First Investigation of Grass Carp Reovirus (GCRV) Infection in Amphioxus: Insights into Pathological Effects, Transmission, and Transcriptomic Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Virus Strains
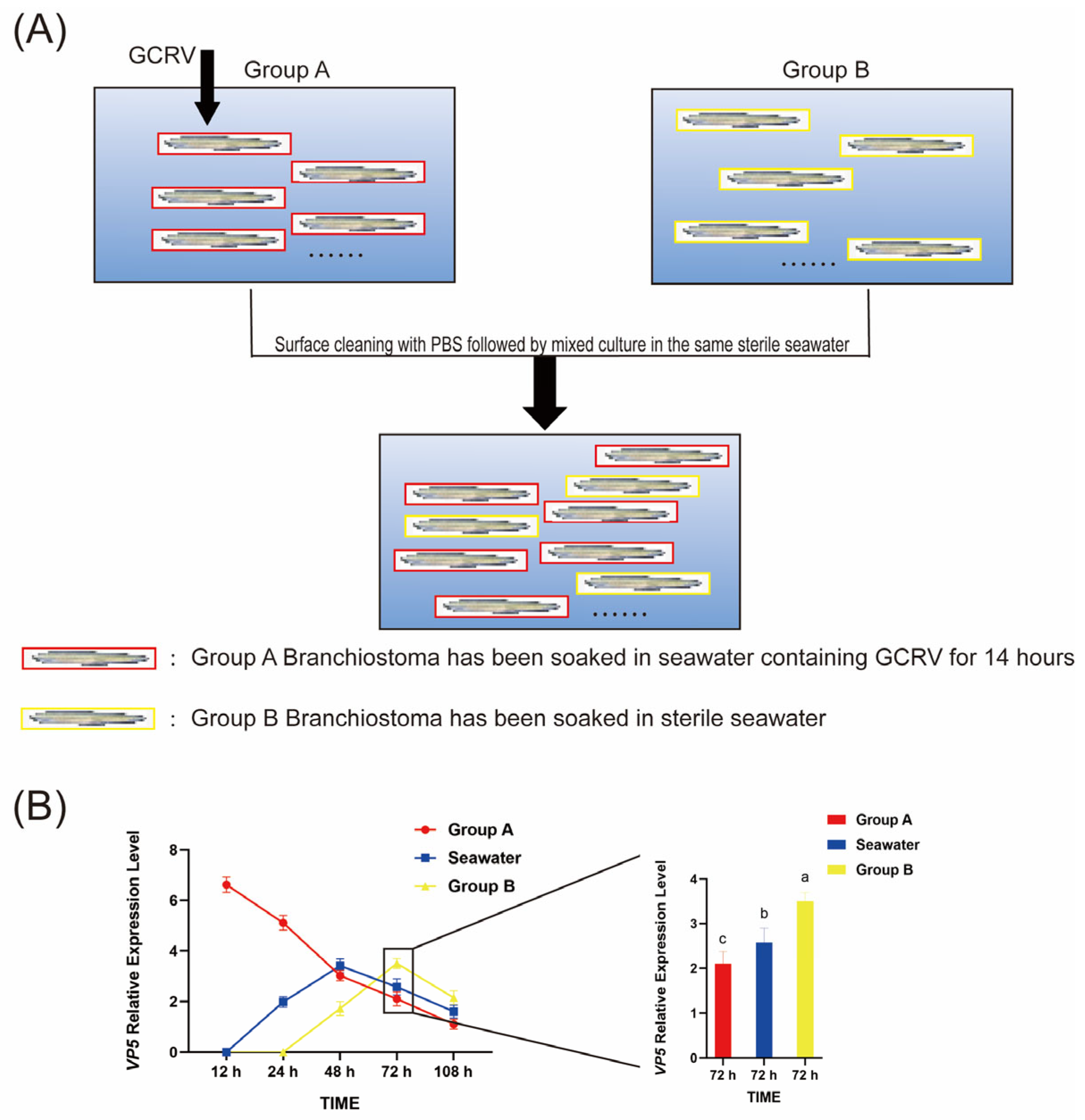
2.2. Infection Modeling and Sample Collection
2.3. qPCR Analysis
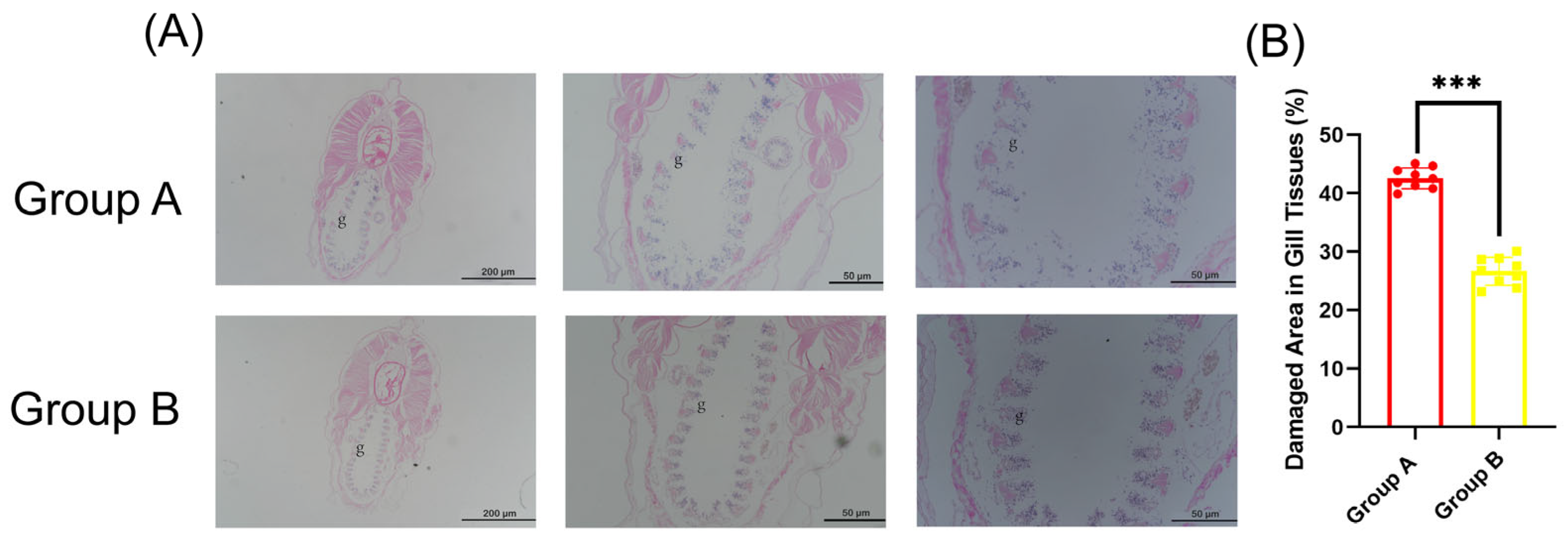
2.4. Histopathology
2.5. Transcriptome Sequencing
2.6. Virus Survival Assay
2.7. Waterborne Transmission Experiment
2.8. Statistical Analysis
3. Results
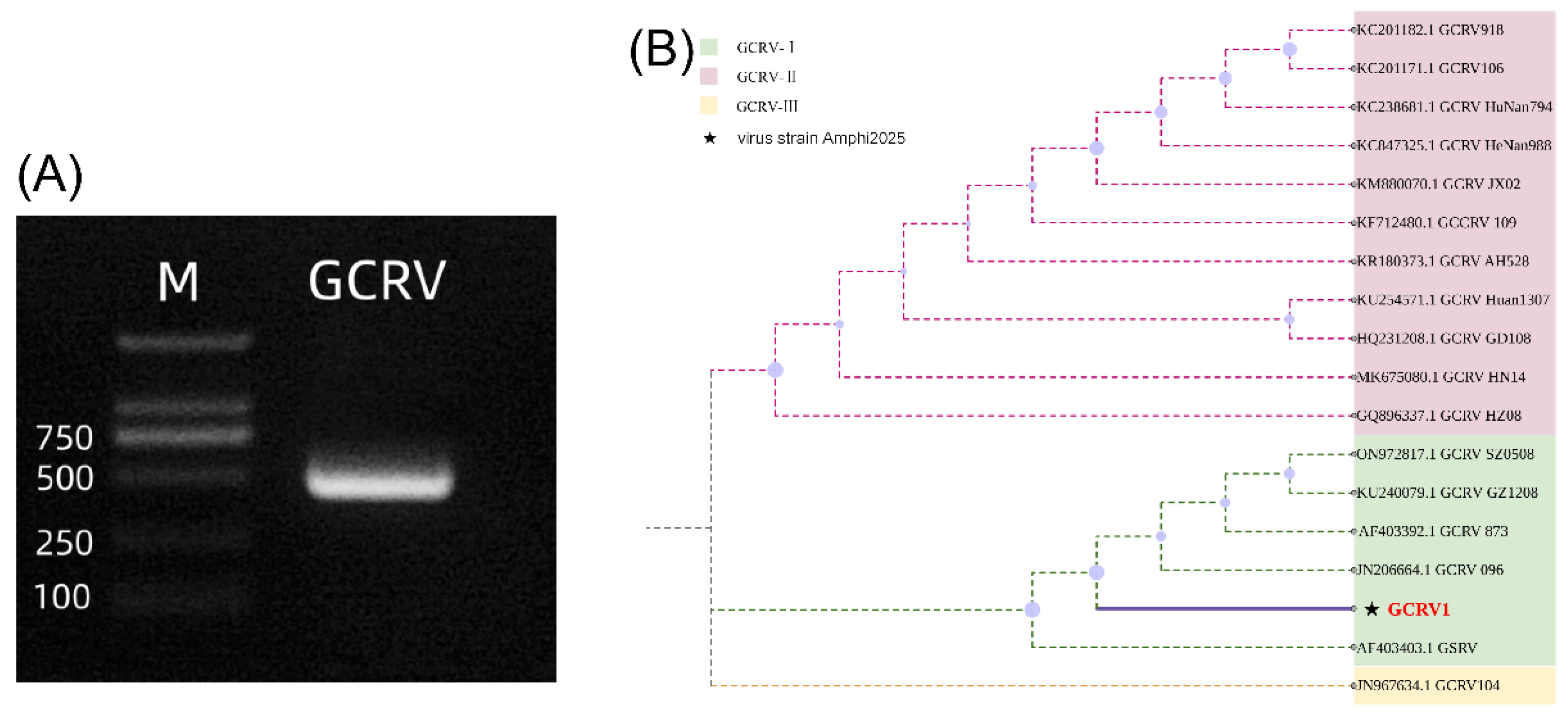
3.1. The GCRV Strain Was Identified as Type I
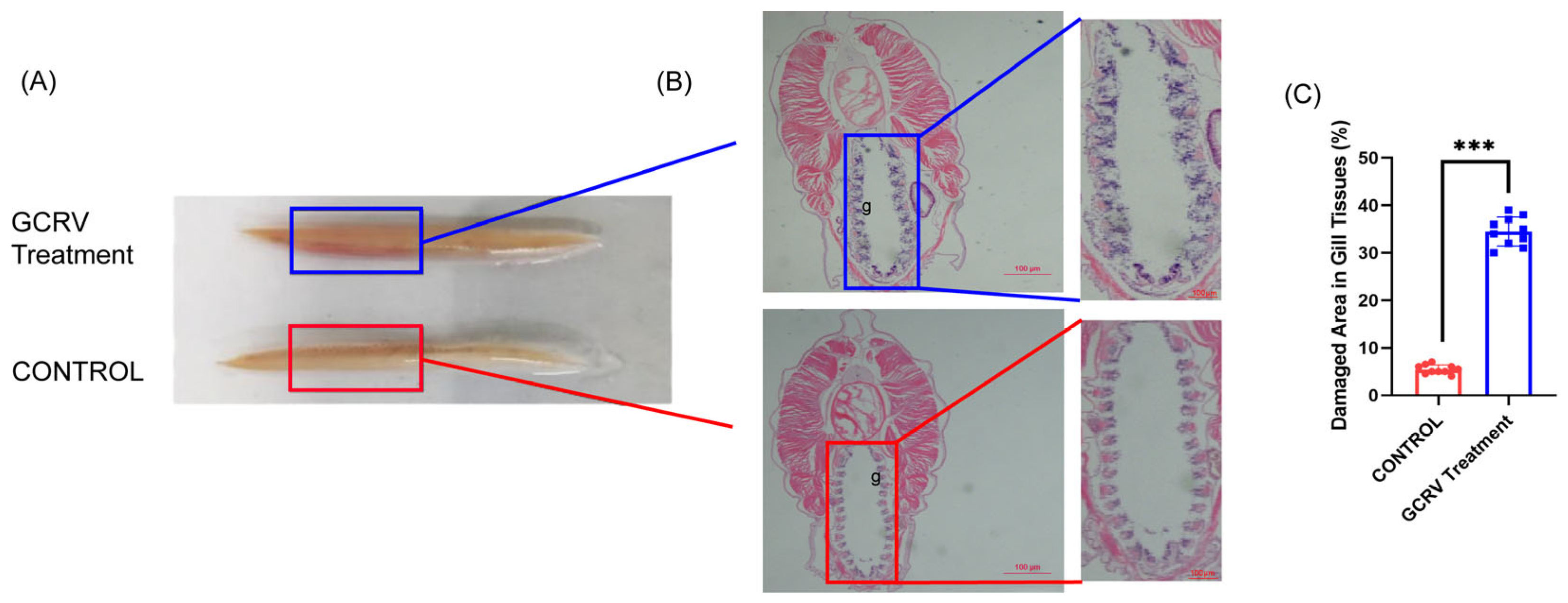
3.2. GCRV Infection Caused Structural Damage of Amphioxus Gill
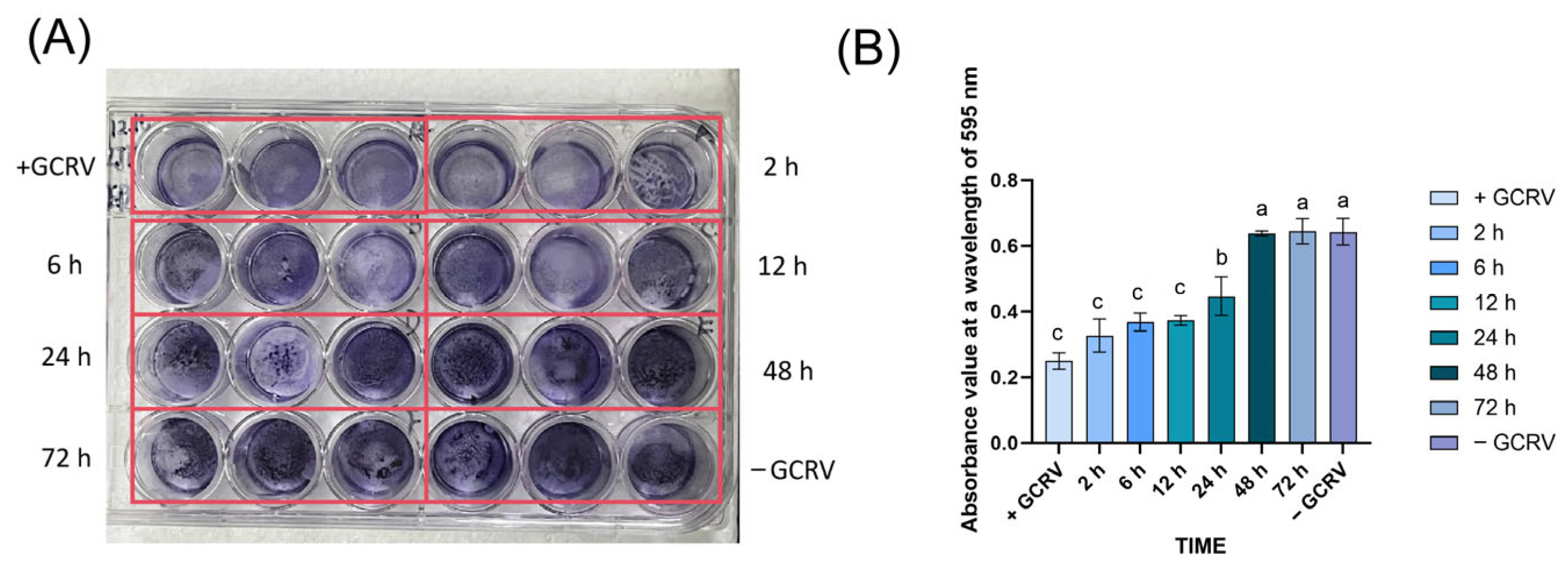
3.3. The GCRV Survived in Seawater for No More than 48 h
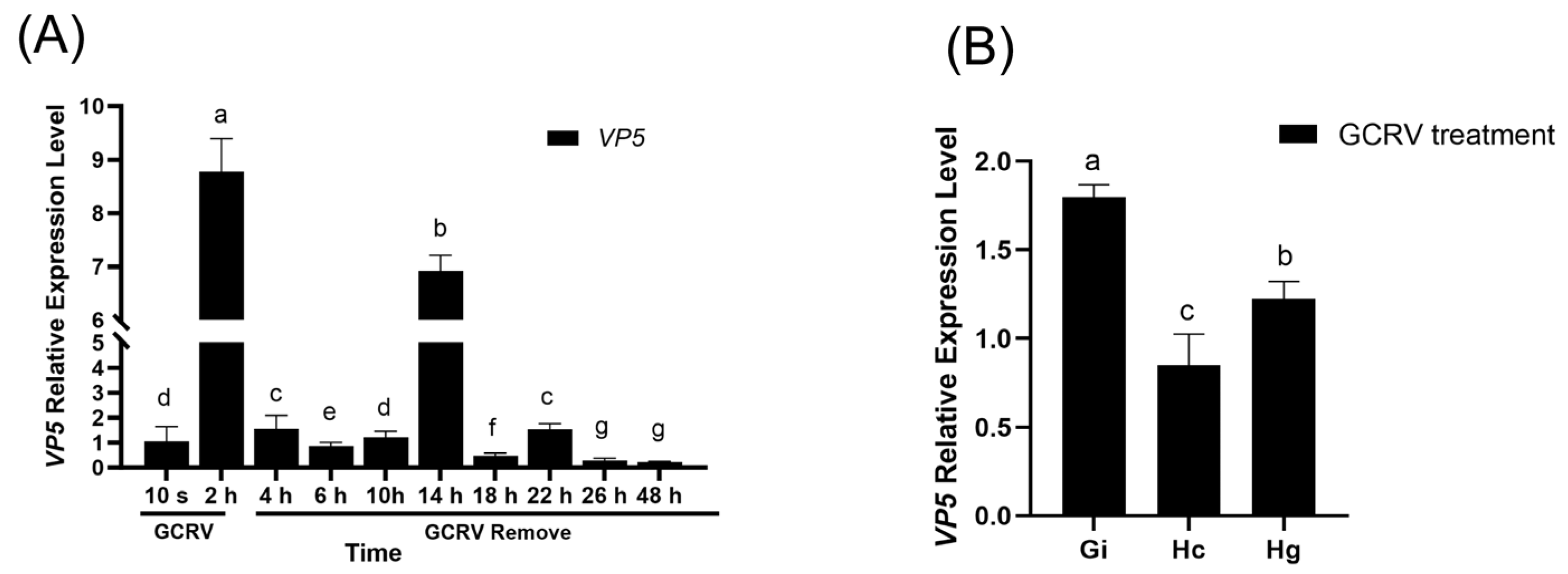
3.4. Infection Kinetics of GCRV on Amphioxus
3.5. GCRV Can Be Transmitted Among Amphioxus Through Co-Culture
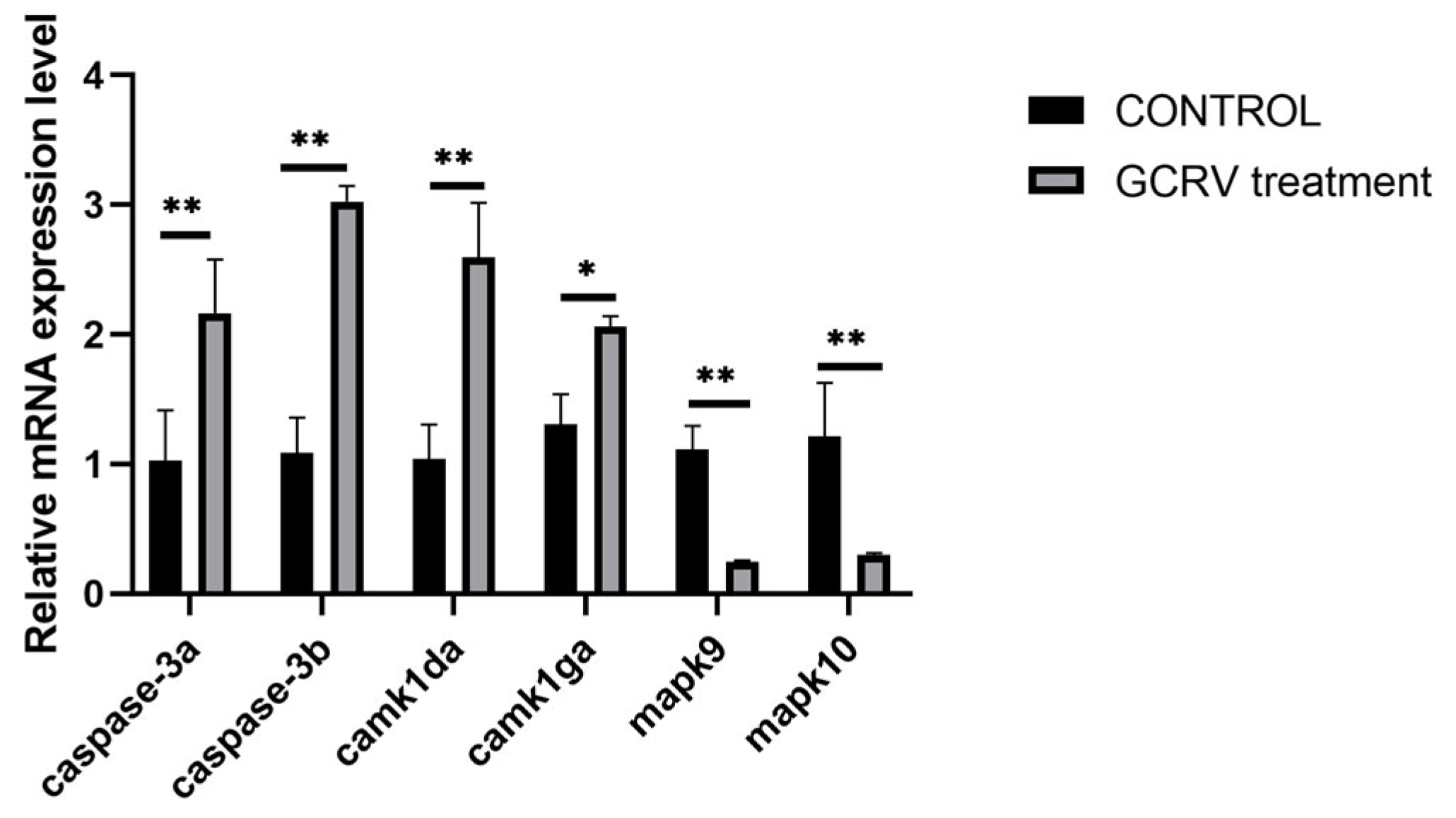
3.6. Transcriptome Analysis Reveals Significant Activation of Immune-Related Pathways
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Xue, M.; Zhou, Y.; Liu, W.; Meng, Y.; Xu, C.; Li, Y.; Jiang, N.; Fan, Y. The Antiviral Efficacy of Andrographolide against Grass Carp Reovirus in Vitro and in Vivo. Fish. Shellfish. Immunol. 2025, 163, 110390. [Google Scholar] [CrossRef]
- Rao, Y.; Su, J. Insights into the Antiviral Immunity against Grass Carp (Ctenopharyngodon Idella) Reovirus (GCRV) in Grass Carp. J. Immunol. Res. 2015, 2015, 670437. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, Y.; Xiong, L.; Jian, J.; Wu, Z. Phylogenetic Analysis of Newly Isolated Grass Carp Reovirus. SpringerPlus 2014, 3, 190. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, A.-Q.; Zhang, C.; Zhang, Y.-A.; Tu, J. Hsp90 Regulates GCRV-II Proliferation by Interacting with VP35 as Its Receptor and Chaperone. J. Virol. 2022, 96, e0117522. [Google Scholar] [CrossRef]
- Gao, Y.; Huo, X.; Wang, Z.; Yuan, G.; Liu, X.; Ai, T.; Su, J. Oral Administration of Bacillus Subtilis Subunit Vaccine Significantly Enhances the Immune Protection of Grass Carp against GCRV-II Infection. Viruses 2021, 14, 30. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, H.; Yuan, Z.; Chen, J.; Zheng, G.; Zou, S. The Effects of GCRV on Various Tissues of Grass Carp (Ctenopharyngodon Idella) and Identification of Differential Interferon-Stimulating Genes (ISGs) through Muscle Transcriptome Analysis. Ecotoxicol. Environ. Saf. 2024, 284, 116956. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Shi, C.; Ouyang, P.; Huang, X.; Chen, D.; Wang, Q.; Yin, J.; Zhang, D.; Geng, Y. The National Surveillance Study of Grass Carp Reovirus in China Reveals the Spatial-Temporal Characteristics and Potential Risks. Aquaculture 2022, 547, 737449. [Google Scholar] [CrossRef]
- D’Aniello, S.; Bertrand, S.; Escriva, H. Amphioxus as a Model to Study the Evolution of Development in Chordates. Elife 2023, 12, e87028. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Yao, Z.; Wang, H.; Dong, X.; Wang, L.; Wang, S.; Gao, Z. Functional Characterization of Interleukin 17 Family Members and Their Receptors in Amphioxus. Int. J. Biol. Macromol. 2025, 311, 143901. [Google Scholar] [CrossRef]
- Chen, G.; Yin, L.; Zhang, H. Isolation and Characterization of Goose Astrovirus Genotype 1 Causing Enteritis in Goslings from Sichuan Province, China. BMC Vet. Res. 2025, 21, 259. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Wang, Y.; Liang, H.; Liu, C.; Song, X.; Shi, C.; Wu, S.; Wang, Q. A One-Step Duplex rRT-PCR Assay for the Simultaneous Detection of Grass Carp Reovirus Genotypes I and II. J. Virol. Methods 2014, 210, 32–35. [Google Scholar] [CrossRef]
- Su, H.; Fan, C.; Liao, Z.; Yang, C.; Clarke, J.L.; Zhang, Y.; Su, J. Grass Carp Reovirus Major Outer Capsid Protein VP4 Interacts with RNA Sensor RIG-I to Suppress Interferon Response. Biomolecules 2020, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Wang, X.-L.; Li, Z.-C.; Zhang, C.; Xu, X.; Cui, B.-J.; Tian, M.-Z.; Zhou, C.-J.; Xu, N.; Wu, Y.; et al. Grass Carp Reovirus VP4 Manipulates TOLLIP to Degrade STING for Inhibition of IFN Production. J. Virol. 2025, 99, e01583-24. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Chen, W.; Lin, Z.; Liu, L.; Zhang, M.; Yang, H.; Liu, Z.; Chen, L. Viral Transmission in Sea Food Systems: Strategies for Control and Emerging Challenges. Foods 2025, 14, 1071. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, W.; Li, Y.; Zhou, Y.; Meng, Y.; Zeng, L.; Vakharia, V.N.; Fan, Y. Isolation, Identification, and Genomic Analysis of a Novel Reovirus from Healthy Grass Carp and Its Dynamic Proliferation in Vitro and in Vivo. Viruses 2021, 13, 690. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, J.; Fan, Y. Epidemiology of the Grass Carp Reovirus. In Aquareovirus; Fang, Q., Ed.; Springer: Singapore, 2021; pp. 133–148. ISBN 978-981-16-1903-8. [Google Scholar]
- Gémez-Mata, J.; Álvarez-Torres, D.; García-Rosado, E.; Alonso, M.C.; Béjar, J. Comparative Analysis of Marine and Freshwater Viral Haemorrhagic Septicaemia Virus (VHSV) Isolates Antagonistic Activity. Comp. Immunol. Microbiol. Infect. Dis. 2020, 69, 101426. [Google Scholar] [CrossRef]
- Jiang, Y. Hemorrhagic Disease of Grass Carp: Status of Outbreaks, Diagnosis, Surveillance, and Research. Isr. J. Aquac.-Bamidgeh 2009, 61, 188–197. [Google Scholar] [CrossRef]
- Zhu, W.; Qiao, M.; Hu, M.; Huo, X.; Zhang, Y.; Su, J. Type II Grass Carp Reovirus Rapidly Invades Grass Carp (Ctenopharyngodon Idella) via Nostril–Olfactory System–Brain Axis, Gill, and Skin on Head. Viruses 2023, 15, 1614. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, J.; Guo, H.; Yan, L.; Chen, Q.; Zhang, F.; Fang, Q. VP5 Autocleavage Is Required for Efficient Infection by in Vitro-Recoated Aquareovirus Particles. J. Gen. Virol. 2015, 96, 1795–1800. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Peng, S.; Zhou, X.; Li, S.; Jin, P. The Amphioxus ERK2 Gene Is Involved in Innate Immune Response to LPS Stimulation. Fish. Shellfish. Immunol. 2019, 86, 64–69. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, D.; Zhang, W.; Cai, Z.; Chen, Z.; Zhang, N.; Mao, B.; Zhang, H. Characterization of the Immune Defense Related Tissues, Cells, and Genes in Amphioxus. Sci. China Life Sci. 2011, 54, 999–1004. [Google Scholar] [CrossRef] [PubMed][Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Yang, M.; Yang, H.; Ji, G.; Liu, Z. First Investigation of Grass Carp Reovirus (GCRV) Infection in Amphioxus: Insights into Pathological Effects, Transmission, and Transcriptomic Responses. Viruses 2025, 17, 1367. https://doi.org/10.3390/v17101367
Lin J, Yang M, Yang H, Ji G, Liu Z. First Investigation of Grass Carp Reovirus (GCRV) Infection in Amphioxus: Insights into Pathological Effects, Transmission, and Transcriptomic Responses. Viruses. 2025; 17(10):1367. https://doi.org/10.3390/v17101367
Chicago/Turabian StyleLin, Jingyuan, Meng Yang, Huijuan Yang, Guangdong Ji, and Zhenhui Liu. 2025. "First Investigation of Grass Carp Reovirus (GCRV) Infection in Amphioxus: Insights into Pathological Effects, Transmission, and Transcriptomic Responses" Viruses 17, no. 10: 1367. https://doi.org/10.3390/v17101367
APA StyleLin, J., Yang, M., Yang, H., Ji, G., & Liu, Z. (2025). First Investigation of Grass Carp Reovirus (GCRV) Infection in Amphioxus: Insights into Pathological Effects, Transmission, and Transcriptomic Responses. Viruses, 17(10), 1367. https://doi.org/10.3390/v17101367




