X.T.; Rollins, L.; Berry, L.; Huang, X.F.; Chen, S.Y. Mucosal and systemic anti-HIV immunity controlled by A20 in mouse dendritic cells. J. Clin. Investig. 2011, 121, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Zammit, N.W.; Siggs, O.M.; Gray, P.E.; Horikawa, K.; Langley, D.B.; Walters, S.N.; Daley, S.R.; Loetsch, C.; Warren, J.; Yap, J.Y.; et al. Denisovan, modern human and mouse TNFAIP3 alleles tune A20 phosphorylation and immunity. Nat. Immunol. 2019, 20, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Bagyinszky, E.; An, S.S.A. Genetic Mutations Associated with TNFAIP3 (A20) Haploinsufficiency and Their Impact on Inflammatory Diseases. Int. J. Mol. Sci. 2024, 25, 8275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).