Abstract
Japanese encephalitis virus (JEV), a mosquito-borne flavivirus, poses a significant public health threat in Asia. Although Culex species are primary vectors, the role of Aedes albopictus in JEV transmission has gained attention under changing ecological conditions. This study evaluated the vector competence of Ae. albopictus for three JEV genotypes: I (GI), III (GIII), and V (GV). Laboratory-reared Ae. albopictus were orally challenged with each genotype, and infection rate (IR), dissemination rate (DR), head–thorax positivity rate (HTR, proxy for potential transmission), and transmission rate (defined as saliva positivity) were assessed at 7 and 14 days post-infection (dpi). Ae. albopictus showed marked genotype-specific differences. By 14 dpi, GV had the highest DR (100.0%) and HTR (71.7%), with viral RNA detected in 36.7% of TR. GIII showed moderate competence (76.9% DR, 39.3% HTR), but low TR (6.6%). In contrast, GI-infected mosquitoes exhibited minimal infection and negligible transmission, with viral RNA rarely detected beyond the midgut. These findings indicate that Ae. albopictus is highly competent for transmitting JEV genotype V and moderately for genotype III, but not genotype I, under laboratory conditions. This highlights its potential role in the transmission dynamics of emerging JEV genotypes and underscores the need for continued surveillance.
1. Introduction
Japanese encephalitis virus (JEV), a mosquito-borne flavivirus, is the leading cause of viral encephalitis in Asia, responsible for an estimated 68,000 cases and over 10,000 deaths annually, predominantly among children in endemic regions [1]. The virus is maintained in a zoonotic transmission cycle involving Culex mosquitoes, particularly Culex tritaeniorhynchus, and vertebrate amplifying hosts, such as pigs and ardeid birds [2]. Human infections are incidental but can lead to outbreaks under favorable ecological and climatic conditions [3,4,5,6].
Among the five recognized JEV genotypes (GI–GV), genotype III (GIII) was the first to be identified, initially isolated in Japan in 1935 [7,8]. Genotype II (GII) and genotype V (GV) were subsequently reported in the 1950s from India and Malaysia, respectively [9,10], while genotype IV (GIV) was detected in Indonesia in 1979. Genotype I (GI), now the dominant strain across much of Asia, emerged in East Asia in the late 1970s [2,11,12,13].
Historically, GIII was the predominant genotype circulating in East and Southeast Asia. However, over the past two decades, GI has largely displaced GIII as the dominant strain in many regions [14]. Notably, GV, once considered extinct following its initial isolation in Malaysia, re-emerged in 2009 when it was detected in Cx. tritaeniorhynchus in China [15]. In the Republic of Korea (ROK), GV was first identified in 2010 in Cx. bitaeniorhynchus, with subsequent detections in other Culex species, including Cx. orientalis in 2020, indicating its sustained circulation [14,15,16]. GIV has also been reported, mainly in Indonesia and parts of Oceania, but its limited geographic distribution and relatively low public health impact have resulted in sparse investigation [17].
Although Culex species remain the principal vectors of JEV, accumulating evidence suggests that Aedes albopictus may also contribute to its transmission, particularly in ecologically altered or urban environments [18,19,20]. Commonly known as the Asian tiger mosquito, Ae. albopictus is a highly adaptable species with a global distribution facilitated by international trade and climate change. In ROK, Ae. albopictus is already widely distributed nationwide [21]. JEV RNA has been detected in field-collected Ae. albopictus in ROK [22]. While repeated isolations of JEV from Ae. albopictus have been reported in Asia, the primary vectors remain Culex species, and the epidemiological significance of Ae. albopictus in natural transmission cycles is still unclear [23,24,25]. The established competence of Ae. albopictus for transmitting other arboviruses—including dengue, chikungunya, and Zika—warrants further investigation into its potential role in JEV transmission [19,20,23,26,27].
Vector competence, the intrinsic ability of a mosquito to acquire, sustain, and transmit a pathogen, varies with both mosquito species and viral genotype. Prior studies have demonstrated that susceptibility to JEV can vary among mosquito species, populations, and viral genotypes, highlighting the importance of investigating local vector competence for both primary and potential supplementary vectors [18,20,21,28]. However, comparative data on Ae. albopictus responses to different JEV genotypes remain limited. In particular, the ability of Ae. albopictus to support replication and transmission of the re-emerging GV genotype is poorly characterized. This gap is especially critical given the recent predominance of GV in ROK, underscoring the need for localized assessments of vector competence.
Given the ongoing genotype shifts in JEV and the expanding ecological range of Ae. albopictus, it is essential to evaluate its potential role in transmitting diverse JEV genotypes. In this study, we assessed the vector competence of laboratory-reared Ae. albopictus for JEV GI, GIII, and GV. Using oral infection assays, we evaluated genotype-specific differences in infection, dissemination, and transmission at multiple time points post-infection. These findings aim to enhance understanding of genotype-dependent transmission dynamics and inform future risk assessments and vector control strategies in the context of evolving JEV ecology.
2. Materials and Methods
2.1. Mosquitoes
Ae. albopictus (F43 generation), originally collected from Incheon, ROK, were used for experimental infections. Following field collection, the colony was continuously maintained under laboratory conditions. Mosquitoes were reared at 27 ± 1 °C, 70 ± 5% relative humidity, and a 12:12 h light–dark photoperiod. Adults were provided with 10% sucrose ad libitum, and females were blood-fed weekly using an artificial membrane feeding system (Hemotek Ltd., Accrington, UK). Each genotype was tested in three independent biological replicates, using newly prepared infectious blood meals and separate cohorts of mosquitoes.
2.2. Production and Titration of JEV Strains for Vector Competence Assays
JEV GI was isolated from Cx. tritaeniorhynchus in 2005 [29], GIII from the same species in 1994 [29], and GV from Cx. orientalis in 2020 [16] (Table 1). Viral stocks were propagated in Vero cells as previously described [19,30]. Briefly, each strain was cultured in Eagle’s Minimum Essential Medium (MEM; Welgene, Gyeongsan, Republic of Korea) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified incubator (Vision Scientific Co.,Ltd., Daejeon, Republic of Korea) with 5% CO2 for 3 days in T75 flasks. Viral replication was confirmed by the presence of cytopathic effects (CPE) observed under a light microscope (ZEISS, Oberkochen, Germany). For virus titration, BHK-21 cells were seeded in six-well plates(SPL, Pocheon, Republic of Korea) 1 day prior to infection. Serial 10-fold dilutions of each viral stock were prepared in MEM containing 2% FBS and 1% penicillin–streptomycin (P/S), and 300 μL of each dilution was inoculated into individual wells. Following 1 h of incubation at 37 °C with 5% CO2, the inoculum was replaced with 4 mL of overlay medium containing 0.5% agarose, 2% FBS, and 1% P/S in MEM. After 5 days of incubation at 37 °C, the cells were fixed with 4% paraformaldehyde for 30 min and stained with 1% crystal violet. Plaques were counted, and viral titers are expressed as plaque-forming units per milliliter (PFU/mL).

Table 1.
Details of Japanese Encephalitis Virus Strains Used for Experimental Infections.
2.3. Oral Infection of JEV
All infection assays were conducted in a Biosafety Level 3 (BSL-3) facility (Permit No. KCDC-18-3-04) at the Division of Vectors and Parasitic Diseases, Korea Disease Control and Prevention Agency (KDCA). Female Ae. albopictus mosquitoes (5–7 days old) were deprived of sucrose for 24 h prior to exposure. Infectious blood meals were prepared by mixing defibrinated sheep blood (Kisan Bio Co., Ltd., Seoul, Republic of Korea) with virus suspension at a 2:1 ratio, supplemented with ATP to a final concentration of 10 nM to stimulate feeding. Viral titers in the blood meals were adjusted to 6.5 × 106–1.0 × 107 PFU/mL for GI, 1.2 × 106–1.0 × 107 PFU/mL for GIII, and 1.0 × 106–1.0 × 107 PFU/mL for GV. Mosquitoes were allowed to feed overnight in a dark incubator at 27 ± 1 °C using a Hemotek membrane feeding system (Hemotek Ltd., Blackburn, UK). Fully engorged females were selected and maintained under standard rearing conditions (27 ± 1 °C, 70 ± 5% relative humidity, 12:12 h light–dark cycle) for the remainder of the experiment.
2.4. Mosquito Dissection and Saliva Collection
To evaluate infection dynamics, mosquitoes were dissected at 7 and 14 days post-infection (dpi) in the BSL-3 insectary under controlled conditions (27 ± 1 °C, 70 ± 5% relative humidity). For each individual, the midgut, legs and wings, and head–thorax were carefully separated and transferred into individual microcentrifuge tubes (SPL, Pocheon, Republic of Korea) containing 300 μL of MEM supplemented with 2% FBS and a ceramic homogenization bead. These tissues were used to assess infection (midgut), dissemination (legs and wings), and head–thorax positivity as a proxy for potential salivary gland invasion. At 14 dpi, saliva was also collected to assess transmissibility. Cold-anesthetized mosquitoes had their legs and wings removed, and their proboscis was inserted into a 10 μL droplet of phosphate-buffered saline (PBS) held within a cut pipette tip under the same environmental conditions (27 ± 1 °C, 70 ± 5% RH). After 30 min of salivation, saliva droplets were collected. All dissected tissues and saliva samples were stored at −80 °C until analysis.
Infection rate (IR), dissemination rate (DR), and transmission rate (TR) were determined based on the detection of viral RNA in specific tissues. IR was defined as the proportion of mosquitoes with viral RNA detected in the midgut, indicating successful initial infection. DR was calculated as the proportion of infected mosquitoes with viral RNA in the legs and wings, representing viral dissemination beyond the midgut barrier. Head–thorax positivity rate (HTR) was defined as the proportion of mosquitoes with viral RNA detected in the head–thorax, serving as a proxy for potential transmission.
In accordance with the accepted definition, TR is defined strictly as the proportion of mosquitoes with viral RNA detected in saliva, reflecting actual transmission potential. In this study, HTR is presented alongside TR as a complementary measure, since saliva-based assays may underestimate transmission due to methodological limitations.
2.5. RNA Extraction and qRT-PCR Detection of JEV
All tissue samples, excluding saliva, were homogenized using the Precellys® Evolution homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France) with two cycles of bead beating at 7500 rpm for 30 s each. Homogenates were centrifuged at 13,000 rpm for 1 min, and 30 μL of the supernatant was transferred to a new tube for RNA extraction. Total RNA was extracted using the Clear-S™ Total RNA Extraction Kit (Invirustech, Gwangju, Republic of Korea) according to the manufacturer’s instructions.
JEV RNA targeting the non-structural protein 5 (NS5) gene was detected using the Clear-MD® Flavivirus Real-Time RT-PCR Detection Kit (Invirustech, Gwangju, Republic of Korea). The qRT-PCR conditions were as follows: reverse transcription at 45 °C for 10 min; enzyme inactivation at 95 °C for 10 min; followed by 40 cycles of amplification consisting of denaturation at 95 °C for 10 s, annealing at 60 °C for 15 s, extension at 72 °C for 10 s, and signal acquisition at 80 °C for 15 s. A melting curve analysis was subsequently performed to confirm product specificity: denaturation at 95 °C for 30 s, annealing at 70 °C for 30 s, followed by gradual heating to 95 °C in 0.5 °C increments every 30 s. Samples with a cycle threshold (Ct) value ≤ 40 and a melting peak between 82 °C and 88 °C were considered positive for JEV RNA. Quantification was calibrated using a standard curve generated from a Vircell JEV RNA quantified control (MBC134-R; AMPLIRUN Japanese Encephalitis RNA control, Granada, Spain, 2024) using 10-fold serial dilutions (105–103 copies per reaction); the resulting standard-curve data are provided in Supplementary Figure S1.
2.6. Viral Titration by TCID50 Assay
To quantify infectious virus, samples from the legs–wings, body, and head–thorax were collected at 7 and 14 dpi. Each tissue homogenate (30 μL) was used for titration via the 50% tissue culture infectious dose (TCID50/mL) assay using BHK-21 cells. BHK-21 cells were seeded into 96-well plates at a density of 1.0 × 104 cells/well in 200 μL of MEM supplemented with 5% FBS and 1% penicillin–streptomycin (P/S), and incubated overnight at 37 °C. Serial 10-fold dilutions of each homogenate were prepared in MEM containing 2% FBS, and 100 μL of each dilution was added to five replicate wells. Plates were incubated at 37 °C for 5 days, after which CPE were visually assessed. Cells were subsequently fixed and stained with crystal violet to confirm CPE. Viral titers were calculated using the Reed–Muench method based on the number of CPE-positive wells at each dilution [3].
2.7. Statistical Analysis
All statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA). Comparisons of viral RNA loads between time points within each tissue were assessed using the nonparametric Mann–Whitney U test. For comparisons across multiple groups (e.g., among JEV genotypes), the Kruskal–Wallis test, followed by Dunn’s multiple comparisons test, was applied. Differences in HTR (proxy for potential transmission) and TR (defined as saliva positivity) between 7 and 14 dpi were analyzed for each genotype using Fisher’s exact test. PTR (population transmission rate) was calculated as the proportion of saliva-positive mosquitoes among blood-fed survivors. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Genotype-Dependent Differences in the Vector Competence of Ae. albopictus
The vector competence of Ae. albopictus for three JEV genotypes (GI, GIII, GV) was assessed based on IR, DR, HTR, and TR (saliva positivity) at 7 and 14 dpi (Table 2). The data revealed pronounced genotype-specific differences. GV-infected mosquitoes displayed the highest competence at 14 dpi, with a DR of 100.0% and HTR of 71.7%. However, the TR, defined as saliva positivity, was 36.7%, indicating that although many mosquitoes showed evidence of potential salivary gland invasion, only about one-third were capable of viral excretion. Mosquitoes infected with GIII showed intermediate competence. At 14 dpi, DR reached 76.9% and HTR was 39.3%, but the corresponding TR was only 6.6%, suggesting inefficient release of virus into saliva despite moderate dissemination and potential salivary gland infection.

Table 2.
Vector Competence of Ae. albopictus for JEV GI, GIII, and GV at 7 and 14 Dpi. IR, DR, HTR and TR of Ae. albopictus orally infected with JEV GI, GIII, and GV at 7 and 14 dpi. Each rate was determined by the detection of JEV RNA in specific mosquito body parts: body (IR), legs and wings (DR), and head–thorax (HTR). TR (Saliva positivity) at 14 dpi is presented separately as a direct indicator of viral excretion. JEV detection was performed using qRT-PCR.
In contrast, GI-infected mosquitoes exhibited minimal competence. At 14 dpi, dissemination was not observed beyond the midgut in most individuals, and the TR was only 4.0%, confirming negligible transmission potential for this genotype in Ae. albopictus. Together, these results demonstrate that while HTR provides an indicator of potential transmission, TR based on saliva positivity represents the conservative and accepted measure of actual transmission capacity, which varied markedly among JEV genotypes.
3.2. Temporal Dynamics of Viral Dissemination and Transmission
Temporal analysis revealed efficient progression of GV infection within mosquito tissues between 7 and 14 dpi. A marked increase in viral RNA detection in both the head–thorax region and saliva was observed at 14 dpi, indicating successful viral escape from tissue barriers and a high probability of transmission. In GIII-infected mosquitoes, dissemination to secondary tissues was already apparent by 7 dpi and remained relatively stable thereafter, with only a modest increase over time. In contrast, GI infection showed no substantial progression during the same period, remaining largely restricted to the midgut. These patterns underscore distinct temporal dynamics in viral replication and dissemination across JEV genotypes (Figure 1).
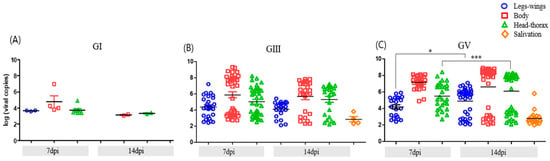
Figure 1.
Viral RNA loads in Ae. albopictus tissues (midgut, legs–wings, head–thorax) and saliva at 7 and 14 dpi with JEV GI (A), GIII (B), and GV (C). Head–thorax RNA positivity was used to calculate transmission rate (TR), while saliva RNA positivity was evaluated separately as an indicator of actual viral excretion. Each dot represents an individual mosquito, and horizontal bars indicate the mean ± standard error of the mean (SEM). Statistical differences between 7 and 14 dpi within each tissue were assessed using the Mann–Whitney U test (* p < 0.05, *** p < 0.001).
3.3. Infectious Viral Titers in Ae. albopictus
To determine the presence of infectious virus, TCID50 assays were performed on mosquito body, legs–wings, and head–thorax samples at 7 and 14 dpi (Table 3). Infectious JEV was consistently detected in GIII-infected mosquitoes. Mean viral titers in the body were 2.39 ± 0.10 log10 TCID50/mL (n = 9) at 7 dpi and 2.30 ± 0.08 log10 TCID50/mL (n = 5) at 14 dpi. Although viral dissemination to the head–thorax was observed in a limited number of mosquitoes, the corresponding titers remained low, 2.33 and 2.35 log10 TCID50/mL at 7 and 14 dpi, respectively.

Table 3.
Infectious virus titers (log10 TCID50/mL) of JEV in Ae. albopictus tissues at 7 and 14 dpi.
3.4. Transmission Potential of Ae. albopictus at the Population Level
To evaluate the population-level transmission potential of Ae. albopictus, viral RNA was quantified in both the head–thorax region (head–thorax positivity rate, indicating potential transmission) and saliva (reflecting actual transmission) (Figure 2). At 14 dpi, GV-infected mosquitoes exhibited the highest HTR (71.7%) based on head–thorax positivity; however, the TR was 36.7%, providing the most accurate indicator of transmission. GIII-infected mosquitoes showed an HTR of 39.3%, but only 6.6% TR, reflecting a strong barrier to viral excretion. GI-infected mosquitoes exhibited minimal competence, with just 4.0% TR, confirming negligible transmission potential. These comparisons highlight that while HTP provides a proxy measure of potential salivary gland invasion, TR based on saliva positivity represents the conservative and accepted indicator of true transmission capacity. These findings indicate a markedly reduced ability of GI to escape midgut and salivary barriers, further highlighting the limited transmission potential of this genotype in Ae. albopictus.
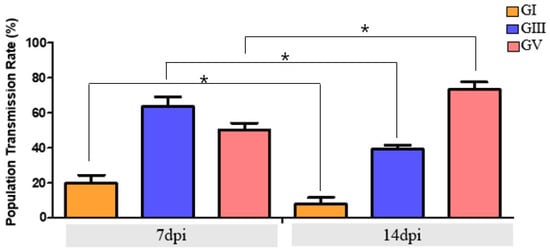
Figure 2.
Comparison of population transmission rates (PTR) of Ae. albopictus orally infected with JEV GI, GIII, and GV at 7 and 14 dpi. PTR was calculated as the proportion of mosquitoes with detectable JEV RNA in the head–thorax (HTR, proxy for potential transmission) or saliva (TR, actual transmission). While HTR reflects possible salivary gland invasion, TR represents the accepted definition of transmission rate based on viral detection in saliva. Statistical differences in PTR-TR between 7 and 14 dpi were evaluated for each genotype using Fisher’s exact test (* p < 0.05).
4. Discussion
This study provides novel insights into the genotype-specific vector competence of Ae. albopictus for JEV, with particular emphasis on the recently re-emerged GV. Under controlled laboratory conditions, Ae. albopictus supported the infection, dissemination, and transmission of JEV GV at levels comparable to, or exceeding, those of GIII, a historically dominant strain. In contrast, GI, currently the predominant circulating strain in East Asia, exhibited markedly reduced vector competence, with limited midgut infection and negligible transmission potential [30,31].
In line with the accepted definition, TR was defined strictly as the proportion of mosquitoes with detectable JEV RNA in saliva [32,33]. To provide additional context on viral dissemination within the vector, we also evaluated population transmission rates based on HTR as a proxy for potential transmission. HTR reflects possible salivary gland invasion but may overestimate actual transmission capacity, since the presence of viral RNA in the head–thorax does not guarantee viral excretion. Conversely, TR offers the most conservative and reliable indicator of true transmission, although it may underestimate potential because forced salivation methods can fail to detect low-level expectoration [31,34,35,36,37,38,39]. Accordingly, HTR and TR should be interpreted as complementary rather than contradictory indicators of vector competence [36].
Our results demonstrated that GV-infected mosquitoes had both high HTR (71.7%) and substantial TR (36.7%), indicating efficient dissemination and partial but effective escape into saliva. For GIII, HTR was 39.3%, but TR was only 6.6%, suggesting a pronounced bottleneck at the level of viral release into saliva. For GI, the TR was just 4.0%, confirming negligible transmission potential. These genotype-dependent differences highlight that viral replication and dissemination kinetics within the mosquito strongly influence ultimate transmission potential [18,19].
A notable finding was the poor vector competence of Ae. albopictus for GI. Despite its predominance in current human and animal infections, GI rarely disseminated beyond the midgut in infected mosquitoes, and transmission was undetectable. This suggests that GI exhibited low replication efficiency in Ae. albopictus and instead relied primarily on other vectors, such as Cx. tritaeniorhynchus, for efficient transmission. Alternatively, genotype-specific immune responses, such as enhanced activation of RNA interference or other antiviral pathways in Ae. albopictus, may suppress GI replication. These findings highlight the complexity of genotype–vector compatibility, which is shaped by the interplay between viral genetics and vector physiology. This interplay is also strongly influenced by ecological and climatic factors [27]. Consistent with this observation, the number of GI-positive mosquitoes was markedly lower at 14 dpi (n = 7), despite a total of 106 individuals being engorged with GI-infected blood meals across three independent replicates. In contrast, 38 and 29 mosquitoes remained positive for GIII and GV, respectively, at 7 dpi. This uneven sample size reflects the inherently reduced replication and persistence of GI in Ae. albopictus rather than insufficient experimental design, further supporting genotype-specific differences in viral replication dynamics.
Temporal infection dynamics further emphasized the differences among the genotypes. GV demonstrated rapid progression from infection to head–thorax positivity (TR) and saliva positivity between 7 and 14 dpi, consistent with high replication efficiency and systemic dissemination. GIII exhibited early dissemination by 7 dpi but showed limited progression thereafter, while GI remained largely restricted to the midgut throughout. These trends were corroborated by our temporal analysis (Section 3.2), which showed a marked increase in viral RNA in the head–thorax and saliva of GV-infected mosquitoes at 14 dpi, indicating effective escape from tissue barriers. In contrast, GIII showed minimal change after initial dissemination, and GI failed to progress meaningfully over time. Together, these findings demonstrate that intra-vector replication kinetics are a critical determinant of genotype-specific transmission potential [2,19]. Such genotype dynamics are also shaped by repeated introductions across regions, as shown by molecular studies indicating frequent incursions of JEV from Southeast and East Asia into Japan [37]. Consistent with saliva assay results, only 4.0% of GI-infected mosquitoes were saliva-positive, further confirming the negligible transmission potential of this genotype in Ae. albopictus.
Taken together, our findings indicate that Ae. albopictus exhibits only low to moderate transmission competence for JEV when evaluated using saliva positivity as the benchmark. This conservative interpretation aligns with entomological surveillance in the ROK where JEV detections in Ae. albopictus have been rare (two positive pools out of >40,000 tested) [16,22]. Although experimental infections have demonstrated that Ae. albopictus is a competent laboratory vector for more than 20 arboviruses [37,38], field evidence supports its major role only for chikungunya virus and, to a lesser extent, dengue and Zika viruses. For JEV, while repeated isolations from Ae. albopictus and other mosquitoes have been reported in Asia [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39], these should be interpreted with caution. Virus isolation alone is insufficient to incriminate a species as a natural vector; this requires supporting evidence of host feeding patterns, natural infection dynamics, and efficient transmission to vertebrate hosts [38]. Thus, while Culex species remain the principal vectors of JEV, the supplementary role of Ae. albopictus—particularly for GV—in urban and peri-urban environments cannot be dismissed [18,37,39,40].
This study has several limitations. First, all experiments were conducted under controlled laboratory conditions using a laboratory-reared mosquito colony; therefore, environmental temperature fluctuations, host availability, microbiota composition, and vector age—factors known to influence vector competence—were not captured. Future investigations should validate these findings under semi-field or field conditions and elucidate the molecular mechanisms that underlie genotype-specific susceptibility in Ae. albopictus.
Second, although inoculated viral titers differed slightly among JEV genotypes, all infectious blood meals were maintained within the biologically relevant range of 106–107 PFU/mL commonly used in vector-competence studies; thus, minor variation within this window is unlikely to have substantially influenced infection outcomes [34]. Third, although saliva positivity (TR) is the most direct measure of transmission, it may underestimate potential due to technical limitations of forced salivation, whereas head–thorax (HTR) may overestimate it; thus, both indicators should be interpreted as complementary rather than contradictory [31,33,34]. Finally, to further assess external transmissibility, future studies will include mammalian infection/challenge experiments to validate transmission potential in vertebrate hosts.
In conclusion, our results demonstrate that Ae. albopictus is a competent but limited vector for JEV GV and GIII, while showing negligible competence for GI, under laboratory conditions. Importantly, this represents the first evidence of GV transmission by Ae. albopictus, underscoring that this globally distributed and ecologically adaptable mosquito could act as a supplementary vector under certain ecological contexts. These findings highlight the need for continued monitoring of JEV genotype–vector interactions, while carefully distinguishing laboratory competence from field incrimination, to better assess their potential impact on future transmission dynamics in the context of ecological change and climate-driven expansion [18,39].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v17101323/s1, Figure S1: tandard curve for the JEV NS5 RT-qPCR generated from a quantified JEV RNA control.
Author Contributions
Conceptualization, H.I.L. and B.-R.Y.; methodology, J.-Y.K.; validation, J.-Y.K.; formal analysis, J.-Y.K.; investigation, J.-Y.K.; resources, B.-R.Y. and J.-Y.K.; data curation, J.-Y.K.; writing—original draft preparation, B.-R.Y. and J.-Y.K.; writing—review and editing, B.-R.Y. and J.-Y.K.; visualization, J.-Y.K.; supervision, D.K. and H.I.L.; project administration, D.K. and H.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data are in the manuscript.
Acknowledgments
The authors thank Hyun Hee Jung for rearing and providing Ae. albopictus mosquitoes used in this study, and Ga Eun Kim for her assistance with the TCID50 assays.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Ae. | Aedes (mosquito genus) |
| BHK | Baby hamster kidney-21 cells |
| Ct | Cycle threshold (qRT-PCR) |
| CPE | Cytopathic effect |
| DR | Dissemination rate |
| dpi | Days post-infection |
| GI | Genotype I Japanese encephalitis virus |
| GII | Genotype II Japanese encephalitis virus |
| GIII | Genotype III Japanese encephalitis virus |
| GIV | Genotype IV Japanese encephalitis virus |
| GV | Genotype V Japanese encephalitis virus |
| IR | Infection rate |
| JEV | Japanese encephalitis virus |
| KDCA | Korea Disease Control and Prevention Agency |
| KCDC | Korea Centers for Disease Control and Prevention |
| NS5 | NON-STRUCTURAL PROTEIN 5 of flaviviruses |
| P/S | Penicillin–streptomycin |
| PTR | Population transmission rate |
| ROK | Republic of Korea |
| TCID50 | 50% Tissue-culture infectious dose |
| TR | Transmission rate |
References
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774. [Google Scholar] [CrossRef]
- Ricklin, M.E.; Garcìa-Nicolàs, O.; Brechbühl, D.; Python, S.; Zumkehr, B.; Posthaus, H.; Oevermann, A.; Summerfield, A. Japanese encephalitis virus tropism in experimentally infected pigs. Vet. Res. 2016, 47, 34. [Google Scholar] [CrossRef]
- Pham, T.T.; Meng, S.; Sun, Y.; Lv, W.; Bahl, J. Inference of Japanese encephalitis virus ecological and evolutionary dynamics from passive and active virus surveillance. Virus Evol. 2016, 2, vew009. [Google Scholar] [CrossRef] [PubMed]
- Furlong, M.; Adamu, A.M.; Hoskins, A.; Russell, T.L.; Gummow, B.; Golchin, M.; Hickson, R.I.; Horwood, P.F. Japanese encephalitis enzootic and epidemic risks across Australia. Viruses 2023, 15, 450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, Y.; Fu, S.; Liu, M.; Li, F.; Liu, C.; Yu, J.; Rui, L.; Wang, D.; Wang, H. Environmental factors and spatiotemporal distribution of Japanese encephalitis after vaccination campaign in Guizhou Province, China (2004–2016). BMC Infect. Dis. 2021, 21, 1172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cao, M.; Feng, H.H.; Fan, H.; Chen, F.; Feng, Z.; Zhou, X.H. Japanese encephalitis risk and contextual risk factors in southwest China: A Bayesian hierarchical spatial and spatiotemporal analysis. Int. J. Environ. Res. Public Health 2014, 11, 4201–4217. [Google Scholar] [CrossRef]
- Solomon, T.; Dung, N.M.; Kneen, R.; Gainsborough, M.; Vaughn, D.W.; Khanh, V.T. Japanese encephalitis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 405–415. [Google Scholar] [CrossRef]
- Erlanger, T.E.; Weiss, S.; Keiser, J.; Utzinger, J.; Wiedenmayer, K. Past, present, and future of Japanese encephalitis. Emerg. Infect. Dis. 2009, 15, 1. [Google Scholar] [CrossRef]
- Schuh, A.J.; Li, L.; Tesh, R.B.; Innis, B.L.; Barrett, A.D. Genetic characterization of early isolates of Japanese encephalitis virus: Genotype II has been circulating since at least 1951. J. Gen. Virol. 2010, 91, 95–102. [Google Scholar] [CrossRef]
- Li, M.H.; Fu, S.H.; Chen, W.X.; Wang, H.Y.; Guo, Y.H.; Liu, Q.Y.; Liang, G.D. Genotype V Japanese encephalitis virus is emerging. PLoS Negl. Trop. Dis. 2011, 5, e1231. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Wang, H.; Fu, S.; Guo, Z.; Liang, G. Southernmost Asia is the source of Japanese encephalitis virus (genotype 1) diversity from which the viruses disperse and evolve throughout Asia. PLoS Negl. Trop. Dis. 2013, 7, e2459. [Google Scholar] [CrossRef]
- Schuh, A.J.; Ward, M.J.; Leigh Brown, A.J.; Barrett, A.D. Dynamics of the emergence and establishment of a newly dominant genotype of Japanese encephalitis virus throughout Asia. J. Virol. 2014, 88, 4522–4532. [Google Scholar] [CrossRef]
- Pan, X.L.; Liu, H.; Wang, H.Y.; Fu, S.H.; Liu, H.Z.; Zhang, H.L.; Liang, G.D. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J. Virol. 2011, 85, 9847–9853. [Google Scholar] [CrossRef]
- Takhampunya, R.; Kim, H.-C.; Tippayachai, B.; Kengluecha, A.; Klein, T.A.; Lee, W.-J.; Grieco, J.; Evans, B.P. Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virol. J. 2011, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yin, Q.; Wang, H.; Liang, G. The reemerging and outbreak of genotypes 4 and 5 of Japanese encephalitis virus. Front. Cell. Infect. Microbiol. 2023, 13, 1292693. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.G.; Lee, H.S.; Yang, S.C.; Noh, B.E.; Kim, T.K.; Lee, W.G.; Lee, H.I. National monitoring of mosquito populations and molecular analysis of flavivirus in the Republic of Korea in 2020. Microorganisms 2021, 9, 2085. [Google Scholar] [CrossRef]
- Sikazwe, C.; Neave, M.J.; Michie, A.; Mileto, P.; Wang, J.; Cooper, N.; Williams, D.T. Molecular detection and characterisation of the first Japanese encephalitis virus belonging to genotype IV acquired in Australia. PLoS Negl. Trop. Dis. 2022, 16, e0010754. [Google Scholar] [CrossRef] [PubMed]
- de Wispelaere, M.; Desprès, P.; Choumet, V. European Aedes albopictus and Culex pipiens are competent vectors for Japanese encephalitis virus. PLoS Negl. Trop. Dis. 2017, 11, e0005294. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Folly, A.J.; Sewgobind, S.; Lean, F.Z.X.; Ackroyd, S.; Nuñez, A.; Delacour, S.; Drago, A.; Visentin, P.; Mansfield, K.L.; et al. Susceptibility of Aedes albopictus and Culex quinquefasciatus to Japanese encephalitis virus. Parasites Vectors 2022, 15, 210. [Google Scholar] [CrossRef]
- Hardy, J.L.; Houk, E.J.; Kramer, L.D.; Reeves, W.C. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu. Rev. Entomol. 1983, 28, 229–262. [Google Scholar] [CrossRef]
- Shin, J.; Jung, J. Comparative population genetics of the invasive mosquito Aedes albopictus and the native mosquito Aedes flavopictus in the Korean peninsula. Parasites Vectors 2021, 14, 377. [Google Scholar] [CrossRef]
- Yun, B.R.; Kwon, J.Y.; Noh, B.E.; Cho, S.; Kwak, D.; Lee, H.I. Genetic shifts of Japanese encephalitis virus (JEV) in mosquitoes in the Republic of Korea, 2017–2022. PLoS Negl. Trop. Dis. 2025, 19, e0013258. [Google Scholar] [CrossRef] [PubMed]
- Lühken, R.; Rauhöft, L.; Pluskota, B.; Lange, U.; Helms, M.; Becker, N.; Heitmann, A. High vector competence for chikungunya virus but heavily reduced locomotor activity of Aedes albopictus from Germany at low temperatures. Parasites Vectors 2024, 17, 502. [Google Scholar] [CrossRef] [PubMed]
- Vythilingam, I.; Tan, C.H.; Chong, C.Y.; Chee, H.Y.; Ng, L.C. Isolation of Japanese encephalitis virus from mosquitoes collected in Sabak Bernam, Selangor, Malaysia in 1992. J. Am. Mosq. Control Assoc. 1995, 11, 94–98. [Google Scholar]
- Weng, M.H.; Lei, H.Y.; Lin, Y.S.; Liu, H.S.; Chen, C.H.; Yeh, T.M. Isolation of Japanese encephalitis virus from mosquitoes collected in Northern Taiwan between 1995 and 1996. J. Microbiol. Immunol. Infect. 1999, 32, 9–13. [Google Scholar]
- Vega-Rúa, A.; Lourenco-de-Oliveira, R.; Mousson, L.; Vazeille, M.; Fuchs, S.; Yébakima, A.; Failloux, A.B. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl. Trop. Dis. 2015, 9, e0003780. [Google Scholar] [CrossRef]
- Le Flohic, G.; Porphyre, V.; Barbazan, P.; Gonzalez, J.P. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Negl. Trop. Dis. 2013, 7, e2208. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.; Beebe, N.W.; Whelan, P.I.; Ritchie, S.A. Aedes albopictus (Diptera: Culicidae) as a potential vector of endemic and exotic arboviruses in Australia. J. Med. Entomol. 2014, 51, 661–668. [Google Scholar] [CrossRef]
- Yun, S.M.; Cho, J.E.; Ju, Y.R.; Kim, S.Y.; Ryou, J.; Han, M.G.; Choi, W.Y.; Jeong, Y.E. Molecular epidemiology of Japanese encephalitis virus circulating in South Korea, 1983–2005. Virol. J. 2010, 7, 127. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, Y.; Zhang, Y.; Zhou, L.; Ma, X.; Xiao, C.; Ma, Z. Shift in dominant genotypes of Japanese encephalitis virus and its impact on current vaccination strategies. Front. Microbiol. 2023, 14, 1302101. [Google Scholar] [CrossRef]
- Honório, N.A.; Câmara, D.C.P.; Wiggins, K.; Eastmond, B.; Alto, B.W. High-Throughput method for detection of Arbo-virus infection of saliva in mosquitoes Aedes aegypti and Ae. albopictus. Viruses 2020, 12, 1343. [Google Scholar] [CrossRef]
- Kim, J.D.; Lee, A.R.; Moon, D.H.; Chung, Y.U.; Hong, S.Y.; Cho, H.J.; Seo, S.U. Efficacy of genotype-matched vaccine against re-emerging genotype V Japanese encephalitis virus. Emerg. Microbes Infect. 2024, 13, 2343910. [Google Scholar] [CrossRef]
- Gloria-Soria, A.; Brackney, D.E.; Armstrong, P.M. Saliva Collection via Capillary Method May Underestimate Arboviral Transmission by Mosquitoes. Parasit. Vectors 2022, 15, 103. [Google Scholar] [CrossRef]
- Eynde, C.V.D.; Sohier, C.; Matthijs, S.; De Regge, N. Japanese Encephalitis Virus Interaction with Mosquitoes: A Review of Vector Competence, Vector Capacity and Mosquito Immunity. Pathogens 2022, 11, 317. [Google Scholar] [CrossRef]
- Karna, A.K.; Bowen, R.A. Experimental evaluation of the role of ecologically-relevant hosts and vectors in Japanese encephalitis virus genotype displacement. Viruses 2019, 11, 32. [Google Scholar] [CrossRef]
- Faizah, A.N.; Kobayashi, D.; Azerigyik, F.A.; Itokawa, K.; Matsumura, R.; Kai, I.; Isawa, H. Vector competence of two globally distributed mosquito species originated from Japan in transmitting Japanese encephalitis virus—Analyses according to their respective insect-specific virus status. Microbe 2024, 2, 100037. [Google Scholar] [CrossRef]
- Gratz, N.G. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004, 18, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rúa, A.; Marconcini, M.; Madec, Y.; Manni, M.; Carraretto, D.; Gomulski, L.M.; Gasperi, G.; Failloux, A.-B.; Malacrida, A.R. Vector competence of Aedes albopictus populations for chikungunya virus is shaped by their demographic history. Commun. Biol. 2020, 3, 326. [Google Scholar] [CrossRef]
- Auerswald, H.; Maquart, P.O.; Chevalier, V.; Boyer, S. Mosquito vector competence for Japanese encephalitis virus. Viruses 2021, 13, 1154. [Google Scholar] [CrossRef]
- Krambrich, J.; Akaberi, D.; Lindahl, J.F.; Lundkvist, Å.; Hesson, J.C. Vector competence of Swedish Culex pipiens mosquitoes for Japanese encephalitis virus. Parasites Vectors 2024, 17, 220. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).