Abstract
(1) Objective: This systematic review summarizes current knowledges about maternal and neonatal outcomes following COVID-19 vaccination during pregnancy and breastfeeding. (2) Study design: PubMed, Cochrane Library, and the Education Resources Information Center (ERIC) were searched up to 27 October 2021. The primary outcome was to estimate how many pregnant and lactating women were reported to be vaccinated and had available maternal and neonatal outcomes. (3) Results: Forty-five studies sourcing data of 74,908 pregnant women and 5098 lactating women who received COVID-19 vaccination were considered as eligible. No major side-effects were reported, especially during the second and third trimester of pregnancy and during breastfeeding. Conversely, available studies revealed that infants received specific SARS-CoV-2 antibodies after maternal vaccination. (4) Conclusions: Vaccination against the SARS-CoV-2 virus should be recommended for pregnant women, after the pros and cons have been adequately explained. In particular, given the still limited evidence and considering that fever during the first months of gestation increases the possibility of congenital anomalies, they should be carefully counseled. The same considerations apply to breastfeeding women, also considering the immune responses that mRNA vaccines can generate in their human milk.
Keywords:
neonates; COVID-19; infants; mothers; pregnancy; fetuses; miscarriage; malformations; women 1. Introduction
Physiological, mechanical, and immunologic changes in pregnant women could influence their possibility of being attacked by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Main symptoms of the disease (COVID-19) are related to an impaired microcirculatory function; indeed, infected pregnant women are at increased risk of preeclampsia (PE)-like symptoms [2] and of need for hospitalization and intensive care unit admission [3]. Given that the incidence of obstetrical complications, such as preterm birth, appears to be proportional to the severity of the infection, infants born to infected mothers with a more severe clinical course may have a worse outcome, mainly due to neonatal morbidity and mortality associated with prematurity. Therefore, the immunization of pregnant women against SARS-CoV-2 appears to be justified [4].
Although no conclusive evidence is yet completely available regarding the effectiveness and safety of COVID-19 vaccines in pregnancy, due to the non-inclusion of these women in clinical trials evaluating vaccines, the studies performed to date have allowed detecting a significant lower risk of contracting SARS-CoV-2 infection among vaccinated than unvaccinated pregnant women. The number of pregnant women vaccinated against COVID-19 to date, globally, has exceeded hundreds of thousands with no adverse event reports in excess of the nonpregnant population [5]. The same goes for the efficacy of vaccination during breastfeeding, which is considered to be similar to that among nonpregnant women. Currently there is unanimous consensus that there is no biological plausibility in support of a possible harm to infants nursed by vaccinated mothers [6]. Regarding the fertility of women who undergo vaccination against COVID-19, public health agencies and scientific societies internationally ruled out a possible association between vaccine and fertility problems [7].
Our aim was to summarize the evidence in the literature on the outcomes of vaccination against SARS-CoV-2 in pregnant women and in women who have recently delivered, as well as the possible effects of vaccines currently used (Figure 1) on their newborns.
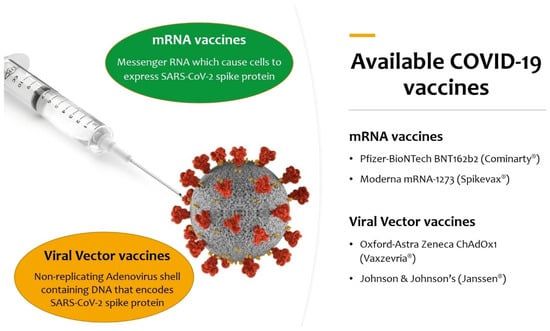
Figure 1.
Available COVID-19 vaccines authorized for use by European Medicines Agency (EMA) at the moment of the literature search.
2. Materials and Methods
2.1. Search Strategy and Study Selection
This systematic review was performed following PRISMA guidelines [8]. Search terms included “SARS-CoV-2” OR “COVID-19” AND “vaccination” OR “vaccine” AND “pregnancy” OR “pregnant” OR “breastfeeding”. We considered studies providing information about maternal and/or child outcomes after maternal SARS-CoV-2 vaccination with a mRNA-based vaccine published after 1 January 2021. We did not apply limitations on study design to include all available literature, but preprint studies were not considered. The selection of studies was made through PubMed (http://www.ncbi.nlm.nih.gov/pubmed/, accessed on 27 October 2021), the Cochrane Library (https://www.cochranelibrary.com/advanced-search, accessed on 27 October 2021), and the Education Resources Information Center (ERIC, https://eric.ed.gov/, accessed on 27 October 2021) (Box 1).
The search was conducted as follows: Dr. De Rose (D.U.D.R.) identified relevant studies by reading the abstract and searching for additional studies through the reference lists of the selected papers. Then, Dr. De Rose (D.U.D.R.) and Dr. Auriti (C.A.) independently reviewed the studies by checking titles and abstracts of the articles and by deciding whether to include each article or not.
Box 1. Different COVID-19 vaccine types to date.
Different types of vaccines are under research to develop an effective vaccine against COVID-19:
- mRNA vaccines: based on messenger RNA (mRNA) or a self-replicating RNA that provides the genetic information required to produce the spike protein: Pfizer–BioNTech BNT162b2 (Comirnaty®) and Moderna mRNA-1273 (Spikevax®);
- Viral-vector vaccines: an existing virus that is incompetent for replication but contains DNA that encodes for the spike protein. In the case of ChAdOx-1S, developed by the University of Oxford and AstraZeneca (Vaxzevria®), the vector is a modified chimpanzee adenovirus; in the case of Johnson & Johnson’s Ad26.COV2.S (Janssen®), the vector is a recombinant human adenovirus (Ad 26–serotype 26); in the case of the Russian Gam-COVID-Vac (Sputnik V®), two recombinant replication-defective human adenoviruses were used (Ad26 and Ad5–serotype 5); in the case of Ad5-nCov (Convidecia®), the vector is similarly the human adenovirus serotype 5 (Ad5);
- Recombinant protein vaccines: based on the laboratory synthesis of the spike protein, the receptor-binding domain (RBD), or virus-like particles. This category includes the American Nuvaxovid®/Indian Covovax ®, the Russian EpiVacCorona®, the Chinese ZF2001 (Zifivax®), the Cuban Soberana-2®, and the Sanofi-GSK VAT00008;
- Inactivated viral vaccines: the SARS-CoV-2 virus has been cultivated in cell cultures and chemically inactivated. This category includes the Chinese CoronaVac® and the Indian Covaxin®;
- Live-attenuated virus vaccines: a genetically weakened variant of the virus that replicates to a limited amount but does not cause illness while eliciting an immune response, as for measles, mumps, and rubella (MMR) vaccine;
- DNA vaccines: modified plasmids that carry genes that typically code for the spike protein, which is then produced in the vaccinated individual, as in Indian ZyCoV-D® COVID vaccine.
How do current available vaccines work?
The Pfizer–BioNTech BNT162b2 vaccine and Moderna mRNA-1273 vaccine contain mRNA encoding for spike membrane proteins, encapsulated in lipid nanoparticles. Oxford–AstraZeneca ChAdOx1 contains SARS-CoV-2 virus cDNA fragments encapsulated in a viral vector. Both are non-replicants and self-destroying. When administered, macrophages and dendritic cells grab particles and convert them into proteins which are degraded into peptides and then exposed as antigens. Antigenic viral peptides are presented to major histocompatibility complexes (MHC) I and II and identified by helper T lymphocytes, causing B cells to synthesize neutralizing antibodies and cytotoxic T lymphocytes that kill infected cells.
Neutralizing antibodies directed to the virus membrane glycoproteins such as the spike protein and nucleocapsid proteins drive the humoral immune response to the SARS-CoV-2 virus. These antibodies prevent the virus from entering cells and, hence, its infectious capacity. However, not all antibodies have neutralizing activity, and some can increase the activity of the virus. Therefore, to solve this problem, mRNA fractions were isolated and encapsulated within lipid nanoparticles to create mRNA vaccines. COVID-19 infection is the first infection to be prevented with mRNA vaccines, and the vaccine underwent many tests quickly before being administered to humans. Both viral-vector vectors and mRNA vaccines induce a cell-mediated response by cytotoxic Th1 and CD8+ lymphocytes that recognize and digest viral antigens exposed by MCH class I molecules. As of yet, the induction of regulatory T cells in the vaccine-induced immune response has not been reported. Other COVID-19 vaccine types are now being studied in phase 1, 2, and 3 clinical trials (https://covid19.trackvaccines.org/vaccines/).
2.2. Assessment of Risk of Bias
We evaluated the quality of included cohort studies and the risk of bias using the Newcastle–Ottawa Scale (NOS). The NOS comprises “participant selection”, “comparability of study groups,” and “assessment of outcome or exposure”. A score above 6–7 denotes a reasonable quality.
3. Results
3.1. Study Selection Process
The searches identified 1345 potentially relevant papers and studies, while 576 after duplicates were removed. After title and abstract screening, 60 full-text studies were considered potentially eligible for inclusion. A flowchart of study selection process is reported in Figure 2. We considered 46 records: 30 of them included pregnant mothers [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38], two studies included both pregnant and lactating mothers [39,40], and 14 studies included lactating mothers [41,42,43,44,45,46,47,48,49,50,51,52,53,54]. Most studies were conducted in the United States (41.3%) and Israel (26.1%), along with three in Italy (6.5%), two in Spain (4.3%) and in Poland (4.3%), and the remaining (17.2%) in the United Kingdom, Qatar, Belgium, Germany, Norway, Portugal, the Netherlands, and Singapore.
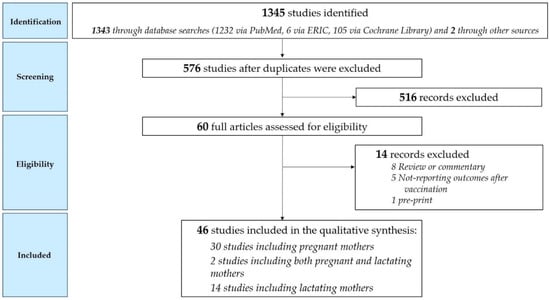
Figure 2.
Study selection process.
Most cohort studies including pregnant women had a reasonable quality, as reported in Figure 3 (case series and case reports without a nonexposed group could not be considered).
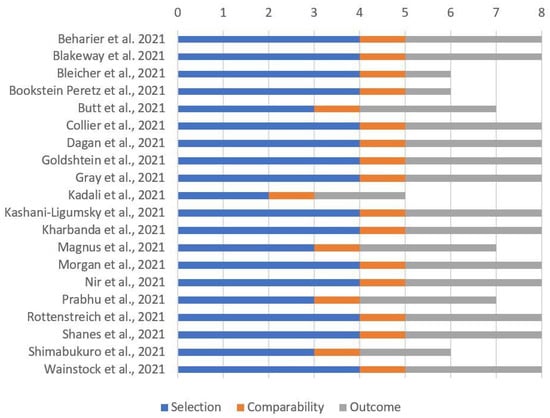
Figure 3.
Quality assessment of included cohort studies involving pregnant women through “Newcastle–Ottawa Scale for cohort studies”.
Most studies including breastfeeding mothers reported only data about exposed women, without a control group; the remaining six studies had a reasonable quality, as reported in Figure 4.
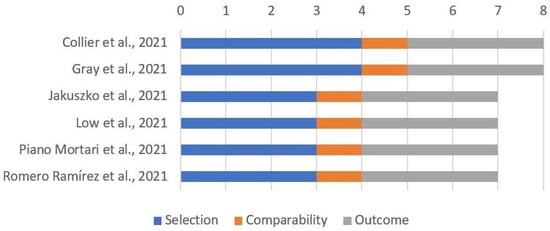
Figure 4.
Quality assessment of included cohort studies involving breastfeeding mothers through “Newcastle–Ottawa Scale for cohort studies”.
3.2. Synthesis of Results in Pregnant Women
The characteristics and most relevant findings of the included studies about vaccination during pregnancy are reported in Table 1. The most relevant question concerns the safety of vaccination administered during pregnancy. No major adverse effects during pregnancy were reported.

Table 1.
List of the 32 studies including pregnant mothers who received SARS-CoV-2 vaccination.
To date, the largest study on the safety profile of mRNA vaccines during pregnancy is a cross-sectional survey published by Shimabukuro et al. in the New England Journal of Medicine, who reported preliminary findings from three US vaccine safety monitoring systems [34]. From 14 March 2020 to 28 February 2021 the authors surveyed over 35,000 women aged 16 to 54, identified as pregnant from their inclusion in various pregnancy registries. Among those women, 4000 provided information on the outcome; 827 of these delivered, and 29,000 reported post-vaccination symptoms. The limitation of the study is that all pregnant women were vaccinated in the third trimester, and this ruled out the possibility of evaluating some of the possible adverse effects of vaccinations on the progress of pregnancy and on newborns. However, although not fully comparable, the estimate of obstetrical and neonatal complications of maternal vaccination (n = 827) is similar to that described in studies on pregnant women conducted in the pre-COVID-19 period [30].
Comparing adverse effects in pregnant women and nonpregnant women, according Bookstein Peretz’s data, there were no additional adverse effects of vaccination during pregnancy. Furthermore, the rate and the severity of adverse effects were unaffected by the timing of immunization during pregnancy [12].
Goldshtein et al. confirmed this trend, with only 68/7530 women vaccinated during pregnancy (0.9%) reporting possible vaccine-related adverse events; none of them were serious. Headache (0.1%), overall asthenia (0.1%), unspecified pain (<0.1%), and stomachache (<0.1%) were the most often reported side-effects. Furthermore, mRNA immunization was linked to a considerably decreased probability of SARS-CoV-2 infection when compared to no immunization [19].
Two very large Israeli cohort studies provided data that support the safety of vaccination in pregnancy. The first is a retrospective study by Goldshtein et al. that looked at the relationship between immunization with Pfizer vaccine and the risk of infection in pregnant women who were vaccinated in the second and third trimesters of gestation. The primary outcome was SARS-CoV-2 infection demonstrated by the positivity of the molecular swab at 28 days after the initial vaccination dose. The cumulative incidence of infection in vaccinated women was significantly lower than in unvaccinated women. SARS-CoV-2 hospitalization rates were 0.2% in the protected women and 0.3% in the unprotected peers. During the study’s follow-up period, 18% of the vaccinated group and 18.9% of the unvaccinated group completed the pregnancy [19].
Dagan et al. in the second Israeli study compared a cohort of 10,861 pregnant women vaccinated with BNT162b2 mRNA matched 1:1 with 10,861 unvaccinated pregnant women. Twenty-six percent were immunized during the first trimester, 48% were immunized during the second trimester, and 26% were immunized during the third trimester. The results reflect the effectiveness of the vaccine against the alpha variant, which was predominantly circulating in Israel during the study period. The primary outcome was to determine the incidence of SARS-CoV-2 infection documented by a positive molecular swab and the incidence of symptoms and hospitalization. The periods in which the cumulative incidence was calculated were 14–20 days and 21–27 days following the first administration and 7–56 days following the second injection. The incidence of infection, as well as the risk of severe illness and hospitalization, was considerably greater in the unvaccinated group compared to the vaccinated group [15].
A very relevant question relates to the protective capacity of vaccination or natural infection for newborns. Several studies on this question have been performed. The most interesting was carried out in Boston on a cohort of 103 women protected with mRNA-1273 (Moderna) or BNT162b2 (Pfizer–BioNTech) vaccine, as well as 28 women with confirmed SARS-CoV-2 infection [39]: 30 vaccinated women were pregnant; 16 vaccinated women were post breastfeeding; 57 vaccinated women were neither pregnant nor breastfeeding. Among the 28 unvaccinated women, 22 were infected with SARS-CoV-2, while six were infected with SARS-CoV-2 and were breastfeeding. The authors measured the anti-SARS-CoV-2 receptor-binding domain (RBD) antibody titer and the neutralizing and non-neutralizing antibody titer after vaccination and after the natural infection. The presence of neutralizing and non-neutralizing antibodies and the response of CD4+ and CD8+ T-cells were detectable in all subgroups and were also observed in the umbilical cord blood and human milk following immunization. The level of antibodies against variants B.1.1.7 and B.1.351 was decreased, but the cellular T response was conserved against these variants.
The presence of neutralizing antibodies in pregnant women and newborns following vaccination was confirmed in all cohorts. Interestingly, a surprising discovery is that the transfer ratio appears to rise with latency from immunization; these findings imply that earlier immunization, at least among women in their third trimester, may create a stronger protection in the baby, the immunobiology of which deserves additional study [26].
3.3. Synthesis of Results in Lactating Women
Questions are often asked about the presence of antibodies and mRNA in breast milk after COVID vaccine. The characteristics and most relevant findings of the included studies on vaccination during breastfeeding are reported in Table 2. No major adverse reactions needing emergency treatment or hospitalization were described in mothers and infants.

Table 2.
List of the 16 studies including pregnant mothers who received SARS-CoV-2 vaccination.
It seems clear that, in the human milk of women protected with mRNA vaccines (currently preferentially indicated in Italy for women of reproductive age), there is a constant presence of anti-spike antibodies, particularly of the IgA and IgG type, as also shown by preliminary data from a study still in progress at the “Bambino Gesù” Children’s Hospital in Rome [53].
The relationship between milk IgA antibodies and immunization timing during breastfeeding has to be researched further, considering the drop in IgA levels recently observed of up to 25% in a cohort of lactating women (probably deriving from the different timing of withdrawals) [55].
To date, the largest study is a cross-sectional survey performed by McLaurin-Jiang et al., who recruited 4455 vaccinated breastfeeding mothers. According to their findings, vaccination appears to have few negative effects on lactation despite the reported short-term side-effects (such as fever, fatigue, or headache) [46], with resolution within 72 h after vaccination [51].
An increase in milk IgA levels is seen 2 weeks after the first dose of vaccine, and they are detectable in 86% of cases 2 weeks after the second dose; IgG was already detected just 1 week after the second dose [40,48,52].
When compared to detectable levels following the first dose, maternal blood IgG levels rose sixfold following the second dose; a similar pattern of increase was observed for milk IgG levels, although whether this could stimulate adequate infant immunity or not still requires further studies [55].
No mRNA presence was detected, highlighting the fact that there is no transfer of mRNA to the baby via breast milk and no reason to discontinue breastfeeding for this reason at the time of vaccine administration [44].
To exclude that mRNA vaccine components could get into milk after immunization, polyethylene glycol was measured by Golan et al. in milk samples collected before and after vaccine administration, and its concentration did not significantly change [55].
4. Discussion
Primary prevention of infections in pregnancy through vaccination is one of the most successful public health programs of the last 10 years. It has led to a significant decrease in maternal and perinatal morbidity and deaths from influenza and whooping cough [56]. The frequency of COVID-19 vaccination is still relatively low among pregnant women worldwide. Shamshirsaz et al. demonstrated that vaccination acceptance was significantly associated with the history of influenza or pertussis vaccine administration during pregnancy (OR = 3.03; 95% CI: 1.37–6.73; p = 0.006) [57].
Therefore COVID-19 infection in pregnancy has a course that is certainly more severe than in other periods of women’s life. It can cause adverse effects on the course of pregnancy, such as preterm birth, even if the real possibility and frequency of maternal–fetal transmission are still under study.
According to Ciapponi et al., COVID-19 pregnant women had a twofold higher risk of requiring mechanical ventilation, whereas their neonates had a threefold higher risk of being hospitalized in the neonatal intensive care unit (NICU) [58]. Kazemi et al. noted a marked risk of abortion in infected mothers; one possible mechanism could be placental inflammation induced by viral particles [59]. Most infections in neonates and children arise from family clusters [60], linked to infected adult patients; they often exhibit only milder clinical signs [61]. Recently, a few case reports of neonates presenting with a multisystem inflammatory syndrome after maternal SARS-CoV-2 infection (MIS-N) were reported [62].
Lumbreras-Marquez et al. assessed that, if all pregnant women received immunization during the two months of May and June 2021, the total expected maternal deaths in Mexico would have firmly decreased [63].
Data from mRNA vaccine studies show that the vaccine safety, tolerability, and efficacy in immunization are similar in pregnant women and their nonpregnant peers [4]. Since mRNA vaccines appear to stimulate Toll-like receptor 3 and that such activation has been associated with negative gestational outcomes [5], Shanes et al. examined the placentas of 84 women who got a SARS-CoV-2 vaccine during pregnancy and 116 unvaccinated women. The authors found no raised incidence of deciduous arterial disease, fetal vascular dysfunction, chronic low-grade villitis, or chronic histiocytic intervillositis in the vaccinated group [33].
However, the speed with which these vaccines were developed and authorized caused some concerns in the communities, starting from the possible effects on fertility or child outcomes. Long-term statistics are obviously scarce and will remain so for some years. The COVID-19 Vaccines International Pregnancy Exposure Registry (C-VIPER) will systemically estimate the risk of obstetric outcomes, neonatal outcomes, and infant outcomes in pregnant women exposed to a COVID-19 vaccine from 30 days before to the first day of their last menstrual period to end of pregnancy compared to a matched unexposed group. To date, the estimated study completion date is 31 December 2025 [64].
Although pregnant women were excluded from the first trials, at the time of writing, there is absolutely no evidence or theoretical reason to believe that any of the COVID-19 vaccinations could impair future fertility to date [7].
Concerns have also been raised about the chance of spontaneous abortion (pregnancy loss that occurs at fewer than 20 weeks of gestational age), a frequent event that affects 11 to 22 percent of identified pregnancies. Analyzing the data of the CVC v-safe COVID-19 pregnancy registry, the cumulative risk was within the expected risk range when data were compared to two historical cohorts that represent the “normal” lower and upper ranges of spontaneous abortion risk [37].
This systematic review is limited by the statistical heterogeneity in results of previous studies (ranging from single case reports to multicenter studies) and the relatively small sample size of pregnant women available in the literature, considering that more than 9.5 billion shots of COVID-19 vaccines have already been administered globally, at the time of writing.
However, we described the outcomes of a global cohort of 74,908 pregnant women and 5082 lactating women who received COVID-19 vaccination, and this can be considered an adequate sample to rule out any adverse effect in them and their infants.
Scientific societies agree in declaring these vaccines safe during pregnancy and breastfeeding.
The American College of Obstetricians and Gynecologists (ACOG) recommends that individuals over the age of 12, including pregnant and lactating women, receive a COVID-19 vaccine or a series of vaccines [65]. The European Board and College of Obstetrics and Gynecology (EBCOG) also said that immunization should be offered to all pregnant women, after they have been adequately informed of the benefits, especially in pregnant women at high risk for the presence of comorbidities (obesity, diabetes, heart disease, lung disease). Furthermore, the EBCOG recommends immunization against COVID-19 for all breastfeeding women, in the absence of a particular contraindication [6].
Considering the still limited evidence of vaccine safety during the first trimester of pregnancy, the Italian National Institute of Health and the Italian Obstetric Surveillance System recently updated guidelines for COVID-19 vaccinations during pregnancy; they cautiously stated that pregnant women who desire to be vaccinated should consider benefits and risks, given that fever is one of the possible reactions to the vaccine [66], and that this can cause an increased risk of birth defects [67], more so than the vaccine itself.
5. Conclusions
In this review we summarize the current knowledge about maternal and neonatal outcomes after COVID-19 vaccination, in order to help clinicians be thoroughly informed and fight misinformation. Benefits of COVID-19 vaccination outweigh the risks during pregnancy for both mothers and infants. Indeed, available studies report a good maternal immune response, as well as the transfer of maternal antibodies to confer passive protection against SARS-CoV-2 in newborns following maternal vaccination. The existence of anti-SARS-CoV-2 antibodies in breast milk suggests a possible specific protective effect on the newborn-infant after both maternal infection and vaccination, even if only clinical trials can provide scientific evidence.
It should be noted that the ability to protect oneself is not limited to the presence of circulating antibodies; next to these there are memory cells, which play a fundamental role in the response against SARS-CoV-2 in the event of virus exposure. Therefore, the potentiality of the cellular component (primarily lymphocytes) of breast milk to react to SARS-CoV-2 must be investigated.
Author Contributions
Conceptualization, D.U.D.R. and C.A.; methodology, D.U.D.R. and C.A.; software, D.U.D.R.; formal analysis, D.U.D.R. and C.A.; data curation, D.U.D.R. and C.A.; writing—original draft preparation, D.U.D.R.; writing—review and editing, G.S., A.D. and C.A.; supervision, G.S., A.D. and C.A. All authors read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors sincerely thank Roberto Salvatori (Johns Hopkins Hospital, Baltimore, US) for the linguistic revision of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jamieson, D.J.; Rasmussen, S.A. An update on COVID-19 and pregnancy. Am. J. Obstet. Gynecol. 2021, 226, 177–186. [Google Scholar] [CrossRef]
- Arthurs, A.L.; Jankovic-Karasoulos, T.; Roberts, C.T. COVID-19 in pregnancy: What we know from the first year of the pandemic. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166248. [Google Scholar] [CrossRef]
- Ellington, S.; Strid, P.; Tong, V.T.; Woodworth, K.; Galang, R.R.; Zambrano, L.D.; Nahabedian, J.; Anderson, K.; Gilboa, S.M. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status-United States, 22 January–7 June 2020. MMWR Morb. Mortal. Wkly Rep. 2020, 69, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Is the immunization of pregnant women against covid-19 justified? Vaccines 2021, 9, 970. [Google Scholar] [CrossRef] [PubMed]
- Shook, L.L.; Fallah, P.N.; Silberman, J.N.; Edlow, A.G. COVID-19 Vaccination in Pregnancy and Lactation: Current Research and Gaps in Understanding. Front. Cell. Infect. Microbiol. 2021, 11, 899. [Google Scholar] [CrossRef]
- Martins, I.; Louwen, F.; Ayres-de-Campos, D.; Mahmood, T. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 262, 256–258. [Google Scholar] [CrossRef]
- Schaler, L.; Wingfield, M. COVID-19 vaccine—Can it affect fertility? Ir. J. Med. Sci. 2021, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Beharier, O.; Plitman Mayo, R.; Raz, T.; Nahum Sacks, K.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin. Investig. 2021, 131, e150319. [Google Scholar] [CrossRef]
- Blakeway, H.; Prasad, S.; Kalafat, E.; Heath, P.T.; Ladhani, S.N.; le Doare, K.; Magee, L.A.; O’Brien, P.; Rezvani, A.; von Dadelszen, P.; et al. COVID-19 vaccination during pregnancy: Coverage and safety. Am. J. Obstet. Gynecol. 2021, 226, 236.e1–236.e14. [Google Scholar] [CrossRef]
- Bleicher, I.; Kadour-Peero, E.; Sagi-Dain, L.; Sagi, S. Early exploration of COVID-19 vaccination safety and effectiveness during pregnancy: Interim descriptive data from a prospective observational study. Vaccine 2021, 39, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Bookstein Peretz, S.; Regev, N.; Novick, L.; Nachshol, M.; Goffer, E.; Ben-David, A.; Asraf, K.; Doolman, R.; Levin, E.G.; Regev Yochay, G.; et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet. Gynecol. 2021, 58, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Chemaitelly, H.; al Khal, A.; Coyle, P.V.; Saleh, H.; Kaleeckal, A.; Latif, A.N.; Bertollini, R.; Abou-Samra, A.B.; Abu-Raddad, L.J. SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J. Clin. Investig. 2021, 131, e153662. [Google Scholar] [CrossRef] [PubMed]
- Cassaniti, I.; Percivalle, E.; Zelini, P.; Nanhorngue, K.N.; Parolo, A.; Bernardi, V.; Jorizzo, G.; Santer, P.; Perotti, F.; Spinillo, A.; et al. Both SARS-CoV-2 infection and vaccination in pregnancy elicited neutralizing antibodies in pregnant women and newborns. Clin. Microbiol. Infect. 2021, 27, 1708–1709. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Biron-Shental, T.; Makov-Assif, M.; Key, C.; Kohane, I.S.; Hernán, M.A.; Lipsitch, M.; Hernandez-Diaz, S.; Reis, B.Y.; et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 2021, 27, 1693–1695. [Google Scholar] [CrossRef]
- Douxfils, J.; Gillot, C.; de Gottal, É.; Vandervinne, S.; Bayart, J.L.; Dogné, J.M.; Favresse, J. Efficient Maternal to Neonate Transfer of Neutralizing Antibodies after SARS-CoV-2 Vaccination with BNT162b2: A Case-Report and Discussion of the Literature. Vaccines 2021, 9, 907. [Google Scholar] [CrossRef]
- Gill, L.; Jones, C.W. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibodies in Neonatal Cord Blood After Vaccination in Pregnancy. Obstet. Gynecol. 2021, 137, 894–896. [Google Scholar] [CrossRef]
- Gloeckner, S.; Hornung, F.; Heimann, Y.; Schleussner, E.; Deinhardt-Emmer, S.; Loeffler, B.; Zoellkau, J. Newborns’ passive humoral SARS-CoV-2 immunity following heterologous vaccination of the mother during pregnancy. Am. J. Obstet. Gynecol. 2021, 226, 261–262. [Google Scholar] [CrossRef]
- Goldshtein, I.; Nevo, D.; Steinberg, D.M.; Rotem, R.S.; Gorfine, M.; Chodick, G.; Segal, Y. Association Between BNT162b2 Vaccination and Incidence of SARS-CoV-2 Infection in Pregnant Women. JAMA 2021, 326, 728–735. [Google Scholar] [CrossRef]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.R.; Racherla, S.; Tirumala, R.; Madathala, R.R.; Gajula, V. Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: A cross-sectional study on healthcare workers with detailed self-reported symptoms. Am. J. Obstet. Gynecol. 2021, 225, 458–460. [Google Scholar] [CrossRef]
- Kashani-Ligumsky, L.; Lopian, M.; Cohen, R.; Senderovich, H.; Czeiger, S.; Halperin, A.; Chaim, A.B.; Kremer, I.; Lessing, J.B.; Somekh, E.; et al. Titers of SARS-CoV-2 antibodies in cord blood of neonates whose mothers contracted SARS CoV-2 (COVID-19) during pregnancy and in those whose mothers were vaccinated with mRNA to SARS CoV-2 during pregnancy. J. Perinatol. 2021, 41, 2621–2624. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, E.O.; Haapala, J.; DeSilva, M.; Vazquez-Benitez, G.; Vesco, K.K.; Naleway, A.L.; Lipkind, H.S. Spontaneous Abortion Following COVID-19 Vaccination During Pregnancy. JAMA 2021, 326, 1629–1631. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Gjessing, H.K.; Eide, H.N.; Wilcox, A.J.; Fell, D.B.; Håberg, S.E. Covid-19 Vaccination during Pregnancy and First-Trimester Miscarriage. N. Engl. J. Med. 2021, 385, 2008–2010. [Google Scholar] [CrossRef] [PubMed]
- Mangat, C.; Milosavljevic, N. BNT162b2 Vaccination during Pregnancy Protects Both the Mother and Infant: Anti-SARS-CoV-2 S Antibodies Persistently Positive in an Infant at 6 Months of Age. Case Rep. Pediatr. 2021, 2021, 6901131. [Google Scholar] [CrossRef]
- Mehaffey, J.H.; Arnold, M.; Huffstetler, E.; Mehaffey, R.L.; Quillian, H.; Mehaffey, J.H. Successful vertical transmission of SARS-CoV-2 antibodies after maternal vaccination. Birth 2021, 48, 451–452. [Google Scholar] [CrossRef]
- Mithal, L.B.; Otero, S.; Shanes, E.D.; Goldstein, J.A.; Miller, E.S. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am. J. Obstet. Gynecol. 2021, 225, 192–194. [Google Scholar] [CrossRef]
- Morgan, J.A.; Biggio, J.R.; Martin, J.K.; Mussarat, N.; Chawla, H.K.; Puri, P.; Williams, F.B. Maternal Outcomes After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Vaccinated Compared With Unvaccinated Pregnant Patients. Obstet. Gynecol. 2022, 139, 107–109. [Google Scholar] [CrossRef]
- Nir, O.; Schwartz, A.; Toussia-Cohen, S.; Leibovitch, L.; Strauss, T.; Asraf, K.; Doolman, R.; Sharabi, S.; Cohen, C.; Lustig, Y.; et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am. J. Obstet. Gynecol. MFM 2021, 4, 100492. [Google Scholar] [CrossRef]
- Paul, G.; Chad, R. Newborn antibodies to SARS-CoV-2 detected in cord blood after maternal vaccination—A case report. BMC Pediatr. 2021, 21, 138. [Google Scholar] [CrossRef]
- Prabhu, M.; Murphy, E.A.; Sukhu, A.C.; Yee, J.; Singh, S.; Eng, D.; Zhao, Z.; Riley, L.E.; Yang, Y.J. Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage Into Cord Blood. Obstet. Gynecol. 2021, 138, 278–280. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Zarbiv, G.; Oiknine-Djian, E.; Zigron, R.; Wolf, D.G.; Porat, S. Efficient maternofetal transplacental transfer of anti-SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin. Infect. Dis. 2021, 73, 1909–1912. [Google Scholar] [CrossRef] [PubMed]
- Rottenstreich, M.; Sela, H.Y.; Rotem, R.; Kadish, E.; Wiener-Well, Y.; Grisaru-Granovsky, S. Covid-19 vaccination during the third trimester of pregnancy: Rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG 2022, 129, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Shanes, E.D.; Otero, S.; Mithal, L.B.; Mupanomunda, C.A.; Miller, E.S.; Goldstein, J.A. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccination in Pregnancy: Measures of Immunity and Placental Histopathology. Obstet. Gynecol. 2021, 138, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef]
- Trostle, M.E.; Aguero-Rosenfeld, M.E.; Roman, A.S.; Lighter, J.L. High antibody levels in cord blood from pregnant women vaccinated against COVID-19. Am. J. Obstet. Gynecol. MFM 2021, 3, 100481. [Google Scholar] [CrossRef]
- Wainstock, T.; Yoles, I.; Sergienko, R.; Sheiner, E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine 2021, 39, 6037–6040. [Google Scholar] [CrossRef]
- Zauche, L.H.; Wallace, B.; Smoots, A.N.; Olson, C.K.; Oduyebo, T.; Kim, S.Y.; Petersen, E.E.; Ju, J.; Beauregard, J.; Wilcox, A.J.; et al. Receipt of mRNA Covid-19 Vaccines and Risk of Spontaneous Abortion. N. Engl. J. Med. 2021, 385, 1533–1535. [Google Scholar] [CrossRef]
- Zdanowski, W.; Waśniewski, T. Evaluation of SARS-CoV-2 Spike Protein Antibody Titers in cord blood after COVID-19 vaccination during pregnancy in Polish healthcare workers: Preliminary results. Vaccines 2021, 9, 675. [Google Scholar] [CrossRef]
- Collier, A.R.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Apraku Bondzie, E.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370–2380. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef]
- Baird, J.K.; Jensen, S.M.; Urba, W.J.; Fox, B.A.; Baird, J.R. SARS-CoV-2 Antibodies Detected in Mother’s Milk Post-Vaccination. J. Hum. Lact. 2021, 37, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, K.; Honerkamp-Smith, G.; Chambers, C.D. Maternal and Child Outcomes Reported by Breastfeeding Women following Messenger RNA COVID-19 Vaccination. Breastfeed. Med. 2021, 16, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Charepe, N.; Gonçalves, J.; Juliano, A.M.; Lopes, D.G.; Canhão, H.; Soares, H.; Serrano, E.F. COVID-19 mRNA vaccine and antibody response in lactating women: A prospective cohort study. BMC Pregnancy Childbirth 2021, 21, 632. [Google Scholar] [CrossRef] [PubMed]
- Golan, Y.; Prahl, M.; Cassidy, A.; Lin, C.Y.; Ahituv, N.; Flaherman, V.J.; Gaw, S.L. Evaluation of Messenger RNA From COVID-19 BTN162b2 and mRNA-1273 Vaccines in Human Milk. JAMA Pediatr 2021, 175, 1069–1071. [Google Scholar] [CrossRef]
- Guida, M.; Terracciano, D.; Cennamo, M.; Aiello, F.; la Civita, E.; Esposito, G.; Gargiulo, V.; Maruotti, G.M.; Portella, G.; Sarno, L. COVID-19 Vaccine mRNABNT162b2 Elicits Human Antibody Response in Milk of Breastfeeding Women. Vaccines 2021, 9, 785. [Google Scholar] [CrossRef]
- Jakuszko, K.; Kościelska-Kasprzak, K.; Żabińska, M.; Bartoszek, D.; Poznański, P.; Rukasz, D.; Rukasz, D.; Kłak, R.; Królak-Olejnik, B.; Krajewska, M. Immune response to vaccination against covid-19 in breastfeeding health workers. Vaccines 2021, 9, 663. [Google Scholar] [CrossRef]
- Juncker, H.G.; Mulleners, S.J.; van Gils, M.J.; de Groot, C.J.M.; Pajkrt, D.; Korosi, A.; van Goudoever, J.B.; van Keulen, B.J. The Levels of SARS-CoV-2 Specific Antibodies in Human Milk Following Vaccination. J. Hum. Lact. 2021, 37, 477–484. [Google Scholar] [CrossRef]
- Kelly, J.C.; Carter, E.B.; Raghuraman, N.; Nolan, L.S.; Gong, Q.; Lewis, A.N.; Good, M. Anti-severe acute respiratory syndrome coronavirus 2 antibodies induced in breast milk after Pfizer-BioNTech/BNT162b2 vaccination. Am. J. Obstet. Gynecol. 2021, 225, 101–103. [Google Scholar] [CrossRef]
- Lechosa-Muñiz, C.; Paz-Zulueta, M.; Mendez-Legaza, J.M.; Irure-Ventura, J.; Cuesta González, R.; Calvo Montes, J.; López-Hoyos, M.; Llorca, J.; Cabero-Pérez, M.J. Induction of SARS-CoV-2-Specific IgG and IgA in Serum and Milk with Different SARS-CoV-2 Vaccines in Breastfeeding Women: A Cross-Sectional Study in Northern Spain. Int. J. Environ. Res. Public Health 2021, 18, 8831. [Google Scholar] [CrossRef]
- Low, J.M.; Gu, Y.; Ng, M.S.F.; Amin, Z.; Lee, L.Y.; Ng, Y.P.M.; Shunmuganathan, B.D.; Niu, Y.; Gupta, R.; Tambyah, P.A.; et al. Codominant IgG and IgA expression with minimal vaccine mRNA in milk of BNT162b2 vaccinees. NPJ Vaccines 2021, 6, 105. [Google Scholar] [CrossRef]
- McLaurin-Jiang, S.; Garner, C.D.; Krutsch, K.; Hale, T.W. Maternal and Child Symptoms following COVID-19 Vaccination among Breastfeeding Mothers. Breastfeed. Med. 2021, 16, 702–709. [Google Scholar] [CrossRef]
- Perl, S.H.; Uzan-Yulzari, A.; Klainer, H.; Asiskovich, L.; Youngster, M.; Rinott, E.; Youngster, I. SARS-CoV-2-Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA 2021, 325, 2013–2014. [Google Scholar] [CrossRef] [PubMed]
- Piano Mortari, E.; Russo, C.; Vinci, M.R.; Terreri, S.; Fernandez Salinas, A.; Piccioni, L.; Alteri, C.; Colagrossi, L.; Coltella, L.; Ranno, S.; et al. Highly Specific Memory B Cells Generation after the 2nd Dose of BNT162b2 Vaccine Compensate for the Decline of Serum Antibodies and Absence of Mucosal IgA. Cells 2021, 10, 2541. [Google Scholar] [CrossRef] [PubMed]
- Romero Ramirez, D.S.; Lara Pérez, M.M.; Carretero Pérez, M.; Suarez Hernandez, M.I.; Martin Pulido, S.; Pera Villacampa, L.; Fernández Vilar, A.M.; Rivero Falero, M.; González Carretero, P.; Reyes Millán, B.; et al. SARS-CoV-2 Antibodies in Breast Milk After Vaccination. Pediatrics 2021, 148, e2021052286. [Google Scholar] [CrossRef] [PubMed]
- Golan, Y.; Prahl, M.; Cassidy, A.G.; Gay, C.; Wu, A.H.B.; Jigmeddagva, U.; Lin, C.Y.; Gonzalez, V.J.; Basilio, E.; Chidboy, M.A.; et al. COVID-19 mRNA Vaccination in Lactation: Assessment of Adverse Events and Vaccine Related Antibodies in Mother-Infant Dyads. Front. Immunol. 2021, 12, 777103. [Google Scholar] [CrossRef] [PubMed]
- Mackin, D.W.; Walker, S.P. The historical aspects of vaccination in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Shamshirsaz, A.A.; Hessami, K.; Morain, S.; Afshar, Y.; Nassr, A.A.; Arian, S.E.; Asl, N.M.; Aagaard, K. Intention to Receive COVID-19 Vaccine during Pregnancy: A Systematic Review and Meta-analysis. Am. J. Perinatol. 2021. [Google Scholar] [CrossRef]
- Ciapponi, A.; Bardach, A.; Comandé, D.; Berrueta, M.; Argento, F.J.; Rodriguez Cairoli, F.; Zamora, N.; Santa María, V.; Xiong, X.; Zaraa, S.; et al. COVID-19 and pregnancy: An umbrella review of clinical presentation, vertical transmission, and maternal and perinatal outcomes. PLoS ONE 2021, 16, e0253974. [Google Scholar] [CrossRef]
- Kazemi, S.N.; Hajikhani, B.; Didar, H.; Hosseini, S.S.; Haddadi, S.; Khalili, F.; Mirsaeidi, M.; Nasiri, M.J. COVID-19 and cause of pregnancy loss during the pandemic: A systematic review. PLoS ONE 2021, 16, e0255994. [Google Scholar] [CrossRef]
- Olivini, N.; Calò Carducci, F.I.; Santilli, V.; de Ioris, M.A.; Scarselli, A.; Alario, D.; Geremia, C.; Lombardi, M.H.; Marabotto, C.; Mariani, R.; et al. A neonatal cluster of novel coronavirus disease 2019: Clinical management and considerations. Ital. J. Pediatr. 2020, 46, 180. [Google Scholar] [CrossRef]
- Garazzino, S.; Montagnani, C.; Donà, D.; Meini, A.; Felici, E.; Vergine, G.; Bernardi, S.; Giacchero, R.; Lo Vecchio, A.; Marchisio, P.; et al. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Eurosurveillance 2020, 25, 2000600. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Hudak, M.; Dimitriades, V.; Higgins, R. Multisystem Inflammatory Syndrome (MIS-C) in Neonates (MIS-N) Following Maternal SARS CoV-2 COVID-19 Infection. Am. J. Perinatol. 2021. [Google Scholar] [CrossRef]
- Lumbreras-Marquez, M.I.; Fields, K.G.; Campos-Zamora, M.; Rodriguez-Bosch, M.R.; Rodriguez-Sibaja, M.J.; Copado-Mendoza, D.Y.; Acevedo-Gallegos, S.; Farber, M.K. A forecast of maternal deaths with and without vaccination of pregnant women against COVID-19 in Mexico. Int. J. Gynaecol. Obstet. 2021, 154, 566–567. [Google Scholar] [CrossRef]
- COVID-19 Vaccines International Pregnancy Exposure Registry (C-VIPER)—ClinicalTrials.gov—Identifier: NCT04705116. 2021. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04705116 (accessed on 22 November 2021).
- The American College of Obstetricians and Gynecologists (ACOG). COVID-19 Vaccination Considerations for Obstetric-Gynecologic Care. 2021. Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care (accessed on 22 November 2021).
- Italian National Institute of Health and Italian Obstetric Surveillance System, Rome (Italy). Ad Interim Indications on “COVID-19 Vaccination during Pregnancy and Breastfeeding” (Update of 2021, September 22). Available online: https://www.epicentro.iss.it/en/vaccines/covid-19-pregnancy-breastfeeding (accessed on 22 November 2021).
- Graham, J.M., Jr. Update on the gestational effects of maternal hyperthermia. Birth Defects Res. 2020, 112, 943–952. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).