Abstract
Coronavirus disease (COVID-19) and its outcomes remain one of the most challenging problems today. COVID-19 in children could be asymptomatic, but can result in a fatal outcome; therefore, predictions of the disease severity are important. The goal was to investigate the human genetic factors that could be associated with COVID-19 severity in children. Single-nucleotide polymorphisms of the following genes were studied: ACE2 (rs2074192), IFNAR2 (rs2236757), TYK2 (rs2304256), OAS1 (rs10774671), OAS3 (rs10735079), CD40 (rs4813003), FCGR2A (rs1801274) and CASP3 (rs113420705). In the case–control study were 30 children with mild or moderate course of the disease; 30 with severe COVID-19 symptoms and multisystem inflammatory syndrome in children (MIS-C) and 15 who were healthy, and who did not have SARS-CoV-2 (PCR negative, Ig G negative). The study revealed that ACE2 rs2074192 (allele T), IFNAR2 rs2236757 (allele A), OAS1 rs10774671 (allele A), CD40 rs4813003 (allele C), CASP3 rs113420705 (allele C) and male sex contribute to severe COVID-19 course and MIS-C in 85.6% of cases. The World Health Organization reported that new SARS-CoV-2 variants may cause previously unseen symptoms in children. Although the study has limitations due to cohort size, the findings can help provide a better understanding of SARS-CoV-2 infection and proactive pediatric patient management.
1. Introduction
Coronavirus disease (COVID-19) and its outcomes remain amongst the most pressing problems today. In October 2023, the total count of confirmed COVID-19 cases reached 771 million worldwide. Notwithstanding vaccination, the mortality rate is still high. As of the end of September 2023, 13.5 billion vaccinations had been given against SARS-CoV-2; 66.12% of adults had completed the primary series of vaccinations; 31.74% of the world population received boosters or additional dosages, but from January till October 2023 more than 237 thousand deaths related to COVID-19 were registered [1]. Despite the assumption that COVID-19 in children and adolescents has an asymptomatic course, fatal outcomes are also noticed in the pediatric population. Based on UNICEF Data, during the COVID-19 pandemic (till October 2023) there were 17,490 COVID-19 deaths among children and adolescents—58.75% were registered in the age group 10–19 and 41.25% in children aged 0–9 years [2]. COVID-19 in childhood can also lead to multisystem inflammatory syndrome in children (MIS-C). Data from the USA reported an MIS-C frequency of 316 cases per million confirmed SARS-CoV-2 episodes in persons aged <21 [3]. Recent studies underline that critical illness or even death among children with COVID-19 is most commonly seen in patients with comorbidities (respiratory, cardiovascular disorders, obesity, neurological or oncological disease) and the presence of co-infections [3,4,5]. In addition, age is an important risk factor for disease severity—premature babies, infants and adolescents are at greater risk for poor COVID-19 outcomes [3,5,6].
The course of any viral infection as well as COVID-19 will depend on the characteristics of the pathogen (its structure, viral load and the site of penetration into the host’s cells), the type of host immune response and transmission environment, along with its influencing factors [6,7]. It must be mentioned that external factors (stress, sleep, nutrition and microbiota type) can undergo corrections, but the host genetic factor cannot be corrected or changed, and has a very significant impact on the course of the viral infection [6].
Therefore, the research goal was to investigate human genetic factors that could be associated with COVID-19 severity in the pediatric population. Single-nucleotide polymorphisms (SNPs) of the following genes were studied: ACE2 (rs2074192); IFNAR2 (rs2236757); TYK2 (rs2304256); OAS1 (rs10774671); OAS3 (rs10735079); CD40 (rs4813003); FCGR2A (rs1801274) and CASP3 (rs113420705).
The angiotensin converting enzyme 2 (ACE2) gene has several single-nucleotide polymorphisms that are associated with communicable and noncommunicable diseases. ACE2 alleles are risk factors for cardiovascular diseases (hypertension, coronary artery disease, heart failure, atherosclerosis), respiratory diseases (pulmonary hypertension, chronic obstructive pulmonary disease, asthma, acute lung injury, acute respiratory distress syndrome, lung cancer, pulmonary sarcoidosis) and endocrine diseases (type 2 diabetes mellitus, obesity) [8,9,10]. Since 2019, SARS-CoV-2 infection has become the most commonly discussed communicable disease related to ACE2 gene expression. Recent studies show that intronic SNP rs2074192 (variant g42403) is related to COVID-19 severity as well as concomitant comorbidities [11].
The interferon system is extremely powerful in the context of antiviral defense. The action of the type I interferon is mediated by the interaction with interferon alpha and beta receptor subunit 1 (IFNAR1) and interferon alpha and beta receptor subunit 2 (IFNAR2) receptors. The expression of IFNAR2 is regulated by the corresponding genes. Recent studies suggest the influence of IFNAR2 gene polymorphism on the course and severity of SARS-CoV-2 infection, especially its risk allele A [12,13].
The interferon system is closely related to tyrosine kinase 2 (TYK2). TYK2, as part of the Jak-family, is discussed as one of the key pathogenetic substances in immune-mediated inflammatory disease development [14]. Current knowledge suggests that changes in TYK2 regulation lead to deviations in interferons α and β response. The downregulation of TYK2 with other Jak-family members (Jak1, Jak2, Jak3) is linked to cytokines action—IL-6, IL-10, IL-11, IL-12, IL-19, IL-20, IL-22 and IL-23 [14,15]. The consequences of the TYK2 gene’s (rs2304256) influence on COVID-19 are still controversial. Previous research has shown discordant results. Dieter C. et al. and Benmansour R. with their colleagues demonstrated a tendency in the association of the AA genotype TYK2 gene (rs2304256) with severe and lethal outcomes in adult patients with COVID-19 [16,17]. At the same time, Risi et al. reported an association of the A allele carrier with mild disease course, and underlined its protective properties [18]. Therefore, the influence of genotypes and alleles of the TYK2 gene (rs2304256) remains a point of concern.
Among interferon-induced enzymes, 2′,5′-oligoadenylate synthetase (OAS) must be mentioned, as it has a great role in antiviral immunity. Much research suggests that OAS1 rs10774671 with the risk allele A plays a dominant role in the regulation of OAS enzymatic activity and SARS-CoV-2 elimination [19]. Single nucleotide variant rs10735079 (A > G) is responsible for gene clusters OAS1, OAS2 and OAS3, and is among the COVID-19 genes [12,20]. Studies in the adult population suggest the association of rs10735079 (risk allele G) with the severity of SARS-CoV-2 infection (odds ratio (OR) = 1.3) [12,21].
MIS-C and Kawasaki disease (KD) show similarities in their pathogenesis (autoimmune pattern), clinical course (fever, rash, no purulent conjunctivitis, cardiovascular involvement) and laboratory findings (elevated pro-inflammatory markers and evidence of coagulopathy) [22]. Therefore, in differential diagnosis, we assessed genome-wide significant variants associated with KD susceptibility [22]. Current studies suggest 63 genes associated with KD [23]. Among them, four groups of genes that are related to KD susceptibility were formed: (1) genes that enhance T cell activation (ITPKC, ORAI1, STIM1); (2) genes related to the dysregulation of B cell signaling (CD40, BLK, FCGR2A); (3) genes associated with decreased apoptosis (CASP3) and (4) genes related to altered transforming growth factor beta signaling (TGFB2, TGFBR2, MMP, SMAD) [23,24]. All listed genes have different risk allele frequencies in the European and Asian populations; therefore, we have focused on the genes that have a higher frequency in Europe—CD40 (CD40 molecule), Fc gamma receptor IIa (FCGR2A) and caspase 3 (CASP3) [22]. In addition, these genes are among the most important regulators of antiviral immune response [25]. CD40 as a costimulatory surface receptor is expressed on B-cells, macrophages, monocytes, platelets, dendritic cells and non-hematopoietic cells—vascular endothelial cells, epithelial cells, myofibroblasts and fibroblasts [26,27,28]. Therefore, CD40 contributes to cellular and humoral immunity as well as to vascular remodeling [26,27,29]. FCGR2A (Fc fragment of immunoglobulin G, low-affinity IIa, receptor) is also discussed in the context of immune response and vascular remodeling [30]. Polymorphism FCGR2A rs1801274 is associated with the substitution of histidine by arginine at position 131 (H131R) [30]. As a result, binding affinity with different immunoglobulins G subclasses is changed and the autoimmune response is activated [30]. Caspase-3 is a well-known protein involved in apoptosis and it is encoded by the CASP3 gene. The activation of caspase-3 in COVID-19 patients can be caused by the enhanced production of reactive oxygen species in the presence of oxidative stress. At the same time, the cytokine storm in SARS-CoV-2 infection is described as the result of apoptosis-related cellular death [31].
Therefore, CD40, FCGR2A and CASP3 genes are discussed as the genetic predisposition factors of KD, as well as severe COVID-19 and MIS-C, in children.
Despite the number of genetic studies related to COVID-19 severity and outcome, the results are controversial, with different findings and associations presented. However, no previous analyses regarding the roles of genetic factors in COVID-19 severity in children have been reported. Therefore, it is the focus of our research.
2. Materials and Methods
A total number of 75 children were involved in the case–control study—30 persons with mild or moderate course of the disease; 30 children with severe COVID-19 and multisystem inflammatory syndrome and 15 healthy children who did not have COVID-19 (PCR-negative, Ig G-negative).
Criteria of the Italian Society of Pediatric Infectious Disease [32,33] and COVID-19 Treatment Guidelines (National Institutes of Health) [34] were used to define disease severity. Mild disease severity was diagnosed in cases when upper airway symptoms without radiological/ultrasound findings were present despite the temperature level. Children with pneumonia diagnosed by imaging studies or persons with upper airway symptoms with respiratory distress were defined as patients with moderate disease severity. When patients had a fever or cough with saturation <92% in room air, severe respiratory distress or systemic symptoms (drowsiness, lethargy, seizures, dehydration), severe disease course was diagnosed.
Diagnosis of MIS-C was done based on the World Health Organization (WHO) criteria [35]—fever duration greater than three days in children and adolescents (0–19 years), plus two of the following: (1) rash or bilateral nonpurulent conjunctivitis or mucocutaneous inflammation signs (oral, hands or feet); (2) hypotension or shock; (3) features of myocardial dysfunction, pericarditis, valvulitis or coronary abnormalities (including ECHO findings or elevated Troponin/N-Terminal Pro–B-Type Natriuretic Peptide [NT-proBNP]); (4) evidence of coagulopathy (by prothrombin time, partial thromboplastin time, elevated d-Dimers) or (5) acute gastrointestinal problems (diarrhea, vomiting or abdominal pain). Other evaluations used in MIS-C diagnosis included elevated markers of inflammation such as erythrocyte sedimentation rate, C-reactive protein or procalcitonin, and no other obvious microbial cause of inflammation, including bacterial sepsis, staphylococcal or streptococcal shock syndromes, as well as evidence of COVID-19 (real-time reverse transcription polymerase chain reaction, antigen test or serology positive) or likely contact with patients with COVID-19.
Children enrolled in the study were examined in Ternopil, Ukraine (Ternopil Municipal Children’s Hospital and Ternopil Regional Children’s Clinical Hospital).
The study was conducted and performed under the principles of the Declaration of Helsinki. The Bioethics Committee of I. Horbachevsky Ternopil National Medical University approved this study (Protocol No 71, dated 25 October 2022). Informed consent was obtained from all the children’s caregivers.
Venous blood samples for the genomic study were collected in tubes with ethylenediamine tetra acetic acid. The blood sample volumes used for clinical care and research did not exceed the recommended limit set by the WHO [36]. The Thermo Scientific™ GeneJET™ Whole Blood Genomic DNA Purification Mini Kit Cat. No K0781 (Thermo Fisher Scientific, Waltham, MA, USA, 02451) was used for genomic DNA extraction according to the manufacturer’s instructions. Predesigned TaqMan™ SNP Genotyping Assays, Cat. No. 4351379 (Thermo Fisher Scientific, Waltham, MA, USA, 02451), were used for the following SNPs—ACE2 rs2074192, IFNAR2 rs2236757, TYK2 rs2304256, OAS1 rs10774671, OAS3 rs10735079, CD40 rs4813003, FCGR2A rs1801274 and CASP3 rs113420705. A TaqMan™ Universal Master Mix II, no UNG, 1 × 5 mL, Cat. No 4440040 was used for DNA amplification using real-time polymerase chain reaction.
Statistical analysis was performed with the computer software IBM SPSS Statistics 21.0. Quantitative values are presented as number (n) and frequency (%). Frequency tables 2 × 3 were compared using the chi-square test (χ2), while for tables 2 × 2, the two-tailed Fisher exact test was used; the level of significance for each test was calculated as the p-value and pF, respectively. Correspondence for Hardy–Weinberg equilibrium was assessed for each gene. With the assumption that gene frequency corresponds to the population, a measurement was taken when p > 0.05 in the chi-square test. For outcome prediction, OR with its 95% confidence interval (95% CI) was calculated. Logistic regression was performed to determine the key predictors of COVID-19 and its severity in the pediatric population. A level of statistical significance was assumed with a p-value < 0.05. GeneMANIA network data were used to assess the network cooperation between studied genes [37]. The statistical tool “G*Power 3.1.9.7” was used for sample size calculations and post-hoc power analysis. For determining the total sample size, we used goodness-of-fit tests for contingency tables (χ2 tests). We assumed an effect size at the level of 0.5 (medium effect according to Cohen’s criteria). Statistical power was fixed at 0.8. The degree of freedom was chosen based on two different planned study approaches: (1) three groups (two COVID-19 groups and one control group)—Df = 2; (2) three groups (two COVID-19 groups and one control group) with different 3 genotypes—Df = 4. Based on the obtained results the required total sample size was fixed as 75 persons. The post-hoc power analysis revealed statistical power, which exceeded 0.8 for genes ACE2, IFNAR2, OAS1, OAS3, CD40, and CASP3, and confirmed that the precise number of participants used was the sufficient sample size. The input parameters for the power calculations were total sample size (75 persons), an α error of probability of 0.05, and an effect size index w that was calculated based on the obtained results (w ACE = 0.45; w IFNAR2 = 0.40; w TYK2 = 0.26; w OAS1 = 0.81; w OAS3 = 0.36; w CD40 = 0.35; w FCGR2A = 0.30; w CASP3 = 0.45). The study results demonstrate the statistical powers for eight selected genes—(1) ACE2 rs2074192 power = 0.97; (2) IFNAR2 rs2236757 power = 0.94; (3) TYK2 rs2304256 power = 0.62; (4) OAS1 rs10774671 power = 0.99; (5) OAS3 rs10735079 power = 0.36; (6) CD40 rs4813003 power = 0.86; (7) FCGR2A rs1801274 power = 0.75; and (8) CASP3 rs113420705 power = 0.97.
3. Results
3.1. Study Group’s Characteristic
The demographic characteristics of children involved in the study are presented in Table 1. There was no sex difference in the studied group, while an age difference was revealed. Children with mild or moderate COVID-19 were significantly younger compared to the control group. Age among COVID-19 patients did not vary (Table 1).

Table 1.
Demographic characteristics of children with COVID-19 (n = 60) and healthy children (n = 15).
3.2. Correspondence to Hardy–Weinberg Equilibrium
The frequency of ACE2 rs2074192, IFNAR2 rs2236757, OAS3 rs10735079, FCGR2A rs1801274 and CASP3 rs113420705 genotypes did not follow the Hardy–Weinberg equilibrium (p < 0.05) due to the directional selection made in our study (focusing on patients with COVID-19). In the COVID-19 group, the TYK2 rs2304256, OAS1 rs10774671, CD40 rs4813003 and FCGR2A rs1801274 homozygote and heterozygote frequencies were in accordance with those indicated by the Hardy–Weinberg principle (p > 0.05). Notably, genotype frequencies in healthy children (control group) correspond to the Hardy–Weinberg proportions (p > 0.05) (Supplementary Materials: Tables S1–S3).
3.3. Genotype and Allele Frequencies
3.3.1. ACE2 rs2074192
The genotype frequencies did not vary in the studied group in the codominant model (Figure 1). Analyses of dominant, recessive and overdominant models demonstrate that children with severe COVID-19 or MIS-C are more often the carriers of the TT genotype ACE2 rs2074192. Genotype TT rs2074192 increases the risk of having a severe course of SARS-CoV-2 infection by 4.57 times (OR = 4.57; 95% CI 1.07–19.57; p = 0.041) (Table 2).
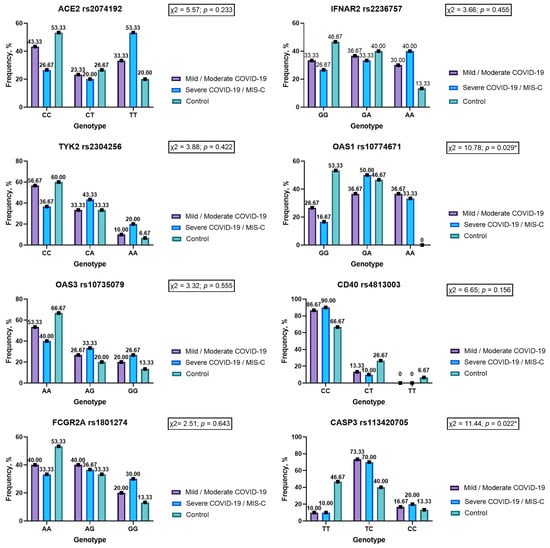
Figure 1.
Genotype frequencies in the codominant model for genes ACE2 rs2074192, IFNAR2 rs2236757, TYK2 rs2304256, OAS1 rs10774671, OAS3 rs10735079, CD40 rs4813003, FCGR2A rs1801274 and CASP3 rs113420705 in children with COVID-19 and noninfected children (ACE 2—angiotensin converting enzyme 2; IFNAR2—interferon alpha and beta receptor subunit 2; TYK2—tyrosine kinase 2; OAS1—2′-5′-oligoadenylate synthetase 1; OAS3—2′-5′-oligoadenylate synthetase 3; CD40—CD40 molecule; FCGR2A—Fc gamma receptor IIa; CASP3—caspase 3; *—statistically significant result.).

Table 2.
Dominant, recessive and overdominant models for ACE2 rs2074192.
Children with severe COVID-19 course and MIS-C are significantly more often allele T carriers ACE2 rs2074192 compared to healthy persons (p = 0.017). Allele T increases the risk of severe disease course by 3.45 times (p = 0.009), while allele C has protective properties (OR = 0.29; 0.12–0.73) (Table 3).

Table 3.
Allele frequencies for genes ACE2 rs2074192, IFNAR2 rs2236757, TYK2 rs2304256, OAS1 rs10774671, OAS3 rs10735079, CD40 rs4813003, FCGR2A rs1801274 and CASP3 rs113420705 in children with different degrees of COVID-19 severity and noninfected children.
The comparison of our results with data from the project “ALFA: Allele Frequency Aggregator” [38] shows that the frequency of alleles C and T in healthy children corresponds to the average frequency in the European population (p = 0.061), while patients with COVID-19 are more often carriers of risk allele T compared to the general population (0.54 versus 0.45; p = 0.048) (Table 4).

Table 4.
Comparison of allele frequencies in children infected by SARS-CoV-2 compared to the general European population.
Notably, the genotype frequency varied significantly between girls and boys in the COVID-19 group—boys showed genotype TT more often compared to girls. In healthy children, this sex difference was not revealed (Table 5). At the same time, a comparison of healthy boys and boys with COVID-19 showed a significant difference in ACE2 rs2074192 genotype frequencies (χ2 = 6.94; p = 0.031); healthy children and those infected with SARS-CoV-2 girls did not differ in terms of genotype frequency (χ2 = 0.68; p = 0.711) (Table 5). Allele T frequency varied between groups—it was found in 60.26% of infected boys compared to 14.29% of noninfected boys (p = 0.002) (Table 5).

Table 5.
Sex differences in genotype frequencies among those infected with SARS-CoV-2 and noninfected children.
3.3.2. IFNAR2 rs2236757
Rare homozygous AA was seen in 40% of patients with severe COVID-19 and MIS-C, while in the control group it was observed in 13.33% of cases. Despite the higher frequency, this difference did not reach the level of statistical significance (p = 0.071) (Figure 1). In the recessive inheritance model, genotype AA IFNAR2 rs2236757 showed a tendency to increase disease severity more than fourfold (OR = 4.33; 95% CI 0.83–22.75; p = 0.083) (Table 6).

Table 6.
Dominant, recessive and overdominant models for IFNAR2 rs2236757.
IFNAR2 rs2236757 risk A allele was revealed significantly more often among patients with severe COVID-19 and MIS-C compared to the control group—56.67% vs. 33.33% (p = 0.046). Carriers of risk allele A are more prone to suffer from severe COVID-19 or MIS-C (OR = 2.62; p = 0.039) (Table 3).
It is important to emphasize that allele A is registered significantly more often in children with COVID-19 compared to data in the general European population (p < 0.001), while the frequencies of alleles G and A in healthy children correspond to it (p = 0.497) (Table 4).
There were no sex differences in genotype or allele frequencies between the COVID-19 group and control group (p > 0.05). However, comparison in terms of the same sex demonstrates a higher frequency of risk allele A in boys with COVID-19 compared to healthy boys—50% and 21.43% (p = 0.049); in girls, such a difference was not revealed (p = 0.365) (Table 5).
3.3.3. TYK2 rs2304256
There were no differences in TYK2 rs2304256 genotype frequencies according to the codominant, dominant, recessive and overdominant models (p > 0.05) (Figure 1, Table 7).

Table 7.
Dominant, recessive and overdominant models for TYK2 rs2304256.
The frequencies of alleles C and A also did not vary between groups with different COVID-19 severity and healthy children (Table 3). Risk allele A was seen in 23.33% of healthy persons vs. 41.67% in patients with severe COVID-19/MIS-C and 26.67% in the mild/moderate group (p > 0.05). Allele frequencies in the COVID-19 group were similar to those in the general population (Table 4).
The TYK2 rs2304256 genotype and allele frequencies were similar between boys and girls, as well as between infected and noninfected children of the same sex (p > 0.05) (Table 5).
3.3.4. OAS1 rs10774671
The codominant model’s study revealed differences in genotype frequencies for gene OAS1 rs10774671 (p < 0.05) (Figure 1). Genotype AA OAS1 rs10774671 was registered in 35% of patients infected by SARS-CoV-2, while in healthy children it was not noticed (p = 0.022). Our study results demonstrate that homozygous GG is more than five times less likely to have a severe COVID-19 course (OR = 0.18) compared to carriers of allele A (heterozygous GA and homozygous AA)—OR = 5.71 (p < 0.05) (Table 8).

Table 8.
Dominant, recessive and overdominant models for OAS1 (rs10774671).
Patients with COVID-19 were often a carrier of allele A (56.67%) compared to healthy children (23.33%) (p = 0.001) (Table 3). Notably, allele A OAS1 is associated with severe COVID-19 course (OR = 4.60; 95% CI 1.71–12.37; p = 0.003). It should be noted that patients in the control group did not match the European population in terms of OAS1 rs10774671 allele G and A frequencies (Table 4).
Frequencies of genes located on the autosome OAS1 rs10774671 genotype and alleles were similar for both sexes (Table 5).
3.3.5. OAS3 rs10735079
There were no statistical differences between OAS3 rs10735079 genotypes and allele frequencies between children with different degrees of COVID-19 severity and healthy children (Figure 1, Table 3 and Table 9). Nevertheless, it should be noticed that the frequencies of allele G and genotypes with allele G (GG + GA) were two times higher in persons with a severe COVID-19 course compared to the control group—43.33% vs. 23.33% for alleles (p = 0.065) and 60% vs. 33.33% for genotypes (p = 0.095).

Table 9.
Dominant, recessive and overdominant models for OAS3 rs10735079.
3.3.6. CD40 rs4813003
The research did not reveal significant differences in codominant, dominant, recessive and overdominant models between study groups for gene CD40 rs4813003 (Figure 1, Table 10). Importantly, genotype CC was typical for 90% of children with severe COVID-19 and MIS-C, while in a healthy group it was registered in 66.67% of cases (p = 0.056). Correspondingly, allele C significantly dominated in the COVID-19 group compared to noninfected children—94.17% vs. 80% (p = 0.024). Based on our study results, we can infer that allele C increases the risk of severe COVID-19 course or MIS-C 4.75 times (p = 0.037) (Table 3).

Table 10.
Dominant, recessive and overdominant models for CD40 rs4813003.
It is important to note that allele C and T frequencies were significantly different here compared not only to the control but also to the general European population (p = 0.011). In noninfected children, the frequencies of both allele C and T correspond to those seen in the general population (p > 0.05) (Table 4).
The comparison between boys and girls did not show any difference in genotype or allele frequency (p > 0.05) (Table 5).
3.3.7. FCGR2A rs1801274
The analysis of all genetic models of inheritance did not reveal significant changes in FCGR2A rs1801274 genotype frequencies between those infected by the SARS-CoV-2 virus and noninfected persons (Figure 1, Table 11).

Table 11.
Dominant, recessive and overdominant models for FCGR2A rs1801274.
Allele A and G frequencies in patients with COVID-19 did not differ significantly from the control group or from the general population (p > 0.05) (Table 3 and Table 4). At the same time, our study results demonstrate a deviation in allele frequencies between healthy children and the European population (p < 0.05) (Table 4).
Genotype and allele frequencies in the male group correspond to the same parameters in the female group of COVID-19 patients and healthy children (p > 0.05) (Table 5).
3.3.8. CASP3 rs113420705
CASP3 rs113420705 genotype TT was registered significantly more often among healthy children (46.67%) compared to patients with COVID-19 of levels of different severity (10%) (p < 0.05) (Figure 1). Notably, TT genotype rs113420705 is protective against severe COVID-19 or MIS-C in the pediatric population (OR = 0.13; p = 0.010) (Table 12). Carriers of allele C in both heterozygous TC and rare homozygous CC have an increased risk of severe SARS-CoV-2 infection (OR = 7.88) (Table 12). Important to note is that despite the absence of a statistically significant difference between COVID-19 groups and the control, the study noted a significantly higher prevalence of allele C in COVID-19 pediatric patients and European allele frequencies (Table 3 and Table 4).

Table 12.
Dominant, recessive and overdominant models for CASP3 rs113420705.
Sex differences were not typical for CASP3 rs113420705 genotypes and allele frequencies (Table 5).
3.3.9. Allele Associations and Gene Interrelationships in Children with COVID-19
Our research has revealed that children not infected by SARS-CoV-2 are often the carriers of two or three risk alleles (p < 0.001). However, 30% of COVID-19 patients are carriers of seven to eight risk alleles, which is not typical for healthy children (p = 0.034) (Table 13).

Table 13.
Association of risk alleles in children with COVID-19 and healthy control.
The logistic regression model for COVID-19 prediction based on the child’s sex and alleles was statistically significant (χ2 = 45.96; p < 0.001 *) (Table 14). The proposed model explained 41.7% (Nagelkerker R Square) of the variation in COVID-19 susceptibility and correctly predicted 85.3% of cases. Therefore, based on the model, the key components of COVID-19 susceptibility in childhood are alleles of genes IFNAR2 rs2236757, OAS1 rs10774671, OAS3 rs10735079, CD40 rs4813003 and CASP3 rs113420705.

Table 14.
Logistic regression analysis for COVID-19 susceptibility prediction in the pediatric population.
Such indicators as male sex, ACE2 rs2074192 allele T, IFNAR2 rs2236757 allele A, OAS1 rs10774671 allele A, CD40 rs4813003 allele C and CASP3 rs113420705 allele C can predict severe COVID-19 course and MIS-C in the pediatric population in 85.6% of cases (Table 15). The proposed model is significant (χ2 = 56.85; p < 0.001) and demonstrates strong goodness of fit—Nagelkerke R square 0.65.

Table 15.
Logistic regression analysis for severe COVID-19 course and MIS-C prediction in the pediatric population.
Network data from GeneMANIA [37] confirm the interrelationship between the studied genes (Figure 2). It should be emphasized that in immune protection, many genes are involved. With the exception of eight studied genes (ACE2, IFNAR2, TYK2, OAS1, OAS3, CD40, CASP3, FCGR2A), we must also focus on the genes angiotensin I converting enzyme (ACE), angiotensinogen (AGT), collectrin, amino acid transport regulator (CLTRN), ghrelin and obestatin prepropeptide (GHRL), 2′-5′-oligoadenylate synthetase 2 (OAS2), 2′-5′-oligoadenylate synthetase like (OASL), poly(A) polymerase beta (PAPOLB), poly(A) polymerase gamma (PAPOLG), terminal nucleotidyltransferase 4B (TENT4B), TNF receptor associated factor 2 (TRAF2), TNF receptor associated factor 3 (TRAF3), CASP8 and FADD like apoptosis regulator (CFLAR), thioredoxin (TXN), neogenin 1 (NEO1), glycoprotein VI platelet (GP6), NADH: ubiquinone oxidoreductase core subunit S1 (NDUFS1), Janus kinase 1 (JAK1), Janus kinase and microtubule interacting protein 1 (JAKM1P1), receptor for activated C kinase 1 (RACK1) and interleukin 12 receptor subunit beta 1 (IL12RB1) (Figure 2).
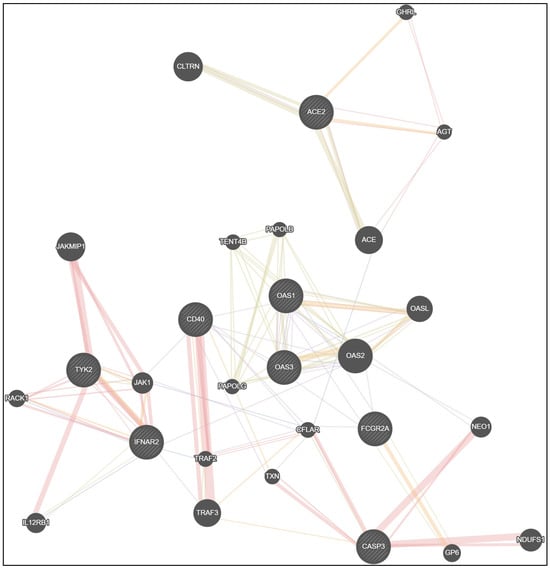
Figure 2.
Network data for gene interactions between studied genes ACE2, IFNAR2, TYK2, OAS1, OAS3, CD40, CASP3, FCGR2A and other genes generated by GeneMANIA.
Physical interactions between genes in the proposed network model were registered in 35.92% of cases, co-expression in 30.46%, shared protein domains in 17.70%, co-localization in 4.55% and predicted functional relationships between genes in 11.37% of cases (Table 16).

Table 16.
Gene network interactions.
4. Discussion
The research conducted suggests the importance of host genetic factors in antiviral immunity against SARS-CoV-2 in children. It is clear that genetic patterns are stable during the whole of life; despite this, the influence of SNPs on COVID-19 severity was studied first. We focused on the key genes, which play the most significant role in the immune response in COVID-19, and we studied genes related to Kawasaki disease.
Our study results confirm certain genes’ interrelationships and how they predict the course of SARS-CoV-2 infection. Regression analysis showed that COVID-19 susceptibility in children increases in cases of interactions of IFNAR2 rs2236757, OAS1 rs10774671, OAS3 rs10735079, CD40 rs4813003 and CASP3 rs113420705. Crucial factors for the development of severe COVID-19 or MIS-C are sex (male gender), ACE2 rs2074192, IFNAR2 rs2236757, OAS1 rs10774671, CD40 rs4813003 and CASP3 rs113420705.
More often, pediatric patients with COVID-19 were found to be the carriers of a combination of seven or eight risk alleles. Noninfected children were more likely to be the carriers of three or fewer risk alleles compared to patients with COVID-19 of different severity.
Importantly, our study has demonstrated the significant prevalence of risk alleles among pediatric COVID-19 groups compared to relevant data for the European population. These frequency differences were revealed for four of the studied genes—ACE2 rs2074192 allele T (54% vs. 45%), IFNAR2 rs2236757 allele A (52% vs. 29%), CD40 rs4813003 allele C (94% vs. 86%) and CASP3 rs113420705 allele C (54% vs. 28%). This finding is of great importance in the confirmation of genetic susceptibility to SARS-CoV-2 infection.
Gene ACE2 encodes cognominal type 1 membrane protein ACE2 [8]. The ACE2 gene is located on the X chromosome (Xp22.2), locus NG_012575 [39,40]. Numerous studies suggest that single-nucleotide polymorphisms (SNPs) in the gene ACE2 are related to the expression of ACE2 and the following binding affinity of SARS-CoV-2 [41,42].
Our research revealed sex differences in risk allele T frequency—boys are more often the carriers of it; therefore, they are more susceptible to SARS-CoV-2 infection. Such sex differences can be explained by the location of ACE2 rs2074192 on the X chromosome. The study suggests that such Xp22 gene location encompasses an area that is not under physiological X-inactivation [8]. X-inactivation normally happens in females to ensure population basis gene distributions. Escaping from X-inactivation leads to different phenotypic patterns and sex tissue-specific differences [8].
Allele T of ACE2 rs2074192 shows a higher frequency in symptomatic COVID-19 patients compared with the asymptomatic group, and it is more often associated with severe outcomes [43]. Previous meta-analyses performed by K. Gupta et al. demonstrated genotype contrasts between the TT and CT genotypes ACE2 rs2074192 in severe COVID-19 prediction in an adult population, but no allele differences [44]. Our research received similar results. However, our study also demonstrated the influence of allele differences and risk allele T on the SARS-CoV-2 infection course in children. In L. E. Martinez-Gomez et al.’s study, codominant, dominant and recessive models did not reveal significant differences between severe and critical COVID-19 patients [45]. Research in Spain demonstrated the protective effect of ACE2 rs2074192 in relation to female hospitalization during COVID-19 [40].
Therefore, data regarding ACE2 rs2074192’s impact on SARS-CoV-2 are still controversial, and future studies must be carried out.
The SARS-CoV-2 virus has a direct stimulatory effect on interferon-stimulated genes (ISG), and also causes the activation of immune cells. ISGs cause an increase in the expression of pro-inflammatory genes. Type I interferon response is among the key pathogenetic bases for antiviral host defense. The course of COVID-19 is characterized by the reduced production of IFN-I in the early stages of the disease. At the same time, studies have emphasized the role of the interferon system in inducing a “cytokine storm” by activating the synthesis of TNF/IL-1β [46]. Hadjadj et al. demonstrated that patients with severe COVID-19 display a downregulation of IFN-stimulated genes (MX1, IFITM1 and IFIT2) compared to patients with mild and moderate disease severity [47,48,49]. Research has suggested that a low type I IFN response manifests a worsening of the clinical condition up to the critical state [17,47]. At the same time, endogenous or exogenous steroids can suppress IFN signaling that manifest impaired antiviral immune responses and increasing disease severity. This is crucial to our understanding of the COVID-19 course, because glucocorticoids are used in the medical management of MIS-C, acute respiratory distress syndrome and sepsis [50]. Therefore, the INF pathway and its genetic control should be assessed in the pediatric population.
Intron variant IFNAR2 rs2236757 is located on chromosome 21q22.1 (chr21:34,624,917) [12]. Fricke-Galindo et al. showed the association of IFNAR2 rs2236757 with disease severity and mortality risk in adults with COVID-19 [13]. The study of genetic mechanisms critical to COVID-19 also underlined that the low expression of IFNAR2 is associated with life-threatening disease, while its high expression reduces the odds of severe COVID-19 [12]. The association of the AA genotype rs2236757 with intensive care admission was shown in adult patients infected by SARS-CoV-2 [16,17].
Despite the fact that mortality rate and intensive care unit admission were not the focus of our research, our findings in a pediatric population are in line with public data regarding disease severity—allele A increases the risk of severe COVID-19 and MIS-C in single-gene studies and in cases of multigene interaction.
Tyrosine kinase 2 (TYK2) is a protein functioning as a Janus kinase/signal transducer and an activator of transcription (Jak-STAT) pathways [14]. TYK2 activation is carried out by IFN-α, which leads to the phosphorylation of STAT1 and STAT2 and the subsequent dimerization of activated STATs. The subsequent translocation of dimerized STATs in the cell nucleus leads to the induction of interferon-stimulated gene expression and the activation of antiviral defense [15,51]. TYK2 also stimulates nontraditional pathways of antiviral protection, such as NF-κB signaling and the mitogen-activated protein kinase pathway [51]. TYK2 regulates the activity and function of T-helpers of type 1 and T-helpers of type 17 through a functional connection with IL-12 and IL-23 [15,52]. The differentiation of CD4+ T cells, activated by the interaction of IL-23 with TYK2/Jak2, occurs with the participation of IL-6, IL-1β and TGF-β, which is extremely important in the context of the cytokine storm in COVID-19 [52].
TYK2 (rs2304256) is a gene that encodes a non-receptor tyrosine-protein kinase; it is a nonsynonymous variant that is located on chromosome 19 in exon 8 (chr19:10,350,533–10,380,608) [53,54]. It is suggested that the TYK2 gene is associated with susceptibility to inflammatory and autoimmune disorders [53]. In the European population, the protective role of minor allele A was demonstrated in systemic lupus erythematosus, type 1 diabetes, psoriasis and idiopathic inflammatory myopathies [53]. Associations with autoimmune diseases can be explained by the rs2304256-related modification of expression of less common disease variants rs34536443 (P1104A) and rs12720356 (I684S) [53].
Our research did not find any differences in genotype or allele frequencies in TYK2 rs2304256 depending on COVID-19 severity. This can be explained by the previously reported absence of altered TYK2 function after acid substitution caused by rs2304256 [53]. Therefore, based on our study, TYK2 did not impact COVID-19 outcome or the autoimmune regulatory mechanism that can be defined in MIS-C, but studies are ongoing, and other regulatory pathways could be found.
The OAS gene family is closely related with interferon-stimulated genes (ISG) [46]. OAS genes are located on chromosome 12 (12q24.13 region) and encode the synthesis of oligoadenylate OAS1, OAS2 and OAS3 [19].
Activated by viral RNA (mainly double-stranded), oligoadenylate catalyzes ATP polymerization and the activation of latent ribonuclease (RNase L) [55]. Direct Rnase L action leads to viral RNA destruction [19,56]. OAS–Rnase L cleaves viral messenger RNA, and as a result, viral replication is impossible [55]. RNase L activation leads to ribosomal and mitochondrial RNA degradation, and then apoptosis [57].
The most recent studies suggest that single-gene inborn errors of the OAS-RNase L lead to the uncontrolled production of pro-inflammatory cytokines by mononuclear phagocytes, which can predispose one to MIS-C development [58]. Lee et al. revealed that approximately 1% of patient with MIS-C had autosomal recessive deficiencies of OAS1, OAS2 or RNase L [58].
The results of our study demonstrate the protective effect of GG genotype OAS1 (rs10774671) in a dominant inheritance model of severe COVID-19 (OR = 0.18; p < 0.05), while carriers of allele A showed a higher risk of worse COVID-19 outcome (OR = 5.71; p < 0.05). The influences of allele A OAS1 rs10774671 on COVID-19 susceptibility and its severe course were confirmed by two logistic models. Our data correspond to the previously presented data from an adult population, where the involvement of rs10774671 in SARS-Co-V-2 pathogenesis was demonstrated [55]. The presence of allele A is associated with two mRNA variants—p48 and p52, with low OAS activity. Allele G leads to the production of the p46 form with high OAS activity [55].
Studies show that the presence of the risk allele G rs10735079 gene leads to reductions in the OAS1 level, which proves the existence of a negative inverse relationship between the severity of the course of COVID-19 and the level of OAS1 [21]. The level of OAS1 is directly related to hospitalization frequency in cases of diagnosed pneumonia [5,21]. However, the role of oligoadenylate synthases in the elimination of single-stranded viral RNAs remains debatable, and requires further study. Before now, results regarding OAS3 rs10735079 have been controversial. Horowitz et al. performed comparisons between SARS-CoV-2-positive patients vs. SARS-CoV-2-negative or unknown, and reported that allele G was the allele affecting COVID-19 pathogenesis [59]. At the same time, Pairo-Castineira et al. as well as Pietro et al. presented the influence of allele A rs10735079 on COVID-19 manifestations in adults and in children [5,12].
Our study did not reveal any differences in the genetic models between the studied OAS3 rs10735079 groups. However, in the case of gene interactions, the significant impact of OAS3 rs10735079 was shown on SARS-CoV-2 susceptibility, but it was not shown to be involved in the prediction of MIS-C or severe COVID-19 development. Importantly, the frequency of minor allele G in our research was significantly lower in healthy children compared to the European population. This diversity can be explained by the small sample in our control group (n = 15). Therefore, the following comparisons between allele frequencies in infected and noninfected groups were difficult.
CD40 is a 48-kDa type I transmembrane protein and belongs to the tumor necrosis factor (TNF) receptor (TNFR) family [26]. It was revealed that cytokine production is stimulated by CD40 engagement on the surface of dendritic cells [26]. Cell immunity is also mediated through CD40 signaling, and as a result, T-cell activation and differentiation are achieved [26]. At the same time, CD40 is involved in the regulation of the humoral immune response. B-cell activation by CD40 stimulates immunoglobulin (Ig) isotype switching and Ig somatic hypermutation [26]. As a result, the affinity of Ig to antigen is increased. The CD40-CD40L pathway is known as the regulator of the production of IL-10 and IL-12 by monocytes and macrophages [60]. Notwithstanding this, CD40-CD40L signaling is related to the formation of memory B cells, as well as long-lived plasma cells and their survival [26,61]. The dysregulation of CD40 is associated with the expansion of autoreactive B cells instead of their elimination, and leads to impaired immune response and autoimmune processes [60].
The CD40 molecule is encoded by the intergenic variant rs4813003 and is located on chromosome 20 (20:46134645; cytogenetic region 20q13.12) [62]. Current research suggests the rs4813003 TT genotype reduces the risk of KD (OR = 0.64), while genotypes CC and CT increase it [28]. Studies confirmed that the association of CD40 with KD is mainly related to the East Asian population, wherein risk allele C increases the risk of KD by 1.41 times [23,27,63]. In our research, CD40 rs4813003 genotype frequencies did not differ between study groups and the control, while allele C was seen significantly more often among patients with COVID-19 compared with the control (p < 0.05). Our study results also demonstrate the significant impact of allele C on severe COVID-19 course or MIS-C in both single-allele analyses (OR = 4.75; p = 0.037) and in case of allele interactions (OR = 264.57; p = 0.004).
Risk allele C increases not only CD40 function, but also increases the expression of its ligand CD40L [23,26]. Soluble CD40L is associated with vasculitis and vascular remodeling [64]. Most of the soluble sCD40L is of platelet origin, which indicates the activation of platelets in the lungs’ microcirculation during SARS-CoV-2 infection. sCD40L is able to locally activate endothelial cells, pericytes and smooth muscle cells, stimulate the expression of FGF-2, and, as a result, induce vascular wall remodeling [64]. In this regard, studies of CD40 rs4813003 polymorphism are important in relation to COVID-19 and MIS-C because cardiovascular system involvement is typical for both of them, especially MIS-C.
Missense variant rs1801274 encodes transmembrane glycoprotein Fc gamma (γ) receptor IIa and is located on chromosome 1 (1:161505430–161524013, cytogenetic region 1q23.3) in the EC2 domain [62,65,66].
Fc γ receptor (FcγR) presents on the immune cells and can bind with specific antibodies. Complex FcγR and Ig G stimulate the release of cytokines, the production of reactive oxygen species, as well as antibody-dependent cellular cytotoxicity and phagocytosis [65,66]. Based on its affinity to different Ig G subtypes, FcγR is divided into three types: FCGR1, FCGR2 and FCGR3. FCGR2 has the lowest affinity to Ig G. Three different subtypes of FCGR2 were revealed—A, B and C. FCGR2 is presented on a variety of immune competent cells, such as natural killers, macrophages and neutrophils [65]. Variable responses to infection depend on Ig F’s binding affinity. Polymorphism in the rs1801274 variant leads to the formation of two different isoforms of FCGR2—FcγRIIA-Arg and FcγRIIA-His [66]. These two forms can be made via the substitution of histidine for arginine at position 131 of the FCGR2A protein [67]. FcγRIIA-His has a higher affinity to Ig G1 and Ig G2 compared to FcγRIIA-Arg, while the binding capacities of Ig G3 and Ig G4 are almost the same in both isoforms [66]. Therefore, any deviations in FCGR2A expression can lead to the implicated Ig G binding affinity and, consequently, to immune dysregulation. It is suggested that FCGR2A is responsible for the modulation of severity of infection and autoimmune response—it is not only a marker of KD [66]. It has also been proposed to use genetic studies of FCGR in disease management, especially in the case of the prescription of therapeutic antibodies [66].
Study results have shown that risk allele G of gene FCGR2A rs1801274 increases the risk of KD under both the homozygous (AA) and heterozygous (AG) models in Asian and Caucasian populations, while allele A has a protective effect [65,68]. At the same time, controversial results were demonstrated by Zhang et al., whereby the estimation of the association between the FCGR2A rs1801274 polymorphism in Asian and Caucasian populations revealed that risk allele A is related to KD (OR = 1.41; p < 0.001) [69]. Research on adults of European ancestry demonstrated that allele G carriers (FcγRIIA-Arg) are more prone to lethal outcome of COVID-19 compared to homozygous AA carriers (OR = 2.2; p = 0.01) [66]. Greek researchers (Chatzikyriakidou et al.) did not reveal any genotype or allele differences between groups that suffered from KD and a healthy control [30]. Cardiovascular events were also not related to rs1801274 polymorphism [30]. Analyses of the previous thirteen case–control studies did not reveal a significant association between FCGR2A rs1801274 and overall pneumonia risk [67].
Our results correspond to those of European studies. The absence of a difference between the control group and the COVID-19 group, as well as the absence of a difference between COVID-19 patients and European FCGR2A allele frequencies, demonstrate that this gene is not related to disease severity.
Caspase 3 (CASP3) is a protein in the cysteine-aspartic acid protease (Caspase) family [66]. CASP3 with caspase-2, -6, -7, -8 and -9 is an apoptotic caspase [70]. An increase in CASP3 level is associated with increased apoptosis [70,71]. CASP3 could restrain IFN production. CASP3 deficiency is related to enhanced type I IFN secretion and the stimulation of innate immune response, and as a result, makes cells virally resistant [70]. CASP3 is also responsible for cell growth and differentiation, as well as for cytokine expression [68,72].
CASP3 is the gene located on chromosome 4 (4:184627696–184650062; cytogenetic region 4q35.1) that encodes protein caspase 3 [62]. Alterations in CASP3 expression lead to increased susceptibility to KD in European American and Asian populations [68]. A case–control study in North India demonstrated that persons with the CT genotype rs113420705 of CASP3 are more prone to have KD, and carriers of minor allele C most often have coronary artery aneurysm compared with T allele carriers [73]. Our study showed a significantly higher frequency of allele C rs113420705 in children with COVID-19 compared to those of European ancestry.
CASP3 gene is also important for the prevention of injuries caused by viral infection (SARS-CoV-2, hepatitis C virus, Enterovirus 71 infection, H-1 parvovirus) because CASP3 maintains cellular homeostasis and viability [72]. Therefore, it is suggested that the caspase’s level and its gene expression could be used to predict viral disease severity. In addition, hematological and immunological parameters of COVID-19 can be associated with dysregulated caspase activation [69]. In COVID-19 patients, CASP3 activity is upregulated in red blood cells, and platelet cell death can also be related to CASP3 [71].
The results of our study show the significant impact of CASP3 rs113420705 on SARS-CoV-2 susceptibility, and lead to a worse COVID-19 outcome in children.
5. Conclusions
COVID-19 shows different severity and outcomes in children. The clinical course varies from asymptomatic to mild and severe COVID-19 symptoms, and can even lead to MIS-C. Among the influencing factors, genetics is one of the most important. The carriers of pathogenic risk alleles are more prone to suffer from severe COVID-19. Allele T ACE2 rs2074192 (OR = 3.45; p = 0.009), allele A IFNAR2 rs2236757 (OR = 2.62; p = 0.039), allele A OAS1 rs10774671 (OR = 4.60; p = 0.003) and allele C CD40 rs4813003 (OR = 4.75; p = 0.037) are associated with severe COVID-19 that could advance to MIS-C. The multifactorial analysis shows that, in addition to ACE2, IFNAR2, OAS1, and CD40 genes, the CASP3 rs113420705 gene (allele C) makes the clinical outcome of COVID-19 in males worse. The findings of pathogenic risk alleles can help in the management of SARS-CoV-2 infections in children, and enhance COVID-19 prevention.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15102093/s1, Table S1: Test for Hardy–Weinberg equilibrium for ACE2 rs2074192, IFNAR2 rs2236757 and TYK2 rs2304256. Table S2: Test for Hardy–Weinberg equilibrium for OAS1 rs10774671 and OAS3 rs10735079. Table S3: Test for Hardy–Weinberg equilibrium for CD40 rs4813003, FCGR2A rs1801274 and CASP3 rs113420705.
Author Contributions
Conceptualization, K.K., H.P. and S.G.V.; methodology, K.K., S.G.V. and O.K.; software, K.K.; investigation, resources, K.K. and H.P.; data curation, writing—original draft preparation, K.K.; writing—review and editing, K.K., S.G.V., H.P., O.K., O.S. and M.K.; visualization, K.K.; supervision, S.G.V. and M.K.; project administration, S.G.V., O.S., M.K., O.K. and K.K.; funding acquisition, K.K. and O.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by RECOOP Fusion Grant # 030 “COVID-19 Severity and Gene Polymorphism in Children and Adults”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of I. Horbachevsky Ternopil National Medical University (Protocol No 71, dated 25 October 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article and Supplementary Material.
Acknowledgments
We thank the Cedars-Sinai Medical Center International Research and Innovation in Medicine Program, and the Association for Regional Cooperation in the Fields of Health, Science and Technology (RECOOP HST Association) for their support.
Conflicts of Interest
The authors declare no conflict of interest.
Limitations of the Study
Study limitations—small sample size (n = 75). It was a pilot study with a fixed effect size of 0.5.
References
- World Health Organization. Available online: https://covid19.who.int/ (accessed on 7 October 2023).
- UNICEF. Available online: https://data.unicef.org/resources/covid-19-confirmed-cases-and-deaths-dashboard/ (accessed on 7 October 2023).
- Nathanielsz, J.; Toh, Z.Q.; Do, L.A.H.; Mulholland, K.; Licciardi, P.V. SARS-CoV-2 Infection in Children and Implications for Vaccination. Pediatr. Res. 2023, 93, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Kozak, K.V.; Pavlyshyn, H.A.; Avramenko, I.Y.; Dyvonyak, O.M.; Hlushko, K.T. SARS-CoV-2 Infection and Thyroid Dysfunction in Children. Ukr. Biochem. J. 2023, 95, 12–21. [Google Scholar] [CrossRef]
- Di Pietro, G.M.; Ronzoni, L.; Meschia, L.M.; Tagliabue, C.; Lombardi, A.; Pinzani, R.; Bosis, S.; Marchisio, P.G.; Valenti, L. SARS-CoV-2 Infection in Children: A 24 Months Experience with Focus on Risk Factors in a Pediatric Tertiary Care Hospital in Milan, Italy. Front. Pediatr. 2023, 11, 1082083. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, L.; Rivalta, B.; Leone, F.; Cancrini, C.; Caffarelli, C.; Marseglia, G.L.; Cardinale, F. Host Defenses to Viruses: Lessons from Inborn Errors of Immunity. Medicina 2022, 58, 248. [Google Scholar] [CrossRef]
- Sánchez-González, M.T.; Cienfuegos-Jiménez, O.; Álvarez-Cuevas, S.; Pérez-Maya, A.A.; Borrego-Soto, G.; Marino-Martínez, I.A. Prevalence of the SNP Rs10774671 of the OAS1 Gene in Mexico as a Possible Predisposing Factor for RNA Virus Disease. Int. J. Mol. Epidemiol. Genet. 2021, 12, 52–60. [Google Scholar]
- Burrell, L.M.; Harrap, S.B.; Velkoska, E.; Patel, S.K. The ACE2 Gene: Its Potential as a Functional Candidate for Cardiovascular Disease. Clin. Sci. 2013, 124, 65–76. [Google Scholar] [CrossRef]
- Gintoni, I.; Adamopoulou, M.; Yapijakis, C. The Impact of ACE and ACE2 Gene Polymorphisms in Pulmonary Diseases Including COVID-19. In Vivo 2022, 36, 13–29. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Arendt-Nielsen, L.; Díaz-Gil, G.; Gómez-Esquer, F.; Gil-Crujera, A.; Gómez-Sánchez, S.M.; Ambite-Quesada, S.; Palomar-Gallego, M.A.; Pellicer-Valero, O.J.; Giordano, R. Genetic Association between ACE2 (Rs2285666 and Rs2074192) and TMPRSS2 (Rs12329760 and Rs2070788) Polymorphisms with Post-COVID Symptoms in Previously Hospitalized COVID-19 Survivors. Genes 2022, 13, 1935. [Google Scholar] [CrossRef]
- Ong, S.Y.Q.; Flyamer, I.M.; Bickmore, W.A.; Biddie, S.C. From Bedside to Bench: Regulation of Host Factors in SARS-CoV-2 Infection. Exp. Mol. Med. 2021, 53, 483–494. [Google Scholar] [CrossRef]
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D.; et al. Genetic Mechanisms of Critical Illness in COVID-19. Nature 2021, 591, 92–98. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; Martínez-Morales, A.; Chávez-Galán, L.; Ocaña-Guzmán, R.; Buendía-Roldán, I.; Pérez-Rubio, G.; Hernández-Zenteno, R.d.J.; Verónica-Aguilar, A.; Alarcón-Dionet, A.; Aguilar-Duran, H.; et al. IFNAR2 Relevance in the Clinical Outcome of Individuals with Severe COVID-19. Front. Immunol. 2022, 13, 949413. [Google Scholar] [CrossRef] [PubMed]
- Rusiñol, L.; Puig, L. Tyk2 Targeting in Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 3391. [Google Scholar] [CrossRef] [PubMed]
- Muromoto, R.; Oritani, K.; Matsuda, T. Current Understanding of the Role of Tyrosine Kinase 2 Signaling in Immune Responses. World J. Biol. Chem. 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dieter, C.; de Almeida Brondani, L.; Lemos, N.E.; Schaeffer, A.F.; Zanotto, C.; Ramos, D.T.; Girardi, E.; Pellenz, F.M.; Camargo, J.L.; Moresco, K.S.; et al. Polymorphisms in ACE1, TMPRSS2, IFIH1, IFNAR2, and TYK2 Genes Are Associated with Worse Clinical Outcomes in COVID-19. Genes 2023, 14, 29. [Google Scholar] [CrossRef]
- Benmansour, R.; Tagajdid, M.R.; Elkochri, S.; Aabi, R.; Elannaz, H.; Laraqui, A.; El Mchichi, B.; Touil, N.; Ennibi, K.; Amine, I.L. Genetic Susceptibility to Severe Forms of COVID-19: What We Learned in 2022. Saudi J. Pathol. Microbiol. 2023, 8, 90–98. [Google Scholar] [CrossRef]
- Zabihi Rizi, F.; Ghorbani, A.; Zahtab, P.; Darbaghshahi, N.N.; Ataee, N.; Pourhamzeh, P.; Hamzei, B.; Dolatabadi, N.F.; Zamani, A.; Hooshmand, M. TYK2 Single-Nucleotide Variants Associated with the Severity of COVID-19 Disease. Arch. Virol. 2023, 168, 119. [Google Scholar] [CrossRef]
- Rouf Banday, A.; Stanifer, M.L.; Florez-Vargas, O.; Onabajo, O.O.; Papenberg, B.W.; Zahoor, M.A.; Mirabello, L.; Ring, T.J.; Lee, C.-H.; Albert, P.S.; et al. Genetic Regulation of OAS1 Nonsense-Mediated Decay Underlies Association with COVID-19 Hospitalization in Patients of European and African Ancestries. Nat. Genet. 2022, 54, 1103–1116. [Google Scholar] [CrossRef]
- Raza, R.Z.; Abbasi, S.W. An Evolutionary Insight Into the Heterogeneous Severity Pattern of the SARS-CoV-2 Infection. Front. Genet. 2022, 13, 859508. [Google Scholar] [CrossRef]
- Steffen, B.T.; Pankow, J.S.; Lutsey, P.L.; Demmer, R.T.; Misialek, J.R.; Guan, W.; Cowan, L.T.; Coresh, J.; Norby, F.L.; Tang, W. Proteomic Profiling Identifies Novel Proteins for Genetic Risk of Severe COVID-19: The Atherosclerosis Risk in Communities Study. Hum. Mol. Genet. 2022, 31, 2452–2461. [Google Scholar] [CrossRef]
- Sancho-Shimizu, V.; Brodin, P.; Cobat, A.; Biggs, C.M.; Toubiana, J.; Lucas, C.L.; Henrikson, S.E.; Belot, A.; Tangye, S.G.; Milner, J.D.; et al. SARS-CoV-2-Related MIS-C: A Key to the Viral and Genetic Causes of Kawasaki Disease? J. Exp. Med. 2021, 218, e20210446. [Google Scholar] [CrossRef]
- Kumrah, R.; Vignesh, P.; Rawat, A.; Singh, S. Immunogenetics of Kawasaki Disease. Clin. Rev. Allergy Immunol. 2020, 59, 122–139. [Google Scholar] [CrossRef]
- Onouchi, Y. The Genetics of Kawasaki Disease. Int. J. Rheum. Dis. 2018, 21, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, H.; Nameirakpam, J.; Kumrah, R.; Pandiarajan, V.; Suri, D.; Rawat, A.; Singh, S. Biomarkers for Kawasaki Disease: Clinical Utility and the Challenges Ahead. Front. Pediatr. 2019, 7, 242. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Benson, M.J.; De Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular Mechanism and Function of CD40/CD40L Engagement in the Immune System. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef]
- Chen, M.R.; Chang, T.Y.; Chiu, N.C.; Chi, H.; Yang, K.D.; Chang, L.; Huang, D.T.N.; Huang, F.Y.; Lien, Y.P.; Lin, W.S.; et al. Validation of Genome-Wide Associated Variants for Kawasaki Disease in a Taiwanese Case–Control Sample. Sci. Rep. 2020, 10, 11756. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Zhong, R.; Shen, N.; Lu, X.Z.; Ke, J.T.; Duan, J.Y.; Qi, Y.Q.; Wang, Y.J.; Zhang, Q.; Wang, W.; et al. Systematic Confirmation Study of GWAS-Identified Genetic Variants for Kawasaki Disease in A Chinese Population. Sci. Rep. 2015, 5, 8194. [Google Scholar] [CrossRef]
- Hara, T.; Yamamura, K.; Sakai, Y. The Up-to-Date Pathophysiology of Kawasaki Disease. Clin. Transl. Immunol. 2021, 10, 1–14. [Google Scholar] [CrossRef]
- Chatzikyriakidou, A.; Aidinidou, L.; Giannopoulos, A.; Papadopoulou-Legbelou, K.; Kalinderi, K.; Fidani, L. Absence of Association of FCGR2A Gene Polymorphism Rs1801274 with Kawasaki Disease in Greek Patients. Cardiol. Young 2015, 25, 681–683. [Google Scholar] [CrossRef]
- Uzuncakmak, S.K.; Dirican, E.; Naldan, M.E.; Can, F.K.; Halıcı, Z. Investigation of CYP2E1 and Caspase-3 Gene Expressions in COVID-19 Patients. Gene. Rep. 2022, 26, 101497. [Google Scholar] [CrossRef]
- Venturini, E.; Montagnani, C.; Garazzino, S.; Donà, D.; Pierantoni, L.; Lo Vecchio, A.; Krzysztofiak, A.; Nicolini, G.; Bianchini, S.; Galli, L.; et al. Treatment of Children with COVID-19: Update of the Italian Society of Pediatric Infectious Diseases Position Paper. Ital. J. Pediatr. 2021, 47, 199. [Google Scholar] [CrossRef]
- Dona’, D.; Montagnani, C.; Di Chiara, C.; Venturini, E.; Galli, L.; Lo Vecchio, A.; Denina, M.; Olivini, N.; Bruzzese, E.; Campana, A.; et al. COVID-19 in Infants Less than 3 Months: Severe or Not Severe Disease? Viruses 2022, 14, 2256. [Google Scholar] [CrossRef]
- National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/ (accessed on 5 August 2023).
- World Health Organization. Available online: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 5 August 2023).
- Howie, S.R.C. Blood Sample Volumes in Child Health Research: Review of Safe Limits. Bull. World Health Organ. 2011, 89, 46–53. [Google Scholar] [CrossRef]
- GeneMANIA. Available online: https://genemania.org/ (accessed on 14 August 2023).
- Phan, L.; Jin, Y.; Zhang, H.; Qiang, W.; Shekhtman, E.; Shao, D.; Revoe, D.; Villamarin, R.; Ivanchenko, E.; Kimura, M.; et al. ALFA: Allele Frequency Aggregator; National Center for Biotechnology Information, U.S. National Library of Medicine: Bethesda, MD, USA, 2020.
- Gómez, J.; Albaiceta, G.M.; García-Clemente, M.; López-Larrea, C.; Amado-Rodríguez, L.; Lopez-Alonso, I.; Hermida, T.; Enriquez, A.I.; Herrero, P.; Melón, S.; et al. Angiotensin-Converting Enzymes (ACE, ACE2) Gene Variants and COVID-19 Outcome. Gene 2020, 762, 145102. [Google Scholar] [CrossRef] [PubMed]
- Sabater Molina, M.; Nicolás Rocamora, E.; Bendicho, A.I.; Vázquez, E.G.; Zorio, E.; Rodriguez, F.D.; Gil Ortuño, C.; Rodríguez, A.I.; Sánchez-López, A.J.; Jara Rubio, R.; et al. Polymorphisms in ACE, ACE2, AGTR1 Genes and Severity of COVID-19 Disease. PLoS ONE 2022, 17, e0263140. [Google Scholar] [CrossRef]
- Möhlendick, B.; Schönfelder, K.; Breuckmann, K.; Elsner, C.; Babel, N.; Balfanz, P.; Dahl, E.; Dreher, M.; Fistera, D.; Herbstreit, F.; et al. ACE2 Polymorphism and Susceptibility for SARS-CoV-2 Infection and Severity of COVID-19. Pharmacogenet. Genom. 2021, 31, 165–171. [Google Scholar] [CrossRef]
- Buchynskyi, M.; Oksenych, V.; Kamyshna, I.; Vari, S.G.; Kamyshnyi, A. Genetic Predictors of Comorbid Course of COVID-19 and MAFLD: A Comprehensive Analysis. Viruses 2023, 15, 1724. [Google Scholar] [CrossRef] [PubMed]
- Cafiero, C.; Rosapepe, F.; Palmirotta, R.; Re, A.; Ottaiano, M.P.; Benincasa, G.; Perone, R.; Varriale, E.; D’Amato, G.; Cacciamani, A.; et al. Angiotensin System Polymorphisms’ in SARS-CoV-2 Positive Patients: Assessment Between Symptomatic and Asymptomatic Patients: A Pilot Study. Pharmacogenom. Pers. Med. 2021, 14, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Kaur, G.; Pathak, T.; Banerjee, I. Systematic Review and Meta-Analysis of Human Genetic Variants Contributing to COVID-19 Susceptibility and Severity. Gene 2022, 844, 146790. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, L.E.; Herrera-López, B.; Martinez-Armenta, C.; Ortega-Peña, S.; del Carmen Camacho-Rea, M.; Suarez-Ahedo, C.; Vázquez-Cárdenas, P.; Vargas-Alarcón, G.; Rojas-Velasco, G.; Fragoso, J.M.; et al. ACE and ACE2 Gene Variants Are Associated with Severe Outcomes of COVID-19 in Men. Front. Immunol. 2022, 13, 812940. [Google Scholar] [CrossRef]
- Gao, L.J.; He, Z.M.; Li, Y.Y.; Yang, R.R.; Yan, M.; Shang, X.; Cao, J.M. Role of OAS Gene Family in COVID-19 Induced Heart Failure. J. Transl. Med. 2023, 21, 212. [Google Scholar] [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired Type I Interferon Activity and Inflammatory Responses in Severe COVID-19 Patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Buchynskyi, M.; Kamyshna, I.; Lyubomirskaya, K.; Moshynets, O.; Kobyliak, N.; Oksenych, V.; Kamyshnyi, A. Efficacy of Interferon Alpha for the Treatment of Hospitalized Patients with COVID-19: A Meta-Analysis. Front. Immunol. 2023, 14, 1069894. [Google Scholar] [CrossRef] [PubMed]
- Kamyshnyi, A.; Koval, H.; Kobevko, O.; Buchynskyi, M.; Oksenych, V.; Kainov, D.; Lyubomirskaya, K.; Kamyshna, I.; Potters, G.; Moshynets, O. Therapeutic Effectiveness of Interferon-α2b against COVID-19 with Community-Acquired Pneumonia: The Ukrainian Experience. Int. J. Mol. Sci. 2023, 24, 6887. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, J.; Khan, S.; Elima, K.; Huttunen, T.; Wang, N.; Hollmén, M.; Elo, L.L.; Jalkanen, S. Polymorphism in Interferon Alpha/Beta Receptor Contributes to Glucocorticoid Response and Outcome of ARDS and COVID-19. Crit. Care 2023, 27, 112. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The Signal Pathways and Treatment of Cytokine Storm in COVID-19. Sig. Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Augustin, M.; Baraliakos, X.; Krönke, G.; Schneider, M.; Schreiber, S.; Schulze-Koops, H.; Zeißig, S.; Thaçi, D. TYK2 Inhibition and Its Potential in the Treatment of Chronic Inflammatory Immune Diseases. J. Dtsch. Dermatol. Ges. 2021, 19, 1409–1420. [Google Scholar] [CrossRef]
- Li, Z.; Rotival, M.; Patin, E.; Michel, F.; Pellegrini, S. Two Common Disease-Associated TYK2 Variants Impact Exon Splicing and TYK2 Dosage. PLoS ONE 2020, 15, e0225289. [Google Scholar] [CrossRef]
- GeneCards. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=TYK2 (accessed on 1 August 2023).
- Gokul, A.; Arumugam, T.; Ramsuran, V. Genetic Ethnic Differences in Human 2′-5′-Oligoadenylate Synthetase and Disease Associations: A Systemic Review. Genes 2023, 14, 527. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, H.; Ji, Y.; Chen, X. Evaluate the Relationship between Polymorphisms of OAS1 Gene and Susceptibility to Chronic Hepatitis C with High Resolution Melting Analysis. Clin. Exp. Med. 2013, 13, 171–176. [Google Scholar] [CrossRef]
- Drappier, M.; Michiels, T. Inhibition of the OAS/RNase L Pathway by Viruses. Curr. Opin. Viral 2015, 15, 19–26. [Google Scholar] [CrossRef]
- Lee, D.; Le Pen, J.; Yatim, A.; Dong, B.; Aquino, Y.; Ogishi, M.; Pescarmona, R.; Talouarn, E.; Rinchai, D.; Zhang, P.; et al. Inborn Errors of OAS-RNase L in SARS-CoV-2-Related Multisystem Inflammatory Syndrome in Children. Science 2023, 379, eabo3627. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.E.; Kosmicki, J.A.; Damask, A.; Sharma, D.; Roberts, G.H.L.; Justice, A.E.; Banerjee, N.; Coignet, M.V.; Yadav, A.; Leader, J.B.; et al. Genome-Wide Analysis Provides Genetic Evidence That ACE2 Influences COVID-19 Risk and Yields Risk Scores Associated with Severe Disease. Nat. Genet. 2022, 54, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Marengo, M.; Migliori, M.; Merlotti, G.; Fabbrini, P.; Panichi, V.; Cantaluppi, V. Role of the CD40-CD40 Ligand Pathway in Cardiovascular Events, Neurological Alterations, and Other Clinical Complications of Chronic Hemodialysis Patients: Protective Role of Adsorptive Membranes. Blood Purif. 2023, 1–16. [Google Scholar]
- Ara, A.; Ahmed, K.A.; Xiang, J. Multiple Effects of CD40–CD40L Axis in Immunity against Infection and Cancer. Immunotargets Ther. 2018, 7, 55–61. [Google Scholar] [CrossRef] [PubMed]
- GWAS Catalog. Available online: https://www.ebi.ac.uk/gwas/home (accessed on 1 August 2023).
- Onouchi, Y.; Ozaki, K.; Burns, J.C.; Shimizu, C.; Terai, M.; Hamada, H.; Honda, T.; Suzuki, H.; Suenaga, T.; Takeuchi, T.; et al. A Genome-Wide Association Study Identifies Three New Risk Loci for Kawasaki Disease. Nat. Genet. 2012, 44, 517–521. [Google Scholar] [CrossRef]
- Petrey, A.C.; Qeadan, F.; Middleton, E.A.; Pinchuk, I.V.; Campbell, R.A.; Beswick, E.J. Cytokine Release Syndrome in COVID-19: Innate Immune, Vascular, and Platelet Pathogenic Factors Differ in Severity of Disease and Sex. J. Leukoc. Biol. 2021, 109, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Geng, P.L. CD32a Polymorphism Rs1801274 Affects the Risk of Kawasaki Disease. Artif. Cells Nanomed. Biotechnol. 2020, 48, 620–626. [Google Scholar] [CrossRef]
- López-Martínez, R.; Albaiceta, G.M.; Amado-Rodríguez, L.; Cuesta-Llavona, E.; Gómez, J.; García-Clemente, M.; Vázquez-Coto, D.; Alvarez, V.; Coto, E. The FCGR2A Rs1801274 Polymorphism Was Associated with the Risk of Death among COVID-19 Patients. Clin. Immunol. 2022, 236, 108954. [Google Scholar] [CrossRef]
- Shi, X.; Ma, Y.; Li, H.; Yu, H. Association between FCGR2A Rs1801274 and MUC5B Rs35705950 Variations and Pneumonia Susceptibility. BMC Med. Genet. 2020, 21, 71. [Google Scholar] [CrossRef]
- Ferdosian, F.; Dastgheib, S.A.; Hosseini-Jangjou, S.H.; Nafei, Z.; Lookzadeh, M.H.; Noorishadkam, M.; Mirjalili, S.R.; Neamatzadeh, H. Association of TNF-α Rs1800629, CASP3 Rs72689236 and FCGR2A Rs1801274 Polymorphisms with Susceptibility to Kawasaki Disease: A Comprehensive Meta-Analysis. Fetal Pediatr. Pathol. 2021, 40, 320–336. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Zhang, H.; Wei, L.; Guo, S. Association of FCGR2A Rs1801274 Polymorphism with Susceptibility to Autoimmune Diseases: A Meta-Analysis. Oncotarget 2016, 7, 39436–39443. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Wang, Y.; Jing, M.; Sha, M.; Lv, M.; Gao, P.; Zhang, R.; Huang, X.; Feng, J.M.; Jiang, Z. Apoptotic Caspases Suppress Type I Interferon Production via the Cleavage of CGAS, MAVS, and IRF3. Mol. Cell 2019, 74, 19–31.e7. [Google Scholar] [CrossRef] [PubMed]
- Premeaux, T.A.; Yeung, S.T.; Bukhari, Z.; Bowler, S.; Alpan, O.; Gupta, R.; Ndhlovu, L.C.; Tincati, C. Emerging Insights on Caspases in COVID-19 Pathogenesis, Sequelae, and Directed Therapies. Front. Immunol. 2022, 13, 842740. [Google Scholar] [CrossRef]
- Yildiz Gulhan, P.; Eroz, R.; Ataoglu, O.; İnce, N.; Davran, F.; Öztürk, C.E.; Gamsızkan, Z.; Balbay, O.A. The Evaluation of Both the Expression and Serum Protein Levels of Caspase-3 Gene in Patients with Different Degrees of SARS-CoV2 Infection. J. Med. Virol. 2022, 94, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Das, K.G.; Bhattarai, D.; Kaur, A.; Kaur, A.; Kumrah, R.; Srivastava, P.; Rawat, A.; Singh, S. Association of Single Nucleotide Polymorphism Rs113420705 of CASP3 in Children with Kawasaki Disease from North India. J. Family Med. Prim. Care 2022, 11, 5404–5409. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).